Abstract
Jaw dystonia and laryngospasm in the context of subacute brainstem dysfunction have been described in a small number of diseases, including antineuronal nuclear antibody type 2 (ANNA-2, also known as anti-Ri) paraneoplastic neurologic syndrome. Severe episodes of laryngospasms causing cyanosis are potentially fatal. Jaw dystonia can also cause eating difficulty, resulting in severe weight loss and malnutrition. In this report, we highlight the multidisciplinary management of this syndrome associated with ANNA-2/anti-Ri paraneoplastic neurologic syndrome and discuss its pathogenesis.
Case Presentation
A 61-year-old White woman presented with horizontal binocular diplopia, progressive trismus with jaw pain, and jaw spasms causing tongue biting over 5 weeks. The trismus worsened to the point that she could only eat pureed food and had severe weight loss. She also experienced intermittent pressure headache over the left temporal area, loss of taste, and sometimes metallic taste. Her medical history included depression treated with duloxetine, a left ovarian cyst, a thyroid nodule, and shingles over the left eyelid 2 months earlier. She had never smoked, and her family history was unremarkable. On examination, she had severe horizontal gaze restriction of both eyes with preserved vertical gaze and mild bilateral ptosis but normal pupillary response. Limited mouth opening and intermittent jaw spasms were also observed. The rest of the cranial nerves were intact. She had full motor power and no sensory deficit. Reflexes were decreased in the lower extremities, and plantar response was down-going bilaterally. She had mild left hemiataxia on finger-to-nose testing, dysdiadochokinesia, and heel-to-shin dysmetria.
Brain and spine MRIs, CSF analysis, and extensive blood tests were obtained. An examination of the brain MRI revealed T2-hyperintense lesions in the dorsal pons and medulla with no associated enhancement or restricted diffusion (Figure 1, A and B). The spine MRI was unremarkable. CSF analysis showed no white cells (normal, 0–5/µL) but showed mildly elevated protein, 70 mg/dL (normal, 0–35 mg/dL), and 4 oligoclonal bands that were unmatched with serum (normal range, 0–1). CSF Gram stain, VZV IgG, HSV PCR, VDRL, IgG index, and cytology showed negative results. Blood test showed negative results for ACE, aquaporin-4 (AQP4), and interferon-gamma release assay. She was found to have a low thiamine level, suspected to result from difficulty eating due to jaw-opening dystonia.
Figure 1. Brain MRI of the Patient Before and After Acute Treatment With IV Methylprednisolone.
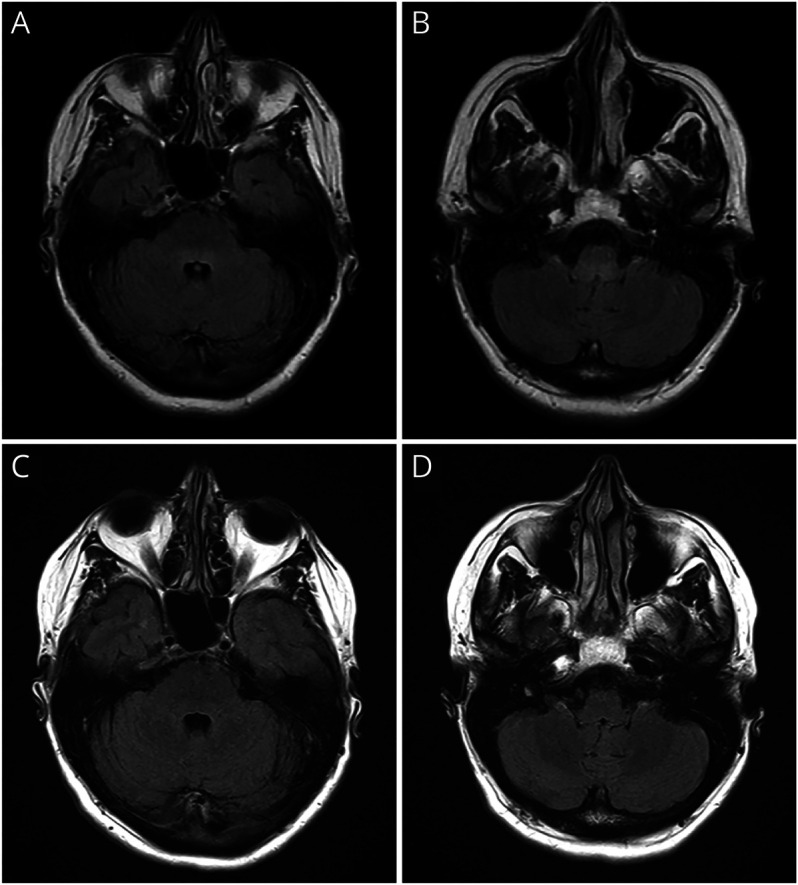
(A and B) Initial brain MRI showing T2-hyperintense lesions in the dorsal pons and medulla. There was no associated enhancement (images not shown); (C and D) The T2-hyperintense lesions disappear after acute treatment with IV methylprednisolone.
