Key Points
Question
How effective is family-based behavioral treatment for childhood overweight and obesity when implemented in pediatric primary care settings?
Finding
In this randomized trial with follow-up over 24 months, children, parents, and siblings with overweight or obesity assigned to undergo family-based treatment had significantly better weight loss outcomes, measured by percentage above the median body mass index for their age and sex, than those assigned to undergo usual care.
Meaning
Family-based treatment can be successfully implemented in pediatric primary care settings and leads to improved weight loss outcomes among participating children and their parents and siblings.
Abstract
Importance
Intensive behavioral interventions for childhood overweight and obesity are recommended by national guidelines, but are currently offered primarily in specialty clinics. Evidence is lacking on their effectiveness in pediatric primary care settings.
Objective
To evaluate the effects of family-based treatment for overweight or obesity implemented in pediatric primary care on children and their parents and siblings.
Design, Setting, and Participants
This randomized clinical trial in 4 US settings enrolled 452 children aged 6 to 12 years with overweight or obesity, their parents, and 106 siblings. Participants were assigned to undergo family-based treatment or usual care and were followed up for 24 months. The trial was conducted from November 2017 through August 2021.
Interventions
Family-based treatment used a variety of behavioral techniques to develop healthy eating, physical activity, and parenting behaviors within families. The treatment goal was 26 sessions over a 24-month period with a coach trained in behavior change methods; the number of sessions was individualized based on family progress.
Main Outcomes and Measures
The primary outcome was the child’s change from baseline to 24 months in the percentage above the median body mass index (BMI) in the general US population normalized for age and sex. Secondary outcomes were the changes in this measure for siblings and in BMI for parents.
Results
Among 452 enrolled child-parent dyads, 226 were randomized to undergo family-based treatment and 226 to undergo usual care (child mean [SD] age, 9.8 [1.9] years; 53% female; mean percentage above median BMI, 59.4% [n = 27.0]; 153 [27.2%] were Black and 258 [57.1%] were White); 106 siblings were included. At 24 months, children receiving family-based treatment had better weight outcomes than those receiving usual care based on the difference in change in percentage above median BMI (−6.21% [95% CI, −10.14% to −2.29%]). Longitudinal growth models found that children, parents, and siblings undergoing family-based treatment all had outcomes superior to usual care that were evident at 6 months and maintained through 24 months (0- to 24-month changes in percentage above median BMI for family-based treatment and usual care were 0.00% [95% CI, −2.20% to 2.20%] vs 6.48% [95% CI, 4.35%-8.61%] for children; −1.05% [95% CI, −3.79% to 1.69%] vs 2.92% [95% CI, 0.58%-5.26%] for parents; and 0.03% [95% CI, −3.03% to 3.10%] vs 5.35% [95% CI, 2.70%-8.00%] for siblings).
Conclusions and Relevance
Family-based treatment for childhood overweight and obesity was successfully implemented in pediatric primary care settings and led to improved weight outcomes over 24 months for children and parents. Siblings who were not directly treated also had improved weight outcomes, suggesting that this treatment may offer a novel approach for families with
multiple children.
Trial Registration
ClinicalTrials.gov Identifier: NCT02873715
This randomized clinical trial evaluates the effects of family-based treatment implemented in primary care pediatric settings on weight change for children with overweight or obesity.
Introduction
Obesity runs in families,1 and child and parent obesity is associated with cardiometabolic diseases.2 Family-based treatment is an evidence-based, cost-effective3 treatment associated with significant4,5 weight changes in both children and parents.6 Currently, family-based treatment is primarily available in specialty clinics, but most children receive medical care in pediatric primary care settings.7 The American Academy of Pediatrics has recommended that children with obesity be offered intensive behavioral interventions,8 but gaps exist in the availability of these interventions in primary care settings due to limited time and lack of behavioral knowledge and onsite specialists.
Preliminary research has shown family-based treatment can also reduce the weight of siblings.9 For families with multiple children with overweight or obesity, behavior change in a child and parent undergoing treatment may be a pathway for change in a sibling who is not being treated. Parents can model healthy behaviors10,11,12,13 and children may spend more time with their siblings than with parents or friends.14
This study’s primary objective was to evaluate the effects of family-based treatment implemented in primary care pediatric settings on weight change for children with overweight or obesity. A secondary objective was to assess similar changes for parents/caregivers (hereafter referred to as parents) and siblings with overweight or obesity.
Methods
Trial Design, Participants, and Oversight
The Primary Care Pediatrics, Learning, Activity, and Nutrition With Families (PLAN) study was a US-based randomized clinical trial comparing the effectiveness of family-based treatment with usual care for families with a child aged 6 through 12 years with overweight or obesity (>85th percentile body mass index [BMI]), a parent with overweight or obesity (BMI >25), and, when available, siblings aged 2 through 18 years with overweight or obesity. The study took place in practice-based research networks in Buffalo, New York (9 practices; 109 participants randomized), Rochester, New York (6 practices; 93 participants randomized), St Louis, Missouri (6 practices; 134 participant randomized), and Columbus, Ohio (15 practices; 116 participants randomized). Practice characteristics are further described in eTable 1 in Supplement 1. The study was implemented between November 2017 and August 2021.
In addition to the above weight criteria, inclusion criteria included both the child and parent being interested in participating and having no dietary restrictions and the child to be living with the participating parent at least 50% of the time. Child-parent dyads were excluded if either was taking medication or had a health condition that altered growth or nutritional status, if either had unmanaged psychiatric illnesses or inability to engage in regular exercise, or if the parent planned to have weight loss surgery. Participating parents were asked to report their race and ethnicity, as well as to report these for the participating child, nontargeted participating sibling, and nonparticipating parent. Race and ethnicity were assessed using fixed categories to evaluate whether they were related to treatment effects. These data were not collected for any additional members of the family. The published protocol includes additional detail.15
The trial was approved by the institutional review board at the University at Buffalo and included a data and safety monitoring board and reliance agreements across sites. The National Heart, Lung, and Blood Institute provided administrative oversight and statistical advice.15 Details of the trial protocol and statistical analytic plan have been published and are included in Supplement 2.15
Screening and Group Assignment
Screening and informed consent of family members older than 18 years and assent by children and siblings younger than 18 years was conducted by a coach or recruitment specialist using instruments described in the published protocol.15 If there were multiple eligible children in a family, the program was first offered to the oldest child.9 Parents were invited, but not required, to enroll any eligible siblings.
