Abstract
SMARCA4-deficient undifferentiated tumors (SMARCA4-dUT) are infrequently occurring aggressive neoplasms found predominantly in young male smokers. These tumors are distinguished by the loss of expression of Brahma-related gene 1 (BRG1) due to a deactivating mutation of SMARCA4. Immunophenotype can be variable but characteristically lack the expression of BRG1. SMARCA4-dUT has a poor prognosis and generally progresses or recurs. The median survival is around 6 months. Here, we report a case of a 36-year-old male smoker who presents with multiple right-sided lung masses. The patient was found to have a loss of SMARAC4 and SMARCA2 along with the absence of markers of vascular, melanocytic, lymphoid, keratin, or myogenic origin. Tumor size was reduced significantly after 3 cycles of carboplatin and 1 cycle of pembrolizumab. From reviewing the literature and the clinical course in our case, we suggest combination chemotherapy plus immune checkpoint inhibitor (ICI) therapy to be the first choice of therapy for treating SMARCA4-dUT of lungs. Further research and studies are needed to evaluate the response to ICI therapy alone or combination therapy (chemotherapy plus ICI).
Keywords: SMARCA4, SMARCA4-dUT, immune checkpoint inhibitors, significant response
Introduction
Le Loarer et al, 1 in 2015, reported a new subtype of thoracic sarcoma that has an SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily A, member 4 (SMARCA4) deficiency, with undifferentiated morphology and an aggressive clinical course. They were dubbed as SMARCA4-deficient thoracic sarcoma (SMARCA4-dTS). 2 These tumors have a loss of the SMARCA4 gene and the Brahma-related gene-1 (BRG1) gene and are seen in up to 5% of non–small cell lung carcinomas (NSCLCs).2,3 The patients are mostly young male smokers. Recently, in addition to sarcomas, there is another entity of poorly differentiated carcinoma, predominantly occurring in lungs, that is associated with the loss of SMARCA4/BRG1.4-7 These carcinomas are called SMARCA4-deficient undifferentiated tumors (dUTs). Patients present primarily with lung masses with pathology ranging from well-differentiated adenocarcinomas to poorly differentiated tumors. In the World Health Organization (WHO) Classification of Tumours of the Lung, Pleura, Thymus and Heart tumors, revision of 2021, tumors that were earlier classified as SMARCA4-dTS were reclassified as SMARCA4-deficient undifferentiated tumors (SMARCA4-dUT). Despite gene expression profiles being similar to malignant rhabdoid tumors (MRTs) and small cell carcinoma of the ovary, hypercalcemic type (SCCOHT), SMARCA4-dUT show striking genomic overlap with SMARCA4-mutant NSCLC with frequent TP53, STK11, KEAP1, and KRAS mutations, high tumor mutation burden (TMB), and the presence of smoking-related molecular signatures in tumor cells. 3 However, these tumors are considered distinct from SMARCA4-deficient NSCLCs. 8 SMARCA4-dUT show uniformly poor survival, which often leads to death within only 4 to 7 (range, 1-13) months.1,9,10 No clear guidelines have been established for treating SMARCA4-dUT. They are unresponsive to conventional therapies.1,9,11 All patients essentially have either disease progression or relapse, with the cause of death being local complications due to disease burden.1,9,11 There is limited response to chemotherapy and surgery. 11 After review of the literature, we found patients of SMARCA4-dUT showing partial response who were treated with immune checkpoint inhibitors (ICI) with or without chemotherapy, with ICI being the first-line therapy or being used after failure of chemotherapy.12-16
Here, we report a case of a 36-year-old smoker with right-sided chest pain, who was found to have right-sided lung masses eroding into surrounding structures with the histological finding of SMARCA4-dUT. After receiving 3 cycles of chemotherapy carboplatin and paclitaxel and 1 cycle of pembrolizumab, the tumor burden decreased significantly by approximately 65%. This is the second case of SMARCA4-dUT which showed an excellent response after just 1 cycle of pembrolizumab with chemotherapy as initial therapy, as per our literature search.
Case Presentation
We describe the case of a 36-year-old male active smoker with no significant medical history and with history of cancer in the mother, type unknown. He presented with right-sided chest pain for 3 months, which had been getting progressively worse for 3 weeks. The patient had presented to the emergency department 3 months back when the pain started and was discharged after the pain resolved with ibuprofen. A chest x-ray (CXR) performed 3 months earlier was clear.