Localization and Differential Diagnosis
The patient presented with subacute progressive bilateral horizontal ophthalmoplegia, bilateral ptosis, jaw dystonia and spasms, and dysgeusia, which could localize to the brainstem or cranial nerves. Within the brainstem, bilateral horizontal ophthalmoplegia could localize to the paramedian pontine reticular formation or the abducens nuclei in the dorsal pons, while bilateral ptosis localized to the central caudal nucleus, a midline subnucleus within the oculomotor nuclear complex (Figure 2). Excitation in the brainstem tegmentum could cause jaw dystonia.1 Left hemiataxia suggested involvement of the left cerebellar hemisphere or spinocerebellar tracts. Headache could either be associated with trismus or irritation of pain-sensitive structures, such as the dura, large blood vessels, or the cranial nerves. The combination of bilateral external ophthalmoplegia and headache could either result from direct involvement of the brainstem or be a false localizing sign from increased intracranial pressure. Considering the age at onset and the subacute progression of brainstem syndrome, differential diagnoses are outlined in the Table.2-4
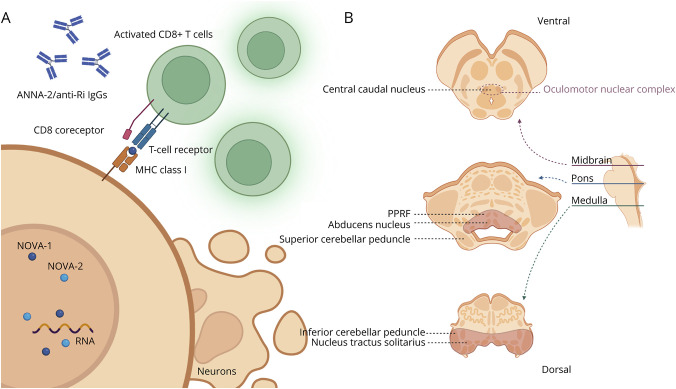
Figure 2. Mechanism and Localization of ANNA-2/anti-Ri Paraneoplastic Neurologic Syndrome in This Patient.
(A) ANNA-2/anti-Ri IgGs are markers of a CD8 T-cell–mediated cytotoxicity process. The antigenic targets of ANNA-2/anti-Ri are NOVA-1 and NOVA-2, which are RNA-binding proteins responsible for alternative splicing, locating in the nucleus of neurons, particularly in the brainstem and cerebellum. Theoretically, these antigens are also present in tumor cells (onconeural antigens), and thus B and T cells are activated in the periphery. Because these antigens are intracellular, CD8 T cells can only detect them when presented by MHC class I molecules before initiating cell killing. (B) Localization of the symptoms and signs in the patient are shown. The central caudal nucleus is a midline nucleus within the oculomotor nuclear complex. It controls bilateral levator palpebrae superioris muscles. The PPRF and abducens nucleus are responsible for horizontal gaze. Nucleus tractus solitarius contains the gustatory nucleus, which receives tastes. The specific localization of jaw dystonia and laryngospasms are less clear but is believed to be excitation or disinhibition in the pontine tegmentum. The T2-hyperintense lesions on the MRI are highlighted in red in this diagram. ANNA = antineuronal nuclear antibody; MHC = major histocompatibility complex; NOVA = neuro-oncological ventral antigen; PPRF = paramedian pontine reticular formation.
