Abstract
Introduction
Sarcopenia is a prevalent risk factor for falls and fractures, and it affects the physical function and mortality of older people. The present study was performed to assess the prevalence of sarcopenia in patients who underwent rehabilitation after hip fracture surgery and to examine the association of sarcopenia with physical and cognitive function outcomes.
Methods
This case–control study involved 132 patients who were admitted to a convalescent rehabilitation ward at a single hospital after surgical treatment of hip fractures from April 2018 to March 2020. The skeletal muscle mass index was examined using whole-body dual-energy X-ray absorptiometry. The Asian Working Group for Sarcopenia 2019 diagnostic criteria were applied on admission. We compared the walking speed, Mini-Mental State Examination (MMSE) score, and Functional Independence Measure (FIM) score between the sarcopenia group and non-sarcopenia group on admission and on discharge.
Results
The prevalence of sarcopenia was 59.8%. In the non-sarcopenia group, the walking speed, MMSE score, FIM total score, FIM motor score, and FIM cognitive score were significantly lower on admission than those on discharge (P < .05). In the sarcopenia group, the walking speed, MMSE score, FIM total score, and FIM motor score were significantly lower on admission than those on discharge (P < .05); there was no significant difference in the FIM cognitive score between admission and discharge. On both admission and discharge, the MMSE score, FIM total score, FIM motor score, and FIM cognitive score were significantly better in the non-sarcopenia group than those in the sarcopenia group.
Conclusions
After postoperative rehabilitation of hip fractures in patients with and without sarcopenia, physical and cognitive function outcomes on discharge were significantly better than those on admission. Patients with sarcopenia had significantly worse physical and cognitive function outcomes than patients without sarcopenia both on admission and on discharge.
Keywords: functional independence measure, handgrip strength, hip fracture, mini-mental state examination, sarcopenia
Introduction
More than one-third of people older than 65 years fall each year, 1 and 4.1% of these falls result in fractures. 2 Among these fractures, hip fractures are very common, with approximately 150,000 such fractures per year in Japan 3 and 340,000 per year in the United States. 4 Advanced age, female sex, and declining cognitive and physical function have been cited as causes of falls in older adults.5,6
Advanced age, lower body weight, osteoporosis, dementia, and several other risk factors are associated with hip fracture.7,8 Sarcopenia is the loss of skeletal muscle mass due to aging 9 and is a predictor of mortality of all causes among older adults living in the community. 10 Older adults with sarcopenia are at high risk for falls and hip fractures11,12 because of muscle weakness and poor balance. 13 Sarcopenia, which results in a decrease in skeletal mechanical loading and adaptive bone remodeling, is associated with reduced bone density and osteoporosis. 14 All of these adverse effects of sarcopenia are responsible for the increased risk of hip fracture in the geriatric population. However, the relationship between sarcopenia and hip fractures is unclear.
Sarcopenia increases the risks of a bedridden status and falls, 15 and the presence of sarcopenia is thought to be important to the outcome after hip fracture surgery. In addition, because cognitive decline is 1 of the causes of falls, 5 the improvement of cognitive function as well as physical function is important to prevent re-fracture and to improve future activities of daily living. 16 Generally, rehabilitation can improve patients’ physical functioning after surgical treatment of hip fractures, 17 but whether the presence of sarcopenia affects the clinical outcome of postoperative rehabilitation remains unknown.
This study was performed to evaluate the prevalence of sarcopenia and investigate its association with physical and cognitive function outcomes in patients with hip fractures who underwent rehabilitation after hip fracture surgery.
Material and Methods
Study Design and Participants
We retrospectively enrolled patients who were admitted to a convalescent rehabilitation ward at a single hospital after surgical treatment of hip fractures from April 2018 to March 2020. Ethics approval was obtained from the ethics review board of our hospital. The present study was performed in accordance with the World Medical Association Declaration of Helsinki principles. We calculated the necessary sample size for an alpha value of .05 and power of .80 using G*Power 3 statistical software18,19 and found it to be 110. The inclusion criterion was surgical treatment of a hip fracture (femoral neck, intertrochanteric, or subtrochanteric fracture). Patients younger than 65 years, with a history of contralateral hip fracture, with pathological fracture, with no measures of fitness, with multiple fractures after multiple traumas, undergoing dialysis, and with paralysis or significant declines in activities of daily living after stroke were excluded from the study. Patients who were admitted to an acute care hospital for >60 days after hip fracture and those who were admitted to a convalescent rehabilitation ward for ≥91 days were also excluded because public medical insurance did not cover acute care or rehabilitation beyond these times.
