Abstract
Several studies have shown improved efficacy of cholesteryl-conjugated phosphorothioate antisense oligodeoxynucleotides. To gain insight into the mechanisms of the improved efficacy in vivo, we investigated the disposition of ISIS-9388, the 3′-cholesterol analog of the ICAM-1-specific phosphorothioate oligodeoxynucleotide ISIS-3082, in rats. Intravenously injected [3H]ISIS-9388 was cleared from the circulation with a half-life of 49.9 ± 2.2 min (ISIS-3082, 23.3 ± 3.8 min). At 3 h after injection, the liver contained 63.7 ± 3.3% of the dose. Compared to ISIS-3082, the hepatic uptake of ISIS-9388 is ∼2-fold higher. Endothelial, Kupffer and parenchymal cells accounted for 45.7 ± 5.7, 33.0 ± 5.9 and 21.3 ± 2.6% of the liver uptake of [3H]ISIS-9388, respectively, and intracellular concentrations of ∼2, 75 and 50 µM, respectively, could be reached in these cells (1 mg/kg dose). Preinjection with polyinosinic acid or polyadenylic acid reduced the hepatic uptake of [3H]ISIS-9388, which suggests the involvement of (multiple) scavenger receptors. Size exclusion chromatography of mixtures of the oligonucleotides and rat plasma indicated that ISIS-9388 binds to a larger extent to high molecular weight proteins than ISIS-3082. Analysis by agarose gel electrophoresis indicated that ISIS-9388 binds more tightly to plasma proteins than ISIS-3082. The different interaction of the oligonucleotides with plasma proteins possibly explains their different dispositions. We conclude that cholesterol conjugation results in high accumulation of phosphorothioate oligodeoxynucleotides in various liver cell types, which is likely to be beneficial for antisense therapy of liver-associated diseases.
INTRODUCTION
Antisense oligodeoxynucleotides can highly selectively inhibit the expression of target genes by hybridizing with their specific ‘sense’ sequences in mRNA or DNA (1–3). The initial drawback of the rapid degradation of oligonucleotides in a biological environment has largely been overcome by the synthesis of a variety of nuclease-resistant analogs, of which the phosphorothioate analog is presently the most widely applied (2,3). Phosphorothioate antisense oligodeoxynucleotides have been shown to be potent inhibitors of the expression of their target genes in vitro and in vivo (2,3). Several have entered clinical trials and one, Vitravene™, has been approved for marketing. In spite of their success, the mechanisms of uptake of phosphorothioate oligonucleotides, their protein binding characteristics and their (sub)cellular distribution have not been extensively studied. In addition, the efffects of chemical modifications of phosphorothioate oligodeoxynucleotides on the above characteristics are not well understood (4,5).
Several modifications have been explored to enhance cellular uptake and it was found that coupling to cholesterol enhances the antisense activity of phosphorothioate oligodeoxynucleotides. Improved efficacy of cholesteryl-conjugated phosphorothioate oligodeoxynucleotides has been observed in cell culture and also in vivo, in particular in the liver (6–10). Furthermore, it has recently also been claimed that conjugation with cholesterol enhances the oral bioavailability of phosphorothioate oligodeoxynucleotides (10). The mechanisms of the improved efficacy of cholesteryl-conjugated oligonucleotides are not entirely clear. It has been reported that conjugation with cholesterol enhances the association of phosphorothioate oligonucleotides with cells in culture, which may explain the improved efficacy of these modified oligonucleotides in vitro (7,9,11).
For an understanding of the mechanisms of the improved efficacy of cholesteryl-conjugated oligonucleotides in vivo, it is necessary to evaluate their disposition. In the present study we therefore investigated the disposition of ISIS-9388 in the rat. ISIS-9388 is the 3′-cholesteryl-conjugated analog of ISIS-3082, a phosphorothioate oligodeoxynucleotide specific for murine ICAM-1. It was recently found that conjugation of ISIS-3082 with cholesterol results in a more effective down-regulation of the hepatic expression of ICAM-1 (Manoharan et al., manuscript in preparation). We earlier examined the disposition of ISIS-3082 and found that the liver plays a major role in clearance of this oligonucleotide (12). A direct comparison of the disposition of ISIS-9388 and ISIS-3082 will provide insight into the effects of cholesterol modification of phosphorothioate oligodeoxynucleotides on their behaviour in vivo.
MATERIALS AND METHODS
Reagents
Polyinosinic acid (5′), polyadenylic acid (5′) and rat serum albumin were from Sigma (St Louis, MO). Emulsifier Safe™ and Hionic Fluor™ scintillation cocktails and Soluene-350 were from Packard (Downers Grove, IL). Na125I (carrier-free) was from Amersham International (Amersham, UK). Nonidet P-40 and proteinase K were purchased from Boehringer Mannheim (Mannheim, Germany). All other reagents were of analytical grade.
Oligonucleotide synthesis and purification
ISIS-3082, a 20mer phosphorothioate oligodeoxynucleotide specific for murine ICAM-1 (sequence 5′-TGC ATC CCC CAG GCC ACC AT-3′), was synthesized as described before (13). ISIS-9388 has the same sequence as ISIS-3082 except that a 3′-modified uridine is the 3′-end base instead of thymidine. For the synthesis of ISIS-9388, 3′-O-(6-aminohexyl)-uridine was condensed with cholesteryl chloroformate and the resulting conjugate was attached to a solid support. Subsequent synthesis of the ISIS-3082 sequence starting from this modified solid support provided ISIS-9388. To allow monitoring of the biological fate of both oligonucleotides, they were radiolabeled with 3H by heat-catalyzed exchange at the C8 positions of the purine nucleotides as described earlier (14). [3H]ISIS-9388 was purified by reversed phase HPLC, using a Waters C4 column (5 µm, 300 Å, 300 × 3.9 mm), at a flow rate of 1 ml/min using the following mobile phases: A, 50 mM triethyl ammonium acetate (pH 7.0); B, acetonitrile. After injection of the samples (0.5 ml), the column was eluted for 5 min with 10% B, followed by a gradient of 10–90% B (25 min). Subsequently, the column was eluted for 10 min with 90% B. The retention time of ISIS-9388 under these conditions was ∼25 min (ISIS-3082, 13 min). The radiolabeled oligonucleotide was precipitated as the sodium salt by adding 10 vol 3% (w/v) NaClO4 in acetone as described earlier (15). The specific radioactivity of [3H]ISIS-9388 was ∼50 × 106 d.p.m./mg and the radiochemical purity >98%.
Isolation of rat lipoproteins
Rat low density lipoprotein (LDL) (density 1.024–1.063 g/ml) and rat high density lipoprotein (HDL) (density 1.063–1.210 g/ml) were isolated by two consecutive density gradient centrifugation steps, as described earlier (16).
Radioiodination of lipoproteins and serum albumin
Rat LDL, rat HDL and rat serum albumin were radioiodinated with carrier-free Na125I as described earlier (17). Less than 2% of the radioactivity in the labeled protein preparations was trichloroacetic acid-soluble.