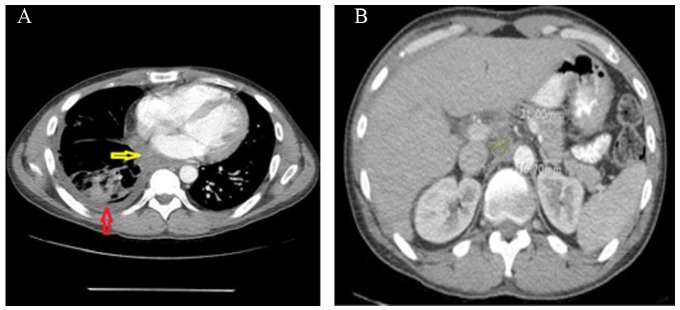
In this presentation, vital signs were within normal limits. On physical examination, there were decreased breath sounds over the right lung on auscultation. Other examinations were unremarkable. Laboratory tests were significant for platelets 729 000/µL, international normalized ratio 1.46, prothrombin time 16.5 seconds, partial thromboplastin time 38.8 seconds, and D-dimer 995 ng/mL. The CXR showed a large right-sided pleural effusion (Figure 1). Computed tomography (CT) angiogram of the chest showed multiple right-sided extra-pleural versus pleural-based masses with an associated large right pleural effusion, with masses eroding adjacent ribs, lateral margin of the T6 vertebral body, possibly involving the adjacent neural foramen, and partially extending through intercostal spaces. A large part of the mass-like process was indenting the heart and displacing it to the left; some elements of cardiac invasion could not be excluded (Figure 2A and B). The patient underwent right-sided thoracentesis. Pleural fluid cytology showed severe mixed acute and chronic inflammation inclusive of scattered uninucleate epithelioid-type histiocytic cells with an isolated large Langhans-type multinucleated histiocytic cell supportive of a granulomatous inflammatory process.
Figure 1.
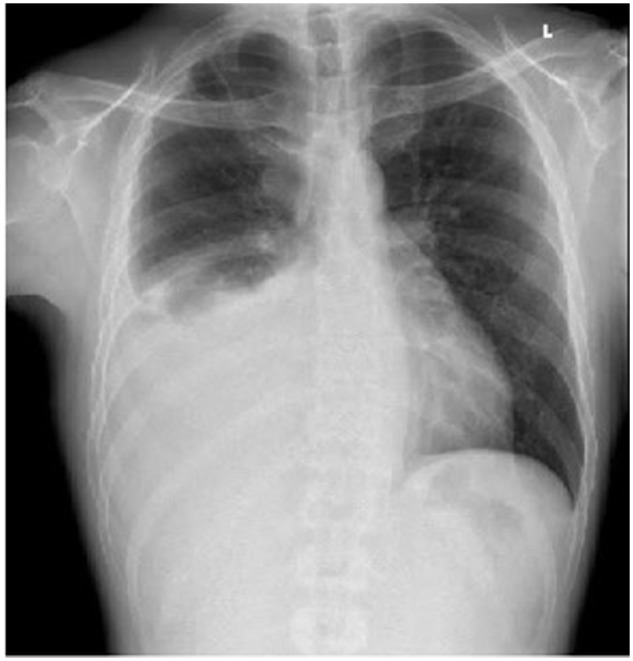
Chest x-ray showing right-sided pleural effusion.
Figure 2.
(A) CT angiogram of the chest showing multiple right-sided extra-pleural versus pleural-based masses eroding adjacent ribs (red arrow), with an associated large right pleural effusion (green arrow), a large part of the mass-like process indenting the heart and displacing it to the left (blue arrow). (B) CT angiogram of the chest showing mass eroding lateral margin of the T6 vertebral body.
Abbreviation: CT, computed tomography.
Magnetic resonance imaging (MRI) of the brain and cervical/thoracic/lumbar spine and CT of the abdomen/pelvis were done for staging. The MRI of the brain was negative for metastases as was MRI of the cervical and lumbar spine. The MRI of the thoracic spine showed a right paraspinal soft tissue mass with direct invasion of the right side of the T6 vertebral body (Figure 3). Computed tomography and pelvis with intravenous and oral contrast with liver protocol showed porta hepatis lymphadenopathy, measuring 3.1 cm (Figure 4). As the patient did not have any clinical and radiological signs of cord compression, no neurosurgical intervention was done for the right paraspinal soft tissue mass with direct invasion of the right side of the T6 vertebral body. An echocardiogram showed a large, posterior extracardiac mass compressing both atria with a small pericardial effusion mostly along the right ventricular free wall with diastolic invagination of the right ventricular free wall. The CT-guided lung mass biopsy was done. The pathology (Figure 5) showed invasive poorly differentiated carcinoma and a high grade of uncertain histogenesis. Immunohistochemistry was positive for EPCAM (MOC 31), AE-1/AE-3, CAM5.2 (focally), DIM staining with p40 (negative CK5/6), synaptophysin, and CDX2 (focally) and negative for chromogranin A, CD56, CK7, CK20, TTF-1, NAPSIN A, GATA-3, S-100, SOX-10, and PAX8. Ki67 was 70%-80%, and the programmed death ligand (PDL)-1 tumor proportion score was 45%-50%. In summary, the stain results showed vague/inconclusive evidence of mixed histogenesis.
Figure 3.
