Abstract
The Keap1-Nrf2-ARE pathway plays an important role in responding to oxidative stress and maintaining the redox homeostasis. Small molecule inhibitors targeting directly the Keap1-Nrf2 protein-protein interaction (PPI) can potentially be developed into effective preventive and therapeutic agents for numerous chronic inflammatory diseases. To improve the drug-like properties and inhibitory potency of these inhibitors, a series of 1,4-bis(arylsulfonamido)benzene or naphthalene-N,N'-diacetic acids with varying substituents at C-2 position of the benzene or naphthalene core were designed and synthesized. Among them, compound 12d with 2-(4-fluorobenzyloxy) group was the most potent direct inhibitor of Keap1-Nrf2 PPI with an IC50 of 64.5 nM in the fluorescent polarization (FP) assay and 14.2 nM in a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. Moreover, cell-based biological assay showed that 12d significantly increased the mRNA levels of Nrf2 downstream genes, GSTM3, HMOX2 and NQO1, through Nrf2 activation. The discovery of the new scaffolds possessing diverse O-linked fragments at the C2 position offers opportunities to further modify the chemical structures of Keap1-Nrf2 PPI inhibitors to improve their pharmacokinetic, efficacy and safety profiles.
Keywords: Keap1, Nrf2, Keap1-Nrf2 interaction, Protein-protein interaction inhibitor, Oxidative stress, Structure-activity relationship, Structural diversity
Graphical Abstract

INTRODUCTION
A variety of endogenous (e.g., cellular metabolism) and exogenous (e.g., radiation, chemicals, and toxins) factors are known to cause excessive production of reactive electrophilic substances, including reactive oxygen species (ROS, e.g., superoxide anion and hydroxyl radical) and reactive nitrogen species (RNS, e.g., peroxynitrite).[1, 2] This results in impaired redox homeostasis between the ROS/RNS exposures and antioxidants, eventually generating oxidative stress conditions.[2] The antioxidant defense system is an essential protective response against oxidative stress by maintaining physiological homeostasis.[1],[3] The induction of metabolizing and antioxidative enzymes, such as glutathione S-transferase (GST), catalase, glutathione peroxidase (GPx), superoxide dismutase (SOD), NAD(P)H quinone oxidoreductase I (NQO1), heme-oxygenase-1 (HO-1), glutamate-cysteine ligase (GCL), and thioredoxin (TRX), can counteract and neutralize overall ROS levels.[4-9]
Activation of the cytoprotective enzymes could be controlled by three cellular components: Kelch-like ECH-associated protein 1 (Keap1), nuclear factor erythroid 2-related factor 2 (Nrf2) and antioxidant response element (ARE), providing a pathway that senses and responds to the status of oxidative stress in cells.[10, 11] Under basal physiological conditions, Keap1 acts as an adaptor protein that takes part in the assembly of the Cullin3 (Cul3)-based ubiquitin E3 ligase complex through Keap1’s BTB domain and Nrf2 through Keap1’s DGR domain. In the Keap1-Nrf2-Cul3 complex, Nrf2 becomes closer to the E3 complex, which serves as the sensor for the Nrf2 ubiquitination machine.[12-14] Subsequently, Nrf2 is rapidly degraded by 26S proteasome, resulting in a low basal level of Nrf2. In a state of oxidative stress, oxidative and electrophilic species covalently modify cysteine residues in the BTB and IVR domains of Keap1,[15, 16] potentially leading to dissociation of Cul3 (in the Cul3 dissociation model),[17-19] disruption of Keap1-Nrf2 DLG motif interaction (in the hinge and latch model),[20, 21] and/or conformational changes of Keap1 (in the conformation cycling model).[22] These mechanisms ultimately perturb the ubiquitination of Nrf2, allowing newly formed Nrf2 to accumulate and translocate into the nucleus.[23, 24] Nrf2 forms heterodimers with small Maf proteins,[4, 25] and binds to ARE to activate the transcription of downstream antioxidant and detoxification genes.[26]
Chronic oxidative stress accelerates oxidative damages to lipids, proteins and DNA in the human body, which may contribute to the pathogenesis of many chronic inflammatory diseases.[27] Thus, Nrf2 activation through the inhibition of the Keap1-Nrf2 protein-protein interaction (PPI) has been proposed as a promising preventive and therapeutic approach for a number of diseases and conditions, including cancer, chronic obstructive pulmonary disorder (COPD), multiple sclerosis (MS), and neurodegenerative diseases.[16, 28-33] The majority of Nrf2 activators are electrophilic modulators that covalently alter Keap1 cysteine residues, which eventually lead to the interruption of Keap1-Nrf2 PPI. Among the well-known Nrf2 activators are dimethyl fumarate, bardoxolone methyl (CDDO-Me), and sulforaphane (Figure 1A).[16, 34-36] These covalent cysteine modifiers may not be selective and specific for Keap1 protein in whole cells, which would cause unwanted toxicity. For instance, bardoxolone methyl was under phase III clinical trial in patients with chronic kidney disease (CKD) and type 2 diabetes mellitus, but its development was halted due to adverse cardiovascular effects.[34, 37] The severe safety issues may be associated with possible off-target activities, through reactions with cysteine residues in other proteins and enzymes.
