Abstract
Breast cancer and lung cancer remain the top two leading causes of cancer death in women. Due to limited success in reducing the high mortality of these diseases, new drugs and approaches are desperately needed. Cancer prevention is one such promising strategy that is effective in both preclinical and clinical studies. I-BET 762 is a new bromodomain inhibitor that reversibly targets BET (bromodomain and extra-terminal) proteins and impairs their ability to bind to acetylated lysines on histones, thus interrupting downstream transcription. This inhibitor has anti-inflammatory effects and induces growth arrest in many cancers and is currently under clinical trials for treatment of cancer. However, few studies have investigated the chemopreventive effects of bromodomain inhibitors. Here, we found that I-BET 762 significantly delayed tumor development in preclinical breast and lung cancer mouse models. This drug not only induced growth arrest and downregulated c-Myc, pSTAT3 and pERK protein expression in tumor cells in vitro and in vivo but also altered immune populations in different organs. These results demonstrate the promising potential of using I-BET 762 for cancer prevention and suggest the striking effects of I-BET 762 are the result of targeting both tumor cells and the tumor microenvironment.
Keywords: Bromodomain inhibitor, I-BET 762, growth arrest, breast cancer, lung carcinogenesis, immune infiltration
Introduction
Breast cancer and lung cancer are the top two leading causes of cancer deaths in women (1). Even though the survival rates of estrogen receptor positive (ER+) breast cancer have gradually improved, primarily because of the benefits of endocrine therapy, the incidence and survival rates for ER-negative (ER−) breast cancer have not noticeably shifted over the past 30 years (2). Lung cancer is responsible for more cancer deaths than breast, prostate, colon and pancreatic cancer combined, and the 5-year survival rates for lung cancer remain a disappointing 18% (1). Therefore, new drugs as well as new approaches are greatly needed to reduce both the incidence and mortality of both of these diseases.
Prevention and early intervention in cancer are two underutilized strategies that have proven effective in both preclinical and clinical studies (3). Fisher et al. reported a 49% reduction in the incidence of breast cancer in women taking tamoxifen compared to a placebo (4). Raloxifene, a second generation selective estrogen receptor modulator (SERM), reduced the incidence of breast cancer by approximately 80% in early clinical trials (5). Abundant clinical data confirm that anti-estrogens or SERMs can prevent ER+ breast cancer in women (6–9). However, tamoxifen can induce rare but serious side effects such as increasing the risk of uterine cancer (10), and there are no approved drugs available for the prevention of ER- breast cancer. In contrast to breast cancer, drugs have been tested for the prevention of lung cancer in a variety of preclinical models, but these efforts have not been successfully translated into the clinic. Because smokers are at high risk of developing lung cancer, developing effective drugs with a good safety profile for the chemoprevention of lung cancer could greatly reduce the overall number of cancer deaths (11).
In addition to known genetic mutations that drive carcinogenesis, epigenetic events are also highly involved in the initiation and progression of cancer (12). Unlike genetic mutations, epigenetic alterations are considered reversible, which makes them an appealing therapeutic target. A number of epigenetic drugs have been developed that can reverse the aberrations of DNA methylation or other histone modifications in cancer. Inhibitors of DNA methylation were the first epigenetic drugs designed for treatment of cancer; these nucleoside analogs inhibit DNA methylation by trapping DNA methyltransferases (13). Drugs targeting histone modifications were then developed, and the HDAC (histone deacetylase) inhibitor suberoylanilide hydroxamic acid (SAHA) is approved by the FDA for the treatment of cutaneous T cell lymphoma. Some of these epigenetic drugs have also been tested for chemoprevention (14). The HDAC inhibitor valproic acid is currently being tested in a clinical trial (NCT02608736) for chemoprevention of head and neck squamous cell carcinoma.
Recently, chromatin “readers” have emerged as promising epigenetic targets. In contrast to “writers” (e.g. histone acetyltransferases (HATs) and histone methyltransferases (HMTs)) or “erasers” (e.g. histone deacetylases (HDACs) and lysine demethylases (KDMs)), readers bind to specific epigenetically modified sites. Bromodomains, which contain a conserved binding motif, are the primary readers of acetylated lysine residues, and over 40 human proteins express bromodomains (15). Importantly, distinct small molecules that are specific to the BET (Bromodomain and extraterminal domain) family (BRD-2, 3, 4, T) of bromodomain proteins (16,17) have been developed. The BET inhibitors have shown promising efficacy in preclinical models for treating cancers including NUT-midline carcinoma (18), hematological malignancies (16,19), and pancreatic cancer (20), but little is known regarding the chemopreventive potential of bromodomain inhibitors in cancer.
JQ1 was one of the earliest BET inhibitors identified (17), and it blocks neoplastic transformation induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) (21) or visceral adipose tissue (22) in mouse skin epidermal JB6 P+ cells and suppresses inflammation in many cell types (23,24). However, poor bioavailability and a short half-life make JQ1 an impractical candidate for clinical development. In contrast, the bromodomain inhibitor I-BET 762 (also known as GSK525762) has good potency and pharmacokinetics following oral administration (25). It is currently being evaluated in clinical trials (NCT01587703, NCT01943851, NCT02964507) for the treatment of NUT middle carcinoma and other cancers including breast and lung cancer. In addition to its anti-proliferative effects in cancer cells, I-BET 762 can inhibit cytokine secretion and decrease the number of tumor associated macrophages and myeloid derived suppressor cells in a preclinical model of pancreatic cancer (23). Because of the known anti-inflammatory and immunomodulatory effects of the bromodomain inhibitors, we tested the efficacy of I-BET 762 for the prevention of breast and lung cancer in two clinically relevant mouse models (MMTV-PyMT ER- breast cancer model and vinyl carbarmate induced lung carcinogenesis model) and investigated its effects on both cancer and immune cells.
Materials and Methods
In vivo experiments
All animal studies were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Michigan State University. For the breast cancer studies, MMTV-PyMT mice were obtained from Dr. Jeffrey Pollard (Albert Einstein College of Medicine, Bronx, NY) and were genotyped as described (26). Four-week-old female PyMT mice were randomized into either control group fed powdered 5002 rodent chow or I-BET 762 (60 mg/kg diet) mixed into powdered diet as described (27); I-BET 762 (purity >95%) was synthesized (25) by J-Star Research (South Plainfield, NJ). Mice were palpated for tumors twice a week. For the immune cell infiltration studies, 11-week-old female PyMT mice were gavaged (5% DMSO and 10% Tween 20 in saline) with 60 mg I-BET 762 /kg body weight daily for one week for a short-term protocol. In a longer-term protocol, four-week-old female PyMT mice were fed either powdered control diet or diet containing I-BET 762 (60 mg/kg diet) until 13 weeks of age. For both studies, tissues were harvested for analysis by flow cytometry and evaluation of biomarkers by Western blot or immunohistochemistry.