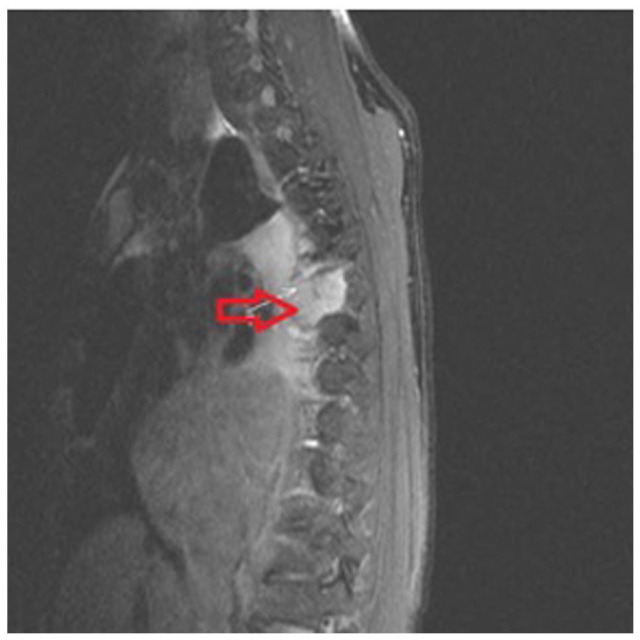
Magnetic resonance imaging of the thoracic spine showing right paraspinal soft tissue mass with direct invasion of the right side of the T6 vertebral body (red and white arrows).
Figure 4.
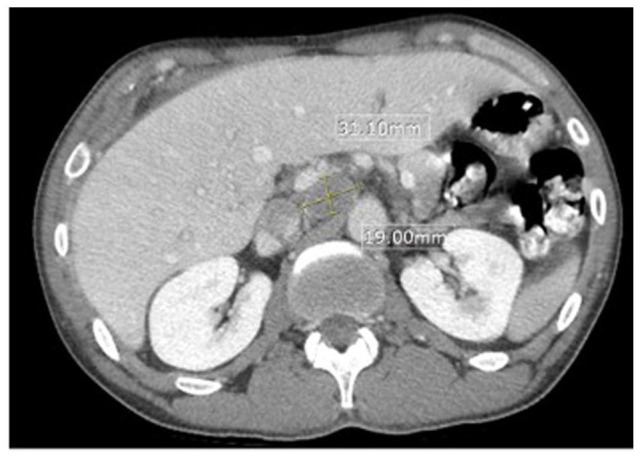
Computed tomography and pelvis with intravenous and oral contrast with liver protocol showing porta hepatis lymphadenopathy, measuring 31 × 19 mm.
Figure 5.
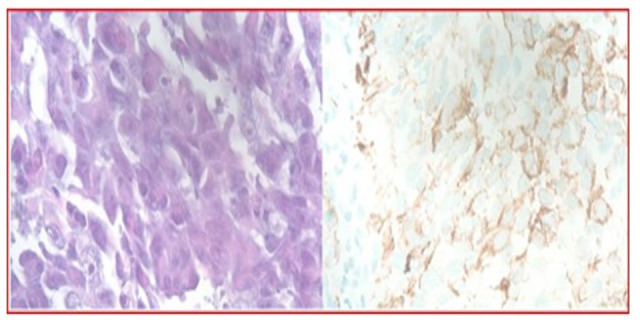
Histopathology shows the carcinoma as poorly differentiated with a partially discohesive growth pattern. The neoplastic cells are polymorphisms with a prominent nucleolus. Some of the neoplastic cells demonstrate the rhabdoid and plasmacytoid features. The neoplastic cells are immunoreactive to carcinoma marker AE1/AE3 (hematoxylin-eosin, original magnifications ×40).
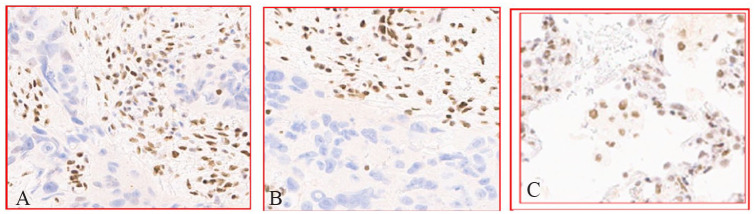
The patient received the first cycle of chemotherapy with carboplatin/paclitaxel. The case was discussed in the tumor board and the decision was made to repeat the biopsy due to the requirement for additional marker tests. A repeat biopsy was performed about 20 days after the initial one. Immunohistochemistry apart from the above was positive for TTF-1(focal) and vimentin, and positive for CEA (polyclonal), Ck5/6, calretinin, D2-40, WT-1, Melan-A, napsin-A, desmin, PLAP, and OCT-4. Histomorphology along with the immunostains made the diagnosis of poorly differentiated carcinoma, favoring adenocarcinoma. PD-L1 tumor proportion score was the same as the previous one: 45%-50%. For the second opinion, a tissue block was sent to Sloan-Kettering Hospital, which showed high-grade undifferentiated morphology with eosinophilic cytoplasm, high nuclear to cytoplasm ratios, somewhat monomorphic nuclei vesicular to coarsely clumped chromatin, and prominent nucleoli. Some tumor cells focally displayed plasmacytoid/rhabdoid-like morphological features with eccentrically placed nuclei, tumor necrosis, and stromal desmoplasia. In addition, claudin-4 showed patchy positivity. Tumor cells showed complete loss of BRM (SMARCA2) and BRG-1 (SMARCA4) expression (Figure 6A and B) with control as lung tissue (Figure 6C). In summary, malignant epithelioid neoplasm with morphological (monomorphic undifferentiated cells with focal rhabdoid features) and immunophenotypic (loss of BRG-1, CO-LOSS of BRM) features was consistent with the diagnosis of SMARCA4-deficient undifferentiated malignant thoracic tumor. The patient was discharged to follow-up in the outpatient clinic.