Determination of the stability of ISIS-9388 in rat blood, serum and plasma
[3H]ISIS-9388 was incubated at 37°C at a concentration of 20 µg/ml with rat blood (clotting prevented by adding EDTA to 4 mM), serum or EDTA–plasma. After 3 h, aliquots of 200 µl of the incubation mixtures were mixed with an equal volume of extraction buffer (25 mM Tris–HCl buffer, pH 8.0, containing 25 mM EDTA, 100 mM NaCl, 0.5% Nonidet P-40 and 1 mg/ml proteinase K) and incubated for a further 2 h at 56°C. Subsequently, the samples were mixed with 400 µl of phenol/isoamyl alcohol/choloroform (25:1:24 v/v/v). After shaking for 10 min, the phases were separated by centrifugation. The organic phase was washed four times with 400 µl of water. The aqueous phases were combined (total extraction efficiciency ∼75%) and dried in a Speed-Vac concentrator. The residues were dissolved in water and 30 µg unlabeled ISIS-9388 was added as marker (final volume 600 µl). An aliquot of 500 µl was subjected to reversed phase HPLC as describred above. Fractions of 1 ml were collected and assayed for radioactivity. It was found that after 3 h incubation of [3H]ISIS-9388 with rat blood, plasma or serum, ∼95% of the radioactivitiy eluted at the position of the unlabled ISIS-9388 marker, indicating that the cholesterol moiety was still attached at the 3′-end of the radiolabeled oligonucleotide. To assess degradation at the 5′-end, the radioactivity peak fractions of the HPLC analysis were pooled and dried in a Speed-Vac concentrator. The residue was dissolved in water and the oligonucleotide precipitated as the sodium salt (see above). The precipitated oligonucleotide was dissolved in electrophoresis buffer (90 mM Tris–borate buffer, pH 8.4, with 2 mM EDTA) containing 7 M urea, heated for 2 min at 95°C and subjected to electrophoresis in a 19% polyacrylamide gel containing 7 M urea. After electrophoresis, the gel was cut into slices, which were counted for radioactivity. Migration of the radioactivity was compared with that of marker oligonucleotides (ISIS-9388, ISIS-3082 and the 3′-truncated ISIS-3082 oligonucleotides described in ref. 12) and it was found that >95% of the radioactivity migrated as intact ISIS-9388.
Determination of plasma clearance and tissue distribution
Male Wistar rats (200–350 g) were used. The animals were anesthesized by i.p. injection of sodium pentobarbital (60 mg/kg body wt) and the abdomen was opened. Radiolabeled oligonucleotide, dissolved in phosphate-buffered saline (10 mM sodium phosphate buffer, pH 7.4, containing 0.15 M NaCl), was injected via the vena penis (2 ml/kg body wt). At the indicated times, blood samples of 0.2–0.3 ml were taken from the inferior vena cava and collected in heparinized tubes. The samples were centrifuged for 2 min at 16 000 g and the plasma assayed for radioactivity. The total amount of radioactivity in plasma was calculated using the equation: plasma volume (ml) = [0.0219 × body wt (g)] + 2.66 (18). At the indicated times, liver lobules were tied off and excised and at the end of the experiment the remainder of the liver was removed. The amount of liver tissue tied off successively did not exceed 15% of the total liver mass. The radioactivity in the liver at each time point was calculated from the radioactivities and weights of the liver samples. Uptake by extrahepatic tissues was determined by removing the tissues at the end of the experiment and counting radioactivity. Radioactivity in the tissues was corrected for radioactivity in plasma present in the tissue at the time of sampling (19).
Determination of the distribution over liver cell types
Rats were anesthesized and injected with radiolabeled oligonucleotide as described above. The liver was perfused at 60 min after injection and parenchymal, Kupffer and endothelial cells were isolated from the liver as described in detail earlier (20). The cell fractions were assayed for radioactivity and protein. Shortly before separation of the cells, a liver lobule was tied off and excised to determine total liver uptake. The contributions of the various cell types to total liver uptake were calculated as described previously (20). As found with other ligands (18,20), no significant amounts of radioactivity were lost from the cells during the isolation procedure. This was checked in each experiment by comparing the calculated liver uptake (i.e. the summation of the contributions of the various cell types) with the value actually measured in the liver lobule.
Determination of association of ISIS-9388 and ISIS-3082 with plasma components: analysis by size exclusion chromatography
[3H]ISIS-9388 and [3H]ISIS-3082 were incubated at 37°C with rat plasma, rat [125I]LDL, rat [125I]HDL or rat [125I]serum albumin. After 30 min, 50 µl aliquots of the incubation mixtures were injected onto a Superose 6 Precision Column (3.2 × 300 mm; SMART system, Pharmacia, Uppsala, Sweden). The column was eluted with phosphate-buffered saline at a flow rate of 50 µl/min. Fractions of 100 µl were collected and assayed for radioactivity, cholesterol and protein.
Determination of association of ISIS-9388 and ISIS-3082 with plasma components: analysis by agarose gel electrophoresis
[3H]ISIS-9388 and [3H]ISIS-3082 were incubated at 37°C with rat plasma. After 30 min, aliquots of the incubation mixtures were subjected to electrophoresis in a 0.75% (w/v) agarose gel at pH 8.8 (75 mM Tris–hippuric acid buffer). Bromophenol blue was used as the front marker. After electrophoresis, the radiolabeled oligonucleotides were detected by cutting the gel into slices that were assayed for radioactivity. Proteins in the gel were detected by fixing the gel in methanol/water/acetic acid (50:47:3 v/v/v) and staining with Coomassie brilliant blue. The gel was subsequently scanned using a flatbed scanner and the stain pattern quantified with Image-Quant software (Molecular Dynamics, Sunnyvale, CA).
Determination of proteins and cholesterol
Protein concentrations in cell suspensions and preparations of LDL, HDL and serum albumin were determined by the method of Lowry et al. (21), with bovine serum albumin as the standard. Total cholesterol in column fractions was determined by an enzymatic assay (Boehringer Mannheim).
Determination of radioactivity
Samples containing 3H were counted in a Packard Tri-Carb 1500 liquid scintillation counter. Liquid samples were counted without further processing by liquid scintillation spectroscopy, using Emulsifier Safe™ or Hionic Fluor™ scintillation cocktails. Polyacrylamide and agarose gel slices were first dissolved in Soluene-350 and N-methyl-2-pyrrolidone, respectively. Tissue samples were processed using a Packard 306 Sample Oxidizer. Some tissues (e.g. bone) were dissolved in 10 M NaOH at 95°C before counting. In samples containing both 125I and 3H, the 125I radioactivity was counted in a Packard Auto-Gamma 5000 counter. The 3H radioactivity was subsequently measured as described above and corrected for the contribution of 125I radioactivity.
