Abstract
Purpose:
Vascular-targeted photodynamic therapy (VTP) with the intravascular photosensitizing agent padeliporfin (WST-11/TOOKAD-Soluble) has demonstrated therapeutic efficacy as an ablative treatment for localized cancer with potential adaptation for endoscopic management of upper tract urothelial carcinoma (UTUC). This phase I trial (NCT03617003) evaluated the safety of VTP with WST-11 in UTUC.
Materials and Methods:
Nineteen patients underwent up to two endoscopic VTP treatments, with follow-up for up to six months. Patients who had residual or recurrent UTUC (any grade/size) failing prior endoscopic treatment or were unable or unwilling to undergo surgical resection were eligible for inclusion. The primary endpoint was to identify the maximally tolerated dose (MTD) of laser light fluence. A dose escalation model was employed, with increasing light fluence (100–200mW/cm) using a modified continual reassessment method. The secondary endpoint was treatment efficacy, defined by absence of visible tumor and negative urine cytology 30 days post-treatment.
Results:
Fourteen (74%) patients received the MTD of 200mW/cm, two (11%) of whom experienced a dose-limiting toxicity. The initial 30-day treatment response rate was 94% (50% complete, 44% partial). Eight patients underwent a second treatment, with a final observed 68% complete response rate. Leading toxicities were flank pain (79%) and hematuria (84%), which were transient. No ureteral strictures associated with treatment were identified during follow-up.
Conclusions:
VTP with WST-11 has an acceptable safety profile with strong potential as an effective, kidney-sparing endoscopic management option for UTUC. The recently initiated multi-center phase 3 ENLIGHTED trial (NCT04620239) is expected to provide further evidence on this therapy.
Keywords: phototherapy, ureteroscopy, urogenital neoplasms
Introduction
Upper tract urothelial carcinoma (UTUC) accounts for 5% of all urothelial neoplasms1 and 10% of renal tumors,2 with approximately two-thirds occurring in the renal pelvis.3 Radical nephroureterectomy as extirpative management is associated with significant morbidity, including the development of chronic kidney disease or end-stage renal disease in up to 80% of patients.4 In cases of low-grade tumors with minimal risk of metastatic progression, and even some early high-grade superficial tumors, this surgery is drastically overutilized.5 As such, there is increased interest in organ-sparing approaches, including endoscopic management techniques, to preserve renal function. Endoscopic approaches are technically challenging and are associated with a 60% rate of recurrence.6 Multiple treatments are often necessary, which increases the risk of procedure-related complications such as perforation, bleeding, infection, and strictures.6 Up to 20% of patients undergoing endoscopic management of UTUC will eventually require nephrectomy, and 30% will recur in the bladder.7 There is a clear unmet need to improve kidney-sparing endoscopic management techniques for patients with UTUC.
Photodynamic therapy, which utilizes a photosensitizing drug and light application to create tissue damage, is an effective and Food and Drug Administration–approved localized treatment modality for several malignancies, including lung, esophageal, and skin cancers, as well as the endoluminal treatment of urothelial cancers.8 In the esophagus, for example, certain obstructing tumors may not be excisable using a neodymium yttrium-aluminum-garnet (Nd:YAG) laser, but photodynamic therapy may offer palliation in lieu of total esophagectomy.8 However, its uptake has been limited by prolonged patient light sensitivity and non-selective tissue destruction.
Padeliporfin (WST-11/TOOKAD Soluble; STEBA Biotech, Luxembourg) is a new investigational short-acting photodynamic agent to produce a novel form of vascular-targeted photodynamic treatment (VTP), which has been proven to be effective against several cancers in preclinical studies and clinical trials.9 10 11 In prostate cancer, VTP has been utilized as focal therapy option for unilateral low-risk disease.11 After intravenous administration of WST-11, near-infrared light delivered through an optical fiber illuminates the tumor tissues and activates the agent to generate reactive oxygen and nitrogen species (free radicals). Tumor vasculature then collapses, and a propagation of cytotoxic effects to surrounding tumor cells results in athermal tumor ablation, which is histologically evident as coagulative necrosis within 24–48 hours.12 13 14 15 The cytotoxic ablative effect is highly localized and transient, as WST-11 is rapidly cleared from the circulation in minutes. As a result, patient light sensitivity is minimized.9 Following success utilizing WST-11–mediated VTP in preclinical models,15 we undertook a prospective phase 1 study to evaluate this approach as an endoscopic treatment in patients with UTUC. While toxicities in prior studies were minimal (i.e. hematuria, pain, dysuria)11, the effective light dose for the treatment of UTUC has not yet been determined, and so we hypothesized that a maximum of 200 mW/cm would have a toxicity rate that does not exceed an acceptable threshold of 20%. The primary objective was to determine the maximum tolerated dose (MTD) of laser light fluence, and the secondary objective was to assess the efficacy of VTP as a primary treatment for UTUC.
