Key Points
Question
Among patients with cancer and a venous thromboembolism (VTE) event, are direct oral anticoagulants noninferior to low-molecular-weight heparin for preventing recurrent VTE events?
Findings
In this pragmatic, noninferiority randomized clinical trial that included 638 patients from 67 centers with cancer and a new VTE, direct oral anticoagulants, compared with low-molecular-weight heparin, resulted in a recurrent VTE rate of 6.1% vs 8.8% at 6 months. The upper confidence limit around the difference was less than the noninferiority margin of 3%.
Meaning
Among adults with cancer and VTE, direct oral anticoagulants were noninferior to low-molecular-weight heparin for preventing recurrent VTE over 6-month follow-up.
Abstract
Importance
In patients with cancer who have venous thromboembolism (VTE) events, long-term anticoagulation with low-molecular-weight heparin (LMWH) is recommended to prevent recurrent VTE. The effectiveness of a direct oral anticoagulant (DOAC) compared with LMWH for preventing recurrent VTE in patients with cancer is uncertain.
Objective
To evaluate DOACs, compared with LMWH, for preventing recurrent VTE and for rates of bleeding in patients with cancer following an initial VTE event.
Design, Setting, and Participants
Unblinded, comparative effectiveness, noninferiority randomized clinical trial conducted at 67 oncology practices in the US that enrolled 671 patients with cancer (any invasive solid tumor, lymphoma, multiple myeloma, or chronic lymphocytic leukemia) who had a new clinical or radiological diagnosis of VTE. Enrollment occurred from December 2016 to April 2020. Final follow-up was in November 2020.
Intervention
Participants were randomized in a 1:1 ratio to either a DOAC (n = 335) or LMWH (n = 336) and were followed up for 6 months or until death. Physicians and patients selected any DOAC or any LMWH (or fondaparinux) and physicians selected drug doses.
Main Outcomes and Measures
The primary outcome was the recurrent VTE rate at 6 months. Noninferiority of anticoagulation with a DOAC vs LMWH was defined by the upper limit of the 1-sided 95% CI for the difference of a DOAC relative to LMWH of less than 3% in the randomized cohort that received at least 1 dose of assigned treatment. The 6 prespecified secondary outcomes included major bleeding, which was assessed using a 2.5% noninferiority margin.
Results
Between December 2016 and April 2020, 671 participants were randomized and 638 (95%) completed the trial (median age, 64 years; 353 women [55%]). Among those randomized to a DOAC, 330 received at least 1 dose. Among those randomized to LMWH, 308 received at least 1 dose. Rates of recurrent VTE were 6.1% in the DOAC group and 8.8% in the LMWH group (difference, −2.7%; 1-sided 95% CI, −100% to 0.7%) consistent with the prespecified noninferiority criterion. Of 6 prespecified secondary outcomes, none were statistically significant. Major bleeding occurred in 5.2% of participants in the DOAC group and 5.6% in the LMWH group (difference, −0.4%; 1-sided 95% CI, –100% to 2.5%) and did not meet the noninferiority criterion. Severe adverse events occurred in 33.8% of participants in the DOAC group and 35.1% in the LMWH group. The most common serious adverse events were anemia and death.
Conclusions and Relevance
Among adults with cancer and VTE, DOACs were noninferior to LMWH for preventing recurrent VTE over 6-month follow-up. These findings support use of a DOAC to prevent recurrent VTE in patients with cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT02744092
This randomized trial assesses the comparative effectiveness of direct oral anticoagulants vs low-molecular-weight heparin on rate of 6-month recurrence of venous thromboembolism (VTE) among patients with cancer who had a new diagnosis of VTE.
Introduction
Patients with cancer have up to a 7-fold excess risk of venous thromboembolism (VTE).1 Long-term anticoagulation is necessary to treat VTE and prevent recurrence.2,3,4 Practice guidelines recommend that patients with cancer and VTE receive treatment with low-molecular-weight heparin (LMWH) based on efficacy studies demonstrating superiority compared with warfarin.5 However, LMWH requires subcutaneous administration, which can be burdensome. Between 2012 and 2015, 4 direct oral anticoagulants (DOACs), dabigatran, rivaroxaban, apixaban, and edoxaban, were approved by the US Food and Drug Administration for treatment of VTE based on randomized clinical trials that demonstrated similar efficacy and safety compared with warfarin in patients with atrial fibrillation or patients undergoing orthopedic surgery.6,7,8,9 However, these trials either excluded patients with cancer or included few people with cancer. Therefore, the effectiveness and safety of DOACs for patients with cancer and VTE remain unclear.5
The CANVAS trial (Cancer Related VTE Anticoagulation Strategies) compared effectiveness of 2 alternative anticoagulant strategies, DOACs vs LMWH, for treating VTE in patients with cancer. In contrast to clinical trials that compared specific DOACs with specific LMWHs in people with cancer,10,11,12,13 this study used a pragmatic design in which patients and physicians selected treatment either from among DOACs or from among LMWH or fondaparinaux. A noninferiority design was selected because DOACs are more convenient compared with subcutaneously administered LMWH.
Methods
Trial Design
The protocol was approved by a centralized institutional review board (Advarra) or by site institutional review boards and was conducted at 67 US member centers of the Alliance for Clinical Trials in Oncology research network. All patients provided written informed consent. The trial protocol and statistical analysis plan are available in Supplement 1. This unblinded pragmatic effectiveness study included a randomized cohort and a preference cohort. The preference cohort consisted of patients who declined randomization but agreed to be followed up longitudinally for the outcomes collected in the randomized clinical trial. The preference cohort enrolled participants until 1 of the following criteria was met: (1) more than 190 participants chose the preference cohort or (2) more than 80% of participants in the preference cohort chose participation in either group. Enrollment for the preference cohort ended in December 2017, when more than 80% of study participants chose DOAC therapy.
Trial Oversight
An independent, unmasked data monitoring committee and Alliance Foundation Trials LLC research staff monitored trial data. Data were collected using standardized case-report forms completed by trained research staff using REDCap software and from participants’ electronic health records. Treating physicians reported VTE events, bleeding, deaths, and serious adverse events prospectively. These events were centrally reviewed and adjudicated using electronic health records.