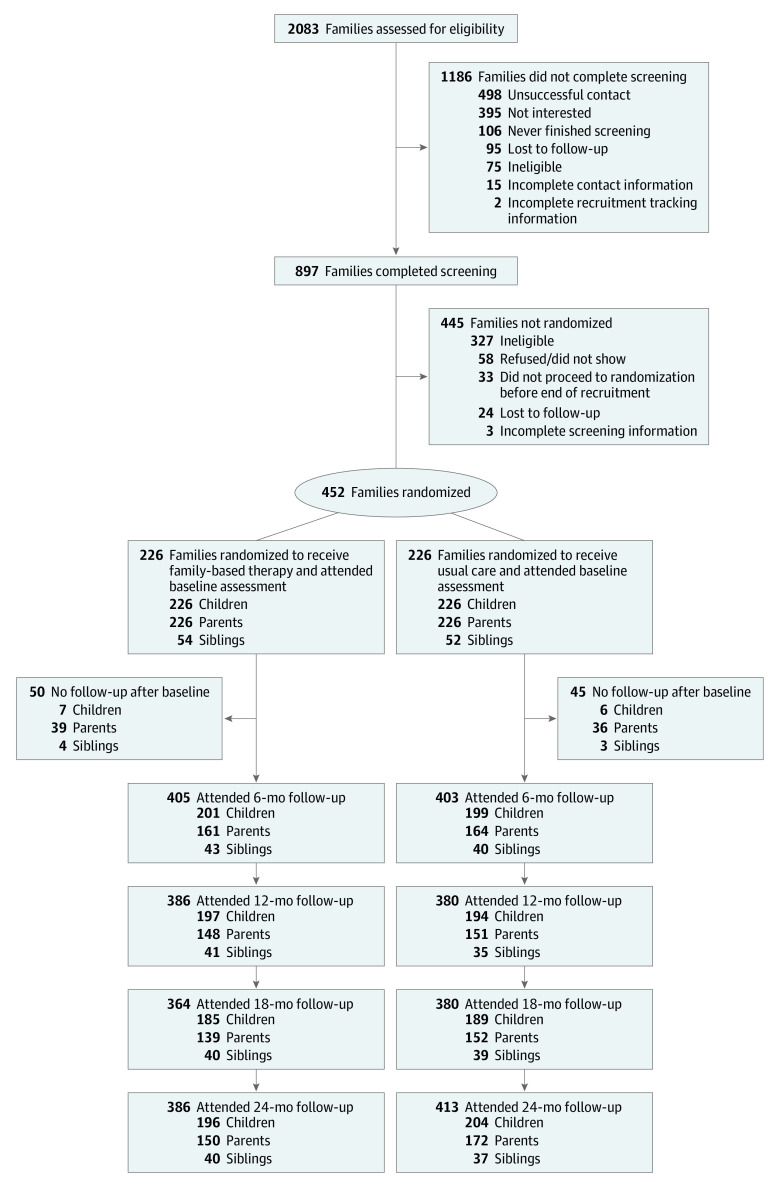
Families were randomly assigned in a 1:1 ratio to undergo usual care or usual care plus family-based treatment (Figure 1). Randomization was stratified by clinic site and was blocked using a range of 4 to 8 random block sizes. Investigators, study personnel performing assessments, pediatricians, and primary care staff were masked to group assignment.
Figure 1. Recruitment, Randomization, and Follow-up in the PLAN Trial of Childhood Obesity.
PLAN indicates Primary Care Pediatrics, Learning, Activity, and Nutrition With Families.
Interventions
Pediatricians in both the intervention and usual care groups were advised to follow standard recommendations for the treatment of overweight and obesity in children and adolescents.16 The family-based treatment4,17 intervention included materials that covered the Traffic Light Eating (eTable 2 in Supplement 1) and Activity Plans, parenting and behavioral techniques, and facilitation of support in family and peer environments.18 Families were seen in individual sessions that incorporated parent and child weigh-ins, review of eating and activity self-monitoring in habit books, review of weight change and problem solving and goal setting for the next meeting in relationship to behavior change, and review of treatment manuals and handouts. The treatment was implemented by people with a variety of backgrounds, with the emphasis on experience working with families. In all cases but 1, individuals either had master’s degrees in psychology, counseling, or social work or were master’s degree–level registered dietitians.
Weekly meetings were planned during the first 4 months as families learned the program, shifting to biweekly for 2 months and then to monthly meetings. The goal was for families to attend 26 sessions during the 24-month intervention and follow-up period, but the frequency of sessions could be increased or decreased based on family progress in meeting weight, eating, physical activity, and parenting behavior goals or challenges in attending meetings.
Recognizing that families make changes in behavior at different rates, behavioral goals were based on demonstrating mastery of behavior and weight change and behavior change concepts.19 To ensure that coaches followed the protocol, they used checklists and had access to a family dashboard that provided cumulative behavior and weight changes to guide treatment sessions. Treatment dose targets were within the American Academy of Pediatrics recommendation of 26 or more hours of intensive intervention.8
Because this study was designed to test implementation of family-based treatment in primary care, it was implemented using coaches who had clinical experience but no prior familiarity with family-based treatment. Coaches were provided a 30-hour certification process combining education, interactive roleplay, and simulations. Coaches had to demonstrate mastery of relevant material before implementing pilot treatment with 2 families.
To ensure fidelity to the intervention protocol, recordings of a subset of treatment sessions were reviewed by supervisors and discussed with the coach. Treatment fidelity was rated and booster training was provided as appropriate. The mean adherence rating to the protocol was 96% and the mean quality rating was 2.61 on a 3-point scale. Additional details of the intervention, coach training (eTable 3 in Supplement 1), and measures of protocol fidelity are presented in the protocol paper15 and eTable 4 in Supplement 1.
Study Adaptations for COVID-19
Approximately 28 months after the study began, treatment and assessment procedures were modified for telephone and video administration due to the COVID-19 pandemic. After initiation of remote treatments, remote assessments were initiated in which digital scales, carpenter’s squares, and metal tape measures were mailed to families to conduct weight and height measurements similar to previous office-based assessment procedures. Testing confirmed high interrater reliability; additional details about adaptations to the COVID-19 pandemic are provided in the study protocol.15
Data Collection, Outcome Measures, and Covariates
Height and weight measures were collected by study staff at baseline and at 6, 12, 18, and 24 months. Data on baseline covariates were collected by trained staff and the number of months that families experienced COVID-19 when the study interventions were delivered remotely was determined by examination of exposure at the conclusion of data collection. Remote interventions began in March 2020. Data were entered using REDCap electronic data capture.20
The primary outcome for children and siblings was the percentage above the median BMI normalized for child age and sex.21,22 The primary outcome for parents was BMI. For analyses of parent, child, and sibling changes within the family, parent percentage above median BMI was calculated using median BMI at age 20 years, normalized for sex.22 The secondary outcome of clinically meaningful change was defined as a BMI z score reduction of 0.25 or more for children and siblings23 and as weight loss of 5% or more for parents.