Figure 6.
(A) Immunohistochemical (IHC) stain demonstrating loss of SMARCA4/BRG1, original magnification ×20. (B) IHC stain demonstrating loss of SMARCA2/BRM 1, original magnification ×20. (C) Positive controls of lung tissue.
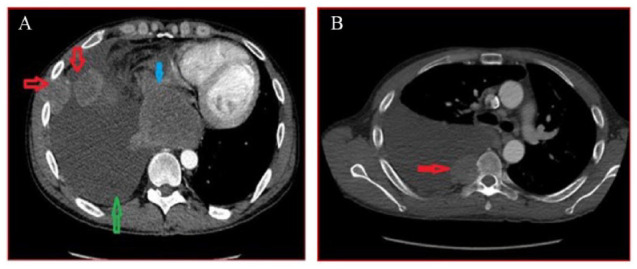
As the PDL-1 tumor proportion was high, with the third cycle of chemotherapy, pembrolizumab was also given. As per Response Evaluation Criteria in Solid Tumors (RECIST) criteria 1.1, this is a partial response because much more than a 30% decrease in the sum of the longest diameter was seen. The patient achieved excellent response with a combination of chemotherapy and ICI. The CT of the chest repeated after the third cycle of chemotherapy showed previously described right-sided pleural-based metastases resolved, soft tissue mass in subcarinal space extending to the mediastinal side of pleura significantly decreased in size (Figure 7A), and enlarged upper abdominal lymph node significantly decreased in size (21 × 10 mm) from the prior CT scan (prior size ~31 × 19 mm) (Figure 7B). The patient was stable and alive at the time of this paper writing and was actively pursuing treatment in different hospitals due to insurance issues.
Figure 7.
(A) CT of the chest repeated after the third cycle of chemotherapy and one cycle of pembrolizumab shows that the previously described right-sided pleural-based metastases resolved, resolving right-sided pleural effusion (red arrow), and soft tissue mass in subcarinal space extending to the mediastinal side of pleura significantly decreased in size (yellow arrow). (B) CT scan repeated after the third cycle of chemotherapy and one cycle of pembrolizumab shows that the upper abdominal lymph node significantly decreased in size (21 × 10 mm) from the prior CT scan (prior size ~31 × 19 mm).
Abbreviation: CT, computed tomography.
Discussion
SMARCA4-dUT, as historically reported, are rare aggressive tumors, typically affecting patients in the age group of 30-59 years with a male:female ratio of 9:1.2,10,17 The characteristic of this tumor is loss of Brahma (BRM) that is encoded by SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily A, member 2 (SMARCA2) or Brahma-related gene 1 (BRG1) encoded by SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily A, member 4 (SMARCA4), respectively.2,4,13 The most common sites of involvement in descending order are anterior mediastinum (61%), pleura (29%), and lung. Often, the tumors are larger at presentation, extending from the mediastinum to the lungs, pleura, neck, and ribs, as in our case. Several complications including post-obstructive lung atelectasis, superior vena cava syndrome, and esophageal invasion are documented due to involvement of large airways and vascular encasement.9-11
On the morphological level, we observed progression-free SMARCA4-dUT tumors are characterized by undifferentiated epithelioid cells with poorly defined nuclear borders, prominent nucleoli, increased mitotic activity, and extensive necrosis. It may exhibit rhabdoid morphology. 17 The tumor cells grow in non-cohesive clusters and sheets. The immunohistochemical profiles of these tumors can vary. Overexpression of MYC is associated with loss of SMARCA4. SMARCA4-dUT is positive for SOX2, CD34, and SALL4 that helps to differentiate it from SMARCA4-NSCLC.1,7,10,18 Tumor cells are also CD30 and CD138 focally positive. 9 The markers found to be negative in SMARCA4-dUT are desmin, S100, NUT, WT-1, keratin, and p40.10,17 There was no report of germline mutation in SMARCA4-dUT. 19
The differential diagnoses for SMARCA4-dUT include malignant rhabdoid tumor, metastatic SCCOHT to the thorax, atypical teratoid/rhabdoid tumor, NUT carcinoma, SMARCA4-dNSCLC, lymphoma, mediastinal germ-cell tumor, malignant mesothelioma, and sarcoma. 17 SMARCA4-dNSCLC has the presence of squamous and solid components that differentiate it morphologically from SMARCA4-dUT. As stated earlier, immunohistochemically, SMARC4-dUT has a strong expression of SOX2, SALL4, and CD34 with characteristic loss of SMARCA4 and SMARCA2. 10 Histology as shown in Figure 5 aids in diagnosing as seen in our patient showing highly undifferentiated rhabdoid cells growing in clusters and sheets. Malignant rhabdoid tumors typically have a younger age range and are not affected by Sox2, p53 (which is present in SMARCA4-dUT), or INI-1 overexpression. 17 Patients with metastatic SCCOHT primarily present with intra-abdominal tumor with or without hypercalcemia. 15 These tumors occasionally express SOX2 but not in diffuse pattern. 9