Outcome Measurements
The patients’ baseline clinical and demographic data were collected, including age, sex, height, weight, and body mass index at the time of admission to our hospital. The skeletal muscle mass index (SMI) was measured using whole-body dual-energy X-ray absorptiometry (Hologic Horizon-WI densitometer; Hologic Inc, Marlborough, MA, USA). The handgrip strength of both upper extremities was measured using a digital hand dynamometer (TKK 5401; Takei Scientific Instruments, Niigata, Japan). Two tests were performed with the right and left hands. The best performance of the trials was used for the analysis. 20 Gait speed was measured using the 10-m walk test. 20 The length of stay in the convalescent rehabilitation ward was also recorded. The walking speed of patients who could not perform this test was recorded as 0 m/s. Total protein, albumin, and HbA1c were also measured as general blood indices.
All patients’ cognitive level was evaluated with the Mini-Mental State Examination (MMSE) 21 within 1 week after admission to the rehabilitation ward and on discharge, by physical therapists or occupational therapists.
The Functional Independence Measure (FIM) 22 is a tool used to measure a patient’s level of disability at admission and on discharge; it is an 18-item measurement tool that examines motor and cognitive function. The motor subscale consists of 13 items (eating, grooming, bathing, dressing of the upper body, dressing of the lower body, toileting, bladder management, bowel management, transferring between bed and chair, transferring in toilet, transferring in shower, walking, and climbing stairs), and the cognitive subscale consists of 5 items (comprehension, expression, social interaction, problem solving, and memory). Each item is rated on a 7-point ordinal scale ranging from a score of 1 (“total assistance with helper”) to 7 (“complete independence with no helper”). A higher score means that the patient is more independent in performing the tasks associated with that item. All FIM scores in the rehabilitation department were recorded by physical therapists or occupational therapists within 1 week after admission to the rehabilitation ward and at discharge.
Sarcopenia Definition
Sarcopenia was diagnosed based on the Asian Working Group for Sarcopenia 2019 diagnostic criteria. The cutoff values for hand grip strength for men and women were <26 kg and <18 kg, respectively. Low walking speed was defined as <1.0 m/s. The cutoff values of the SMI for men and women were <7.0 kg/m2 and <5.4 kg/m2, respectively.
In addition, the patients were classified into 2 groups at the time of admission: the sarcopenia group and the non-sarcopenia group.
Rehabilitation Dose
In our convalescent rehabilitation ward, the patients were able to undergo inpatient rehabilitation for 80 minutes (40 minutes × 2 times) per day for 2 months after transfer to a postoperative rehabilitation hospital. The physical therapists mainly changed the rehabilitation program in a stepwise manner according to the patient’s postoperative condition. First, acupressure and massage were used to relax the muscles of the lower limbs. This was followed by range-of-motion training of the hip and knee joints. Second, muscle strengthening was performed, focusing on the lower limbs. Finally, pick-up walkers and four-wheeled walkers were used for gait training, leading to walking with a T-shaped cane, and then to independent walking training. Furthermore, before the patients returned home, physical therapists and occupational therapists evaluated the patients and provided instruction regarding how to manage stairs and steps, bathing, and toileting. The time allocation for each rehabilitation session could be adjusted according to the patient’s needs.
Statistical Analysis
Continuous data are presented as mean and standard deviation. All assessment results were subjected to the Shapiro–Wilk test for normality, and subsequent tests were selected based on the results. Data for all cases were examined by the Mann–Whitney U test using SPSS for Windows version 25.0 (IBM Corp, Armonk, NY, USA). A P value of <.05 was considered statistically significant.
