Abstract
Objective
To determine whether the frequency of paraneoplastic or autoimmune encephalitis antibodies examined in a referral center changed during the COVID-19 pandemic.
Methods
The number of patients who tested positive for neuronal or glial (neural) antibodies during pre–COVID-19 (2017–2019) and COVID-19 (2020–2021) periods was compared. The techniques used for antibody testing did not change during these periods and included a comprehensive evaluation of cell-surface and intracellular neural antibodies. The chi-square test, Spearman correlation, and Python programming language v3 were used for statistical analysis.
Results
Serum or CSF from 15,390 patients with suspected autoimmune or paraneoplastic encephalitis was examined. The overall positivity rate for antibodies against neural-surface antigens was similar in the prepandemic and pandemic periods (neuronal 3.2% vs 3.5%; glial 6.1 vs 5.2) with a mild single-disease increase in the pandemic period (anti-NMDAR encephalitis). By contrast, the positivity rate for antibodies against intracellular antigens was significantly increased during the pandemic period (2.8% vs 3.9%, p = 0.01), particularly Hu and GFAP.
Discussion
Our findings do not support that the COVID-19 pandemic led to a substantial increase of known or novel encephalitis mediated by antibodies against neural-surface antigens. The increase in Hu and GFAP antibodies likely reflects the progressive increased recognition of the corresponding disorders.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative virus of COVID-19, is associated with a wide range of neurologic symptoms, with encephalopathy the most frequent among patients admitted to hospitals. Activation of the immune system by the virus has emerged as a potential contributor to the complications of some patients. In particular, broad humoral immune activation seems to be frequent in patients with COVID-19,1 and some studies have demonstrated a high prevalence of neuronal or glial (neural) antibodies in the CSF of patients with neurologic manifestations.2,3 However, the scarce epidemiologic data on patients with COVID-19 do not support an increase in the incidence of autoimmune encephalitis (AE).4-6 A problem in assessing whether there is a causal relationship between SARS-CoV-2 and AE is that the frequency of AE in general is very low, and therefore, small studies are not powered enough to address this question.
We reasoned that any substantial association between COVID-19 and AE would be reflected by the pattern of case referrals to a clinical laboratory focused on the study of paraneoplastic and AE. To test this hypothesis, we assessed the frequency and type of neural antibodies in a cohort of 15,390 patients with suspected encephalitis over a period of 5 years that covered the prepandemic and pandemic periods.
Methods
Patients with suspected AE whose serum or CSF was sent to the Neuroimmunology Clinical Laboratory of Hospital Clinic of Barcelona for antibody testing between January 1, 2017, and December 31, 2021, were included. The repertoire of techniques used to determine neural antibodies has previously been reported and did not change over the study period.7 These included immunohistochemistry (IHC), cell-based assays (CBA) with HEK293 expressing 18 distinct neuronal surface antigens (NSA), 2 glial surface antigens (GSA: MOG, AQP4), or immunoblots containing 12 intracellular neural antigens. Samples that produced brain tissue reactivity without identification of the specific antigen were further examined with live neuronal immunofluorescence to determine antibodies against potentially novel surface antigens. Determination of antibodies against GFAP was not systematically performed unless the syndrome or IHC suggested the presence of these antibodies.
We compared the frequency of antibody-positive patients from pre–COVID-19 (2017–2019) and COVID-19 (2020–2021) periods. The chi-square test was used to compare proportions. Spearman correlation was used to analyze a temporal trend during the 5 years of study of positivity rates, where rho is the measure for strength of association; positive values indicate monotonic increase, and negative values indicate a monotonic decrease. A p-value under 0.05 was considered significant. Statistical analyses and visualization were performed with Python programming language v3 (python.org/).
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the local ethics board.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Samples of 15,390 patients were included in the study: 7,151 were tested for antibodies against NSA, 6,718 for antibodies against intracellular antigens (ICA), and 5,922 for antibodies against GSA. Testing for more than one group of autoantibodies was performed for 4,051 patients, most commonly NSA + ICA (3,323 patients). Overall, CSF antibody studies were obtained in 4,486 of 7,151 (63%) patients tested for NSA, 3,603 of 6,718 (54%) tested for ICA, and 712 of 5,922 (12%) tested for GSA.