Figure 1.
Structures of representative (A) indirect and (B) direct inhibitors of Keap1-Nrf2 PPI
The direct and noncovalent inhibitors of Keap1-Nrf2 PPI would have certain advantages over electrophilic compounds in terms of target selectivity. A Keap1 homodimer forms an interaction with Nrf2 through two binding sites, ETGE and DLG motifs, located in the Neh2 domain of Nrf2.[20, 38] The binding of non-electrophilic small molecules to the Keap1 Kelch domain is more specific in disrupting the interactions between Keap1 and Nrf2 proteins. Recently, several small molecule inhibitors have been reported through strategies like high-throughput screening (HTS) and fragment-based drug discovery (FBDD).[9, 39] As presented in Figure 1B, these direct inhibitors include tetrahydroisoquinoline (THIQ),[40] 1,4-diaminonaphthalene,[41-43] 3-phenylpropionic acid,[44] 4-amino-1-naphthol,[45, 46] and 1,2-xylylenediamine derivatives.[47] Among them, the symmetric 1,4-diaminonaththalene scaffold like 2a exhibits the best potency. Although some of these non-electrophilic activators possess strong target binding affinity, their cellular activity and drug-like properties need further improvement as potential preventive and therapeutic agents.
Herein, we report the design, synthesis and evaluation of the 2-substituted derivatives on 1,4-bis(arylsulfonamido)benzene or naphthalene-N,N'-diacetic acids 6 and 2a as inhibitors of Keap1-Nrf2 PPI.[41, 42] The 2-alkoxy or aryloxy substitution occupies another binding pocket and could be optimized in terms of potency, physicochemical and PK properties. Our investigation revealed that the naphthalene scaffold 12d with 2-(4-fluorobenzyloxy) substitution led to the most potent Keap1-Nrf2 PPI inhibition in both our FP and TR-FRET assays (IC50 = 64.5 nM and 14.2 nM, respectively). In addition, 12d was found to effectively stimulate transcription of Nrf2-derived genes (GSTM3, HMOX2 and NQO1), suggesting that it acts as a Nrf2 activator via the inhibition of Keap1-Nrf2 PPI. The new 2-alkoxy or aryloxy derivatives provided more structural diversity to the 1,4-bis(arylsulfonamido)benzene or naphthalene-N,N'-diacetic acid series and serve as good leads for further optimization.
RESULTS AND DISCUSSION
Design strategy.