For the lung cancer studies, eight-week old female A/J mice from Jackson Laboratories were injected i.p. with 0.32 mg vinyl carbamate (Toronto Research Chemicals) dissolved in saline. One week after injection, the mice were randomized into either control group fed AIN-93G semi-synthetic diet (BioServ), I-BET 762 (40–120 mg/kg diet), LG100268 (40 mg/kg diet) or the combination of I-BET 762 and LG100268 (40 mg of each drug/kg diet). After 16 weeks on diet, lungs were harvested and inflated with PBS. Left lungs were fixed in neutral buffered formalin (NBF) for histopathology. Right lungs were used immediately for flow cytometry (two lobes) or were flash frozen (the other two lobes). Samples were coded with random numbers to blind the investigators to the identity of the treatment group during analysis. Then tumor number, size and histopathology were assessed on two separate sections of the left lung by two independent investigators using published criteria (28).
Flow cytometry
The same two mammary glands or two lobes of the right lung were harvested from each PyMT mouse or A/J mouse, respectively, for flow cytometry. Fresh tissues were homogenized and incubated in digestion media containing 300 U/ml collagenase (Sigma), 1 U/ml dispase (Worthington), and 2 U/ml DNAse (EMD Millipore) for 30 minutes at 37°C. Cells were passed through a 40 μm cell strainer (Falcon) to obtain a single cell suspension and treated with a lysing solution (eBioscience) to eliminate red blood cells. Cells were then stained with 5 μg/ml anti-mouse Fc block (Biolegend) and two panels of validated antibodies(23) for 30 minutes on ice. Panel 1: CD45-VioGreen (3 μg/ml, Miltenyi), Gr-1-PE (3 μg/ml, Miltenyi), CD11b-FITC (3 μg/ml, Miltenyi). Panel 2: CD45-VioGreen (3 μg/ml, Miltenyi), CD3-PE (2 μg/ml, BioLegend), CD4-FITC (3 μg/ml, Miltenyi), CD8-PerCP/Cy5.5 (2 μg/ml, BioLegend), CD25-PE/Cy7 (2 μg/ml, BD Biosciences). Flow cytometry was performed using a LSR II flow cytometer with three laser sources (488 nm, 633 nm, 407 nm) and DIVA 6.2 software (BD); data were analyzed by FlowJo x.10.0.7r2 software (Tree Star).
Immunohistochemistry
Upper right mammary glands and left lungs inflated with PBS were fixed in 10% NBF for 48 h for histopathology and immunohistochemistry. Citrate or EDTA buffer was used for antigen retrieval, and hydrogen peroxide was used to quench endogenous peroxidase activity. Sections were immunostained with pSTAT3 or pERK (1:50, Cell Signaling) and biotinylated anti-rabbit secondary (Cell Signaling), CD45 (1:100, BioScience) and anti-rat secondary (Vector), or PCNA (1:100 Biolegend) antibodies and biotinylated anti-mouse secondary (Cell Signaling). Signal was detected using a DAB kit (Cell Signaling). Sections were counterstained with hematoxylin (Vector).
Cell culture
MDA-MB-231 and A549 cells (ATCC) were cultured in DMEM or RPMI 1640, respectively, with 10% FBS and 1% Pen/Strep. Primary PyMT tumor cells were isolated from female PyMT mice. Mammary tumors were dissected, homogenized and digested in DMEM with 10% FBS, 300 U/ml collagenase, 1.0 U/ml dispase and 2 U/ml DNase for 30 min at 37°C. The cell suspension was passed through a 40 μM cell strainer. After centrifugation at 220 g for 10 min, cells were plated in DMEM with 10% FBS and 1% Pen/Strep. All experiments were performed within 1 week of isolation. VC-1 cells were derived from lung tumors in A/J mice injected with vinyl carbamate, using the same protocol (28,29). Media and supplements were purchased from Corning Cellgro (Mediatech, Manassas, VA).
Western Blotting
Cells treated with different concentration of I-BET 762 were lysed in RIPA buffer (1 M Tris-Cl, 5 M NaCl, pH 7.4, 0.5 M EDTA, 25 mM deoxycholic acid, 1% triton-X, 0.1% SDS) with protease inhibitors. Mammary glands or lung tissues were homogenized and incubated in EBC buffer (5 M NaCl, 1 M Tris pH 8) with protease inhibitors and 10% NP-40 for 30 min. The BCA assay (Sigma-Aldrich) was performed to determine protein concentrations. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. p27KIP1, Cyclin D1, c-Myc, p-Erk 1/2, pSTAT3 and vinculin (all from Cell Signaling) primary antibodies were used to analyze the corresponding proteins; secondary antibodies were purchased from Cell Signaling. ImageJ was used to quantify the immunoblots. Images shown are representative of 2–3 independent experiments.
Statistical analysis
The in vitro experiments were repeated in at least three independent experiments. Results were analyzed using the t-test or one-way ANOVA (Prism 6). In vivo data were analyzed by one-way ANOVA followed by a Tukey test, or one-way ANOVA on ranks and the Dunn test if the data did not fit a normal distribution (SigmaStat 3.5). Results were expressed as the mean±SE unless otherwise noted. p<0.05 was considered statistically significant.
Results
The bromodomain inhibitor I-BET 762 delays tumor development in the MMTV-PyMT model of breast cancer
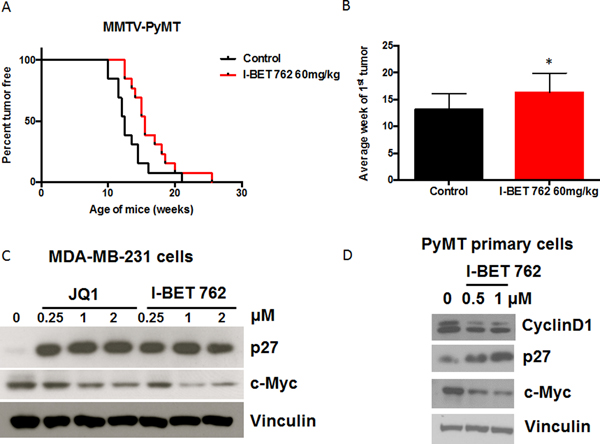
To investigate whether I-BET 762 affects tumor development, we first tested this drug in MMTV-PyMT mice. In this ER- breast cancer model, expression of the oncogenic PyMT protein is targeted to the mammary epithelium by the MMTV promoter (26). This model mimics several key features of human breast cancer including disease progression from hyperplasia to invasive carcinoma and the early infiltration of Tumor Associated Macrophages (TAMs) (30). Female PyMT mice were fed control diet or diet containing I-BET 762 (60 mg/kg diet, which is approximately 15 mg/kg body weight) starting at 4 weeks of age. Treatment with I-BET 762 significantly (p<0.05) delayed the development of mammary tumors (Fig. 1A). The average time of the first palpable tumor increased from 13.1±0.8 weeks in the control group to 16.3±1.0 weeks with the I-BET 762 treatment (Fig. 1B; p<0.05). I-BET 762 was well tolerated at this dose, with no significant differences in weight between groups (average body weight of 24.1±1.6 g in control group vs. 24.5±1.8 g in I-BET 762 treated group at 17 weeks of age).