Sample Size and Statistical Methods
The target sample size was 528 families, which was calculated based on previous research4,24 and the expectation that translation of treatments developed in specialty clinics to clinical settings would lead to a smaller magnitude effect.25,26 In previous studies in specialty clinics,27,28,29,30,31,32 the reduction in mean (SD) percentage above median BMI was 10.7% (15.5%; range, −13.3% to −6.02%) and 45% of children (range, 34%-55%) had clinically meaningful weight change. This study’s sample size assumed that the effect of treatment implemented in primary care settings would be 50% to 70% of that achieved in specialty clinics with more experienced interventionists. Sample size computations were for 2-sided tests at a significance level of .05 and assumed an intraclass correlation coefficient of 0.01 in the control group and 0.04 in the intervention group. Full details of the power and sample size computations based on previous family-based treatment studies implemented in specialty clinics27,28,29,30,33,34 are available in eTable 5 in Supplement 1.
The primary analysis used intention-to-treat assignment and assessed the change in the child’s percentage above the median BMI at 24 months using analysis of covariance adjusted by site and pooled across 10 multiply imputed data sets with each intervention group participant (child) nested within their coach in the intervention group. This was an individually randomized group treatment trial35 with children, parents, and siblings nested within coaches in the intervention group; no similar nesting occurred in the usual care group. Thus, covariance structures were determined separately for each treatment group. Secondary analyses adjusted for covariates, including sex, age, and race of the child; family income; educational level of the parent; the number of months between baseline and follow-up measurements (which varied due to the pandemic and the resulting decision to permit assessments up to 30 months); and the number of months after the start of the pandemic.
To study simultaneous child, parent, and sibling changes from baseline to 6, 12, 18, and 24 months, longitudinal random coefficient growth curve models were developed, using the changes as the dependent measure, controlling for site and for baseline values of the primary outcome. A dummy variable reflected whether the observation was from the child, sibling, or parent, with children, siblings, and parents nested within family units and within coaches in the intervention group. The overall effect of treatment in the full family unit was first determined based on the coefficient of the treatment variable and the stratified effect of treatment for each family member was subsequently calculated by including an interaction term between treatment and family role dummy variable.
Secondary analyses adjusted for sex, age, and each individual family member’s race (parent, index child, sibling), number of months between baseline and follow-up, months of follow-up after start of the COVID-19 pandemic, family income, and educational level of the participating parent. A time-varying covariate reflected the number of months that the follow-up visit occurred after the start of the COVID-19 pandemic. Because the follow-up time after the start of the COVID-19 pandemic and race were statistically significant covariates, differential effects of COVID-19 or race on the patterns of change were assessed using interaction terms between each of these variables and the family role variable. For race, only comparisons between Black and White individuals were assessed due to limited sample sizes in other racial groups. Between-group differences in the percentage of children, parents, and siblings who met clinically meaningful changes at 24 months was tested using χ2 testing. The analytic plans to assess the relationships between child, parent, and sibling change; the association between attendance and weight outcome; and 2 sensitivity analyses to assess whether analytic approaches affected study findings were implemented. These plans were to assess whether analysis of the primary outcome differed when participants who provided no follow-up data were excluded and whether a modified approach to imputation would yield the same results, in which controlled imputation was used. Missing data were handled using multiple imputations. Analyses were implemented in SAS, version 9.4 (SAS Institute).
Results
Of the 2083 families referred by pediatricians, 898 were screened and 452 were enrolled, with 226 assigned to undergo family-based treatment and 226 assigned to undergo usual care (Figure 1). A total of 106 siblings from 94 families were included in the analysis. The percentages of missing follow-up weight data at 24 months were 11.5% for children, 28.8% for parents, and 27.4% for siblings. There were less missing child data due to the ability to obtain weight data for participating children from the pediatric primary care practices.
The mean (SD) age among children enrolled was 9.8 (1.9) years and the mean (SD) percentage above median BMI at baseline was 59.4% (27.0%); 123 children (27.2%) were Black, 258 (57.1%) were White, and 40 (8.8%) were Hispanic. Parents had a mean (SD) age of 41.4 (7.5) years and a mean (SD) BMI of 37.0 (7.8); siblings had a mean (SD) age of 10.0 (3.7) years and a mean (SD) percentage above median BMI of 49.6% (30.8%). Other participant characteristics are presented in Table 1. Comparison of families who did or did not enroll siblings showed that those enrolling siblings had larger family size (eTable 6 in Supplement 1), but were similar in other respects.
Table 1. Index Child and Parent Descriptive Information in the Trial of Family-Based Behavioral Treatment for Childhood Obesity .