Table.
Differential Diagnoses of Adult-Onset Subacute Brainstem Syndrome
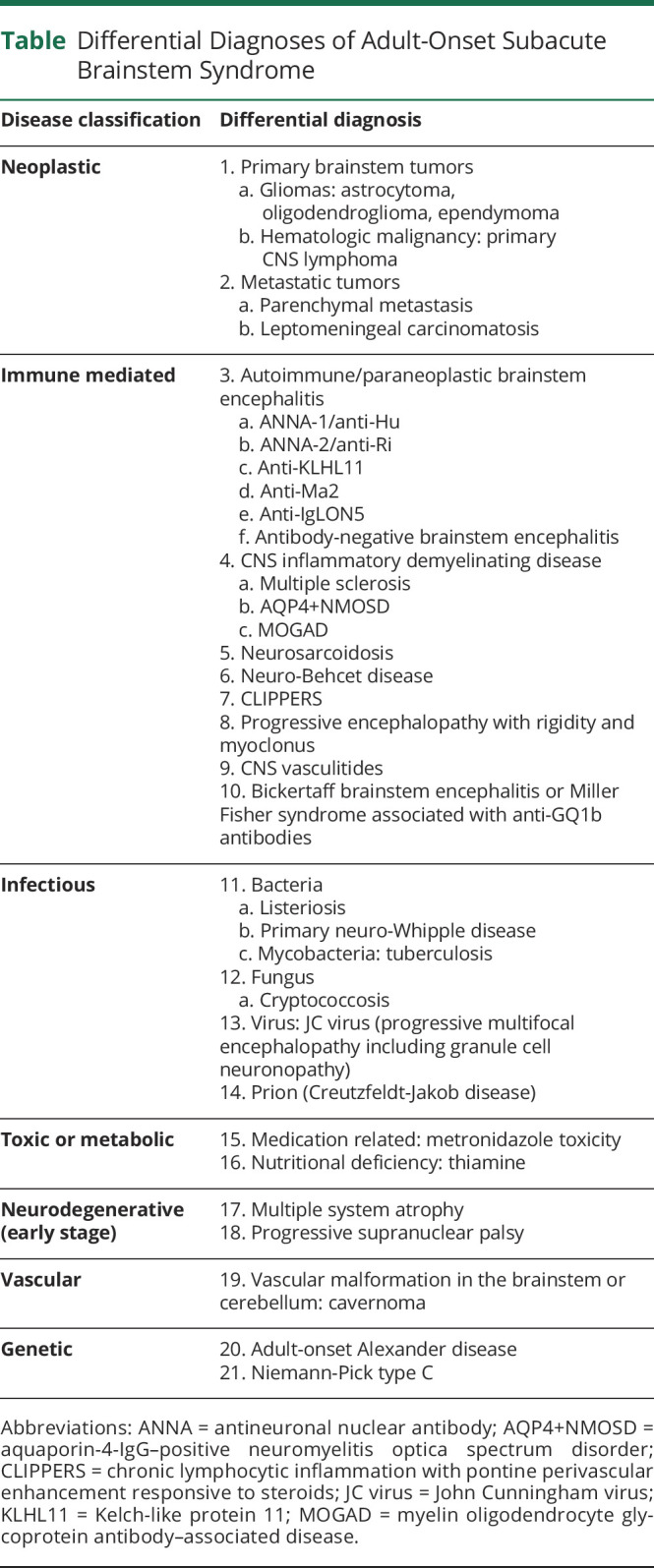
The nonenhancing homogeneous T2-hyperintense lesion in the pons and medulla and inflammatory CSF profile made neurodegenerative causes less likely. The absence of supratentorial abnormalities also made multiple sclerosis, toxic/metabolic, prion, and genetic diseases less likely. The indistinct border of the lesion and absence of enhancement was not typical for tuberculosis, fungal infection, metastatic cancer, or lymphoma, which were often well-circumscribed. Primary brainstem tumor remained a possibility. The patient had no risk factors for an immunocompromised status and no travel history to suggest any specific infection. Among autoimmune and paraneoplastic brainstem encephalitis, jaw dystonia is a rare but characteristic feature of ANNA-2/anti-Ri paraneoplastic neurologic syndrome (PNS).1 Some patients with anti-Ma2 encephalitis can also develop jaw dystonia (Josep Dalmau, personal communication). Moreover, jaw dystonia and laryngospasm may occur with paraneoplastic brainstem encephalitis in the setting of lung cancer but without detectable antibodies (Francesc Graus, personal communication). Isolated brainstem syndromes can occur in AQP4-IgG–positive neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), which can be diagnosed by the presence of AQP4-IgG or MOG-IgG in the serum.3 On the contrary, serum ACE is insensitive for neurosarcoidosis, and thus, a negative result did not exclude the possibility. Neuro-Behcet disease also has a predilection for the brainstem.
Six weeks after the onset of neurologic symptoms, she noticed a lump in her right breast. Ultrasound and mammogram revealed an infiltrative mass in her right breast and axilla, which was pathologically confirmed to be a stage 2 invasive ductal carcinoma with estrogen receptor positive, progesterone receptor positive, and human epidermal growth factor receptor 2 (HER2) negative results.