Computational analysis performed by You and coworkers[48] divided the Nrf2-binding cavity of Keap1 Kelch domain into five sub-pockets: polar subpockets (P1 and P2), nonpolar subpockets (P4 and P5), and central subpocket P3.[48, 49] An inhibitor that can occupy all five of these subpockets would more likely lead to potent inhibition of Keap1-Nrf2 PPI, which is demonstrated by the cocrystal structure of Keap1 Kelch domain with compound 2b as shown in Figure 2C.[41] However, compound 6 bearing a benzene core exhibited dramatically reduced potency with an IC50 value of 1.4 μM, that is about 50 fold less potent as compared to analog 2a with a naphthalene core. As reported previously, the replacement of naphthalene core with a benzene core as in 6 leads to weaker hydrophobic interactions as well as a weaker π-cation interaction with Arg415 in the P3 subpocket.[41, 42] To improve the structural diversity of the core moiety without affecting potency, we were interested in replacing the outer benzene ring of the naphthalene core with varying O-linked aromatic ring fragments (Figure 2A). Importantly, the binding mode of compound 4a indicates that its p-cumenesulfonamide is inserted deep into the pore behind the P3 pocket, supporting that there is enough chemical space to introduce an additional fragment at the back of P3 pocket (Figure 2D).[45, 50, 51] Most recently, Zhong and coworkers suggested that hot spot A, located in the 6 Å diameter tunnel at the center of the binding pocket, is one of the strong binding hot spots, using FTMap.[52] Accordingly, we hypothesized C2-substituents of 1,4-bis(arylsulfonamido)benzene-N,N'-diacetic acid could play significant roles in maintaining high potency by occupying the deep hole behind the binding site. In an effort to explore the space at the back of the central P3 subpocket where the C2-fragment could reach, five compounds 7a, 7d, 7e, 7k and 7l with different size of substituents ranging from benzene to O-linked biphenyl ring were designed and docked within the binding site of the Keap1 Kelch domain (PDB code: 4XMB), as outlined in Figure 2E. As expected, it revealed that the deep back side of the P3 subpocket is wide enough to accommodate bulky moieties including small benzene rings (7a, 7d and 7e), a naphthalene (7k) and a biphenyl ring (7l). Therefore, we focused our optimization around the C2-substituents on 1,4-bis(arylsulfonamido)benzene or naphthalene-N,N'-diacetic acids by exploring and expanding the chemical space behind the P3 subpocket.
Figure 2.
Design strategy of 2-substituted 1,4-bis(arylsulfonamido)benzene or naphthalene-N,N'-diacetic acids. The Keap1 Kelch domain contains five subpockets (P1 to P5), and P3 is the central subpocket where the core moiety of ligands (represented in a blue circle) occupies. (A) Optimization process of 1,4-diaminonaphthalene derivative 2a by the introduction of a new fragment to 6. (B) Co-crystal structure of the Kelch domain in complex with compound 6 (derived from PDB code: 4IQK). (C) Co-crystal structure of the Kelch domain in complex with compound 2b (PDB code: 4XMB). (D) Docking pose of compound 4a in the active site of Keap1 Kelch domain reported by Zhuang’s group (derived from PDB code: 4IQK). (E) Docking pose of target compounds 7a, 7d, 7e, 7k, 7l with different sizes of new fragments.
Chemistry.
As shown in Scheme 1, key intermediates 16a-y bearing two benzenesulfonamide moieties were prepared by three different routes. The biphenyl analog 16a was obtained through selective bromination of 4-nitroaniline,[53] iron-catalyzed nitro reduction, Suzuki coupling with phenylboronic acid, and subsequent sulfonamide formation with 4-methoxy benzenesulfonyl chloride. To introduce various fragments on the core moiety (15b and 15d-o), 2-fluoro-4-nitroaniline was initially treated with piperidine, aryl or aliphatic alcohols under three reaction conditions using different bases, such as TEA, K2CO3 and NaH. After iron or palladium-catalyzed nitro reduction of 15b and 15d-o, 1,4-diamine intermediates were subsequently reacted with the corresponding benzenesulfonyl chloride reagents to yield the sulfonamide derivatives 16b and 16d-y. Lastly, the synthesis of N-linked analog 16c was accomplished in four steps from commercially available 4-bromo-2-fluoro-1-nitrobenzene. Treatment with N-benzylmethylamine followed by the conversion of the bromo to amino group through a copper-catalyzed amination reaction gave 15c,[54] which was reduced by iron powder and subsequently N-sulfonylated to afford 16c. The resulting intermediates 16a-y were alkylated with ethyl bromoacetate, and then hydrolyzed under basic conditions to obtain the target compounds 7a-y.