| Group assignment | Mean (SD) | |||||
|---|---|---|---|---|---|---|
| Index child | Parents | Siblings | ||||
| Family-based treatment (n = 226) | Usual care (n = 226) | Family-based treatment (n = 226) | Usual care (n = 226) | Family-based treatment (n = 54) | Usual care (n = 52) | |
| Age, y | 9.8 (1.9) | 9.7 (1.9) | 41.2 (7.8) | 41.4 (7.0) | 9.7 (3.6) | 10.4 (3.8) |
| Height, in | 56.9 (5.2) | 56.6 (5.2) | 65.2 (3.2) | 65.7 (3.4) | 55.1 (8.3) | 57.5 (8.5) |
| Weight, lb | 126.8 (42.2) | 126.5 (39.5) | 221.4 (59.9) | 230.3 (52.5) | 112.8 (55.9) | 138.1 (71.0) |
| BMI | 26.7 (5.0) | 26.9 (5.2) | 36.5 (7.6) | 37.5 (7.9) | 24.4 (5.6) | 27.4 (7.9) |
| Child BMI percentile | 97.3 (2.8) | 97.6 (2.6) | 95.5 (4.1) | 96.5 (3.6) | ||
| BMI category, No. (%) | ||||||
| Normal | 1 (0.4) | 2 (0.9) | 0 | 0 | 0 | 0 |
| Overweight | 35 (15.5) | 20 (8.9) | 47 (20.8) | 40 (17.7) | 18 (33.3) | 14 (26.9) |
| Obese | 190 (84.1) | 204 (90.3) | 179 (79.2) | 186 (82.3) | 36 (66.7) | 38 (73.1) |
| Percentage above median BMIa | 58.6 (27.2) | 60.1 (26.9) | 67.1 (35.1) | 71.2 (36.6) | 43.1 (24.4) | 56.4 (35.2) |
| Child percentage above 95th percentile BMI | 19.0 (20.0) | 20.5 (20.2) | 9.3 (17.5) | 18.1 (24.5) | ||
| Sex, No. (%) | ||||||
| Female | 120 (53) | 122 (54) | 196 (87) | 192 (85) | 30 (56) | 30 (58) |
| Male | 106 (47) | 104 (46) | 30 (13) | 34 (15) | 24 (44) | 22 (42) |
| Race and ethnicity, No. (%) | ||||||
| American Indian | 0 | 1 (0.4) | 0 | 1 (0.4) | 0 | 1 (2) |
| Asian | 5 (2) | 3 (1) | 4 (2) | 3 (1) | 1 (2) | 0 |
| Black | 58 (26) | 65 (29) | 59 (26) | 63 (28) | 14 (26) | 17 (33) |
| White | 131 (58) | 127 (56) | 148 (65) | 143 (63) | 29 (54) | 25 (48) |
| Multiracial | 17 (8) | 22 (10) | 2 (1) | 5 (2) | 5 (9) | 5 (10) |
| Other | 10 (4) | 8 (4) | 9 (4) | 11 (5) | 4 (7) | 3 (6) |
| Missing/refused | 5 (2) | 0 | 4 (2) | 0 | 1 (2) | 1 (2) |
| Hispanic or Latino, No. (%) | 21 (9) | 19 (8) | 17 (8) | 9 (4) | 6 (11) | 5 (10) |
| Site, No. (%) | ||||||
| Buffalo | 54 (23.9) | 55 (24.3) | Same as index child | 10 (18.5) | 9 (17.3) | |
| Columbus | 59 (26.1) | 57 (25.2) | 10 (18.5) | 14 (26.9) | ||
| Rochester | 46 (20.4) | 47 (20.8) | 14 (25.9) | 14 (26.9) | ||
| St Louis | 67 (29.6) | 67 (29.6) | 20 (37.0) | 15 (28.9) | ||
| Relationship of index child to index parent, No. (%) | ||||||
| Biological | 209 (92) | 213 (94) | ||||
| Adopted | 8 (4) | 9 (4) | ||||
| Stepchild | 0 | 1 (<1) | ||||
| Other relative | 8 (4) | 3 (1) | ||||
| Parent education, median (IQR), y | 14 (14-18) | 14 (14-18) | ||||
| Annual income in thousands, median (IQR), $ | 70 (35-125) | 75 (35-125) | ||||
| Annual income category, median (IQR), $ | ||||||
| <20 000 | 20 (8.9) | 11 (4.9) | ||||
| 20 000 to 40 000 | 45 (19.9) | 45 (19.9) | ||||
| 40 000 to <60 000 | 32 (14.2) | 40 (17.7) | ||||
| 60 000 to <80 000 | 25 (11.1) | 26 (11.5) | ||||
| 80 000 to <100 000 | 22 (9.7) | 30 (13.3) | ||||
| 100 000 to <120 000 | 23 (10.2) | 15 (6.6) | ||||
| 120 000 to <140 000 | 16 (7.1) | 19 (8.4) | ||||
| 140 000 to <160 000 | 14 (6.2) | 9 (4.0) | ||||
| 160 000 to <180 000 | 9 (4.0) | 8 (3.5) | ||||
| 180 000 to <200 000 | 7 (3.1) | 8 (3.5) | ||||
| ≥200 000 | 11 (4.9) | 12 (5.3) | ||||
| Missing | 2 (0.9) | 3 (1.3) | ||||
| Receiving public assistance, No. (%) | 9 (4.0) | 4 (1.8) | ||||
| Medicaid health insurance, No. (%) | 48 (21.2) | 45 (19.9) | ||||
Percentage above the age- and sex-specific 50th body mass index (BMI) percentile for kids and percentage above the sex-specific 50th BMI percentile for those aged 20 years for parents. Percentage above median BMI is the difference in observed BMI and the age and sex appropriate median BMI from Centers for Disease Control and Prevention growth charts expressed as a percentage of the median BMI ([BMI – BMI at 50th percentile]/BMI at 50th percentile) × 100. Likewise, percentage above the 95th BMI percentile is expressed relative to the 95th BMI percentile cutoff for obesity, so that numbers at 0 or below are nonobese, while numbers greater than 0 represent the degree of obesity.
Intervention Effects
The primary outcome, change in percentage above median BMI for the child at 24 months, was significantly better in the family-based treatment group than the usual care group (between-group difference, −6.21% [95% CI, −10.14% to −2.29%]; P = .002). Figure 2 presents longitudinal analyses stratified by family role and for the overall family. Boxplots are used in Figure 3 to present the distribution of data points also stratified by family role and overall family model. At 6 months, the between-group differences in change in percentage above median BMI between the intervention and usual care groups were −6.5% (−3.2% for family-based treatment vs 3.3% for usual care; 95% CI, −8.1% to −4.9%; P < .001) for children, −4.0% (−4.2% for family-based treatment vs −0.2% for usual care; 95% CI, −5.7% to −2.3%; P < .001) in parents, and −5.3% (−3.1% vs 2.2%; 95% CI, −8.5% to −2.2%; P = .001) in siblings. Differences between the intervention and usual care groups were maintained at 12, 18, and 24 months, with no significant interactions between intervention and follow-up time or between intervention, family role, and time from randomization to follow-up. The 0- to 24-month changes in percentage above median BMI for family-based treatment and usual care were 0.00% (95% CI, −2.20% to 2.20%) vs 6.48% (95% CI, 4.35%-8.61%) for children; −1.05% (95% CI, −3.79% to 1.69%) vs 2.92% (95% CI, 0.58%-5.26%) for parents; and 0.03% (95% CI, −3.03% to 3.10%) vs 5.35% (95% CI, 2.70%-8.00%) for siblings. Family-based treatment resulted in greater reductions in parent BMI than usual care (between-group difference, −1.1; 95% CI, −1.5 to −0.65, P < .001). Results did not change after adjusting for covariates; thus, the reported results are covariate-adjusted.
Figure 2. Covariance-Adjusted Mean Change From Baseline in Percentage Above Median Body Mass Index (BMI) for Child, Parent, Sibling, and the Family .