Materials and Methods
This is a single-institution, non-randomized open-label phase I study conducted at Memorial Sloan Kettering Cancer Center (MSK), registered under ClinicalTrials.gov Identifier NCT03617003. The protocol was approved on March 19, 2018 and began accrual on August 1, 2018. The study was closed to accrual on April 21, 2021, and the final analysis was performed on June 15, 2022. After institutional review board (IRB) approval (MSK IRB# 18–140), patients were enrolled based on the following inclusion criteria: confirmed tissue diagnosis of UTUC; residual or recurrent cancer following prior endoscopic treatment; and ineligibility or unwillingness to undergo surgical management by resection of the involved kidney and/or ureter. Patients were also required to have a Karnofsky Performance Status ≥50%, adequate organ function (including calculated creatinine clearance ≥40 ml/min), and could not have received any systemic therapy (i.e. chemotherapy, biologic therapy, and/or immunotherapy) ≤4 weeks prior to treatment. Patients with existing ureteral obstruction and/or existing ureteral stent were permitted. Patients were excluded if pregnant or breast-feeding, with T4 tumors involving bowel or major blood vessels, or not surgical candidates due to medical comorbidities. Patients then underwent endoscopy of the involved upper urinary system, with measurement of tumor size (by caliper or visual reference gauge) and determination of location. Visible tumors were treated with VTP based on a prescribed light dose using the escalation protocol described below. Treatment consisted of a ten-minute intravenous administration of WST-11 at a fixed dose of 4 mg/kg, followed by light activation delivered through an INTERmedic (Barcelona, Spain) diode laser (200 micron fiber, 753nm wavelength of light) over ten minutes. The endoscope is positioned proximal to the site of the index tumor to be treated, and the light fiber is advanced through the endoscope and positioned within the collecting system in proximity to the index tumor. The fiber is held in place for the duration of light application, then removed through the endoscope. Per protocol, a ureteral stent was utilized following the initial endoscopic treatment to mitigate the risks inherent to endoscopy, which was removed at the subsequent evaluation. Repeat endoscopic evaluations were performed at 7- and 30-days post-treatment to assess for visual evidence of abnormalities, treatment effect, and evidence of ureteral stricture. Patients were followed for up to six months. Patients were allowed to receive a second VTP treatment within six months of the first if tumors were subsequently identified at the initial target site or at a different site in the upper tract.
The primary objective of this Phase 1 study was to identify the MTD of laser light fluence rate (mW/cm) of light exposure for VTP treatment of urothelial cancer involving the upper urinary tract. The MTD was defined as the dose whose toxicity rate did not exceed an acceptable threshold of 20%. Dose-limiting toxicity (DLT) was a binary outcome where a patient either did or did not experience a DLT. All toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. The trial was monitored using a modification of the continual reassessment method (mCRM),16 which assumes a simple model for the probability of a DLT as a function of dose and uses the occurrence of toxicities in the patients enrolled in the trial to sequentially determine which dose to administer to a newly enrolled patient. Newly enrolled patients were assigned a dose after previously enrolled patients were assessed for DLT. The first enrolled patient received the lowest dose, and dose skipping (eg, from the lowest dose to the highest) was not permitted. Enrollment continued until 18 patients were eligible for the primary study aim to identify the MTD and the secondary aim assessing tumor response.
We studied three dose levels of laser light fluence: 100, 150, and 200 mW/cm. These levels were based on our preclinical large-animal studies, in which levels from 50 to 200 mW/cm were tested. 50 mW/cm produced treatment depth limited to the lamina propria, while 200 mW/cm penetrated muscularis propria.15 Our initial estimates of DLT probabilities were 5%, 10%, and 20% for the three doses, respectively. Thus, our a priori belief was that 200 mW/cm is the MTD. We assumed that the dose-toxicity followed a logistic model where a was the unknown parameter we needed to estimate in order to determine which dose was the MTD, and b was fixed at three.
A value of a=1.0 indicates that our beliefs were correct, while a value of a less than (greater than) 1.0 indicates that the combinations are more (less) toxic than believed. To reflect the uncertainty in our prior probability estimates, we assumed a followed an exponential distribution (prior distribution) with a mean of 1.0. If our a priori estimates of toxicity were correct, the probability that we would correctly identity 200 mW/cm as the MTD was 75%, with an expected 62% of enrolled patients being treated at the MTD.
The secondary objective of this study was to assess the efficacy of VTP as a primary treatment for UTUC, based on the response of the target treatment site. No entry criteria were placed on tumor size or grade in this phase 1 trial. Response was assessed at 30 days after treatment and complete response (CR) was defined as an absence of visible tumor on endoscopic evaluation and absence of malignant cells on cytology from instrumented urine sample from the renal pelvis or ureter. Atypical cytologies were categorized as negative, and suspicious cytologies were categorized as positive. Partial response (PR) was defined as an absence of the index tumor treated but evidence of recurrence outside the treatment area within the ipsilateral renal pelvis or ureter.