Results
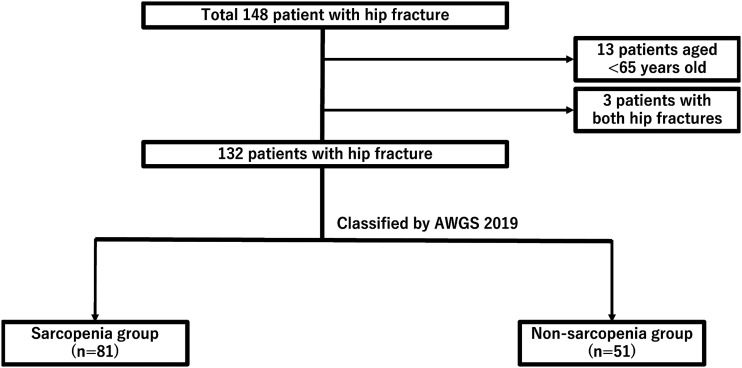
In total, 148 patients underwent rehabilitation in our hospital. We excluded 13 patients younger than 65 years and 3 patients with bilateral hip fractures. Therefore, 132 patients were enrolled in this study (Figure 1). The patients were treated with 53 bipolar hemiarthroplasties and 79 internal fixations. The patients comprised 91 women (68.9%) and 41 men (31.1%) with a mean age of 81.2 ± 7.6 years. Of the 132 patients, 53 patients had no sarcopenia, namely 37 women (69.8%) and 16 men (30.2%), and 79 patients had sarcopenia, namely 54 women (68.4%) and 25 men (31.6%). Sarcopenia was found in 59.8% of the study cohort. Weight, body mass index, handgrip strength, SMI, and total protein were significantly lower in the sarcopenia group compared with the non-sarcopenia group (Table 1). However, there was no significant difference in age, sex, height, length of rehabilitation stay, walking speed, albumin, or HbA1c.
Figure 1.
Flow diagram showing the study design and finalization of the study size.
Table 1.
Comparison of the Characteristics of the Non-Sarcopenia and Sarcopen.ia Groups in the Convalescent Rehabilitation Ward.
| Total n = 132 | Non-sarcopenia Group n = 51 | Sarcopenia Group n = 81 | P Value | |
|---|---|---|---|---|
| Age, years (SD) | 81.8 (7.6) | 80.5 (7.9) | 82.6 (7.3) | .048 |
| Gender, n (%) | ||||
| Female | 91 (68.9) | 37 (72.5) | 54 (66.7) | .479 |
| Male | 41 (31.1) | 14 (27.5) | 27 (33.3) | |
| Height, cm (SD) | 153.0 (9.7) | 153.4 (.1) | 152.8 (.1) | .774 |
| Weight, kg (SD) | 48.9 (11.1) | 54.7 (11.5) | 45.2 (9.2) | <.001 |
| BMI, kg/m2 (SD) | 20.8 (3.9) | 23.2 (4.1) | 19.3 (2.8) | <.001 |
| Length of stay, day (SD) | 55.5 (7.5) | 55.6 (7.1) | 55.5 (7.7) | .827 |
| Handgrip strength, kg (SD) | 16.2 (7.1) | 19.8 (7.9) | 14.0 (5.5) | <.001 |
| Walking speed, m/s (SD) | .38 (.52) | .36 (.46) | .39 (.56) | .958 |
| SMI, kg/m2 (SD) | 5.3 (.9) | 5.8 (.8) | 5.0 (.7) | <.001 |
| Total protein, g/dl (SD) | 6.8 (.6) | 6.9 (.6) | 6.7 (.6) | .014 |
| Albumin, g/dl (SD) | 3.6 (.4) | 3.6 (.5) | 3.5 (.4) | .200 |
| HbA1c, % (SD) | 5.7 (.8) | 5.8 (.7) | 5.7 (.8) | .437 |
Abbreviations: BMI, body mass index; SMI, skeletal muscle mass index.