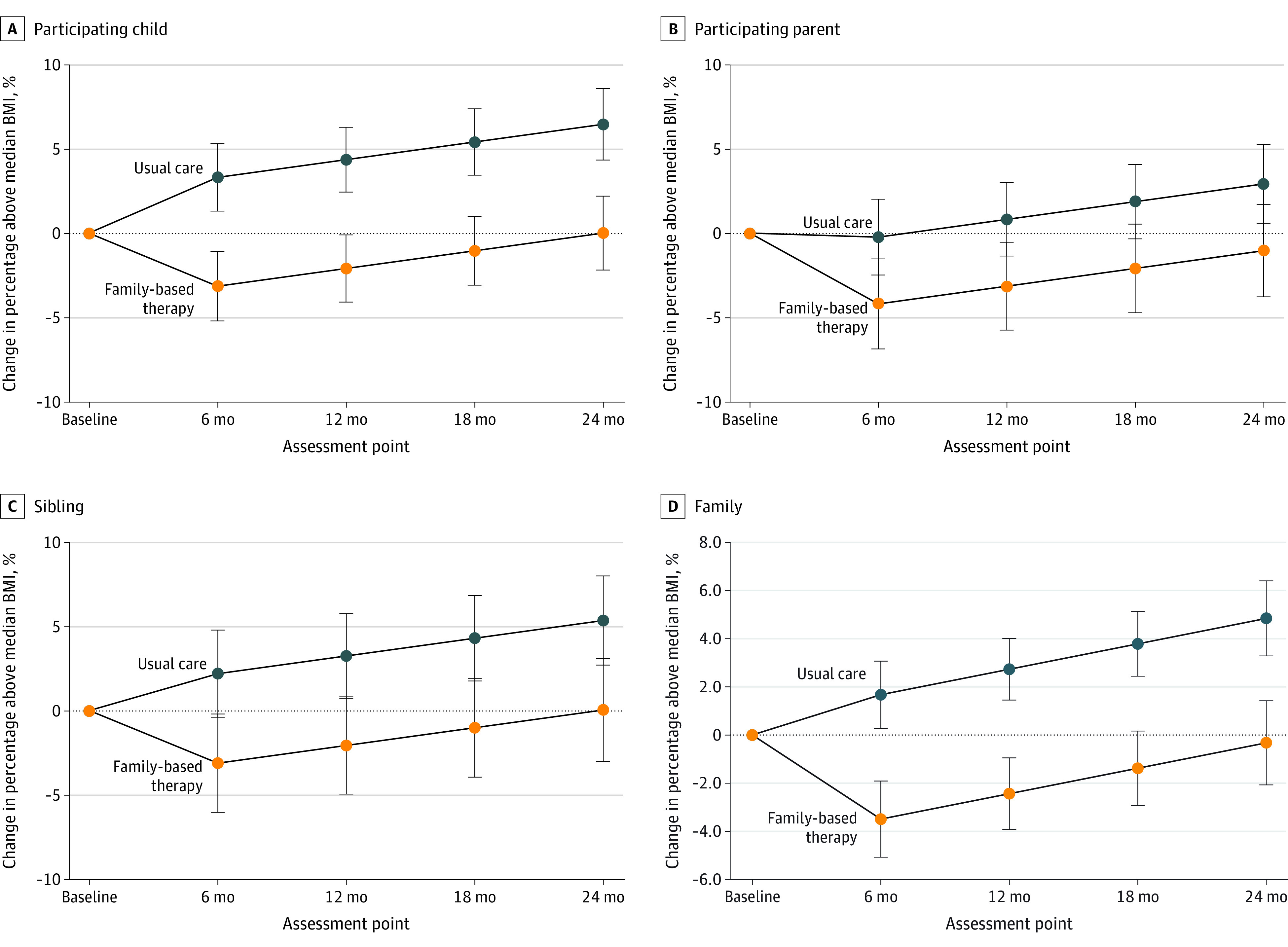
Whiskers indicate 95% CIs.
Figure 3. Distribution of Data Showing Change in Percentage Above Median Body Mass Index (BMI) From Baseline for Child, Parent, Sibling, and the Family.
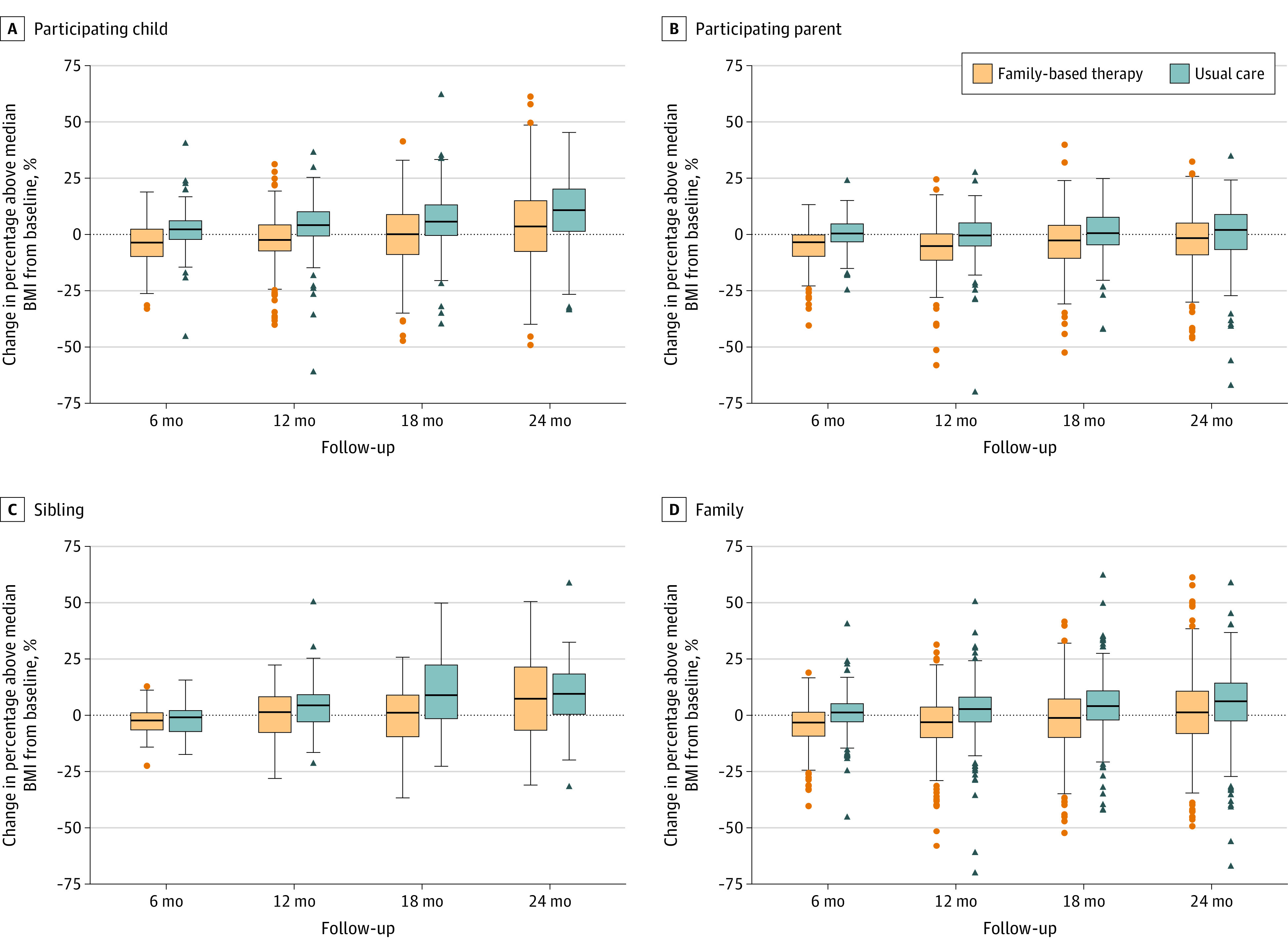
The middle of the boxplot is the median, the box is between the 25th and 75th percentile, and the whiskers are 1.5 times the interquartile range.