The median survival of SMARCA4-dUT patients was very short, 4-7 (range, 1-13) months. The disease progresses and relapses in virtually all patients, which increases mortality, primarily from local complications.1,11 Treatment options are limited in the context of poor response to chemotherapy and surgery; however, after a detailed review of the available cases, we have summarized the response of the tumor to chemotherapy alone, chemotherapy combined with ICI, and ICI alone. Table 1 lists cases from case reports/series that showed the use of combined modalities—chemotherapy with ICI.13-16,20,21 Tanaka et al 13 reported 1 patient with 10% PD-L1 expression, who was given combined first-line agents with carboplatin, pemetrexed, and pembrolizumab, resulting in partial response (PR). Shinno et al 21 demonstrated the response in case series of 12 patients with SMARCA4-deficient thoracic tumor treated with ICI with or without chemotherapy and ICI as first-line therapy in 5 cases with partial response, 1 with stable disease, and 1 with progressive disease (PD). Other remaining patients where ICI was given as second, third, or fourth line had PD. Kawachi et al 15 reported a case series of combination chemotherapy—atezolizumab, bevacizumab, paclitaxel, and carboplatin (ABCP) with atezolizumab (PD-L1 expressions being 40%, 10%, and 80%). Partial response was achieved in 2 cases with disease progression in 1 case. Kunimasa et al 16 reported a case, with 0% PD-L1, given ABCP and atezolizumab but ultimately needed surgery. Henon et al 21 described a case of SMARCA4-dUT with 0% PD-L1 who received carboplatin, paclitaxel, and radiotherapy as the first-line followed by pembrolizumab as the second-line agent. The patient showed stable PR and was alive at the time of reporting. Iijima et al 14 mentioned the use of carboplatin and paclitaxel as the first-line (PD-L1 expression<1%) and carboplatin and etoposide as the second-line followed by nivolumab as the third-line agent. Complete response was achieved with an undetectable tumor mass with a sustained response for 22 months. Lin et al 22 showed promising efficacy of ICI with chemotherapy for SMARCA4-deficient thoracic sarcomatoid tumors, which can also play a pivotal role in the treatment of SMARCA4-dUT.
Table 1.
Cases of SMARCA4-dUT Treated With Chemotherapy Followed by ICI.
| Authors | Age/sex | PD-L1 expression | Chemotherapy | ICI (doses) (first/second/third line of therapy) |
Response (PR) (CR) |
Remarks | |
|---|---|---|---|---|---|---|---|
| Tanaka et al 13 | 58/M | 10% | Carboplatin and pemetrexed (first line) (received 3 cycles every 3 wk) (discontinued after 3 cycles due to grade III anorexia) | Pembrolizumab (8 doses) (first line) | PR | Clinical improvement and decrease in size of tumor after 3 mo of ICI. The patient survived. | |
| Shinno et al 20 | 76/M | NA | Cytotoxic chemotherapy | Nivolumab (unknown) (third line) | PR | ||
| Kawachi et al15 | Case 1 (SMARCA4-DTS) |
73/F | 40% | Atezolizumab, bevacizumab, paclitaxel and carboplatin (ABCP). CT discontinued after 3 cycles due to fatigue and nausea | Atezolizumab (3 cycles initially)) (first line) × 17 mo | PR | PR achieved after 2 courses of ABCP. Rx was continued after 17 mo of initial Rx. |
| Case 2 (SMARCA4-DTS) |
59/ M | 10% | ABCP CT discontinued due to grade 3 peripheral neuropathy after 3 cycles |
Atezolizumab (dose NA) (first line) Maintenance Rx for 10 mo |
PR | PR achieved after 2 courses of induction. The disease progressed with bilateral adrenal gland and abdominal lymph node metastases | |
| Case 3 | 64/ F | 80% | ABCP (first line) After 3 cycles of induction—PR After 4 cycles—brain metastasis RT given with maintenance with AB × 2 mo—disease progressed to mediastinal LN metastasis—ICI stopped |
Atezolizumab (dose NA) (first line) | PR | ||
| Kunimasa et al 16 | 51/M | 0% | ABCP (first line) × 6 courses in total | Atezolizumab (6) (first line) | PR | Surgery done after 6 courses | |
| Henon et al 21 | 58/F | 0% | Carboplatin with paclitaxel (First line) RT × 3 cycles |
Pembrolizumb (second line) PR after 2 injections |
PR | Continue to receive ICI × 11 mo with stable PR | |
| Iijima et al 14 | 76/M | <1% | Carboplatin and paclitaxel (first line) Carboplatin and etoposide (second line after femoral metastases) |
Nivolumab (third line) | CR (undetectable tumor mass) |
Sustained response × 22 mo |
Abbreviations: dUT, deficient undifferentiated tumors; ICI, immune checkpoint inhibitor; PDL-1, programmed death ligand 1; CR, complete response; PR, partial response; NA, not available; F, female; M, male; RT, radiotherapy; AB, atezolizumab, bevacizumab; CT, computed tomography; LN, lymph node.