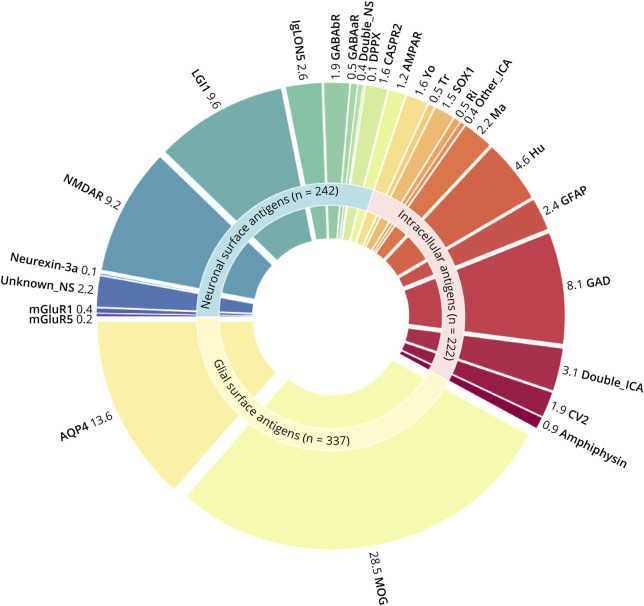
Considering all types of antibodies, there were 801 positive results from 794 different patients: 235 NSA, 216 ICA, 336 GSA, 6 NSA + ICA, and 1 NSA + GSA. This finding represents an overall positivity rate of 5.2% (95% CI 4.8%–5.5%) for any type of neural antigen over the 5-year period. The most frequent antibodies (>20 cases each) in order of frequency were MOG, AQP4, LGI1, NMDAR, GAD, Hu, double intracellular, and IgLON5 (Figure 1).
Figure 1. Antibody Findings During the Study Period (2017–2022).
Donut pie chart illustrating the distribution of specific antigenic reactivities among 801 positive results. Numbers indicate the percentage of particular antigenic specificities among patients with neural antibodies.
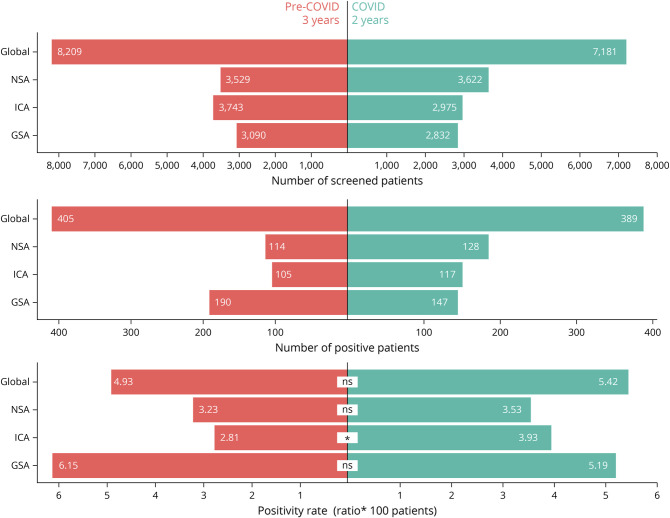
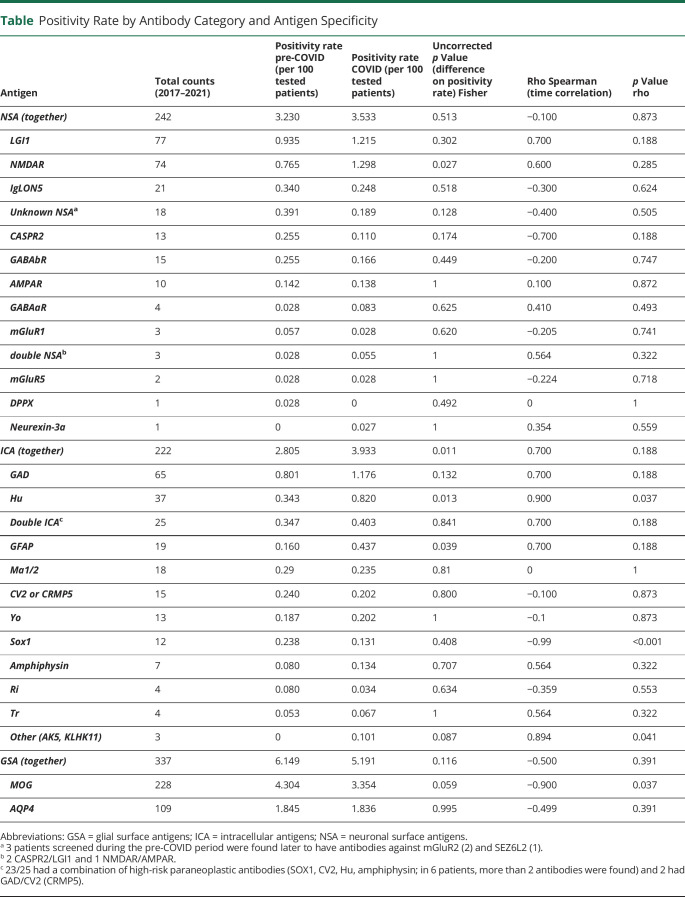
The number of patients examined during the pre–COVID-19 period was 8,209 and during COVID-19 was 7,181 (Figure 2). Therefore, the annualized mean number of patients tested increased 1.3 times during the COVID-19 period (p < 0.001; 1.2 ICA, 1.4 GSA, and 1.5 NSA). Compared with the pre–COVID-19 period, the overall positivity rate changed from 4.9% to 5.4% (p = 0.18) for any type of neural antibody during COVID-19. This rate change varied depending on the type of antibody (Figure 2). The overall NSA positivity rate did not significantly change during COVID-19 (p = 0.51), but subanalysis of NSA for different specificities showed a mild but significant increase of patients with anti-NMDAR encephalitis (p = 0.03). The overall GSA positivity rate was also similar across both periods. By contrast, the overall rate of ICA showed a 1.4-fold increase (p = 0.01), particularly for Hu and GFAP antibodies (Table).