Fig 1: The bromodomain inhibitor I-BET 762 delays tumor development in the MMTV-PyMT model of ER- breast cancer.
Female PyMT mice were started on either control diet or I-BET 762 (60 mg/kg in diet) at 4 weeks old. Mice were palpated twice a week for tumors. A. Treatment with I-BET 762 significantly (p<0.05) delayed development of the initial tumor compared to the control group. n=13 per group. B. Average time (in weeks) of the first tumor appearance. *, p<0.05 vs. control. Results are represented as Mean±SD. C. MDA-MB-231 cells were treated with different concentrations of JQ1 or I-BET 762 for 48 hrs. p27 and c-Myc protein expression were detected by western blotting. D. Primary tumor cells were isolated from tumors in PyMT mice. Cells were treated with I-BET 762 for 48 hrs and lysates were immunoblotted with antibodies against Cyclin D1, p27, and c-Myc.
I-BET 762 induces growth arrest and downregulates c-Myc expression in breast cancer cells
Bromodomain inhibitors can inhibit c-Myc and induce growth arrest in many cell types (23,31,32). In our studies, I-BET 762 inhibited proliferation of MDA-MB-231, a triple negative human breast cancer cell line, with an IC50 of 0.46±0.4 μM in the MTT assay (Suppl Fig. 1A). At concentrations as low as 0.25 μM, both I-BET 762 and JQ-1 upregulated p27 and downregulated c-Myc protein expression (Fig. 1C) in these cells. Next, primary cells were isolated from mammary tumors in PyMT mice and treated with I-BET 762 for 48 hrs. In these cells, treatment with I-BET 762 increased protein levels of p27 and decreased Cyclin D1 (Fig. 1D); these changes are hallmarks of G1 phase cell cycle arrest (33). I-BET 762 does arrest these cells in G1, as confirmed by flow cytometry (Suppl Fig. 1B). C-Myc protein expression was also decreased by I-BET 762 (Fig. 1D).
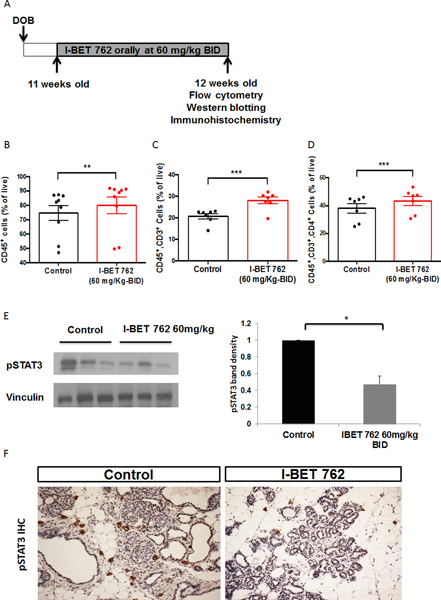
I-BET 762 modulates T cell populations in the mammary gland and spleen and decreases pSTAT3 expression in the mammary gland
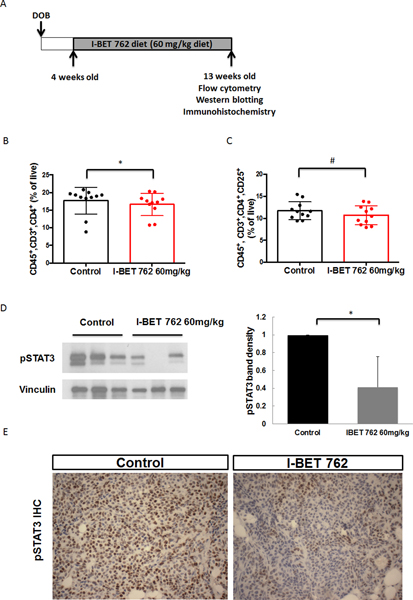
In addition to the anti-proliferative role of I-BET 762 on tumor cells, this drug also modulates the tumor microenvironment. We and others have shown that bromodomain inhibitors suppress the production of pro-inflammatory molecules by macrophages (34) and have anti-inflammatory effects in pancreatic cancer (20,23). Because epigenetic modulators can have rapid effects on cells, a short-term in vivo study was performed. In the PyMT model, 11 week-old female PyMT mice without palpable tumors were treated daily with either vehicle or I-BET 762 (60 mg/kg) by gavage for one week (short treatment protocol, Fig. 2A). This dose of I-BET 762 altered immune cell populations in a model of pancreatic cancer (23). The time point was selected based on previous studies indicating that the highest infiltration of immune cells into the mammary gland occurred at 12–13 weeks of age in PyMT mice (35). Both the mammary gland and spleen were harvested and analyzed by flow cytometry to obtain an overview of the immune cell populations in these tissues. The percentages of CD45+ immune cells, total T cells (CD45+, CD3+), CD4 helper T cells (CD45+, CD3+, CD4+) and CD8 cytotoxic T cells (CD45+, CD3+, CD8+) and macrophages (CD45+, CD11b+, F4/80+) were analyzed. After one week of treatment, the percentages of CD45+ immune cells (Fig. 2B), total T cells (Fig. 2C), and CD4 helper T cells (Fig. 2D) were all significantly (p<0.05) higher in the spleen of the PyMT mice treated with I-BET 762. There were no significant changes in immune cell populations in the mammary glands after this limited treatment (Suppl Fig. 2A). We then used a longer-term treatment protocol (Fig. 3A) to evaluate the chronic effects of I-BET 762 on immune cell populations. Four-week old female PyMT mice were fed with either control diet or diet containing I-BET 762 (60 mg/kg diet) until 13 weeks of age. In these experiments, there was a small but significant (p<0.05) decrease of CD4 helper T cells in the mammary gland of mice treated with I-BET 762 (Fig. 3B). Activation of CD4 helper T cells was then evaluated by expression of the CD25 surface marker. A lower percentage of activated CD4 T cells was detected in I-BET 762 treated group (p=0.08, Fig. 3C). No other changes in immune cells in the mammary gland or the spleen were observed at this time point (Suppl Fig. 2B).
Fig 2: Short-term treatment with I-BET 762 increases CD45+ immune cells and T cell infiltration in the spleen and decreases pSTAT3 expression in the mammary gland.
A. Diagram of short-term treatment protocol by gavage (n=9 per group). The immune cell populations in mammary glands or spleen from PyMT mice were analyzed by flow cytometry. Percentages of total immune cells (CD45+), total T cells (CD45+, CD3+), and T helper cells (CD45+, CD3+, CD4+) in the spleen are shown from B to D respectively. **, p<0.01; ***, p<0.001. E. Another mammary gland from the same mice was used to isolate total proteins. Lysates were immunoblotted with anti-pSTAT3 antibodies. Representative immunoblots are shown on the left and quantification of all samples by ImageJ is shown on the right. Results were normalized to vinculin and then expressed as fold control. *, p<0.05 vs control. F. Another mammary gland in the mice was fixed in formalin and sectioned for immunohistochemistry (IHC). pSTAT3 staining is indicated in brown. Representative images are shown in F (200X magnification).