Paraneoplastic panel revealed antineuronal nuclear antibody type 2 (ANNA-2, also known as anti-Ri) positivity in the serum (titer 1:7680) and CSF (titer 1:128) by tissue immunofluorescence, and both were confirmed with western blot positivity. She was also found to have a low thiamine level, suspected to result from difficulty eating due to jaw-opening dystonia. Subsequently, she reported a 60- to 90-second episode of cyanosis suspected to be secondary to laryngospasm. There were also episodes of coughing unrelated to oral intake.
Final Diagnosis and Treatment
This patient was diagnosed with ANNA-2/anti-Ri antibody–associated paraneoplastic brainstem encephalitis with a stage 2 breast carcinoma. The presence of a high-risk antibody accompanied by classical phenotypes and cancer for ANNA-2/anti-Ri consolidated the definite PNS diagnosis according to the 2021 Updated Diagnostic Criteria for PNS.5
The principles of management of PNS are early treatment of underlying cancer and immunotherapy.6 An oncologist, a general surgeon, and a radiation oncologist were consulted for the treatment of breast cancer. For immunotherapy, she received IV methylprednisolone for 5 days with minimal improvement and continued on 60 mg/d of oral prednisone. Repeated brain MRI showed resolution of lesions (Figure 1, C and D), so prednisone was tapered down to 10 mg/d before surgery. She underwent right mastectomy and axillary lymph node dissection, followed by adjuvant chemotherapy (docetaxel and cyclophosphamide for 4 cycles) and radiation therapy.
Symptomatic management with a multidisciplinary approach should be performed concurrently. A speech therapist was consulted for swallowing evaluation and oral diet modification to accommodate her jaw opening difficulty. Her weight was closely monitored, and thiamine was prescribed to supplement for its deficiency. Antipasticity medication for her jaw-opening dystonia included meloxicam, diazepam, and cyclobenzaprine, which did not provide improvement. Movement disorder specialists recommended botulinum toxin injection to the masseter, temporalis, medial pterygoid, and platysma, which enabled her to open her mouth to the point that she could properly brush her teeth for the first time. The dose of botulinum toxin injection was gradually titrated at each visit. Baclofen and clonazepam also provided some small additional benefits.