Figure 2. Distribution of Antibody Categories Studied During Pre-COVID and COVID-19 Periods.
Bar charts comparing the number of unique patients screened for NSA, ICA, and GSA antibodies during the pre-COVID (3 years) and COVID-19 (2 years) periods, showing also the number with positive results and the positivity rates. *p = 0.01. GSA = glial surface antigens; ICA = intracellular antigens; ns = not significant; NSA = neuronal surface antigens.
Table.
Positivity Rate by Antibody Category and Antigen Specificity
To explore the temporal trend of the positivity rate, a correlation analysis was performed. The global positivity rate progressively increased starting in the pre–COVID-19 period (rho = 0.99, p < 0.001). For the different specificities, this analysis showed an increasing tendency of positivity for Hu antibodies and a decreasing tendency for MOG and SOX1 antibodies, whereas no significant trends were observed for the rest of antibodies including those against unknown surface antigens (rho = −0.4, p = 0.5) (Table).
Discussion
This study shows that during the COVID-19 pandemic, we did not experience a significant increase of referral samples from patients with encephalitis mediated by antibodies against NSA. In this category, the only exception was patients with anti-NMDAR encephalitis for whom referrals mildly increased. The implications of this finding are unclear, but we suspect it is unrelated to COVID-19. The involvement of vaccines as potential cause of a selective mild increase of the number of cases with anti-NMDAR encephalitis, without affecting the frequency of other disorders (e.g., MOG-associated syndromes or other encephalitis) seems improbable. Other possibilities seem more likely: During the same period, we were conducting a prospective study of patients with anti-NMDAR encephalitis that could have potentially led to an increase of referrals.8 Moreover, the COVID-19 pandemic probably modified the referral patterns for some of these patients. For example, patients who previously would have been considered as having first-onset psychosis or abnormal behavior and referred to psychiatry facilities may have been investigated for possible viral or vaccine-induced AE during the pandemic, as suggested by the increase of samples referred for testing of NMDAR and other NSA.
During the pandemic, we did not find an increase of samples with GSA antibodies (MOG, AQP4). This finding is important in the context of COVID-19 because brain demyelinating lesions resembling acute disseminated encephalomyelitis (ADEM) have been described in patients with COVID-19.9 By contrast, the frequency of antibodies against ICA showed a 1.4-fold increase, particularly for the Hu antibody and GFAP (which was first described in 2016).10 This observation may be related, in part, to a steady increased recognition of patients harboring these antibodies that had already been noted pre–COVID-19,11 or a true rise in incidence, possibly because of the increased use of immune checkpoint inhibitors.12 There is no previous evidence of Hu antibodies triggered by viral infections, and therefore, this also seems unlikely for SARS-CoV-2, but we cannot exclude a causal relationship with the COVID-19 pandemic or vaccination.
In our center, around 7,000 patients were admitted for COVID-19 from onset of the pandemic until December 2021. These patients were not systematically tested for neuronal antibodies unless they had symptoms of encephalitis. None of these patients developed encephalitis associated with antibodies against neuronal surface or intracellular antigens. Moreover, 39 patients with COVID-19 were admitted for encephalopathy or encephalitis of unclear etiology, and none had antibodies against cell surface or intracellular neural proteins.6 These patients were comprehensively tested with all the indicated techniques that also showed the absence of immunoreactivity against blood vessels, ependymal cells, oligodendrocytes, or microglia.6 Thus, we did not find any emerging novel or atypical antibody reactivity during the COVID-19 pandemic compared with our experience over the years with thousands of patients examined for antibodies against neural antigens or cases with AE postviral infections (i.e., herpes simplex encephalitis), patients with psychosis, patients with schizophrenia, patients with neurodegenerative diseases, or healthy participants.13,14
Our study does not exclude the possibility of antibodies not detectable by the current techniques; however, these are the same techniques that led us to discover 12 of the currently known antibodies against neuronal surface proteins.15 A recent study examining a potential change in the serum positivity rate of 5 antibodies (MOG, AQP4, NMDAR, LGI1, and Caspr2) during the COVID-19 pandemic did not find any significant increases in positivity but did find a decrease positivity rate of LGI1 antibodies,16 which we did not observe in our study.