Fig 3: Long-term treatment with I-BET 762 reduces the infiltration of CD4+ T helper cells and decreases pSTAT3 expression in the mammary gland.
A. Timeline of the longer-term treatment protocol (n=11 per group). The immune cell populations in the mammary glands from PyMT mice were analyzed by flow cytometry. B. The percentage of T helper cells (CD45+, CD3+, CD4+) in the mammary gland is significantly (*, p<0.05) lower in the group treated with I-BET 762 than controls. C. Percentage of activated T helper cells (CD45+, CD3+, CD4+, CD25+) in the mammary gland is also decreased (#, p=0.08 vs. controls). D. Protein extracts from the mammary gland were used to detect pSTAT3 expression by western blotting. Representative immunoblots are shown on the left and quantification of all samples by ImageJ is shown on the right. Results were normalized to vinculin and expressed as fold control. *, p<0.05. E. pSTAT3 expression was confirmed by immunohistochemistry (IHC) on sections of mammary gland. Positive staining is indicated in brown. Representative pictures are shown at 400X magnification.
STAT3 plays a critical role in the crosstalk between cancer and immune cells (36). As a point of convergence of many important oncogenic signaling pathways, STAT3 is constitutively activated in cell types. Activation of STAT3 inhibits the activation of dendritic cells, CD8+ T cells and natural killer cells (NK cells) and enhances the production of immunosuppressive factors, which are associated with poor prognosis (37). Hence, pSTAT3 expression was evaluated in the mammary gland by both western blotting and immunohistochemistry. Treatment with I-BET 762 significantly (p<0.05) decreased expression of pSTAT3 in both the short (Fig. 2E) and longer-term (Fig. 3D) protocols. With short-term treatment, decreased pSTAT3 expression (Fig. 2F) was mainly observed in immune cells surrounding early lesions, while pSTAT3 was also greatly decreased within tumor cells in the longer-term protocol (Fig. 3E).
I-BET 762 prevents lung carcinogenesis in A/J mice challenged with vinyl carbamate
The anti-tumor effect of bromodomain inhibitors is not limited to a single cancer type. Besides breast cancer, these compounds have been shown to suppress growth of lung cancer cell lines and sensitize these cells to pro-apoptotic agents (38,39). However, the efficacy of bromodomain inhibitors for prevention of lung cancer has not been investigated. The A/J mouse model is widely used to evaluate chemopreventive agents for lung cancer (40,41). These mice can develop adenomas spontaneously in the lung, but injection of the carcinogen vinyl carbamate can induce invasive lung adenocarcinomas (28). Vinyl carbamate induces Kras mutations (42), which are the most frequent mutations found in human lung cancers (43), especially in smokers and Caucasian patients (44). Because urethane and carcinogens found in cigarette smoke such as NNK (a nitrosamine ketone derived from nicotine) only induce adenomas, the use of vinyl carbamate in A/J mice is more clinically relevant. In this study, female A/J mice were injected i.p. with 0.32 mg vinyl carbamate to induce lung adenocarcinomas. One week after initiation, the mice were fed control diet or I-BET 762 in diet for 16 weeks. Then tumor number, size and histopathology were evaluated using published criteria (28). In our first study, two doses of I-BET 762 (60 and 120 mg/kg diet or approximately 15 and 30 mg/kg body weight) were tested (Table 1). Strikingly, treatment with I-BET 762 significantly (p<0.05) reduced the average number of grossly visible tumors on the inflated left lung from 12.4±0.6 tumors in the control group to only 3.7±0.6 in mice fed the highest concentration of I-BET 762, a reduction of 70%. Although the percentage of grossly visible small tumors (< 0.5 mm in diameter) was only 2.5% in the control group, 63% of the tumors in the I-BET 762 120 group were small (p<0.05 vs. control). Notably, not a single tumor in either group treated with I-BET was larger than 1 mm in diameter, while nearly 10% of the control tumors had grown to this size. To evaluate the histopathology, lungs were sectioned, randomized and the groups blinded. Impressively, the high dose of I-BET 762 significantly (p<0.05) reduced the average tumor number by 78% (from 3.84±0.25 to 0.86±0.19 per slide), average tumor size by 83% (from 0.33±0.03 mm3 to 0.06±0.01 mm3), and average tumor burden by 96% (from 1.26±0.14 mm3 to 0.05±0.01 mm3). Even with half of this dose (with 60 mg/kg diet), I-BET 762 still significantly (p<0.05) reduced the grossly visible tumors on the surface of left lung, and no large tumors (> 1mm in diameter) were found. At this lower dose, I-BET 762 also caused a 64% reduction in tumor size and a nearly 80% decrease in average tumor burden (p<0.05 vs. control for both characteristics).
Table 1:
I-BET 762 reduces lung carcinogenesis in A/J mice
| Control | I-BET 762 | I-BET 762 | |
|---|---|---|---|
| 60 mg/kg diet | 120 mg/kg diet | ||
| Inflated lungs: | |||
| # of mice/group | 28 | 12 | 11 |
| # of tumors/group | 348 | 118 | 41 |
| # of tumors/lung (% control) | 12.4 ± 0.6 (100%) | 9.8 ± 0.9 * (79%) | 3.7 ± 0.6 ** (30%) |
| # of tumors < 0.5 mm (% of total tumors) | 9 (2.5%) | 20 (17%) ** | 26 (63%) ** |
| # of tumors > 1 mm (% of total tumors) | 33 (9.5%) | 0** | 0ǂ |
| Slides: | |||
| # of slides/group | 56 | 24 | 22 |
| Average # of tumors/slide (% control) | 3.84 ± 0.25 (100%) | 2.25 ± 0.36 * (58.6%) | 0.86 ± 0.19 * (22.5%) |
| Average tumor size, mm3 (% control) | 0.33 ± 0.03 (100%) | 0.12 ± 0.02 * (35.9%) | 0.06 ± 0.01 * (17.2%) |
| Average tumor burden, mm3 (% control) | 1.26 ± 0.14 (100%) | 0.27 ± 0.06 * (21%) | 0.05 ± 0.01 * (3.9) |
| Histopathology: | |||
| Total # LL Grade (% of total tumors) | 4 (2%) | 15 (28%) ** | 6 (32%) *** |
| Total # LH/HL Grade (% of total tumors) | 102 (47%) | 32 (59%) | 12 (63%) |
| Total # HH Grade (% of total tumors) | 109 (51%) | 7 (13%) ** | 1 (5%) ** |
Note: Female A/J mice were injected i.p. with 0.32 mg vinyl carbamate and were randomized into either control group fed AIN-93G diet or treatment groups fed I-BET 762 in the diet, starting one week after initiation with vinyl carbamate. After 16 weeks of treatment, lungs were harvested. The tumor number, size and histopathology were assessed in a blinded fashion by two independent investigators. Results are presented as mean±SEM.
p < 0.05 vs. control
p < 0.001 vs. control
p = 0.078 vs. control.