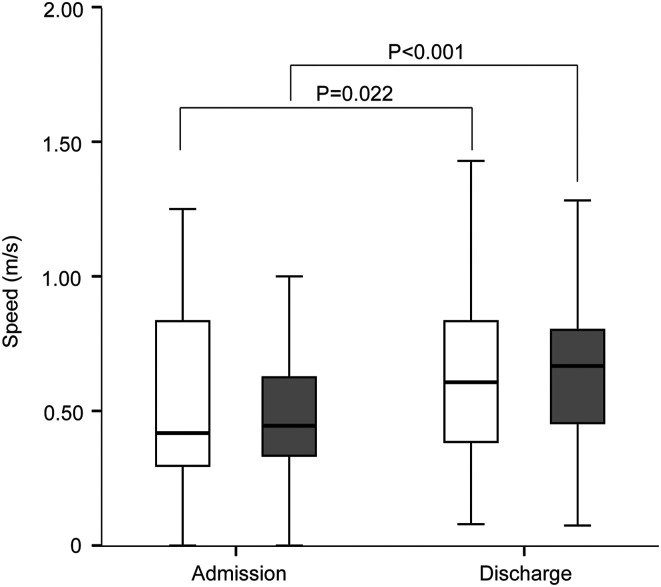
In the non-sarcopenia group, the median walking speed was higher on discharge than that on admission (Figure 2; Table 2). In the sarcopenia group, the median walking speed was higher on discharge than that on admission. There was no significant difference in the walking speed between the sarcopenia group and non-sarcopenia group on either admission or discharge.
Figure 2.
Improvement in walking speed between the sarcopenia group and non-sarcopenia group on admission and discharge. White boxes, non-sarcopenia group; gray boxes, sarcopenia group. *P < .05, **P < .005, ***P < .001.
Table 2.
Comparison of the Non-Sarcopenia and Sarcopenia Groups with Regard to Differences Between Admission and Discharge.
| Outcomes | Non-Sarcopenia Group (n = 51) | Sarcopenia Group (n = 81) | ||||
|---|---|---|---|---|---|---|
| Admission | Discharge | P Value | Admission | Discharge | P Value | |
| Walking speed, m/s (SD) | .36 (.46) | .41 (.43) | .025 | .39 (.57) | .45 (.51) | <.001 |
| MMSE score (SD) | 24.8 (5.0) | 25.8 (5.0) | .003 | 22.2 (5.3) | 22.8 (5.1) | .021 |
| FIM score | ||||||
| Total score (SD) | 86.2 (15.2) | 105.2 (16.9) | <.001 | 75.6 (19.8) | 93.7 (20.0) | <.001 |
| FIM motor score (SD) | 58.1 (12.0) | 75.8 (12.3) | <.001 | 51.7 (15.6) | 68.1 (15.2) | <.001 |
| FIM cognitive score (SD) | 28.2 (5.1) | 29.4 (5.6) | .001 | 23.9 (7.0) | 25.6 (6.4) | <.001 |
Abbreviations: FIM, Functional Independence Measure; MMSE, Mini-Mental State Examination.
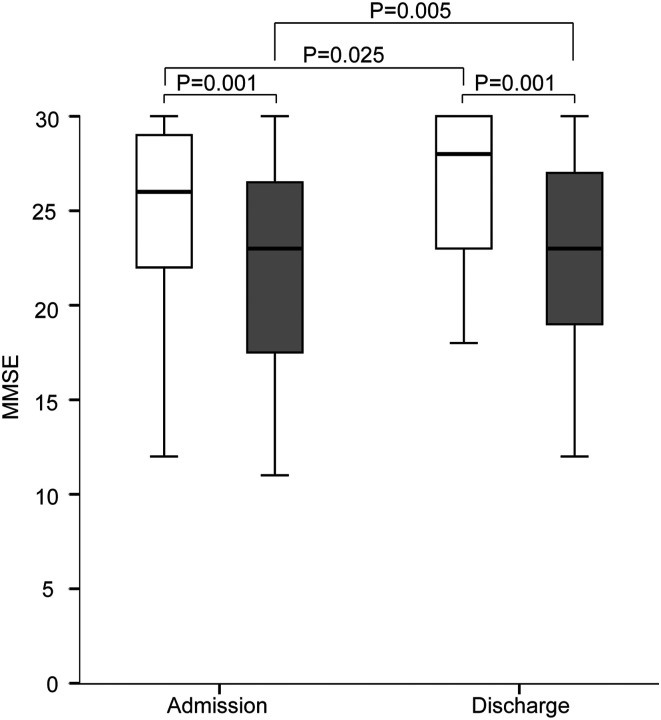
The MMSE score was significantly higher in the non-sarcopenia group than that in the sarcopenia group on admission and discharge (Figure 3). In both groups, the MMSE score was higher on discharge than that on admission.
Figure 3.
Improvement in Mini-Mental State Examination (MMSE) score between the sarcopenia group and non-sarcopenia group on admission and discharge. White boxes, non-sarcopenia group; gray boxes, sarcopenia group. *P < .05, **P < .005, ***P < .001.