Eligibility Criteria
Adults aged 18 years or older with solid tumors, lymphoma, chronic lymphocytic leukemia, or multiple myeloma were eligible if they had advanced disease or were diagnosed within the past 12 months. Patients with either symptomatic or asymptomatic VTE detected on imaging studies within 30 days prior to enrollment were eligible regardless of the type of anticoagulant initiated prior to enrollment. Additional eligibility criteria were a platelet count of 50 000/μL or greater and a creatinine clearance of 15 mL/min/1.75 m2 or greater within 7 days of enrollment. Exclusion criteria included acute leukemia, recent or planned stem cell transplant, ongoing clinically significant bleeding, pregnancy or breastfeeding, use of medications that interfered with DOAC metabolism, life expectancy of less than 3 months, or receipt of therapeutic anticoagulation at the time the new VTE was diagnosed. Race and ethnicity data were collected using self-report and categorized into fixed categories because race and ethnicity are associated with differential outcomes in people with cancer. Comorbid illness and smoking history were collected by self-report. Body mass index was ascertained from electronic health records.
Randomization
Participants were randomized 1:1 to either a DOAC or LMWH with a computer-generated random sequence and an interactive web-based system using a permuted block design with varying block sizes (2, 4, and 6). Treating physicians were blinded to group assignment.
Study Intervention and Trial Procedures
Within 14 days of randomization, physicians selected and prescribed any DOACs or LMWH based on availability, formulary preference, insurance coverage, drug metabolism, or drug-drug interactions. Participants obtained treatment from retail or hospital-affiliated pharmacies. The LMWH group could receive either LMWH or fondaparinux. Decisions about drug doses and temporary discontinuation of anticoagulation were made by the treating physicians (Supplement 1). Participants randomized to LMWH who opted to discontinue treatment due to dissatisfaction with injections were offered warfarin therapy titrated to attain an international normalized ratio of 2 to 3. Participants completed questionnaires electronically or by telephone at baseline, 3 months, and 6 months.
Primary Outcome
The primary study outcome was the cumulative incidence of recurrent nonfatal VTE at 6-month follow-up. Data on recurrent VTE were ascertained by treating clinicians based on imaging studies revealing a new thrombus and adjudicated by investigators without knowledge of assigned treatment.
Secondary Outcomes
The 6 secondary outcomes were (1) cumulative incidence of major bleeding (grade ≥3) at 6 months using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 5.0; (2) clinically significant bleeding (grade 2); (3) minor bleeding (grade 1); (4) cumulative incidence of death and restricted mean survival time at 6 months; (5) health-related quality of life, measured using the 12-Item Short Form Health Survey (SF-12; score range, 0-100; minimum clinically important difference [MCID], 214,15); and (6) participants’ perceptions of the burdens and benefits of anticoagulation treatment on the Anti-Clot Treatment Scale at 6-month follow-up. The Anti-Clot Treatment Scale measures perceptions of anticoagulation treatment (burden score range, 12-60, with 60 being best; MCID, 6.7; benefits score range, 3-15, with 15 being best; MCID corresponding to differences greater than 2.5 or 1 SD16). Bleeding outcomes were adjudicated based on medical record data by investigators unaware of assigned treatment.
Additional Outcomes
Other prespecified outcomes were (1) serious adverse event rates; (2) severe serious adverse event rates (categorized as grade >3 on the CTCAE); (3) adherence to assigned anticoagulant therapy; and (4) adherence to any anticoagulant therapy based on patient self-report and prescriptions.
Sample Size Calculation
The original power calculation determined that 750 patients were needed for the primary outcome population to provide 93% power for noninferiority and 80% for superiority testing, at a 1-sided α = .05, assuming that the recurrent VTE event rates at 6 months for DOACs and LMWH were 5.0% and 7.5%, respectively. The COVID-19 pandemic impaired enrollment and the protocol was amended in May 2020 to reduce the sample size from 750 to 670, which provided 89% power for noninferiority and 74% for superiority. In April 2020, the data and safety monitoring board made the decision to stop randomization due to the COVID-19 pandemic and because a clinical trial was published demonstrating noninferiority for apixaban compared with dalteparin10 without differences in major bleeding, resulting in loss of equipoise. The prespecified noninferiority margin was 3% for the cumulative event rate at 6 months, selected based on patient responses to focus groups combined with input from 18 oncologists attending the May 2016 meeting of the Alliance for Clinical Trials in Oncology. A 3.0% noninferiority margin was regarded as clinically meaningful to support substituting DOACs for LMWH for treatment of VTE for patients with cancer.
Statistical Analysis
Analysis Population
The primary analysis population consisted of all randomized participants who initiated assigned treatment (the as-treated population). Analyses were repeated among all randomized participants regardless of therapy received. Because participants who did not take their assigned treatment could bias results toward noninferiority, primary analyses were performed in the as-treated population.
Primary Outcome
A 1-sided 95% CI for the difference (DOACs vs LMWH) in cumulative incidence rate of recurrent VTE at 6 months was constructed based on the nonparametric estimation of the cumulative incidence function, where deaths were handled as a competing risk of recurrent VTE. If the upper bound of the 1-sided 95% CI was less than 3.0%, noninferiority was concluded. If the upper bound of the 1-sided 95% CI was less than 0%, superiority was concluded. Participants with missing data at 6 months were handled as censored observations using the last date of available follow-up. Because fatal VTE events were clinically indistinguishable from deaths due to cancer progression, and autopsies were not performed, the primary end point included only nonfatal VTEs.
Secondary Outcomes: Deaths and Bleeding Events
Time from randomization to all-cause death was calculated using Kaplan-Meier analysis, with between-group difference in all-cause mortality at 6 months constructed with a corresponding 1-sided 95% CI. Restricted mean survival times between groups were compared.17 The truncation time point for calculating the restricted mean survival time was 6 months and the difference and corresponding 1-sided 95% CI were estimated.18 These methods were prespecified as the primary analyses for mortality and do not require the proportional hazards assumption. Major bleeding was analyzed using methods used for the primary end point. The noninferiority margin for major bleeding was derived from the hazard ratio criterion used for this end point in the Hokusai-VTE Study.6 The hazard ratio of 1.5 was translated into the absolute difference in the assumed cumulative event rate for major bleeding of 2.5%. If the upper bound of the 1-sided 95% CI for the difference in the cumulative incidence probability at 6 months was less than 2.5%, DOACs were considered noninferior to LMWH for major bleeding. Cumulative event rates for clinically significant nonmajor and minor bleeding at 6 months were calculated for each group, between groups, and for the corresponding 1-sided 95% CIs. Because of the potential for type I error due to multiple comparisons, findings for secondary end points should be interpreted as exploratory.