RESULTS
Plasma clearance and tissue uptake of ISIS-9388
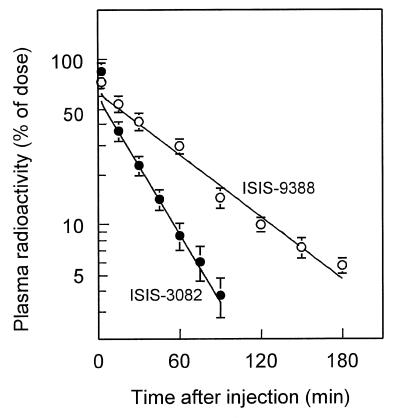
The disposition of ISIS-9388 was studied after a bolus injection of the radiolabeled oligonucleotide into rats. The dose, 1 mg/kg body wt, was in the range of doses of ICAM-1-directed antisense oligonucleotides that have been found to be effective in preclinical models and in patients (13,22–24). Figure 1 shows the plasma clearance of radioactivity after injection of [3H]ISIS-9388 or [3H]ISIS-3082. Both oligonucleotides displayed rapid initial distribution phases. The distribution volumes (52.6 ± 4.4 and 63.9 ± 9.3 ml/kg for ISIS-9388 and ISIS-3082, respectively) were not significantly different. The plasma clearance of ISIS-9388 was followed for 3 h. In vitro incubation studies with rat plasma, serum and whole blood indicate that ISIS-9388 remains >90% intact (i.e. full-length and cholesterol attached) during this time period. The cholesteryl-derivatized oligonucleotide is more slowly cleared from the circulation than its non-conjugated counterpart ISIS-3082 (half-lives 49.0 ± 2.2 and 23.3 ± 3.8 min, respectively).
Figure 1.
Plasma clearance of i.v. injected [3H]ISIS-9388 and [3H]ISIS-3082. Rats were i.v. injected with [3H]ISIS-9388 (open circles) or [3H]ISIS-3082 (closed circles), both at a dose of 1 mg/kg body wt. Blood samples were taken at the indicated times and the radioactivity in the plasma was determined. Values are means ± SEM of four rats.
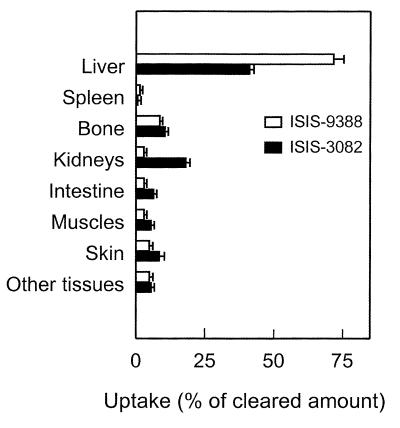
At 3 h after injection, when ∼90% of the injected dose of [3H]ISIS-9388 had been cleared from the circulation, the distribution of the radioactivity over the body was determined. The results are shown in Table 1. A small amount of the administered radioactivity (1.1 ± 0.2% of the dose) was recovered in the urine. The liver contained 63.7 ± 3.3% of the dose and is thus mainly responsible for clearance of the oligonucleotide. The remainder of the radioactivity was distributed over a large number of tissues. Bulky tissues, like muscle and skin contained 2.5 ± 0.9 and 4.2 ± 1.1% of the dose, respectively. The bone contained 7.6 ± 0.2% of the dose, which was mainly present in the marrow. The specific uptake, expressed as relative specific radioactivity, was highest in the reticuloendothelial system (liver, spleen and bone marrow). Figure 2 compares the tissue distribution of ISIS-9388 with that of ISIS-3082. The most striking difference in the distribution of the two oligonucleotides is the much higher uptake of ISIS-9388 by the liver. More than 70% of the cleared amount of ISIS-9388 was recovered in the liver versus ∼40% of the cleared amount of ISIS-3082. Uptake of ISIS-9388 by extrahepatic tissues, most notably the kidneys, was much lower than that of ISIS-3082.
Table 1. Tissue distribution of i.v. injected [3H]ISIS-9388.
| Tissue | Radioactivity (% of recovered) | Relative specific radioactivity |
|---|---|---|
| Blood plasma | 11.1 ± 2.3 | |
| Urine | 1.1 ± 0.2 | |
| Liver | 63.7 ± 3.3 | 12.5 ± 1.1 |
| Spleen | 1.2 ± 0.1 | 4.8 ± 0.5 |
| Bone marrow | 7.0 ± 0.6 | |
| Kidneys | 2.5 ± 0.4 | 2.8 ± 0.4 |
| Intestines | 2.6 ± 0.6 | 0.3 ± 0.1 |
| Muscles | 2.5 ± 0.9 | 0.1 ± 0.0 |
| Skin | 4.2 ± 1.1 | 0.3 ± 0.1 |
| Bone (including marrow) | 7.6 ± 0.2 | 0.3 ± 0.1 |
| All other tissues | 3.4 ± 1.0 | 0.3 ± 0.1 |
Rats were i.v. injected with [3H]ISIS-9388 at a dose of 1 mg/kg body wt. At 3 h after injection, the distribution of radioactivity over all tissues was determined. The results are expressed as percentages of the recovered amount of radioactivity and as relative specific activity (% recovered radioactivity divided by % recovered weight). Recoveries of radioactivity and tissues were 115.2 ± 4.8 and 96.3 ± 0.8%, respectively. Only tissues containing >1.0% of the recovered dose and/or a relative specific radioactivity >2.0 are listed. Values are means ± SEM of three rats.
Figure 2.
Comparison of tissue uptake of i.v. injected [3H]ISIS-9388 and [3H]ISIS-3082. Rats were i.v. injected with [3H]ISIS-9388 (open bars) or [3H]ISIS-3082 (closed bars), both at a dose of 1 mg/kg body wt. The distribution of radioactivity over all tissues was determined at 180 and 90 min after injection, respectively. Tissue uptakes are expressed as percentages of amounts of radioactivity cleared from the circulation (88.9 ± 2.3 and 97.9 ± 0.3% of the dose for ISIS-9388 and ISIS-3082, respectively). Values are means ± SEM of three rats.