Histopathology was evaluated based on two criteria (histological and nuclear), as published previously (28). The percentage of high-grade tumor (HH), high for both histological and nuclear characteristics, such as fused trabecular architecture, distinct nucleoli and conspicuous mitoses was 51% in the control group but only 5–13% in mice fed I-BET 762 (p<0.05). The percentage of low-grade tumors (LL) was also significantly higher in the treated groups (28–32%) vs. the control group (2%). These doses were well-tolerated with no observed adverse effects; average body weights at the end of the study were 21.7±2.4 g in the controls, 22.5±1.7 g in the I-BET 60 group, and 20.7±1.5 g in the I-BET 120 group.
Because impressive efficacy was observed in this first lung experiment, a lower dose of I-BET 762 (40 mg/kg diet or approximately 10 mg/kg body weight) was tested, alone and in combination with the rexinoid LG100268 (40 mg/kg). Rexinoids are selective ligands for RXRs (Retinoid X Receptors) with known anti-inflammatory and anti-tumor effects (29,45). LG100268 potently suppressed tumor development in this lung cancer model (27). In addition, the combination of I-BET 762 and LG100268 was more effective than either drug alone for suppressing inducible nitric oxide synthase in macrophage-like cells (Suppl Fig. 3). This in vitro assay correlates well with efficacy in the A/J lung carcinogenesis model. The combination of I-BET 762 and LG100268 was therefore tested to investigate potential synergistic effects in vivo. Even though the concentration of I-BET 762 was reduced to only 40 mg/kg diet, a significant (p<0.05) reduction in tumor number (52%), size (60%), and burden (81%) were still observed in the mice (Table 2 and Fig. 4A). The efficacy in the combination group was not significantly different than I-BET 762 or LG100268 alone; lower doses of both drugs should be evaluated in future studies. The I-BET diets were well-tolerated with no significant differences in body weight (23.6±3.4 g in control group vs. 24.1±2.5 g in the I-BET 762 group after 16 weeks on diet).
Table 2:
The combination of I-BET 762 and the rexinoid LG100268 for prevention of lung carcinogenesis
| Control | IBET 762 40 mg/kg diet | LG100268 40 mg/kg diet | I-BET762+LG268 40 mg each drug/kg diet | |
|---|---|---|---|---|
| Inflated lungs: | ||||
| # of mice/group | 24 | 12 | 12 | 12 |
| # of tumors/group | 188 | 60 | 62 | 57 |
| # of tumors/lung (% control) | 7.8±0.5 (100%) | 5.0±0.5* (63.8%) | 5.2±0.6*(66%) | 4.8±0.7* (60.6%) |
| # of tumors < 0.5 mm (% of total tumors) | 5 (2.7%) | 7* ( 11.7%) | 9** (14.5%) | 13** (22.8%) |
| # of tumors > 1 mm (% of total tumors) | 21 (11.2%) | 1* (1.7%) | 6 (9.7%) | 1 (1.8%) |
| Slides: | ||||
| # of slides/group | 48 | 24 | 24 | 24 |
| # of tumors/group | 92 | 22 | 39 | 19 |
| Average # of tumors/slide (% control) | 1.92±0.25 (100%) | 0.92±0.15* (47.8%) | 1.63±0.32 (84.8%) | 0.79±2* (41.3%) |
| Average Tumor Size, mm3 (% control) | 0.24±0.09 (100%) | 0.10±0.02* (39.8%) | 0.11±0.02* (45.8%) | 0.10±0.03* (42.5%) |
| Average Tumor Burden, mm3 (% control) | 0.47±0.19 (100%) | 0.09±0.02* (19.1%) | 0.18±0.04 (38.8%) | 0.08±0.03* (17.5%) |
| Histopathology: | ||||
| Total # LL Grade (% of total tumors) | 4 (4%) | 1 (4%) | 1 (3%) | 3 (16%) |
| Total # LH/HL Grade (% of total tumors) | 53 (58%) | 16 (73%) | 22 (56%) | 12 (63%) |
| Total # HH Grade (% of total tumors) | 35 (38%) | 5 (23%) | 16 (41%) | 4 (21%) |
Note: The same model was used as Table 1, but a lower concentration of I-BET 762 and a potent rexinoid (LG100268) were tested. The tumor number, size and histopathology were again assessed in a blinded fashion by two independent investigators. Results are shown as mean±SEM.
p < 0.05 vs. control
p < 0.001 vs. control.
Fig 4: I-BET 762 prevents lung carcinogenesis in A/J mice.
Female A/J mice were initiated with vinyl carbamate. The mice were then randomized and fed control diet or IBET 762 (40 mg/kg diet), starting one week after initiation with vinyl carbamate. After 16 weeks of treatment, lungs were harvested to examine tumor burden. A. Representative images of left lungs in each group (10X magnification). A549 lung cancer cells (B), primary VC-1 cells (C) isolated from lung tumors in A/J mice, or lung extracts (D) were treated with different concentrations of I-BET 762 for 48 or 24 hrs, respectively and protein expression analyzed by western blotting. Changes in pERK and PCNA expression in the tumor were confirmed by IHC (E); positive staining is indicated in brown. Representative pictures are shown at 400X magnification, n=5 lungs per group.
I-BET 762 induces growth arrest and downregulation of pSTAT3 and pERK in lung cancer in vitro and in vivo
To elucidate biomarkers of I-BET 762 efficacy in lung cancer, tumor cells were first evaluated. Both A549 (a human NSCLC cell line) and VC-1 (primary cells isolated from a lung tumor in an A/J mouse) cells were treated with I-BET 762 for 24–48 hrs. With I-BET 762 treatment, protein levels of p27 increased while Cyclin D1 and c-Myc expression decreased (Fig. 4B and C). Lungs from the mouse model were homogenized and immunoblotted. Cyclin D1, pSTAT3, pERK, and PCNA were all significantly (p<0.05, quantification of western blots is shown in Suppl Fig. 4) decreased in the lungs of mice treated with I-BET 762 (Fig. 4D). C-Myc was expressed at very low levels in lung tissue, and no differences were detected between groups (Suppl Fig. 5). Kras mutations induced by vinyl carbamate activate downstream targets like ERK, and pERK expression was significantly decreased by I-BET 762 in the lung tumors (Fig. 4D), as confirmed by IHC (Fig. 4E). Proliferation in the tumor was also significantly (p<0.05, Suppl Fig. 6) inhibited by I-BET 762 treatment, as shown by PCNA staining (Fig. 4E).