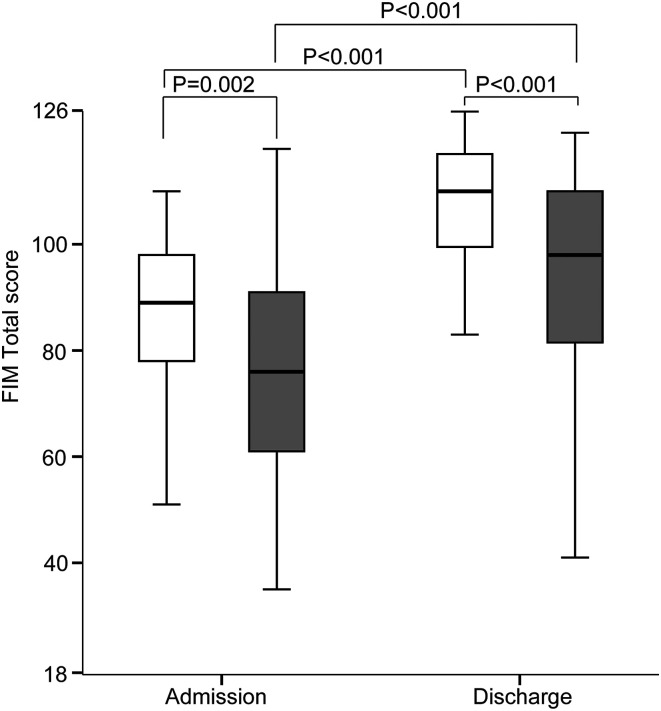
The FIM total score was significantly higher in the non-sarcopenia group than that in the sarcopenia group on admission and discharge (Figure 4). In both groups, the FIM total score was higher on discharge than that on admission.
Figure 4.
Improvement in functional independence measure (FIM) total score between the sarcopenia group and non-sarcopenia group on admission and discharge. White boxes, non-sarcopenia group; gray boxes, sarcopenia group. *P < .05, **P < .005, ***P < .001.
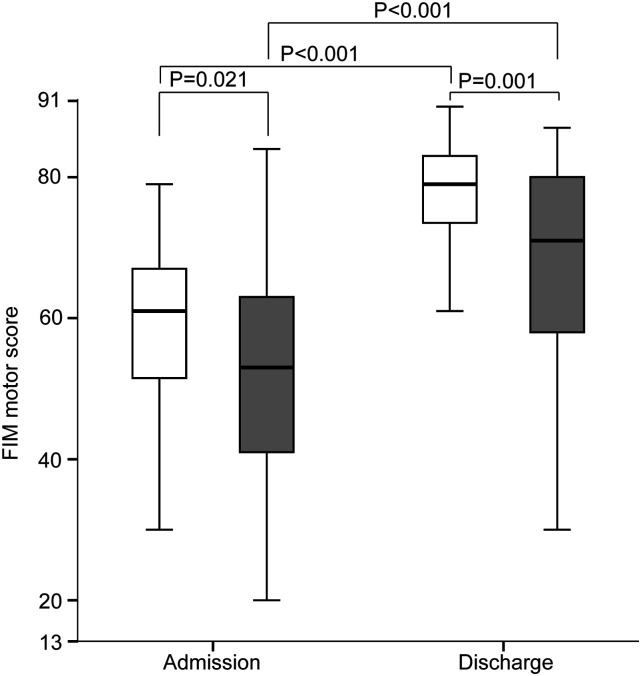
The FIM motor score was significantly higher in the non-sarcopenia group than that in the sarcopenia group on admission and discharge (Figure 5). In both groups, the FIM motor score was higher on discharge than that on admission.
Figure 5.
Improvement in functional independence measure (FIM) motor score between the sarcopenia group and non-sarcopenia group on admission and discharge. White boxes, non-sarcopenia group; gray boxes, sarcopenia group. *P < .05, **P < .005, ***P < .001.