First-line median progression-free survival (PFS) was significantly higher for patients with ICI plus chemotherapy than those treated with only chemotherapy23-25 as shown in Table 2. Sauter et al 24 described a case of 69-year-old woman with SMARCA4-dUT who was treated with only chemotherapy and radiotherapy but ultimately succumbed to the disease. Mehta et al 25 reported 2 case reports of SMARCA4-dUT with PD-L1 expression <1% who were treated with platinum doublet at another center; however, the status of objective response and the nature of therapy were not known. Similarly, Okazaki et al 23 described a case of SMARCA4-deficient thoracic tumor in which chemotherapy resulted only in a temporary response, and the patient’s condition worsened over several months.
Table 2.
Cases of SMARCA4-dUT Treated With Chemotherapy Alone Without the Use of ICI.
| Authors | Age/sex | PD-L1 expression | Chemotherapy | ICI (doses) (first/second/third line of therapy) |
Response (PR) (CR) |
Remarks | |
|---|---|---|---|---|---|---|---|
| Sauter et al 24 | 69/F | NA | Chemotherapy and RT | Not given | Death | ||
| Mehta et al 25 | Case 1 | 49/M | <1% | Platinum double | NA | Not known | Alive |
| Case 2 | 46/M | <1% | Platinum double | NA | Not known | Alive | |
| Okazaki et al 23 | Case 1 | 74/M | NA | Nature of agents not mentioned | Not given | Temporary response | Worsening of disease |
Abbreviations: dUT, deficient undifferentiated tumors; ICI, immune checkpoint inhibitor; PDL-1, programmed death ligand 1; CR, complete response; PR, partial response; NA, not available; F, female; M, male; RT, radiotherapy.
Several case reports and series have been published showing some of the effectiveness of ICI,9,12,14,15 with the theory that the higher the PD-L1 expression more successful the treatment. However, the dilemma of treating the tumor with ICI alone still ponders the mind of oncologists. Table 3 shows a case report of SMARCA4-deficient thoracic sarcoma (PD-L1 expression >60%) with the use of pembrolizumab alone (8 cycles). After 8 cycles of pembrolizumab, a repeat CT scan demonstrated a sustained durable PR response with no adverse events. 12
Table 3.
Cases of SMARCA4-dUT Treated With ICI Alone Without the Use of Chemotherapy.
| Authors | Age/sex | PD-L1 expression | Chemotherapy | ICI (doses) (first/second/third line of therapy) |
Response (PR) (CR) |
Remarks |
|---|---|---|---|---|---|---|
| Takada et al 12 | 70 /F | >60% | Not given | Pembrolizumab (1 dose) (first line) | PR | Sustained PR after 8 cycles of ICI |
Abbreviations: dUT, deficient undifferentiated tumors; ICI, immune checkpoint inhibitor; PDL-1, programmed death ligand 1; CR, complete response; PR, partial response; F, female.
The overall response noted with the combined modality (chemotherapy with ICI) was that 87.5% (7 of 8 patients) achieved partial remission, while 1 of 8 patients (~12.5%) had complete remission. With the use of chemotherapy alone, 20% (1 out of 4 patients) had the worsening disease, 50% (2 of 4) were alive and 20% (1 of 4 patients) succumbed to death.