PNSs associated with antibodies to intracellular antigens, such as ANNA-2/anti-Ri, have a variable prognosis but are generally less favorable than those associated with antibodies to cell surface antigens. Subsequently, she reported deterioration of trismus and a 60- to 90-second episode of cyanosis suspected to be secondary to laryngospasm. There were also episodes of coughing unrelated to oral intake. This progression prompted initiation of maintenance immunotherapy, so she was prescribed intravenous immunoglobulin (IVIg) every 3 weeks, before transitioning to rituximab. Cyclophosphamide, already given as a part of chemotherapy for breast cancer, was extended to 6 months. A trial of 5 cycles of plasma exchange was also undertaken.
Despite cancer treatment and immunotherapy, she continued to have 2–3 episodes of laryngospasm lasting 15–20 seconds a day, with occasional cyanosis. She had never lost consciousness or required intubation because of this. A whole-body fluorodeoxyglucose PET (FDG-PET) and CT scan showed no residual breast cancer.
Laryngospasm was the most concerning symptom for this patient that could potentially cause critical hypoxia. She felt that clonazepam helped her laryngospasms, but the mainstay of management is tracheostomy. The neuroimmunology team collaborated with otorhinolaryngologists, and tracheostomy and alternative management options including botulinum toxin injections in the larynx were being considered.
At the last follow-up, she had persistent bilateral horizontal ophthalmoplegia, ataxia, and jaw-opening dystonia with difficulty eating but to a milder degree. Treatment for breast cancer was completed with success, but she continued surveillance imaging as planned. Maintenance immunotherapy was continued with rituximab every 6 months and low-dose oral prednisone.
Discussion
ANNA-2/anti-Ri is a marker of CD8 T-cell–mediated PNS with a strong cancer association.5 Classically, patients present with neurologic syndromes involving the brainstem or cerebellum, such as ataxia, ophthalmoplegia, opsoclonus-myoclonus syndrome, jaw dystonia, and laryngospasm. The stepwise disease progression and variety of presentations can lead to delayed diagnosis. More recently, isolated confusion, syndrome of inappropriate antidiuretic hormone secretion, and dysautonomia with central hypoventilation have been described in association with ANNA-2/anti-Ri PNS. The wide clinical spectrum involving cardiovascular and respiratory system dysfunction make multidisciplinary care essential for patients with ANNA-2/anti-Ri PNS, especially those with brainstem involvement.4 More than 70% of patients have a certain degree of neurologic improvement after cancer treatment and immunotherapy, with variable survival ranging from a few months to several years.4,7
ANNA-2/anti-Ri PNS has a female preponderance, and the commonly associated cancers are breast cancer in women and neuroendocrine tumors of the lung or bladder in men. Of interest, none of the reported patients with breast cancer, including the present case, had HER2 expression.4 Autopsies of ANNA-2/anti-Ri PNS patients had shown prominent CD8 T-lymphocyte infiltration, neuronal cell loss with astrogliosis in the brainstem, and Purkinje cell loss with Bergmann gliosis in the cerebellum.1,8,9 These regions have a high expression of neuro-oncologic ventral antigen (NOVA)-1 and NOVA-2, which are the targets of ANNA-2/anti-Ri.10 NOVA-1 and NOVA-2 are RNA-binding proteins acting as alternative splicing regulators responsible for cancer cell proliferation, invasion, and angiogenesis.11,12
Management of PNS requires cancer treatment and immunotherapy. While the rarity of PNS precludes randomized controlled trials, expert opinion suggests that acute immunotherapy with IV methylprednisolone is often considered as first line. Second-line treatment is guided by the target antigen location, either cell surface or intracellular, which determines the underlying mechanism. In those with antibodies to cell surface targets, additional treatments aimed at antibody depletion (plasma exchange), neutralization (IVIg), and reducing antibody production (anti-CD20 B cell depleting agents such as rituximab) are often favored. Antibodies targeting cell surface antigens are considered pathogenic by inducing antibody-dependent cellular cytotoxicity, complement activation, or functional blockade. Cyclophosphamide is a cytotoxic agent more often considered in those with antibodies to intracellular targets, in whom a CD8 T-cell–mediated process is suspected, particularly with fulminant presentations.6 However, T cells and B cells work in tandem, and thus, these treatments may sometimes be used in combination, as in this case.