Analyses were conducted using R 4.1.0 utilizing the tidyverse (v1.3.1), gtsummary (v1.4.1), and CRM (v1.2.4) packages.17 18 19 20
Results
A total of 22 patients were enrolled into the study. Three patients were removed after further screening per protocol, due to absence of visible tumor at the time of planned experimental treatment, leaving 19 patients who received the VTP treatment (Table 1). Of these 19 patients, all were eligible for the MTD assessment, and 18 were eligible for the secondary response endpoint. One patient was removed from the study two weeks after treatment for non-compliance (illicit drug use) (Supplemental Figure 1).
Table 1.
Patient summary
| Characteristic | N = 191 |
|---|---|
| Age | 65 (60, 70) |
| Sex | |
| Female | 6 (32%) |
| Male | 13 (68%) |
| Race | |
| White | 16 (84%) |
| Black or African American | 2 (11%) |
| Other | 1 (5.3%) |
| BMI | 28 (26, 31) |
| Unknown | 2 |
| Smoking Status | |
| Current | 4 (22%) |
| Former | 9 (50%) |
| Never | 5 (28%) |
| Unknown | 1 |
| Prior Radiation | 0 (0%) |
| Prior Surgery2 | 11 (58%) |
| Tumor Grade | |
| High Grade | 5 (26%) |
| Low Grade | 14 (74%) |
| Laterality | |
| Left | 9 (47%) |
| Right | 10 (54%) |
| Retreated | 8 (42%) |
| VTP Dose Received | |
| 100 mW/cm | 1 (5.3%) |
| 150 mW/cm | 4 (21%) |
| 200 mW/cm | 14 (74%) |
| DLT | 2 (11%) |
| Max. Tumor Diameter, mm | 10 (5, 20) |
| < 15 | 11 (58%) |
| ≥ 15 | 8 (42%) |
| Treatment Response | |
| No response | 1 (5.6%) |
| Partial response | 8 (44%) |
| Complete response | 9 (50%) |
| Unknown | 1 |
Median (IQR); n (%),
ureteroscopy and ablation
One (5%) patient received a dose of 100 mW/cm, four (21%) patients received a dose of 150 mW/cm, and 14 (74%) patients received the highest dose of 200 mW/cm. No patients treated with 100 or 150 mW/cm experienced a DLT. Two patients treated with 200 mW/cm experienced a DLT (2/14; 14%). Both toxicities were pain-related and were graded 2 and 3 because they required additional intravenous medication for management and overnight hospital stay instead of outpatient discharge immediately after the procedure, respectively. These two DLTs were attributed to the procedure, likely due to the stent, and not the drug or device (Supplemental Table 1). The final recommended dose based on the mCRM study design was 200 mW/cm.
The rate of CR among patients eligible for the 30-day response assessment was 9/18 (50%; exact binomial 95% CI 26%, 74%) (Table 2). Treatment efficacy stratified by tumor size yielded CR in 55% and 43% of tumors < 15 and ≥ 15 mm, respectively. When stratified by tumor grade, the CR was 40% and 54% in high- and low-grade tumors, respectively. The rate of PR was 8/18 (44%), after which all eight patients underwent a second treatment, with a final 68% CR rate.
Table 2.
Response status by dose
| Group | ||||
|---|---|---|---|---|
| Overall | 100 mW/cm | 0 (0%) | 0 (0%) | 1 (100%) |
| 150 mW/cm | 0 (0%) | 2 (50%) | 2 (50%) | |
| 200 mW/cm | 1 (7.7%) | 6 (46%) | 6 (46%) | |
| Overall | 1 (5.6%) | 8 (44%) | 9 (50%) | |
| Max. tumor diameter < 15 mm | 100 mW/cm | 0 (0%) | 0 (0%) | 1 (100%) |
| 150 mW/cm | 0 (0%) | 2 (67%) | 1 (33%) | |
| 200 mW/cm | 0 (0%) | 3 (43%) | 4 (57%) | |
| Overall | 0 (0%) | 5 (45%) | 6 (55%) | |
| Max. tumor diameter ≥ 15 mm | 100 mW/cm | — | — | — |
| 150 mW/cm | 0 (0%) | 0 (0%) | 1 (100%) | |
| 200 mW/cm | 1 (17%) | 3 (50%) | 2 (33%) | |
| Overall | 1 (14%) | 3 (43%) | 3 (43%) | |
| High Grade | 100 mW/cm | — | — | — |
| 150 mW/cm | 0 (0%) | 1 (100%) | 0 (0%) | |
| 200 mW/cm | 1 (25%) | 1 (25%) | 2 (50%) | |
| Overall | 1 (20%) | 2 (40%) | 2 (40%) | |
| Low Grade | 100 mW/cm | 0 (0%) | 0 (0%) | 1 (100%) |
| 150 mW/cm | 0 (0%) | 1 (33%) | 2 (67%) | |
| 200 mW/cm | 0 (0%) | 5 (56%) | 4 (44%) | |
| Overall | 0 (0%) | 6 (46%) | 7 (54%) | |
Serum creatinine and eGFR measures taken at days 2, 7, 14, and 30 following treatment did not show significant change from baseline (Table 3).