As per the review of the previously published cases that showed some response to ICI, PD-L1 ranged from 0 to 100%.12-16,26,27 The theory that treatment response depends on PD-L1 expression does not seem to hold. But this is a very small sample size to conclude and requires more evidence. Deficiency in other components of the SWI/SNF complex has been found to result in increased T-cell cytotoxicity induced by interferon-gamma. This deficiency has shown better results in anti-PD1 therapy in other types of tumors.17,21,28 SMARCA4-deficient tumors usually harbor KEAP1/STK11 co-mutations. The ICI therapy has not shown promising results in that subset. In our case, whether there is co-mutation of KEAP1/STK11 or not is unclear, and the exceptional response to the combined treatment could be due to the loss of this mutation. Verification of the presence of the mutation with comprehensive genome profiling (CGP) assay is needed to get further answers and should be studied in the future. As of the present, such mutations have not yet been seen in ICI-sensitive cases. 14 As ICI-resistant cases exist, chemotherapy is generally used along with ICI. 13 In NSCLC, a higher TMB of a nonsynonymous mutation was found to be associated with the clinical effectiveness of pembrolizumab. High somatic TMB looks like a promising predictive marker for ICI sensitivity.9,29 There are ongoing preliminary clinical trials with the H3K27 histone methyltransferase EZH2 inhibitor related to the SWI/SNF complex, which also looks promising.1,9,30
Conclusion
A SMARCA4-dUT was successfully treated with carboplatin, paclitaxel, and pembrolizumab with an exceptionally good response. Our case substantiates combination chemotherapy plus immunotherapy as the possible best treatment modality for this type of carcinoma. Further clinical trials and studies are warranted to establish an optimal treatment strategy.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Ethics approval to report this case was obtained from Brookdale Hospital Institutional Review Board. Our institution does not require ethical approval for reporting individual case reports.
Informed Consent: The patient expired before consent could be taken. Brookdale Hospital Institutional Review Board does not need consent for publishing case reports.
ORCID iDs: Akriti Pokhrel  https://orcid.org/0000-0002-6628-1875
https://orcid.org/0000-0002-6628-1875
Ruchi Yadav  https://orcid.org/0000-0002-8207-2424
https://orcid.org/0000-0002-8207-2424
Jen C. Wang  https://orcid.org/0000-0002-9623-6645
https://orcid.org/0000-0002-9623-6645
References
- 1.Le Loarer F, Watson S, Pierron G, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet. 2015;47(10):1200-1205. doi: 10.1038/ng.339 [DOI] [PubMed] [Google Scholar]
- 2.Yadav R, Sun L, Salyana M, Eric M, Gotlieb V, Wang JC.SMARCA4-deficient undifferentiated tumor of lung mass—a rare tumor with the rarer occurrence of brain metastasis: a case report and review of the literature. J Investig Med High Impact Case Rep. 2022;10. doi: 10.1177/23247096221074864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nambirajan A, Jain D.Recent updates in thoracic SMARCA4-deficient undifferentiated tumor. Semin Diagn Pathol. 2021; 38(5):83-89. doi: 10.1053/J.SEMDP.2021.06.001 [DOI] [PubMed] [Google Scholar]
- 4.Herpel E, Rieker RJ, Dienemann H, et al. SMARCA4 and SMARCA2 deficiency in non-small cell lung cancer: immunohistochemical survey of 316 consecutive specimens. Ann Diagn Pathol. 2017;26:47-51. doi: 10.1016/J.ANNDIAGPATH.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 5.Agaimy A, Fuchs F, Moskalev EA, Sirbu H, Hartmann A, Haller F.SMARCA4-deficient pulmonary adenocarcinoma: clinicopathological, immunohistochemical, and molecular characteristics of a novel aggressive neoplasm with a consistent TTF1neg/CK7pos/HepPar-1pos immunophenotype. Virchows Arch. 2017;471(5):599-609. doi: 10.1007/S00428-017-2148-5 [DOI] [PubMed] [Google Scholar]
- 6.Matsubara D, Kishaba Y, Ishikawa S, et al. Lung cancer with loss of BRG1/BRM, shows epithelial mesenchymal transition phenotype and distinct histologic and genetic features. Cancer Sci. 2013;104(2):266-273. doi: 10.1111/CAS.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naito T, Umemura S, Nakamura H, et al. Successful treatment with nivolumab for SMARCA4-deficient non-small cell lung carcinoma with a high tumor mutation burden: a case report. Thorac Cancer. 2019;10(5):1285-1288. doi: 10.1111/1759-7714.13070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatzopoulos K, Boland JM.Update on genetically defined lung neoplasms: NUT carcinoma and thoracic SMARCA4-deficient undifferentiated tumors. Virchows Arch. 2021;478(1):21-30. doi: 10.1007/S00428-020-03011-3 [DOI] [PubMed] [Google Scholar]