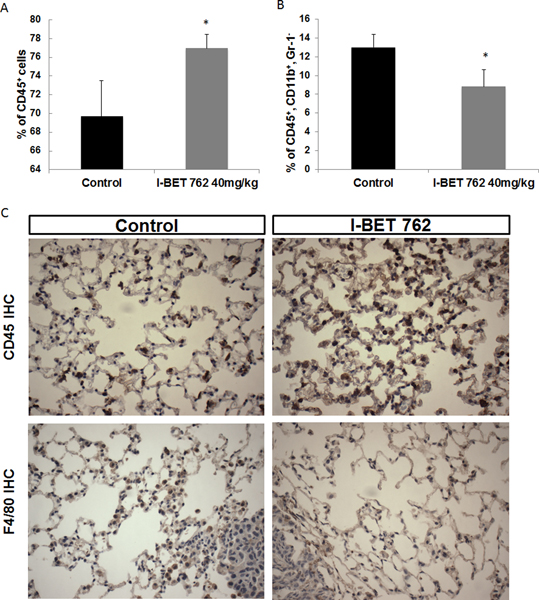
I-BET 762 increases CD45+ immune cells and decreases macrophages in the lung
JQ1 has been shown to attenuate the function of immunosuppressive T regulatory cells in an NSCLC model (46), indicating the potential immunomodulatory role of bromodomain inhibitors. Two lobes of the right lung were freshly homogenized and prepared for flow cytometry as described above. The CD45+ immune cell population (CD45+/live cells) was significantly (p<0.05) higher and the macrophage (CD45+, CD11b+, Gr1-) population lower in the lungs of mice treated with I-BET 762 (Fig. 5A and B) vs. controls, but there were no significant differences in T cell populations (Suppl Fig. 7). The changes in CD45+ (Fig. 5C) and macrophages near tumors (Fig. 5D) were confirmed by immunohistochemistry on the lung sections (quantitation shown in Suppl Fig. 6).
Fig 5: I-BET 762 increases the infiltration of CD45+ immune cells and decreases the percentage of macrophages in the lungs of A/J mice challenged with vinyl carbamate.
Two lobes of lung from A/J mice were isolated and processed immediately for flow cytometry to detect total immune cells (CD45+, A) and macrophages (CD45+, CD11b+ Gr1- in B or F4/80+ in C) in the lung. The changes were confirmed by IHC, as shown in C at 400x magnification. *, p<0.05 vs. control, n = 4–8 lungs per group.
Discussion
Bromodomains are an attractive target for cancer therapeutics and have been intensively studied as treatment in many cancer types (47). Several studies reported the antiproliferative effects of bromodomain inhibitors in breast cancer cell lines and a reduction of tumor mass in mouse xenograft models (48–50). Arrested tumor growth by bromodomain inhibitors has also been shown in preclinical models of lung cancer. These drugs also triggered significant tumor regression and prolonged survival in mouse models of lung cancer initiated by KRAS mutations (51,52). In contrast to these previous treatment studies, our study provides direct evidence of effective chemoprevention by the potent bromodomain inhibitor I-BET 762 in relevant preclinical models of both breast and lung cancer. This bromodomain inhibitor not only significantly induced growth arrest and downregulation of oncoproteins and downstream effector proteins like c-Myc, pSTAT3 and pERK in tumor cells but also altered various immune populations within the tumor microenvironment.
Although I-BET 762 did not prevent the development of all tumors, but only delayed and reduced tumor burden in our models, no drug will be able to completely stop tumor development. The available animal models used to study chemoprevention and carcinogenesis are genetically manipulated or challenged with high doses of carcinogens and thus develop multiple tumors with a very short latency. In humans, tumors are usually not detected until decades after the initiating event, even in smokers, and the incidence is never as high as in animal studies. However, any drug that significantly increases tumor latency or suppresses malignancy is likely to have a profoundly positive impact on the quality of life in people (53).
Interestingly, I-BET 762 was more effective and potent in the lung cancer model than in the breast cancer model. Notably, the lung tumors in A/J mouse model induced by vinyl carbamate are Kras-dependent, while MMTV-PyMT tumors are driven by the polyomavirus middle T-antigen, which is not found in human breast cancer. Activating mutations in Ras drive a variety of cancers including melanomas, gliomas, pancreatic cancer and lung cancer (20,51,54), which are all sensitive to bromodomain inhibitors. In addition, a panel of lung cancer cell lines including non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) harboring different oncogenic mutations have different susceptibilities to bromodomain inhibitors (39), indicating the involvement of the BET protein in different signaling pathways. Further studies are needed to better understand the mechanisms of bromodomain inhibitors in different cancer populations, and biomarkers need to be developed to predict the response in patients.
The immunomodulatory effects of bromodomain inhibitors for cancer prevention need to be emphasized in addition to the more widely studied suppression of oncogenic pathways. Inflammation is known to promote the progression of premalignant lesions and tumor growth (55). As suggested by the “seed and soil” hypothesis, an appropriate host microenvironment (the soil) is necessary for the growth of tumor cells (the seed) (56). In cancer prevention, when tumors are premalignant or too small to be detected, modulation of the stromal microenvironment is extremely important for preventing the development of immune tolerance and tumor progression (57,58). Notably, immunomodulatory activity has been reported for many chemopreventive agents (59), and emerging evidence supports the critical role of targeting the microenvironment for the efficacy of chemoprevention. Jaffee’s group has reported that prevention of murine pancreatic ductal adenocarcinoma is feasible with Listeria vaccine and simultaneous depletion of proinflammatory immune cells (60). In a recent clinical trial targeting MUC1 (Mucin 1, cell surface associated) in patients with premalignant colorectal adenomas, nonresponders had a significantly higher percentage of immunosuppressive cells like myeloid-derived suppressor cells (61). The finding of increased CD45+ cells in the lungs of mice treated with I-BET 762 suggests a more active immune response that could halt or reverse tumor development, and we are designing new experiments to address this question. Although the magnitude of the changes in immune cell numbers are small, they are functionally significant as there is a decrease in activated T helper cells (CD25+), which are immunosuppressive and predict for poor survival in cancer patients (62). In addition, we have previously shown that I-BET 762 decreases expression of markers of M2 tumor-promoting macrophages and promotes skewing of human macrophages toward an anti-tumor M1 phenotype (23). Bromodomain inhibitors also might be useful if used in combination with immunotherapy to enhance the anti-tumor response. JQ-1 has been shown to help maintain the function of CD8+ cytotoxic T cells and enhance the persistence of T cells in adoptive immunotherapy models (63). JQ-1 also downregulates the expression of PD-L1 and its combination with anti-PD-1 antibodies caused synergistic responses in Myc-driven lymphomas (64).
Despite the promising preclinical data with bromodomain inhibitors, some investigators are urging caution with the numerous clinical trials for these drugs and suggest that clinical efforts are “running ahead of the science”(65). Bromodomain inhibitors have been shown to reactivate HIV in human cells (66) and inhibit erythroid maturation in both erythroid cells lines and primary mouse erythroblasts (67). In addition, BET proteins are required for a variety of cellular activities, and each one regulates distinct transcriptional pathways. In order to understand the toxicity profile of bromodomain inhibitors, the role of each BET protein needs to be investigated; synthesis of drugs targeting specific BET protein appear to be feasible (68). Optimized dosing and treatment schedules also need to be further explored.