Table 3.
Laboratory values
| Characteristic | Baseline | Day 2 | Day 7 | Day 14 | Day 30 |
|---|---|---|---|---|---|
| Creatinine, mg/dL | |||||
| N | 19 | 18 | 18 | 18 | 16 |
| Median (IQR) | 1.10 (0.90, 1.35) | 1.10 (0.90, 1.37) | 1.05 (1.00, 1.48) | 1.10 (0.90, 1.40) | 1.05 (0.88, 1.30) |
| Range | 0.80, 1.80 | 0.80, 1.90 | 0.70, 9.00 | 0.80, 2.00 | 0.70, 1.50 |
| BUN, mg/dL | |||||
| N | 19 | 18 | 18 | 18 | 16 |
| Median (IQR) | 18.0 (16.0, 21.0) | 15.5 (12.0, 17.0) | 16.5 (15.2, 19.8) | 18.0 (16.2, 19.0) | 18.0 (15.2, 20.5) |
| Range | 14.0, 28.0 | 9.0, 27.0 | 13.0, 28.0 | 14.0, 33.0 | 10.0, 28.0 |
| Calcium, mg/dL | |||||
| N | 19 | 18 | 18 | 18 | 16 |
| Median (IQR) | 9.50 (9.05, 9.60) | 8.95 (8.70, 9.28) | 9.35 (9.15, 9.70) | 9.30 (9.00, 9.40) | 9.40 (9.00, 9.53) |
| Range | 8.50, 10.60 | 8.10, 10.20 | 8.90, 10.40 | 8.70, 10.30 | 8.80, 10.00 |
| eGFR (MDRD), mL/min/1.73m2 | |||||
| N | 19 | 18 | 18 | 18 | 16 |
| Median (IQR) | 63 (50, 74) | 62 (51, 67) | 64 (51, 75) | 66 (50, 73) | 66 (56, 72) |
| Range | 35, 85 | 36, 84 | 6, 85 | 34, 85 | 47, 85 |
Of note, one patient treated early in the trial was incorrectly recorded as experiencing a Grade 3 treatment-related DLT (urinary tract infection), which was later investigated by a separate medical monitor and determined to be Grade 2 and resulting from follow-up endoscopy. The error was corrected after the final patient had received treatment. Because the mCRM algorithm uses all previously enrolled patients’ information to inform the dose of each newly enrolled patient, the recording error modified the mCRM-recommended doses. However, this did not impact the final recommended MTD in this dose-finding study; if the recording error had not been made and patients’ DLT statuses remained what was observed (Supplemental Figure 2), the final recommended dose would have remained 200 mW/cm.
Discussion
This is the first study demonstrating the safety profile and treatment effects of WST-11 VTP in the endoscopic management of UTUC. According to the mCRM, the majority of patients received the MTD of 200 mW/cm, with no DLTs in the dose-escalation groups, and only two DLTs at the MTD. These two DLTs were pain-related and transient. No DLTs were related to the drug and no phototoxicity events were seen. There were no Grade 4 or 5 toxicities over the course of the study (Supplemental Tables 3–5). Typical for related types of procedures,22 all patients reported at least one adverse event, but the majority were minor (Grade 1–2), involving discomfort, hematuria, voiding symptoms, and fatigue. Severe adverse events were reported in four patients, with two related to the device and the remainder related to the procedure. Ureteral stenting was felt to be a contributor to several of the experienced events, including reports of flank pain, hematuria, and urinary frequency. These toxicities were minor and transient, and no adverse event led to study discontinuation. Renal function was essentially unchanged over the course of the study, and all treated kidneys were preserved at 6 months follow-up. Overall, VTP with WST-11 appears to be a safe endoscopic treatment option for UTUC.
In evaluating efficacy, VTP demonstrated tumor response in the majority (94%) of patients, with CR seen in half of patients after one treatment. Complete response was more often seen in tumors smaller than 15mm but also achieved in tumors larger than 15mm; however, case numbers are limited. Similarly, CR was more common in low-grade than high-grade tumors. An example of the endoscopic appearance of a UTUC tumor before and after therapy is presented in Figure 1, where a papillary lesion is clearly visualized on Day 0, collagenous fibers are present at Day 7, and absence of disease at Day 30. A retrograde pyelogram in a patient with multiple tumors prior to VTP, with complete resolution on follow-up retrograde pyelogram at 30 days, is presented in Figure 2. The histologic appearances of both low and high-grade UTUC tumors, before and after therapy, are presented in Figure 3. Cytology results (Supplemental Table 2) remained relatively unchanged over the course of the study, although the majority were negative at the onset, and the positive results at day 30 reflect tumors with a partial response. All eight patients offered repeat treatment per protocol after their day 30 evaluation elected a second VTP treatment, supporting the acceptability of VTP therapy. All second treatments were performed at the MTD, and an additional four patients (68% overall) achieved a complete response within 30 days of the second treatment.