Liver accumulation of ISIS-9388 and cellular distribution in the liver
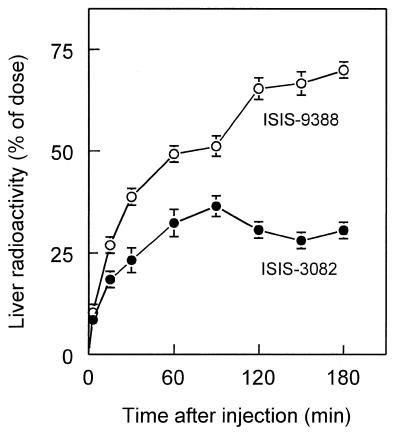
The rate of accumulation of ISIS-9388 in the liver, being the most important organ for disposition, was investigated and compared with that of ISIS-3082. Figure 3 shows that after i.v. injection both oligonucleotides accumulate steadily in the liver and that, eventually, the liver contains about twice as much ISIS-9388 as ISIS-3082. The liver contains several actively endocytosing cell types (25,26) and we demonstrated earlier that ISIS-3082 is predominantly taken up by endothelial liver cells (12). The distribution of ISIS-9388 over liver cell types was determined by injecting rats with the radiolabeled oligonucleotide, followed by isolation of parenchymal, endothelial and Kupffer cells from the liver 60 min later. The cell isolation procedure was performed at low temperature (8°C) to prevent processing of internalized oligonucleotide. Table 2 shows the amounts of ISIS-9388 present in the three isolated liver cell polulations at 60 min after injection and also the calculated total cellular load reached when all oligonucleotide had been cleared from the circulation. Comparison with ISIS-3082 shows that all three liver cell types internalize more ISIS-9388 than ISIS-3082. However, calculation of the uptake indices (which takes into account the different plasma concentrations of the oligonucleotides during the course of the experiments) indicates that the rate of uptake of ISIS-9388 by parenchymal and endothelial cells is not significantly different from that of ISIS-3082. Only Kupffer cells showed a 5-fold increased uptake index. As a result, the contribution of the Kupffer cells to the total liver uptake increased from 4.3 ± 1.7% for ISIS-3082 to 21.3 ± 2.6% for ISIS-9388. Endothelial cells were, however, still the main site of uptake of ISIS-9388 in the liver (45.7 ± 5.7% of total liver uptake). Parenchymal cells (which show a low specific uptake, but constitute >90% of the liver mass) were responsible for 33.0 ± 5.9% of the hepatic uptake.
Figure 3.
Liver accumulation of [3H]ISIS-9388 and [3H]ISIS-3082. Rats were i.v. injected with [3H]ISIS-9388 (open circles) or [3H]ISIS-3082 (closed circles), both at a dose of 1 mg/kg body wt. At the indicated times, the amounts of radioactivity in the liver were determined. Values are means ± SEM of three or four rats.
Table 2. Uptake of [3H]ISIS-9388 and [3H]ISIS-3082 by liver cell types.
| Oligonucleotide | Cell type | ng/mg of cell protein (at 60 min) | Total cellular load (µM) | Uptake index (µl/h/mg protein) | % of total liver uptake |
|---|---|---|---|---|---|
| ISIS-9388 | Parenchymal cells | 33.7 ± 6.5 | 1.9 ± 0.3 | 2.7 ± 0.5a | 33.0 ± 5.9 |
| Kupffer cells | 660.3 ± 19.6 | 47.2 ± 9.5 | 66.8 ± 13.4b | 21.3 ± 2.6 | |
| Endothelial cells | 1260.0 ± 286.9 | 76.3 ± 11.3 | 108.0 ± 16.0a | 45.7 ± 5.6 | |
| ISIS-3082 | Parenchymal cells | 23.0 ± 3.8 | 1.1 ± 0.2 | 3.3 ± 0.5 | 39.6 ± 4.5 |
| Kupffer cells | 90.0 ± 33.8 | 4.4 ± 1.6 | 12.8 ± 4.8 | 4.3 ± 1.7 | |
| Endothelial cells | 901.0 ± 7.5 | 43.6 ± 0.4 | 128.7 ± 1.1 | 56.1 ± 3.0 |
Rats were injected with [3H]ISIS-9388 or [3H]ISIS-3082 (1 mg/kg body wt). Sixty minutes later, parenchymal, endothelial and Kupffer cells were isolated and the cell-associated radioactivity was determined. The amount of oligonucleotide found in each cell type is given per mg cell protein. Total cellular loads (intracellular concentrations reached when all oligonucleotide had been cleared) were calculated by extrapolaton from the amounts of oligonucleotide in the liver at 60 min after injection, taking into account a hepatic cellular protein content of 17.7 ± 1.4% (mean ± SEM of seven determinations) and assuming that 75% of the cellular volume consists of water. Uptake indices (µl plasma cleared/h/mg cell protein) were calculated as described earlier (43). Differences in the uptake indices of ISIS-9388 and ISIS-3082 were tested for significance using Wilcoxon’s two-sample test (44) (anot significant; bP < 0.05). The contribution of each cell type to total liver uptake was calculated from the uptake per mg cell protein and the contribution of each cell type to total liver protein. Values are means ± SEM of three or four rats.
Involvement of scavenger receptors in liver uptake of ISIS-9388
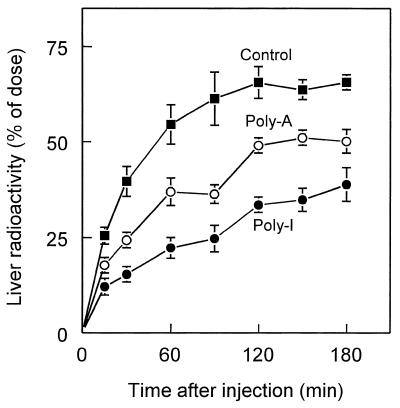
We earlier investigated the role of scavenger receptors in the hepatic uptake of ISIS-3082 (12). Endothelial liver cells abundantly express the scavenger receptor type AI/AII (SR-AI/II), which binds and internalizes a wide variety of polyanionic ligands (20,27). Interaction of ligands with SR-AI/II can be effectively inhibited by polyinosinic acid [poly(I)]. Polyadenylic acid [poly(A)], which has a different tertiary structure, is not inhibitory (28). We showed earlier that liver uptake of ISIS-3082 is inhibited by poly(I), whereas poly(A) has no effect, which suggests the involvement of SR-AI/II in the hepatic uptake of ISIS-3082. To study the possible role of SR-AI/AII in the liver uptake of ISIS-9388, rats were preinjected with poly(I) or poly(A) shortly before injection of [3H]ISIS-9388. The liver uptake of ISIS-9388 liver was substantially inhibited by poly(I) (Fig. 4). Poly(A) also inhibited hepatic uptake of ISIS-9388, albeit that it was less effective than poly(I). These findings suggest that scavenger receptors play a major role in the hepatic uptake of ISIS-9388. The inhibition of uptake of the oligonucleotide by both poly(I) and poly(A) suggests that in addition to SR-AI/II, alternative scavenger receptor systems may be involved.
Figure 4.
Liver uptake of [3H]ISIS-9388: effects of polyanions. Rats were i.v. injected with [3H]ISIS-9388 at a dose of 1 mg/kg body wt. Shortly (1 min) prior to injection of the labeled ligand, the animals received 10 mg/kg poly(I) (closed circles), 10 mg/kg poly(A) (open circles) or an equal volume (2 ml/kg) of saline solvent (closed squares). At the indicated times, the amounts of radioactivity in the liver were determined. Values are means ± SEM of three or four rats.