Clinically meaningful changes in weight outcomes by family role and study group are presented in Table 2. Nearly 3 times as many children receiving family-based treatment met a clinically meaningful weight outcome compared with those receiving usual care (27.0% vs 9.3%; P < .001).
Table 2. Percentage of Children, Siblings, and Parents Who Met Criteria for Clinically Meaningful Changes.
| Family member | Measure | No./total No. (%) | P value | |
|---|---|---|---|---|
| Family-based treatment | Usual care | |||
| Participating child | BMI z score reduction ≥0.25 | 53/196 (27.0) | 19/204 (9.3) | <.001 |
| Sibling | BMI z score reduction ≥0.25 | 11/40 (27.5) | 4/37 (10.8) | .06 |
| Participating parent | ≥5% reduction in weight | 43/150 (28.7) | 38/172 (22.1) | .18 |
In longitudinal analyses of families at 6 months, family-based treatment was associated with an overall familial decrease of 3.5% above median BMI (95% CI, −5.1% to −1.9%; P < .001), compared with an increase of 1.7% (95% CI, 0.3%-3.1%; P = .02) in the usual care group (difference, −5.2% [95% CI, −6.6% to −3.7%]; P < .001). Differences between the intervention and usual care groups were maintained at 12, 18, and 24 months, with no statistically significant interaction between treatment and follow-up time.
Results of analyses of primary outcomes are presented in eTable 7A-D in Supplement 1. Sensitivity analyses did not meaningfully affect results (eTable 8A-H in Supplement 1). No serious adverse events associated with study participation were reported (eTable 9 in Supplement 1).
Influence of Covariates on Intervention Effects
The number of months families experienced the COVID-19 pandemic when treatment was implemented remotely was associated with lowered treatment effectiveness in percentage above median BMI per month for children (0.54% [95% CI, 0.36%-0.73%]; P < .001) and siblings (0.62% [95% CI, 0.31%-0.94%]; P < .001), but not parents (−0.17% [95% CI, −0.37% to 0.03%]; P = .10). White children, compared with Black children, experienced stronger effects of family-based treatment (−6.22% [95% CI, −8.02% to −4.42%]; P < .001). In contrast, no significant difference was observed in Black parents compared with White parents (−0.82% [95% CI, −2.61 to 0.97]; P = .37) or Black siblings compared with White siblings (−2.92% [95% CI, −6.25 to 0.41]; P = .09).
In the intervention group, the mean number of sessions attended was 30.9, with a range of 0 to 92 sessions. Forty-five percent of children and 36% of parents attended 26 or more sessions. White families attended a mean (SD) of 36.7 (32.4) sessions compared with 24.7 (18.8) sessions for Black families (P = .001). Treatment attendance was significantly associated with child outcome at 24 months (r = −0.32; P < .001).
Associations of Weight Changes Among Family Members
Weight changes within families showed associations between change in percentage above median BMI for parents with children (unstandardized β = .07 [95% CI, .01-.12]; P = .02) and children with their siblings (β = .22 [95% CI, .09-.36]; P = .002). The age or sex of the index child relative to the sibling did not affect this relationship. No association was observed between parents and siblings in change in percentage above median BMI (β = .05 [95% CI, −.08-.18]; P = .44). Sibling weight change was significantly associated with weight change in the index child, but not in the parent.
Discussion
This randomized clinical trial found that family-based treatment for childhood overweight and obesity could be successfully implemented in pediatric primary care settings. The intervention led to improved weight outcomes over 24 months for children. In addition, family-based treatment had similar benefits for parents and for siblings who were not directly treated, beginning at 6 months and continuing through 24-month follow-up.
This study goes beyond previous research in that it adapted the family-based treatment intervention for use in primary care settings. Previous studies of intensive behavioral interventions, and subsequent implementation efforts in many US settings, have been based in specialty clinics. In this study, persons without prior skills in implementing behavior change were successfully trained to deliver the intervention in pediatric primary care settings. These findings may suggest a way for more clinical groups to implement national recommendations by the American Academy of Pediatrics8 and the US Preventive Services Task Force23 to offer intensive behavioral interventions for childhood overweight and obesity.
The magnitude of effects (ie, the changes in percentage above median BMI) was smaller than in other studies with highly trained coaches.5 This trial in primary care settings observed a between-group difference of −6.1% in percentage above median BMI, compared with a projected reduction in specialty clinics27,28,29,30,33,34 of −10.6% using the same measure. In addition, 27% of children in this trial’s treatment group had clinically meaningful BMI z score effects,23 compared with 45% when treatment was implemented in specialty clinics.27,28,29,30,31,32
Community-based effectiveness trials typically have attenuated treatment effects compared with treatment implemented in specialty clinics,25,26 and the treatment delivered in this study was adapted in ways that could have diminished its efficacy.4,17,24 In previous studies in specialty clinics, family-based treatment has typically been delivered using a mixed individual plus group format rather than an individualized format. It has typically used a structured schedule (eg, weekly for 8 weeks then biweekly for 16 weeks) rather than individualized treatment made available over 24 months. Treatment in specialty clinics is usually implemented by highly trained staff, while the current study used newly trained coaches. In addition, a large percentage of families in this trial received treatment during the COVID-19 pandemic.