Figure 1.
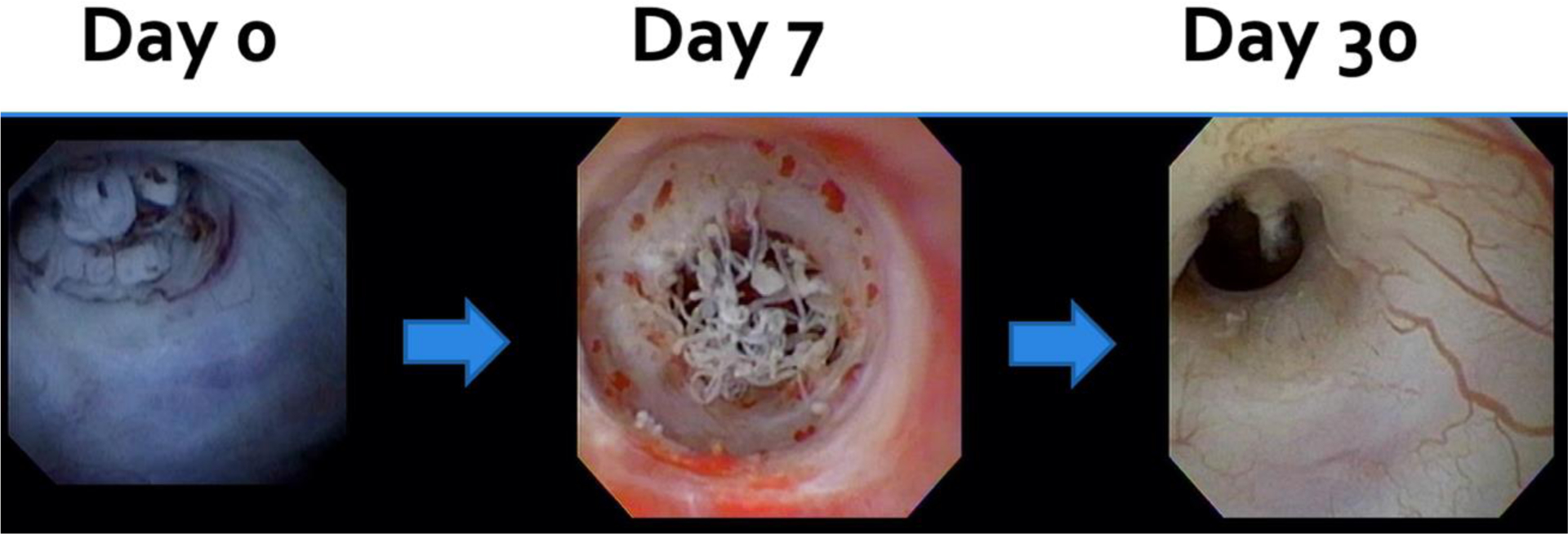
Endoscopic appearance of tumor before and after one vascular-targeted photodynamic therapy treatment
Figure 2.