Patient-Reported Secondary Outcomes
Differences in the SF-12 score from baseline to month 6 were compared between the 2 groups.15,19,20 Patients’ caregivers were allowed to complete patient-reported outcome measures when a patient was unable. Multiple imputation was used for missing observations.21 Ten complete data sets were created and missing values were imputed using chained equations and including measurements at baseline, 3 months, and 6 months. Mean changes and standard errors were estimated for each data set. The Rubin method was used to integrate the results from 10 complete sets and derive an estimate for the difference in the mean change score between groups.22 Using the resulting estimate and the standard error, a z test was used to compare the mean scores between groups.
Adverse Events and Other Prespecified Outcomes
Treatment-emergent serious adverse events were defined as severe if they were grade 3 or greater. Adherence to study treatment was determined using electronic health records.
Sensitivity Analysis
Primary end-point analyses were repeated for the following prespecified subgroups (Supplement 1): (1) highly thrombogenic tumors (pancreatic cancer, gastroesophageal cancer, ovarian cancer, and lung cancer); (2) indwelling central venous catheters; and (3) platelet counts of 150/μL or lower. Sensitivity analyses were performed for center effects using generalized linear mixed-effects models with logit link, where the treatment indicator was included as a fixed effect and participating sites were included as random effects (eTable 1 in Supplement 2). Censored observations were handled using an inverse probability censoring weight technique.23 Subgroup analyses specified in the statistical analysis plan were not performed when characteristics could not be measured at baseline, specifically for adherence. All statistical analyses were performed with R version 4.0.3 (R Foundation) as detailed in Supplement 1.
Interim Analyses
This study design incorporated interim analyses for the comparison of the primary end point using a 1-sided 95% repeated CI (RCI) for the difference in the cumulative incidence of VTE at 6 months (DOACs minus LMWH).24 Critical values were based on the Lan-DeMets error spending function corresponding to the truncated version of O’Brien-Fleming boundaries.25,26
Post Hoc Analyses
In post hoc analyses, analyses for the primary outcome, deaths, major bleeding, and minor bleeding were repeated calculating a 2-sided 95% CI for each outcome.
Results
Trial Participants
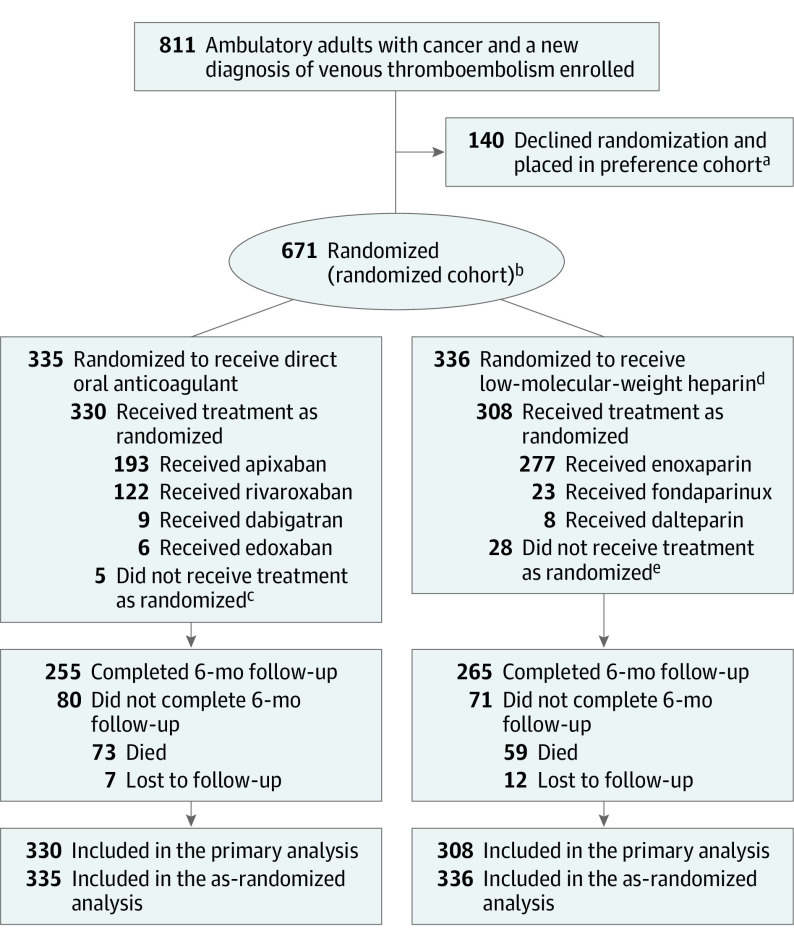
Between December 2016 and April 2020, 811 patients provided written informed consent, including 671 for randomization and 140 for the preference cohort. All participants completed 6-month follow-up. Final follow-up occurred on November 15, 2020. Among the 671 randomized participants, 638 (95.1%) received at least 1 dose of study medication and were included in the primary analysis. Of 335 who were randomized to a DOAC, 330 (98.5%) had at least 1 dose of study drug, and of 336 randomized to LMWH, 308 (91.7%) had at least 1 dose of study drug (Figure 1). Of those randomized to a DOAC, 58.5% received apixaban, 37.0% rivaroxaban, 2.7% dabigatran, and 1.8% edoxaban. Of those randomized to LMWH, 89.9% received enoxaparin, 7.5% fondaparinux, and 2.6% dalteparin.
Figure 1. Participant Flow in a Trial of Anticoagulation for Venous Thromboembolism in Adults With Cancer.
Oncologists in the Alliance for Clinical Trials in Oncology research network screened their ambulatory patients for adults with a new diagnosis of venous thromboembolism and either (1) an advanced solid tumor, lymphoma, multiple myeloma, or chronic lymphocytic leukemia or (2) an early-stage cancer of one of these same types diagnosed within 12 months of enrollment. Screening logs were not mandatory, so the exact number of screened patients was not captured.
aSee eFigure 1 in Supplement 2 for participant flow in the preference cohort.
bPermuted block randomization with varying block sizes was used. No stratification factors were considered at randomization.
cOf those who did not receive their randomized treatment, 2 received enoxaparin, 1 received fondaparinux, and 2 did not receive any treatment.
dPatients randomized to receive low-molecular-weight heparin could transition to warfarin with a target international normalized ratio of 2 to 3.
eOf those who did not receive their randomized treatment, 12 received apixaban, 6 received rivaroxaban, and 10 did not receive any treatment.