In drugs used for prevention, safety must be a top priority. In our studies, effective doses of I-BET 762 (40–60 mg/kg diet, approximately 10–15 mg/kg body weight) were strikingly 3–5 fold lower than used for treatment of cancer in preclinical models (69,70). The doses used for prevention were well-tolerated by the mice with no obvious toxicity, and it is possible that even lower concentrations could be used, especially if I-BET 762 is used in combination with other potent chemopreventive agents that work through different mechanisms. Drug combinations, intermittent dosing, and targeting high risk individuals are some of the strategies that can be used for effective cancer prevention in the clinic (3,71). Additional studies are needed to determine what dose, drug combinations, and molecular characteristics would be most appropriate for intervention or prevention with a safe and effective bromodomain inhibitor.
Supplementary Material
Acknowledgements
We thank Darlene Royce, Charlotte Williams, Nicole Chaaban and Quinn Hanses for their assistance with the in vivo studies and immunohistochemistry. This work was generously supported by a grant from the Breast Cancer Research Foundation (BCRF-16-095) and startup funds from Michigan State University.
Supported by a grant from the Breast Cancer Research Foundation (BCRF-16-095) and startup funds from Michigan State University.
Footnotes
Conflict of interest:
None
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Gompel A. Breast cancer incidence rates in US women are no longer declining. Climacteric 2011;14(6):690–1. [PubMed] [Google Scholar]
- 3.Meyskens FL Jr., Mukhtar H, Rock CL, Cuzick J, Kensler TW Yang CS, et al. Cancer Prevention: Obstacles, Challenges and the Road Ahead. J Natl Cancer Inst 2016;108(2):djv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90(18):1371–88. [DOI] [PubMed] [Google Scholar]
- 5.Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 2004;96(23):1751–61. [DOI] [PubMed] [Google Scholar]
- 6.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011;364(25):2381–91. [DOI] [PubMed] [Google Scholar]
- 7.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3(6):696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet 2013;381(9880):1827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallick S, Benson R, Julka PK. Breast cancer prevention with anti-estrogens: review of the current evidence and future directions. Breast Cancer 2016;23(2):170–7. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst 1994;86(7):527–37. [DOI] [PubMed] [Google Scholar]
- 11.Lung cancer: despite advances, prevention is still best. Lancet 2016;388:533. [DOI] [PubMed] [Google Scholar]
- 12.Coyle KM, Boudreau JE, Marcato P. Genetic Mutations and Epigenetic Modifications: Driving Cancer and Informing Precision Medicine. Biomed Res Int 2017;2017:9620870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis 2010;31(1):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Plass C, Gerhauser C. Cancer chemoprevention by targeting the epigenome. Curr Drug Targets 2011;12(13):1925–56. [DOI] [PubMed] [Google Scholar]
- 15.Chung CW, Witherington J. Progress in the discovery of small-molecule inhibitors of bromodomain--histone interactions. J Biomol Screen 2011;16(10):1170–85. [DOI] [PubMed] [Google Scholar]
- 16.Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature 2011;478(7370):529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature 2010;468(7327):1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French CA. NUT midline carcinoma. Cancer Genet Cytogenet 2010;203(1):16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature 2011;478(7370):524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia PL, Miller AL, Kreitzburg KM, Council LN, Gamblin TL, Christein JD, et al. The BET bromodomain inhibitor JQ1 suppresses growth of pancreatic ductal adenocarcinoma in patient-derived xenograft models. Oncogene 2016;35(7):833–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C, Su ZY, Wang L, Shu L, Yang Y, Guo Y, et al. Epigenetic blockade of neoplastic transformation by bromodomain and extra-terminal (BET) domain protein inhibitor JQ-1. Biochem Pharmacol 2016;117:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakraborty D, Benham V, Bullard B, Kearney T, Hsia HC, Gibbon D, et al. Fibroblast growth factor receptor is a mechanistic link between visceral adiposity and cancer. Oncogene 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leal AS, Williams CR, Royce DB, Pioli PA, Sporn MB, Liby KT. Bromodomain inhibitors, JQ1 and I-BET 762, as potential therapies for pancreatic cancer. Cancer Lett 2017;394:76–87. [DOI] [PubMed] [Google Scholar]
- 24.Hytti M, Tokarz P, Maatta E, Piippo N, Korhonen E, Suuronen T, et al. Inhibition of BET bromodomains alleviates inflammation in human RPE cells. Biochem Pharmacol 2016;110–111:71–9. [DOI] [PubMed] [Google Scholar]
- 25.Mirguet O, Gosmini R, Toum J, Clement CA, Barnathan M, Brusq JM, et al. Discovery of epigenetic regulator I-BET762: lead optimization to afford a clinical candidate inhibitor of the BET bromodomains. J Med Chem 2013;56(19):7501–15. [DOI] [PubMed] [Google Scholar]
- 26.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 1992;12(3):954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao M, Royce DB, Risingsong R, Williams CR, Sporn MB, Liby KT. The Rexinoids LG100268 and LG101506 Inhibit Inflammation and Suppress Lung Carcinogenesis in A/J Mice. Cancer Prev Res (Phila) 2016;9:105–14. [DOI] [PubMed] [Google Scholar]
- 28.Liby K, Royce DB, Williams CR, Risingsong R, Yore MM, Honda T, et al. The synthetic triterpenoids CDDO-methyl ester and CDDO-ethyl amide prevent lung cancer induced by vinyl carbamate in A/J mice. Cancer Res 2007;67(6):2414–9. [DOI] [PubMed] [Google Scholar]
- 29.Liby KT, Sporn MB. Rexinoids for prevention and treatment of cancer: opportunities and challenges. Curr Top Med Chem 2017;17:721–30. [PubMed] [Google Scholar]
- 30.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001;193(6):727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011;146(6):904–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci U S A 2011;108(40):16669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994;78(1):67–74. [DOI] [PubMed] [Google Scholar]
- 34.Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, et al. Suppression of inflammation by a synthetic histone mimic. Nature 2010;468(7327):1119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tran K, Risingsong R, Royce D, Williams CR, Sporn MB, Liby K. The synthetic triterpenoid CDDO-methyl ester delays estrogen receptor-negative mammary carcinogenesis in polyoma middle T mice. Cancer Prev Res (Phila) 2012;5(5):726–34. [DOI] [PubMed] [Google Scholar]
- 36.Huynh J, Etemadi N, Hollande F, Ernst M, Buchert M. The JAK/STAT3 axis: A comprehensive drug target for solid malignancies. Semin Cancer Biol 2017;45:13–22. [DOI] [PubMed] [Google Scholar]
- 37.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007;7(1):41–51. [DOI] [PubMed] [Google Scholar]