Association of ISIS-9388 and ISIS-3082 with plasma proteins
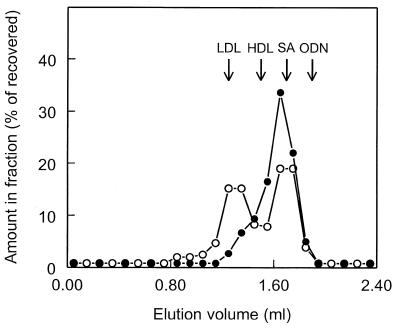
Phosphorothioate oligonucleotides have been reported to bind to plasma proteins (4,29–31), which is likely to affect their pharmacokinetics. To examine the interaction of ISIS-9388 and ISIS-3082 with plasma proteins, both radiolabeled oligonucleotides were incubated with rat plasma (at 20 µg/ml, the plasma concentrations immediately after i.v. injection). After 30 min, the incubation mixtures were subjected to size exclusion chromatography. The results are shown in Figure 5. The chromatographic profiles of [3H]ISIS-9388 and [3H]ISIS-3082 were clearly different. Both oligonucleotides were found to be protein bound; no radioactivity was recovered at the position of free oligonucleotide. [3H]ISIS-3082 was predominantly recovered in fractions eluting at 1.50–1.90 ml, which contain the bulk of the plasma proteins. Only a small proportion (15–20%) was recovered in fractions eluting at 1.10–1.50 ml, which contain high molecular weight proteins. [3H]ISIS-9388 also eluted at 1.50–1.90 ml, but compared to ISIS-3082 a high proportion (∼45%) was found in the fractions containing high molecular weight proteins.
Figure 5.
Association of ISIS-9388 and ISIS-3082 with plasma components: analysis by size exclusion chromatography. [3H]ISIS-9388 (open circles) and [3H]ISIS-3082 (closed circles) were incubated at 37°C with rat plasma at a concentration of 20 µg/ml. After 30 min, aliquots of the incubation mixtures were subjected to size exclusion chromatography on a Superose 6 column. Fractions of 0.1 ml were collected and were assayed for radioactivity. The results are expressed as percentages of the recovered amounts (recoveries >90%). The elution volumes of LDL, HDL, serum albumin (SA) and free oligodeoxynucleotide (ODN) are indicated by arrows.
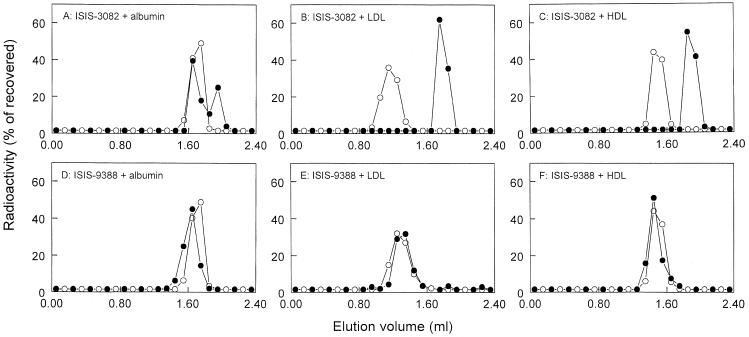
Several plasma proteins may bind ISIS-9388 and ISIS-3082. It has been shown that serum albumin binds various phosphorothioate oligodeoxynucleotides (4,29,30). To assess the interaction with this major plasma protein, [3H]ISIS-9388 and [3H]ISIS-3082 were incubated with radioiodinated serum albumin (25 mg/ml, the concentration in plasma) and the incubation mixtures were analyzed by size exclusion chromatography. Figure 6A and D shows that a substantial proportion (but not all) of ISIS-3082 binds to isolated serum albumin and that ISIS-9388 binds quantitatively. Furthermore, it has been reported that cholesteryl-conjugated oligonucleotides associate with lipopoproteins (15,32,33). To assess interaction with LDL and HDL, [3H]ISIS-9388 was incubated with radioiodinated LDL and HDL (0.2 and 1.0 mg/ml, respectively, the approximate concentrations in rat plasma). The incubation mixtures were analyzed by size exclusion chromatopgraphy. Figure 6 shows that ISIS-9388 readily and quantitatively associates with rat LDL and HDL, whereas ISIS-3082 does not.
Figure 6.
Association of ISIS-9388 and ISIS-3082 with LDL, HDL and serum albumin. [3H]ISIS-3082 and [3H]ISIS-9388 (20 µg/ml) were incubated at 37°C with 25 mg/ml rat [125I]serum albumin (A and D), 0.2 mg/ml rat [125I]LDL (B and E) or 1.0 mg/ml rat [125I]HDL (C and F). After 30 min, aliquots of the incubation mixtures were subjected to size exclusion chromatography on a Superose 6 column. The fractions (0.1 ml) were assayed for 3H radioactivity (closed circles) and 125I radioactivity (open circles). The results are expressed as percentages of the recovered radioactivity (recoveries >70%).
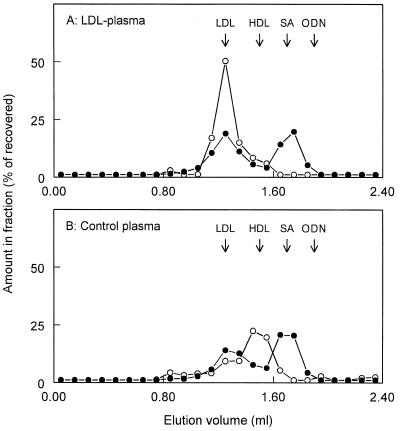
Thus, ISIS-9388 binds to isolated LDL and HDL. But does it also associate with these lipoproteins in plasma? HDL is the major lipoprotein in rat plasma. However, only low amounts of radioactivity were recovered in the HDL-containing fractions after incubation of [3H]SIS-9388 with rat plasma (Fig. 5). A significant proportion of [3H]ISIS-9388 was recovered in fractions containing high molecular weight proteins, including LDL. To examine possible binding of ISIS-9388 to LDL, the radiolabeled oligonucleotide was incubated with rat plasma supplemented with human LDL (which binds ISIS-9388 with the same affinity as rat LDL) and the incubation mixture was subjected to size exclusion chromatography. The additional LDL raised the total LDL concentration from 0.2 to 1.2 mg/ml and resulted in a markedly altered distribution of cholesterol over the column fractions (Fig. 7). However, the amount of [3H]ISIS-9388 that was recovered in the LDL-containing fractions was not appreciably increased upon LDL supplementation (47.7 ± 1.3 versus 39.8 ± 0.4% in control plasma; means ± SEM of two experiments). This finding suggests that, in plasma, ISIS-9388 does not extensively associate with LDL.
Figure 7.
Interaction of ISIS-9388 with LDL-enriched plasma. [3H]ISIS-9388 (20 µg/ml) was incubated at 37°C with rat plasma supplemented with 1 mg human LDL/ml (A) or with control plasma mixed with phosphate-buffered saline instead of LDL (B). After 30 min, aliquots of the incubation mixtures were subjected to size exclusion chromatography on a Superose 6 column. The fractions were assayed for 3H radioactivity (l) and cholesterol (). Values are expressed as percentages of the recovered amounts (recoveries >75%). The elution volumes of LDL, HDL, serum albumin (SA) and free oligodeoxynucleotide (ODN) are indicated by arrows.