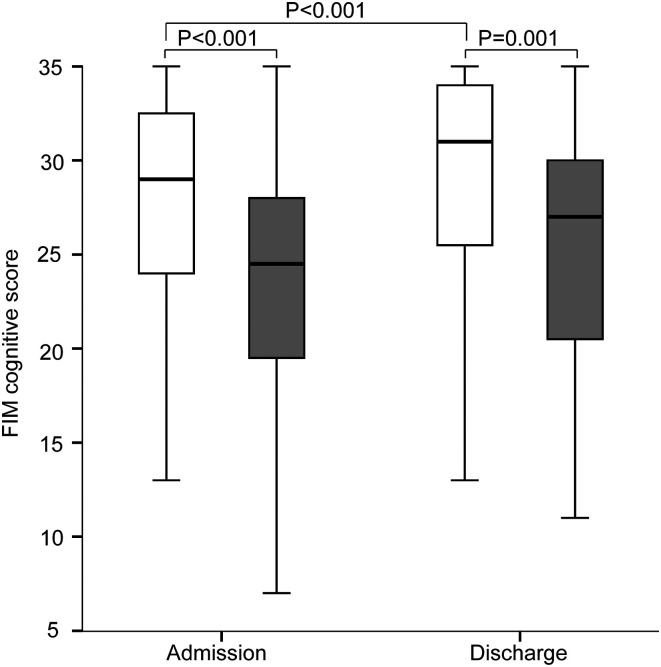
The FIM cognitive score was significantly higher in the non-sarcopenia group than that in the sarcopenia group on admission and discharge (Figure 6). In the non-sarcopenia group, the FIM cognitive score was higher on discharge than that on admission.
Figure 6.
Improvement in functional independence measure (FIM) cognitive score between the sarcopenia group and non-sarcopenia group on admission and discharge. White boxes, non-sarcopenia group; gray boxes, sarcopenia group. *P < .05, **P < .005, ***P < .001.
Discussion
In this study, the prevalence of sarcopenia after a hip fracture was 59.8%. Both on admission and after rehabilitation, cognitive and motor function were worse in patients with sarcopenia compared with patients without sarcopenia. The results of this study showed that rehabilitation improved not only motor function but also cognitive function. Both cognitive and motor function were found to improve even in the presence of sarcopenia.
The prevalence of sarcopenia in patients with hip fractures ranges from 17% to 70%23-25 according to the population, diagnostic tools, and definition of sarcopenia. In this study, sarcopenia was found in 59.8% of the total study population (in 59.3% of women and 65.9% of men with hip fractures). Between 2 studies that used dual-energy X-ray absorptiometry to measure low muscle mass, 1 study revealed a prevalence of 21.8% in women and 86.7% in men, 26 and the other revealed a prevalence of 44.7% in women and 81.1% in men. 27 Other reports focused on the prevalence of sarcopenia in the acute phase; however, our study focused on the subacute phase after surgery at another hospital, which may have changed the proportions.
Rehabilitation after hip fracture surgery in patients with or without sarcopenia improved the walking speed and FIM motor score, in this study. In the published literature, the Minimally Clinically Important Difference for walking speed in hip fracture patients is .10 m/s, 28 whereas in the current study, the difference was less than .10 m/s. However, in 1 study, physical therapy produced significantly greater improvements in strength, gait speed, balance, and perceived health in community-dwelling frail patients with hip fracture compared with controls, although there was no effect on bone mineral density or fat-free mass. 17 In another study, 30 minutes of daily rehabilitation in patients with sarcopenia after hip fracture improved not only physical function but also cognitive function. 29 Our study also showed that the MMSE score improved significantly after rehabilitation in both patients with and without sarcopenia. The non-sarcopenia group showed a significant improvement in FIM cognitive scores after rehabilitation, while the sarcopenia group tended to improve, without a significant difference. In another study, the MMSE score was also reported to be significantly improved after rehabilitation for hip fracture in both patients with and without sarcopenia. 30 These findings suggest that rehabilitation after hip fracture improves physical and cognitive function.
Cognitive function is an important factor influencing rehabilitation outcomes for patients after hip fracture. Studies have provided various findings regarding the impact of cognitive impairment on functional outcomes. Cognitive impairment has been reported as a significant risk factor for worse rehabilitation outcomes. 31 However, the treatment of behavioral psychological symptoms of dementia can provide better functional recovery during rehabilitation after hip fracture. 32
This study had several limitations. First, the observational period was only approximately 2-3 months; the 1- or 2-year outcome should also be evaluated in future studies. However, we were able to evaluate the effects of rehabilitation after hip fracture surgery. Second, the patients’ pre-injury status was unknown and could be evaluated only after fracture surgery. The next study will be a prospective study. Third, the possibility of anesthesia-induced cognitive decline cannot be ruled out. Cognitive function declines to 12% in older patients within 3 months after general anesthesia for surgery. 33 However, the association between anesthesia and cognitive decline is difficult to clarify because of inconsistencies regarding the definitions and diagnoses. Finally, the number of patients was small; therefore, our findings should be confirmed in a large cohort. We plan to perform a large cohort study of patients with hip fracture to examine physical and cognitive outcomes.