Characteristics of participants are shown in Table 1. In the DOAC and LMWH groups, respectively, 34% vs 32% had a highly thrombogenic tumor type; 70% vs 68% had advanced cancer; 58% vs 56% had a pulmonary embolism; and 38% vs 44% had VTE first detected by imaging (Table 1). The disposition of patients in the preference cohort and their characteristics are shown in eFigure 2 and eTable 2 in Supplement 2.
Table 1. Baseline Participant Characteristicsa.
| Characteristics | Direct oral anticoagulants (n = 330) | Low-molecular-weight heparin (n = 308) |
|---|---|---|
| Age, median (IQR), y | 64 (56-70) | 62 (54-68) |
| Aged ≥65 y, No. (%) | 155 (47) | 111 (36) |
| Sex, No. (%) | ||
| Female | 181 (55) | 172 (56) |
| Male | 149 (45) | 136 (44) |
| Ethnicity, No. (%)b | n = 321 | n = 308 |
| Hispanic or Latino | 15 (5) | 15 (5) |
| Not Hispanic or Latino | 306 (95) | 287 (95) |
| Race, No. (%)c | n = 323 | n = 300 |
| American Indian or Alaska Native | 3 (1) | 0 |
| Asian | 4 (1) | 6 (2) |
| Black or African American | 39 (12) | 38 (13) |
| Native Hawaiian or Other Pacific Islander | 0 | 1 (<1) |
| White | 277 (86) | 255 (85) |
| Education level, No. (%) | n = 314 | n = 286 |
| Grade school or less | 14 (4) | 11 (4) |
| High school graduate or GED | 79 (25) | 73 (26) |
| Some vocational, business, or trade school | 27 (9) | 10 (3) |
| Some college | 66 (21) | 69 (24) |
| College graduate and some graduate school without graduate degree | 81 (26) | 68 (24) |
| Graduate or professional degree | 47 (15) | 55 (19) |
| Body mass index, median (IQR)d | 27.8 (24.5-32.2) | 28.0 (24.3-32.6) |
| No. (%) | ||
| <18.5 | 13 (4) | 6 (2) |
| 18.5-24.9 | 83 (25) | 90 (29) |
| 25-29.9 | 107 (32) | 101 (33) |
| ≥30 | 127 (38) | 111 (36) |
| Smoking status, No. (%) | n = 315 | n = 284 |
| Never smoker | 157 (50) | 138 (49) |
| Former smoker | 130 (41) | 131 (46) |
| Current smoker | 28 (9) | 15 (5) |
| Participant-reported ECOG performance status score, No. (%)e | ||
| 0 | 117 (35) | 110 (36) |
| 1 | 155 (47) | 145 (47) |
| ≥2 | 58 (18) | 53 (17) |
| Presence of highly thrombogenic tumor, No. (%)f | 113 (34) | 99 (32) |
| Presence of indwelling central venous catheter, No. (%) | 179 (54) | 177 (57) |
| Platelet count, /μL, No. (%)g | ||
| <100 000 | 41 (12) | 47 (15) |
| 100 000-149 000 | 75 (23) | 55 (18) |
| 150 000-249 000 | 131 (40) | 133 (43) |
| 250 000-449 000 | 80 (24) | 69 (22) |
| ≥450 000 | 3 (1) | 4 (1) |
| Serum albumin, g/dL, No. (%)h | ||
| <3.5 | 109 (33) | 96 (31) |
| ≥3.5 | 221 (67) | 212 (69) |
| Type of cancer, No. (%) | ||
| Gastrointestinal | 115 (35) | 107 (35) |
| Lung | 56 (17) | 59 (19) |
| Breast | 44 (13) | 50 (16) |
| Genitourinary | 30 (9) | 19 (6) |
| Gynecologic | 28 (8) | 18 (6) |
| Lymphoma/multiple myeloma/chronic lymphocytic leukemia | 26 (8) | 24 (8) |
| Head and neck | 11 (3) | 7 (2) |
| Sarcoma | 10 (3) | 13 (4) |
| Skin/melanoma | 6 (2) | 3 (1) |
| Brain | 3 (1) | 4 (1) |
| Neuroendocrine | 1 (<1) | 1 (<1) |
| Unknown primaryi | 0 | 3 (1) |
| Evidence of any metastatic disease, No. (%)j | 231 (70) | 210 (68) |
| Liver | 93 | 84 |
| Lung | 57 | 48 |
| Bone | 51 | 63 |
| Brain | 29 | 27 |
| Other | 91 | 79 |
| Treatment with bevacizumab, No. (%) | 16 (5) | 24 (8) |
| Type of index VTE, No. (%) | ||
| PE with or without DVT | 190 (58) | 174 (56) |
| DVT alone | 134 (41) | 127 (41) |
| Other type | 6 (2) | 7 (2) |
| Mode of presentation of index VTE, No. (%) | ||
| Presentation with symptoms or physical findings | 199 (60) | 167 (54) |
| Presentation detected on imaging | 127 (38) | 137 (44) |
| Uncertain | 4 (1) | 4 (1) |
Abbreviations: GED, General Educational Development test; VTE, venous thromboembolism; PE, pulmonary embolism; DVT, deep vein thrombosis.
This table reports participant characteristics of the primary analysis cohort, which includes all patients who were randomized and began their assigned treatment as randomized. Participant characteristics of the preference cohort are in eTable 1 in Supplement 2.
Ethnicity is based on patient self-report on the baseline survey.
Race is based on patient self-report recorded in electronic health records.
Calculated as weight in kilograms divided by height in meters squared.
The Eastern Cooperative Oncology Group (ECOG) performance status scale assesses the functional status of patients (score range, 0-5; 0 indicates fully active and 5 indicates death).
Highly thrombogenic tumors: pancreas, lung, esophagus, and ovarian.
Reference range: 150 000/μL to 450 000/μL.
Reference range: 3.5 to 5.4 g/dL.
Unknown primary cancers: 2 adenocarcinoma, 1 potential cholangiocarcioma.
Data on locations of metastases are not mutually exclusive.