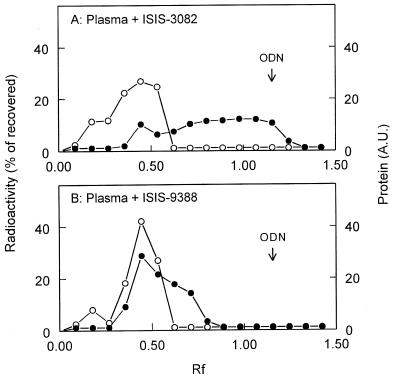
The association of ISIS-9388 and ISIS-3082 with plasma proteins was also investigated by subjecting mixtures of plasma and the radiolabeled oligonucleotides to agarose electrophoresis. Figure 8A shows that [3H]ISIS-3082 migrates only partially with the protein fraction. Most radioactivity was recovered at the position of free ISIS-3082 and between the position of free oligonucleotide and the protein fraction. [3H]ISIS-9388, on the other hand, migrates predominantly with the plasma proteins and no radioactivity was recovered at the position of free ISIS-9388 (Fig. 8B). This finding indicates that ISIS-3082, which does bind to plasma proteins (Fig. 5), is not sufficiently tightly bound to the proteins. The interaction of negatively charged ISIS-3082 and the proteins is disrupted in the electrical field applied during electrophoresis and the oligonucleotide subsequently migrates as the free molecule. The oligonucleotide dissociates from the proteins during electrophoresis. ISIS-9388, on the other hand, remains predominantly associated with the protein fraction during electrophoresis. These findings indicate that ISIS-9388 is more tightly bound to the protein fraction than ISIS-3082.
Figure 8.
Association of ISIS-9388 and ISIS-3082 with plasma components: analysis by agarose gel electrophoresis. [3H]ISIS-9388 (A) and [3H]ISIS-3082 (B) were incubated at 37°C with rat plasma at a concentration of 20 µg/ml. After 30 min, aliquots of the incubation mixtures were subjected to electrophoresis in a 0.75% (w/v) agarose gel at pH 8.8 (75 mM Tris–hippuric acid buffer). Bromophenol blue was used as the front marker. After electrophoresis, protein in the gel was visualized by Coomassie brilliant blue staining and quantified by scanning the gel. The amounts of protein in the gel () are given in arbitrary units (AU). Slices of the gel were assayed for 3H radioactivity (l) and the amounts of radioactivity are given as percentage of the recovered amount (recoveries >60%). Migration is given relative to the migration of Bromophenol blue (5.0 cm). The migration of free oligodeoxynucleotide (ODN) is indicated by the arrow.
DISCUSSION
We found that conjugation of cholesterol to the 3′-end of ISIS-3082, yielding ISIS-9388, enhances liver uptake of the oligonucleotide. This finding agrees with an earlier study showing increased liver uptake of 5′-cholesteryl-derivatized ISIS-3082 (4). In the present study liver uptake was more elaborately examined. The contributions of liver cell types were quantitatively analyzed, which is crucial for evaluation of the therapeutic significance of the cholesterol-induced increase in liver uptake. Further, we examined the role of savenger receptors. Finally, we demonstrate that cholesterol conjugation affects association of oligonucleotides with plasma proteins. Our data indicate that, in contrast to what is generally believed, cholesterol-modified phosphorothioate oligonucleotides do not associate with circulating lipopoproteins.
The uptake of ISIS-9388 by liver cells was examined and high concentrations were found in endothelial and Kupffer cells. By comparing the rates of uptake of ISIS-9388 and ISIS-3082, we found that Kupffer cells are 5-fold more active in uptake of ISIS-9388. The contribution of Kupffer cells to liver uptake rises from 4.3 ± 1.7% for ISIS-3082 to 21.3 ± 2.6% for ISIS-9388. The uptake indices of parenchymal and endothelial cells were similar for both oligonucleotides. Yet, the total cellular load of ISIS-9388 in these cells was found to be almost twice as high as that of ISIS-3082. This can be attributed to the higher availability of ISIS-9388 to the liver, caused by less avid uptake of the oligonucleotide by extrahepatic tissues, most notably the kidneys.
Earlier, we presented data that suggest that scavenger receptors on endothelial cells are implicated in liver uptake of ISIS-3082 (12). Scavenger receptors bind negatively charged ligands and at present six classes of receptors (denoted A–F) are distinguished (34,35). Endothelial liver cells express scavenger receptors typeAI/AII (SR-AI/II), which bind a variety of polyanionic ligands, including polynucleotides (27). The three-dimensional structure of polynucleotides is an important determinant for their affinity for SR-AI/AII (27,28). Poly(I) binds tightly, whereas poly(A) is poorly bound. We showed earlier that liver uptake of ISIS-3082 is inhibited by poly(I), whereas poly(A) has no effect, which may point to a role of SR-AI/AII (12). Butler et al. recently examined the uptake of phosphorothioate oligonucleotides by peritoneal macrophages isolated from SR-AI/II knock-out and wild-type mice (36). Two populations of binding sites were found: high affinity/low capacity and low affinity/high capacity. Compared to wild-type mice, the high affinity component was ∼50% lower in SR-AI/II knock-out mice. This finding indicates that SR-AI/II is involved in the high affinity uptake of oligonucleotides by macrophages and suggests that they may also be involved in uptake by other (liver) cell types. Butler et al. also studied the oligonucleotide tissue distribution in SR-AI/II knock-out and wild-type mice. Compared to wild-type mice, liver uptake in the knock-out mice was slightly (22%) decreased. However, a high dose of oligonucleotide (20 mg/kg) was administered and it is expected that under these conditions the low affinity/high capacity uptake system predominates. At the dose used in our studies (1 mg/kg), the high affinity sites are likely to contribute significantly to tissue uptake. We show inhibition of liver uptake of ISIS-9388 by poly(I) and poly(A), which suggests uptake via systems that can be inhibited by negatively charged compounds like poly(I) and poly(A). These uptake systems may include SR-AI/II, but the involvement of other (scavenger) receptor systems is very likely. The nature of the alternative receptors remains to be established. Various candidate receptors have been described. Endothelial liver cells express scavenger receptors that are different from SR-AI/AII (34,35,37). Kupffer cells express scavenger receptors that recognize oxidized LDL and are likely related to macrosialin/CD68 (38). Kupffer cells account for 21.3 ± 2.6% of the liver uptake of ISIS-9388, and the receptors for oxidized LDL may play a role. Possibly, uptake via these receptors is triggered by the formation of large negatively charged complexes of ISIS-9388 and high molecular weight plasma proteins. We found that ISIS-9388 does not associate to a great extent with LDL (see below). However, it is possible that relatively minor amounts of ISIS-9388 complexes are formed, which are recognized and rapidly taken up by oxidized LDL receptors. ISIS-9388 exchanges rapidly between serum components (39), which may lead to the formation of new ISIS-9388/LDL complexes. In this way, the continuous replacement of removed complexes may result in the continuous generation of ligand for the oxidized LDL receptor.