Outcomes of PNS with antibodies targeting intracellular neural antigens, such as ANNA-2/anti-Ri, are generally less favorable than those with antibodies against cell surface antigens, possibly due to CD8 T–cell associated cytotoxicity. However, prompt early treatment could potentially limit neuronal loss and stabilize neurologic symptoms, and improvement of ANNA-2/anti-Ri PNS with treatment has been observed in some patients.1
Laryngospasm is an uncommon manifestation of brainstem syndromes that has been reported in the context of autoimmune/paraneoplastic, neurodegenerative, viral, and ischemic etiologies.1,13-15 Antibodies reported in association with laryngospasm include ANNA-2/anti-Ri and IgLON5.1,16 In neurodegenerative diseases, laryngospasm has been reported in multiple system atrophy, amyotrophic lateral sclerosis, and progressive supranuclear palsy and could also be an early presentation.14,15,17 The pathophysiology is unclear but could involve either the loss of inhibition of the laryngeal closure reflex or the stimulation of the superior laryngeal nerve.18 Prolonged episodes of laryngospasm could lead to sudden death from asphyxiation; therefore, prophylactic tracheostomy should be discussed.1,16 Caution should be taken in provocative circumstances such as airway manipulation, emergence from general anesthesia, or comorbid gastroesophageal reflux disease.13 Botulinum toxin injection has been explored for the management of spasmodic dysphonia and laryngeal dystonia causing stridor but is not yet standard for laryngospasm.19,20
Symptomatic therapy and comprehensive multidisciplinary care should always accompany PNS treatment. Jaw dystonia could be relieved by botulinum toxin injection and antipasticity medications. Nutritional deficiency from restricted jaw opening, also observed in this patient, is not uncommon.1 Gastrostomy tube might be required to improve intake in severe cases.
In summary, paraneoplastic etiologies should be considered in the differential diagnosis of subacute brainstem syndromes. The accompanying manifestations can be potentially life-threating in the setting of laryngospasm. Jaw dystonia can be complicated by malnutrition and severely affect the quality of life. A multidisciplinary team is required for the optimal management in these patients, from treating the culprit cancer to addressing the downstream complications from PNS.
Acknowledgment
Figure 2 was created with BioRender.com.
Glossary
- AQP4
aquaporin-4
- FDG-PET
fluorodeoxyglucose PET
- HER2
human epidermal growth factor receptor 2
- IVIg
IV immunoglobulin
- MOG
myelin oligodendrocyte glycoprotein
- PNS
paraneoplastic neurologic syndrome
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Pittock SJ, Parisi JE, McKeon A, et al. Paraneoplastic jaw dystonia and laryngospasm with antineuronal nuclear autoantibody type 2 (anti-Ri). Arch Neurol. 2010;67(9):1109-1115. doi: 10.1001/archneurol.2010.209 [DOI] [PubMed] [Google Scholar]
- 2.Tan IL, Mowry EM, Steele SU, et al. Brainstem encephalitis: etiologies, treatment, and predictors of outcome. J Neurol. 2013;260(9):2312-2319. doi: 10.1007/s00415-013-6986-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks SA, Morris PP, Chen JJ, et al. Brainstem and cerebellar involvement in MOG-IgG-associated disorder versus aquaporin-4-IgG and MS. J Neurol Neurosurg Psychiatry. 2020:jnnp-2020-325121. doi: 10.1136/jnnp-2020-325121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simard C, Vogrig A, Joubert B, et al. Clinical spectrum and diagnostic pitfalls of neurologic syndromes with Ri antibodies. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e699. doi: 10.1212/nxi.0000000000000699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graus F, Vogrig A, Muñiz-Castrillo S, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1014. doi: 10.1212/nxi.0000000000001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devine MF, Kothapalli N, Elkhooly M, Dubey D. Paraneoplastic neurological syndromes: clinical presentations and management. Ther Adv Neurol Disord. 2021;14:175628642098532. doi: 10.1177/1756286420985323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Ann Neurol. 2003;53(5):580-587. doi: 10.1002/ana.10518 [DOI] [PubMed] [Google Scholar]
- 8.Hormigo A, Dalmau J, Rosenblum MK, River ME, Posner JB. Immunological and pathological study of anti-Ri-associated encephalopathy. Ann Neurol. 1994;36(6):896-902. doi: 10.1002/ana.410360615 [DOI] [PubMed] [Google Scholar]
- 9.Brieva-Ruíz L, Diaz-Hurtado M, Matias-Guiu X, Márquez-Medina D, Tarragona J, Graus F. Anti-Ri-associated paraneoplastic cerebellar degeneration and breast cancer: an autopsy case study. Clin Neurol Neurosurg. 2008;110(10):1044-1046. doi: 10.1016/j.clineuro.2008.06.016 [DOI] [PubMed] [Google Scholar]
- 10.Buckanovich RJ, Posner JB, Darnell RB. Nova, the paraneoplastic Ri antigen, is homologous to an RNA-binding protein and is specifically expressed in the developing motor system. Neuron. 1993;11(4):657-672. doi: 10.1016/0896-6273(93)90077-5 [DOI] [PubMed] [Google Scholar]
- 11.Qu L, Tian Y, Wang F, Li Z. NOVA1 promotes NSCLC proliferation and invasion by activating Wnt/β-catenin signaling. BMC Cancer. 2022;22(1):1091. doi: 10.1186/s12885-022-10164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giampietro C, Deflorian G, Gallo S, et al. The alternative splicing factor Nova2 regulates vascular development and lumen formation. Nat Commun. 2015;6(1):8479. doi: 10.1038/ncomms9479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gdynia HJ, Kassubek J, Sperfeld AD. Laryngospasm in neurological diseases. Neurocrit Care. 2006;4(2):163-167. doi: 10.1385/ncc:4:2:163 [DOI] [PubMed] [Google Scholar]
- 14.van der Graaff MM, Grolman W, Westermann EJ, et al. Vocal cord dysfunction in amyotrophic lateral sclerosis: four cases and a review of the literature. Arch Neurol. 2009;66(11):1329-1333. doi: 10.1001/archneurol.2009.250 [DOI] [PubMed] [Google Scholar]
- 15.Tipton PW, Ekbom DC, Rutt AL, van Gerpen JA. Vocal fold “paralysis”: an early sign in multiple system atrophy. J Voice. 2020;34(6):940-944. doi: 10.1016/j.jvoice.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 16.Honorat JA, Komorowski L, Josephs KA, et al. IgLON5 antibody: neurological accompaniments and outcomes in 20 patients. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e385. doi: 10.1212/nxi.0000000000000385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panegyres PK, Hillman D, Dunne JW. Laryngeal dystonia causing upper airway obstruction in progressive supranuclear palsy. J Clin Neurosci. 2007;14(4):380-381. doi: 10.1016/j.jocn.2005.12.019 [DOI] [PubMed] [Google Scholar]
- 18.Fink BR. The etiology and treatment of laryngeal spasm. Anesthesiology. 1956;17(4):569-577. doi: 10.1097/00000542-195607000-00007 [DOI] [PubMed] [Google Scholar]
- 19.Benninger MS, Gardner G, Grywalski C. Outcomes of botulinum toxin treatment for patients with spasmodic dysphonia. Arch Otolaryngol Head Neck Surg. 2001;127(9):1083-1085. doi: 10.1001/archotol.127.9.1083 [DOI] [PubMed] [Google Scholar]
- 20.Merlo IM, Occhini A, Pacchetti C, Alfonsi E. Not paralysis, but dystonia causes stridor in multiple system atrophy. Neurology. 2002;58(4):649-652. doi: 10.1212/wnl.58.4.649 [DOI] [PubMed] [Google Scholar]