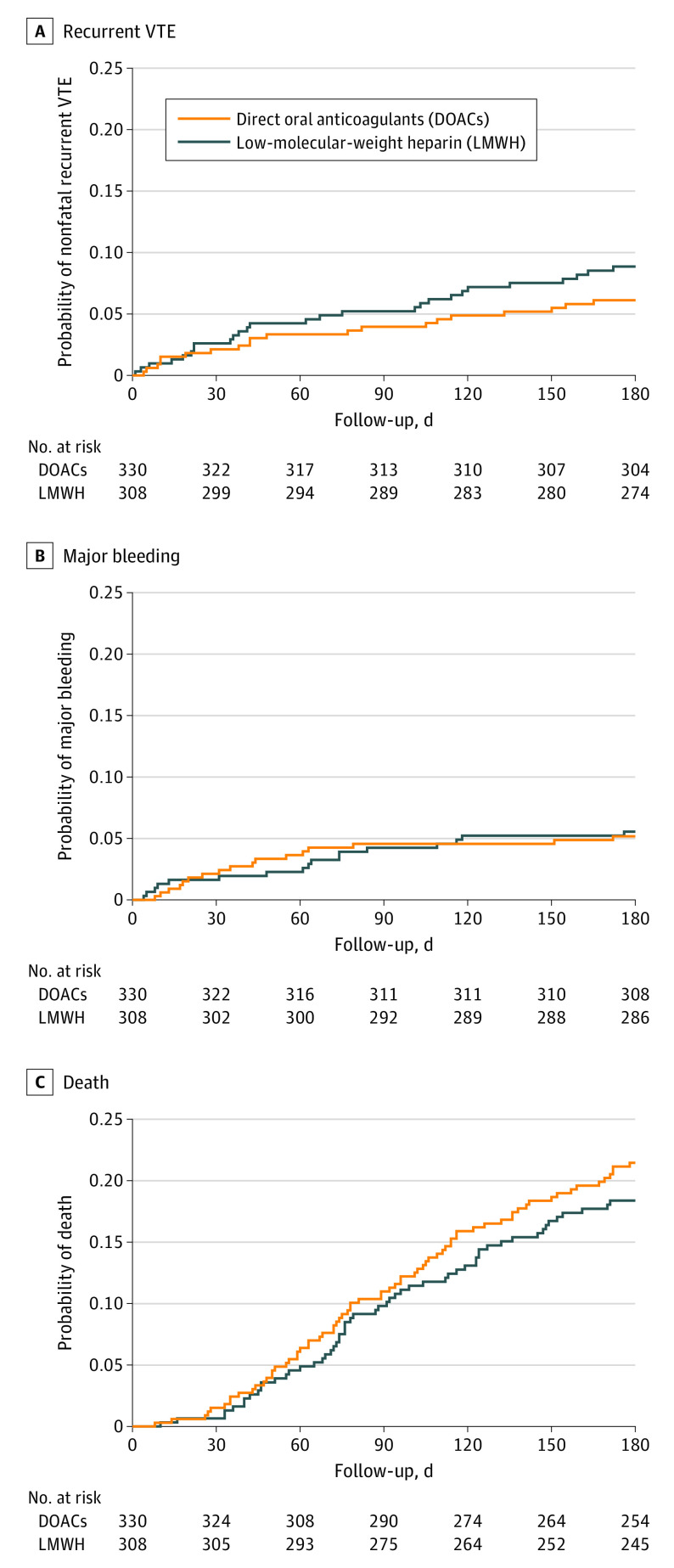
Primary Outcome: Recurrent VTE
At the fourth interim analysis on March 25, 2020, the upper bound of the 1-sided 95% RCI was below the noninferiority threshold of 3.0% but did not support early stopping for superiority. As noninferiority was already demonstrated at the fourth interim analysis, the RCI was not used for the final analysis. At final analysis on February 22, 2021, recurrent VTE had occurred in 6.1% of participants treated with a DOAC and 8.8% of participants treated with LMWH (difference, −2.7%; 1-sided 95% CI, −100% to 0.7%), meeting criterion for noninferiority (Table 2 and Figure 2A). Superiority was not demonstrated for DOACs compared with LMWH according to prespecified criteria. Outcomes for the preference cohort are shown in eTable 3 and propensity score–adjusted models for the preference cohort are shown in eTables 5 and 6 in Supplement 2.
Table 2. Primary and Secondary Clinical Outcomes at 6 Months.
| Outcomesa | Direct oral anticoagulants (n = 330) | Low-molecular-weight heparin (n = 308) | Differenceb | ||
|---|---|---|---|---|---|
| Estimate | 1-Sided 95% CI | 2-Sided 95% CI | |||
| Primary outcome | |||||
| Recurrent nonfatal VTE, %c | 6.1 | 8.8 | −2.7 | −100 to 0.7 | −6.8 to 1.4 |
| Secondary outcomes | |||||
| Death, % | 21.5 | 18.4 | 3.1 | −100 to 8.3 | −3.1 to 9.3 |
| Restricted mean survival time, dd | 161 | 164 | −3 | −8 to 180 | −9 to 4 |
| Bleeding, % | |||||
| Major bleedinge | 5.2 | 5.6 | −0.4 | −100 to 2.5 | −3.9 to 3.1 |
| Clinically relevant nonmajor bleedingf | 5.8 | 2.6 | 3.2 | −100 to 5.8 | 0.1 to 6.3 |
| Minor bleedingg | 8.0 | 11.4 | −3.5 | −100 to 0.4 | −8.1 to 1.1 |
| Other prespecified outcomes | |||||
| Adverse events (safety end point), % | |||||
| Any serious adverse eventsh | 36.0 | 38.7 | −2.7 | −100 to 3.6 | −10.2 to 4.8 |
| Severe serious adverse eventsi | 33.8 | 35.1 | −1.2 | −100 to 5.0 | −8.6 to 6.2 |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; VTE, venous thromboembolism.
Primary analyses were performed for the as-treated population (participants who were randomized and began their assigned treatment as randomized). The cumulative incidence function approach was used to derive event rates (percentages) at 6 months. The outcomes are not binary events but possibly censored time-to-event outcomes, and therefore, absolute numbers of events are not presented.
Differences are unadjusted.
Only VTEs that were nonfatal were considered because of the challenges of attributing cause of death in cancer patients to tumor progression vs VTE. Deaths irrespective of cause were considered as a secondary end point.
A truncation time of 180 days was used for calculation of the restricted mean survival time, which is interpreted as 180-day lifetime expectancy.
Major bleeding was defined as grade ≥3 on the National Cancer Institute’s CTCAE version 5.0 (ie, severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living).
Clinically significant nonmajor bleeding was defined as grade 2 on the CTCAE version 5.0.
Minor bleeding was defined as grade 1 on the CTCAE version 5.0.
Any serious adverse events include VTE events and bleeding events of any grade.
Severe serious adverse events were defined as grade 3 or higher on the CTCAE version 5.0.