The different biological behaviours of ISIS-9388 and ISIS-3082 may be due to different binding to plasma proteins. Analysis by size exclusion chromatography indicates that both oligonucleotides associate with plasma proteins. In contrast to ISIS-3082, a significant proportion of ISIS-9388 elutes with high molecular weight proteins. The elution profiles of ISIS-9388 and ISIS-3082 also show a considerable overlap. This suggests that both oligonucleotides associate with the same plasma proteins. The interaction with the proteins may, however, be different. Size exclusion chromatography of mixtures of the oligonucleotides with serum albumin indicates that ISIS-9388 associates more tightly with albumin than ISIS-3082. Analysis by gel electrophoresis of mixtures of oligonucleotides with plasma also indicates that ISIS-9388 is more tightly associated with plasma proteins. The tight interaction of ISIS-9388 with plasma proteins may explain the retarded disposition into extrahepatic tissues. Further experiments are required to identify plasma proteins involved in the binding of both oligonucleotides.
It has been suggested that cholesteryl-derivatized oligonucleotides associate in plasma with lipoproteins (32,33). Indeed, ISIS-9388 readily associates with isolated LDL and HDL. However, two observations argue against extensive binding of ISIS-9388 to lipoproteins in plasma. HDL is the major lipoprotein in the rat, but we detected no appreciable binding of ISIS-9388 to HDL after incubation with rat plasma. Furthermore, when ISIS-9388 was incubated with rat plasma supplemented with LDL (which raised the LDL concentration 6-fold), the recovery of ISIS-9388 in the LDL-containing fractions was not appreciably increased. These findings suggest that in plasma ISIS-9388 does not associate to a great extent with HDL and/or LDL. We earlier analyzed the association of ISIS-9388 with plasma proteins by density gradient centrifugation and found substantial association with lipoproteins (40). The difference between the present and the earlier data is probably due to the different analytical techniques used. In this study, the association of ISIS-9388 with plasma proteins was analyzed by size exclusion chromatography under physiological running conditions. In the density gradients employed earlier, KBr concentrations in the lipoprotein-free serum fraction were up to 2.5 M. These high salt conditions may induce dissociation of the oligonucleotide from proteins in the lipoprotein-free serum fraction and association with LDL and HDL, which have a lower density and thus partition at lower KBr concentrations.
What are the therapeutic implications of the present findings? Derivatization of ISIS-3082 with cholesterol resulted in increased uptake by all three liver cell types investigated, in particular by Kupffer cells. For liver-associated targets, cholesterol modification of oligonucleotides should therefore be beneficial. For example, ICAM-1 has a crucial role in the adhesion of leukocytes during inflammation and transplant rejection. It is up-regulated on endothelial liver and Kupffer cells under inflammatory conditions and in liver transplants (41,42). Antisense oligonucleotides directed against ICAM-1 down-regulate hepatic ICAM-1 expression and have been shown to be therapeutically active (e.g. suppress transplant rejection) (42). Indeed, it was recently found that conjugation of ISIS-3082 with cholesterol results in a more effective down-regulation of hepatic expression of ICAM-1. In mice in which hepatic expression of ICAM-1 was up-regulated by lipopolysaccharide, administration of 5′-cholesteryl-derivatized ISIS-3082 (10 mg/kg body wt, given 24 and 2 h prior to lipopolysaccharide) resulted in a siginficant reduction in lipopolysaccharide-induced expression of ICAM-1 mRNA in the liver. Underivatized ISIS-3082 had no effect at all at that dosing schedule (Manoharan et al., manuscript in preparation). However, for extrahepatic targets (like ICAM-1 in Crohn’s disease or transplant rejection) the benefits of cholesterol modification are questionable (13,22–24). Several in vitro studies have demonstrated that cholesteryl-conjugated phosphorothioate oligodeoxynucleotides are more readily taken up by cultured cells of extrahepatic origin and have better efficacy than their non-conjugated counterparts (7,9). However, our present data show that in vivo the extrahepatic uptake of cholesteryl-conjugated oligonucleotides is dramatically reduced.
In conclusion, we show that attachment of cholesterol to a phosphorothioate oligonucleotide results in an ∼2-fold increased liver accumulation of the oligonucleotide. Uptake by all three liver cell types investigated was increased, but the uptake by Kupffer cells was particularly increased. In addition, we examined the role of scavenger receptors in hepatic uptake. We further showed that cholesterol conjugation affects the interaction with plasma proteins, which may explain the different biological fates. The high liver accumulation of cholesteryl-conjugated oligonucleotides, compared to non-conjugated oligonucleotides, may at least partly account for the higher in vivo efficacy against hepatic targets, although mechanisms such as more favorable intracellular trafficking may also play a role.
REFERENCES
- 1.Branch A.D. (1996) Hepatology, 24, 1517–1529. [DOI] [PubMed] [Google Scholar]
- 2.Szymkowski D.E. (1996) Drug Discov. Today, 1, 415–428. [DOI] [PubMed] [Google Scholar]
- 3.Akhtar S. and Agrawal,S. (1997) Trends Pharm. Sci., 18, 12–18. [DOI] [PubMed] [Google Scholar]
- 4.Crooke S.T., Graham,M.J., Zuckerman,J.E., Brooks,D., Conklin,B.S., Cummins,L.L., Greig,M.J., Guinosso,C.J., Kornbrust,D., Manoharan,M., Sasmor,H.M., Schleich,T., Tivel,K.L. and Griffey,R.H. (1996) J. Pharmacol. Exp. Ther., 277, 923–937. [PubMed] [Google Scholar]