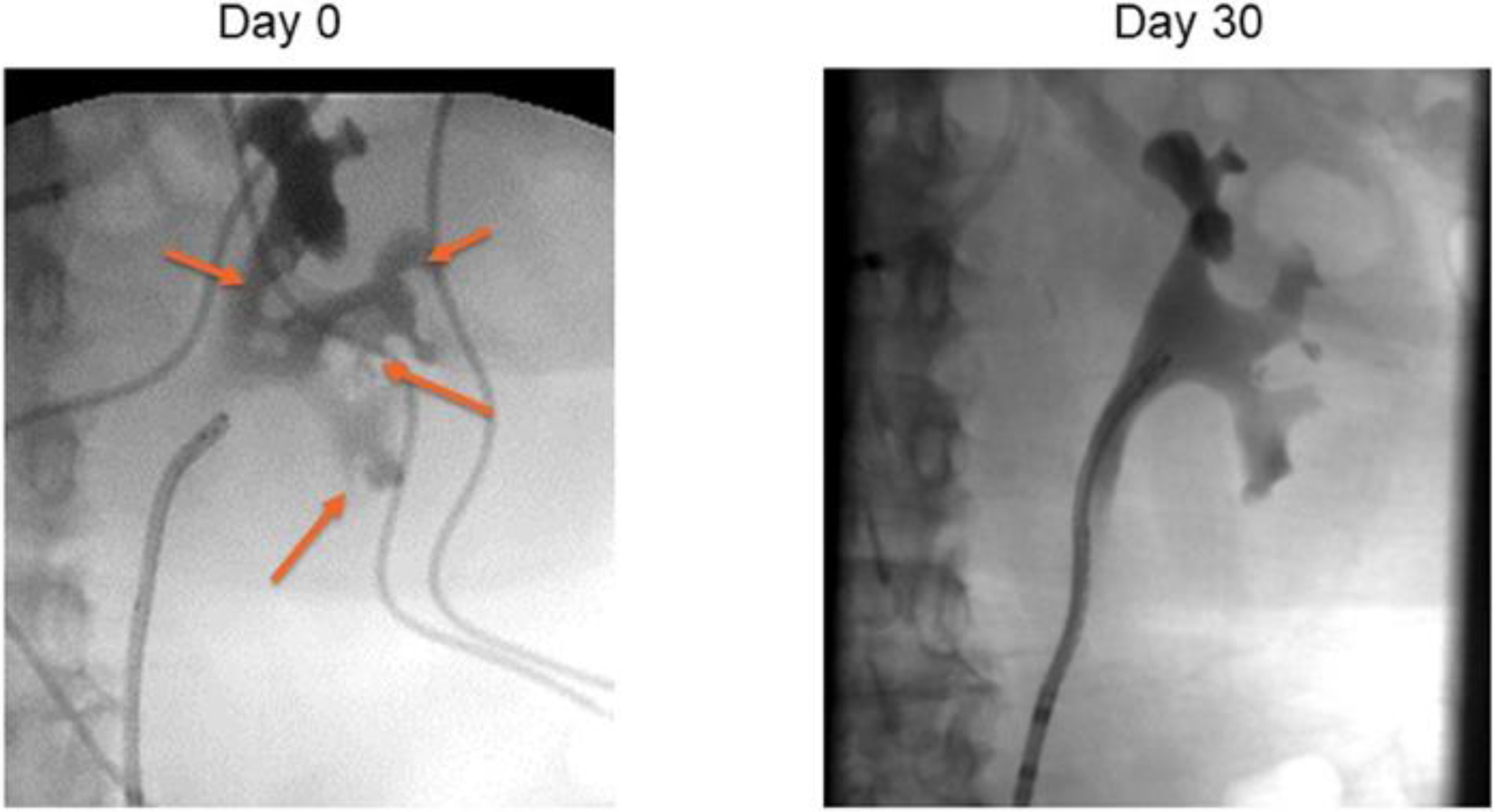
Retrograde pyelogram before and after vascular-targeted photodynamic therapy
Figure 3.
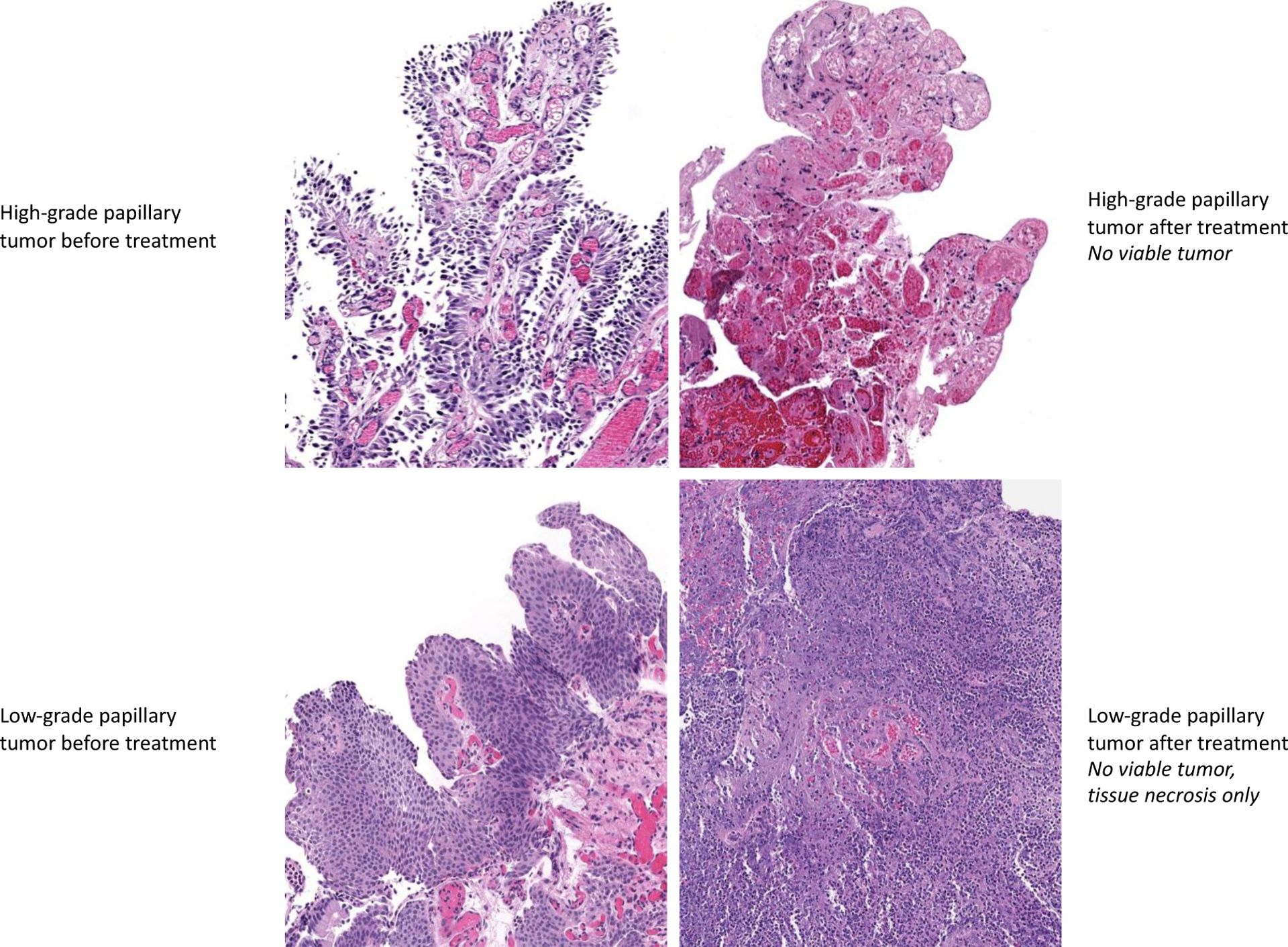
Histologic appearance of tumor before and after one vascular-targeted photodynamic therapy treatment
Regarding tolerability, treatment with WST-11 VTP in the upper urinary tract was not associated with off-target systemic, ureteral, and/or related collateral toxicities, as seen with other local ablative therapies. Endoscopic laser ablation treatments are associated with events such as perforation, stricture, and sepsis,6 as well as major complications reported in over 5% of procedures.21 BCG instillation in the upper tract, either by cutaneous nephrostomy or retrograde ureteral catheterization, has been associated with rates of fever between 56% and 90%, as well as hematuria, hydronephrosis, and back pain. Severe sepsis and acute renal failure have been reported in 5.7% and 2.9% of patients, respectively.22 With mitomycin thermal gel, which requires six weekly catheter-based instillations, events such as acute kidney injury (9%), myelosuppression (9%), ureteral stricture preventing further instillations (5%), and treatment discontinuation (27%) were described in the original report in which a 36% complete response rate was seen.23 In the phase 3 trial of primary chemoablation with mitomycin thermal gel for low-grade UTUC (OLYMPUS), a 59% complete response rate was seen, while 44% of patients developed ureteric stenosis.24 Our study demonstrated similar efficacy, in both low- and high-grade tumors, with no cases of treatment-related ureteral stricture.
Based on this trial’s results, the multi-center phase 3 ENLIGHTED trial (NCT04620239) has been initiated for low-grade disease. The multi-center nature of the ENLIGHTED trial underscores the portability of this treatment option, which can be performed by any urologist trained in ureteroscopy, as additional equipment necessary only includes a laser fiber and light source.