Figure 2. Cumulative Incidence Rates of Outcomes.
Cumulative incidence rates are shown for the as-treated population for (A) the first occurrence of the primary efficacy outcome of recurrent nonfatal venous thromboembolism (VTE), (B) the first occurrence of major bleeding, and (C) death. The median lengths of observation time are 180 (IQR, 159-180), 180 (IQR, 173.5-180), and 180 (IQR, 180-180) days, respectively.
Secondary Outcomes
Bleeding
Major bleeding occurred in 5.2% of the DOAC group and 5.6% of the LMWH group (difference, −0.4%; 1-sided 95% CI, −100% to 2.5%), which did not meet the prespecified criterion for noninferiority (Table 2 and Figure 2B). Clinically significant nonmajor bleeding occurred in 5.8% of the DOAC group and 2.6% of the LMWH group (difference, 3.2%; 1-sided 95% CI, −100% to 5.8%). Minor bleeding occurred in 8.0% of the DOAC group and 11.4% of the LMWH group (difference, −3.5%; 1-sided 95% CI, −100% to 0.4%) (Table 2). Noninferiority tests were not planned for nonmajor or minor bleeding events.
Death
At 6 months, death occurred in 21.5% of patients in the DOAC group and 18.4% in the LMWH group (difference, 3.1%; 1-sided 95% CI, −100% to 8.3%). The restricted mean survival times with a truncation time of 180 days were 161 days in the DOAC group and 164 days in the LMWH group (difference, −3 days; 1-sided 95% CI, −8 to 180 days) (Table 2 and Figure 2C).
Patient-Reported Outcomes
Among participants alive at 3-month follow-up, questionnaires were completed by 72% of patients in the DOAC group and by 70% of patients in the LMWH group. Among those alive at 6 months, questionnaires were completed by 73% of patients in the DOAC group and by 70% of patients in the LMWH group.
Health-Related Quality of Life
At 3 months, mean SF-12 scores for physical function increased from 36.6 to 38.4 (+1.8) for participants assigned to DOACs and from 36.8 to 37.5 (+0.7) for those assigned to LMWH (difference, 1.1; 2-sided 95% CI, −0.6 to 2.8). At 6 months, mean physical function scores increased from 36.9 to 39.3 (+2.4) in the DOAC group vs from 37.0 to 37.7 (+0.7) in the LMWH group (difference, 1.7; 2-sided 95% CI, −0.4 to 3.8). Mean mental health scores on the SF-12 changed by less than 1 point in each group (Table 3).
Table 3. Patient-Reported Secondary Outcomes of Health-Related Quality of Life and Treatment Burden and Other Prespecified Outcomes of Adherence to Anticoagulant Therapy.
| Outcomes | Direct oral anticoagulants | Low-molecular-weight heparin | Between-group difference (95% CI)a | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Difference | Baseline | Follow-up | Difference | ||
| Health-related quality of life, mean scoreb | |||||||
| At baseline and 3-mo follow-up | n = 212/295c | n = 197/280c | |||||
| Physical health | 36.6 | 38.4 | 1.8d | 36.8 | 37.5 | 0.7d | 1.1 (−0.6 to 2.8) |
| Mental health | 49.4 | 49.1 | −0.3d | 48.1 | 48.8 | 0.7d | −1.0 (−3.1 to 1.0) |
| At baseline and 6-mo follow-up | n = 191/262c | n = 177/253c | |||||
| Physical health | 36.9 | 39.3 | 2.4d | 37.0 | 37.7 | 0.7d | 1.7 (−0.4 to 3.8) |
| Mental health | 49.6 | 49.9 | 0.3d | 48.1 | 49.0 | 0.9d | −0.6 (−2.6 to 1.4) |
| Anti-Clot Treatment Scale, mean scoree | |||||||
| At 3-mo follow-up | n = 212/295c | n = 197/280c | |||||
| Benefit | 11.2 | 10.7 | 0.5 (−0.2 to 1.1) | ||||
| Burden | 56.7 | 53.3 | 3.3 (2.3 to 4.4) | ||||
| At 6-mo follow-up | n = 191/262c | n = 177/253c | |||||
| Benefit | 11.6 | 11.3 | 0.3 (−0.3 to 0.8) | ||||
| Burden | 56.5 | 54.1 | 2.3 (1.2 to 3.5) | ||||
| Adherence to anticoagulant therapy, %f | |||||||
| At 3-mo follow-up | |||||||
| Taking anticoagulant as randomized | 82.1 (n = 271/330) | 72.7 (n = 224/308) | 9.4 (2.9 to 15.9) | ||||
| Taking any anticoagulant | 83.9 (n = 277/330) | 85.4 (n = 263/308) | −1.5 (−7.0 to 4.1) | ||||
| At 6-mo follow-up | |||||||
| Taking anticoagulant as randomized | 70.9 (n = 234/330) | 59.4 (n = 183/308) | 11.5 (4.1 to 18.8) | ||||
| Taking any anticoagulant | 72.7 (n = 240/330) | 72.1 (n = 222/308) | 0.6 (−6.3 to 7.6) | ||||
Direct oral anticoagulant group minus low-molecular-weight heparin group.
Health-related quality of life was measured using the 12-Item Short Form Health Survey subscales for physical and mental health (score range, 0-100; higher scores indicate better physical and mental health functioning). Survey content included minor verbiage changes for clarity.
Respondents/nondeceased participants.
Change in score from baseline.
The Anti-Clot Treatment Scale is a 15-item patient-reported measure of satisfaction with anticoagulant treatment. The benefit scale has 3 items, with scores ranging from 3 to 15; higher scores indicate greater satisfaction with treatment. The burden scale has 12 items with scores ranging from 12 to 60; higher scores indicate greater satisfaction (lower burden).
Participants who died prior to month 3 or 6 were considered adherent if they were not known to have stopped treatment prior to death. Adherence to anticoagulant therapy was ascertained based on both participant surveys and electronic health records. Randomized anticoagulants were any of apixaban, dabigatran, edoxaban, or rivaroxban for the direct oral anticoagulant group and any of enoxaparin, dalteparin, or fondaparinux for the low-molecular-weight heparin group. Any anticoagulant was defined as any of the medications listed above plus warfarin. Patients who died or were lost to follow-up at 3 or 6 months were included in the denominator in calculating these proportions.
Benefits and Burdens of Anticoagulant Therapy
Patients reported no clinically meaningful differences in the benefits or burdens of treatment on the Anti-Clot Treatment Scale (Table 3).