Conclusions
The prevalence of sarcopenia was 59.8% in this study. In both the sarcopenia group and non-sarcopenia group, physical and cognitive function outcomes were significantly better on discharge than on admission. These outcomes were significantly worse in the sarcopenia group than those in the non-sarcopenia group both on admission and on discharge. Even in patients with sarcopenia, postoperative rehabilitation of hip fractures significantly improved both these outcomes.
Acknowledgments
We thank Angela Morben, DVM, ELS, and Jane Charbonneau, DVM, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Hirokazu Inoue https://orcid.org/0000-0001-8420-6724
References
- 1.Tinetti ME, Kumar C. The patient who falls: "It's always a trade-off. JAMA. 2010;303:258-266. doi: 10.1001/jama.2009.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison A, Fan T, Sen SS, et al. Epidemiology of falls and osteoporotic fractures: A systematic review. Clinicoecon Outcomes Res. 2013;5:9-18. doi: 10.2147/CEOR.S38721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orimo H, Yaegashi Y, Onoda T, et al. Hip fracture incidence in Japan: Estimates of new patients in 2007 and 20-year trends. Arch Osteoporosis. 2009;4:71-77. doi: 10.1007/s11657-009-0031-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579. doi: 10.1001/jama.2009.1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welmer AK, Rizzuto D, Laukka EJ, et al. Cognitive and Physical function in relation to the risk of injurious falls in older adults: A population-based study. J Gerontol A Biol Sci Med Sci. 2017;72:669-675. doi: 10.1093/gerona/glw141 [DOI] [PubMed] [Google Scholar]
- 6.Gale CR, Westbury LD, Cooper C, et al. Risk factors for incident falls in older men and women: The English longitudinal study of ageing. BMC Geriatr. 2018;18:117. doi: 10.1186/s12877-018-0806-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon JH, Park JH, Oh C, et al. Dementia is associated with an increased risk of hip fractures: A nationwide analysis in Korea. J Clin Neurol. 2019;15:243-249. doi: 10.3988/jcn.2019.15.2.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiklund R, Toots A, Conradsson M, et al. Risk factors for hip fracture in very old people: A population-based study. Osteoporos Int. 2016;27:923-931. doi: 10.1007/s00198-015-3390-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morley JE. Sarcopenia: Diagnosis and treatment. J Nutr Health Aging. 2008;12:452-456. doi: 10.1007/BF02982705 [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Hao Q, Hai S, et al. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: A systematic review and meta-analysis. Maturitas. 2017;103:16-22. doi: 10.1016/j.maturitas.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 11.Aibar-Almazan A, Martinez-Amat A, Cruz-Diaz D, et al. Sarcopenia and sarcopenic obesity in Spanish community-dwelling middle-aged and older women: Association with balance confidence, fear of falling and fall risk. Maturitas. 2018;107:26-32. doi: 10.1016/j.maturitas.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 12.Elhakeem A, Hartley A, Luo Y, et al. Lean mass and lower limb muscle function in relation to hip strength, geometry and fracture risk indices in community-dwelling older women. Osteoporos Int. 2019;30:211-220. doi: 10.1007/s00198-018-4795-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benichou O, Lord SR. Rationale for strengthening muscle to prevent falls and fractures: A review of the evidence. Calcif Tissue Int. 2016;98:531-545. doi: 10.1007/s00223-016-0107-9 [DOI] [PubMed] [Google Scholar]
- 14.He H, Liu Y, Tian Q, et al. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27:473-482. doi: 10.1007/s00198-015-3241-8 [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K, Kanazawa I, Sugimoto T. Reduction in endogenous insulin secretion is a risk factor of sarcopenia in men with type 2 diabetes mellitus. Calcif Tissue Int. 2015;97:385-390. doi: 10.1007/s00223-015-9990-8 [DOI] [PubMed] [Google Scholar]