- 5.Juliano R.L., Alahari,S., Yoo,H., Kole,R. and Cho,M. (1999) Pharm. Res., 16, 494–502. [DOI] [PubMed] [Google Scholar]
- 6.Desjardins J., Mata,J., Brown,T., Graham,D., Zon,G. and Iversen,P. (1995) J. Drug Target., 2, 477–485. [DOI] [PubMed] [Google Scholar]
- 7.Alahari S.K., Dean,N.M., Fisher,M.H., Delong. R., Manoharan,M., Tivel,K.L. and Juliano,R.L. (1996) Mol. Pharmacol., 50, 808–819. [PubMed] [Google Scholar]
- 8.Manoharan M., Tivel,K.L., Condon,T.P., Andrade,L.K., Barber-Peoch,I., Inamati,G., Shah,S., Mohan,V., Graham,M., Bennett,C.F., Crooke,S. and Cook,P.D. (1997) Nucleosides Nucleotides, 16, 1129–1138. [Google Scholar]
- 9.Epa W.R., Rong,P., Bartlett,P.F., Coulson,E.J. and Barrett,G. (1998) Antisense Nucleic Acid Drug Dev., 8, 489–498. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto Y. and Nakano,H. (1999) Hepatol. Res., 13, 252–258. [Google Scholar]
- 11.Temsamani J., Kubert,M., Tang,J., Padmapriya,A. and Agrawal,S. (1994) Antisense Res. Dev., 4, 35–42. [DOI] [PubMed] [Google Scholar]
- 12.Bijsterbosch M.K., Manoharan,M., Rump,E.T., de Vrueh,R.L.A., van Veghel,R., Tivel,K.L., Biessen,E.A.L., Bennett,C.F., Cook,P.D. and van Berkel,Th.J.C. (1997) Nucleic Acids Res., 25, 3290–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stepkowski S.M., Tu,Y., Condon,T.P. and Bennett,C.F. (1994) J. Immunol., 153, 5336–5346. [PubMed] [Google Scholar]
- 14.Graham M.J., Freier,S.M., Crooke,R.M., Ecker,D.J., Maslova,R.N. and Lesnik,E.A. (1993) Nucleic Acids Res., 21, 3737–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rump E.T., de Vrueh,R.L.A., Sliedregt,L.A.J.M., Biessen,E.A.L., van Berkel,Th.J.C. and Bijsterbosch,M.K. (1998) Bioconjugate Chem., 9, 341–349. [DOI] [PubMed] [Google Scholar]
- 16.Redgrave T.G., Roberts,D.C.K. and West,C.E. (1975) Anal. Biochem., 65, 42–49. [DOI] [PubMed] [Google Scholar]
- 17.McFarlane A.S. (1958) Nature, 182, 53–54. [DOI] [PubMed] [Google Scholar]
- 18.Bijsterbosch M.K., Ziere,G.J. and van Berkel,Th.J.C. (1989) Mol. Pharmacol., 36, 484–489. [PubMed] [Google Scholar]
- 19.Caster W.O., Simon,A.B. and Armstrong,W.D. (1955) Am. J. Physiol., 183, 317–321. [DOI] [PubMed] [Google Scholar]
- 20.Nagelkerke J.F., Barto,K.P. and van Berkel,Th.J.C. (1983) J. Biol. Chem., 263, 10221–12227. [Google Scholar]
- 21.Lowry O.H., Rosebrough,N.J., Farr,A.L. and Randall,R.J. (1951) J. Biol. Chem., 193, 265–275. [PubMed] [Google Scholar]
- 22.Bennett C.F., Kornbrust,D., Henry,S., Stecker,K., Howard,R., Cooper,S., Dutson,S., Hall,W. and Jacoby,H.I. (1997) J. Pharmacol. Exp. Ther., 280, 988–1000. [PubMed] [Google Scholar]
- 23.Stepkowski S.M., Wang,M., Condon, TP, Flournoy,S.C., Stecker,K., Graham,M., Qu,X., Chen,W., Kahan,B.D. and Bennett,C.F. (1998) Transplantation, 66, 699–707. [DOI] [PubMed] [Google Scholar]
- 24.Yacyshyn B.R., Bowen-Yacyshyn,M.B., Jewell,L., Tami,J.A., Bennett,C.F., Kisner,D.L. and Shananan,W.R.,Jr (1998) Gastroenterology, 114, 1133–1142. [DOI] [PubMed] [Google Scholar]
- 25.Ashwell G. and Harford,J. (1982) Annu. Rev. Biochem., 51, 531–554. [DOI] [PubMed] [Google Scholar]
- 26.Smedsrod B., de Bleser,P.J., Braet,F., Lovisetti,P., Vanderkerken,K., Wisse,E. and Geerts,A. (1994) Gut, 35, 1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krieger M. and Herz,J. (1994) Annu. Rev. Biochem., 63, 601–637. [DOI] [PubMed] [Google Scholar]
- 28.Pearson A.M., Rich,A. and Krieger,M. (1993) J. Biol. Chem., 268, 3546–3554. [PubMed] [Google Scholar]
- 29.Cossum P.A., Sasmor,H., Dellinger,D., Truong,L., Cummins,L., Owens,S.R., Markham,P.M., Shea,J.P. and Crooke,S. (1993) J. Pharmacol. Exp. Ther., 267, 1181–1190. [PubMed] [Google Scholar]
- 30.Srinivasan S.K., Tewary,H.K. and Iversen,P. (1995) Antisense Res. Dev., 5, 131–139. [DOI] [PubMed] [Google Scholar]
- 31.Nolting A., DeLong,R.K., Fisher,M.H., Wickstrom,E., Pollack,G.M., Juliano,R.L. and Brouwer,K.L.R. (1997) Pharm. Res., 14, 516–521. [DOI] [PubMed] [Google Scholar]
- 32.de Smidt P.C., Le Doan,T., de Falco,S. and van Berkel,Th.J.C. (1991) Nucleic Acids Res., 19, 4695–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krieg A.M., Tonkinson,J., Matson,S., Zhao,Q., Saxon,M., Zhang,L.-M., Bhanja,U., Yakubov,L. and Stein,C.A. (1993) Proc. Natl Acad. Sci. USA, 90, 1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieger M. (1997) Curr. Opin. Lipidol., 8, 275–280. [DOI] [PubMed] [Google Scholar]
- 35.Greaves D.R., Gough,P.J. and Gordon,S. (1998) Curr. Opin. Lipidol., 9, 425–432. [DOI] [PubMed] [Google Scholar]
- 36.Butler M., Crooke,R.M., Graham,M.J., Lemonidis,K.M., Lougheed,M., Murray,S.F., Witchell,D., Steinbrecher,U. and Bennett,C.F. (2000) J. Pharmacol. Exp. Ther., 292, 489–496. [PubMed] [Google Scholar]
- 37.van Berkel,Th.J.C., van Velzen,A., Kruijt,J.K., Suzuki,H. and Kodama,T. (1998) Biochem. J., 331, 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Velzen A.G., Da Silva,R.P., Gordon,S. and van Berkel,Th.J.C. (1997) Biochem. J., 322, 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rump E.T., De Vrueh,R.L.A., Manoharan,M., Waarlo,I.H.E., van Veghel,R., Biessen,E.A.L., van Berkel,Th.J.C. and Bijsterbosch,M.K. (2000) Biochem. Pharmacol., 59, 1407–1416. [DOI] [PubMed] [Google Scholar]
- 40.Bijsterbosch,M.K., Manoharan,M., Tivel. K.L., Rump,E.T., Biessen,E.A.L., De Vrueh,R.L.A., Cook,P.D. and van Berkel,Th.J.C. (1997) Nucleosides Nucleotides, 16, 1165–1168. [Google Scholar]
- 41.van Oosten M., van de Bilt,E., de Vries,H.E., van Berkel,Th.J.C. and Kuiper,J. (1995) Hepatology, 22, 1538–1546. [DOI] [PubMed] [Google Scholar]
- 42.Wong J., Kubes,P., Zhang,Y. and Lee,S.S. (1997) Hepatology, 26, 165A.9214466 [Google Scholar]
- 43.Sinke J., Bouma,J.M.W., Kooistra,T. and Gruber,M. (1979) Biochem. J., 180, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilcoxon F. (1945) Biom. Bull., 1, 80–83. [Google Scholar]