- 38.Klingbeil O, Lesche R, Gelato KA, Haendler B, Lejeune P. Inhibition of BET bromodomain-dependent XIAP and FLIP expression sensitizes KRAS-mutated NSCLC to pro-apoptotic agents. Cell Death Dis 2016;7(9):e2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riveiro ME, Astorgues-Xerri L, Vazquez R, Frapolli R, Kwee I, Rinaldi A, et al. OTX015 (MK-8628), a novel BET inhibitor, exhibits antitumor activity in non-small cell and small cell lung cancer models harboring different oncogenic mutations. Oncotarget 2016;7(51):84675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorelik E, Herberman RB. Susceptibility of various strains of mice to urethan-induced lung tumors and depressed natural killer cell activity. J Natl Cancer Inst 1981;67:1317–22. [PubMed] [Google Scholar]
- 41.Bauer AK, Malkinson AM, Kleeberger SR. Susceptibility to neoplastic and non-neoplastic pulmonary diseases in mice: genetic similarities. Am J Physiol Lung Cell Mol Physiol 2004;287(4):L685–703. [DOI] [PubMed] [Google Scholar]
- 42.You M, Candrian U, Maronpot RR, Stoner GD, Anderson MW. Activation of the Ki-ras protooncogene in spontaneously occurring and chemically induced lung tumors of the strain A mouse. Proc Natl Acad Sci U S A 1989;86(9):3070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackman DM, Miller VA, Cioffredi LA, Yeap BY, Janne PA, Riely GJ, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res 2009;15:5267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guibert N, Ilie M, Long E, Hofman V, Bouhlel L, Brest P, et al. KRAS Mutations in Lung Adenocarcinoma: Molecular and Epidemiological Characteristics, Methods for Detection, and Therapeutic Strategy Perspectives. Curr Mol Med 2015;15:418–32. [DOI] [PubMed] [Google Scholar]
- 45.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 2007;7:357–69. [DOI] [PubMed] [Google Scholar]
- 46.Adeegbe D, Liu Y, Lizotte PH, Kamihara Y, Aref AR, Almonte C, et al. Synergistic Immunostimulatory Effects and Therapeutic Benefit of Combined Histone Deacetylase and Bromodomain Inhibition in Non-small Cell Lung Cancer. Cancer Discov 2017;7:852–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung M, Gelato KA, Fernandez-Montalvan A, Siegel S, Haendler B. Targeting BET bromodomains for cancer treatment. Epigenomics 2015;7(3):487–501. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y, Bai L, Liu L, McEachern D, Stuckey JA, Meagher JL, et al. Structure-Based Discovery of 4-(6-Methoxy-2-methyl-4-(quinolin-4-yl)-9H-pyrimido[4,5-b]indol-7-yl)-3,5-dimethy lisoxazole (CD161) as a Potent and Orally Bioavailable BET Bromodomain Inhibitor. J Med Chem 2017;60(9):3887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vazquez R, Riveiro ME, Astorgues-Xerri L, Odore E, Rezai K, Erba E, et al. The bromodomain inhibitor OTX015 (MK-8628) exerts anti-tumor activity in triple-negative breast cancer models as single agent and in combination with everolimus. Oncotarget 2017;8(5):7598–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.da Motta LL, Ledaki I, Purshouse K, Haider S, De Bastiani MA, Baban D, et al. The BET inhibitor JQ1 selectively impairs tumour response to hypoxia and downregulates CA9 and angiogenesis in triple negative breast cancer. Oncogene 2017;36(1):122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimamura T, Chen Z, Soucheray M, Carretero J, Kikuchi E, Tchaicha JH, et al. Efficacy of BET bromodomain inhibition in Kras-mutant non-small cell lung cancer. Clin Cancer Res 2013;19(22):6183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adeegbe D, Liu Y, Lizotte PH, Kamihara Y, Aref AR, Almonte C, et al. Synergistic Immunostimulatory Effects and Therapeutic Benefit of Combined Histone Deacetylase and Bromodomain Inhibition in Non-small Cell Lung Cancer. Cancer Discov 2017; 7(8):852–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sporn MB, Suh N. Chemoprevention: an essential approach to controlling cancer. Nat Rev Cancer 2002;2(7):537–43. [DOI] [PubMed] [Google Scholar]
- 54.De Raedt T, Beert E, Pasmant E, Luscan A, Brems H, Ortonne N, et al. PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies. Nature 2014;514(7521):247–51. [DOI] [PubMed] [Google Scholar]
- 55.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140(6):883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989;8(2):98–101. [PubMed] [Google Scholar]
- 57.Smit MA, Jaffee EM, Lutz ER. Cancer immunoprevention--the next frontier. Cancer Prev Res (Phila) 2014;7(11):1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer 2007;7(2):139–47. [DOI] [PubMed] [Google Scholar]
- 59.Sharma SH, Thulasingam S, Nagarajan S. Chemopreventive agents targeting tumor microenvironment. Life Sci 2016;145:74–84. [DOI] [PubMed] [Google Scholar]
- 60.Keenan BP, Saenger Y, Kafrouni MI, Leubner A, Lauer P, Maitra A, et al. A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology 2014;146(7):1784–94 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res (Phila) 2013;6(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X, Du Y, Lin X, Qian Y, Zhou T, Huang Z. CD4+CD25+ regulatory T cells in tumor immunity. Int Immunopharmacol 2016;34:244–9. [DOI] [PubMed] [Google Scholar]
- 63.Kagoya Y, Nakatsugawa M, Yamashita Y, Ochi T, Guo T, Anczurowski M, et al. BET bromodomain inhibition enhances T cell persistence and function in adoptive immunotherapy models. J Clin Invest 2016;126(9):3479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hogg SJ, Vervoort SJ, Deswal S, Ott CJ, Li J, Cluse LA, et al. BET-Bromodomain Inhibitors Engage the Host Immune System and Regulate Expression of the Immune Checkpoint Ligand PD-L1. Cell Rep 2017;18(9):2162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andrieu G, Belkina AC, Denis GV. Clinical trials for BET inhibitors run ahead of the science. Drug Discov Today Technol 2016;19:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banerjee C, Archin N, Michaels D, Belkina AC, Denis GV, Bradner J, et al. BET bromodomain inhibition as a novel strategy for reactivation of HIV-1. J Leukoc Biol 2012;92(6):1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stonestrom AJ, Hsu SC, Werner MT, Blobel GA. Erythropoiesis provides a BRD’s eye view of BET protein function. Drug Discov Today Technol 2016;19:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Z, Wang P, Chen H, Wold EA, Tian B, Brasier AR, et al. Drug Discovery Targeting Bromodomain-Containing Protein 4. J Med Chem 2017;60(11):4533–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Imayoshi N, Yoshioka M, Chauhan J, Nakata S, Toda Y, Fletcher S, et al. CG13250, a novel bromodomain inhibitor, suppresses proliferation of multiple myeloma cells in an orthotopic mouse model. Biochem Biophys Res Commun 2017;484(2):262–8. [DOI] [PubMed] [Google Scholar]
- 70.Berenguer-Daize C, Astorgues-Xerri L, Odore E, Cayol M, Cvitkovic E, Noel K, et al. OTX015 (MK-8628), a novel BET inhibitor, displays in vitro and in vivo antitumor effects alone and in combination with conventional therapies in glioblastoma models. Int J Cancer 2016;139(9):2047–55. [DOI] [PubMed] [Google Scholar]
- 71.Raj KP, Zell JA, Rock CL, McLaren CE, Zoumas-Morse C, Gerner EW, et al. Role of dietary polyamines in a phase III clinical trial of difluoromethylornithine (DFMO) and sulindac for prevention of sporadic colorectal adenomas. Br J Cancer 2013;108(3):512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.