Limitations of this phase 1 dose-finding study include its small sample size; limited follow-up duration; and broad patient selection criteria, which did not limit tumor size or grade. Patients in the study were followed for six months to evaluate for late toxicities, but long-term treatment durability and recurrence assessment is limited. Further, the utility of continued maintenance treatment was not included in this trial design. Lastly, the majority of patients in the trial had previously failed other local ablative therapies and thus may represent a more treatment-resistant phenotype, including one patient who failed induction treatment on trial with mitomycin thermal gel but experienced a CR with VTP therapy.
Conclusions
The results of our phase 1 trial demonstrate that WST-11 VTP for UTUC has an acceptable safety profile and promising therapeutic effect. The MTD of 200 mW/cm was reached, and only two DLTs were reported at the highest dose. Safety events were mainly local effects, with relatively negligible systemic toxicity. The procedure led to a 50% complete response rate at 30 days and 68% complete response rate after a second VTP treatment. Overall, VTP with WST-11 has the potential for use as a safe, efficacious, and kidney-sparing treatment option for UTUC. The recently initiated multi-center phase 3 ENLIGHTED trial (NCT04620239) is expected to provide further evidence on this treatment option.
Supplementary Material
Acknowledgements
Funding:
Received from the Thompson Family Foundation, NIH/NCI Cancer Center Support Grant P30 CA008748, and Ruth L. Kirschstein National Research Service Award T32CA082088.
Role of the Funding Source:
The study sponsors had no role in the design of the study; in the analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflicts of Interest: No conflicts of interest.