This study’s results were consistent with a previous study of family-based treatment effects in pediatric primary care for children aged 2 to 5 years and their parents with obesity.36 This study also found that siblings benefited from treatment. Sibling changes in weight outcomes were related to changes of the index child, but not the parent. These results suggest siblings may be modeling the health behaviors of the index children37 rather than the parent. However, changes in parenting38 and the shared family environment11 could simultaneously influence the index child and sibling.
This study’s findings suggest that embedding behaviorally trained interventionists within primary care practices could be tested for other common behavioral issues that may benefit from improving parenting practices.39 Currently, payment for scalable family-based intensive behavioral treatment may pose a barrier for some families, but federal and state initiatives consistent with the recommended US Preventive Services Task Force and American Academy of Pediatrics levels of care8,23 are being implemented.40,41
Limitations
This study has several limitations. First, the intensity of the family-based treatment intervention evaluated here could pose a barrier in time and expertise for many primary care settings.8 It generally requires at least 26 sessions with the family, consistent with previous reports that found that such intensity is required to achieve benefits. Generalizability might have been improved if existing primary care clinic staff had been trained to implement treatment, but this approach might have reduced participation by some clinics due to staffing challenges.
Second, sibling participation was not required to participate in this study, resulting in a smaller sample of siblings than index children. There could have been selection bias toward families who included siblings. However, the only differences in family characteristics who included siblings was family size, which was expected because larger families are more likely to have multiple children with obesity. No data were obtained about nonparticipating parents; such data could provide additional information on treatment generalization within families. Third, the percentage of parents lost to follow-up may limit the ability to generalize parental outcomes to the broader population. Fourth, although this study observed significant intervention effects on participants’ weight outcomes, it was implemented during the COVID-19 pandemic, which, results showed, had a negative influence on the magnitude of change.
Conclusions
Family-based behavioral treatment for childhood overweight and obesity was successfully implemented in pediatric primary care settings and led to better weight outcomes than usual care for index children and parents, as well as their nontreated siblings. These findings suggest this treatment may offer a novel approach for families with 2 or more children with excess body weight.
eMethods
Trial Protocol
Data Sharing Statement
References
- 1.Magarey AM, Daniels LA, Boulton TJ, Cockington RA. Predicting obesity in early adulthood from childhood and parental obesity. Int J Obes Relat Metab Disord. 2003;27(4):505-513. doi: 10.1038/sj.ijo.0802251 [DOI] [PubMed] [Google Scholar]
- 2.Drozdz D, Alvarez-Pitti J, Wójcik M, et al. Obesity and cardiometabolic risk factors: from childhood to adulthood. Nutrients. 2021;13(11):13. doi: 10.3390/nu13114176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein LH, Paluch RA, Wrotniak BH, et al. Cost-effectiveness of family-based group treatment for child and parental obesity. Child Obes. 2014;10(2):114-121. doi: 10.1089/chi.2013.0123 [DOI] [PubMed] [Google Scholar]
- 4.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol. 1994;13(5):373-383. doi: 10.1037/0278-6133.13.5.373 [DOI] [PubMed] [Google Scholar]
- 5.Wilfley DE, Tibbs TL, Van Buren DJ, Reach KP, Walker MS, Epstein LH. Lifestyle interventions in the treatment of childhood overweight: a meta-analytic review of randomized controlled trials. Health Psychol. 2007;26(5):521-532. doi: 10.1037/0278-6133.26.5.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wrotniak BH, Epstein LH, Paluch RA, Roemmich JN. Parent weight change as a predictor of child weight change in family-based behavioral obesity treatment. Arch Pediatr Adolesc Med. 2004;158(4):342-347. doi: 10.1001/archpedi.158.4.342 [DOI] [PubMed] [Google Scholar]
- 7.Klein JD, Sesselberg TS, Johnson MS, et al. Adoption of body mass index guidelines for screening and counseling in pediatric practice. Pediatrics. 2010;125(2):265-272. doi: 10.1542/peds.2008-2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampl SE, Hassink SG, Skinner AC, et al. Clinical practice guidelines for the evaluation and treatment of chidren and adolescents with obesity. Pediatrics. 2023;151(2):e2022060640. doi: 10.1542/peds.2022-060640 [DOI] [PubMed] [Google Scholar]
- 9.Epstein LH, Paluch RA, Raynor HA. Sex differences in obese children and siblings in family-based obesity treatment. Obes Res. 2001;9(12):746-753. doi: 10.1038/oby.2001.103 [DOI] [PubMed] [Google Scholar]
- 10.Natale RA, Messiah SE, Asfour L, Uhlhorn SB, Delamater A, Arheart KL. Role modeling as an early childhood obesity prevention strategy: effect of parents and teachers on preschool children’s healthy lifestyle habits. J Dev Behav Pediatr. 2014;35(6):378-387. doi: 10.1097/DBP.0000000000000074 [DOI] [PubMed] [Google Scholar]
- 11.Rosenkranz RR, Dzewaltowski DA. Model of the home food environment pertaining to childhood obesity. Nutr Rev. 2008;66(3):123-140. doi: 10.1111/j.1753-4887.2008.00017.x [DOI] [PubMed] [Google Scholar]
- 12.Arcan C, Neumark-Sztainer D, Hannan P, van den Berg P, Story M, Larson N. Parental eating behaviours, home food environment and adolescent intakes of fruits, vegetables and dairy foods: longitudinal findings from Project EAT. Public Health Nutr. 2007;10(11):1257-1265. doi: 10.1017/S1368980007687151 [DOI] [PubMed] [Google Scholar]
- 13.Kalakanis LE, Goldfield GS, Paluch RA, Epstein LH. Parental activity as a determinant of activity level and patterns of activity in obese children. Res Q Exerc Sport. 2001;72(3):202-209. doi: 10.1080/02701367.2001.10608953 [DOI] [PubMed] [Google Scholar]