A limitation of our study is that we do not have the clinical information of most of the cases or know the rate of vaccination. However, over 85% of the Spanish population participated in the COVID-19 vaccination program, and most of the samples tested in our center are from Spain.
The strength of this study resides in the use of brain tissue immunostaining that allows detection of antibodies against all intracellular and cell-surface antigens except for those against GlyR, D2R, and some MOG epitopes (which were identified with CBA). Another important contribution is the extensive number of patients whose CSF was tested with the indicated techniques. This is important because in some antibody-mediated encephalitis, such as anti-NMDAR encephalitis, the antibodies may only be identified in CSF.
Overall, our experience does not support that during the COVID-19 pandemic, there was a clinically meaningful group of patients with encephalopathy or encephalitis associated with known or novel neural surface antibodies that was triggered by the viral infection or vaccination.
Appendix. Authors
Study Funding
H. Ariño acknowledges receipt of a “BITRECS” fellowship; the “BITRECS” project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 754550 and from the “La Caixa” Foundation (ID 100010434), under the agreement LCF/PR/GN18/50310006. Instituto de Salud Carlos III—Subdirección General de Evaluación y Fomento de la Investigación Sanitaria and cofunded by European Union, FIS (PI21/00255, RR-G; PI20/00197 JD; PI20/00820 EM-H).
Disclosure
J. Dalmau holds patents for the use of NMDAR, GABAaR, GABAbR, and DPPX, as autoantibody tests. Drs. Dalmau and Graus hold a patent for the use of IgLON5 as an autoantibody test. The rest of the authors have no conflict of interest. Go to Neurology.org/NN for full disclosure.
References
- 1.Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595(7866):283-288. [DOI] [PubMed] [Google Scholar]
- 2.Song E, Bartley CM, Chow RD, et al. Divergent and self-reactive immune responses in the CNS of COVID-19 patients with neurological symptoms. Cell Rep Med. 2021;2(5):100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franke C, Ferse C, Kreye J, et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun. 2021;93:415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valencia Sanchez C, Theel E, Binnicker M, Toledano M, McKeon A. Autoimmune encephalitis after SARS-CoV-2 infection: case frequency, findings, and outcomes. Neurology. 2021;97:e2262-e2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarius S, Pache F, Körtvelyessy P, et al. Cerebrospinal fluid findings in COVID-19: a multicenter study of 150 lumbar punctures in 127 patients. J Neuroinflammation. 2022;19(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guasp M, Muñoz-Sánchez G, Martínez-Hernández E, et al. CSF biomarkers in COVID-19 associated encephalopathy and encephalitis predict long-term outcome. Front Immunol. 2022;13:866153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guasp M, Landa J, Martinez-Hernandez E, et al. Thymoma and autoimmune encephalitis: clinical manifestations and antibodies. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guasp M, Rosa-Justicia M, Muñoz-Lopetegi A, et al. Clinical characterisation of patients in the post-acute stage of anti-NMDA receptor encephalitis: a prospective cohort study and comparison with patients with schizophrenia spectrum disorders. Lancet Neurol. 2022;21:899-910. [DOI] [PubMed] [Google Scholar]
- 9.Manzano GS, McEntire CRS, Martinez-Lage M, Mateen FJ, Hutto SK. Acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis following COVID-19: systematic review and meta-synthesis. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang B, McKeon A, Hinson SR, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol. 2016;73:1297-1307. [DOI] [PubMed] [Google Scholar]
- 11.Shah S, Flanagan EP, Paul P, et al. Population-based epidemiology study of paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. 2021;9(2):e1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farina A, Villagrán-García M, Ciano-Petersen NL, et al. Anti-Hu antibodies in patients with neurologic side effects of immune checkpoint inhibitors. Neurol Neuroimmunol Neuroinflamm. 2022;10(1):e200058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17:760-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara M, Martinez-Hernandez E, Ariño H, et al. Clinical and pathogenic significance of IgG, IgA, and IgM antibodies against the NMDA receptor. Neurology. 2018;90:e1386-e1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. 2017;97:839-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handel AE, Palace J, Bateman E, Waters P, Irani SR. Changes in the rate of leucine-rich glioma-inactivated 1 seropositivity during the COVID-19 lockdown. JAMA Neurol. 2023;80:419-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.