Data Sharing Statement:
Deidentified participant data and related study documents will be made available to other researchers with publication upon request through a formal signed data sharing agreement process.
References
- 1.Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164(5):1523–5. [PubMed] [Google Scholar]
- 2.Redrow GP, Matin SF. Upper tract urothelial carcinoma: epidemiology, high risk populations and detection. Minerva Urol Nefrol. 2016;68(4):350–8. [PubMed] [Google Scholar]
- 3.Favaretto RL, Shariat SF, Chade DC, et al. The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur Urol. 2010;58(4):574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane BR, Smith AK, Larson BT, et al. Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer. 2010;116(12):2967–73. [DOI] [PubMed] [Google Scholar]
- 5.Raman J, Shore ND. Management of Low-grade Upper Tract Urothelial Carcinoma: An Unmet Need. Rev Urol. 2020;22(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Cutress ML, Stewart GD, Tudor EC, et al. Endoscopic versus laparoscopic management of noninvasive upper tract urothelial carcinoma: 20-year single center experience. J Urol. 2013;189(6):2054–60. [DOI] [PubMed] [Google Scholar]
- 7.Cutress ML, Stewart GD, Zakikhani P, et al. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int. 2012;110(5):614–28. [DOI] [PubMed] [Google Scholar]
- 8.Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5(8):497–508. [DOI] [PubMed] [Google Scholar]
- 9.Mazor O, Brandis A, Plaks V, et al. WST11, a novel water-soluble bacteriochlorophyll derivative; cellular uptake, pharmacokinetics, biodistribution and vascular-targeted photodynamic activity using melanoma tumors as a model. Photochem Photobiol. 2005;81(2):342–51. [DOI] [PubMed] [Google Scholar]
- 10.Eymerit-Morin C, Zidane M, Lebdai S, et al. Histopathology of prostate tissue after vascular-targeted photodynamic therapy for localized prostate cancer. Virchows Arch. 2013;463(4):547–52. [DOI] [PubMed] [Google Scholar]
- 11.Taneja SS, Bennett J, Coleman J, et al. Final Results of a Phase I/II Multicenter Trial of WST11 Vascular Targeted Photodynamic Therapy for Hemi-Ablation of the Prostate in Men with Unilateral Low Risk Prostate Cancer Performed in the United States. J Urol. 2016;196(4):1096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleshker S, Preise D, Kalchenko V, et al. Prompt assessment of WST11-VTP outcome using luciferase transfected tumors enables second treatment and increase in overall therapeutic rate. Photochem Photobiol. 2008;84(5):1231–7. [DOI] [PubMed] [Google Scholar]
- 13.Ashur I, Goldschmidt R, Pinkas I, et al. Photocatalytic generation of oxygen radicals by the water-soluble bacteriochlorophyll derivative WST11, noncovalently bound to serum albumin. J Phys Chem A. 2009;113(28):8027–37. [DOI] [PubMed] [Google Scholar]
- 14.Kimm SY, Tarin TV, Monette S, et al. Nonthermal Ablation by Using Intravascular Oxygen Radical Generation with WST11: Dynamic Tissue Effects and Implications for Focal Therapy. Radiology. 2016;281(1):109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray KS, Winter AG, Corradi RB, et al. Treatment Effects of WST11 Vascular Targeted Photodynamic Therapy for Urothelial Cell Carcinoma in Swine. J Urol. 2016;196(1):236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics. 1990;46(1):33–48. [PubMed] [Google Scholar]
- 17.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 18.Wickham H, Averick M, Bryan J, et al. Welcome to the Tidyverse. Journal of Open Source Software 2019. p. 1686. [Google Scholar]
- 19.Sjoberg DD, Whiting K, Curry M, et al. Reproducible summary tables with the gtsummary package. The R Journal 2021. p. 570–80. [Google Scholar]
- 20.Mo Q CRM: Continual reassessment method (CRM) for phase i clinical trials. 2018.
- 21.Gadzinski AJ, Roberts WW, Faerber GJ, et al. Long-term outcomes of nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma. J Urol. 2010;183(6):2148–53. [DOI] [PubMed] [Google Scholar]
- 22.Linehan J, Schoenberg M, Seltzer E, et al. Complications Associated With Ureteroscopic Management of Upper Tract Urothelial Carcinoma. Urology. 2021;147:87–95. [DOI] [PubMed] [Google Scholar]
- 23.Kleinmann N, Wirth G, Lin JS, et al. Thermo Reversible Hydrogel Based Delivery of Mitomycin C (UGN-101) for Treatment of Upper Tract Urothelial Carcinoma (UTUC). Bladder Cancer. 2019;5(1):21–9. [Google Scholar]
- 24.Kleinmann N, Matin SF, Pierorazio PM, et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial. Lancet Oncol. 2020;21(6):776–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant data and related study documents will be made available to other researchers with publication upon request through a formal signed data sharing agreement process.