Other Prespecified Outcomes
At 3 months, 82.1% of participants randomized to DOACs and 72.7% of those randomized to LMWH continued to take their assigned treatment (difference, 9.4%; 2-sided 95% CI, 2.9%-15.9%). At 6 months, 70.9% and 59.4% of participants assigned to DOACs and LMWH, respectively, were still taking their assigned anticoagulant (difference, 11.5%; 2-sided 95% CI, 4.1%-18.8%). Of those assigned to LMWH, 6% (20/338) transitioned to warfarin therapy. Patient-reported outcomes in the preference cohort are shown in eTable 4 in Supplement 2.
Adverse Events
Adverse events including VTE and bleeding occurred in 36.0% of participants assigned to DOACs and 38.7% assigned to LMWH (difference, −2.7%; 2-sided 95% CI, −10.2% to 4.8%). Rates of severe adverse events were 33.8% in the DOAC group and 35.1% in the LMWH group (difference, −1.2%; 2-sided 95% CI, −8.6% to 6.2%) (Table 2). The most common severe adverse event was death (21.5% in the DOAC group and 18.4% in the LMWH group). Other severe adverse events included anemia of grade 3 or higher (n = 10 [3.0%] in the DOAC group and n = 3 [1.0%] in the LMWH group).
Subgroup Analyses
Among participants with highly thrombogenic tumors, the cumulative incidence of recurrent VTE at 6 months was 9.0% for patients in the DOAC group and 12.2% in the LMWH group (difference, −3.3%; 2-sided 95% CI, −11.7% to 5.1%). Among participants with an indwelling central venous catheter, recurrent VTE occurred in 6.2% in the DOAC group and 9.1% in the LMWH group (difference, −2.9%; 2-sided 95% CI, −8.4% to 2.6%). Among patients with a baseline platelet count of 150/μL or lower, recurrent VTE occurred in 6.9% in the DOAC group and 10.8% in the LMWH group (difference, −3.9%; 2-sided 95% CI, −11.5% to 3.7%). Among participants aged 65 years or older, recurrent VTE occurred in 3.9% in the DOAC group and 10.9% in the LMWH group (difference, −7.0%; 2-sided 95% CI, −13.6% to −0.4%) (eFigure 1 in Supplement 2).
Post Hoc Analyses
For the VTE primary end point, the 2-sided 95% CI for the difference of −2.7% was −6.8% to 1.4% (Table 2). For the outcome of major bleeding, the 2-sided 95% CI for the difference of −0.4% was −3.9% to 3.1%. For death, the 2-sided 95% CI for the difference of 3.1% in death rates at 6 months was −3.1% to 9.3%. The 2-sided 95% CI for the difference of −3 days in restricted mean survival times with a truncation time of 180 days was −9 to 4 days (Table 2).
Discussion
This pragmatic, unblinded, comparative effectiveness trial of patients with cancer and acute VTE found that 6 months of DOAC therapy was noninferior to LMWH therapy for preventing VTE recurrence. Rates of major bleeding and death were not significantly different between groups. There were no clinically meaningful differences in patient-reported health-related quality of life or perceived benefits and burdens of taking anticoagulant medication. Compared with patients randomized to LMWH, 11% more patients were taking their assigned treatment in the DOAC group at 6 months. After the start of enrollment, several randomized trials assessing efficacy and safety of individual DOACs compared with dalteparin in patients with cancer-associated VTE were reported. Compared with prior trials, this study was an effectiveness trial that enrolled patients with borderline low platelet counts, brain metastases, and limited functional status.10,11,12,13
In previous efficacy trials,10,11,12,13 rates of major bleeding were significantly higher for edoxaban or rivaroxaban compared with dalteparin but not for apixaban compared with dalteparin. The use of apixaban by almost 58.5% of participants randomized to the DOAC group may explain the lack of difference in major bleeding between the DOAC and LMWH groups, since apixaban is associated with lower bleeding rates than other DOACs. Although the bleeding rates in this trial were higher than those observed for DOAC treatment of VTE in the general population, they were similar to results from studies of anticoagulant therapy in patients with cancer-associated VTE. Consistent with prior clinical trials,10,12,27 results from this trial showed that patients treated with DOACs have a higher risk of clinically relevant nonmajor bleeding compared with patients treated with LMWH.
In contrast to previous randomized clinical trials of DOAC therapy for patients with cancer and VTE, this study was pragmatic and represented routine oncology practice by allowing clinicians and patients to select a drug from within a class and modify doses or stop therapy for procedures. Enrollment criteria were less stringent than in prior trials of DOAC therapy.10,11 Because patients with advanced cancer and brain metastases, impaired performance status, and reduced liver or kidney function were enrolled, results are likely to be more generalizable to patients encountered in oncology practice. Although there was no significant difference between groups in the burdens or benefits of anticoagulation treatment or in health-related quality of life, adherence to the assigned anticoagulation therapy at 6 months was significantly greater for the DOAC group compared with the LMWH group.
Limitations
This study has several limitations. First, participants and physicians were not blinded to treatment assignment. Second, because participants were randomized within 30 days of a new VTE diagnosis, some were treated with different therapy before randomization. Third, in patients with advanced-stage cancer, it was not possible to distinguish VTE from cancer as the cause of death. Fourth, people of Asian race or Hispanic ethnicity were not well represented, and the proportion of participants who were Black was 13%, limiting the generalizability of results. Fifth, detailed medication adherence diaries were not obtained. Sixth, lower adherence rates with LMWH could have biased results toward noninferiority of DOAC therapy. Seventh, the study lacked statistical power for superiority testing. Eighth, the Anti-Clot Treatment Scale may not be sufficiently sensitive to detect differences in burden of treatment between groups.
Conclusions
Among adults with cancer and VTE, DOACs were noninferior to LMWH for preventing recurrent VTE over 6-month follow-up. These findings support use of a DOAC to prevent recurrent VTE in patients with cancer.