- 16.Avci CC, Saglam N, Saka G, et al. Is internal fixation of the intertrochanteric fractures reliable option in patients with cognitive dysfunction? Acta Orthop Belg. 2016;82:1-11. [PubMed] [Google Scholar]
- 17.Binder EF, Brown M, Sinacore DR, et al. Effects of extended outpatient rehabilitation after hip fracture: A randomized controlled trial. JAMA. 2004;292:837-846. doi: 10.1001/jama.292.7.837 [DOI] [PubMed] [Google Scholar]
- 18.Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149-1160. doi: 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- 19.Faul F, Erdfelder E, Lang AG, et al. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175-191. doi: 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
- 20.Inoue H, Watanabe H, Okami H, et al. Handgrip strength correlates with walking in lumbar spinal stenosis. Eur Spine J. 2020;29:2198-2204. doi: 10.1007/s00586-020-06525-1 [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 22.Dodds TA, Martin DP, Stolov WC, et al. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil. 1993;74:531-536. doi: 10.1016/0003-9993(93)90119-u [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Montalvo JI, Alarcon T, Gotor P, et al. Prevalence of sarcopenia in acute hip fracture patients and its influence on short-term clinical outcome. Geriatr Gerontol Int. 2016;16:1021-1027. doi: 10.1111/ggi.12590 [DOI] [PubMed] [Google Scholar]
- 24.Ho AW, Lee MM, Chan EW, et al. Prevalence of pre-sarcopenia and sarcopenia in Hong Kong Chinese geriatric patients with hip fracture and its correlation with different factors. Hong Kong Med J. 2016;22:23-29. doi: 10.12809/hkmj154570 [DOI] [PubMed] [Google Scholar]
- 25.Steihaug OM, Gjesdal CG, Bogen B, et al. Sarcopenia in patients with hip fracture: A multicenter cross-sectional study. PLoS One. 2017;12:e0184780. doi: 10.1371/journal.pone.0184780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Monaco M, Castiglioni C, Vallero F, et al. Sarcopenia is more prevalent in men than in women after hip fracture: A cross-sectional study of 591 inpatients. Arch Gerontol Geriatr. 2012;55:e48-e52. doi: 10.1016/j.archger.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 27.Hida T, Ishiguro N, Shimokata H, et al. High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr Gerontol Int. 2013;13:413-420. doi: 10.1111/j.1447-0594.2012.00918.x [DOI] [PubMed] [Google Scholar]
- 28.Palombaro KM, Craik RL, Mangione KK, et al. Determining meaningful changes in gait speed after hip fracture. Phys Ther. 2006;86:809-816. [PubMed] [Google Scholar]
- 29.Oh MK, Yoo JI, Byun H, et al. Efficacy of combined antigravity treadmill and conventional rehabilitation after hip fracture in patients with Sarcopenia. J Gerontol A Biol Sci Med Sci. 2020;75:e173-e181. doi: 10.1093/gerona/glaa158 [DOI] [PubMed] [Google Scholar]
- 30.Lim SK, Lee SY, Beom J, et al. Comparative outcomes of inpatient fragility fracture intensive rehabilitation management (FIRM) after hip fracture in sarcopenic and non-sarcopenic patients: A prospective observational study. Eur Geriatr Med. 2018;9:641-650. doi: 10.1007/s41999-018-0089-4 [DOI] [PubMed] [Google Scholar]
- 31.Seematter-Bagnoud L, Lecureux E, Rochat S, et al. Predictors of functional recovery in patients admitted to geriatric postacute rehabilitation. Arch Phys Med Rehabil. 2013;94:2373-2380. doi: 10.1016/j.apmr.2013.06.024 [DOI] [PubMed] [Google Scholar]
- 32.Shibasaki K, Asahi T, Mizobuchi K, et al. Rehabilitation strategy for hip fracture, focused on behavioral psychological symptoms of dementia for older people with cognitive impairment: A nationwide Japan rehabilitation database. PLoS One. 2018;13:e0200143. doi: 10.1371/journal.pone.0200143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: What we need to know and do. Br J Anaesth. 2017;119:i115-i125. doi: 10.1093/bja/aex354 [DOI] [PubMed] [Google Scholar]