- 14.Dunifon R, Fomby P, Musick K. Siblings and children’s time use in the United States. Demogr Res. 2017;37:1611-1624. doi: 10.4054/DemRes.2017.37.49 [DOI] [Google Scholar]
- 15.Epstein LH, Schechtman KB, Kilanowski C, et al. Implementing family-based behavioral treatment in the pediatric primary care setting: design of the PLAN study. Contemp Clin Trials. 2021;109:106497. doi: 10.1016/j.cct.2021.106497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120(suppl 4):S254-S288. doi: 10.1542/peds.2007-2329F [DOI] [PubMed] [Google Scholar]
- 17.Wilfley DE, Stein RI, Saelens BE, et al. Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. JAMA. 2007;298(14):1661-1673. doi: 10.1001/jama.298.14.1661 [DOI] [PubMed] [Google Scholar]
- 18.Wilfley DE, Saelens BE, Stein RI, et al. Dose, content, and mediators of family-based treatment for childhood obesity: a multisite randomized clinical trial. JAMA Pediatr. 2017;171(12):1151-1159. doi: 10.1001/jamapediatrics.2017.2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epstein LH, McKenzie SJ, Valoski A, Klein KR, Wing RR. Effects of mastery criteria and contingent reinforcement for family-based child weight control. Addict Behav. 1994;19(2):135-145. doi: 10.1016/0306-4603(94)90038-8 [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flegal KM, Cole TJ. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. Natl Health Stat Report. 2013;(63):1-3. [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC growth charts for the United States: Methods and development. Vital Health Statistics. Series 11. Vol 246. National Center for Health Statistics; 2002:1-90. [PubMed] [Google Scholar]
- 23.Grossman DC, Bibbins-Domingo K, Curry SJ, et al. ; US Preventive Services Task Force . Screening for obesity in children and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317(23):2417-2426. doi: 10.1001/jama.2017.6803 [DOI] [PubMed] [Google Scholar]
- 24.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26(4):381-391. doi: 10.1037/0278-6133.26.4.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacher PM, Kolotourou M, Chadwick PM, et al. Randomized controlled trial of the MEND program: a family-based community intervention for childhood obesity. Obesity (Silver Spring). 2010;18(suppl 1):S62-S68. doi: 10.1038/oby.2009.433 [DOI] [PubMed] [Google Scholar]
- 26.Pagoto SL, Kantor L, Bodenlos JS, Gitkind M, Ma Y. Translating the diabetes prevention program into a hospital-based weight loss program. Health Psychol. 2008;27(1S):S91-S98. doi: 10.1037/0278-6133.27.1.S91 [DOI] [PubMed] [Google Scholar]
- 27.Epstein LH, Wing RR, Koeske R, Andrasik F, Ossip DJ. Child and parent weight loss in family-based behavior modification programs. J Consult Clin Psychol. 1981;49(5):674-685. doi: 10.1037/0022-006X.49.5.674 [DOI] [PubMed] [Google Scholar]
- 28.Epstein LH, Paluch RA, Gordy CC, Dorn J. Decreasing sedentary behaviors in treating pediatric obesity. Arch Pediatr Adolesc Med. 2000;154(3):220-226. doi: 10.1001/archpedi.154.3.220 [DOI] [PubMed] [Google Scholar]
- 29.Epstein LH, Paluch RA, Gordy CC, Saelens BE, Ernst MM. Problem solving in the treatment of childhood obesity. J Consult Clin Psychol. 2000;68(4):717-721. doi: 10.1037/0022-006X.68.4.717 [DOI] [PubMed] [Google Scholar]
- 30.Epstein LH, Paluch RA, Kilanowski CK, Raynor HA. The effect of reinforcement or stimulus control to reduce sedentary behavior in the treatment of pediatric obesity. Health Psychol. 2004;23(4):371-380. doi: 10.1037/0278-6133.23.4.371 [DOI] [PubMed] [Google Scholar]
- 31.Epstein LH, Roemmich JN, Paluch RA, Raynor HA. Physical activity as a substitute for sedentary behavior in youth. Ann Behav Med. 2005;29(3):200-209. doi: 10.1207/s15324796abm2903_6 [DOI] [PubMed] [Google Scholar]
- 32.Epstein LH, Paluch RA, Beecher MD, Roemmich JN. Increasing healthy eating vs reducing high energy-dense foods to treat pediatric obesity. Obesity (Silver Spring). 2008;16(2):318-326. doi: 10.1038/oby.2007.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epstein LH, Wing RR, Koeske R, Valoski A. A comparison of lifestyle exercise, aerobic exercise and calisthenics on weight loss in obese children. Behav Ther. 1985;16:345-356. doi: 10.1016/S0005-7894(85)80002-2 [DOI] [Google Scholar]
- 34.Epstein LH, Roemmich JN, Stein RI, Paluch RA, Kilanowski CK. The challenge of identifying behavioral alternatives to food: clinic and field studies. Ann Behav Med. 2005;30(3):201-209. doi: 10.1207/s15324796abm3003_4 [DOI] [PubMed] [Google Scholar]
- 35.Pals SL, Murray DM, Alfano CM, Shadish WR, Hannan PJ, Baker WL. Individually randomized group treatment trials: a critical appraisal of frequently used design and analytic approaches. Am J Public Health. 2008;98(8):1418-1424. doi: 10.2105/AJPH.2007.127027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quattrin T, Roemmich JN, Paluch R, Yu J, Epstein LH, Ecker MA. Efficacy of family-based weight control program for preschool children in primary care. Pediatrics. 2012;130(4):660-666. doi: 10.1542/peds.2012-0701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brody GH. Sibling relationship quality: its causes and consequences. Annu Rev Psychol. 1998;49:1-24. doi: 10.1146/annurev.psych.49.1.1 [DOI] [PubMed] [Google Scholar]
- 38.Balantekin KN, Anzman-Frasca S, Francis LA, Ventura AK, Fisher JO, Johnson SL. Positive parenting approaches and their association with child eating and weight: a narrative review from infancy to adolescence. Pediatr Obes. 2020;15(10):e12722. doi: 10.1111/ijpo.12722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Haines J, Charlton BM, VanderWeele TJ. Positive parenting improves multiple aspects of health and well-being in young adulthood. Nat Hum Behav. 2019;3(7):684-691. doi: 10.1038/s41562-019-0602-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US Office of Personnel Management . Technical Guidance and Instructions for 2023 Benefit Proposals. Federal Employee Health Benefits Program Carrier Letter 2022-04:25-26. March 16, 2023. https://www.opm.gov/healthcare-insurance/healthcare/carriers/2022/2022-04.pdf
- 41.Missouri Department of Health and Senior Services . MO HealthNet Division. Biopsychosocial treatment for youth and adults. https://health.mo.gov/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Trial Protocol
Data Sharing Statement