Trial Protocol and Statistical Analysis Plan
eTable 1. Adjusted Event Rates of Recurrent VTE, Death, and Major Bleeding at 6 Months in the Randomized Cohort by Generalized Linear Mixed‐Effects Models
eFigure 1. Forest Plot of the Likelihood of Recurrent VTE in Pre‐defined Participant Subgroups in the Randomized Cohort
eFigure 2. Patient Dispositions in the Preference Cohort
eTable 2. Baseline Participant Characteristics in a Study of the Effect of Direct Oral Anticoagulants Versus Low Molecular Weight Heparins for Treatment of Venous Thromboembolic Events in Patients With Cancer (the Preference Cohort)
eTable 3. Clinical Outcomes at 6 Months in the Preference Cohort
eTable 4. Patient-Reported Outcomes in the Preference Cohort: Health-Related Quality of Life, Treatment Burden and Persistence With Anticoagulant Therapy
eTable 5. Propensity Score Model Used for Adjustment of Potential Confounding Factors in the Preference Cohort
eTable 6. Association Between the Treatment Selection and the Baseline Characteristics With and Without Adjustment by the Inverse Probability Weighting by the Propensity Scores in the Preference Cohort
Nonauthor Collaborators. CANVAS Investigators
Data Sharing Statement
References
- 1.Blom JW, Doggen CJM, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715-722. doi: 10.1001/jama.293.6.715 [DOI] [PubMed] [Google Scholar]
- 2.Castellucci LA, Cameron C, Le Gal G, et al. Clinical and safety outcomes associated with treatment of acute venous thromboembolism: a systematic review and meta-analysis. JAMA. 2014;312(11):1122-1135. doi: 10.1001/jama.2014.10538 [DOI] [PubMed] [Google Scholar]
- 3.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ III. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585-593. doi: 10.1001/archinte.158.6.585 [DOI] [PubMed] [Google Scholar]
- 4.Streiff MB, Milentijevic D, McCrae K, et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018;93(5):664-671. doi: 10.1002/ajh.25059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496-520. doi: 10.1200/JCO.19.01461 [DOI] [PubMed] [Google Scholar]
- 6.Büller HR, Décousus H, Grosso MA, et al. ; Hokusai-VTE Investigators . Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406-1415. doi: 10.1056/NEJMoa1306638 [DOI] [PubMed] [Google Scholar]
- 7.Bauersachs R, Berkowitz SD, Brenner B, et al. ; EINSTEIN Investigators . Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499-2510. doi: 10.1056/NEJMoa1007903 [DOI] [PubMed] [Google Scholar]
- 8.Agnelli G, Buller HR, Cohen A, et al. ; AMPLIFY-EXT Investigators . Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699-708. doi: 10.1056/NEJMoa1207541 [DOI] [PubMed] [Google Scholar]
- 9.Schulman S, Kearon C, Kakkar AK, et al. ; RE-COVER Study Group . Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342-2352. doi: 10.1056/NEJMoa0906598 [DOI] [PubMed] [Google Scholar]
- 10.Agnelli G, Becattini C, Meyer G, et al. ; Caravaggio Investigators . Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599-1607. doi: 10.1056/NEJMoa1915103 [DOI] [PubMed] [Google Scholar]
- 11.McBane RD II, Wysokinski WE, Le-Rademacher JG, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2020;18(2):411-421. doi: 10.1111/jth.14662 [DOI] [PubMed] [Google Scholar]
- 12.Raskob GE, van Es N, Verhamme P, et al. ; Hokusai-VTE Cancer Investigators . Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. doi: 10.1056/NEJMoa1711948 [DOI] [PubMed] [Google Scholar]
- 13.Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36(20):2017-2023. doi: 10.1200/JCO.2018.78.8034 [DOI] [PubMed] [Google Scholar]
- 14.Ware J Jr, Kosinski M, Keller SDA. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Kosinski M, Turner-Bowker M, Gandek B. How to Score the SF-12v2 Health Survey: Administration Guide for Clinical Trial Investigators. QualityMetric; 2002. [Google Scholar]
- 16.Cano SJ, Lamping DL, Bamber L, Smith S. The Anti-Clot Treatment Scale (ACTS) in clinical trials: cross-cultural validation in venous thromboembolism patients. Health Qual Life Outcomes. 2012;10:120. doi: 10.1186/1477-7525-10-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32(22):2380-2385. doi: 10.1200/JCO.2014.55.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian L, Zhao L, Wei LJ. Predicting the restricted mean event time with the subject’s baseline covariates in survival analysis. Biostatistics. 2014;15(2):222-233. doi: 10.1093/biostatistics/kxt050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. L Erlbaum Associates; 1988. [Google Scholar]
- 20.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27(3)(suppl):S178-S189. doi: 10.1097/00005650-198903001-00015 [DOI] [PubMed] [Google Scholar]
- 21.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3-15. doi: 10.1177/096228029900800102 [DOI] [PubMed] [Google Scholar]
- 22.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 23.Uno H, Cai T, Tian L, Wei LJ. Evaluating prediction rules for t-year survivors with censored regression models. J Am Stat Assoc. 2007;102(478):527-537. doi: 10.1198/016214507000000149 [DOI] [Google Scholar]
- 24.Jennison C, Turnbull BW. Interim analyses: the repeated confidence interval approach. J R Stat Soc Series B Stat Methodol. 1989;51(3):305-334. doi: 10.1111/j.2517-6161.1989.tb01433.x [DOI] [Google Scholar]
- 25.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659-663. doi: 10.2307/2336502 [DOI] [Google Scholar]
- 26.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549-556. doi: 10.2307/2530245 [DOI] [PubMed] [Google Scholar]
- 27.Lee AY, Levine MN, Baker RI, et al. ; CLOT Investigators . Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153. doi: 10.1056/NEJMoa025313 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. Adjusted Event Rates of Recurrent VTE, Death, and Major Bleeding at 6 Months in the Randomized Cohort by Generalized Linear Mixed‐Effects Models
eFigure 1. Forest Plot of the Likelihood of Recurrent VTE in Pre‐defined Participant Subgroups in the Randomized Cohort
eFigure 2. Patient Dispositions in the Preference Cohort
eTable 2. Baseline Participant Characteristics in a Study of the Effect of Direct Oral Anticoagulants Versus Low Molecular Weight Heparins for Treatment of Venous Thromboembolic Events in Patients With Cancer (the Preference Cohort)
eTable 3. Clinical Outcomes at 6 Months in the Preference Cohort
eTable 4. Patient-Reported Outcomes in the Preference Cohort: Health-Related Quality of Life, Treatment Burden and Persistence With Anticoagulant Therapy
eTable 5. Propensity Score Model Used for Adjustment of Potential Confounding Factors in the Preference Cohort
eTable 6. Association Between the Treatment Selection and the Baseline Characteristics With and Without Adjustment by the Inverse Probability Weighting by the Propensity Scores in the Preference Cohort
Nonauthor Collaborators. CANVAS Investigators
Data Sharing Statement