- 9.Roden AC.Thoracic SMARCA4-deficient undifferentiated tumor-a case of an aggressive neoplasm-case report. Mediastinum. 2021;5:39. doi: 10.21037/MED-20-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rekhtman N, Montecalvo J, Chang JC, et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol. 2020;15(2):231-247. doi: 10.1016/J.JTHO.2019.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crombé A, Alberti N, Villard N, et al. Imaging features of SMARCA4-deficient thoracic sarcomas: a multi-centric study of 21 patients. Eur Radiol. 2019;29(9):4730-4741. doi: 10.1007/S00330-019-06017-X [DOI] [PubMed] [Google Scholar]
- 12.Takada K, Sugita S, Murase K, et al. Exceptionally rapid response to pembrolizumab in a SMARCA4-deficient thoracic sarcoma overexpressing PD-L1: a case report. Thorac Cancer. 2019;10(12):2312-2315. doi: 10.1111/1759-7714.13215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka S, Hayashi S, Isobe Y, et al. Positive outcome of first-line therapy for a SMARCA4-deficient thoracic sarcomatoid tumor. Int Cancer Conf J. 2021;10(2):112-115. doi: 10.1007/S13691-021-00472-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iijima Y, Sakakibara R, Ishizuka M, et al. Notable response to nivolumab during the treatment of SMARCA4-deficient thoracic sarcoma: a case report. Immunotherapy. 2020;12(8): 563-569. doi: 10.2217/IMT-2019-0142 [DOI] [PubMed] [Google Scholar]
- 15.Kawachi H, Kunimasa K, Kukita Y, et al. Atezolizumab with bevacizumab, paclitaxel and carboplatin was effective for patients with SMARCA4-deficient thoracic sarcoma. Immunotherapy. 2021;13(10):799-806. doi: 10.2217/IMT-2020-0311 [DOI] [PubMed] [Google Scholar]
- 16.Kunimasa K, Okami J, Takenaka S, et al. Conversion surgery for advanced thoracic SMARCA4-deficient undifferentiated tumor with atezolizumab in combination with bevacizumab, paclitaxel, and carboplatin treatment: a case report. JTO Clin Res Rep. 2021;2(11):100235. doi: 10.1016/J.JTOCRR.2021.100235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perret R, Chalabreysse L, Watson S, et al. SMARCA4-deficient thoracic sarcomas: clinicopathologic study of 30 cases with an emphasis on their nosology and differential diagnoses. Am J Surg Pathol. 2019;43(4):455-465. doi: 10.1097/PAS.0000000000001188 [DOI] [PubMed] [Google Scholar]
- 18.Yoshida A, Kobayashi E, Kubo T, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol. 2017;30(6):797-809. doi: 10.1038/modpathol.2017.11 [DOI] [PubMed] [Google Scholar]
- 19.Schaefer I-M, Cote GM, Hornick JL.Contemporary sarcoma diagnosis, genetics, and genomics. J Clin Oncol. 2018;36(2): 101-110. [DOI] [PubMed] [Google Scholar]
- 20.Shinno Y, Yoshida A, Masuda K, et al. Efficacy of immune checkpoint inhibitors in SMARCA4-deficient thoracic tumor. Clin Lung Cancer. 2022;23(5):386-392. doi: 10.1016/J.CLLC.2022.03.005 [DOI] [PubMed] [Google Scholar]
- 21.Henon C, Blay JY, Massard C, et al. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann Oncol. 2019;30(8):1401-1403. doi: 10.1093/annonc/mdz160 [DOI] [PubMed] [Google Scholar]
- 22.Lin Y, Zhang Y, Hu Z, et al. Promising efficacy of immune-checkpoint inhibitor with chemotherapy for SMARCA4-deficient thoracic sarcomatoid tumors. J Clin Oncol. 2022; 40(suppl 6):e21074. doi: 10.1200/JCO.2022.40.16_SUPPL.E21074 [DOI] [Google Scholar]
- 23.Okazaki T, Yokoyama K, Tsuchiya J, et al. SMARCA4-deficient thoracic tumor detected by [18F]FDG PET/CT. Eur J Hybrid Imaging. 2021;5:8. doi: 10.1186/s41824-021-00102-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauter JL, Graham RP, Larsen BT, et al. SMARCA4-deficient thoracic sarcoma: a distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Mod Pathol. 2017;30(10):1422-1432. doi: 10.1038/modpathol.2017.61 [DOI] [PubMed] [Google Scholar]
- 25.Mehta A, Bansal D, Tripathi R, Jajodia A.SMARCA4/BRG1 protein-deficient thoracic tumors dictate re-examination of small biopsy reporting in non–small cell lung cancer. J Pathol Transl Med. 2021;55(5):307-316. doi: 10.4132/jptm.2021.05.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jelinic P, Ricca J, van Oudenhove E, et al. Immune-active microenvironment in small cell carcinoma of the ovary, hypercalcemic type: rationale for immune checkpoint blockade. J Natl Cancer Inst. 2018;110(7):787-790. doi: 10.1093/JNCI/DJX277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anžič N, Krasniqi F, Eberhardt AL, Tzankov A, Haslbauer JD.Ipilimumab and pembrolizumab mixed response in a 41-year-old patient with SMARCA4-deficient thoracic sarcoma: an interdisciplinary case study. Case Rep Oncol. 2021;14(2):706-715. doi: 10.1159/000515416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miao D, Margolis CA, Gao W, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359(6377):801-806. doi: 10.1126/science.aan5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-128. doi: 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan-Penebre E, Armstrong K, Drew A, et al. Selective killing of SMARCA2- and SMARCA4-deficient small cell carcinoma of the ovary, hypercalcemic type cells by inhibition of EZH2: in vitro and in vivo preclinical models. Mol Cancer Ther. 2017; 16(5):850-860. doi: 10.1158/1535-7163.MCT-16-0678 [DOI] [PubMed] [Google Scholar]