Scheme 1. Synthesis of various 1,4-diamino substituted benzene ring derivatives 7a-ya,b.
aReagents and conditions: (a) Br2, AcOH, 5 °C, 30 min, 40%; (b) Fe, AcOH, H2O, 80 °C, 30 min; (c) phenylboronic acid, Pd(PPh3)4, K2CO3, dioxane, H2O, 100 °C, overnight, 47% (over 2 steps); (d) R2-substituted benzenesulfonyl chloride, pyridine, DCM, rt, overnight, 15-65% (over 1 step for 16a and 16p-y) and 9-65% (over 2 steps for 16b-o); (e) piperidine, TEA, ACN, rt, overnight, 85% (for 15b); (f) aryl alcohols, K2CO3, DMF, 90 °C, overnight, 73-quant.% (for 15e, m and o); (g) aliphatic alcohols, NaH, THF, 0 °C to rt, 1 h-overnight, 21-63% (for 15d, f-l and n); (h) Pd/C, H2, MeOH, rt, 1 h (for 16b, m and o); (i) N-benzyl methylamine, NaH, THF, 0 °C to rt, 6 h, quant.%; (j) CuI, N,N’-dimethylethyleneamine, DMSO, NH4OH, 130 °C, 3 h, 50%; (k) ethyl bromoacetate, K2CO3, DMF, rt, overnight, 31-quant.%; (l) NaOH, MeOH, H2O, 50-60 °C, 4 h-overnight, 25-87%. bSee Tables 1 and 2 for the chemical structure.
Compound 8 with 2-phenoxymethyl substitution was prepared as shown in Scheme 2. After reducing 2-bromo-5-nitrobenzoic acid, mesylation of the generated primary alcohol and subsequent substitution with phenol produced 18. Buchwald-Hartwig amination of its bromo group with benzophenone imine gave an imine intermediate, allowing for facile reduction of the imine group under acidic conditions using sodium acetate and hydroxylamine to afford the aniline 19.[55] Nitro reduction and reaction with 4-methoxybenzenesulfonyl chloride gave the sulfonamide derivative 20, which upon alkylation of the sulfonamides with ethyl bromoacetate and base-catalyzed hydrolysis of the ethyl esters provided the final compound 8.
Scheme 2. Synthesis of compound 8 with 2-phenoxymethyl substitutiona.
aReagents and conditions: (a) 1M BH3 in THF, THF, 60 °C, 1 h, quant. (b) MsCl, TEA, DCM, 0 °C, 5 min; (c) phenol, NaH, DMF, 0 °C to rt, 5 h, 42% (over 2 steps); (d) benzophenone imine, Pd2(dba)3, BINAP, NaOtBu, toluene, 80 °C, overnight; (e) NaOAc, HONH2 HCl, MeOH, rt, overnight, 26% (over 2 steps); (f) Fe, AcOH, H2O, 80 °C, 30 min; (g) 4-methoxybenzenesulfonyl chloride, pyridine, DCM, rt, overnight, 53% (over 2 steps); (h) ethyl bromoacetate, K2CO3, DMF, rt, overnight, quant.; (i) NaOH, MeOH, H2O, 60 °C, overnight, 72%.
As outlined in Scheme 3, the analogs 9a-b with 2-(3-pyridinyl)methoxy or 2-(4-phenoxybenzyloxy) substitution were prepared in two steps from intermediate 25. First, 4-phenoxybenzyl chloride 24, used as a building block for nucleophilic substitution, was prepared starting from 4-fluorobenzaldehyde in a three-step sequence that includes aromatic nucleophilic substitution with phenol, reduction of the aldehyde 22, and chlorination of 23. The key intermediate 25, which was synthesized by cleavage of the benzyl protecting group in 17d, was alkylated with either 3-(chloromethyl)pyridine hydrochloride or 24 to afford 26a-b. Removal of their ethyl esters by base hydrolysis gave the desired target products 9a-b.
Scheme 3. Synthesis of O-linked benzene ring derivatives 9a-b from key intermediate 25a.
aReagents and conditions: (a) phenol, K2CO3, DMF, 90 °C, 6 h, 59%; (b) NaBH4, MeOH, 0 °C to rt, 1 h, 57%; (c) SOCl2, DCM, rt, 4 h, 94%; (d) TFA, rt, overnight, 38%; (e) 3-(chloromethyl)pyridine hydrochloride or 24, K2CO3, DMF, rt-90 °C, overnight, 20-61%; (f) NaOH, MeOH, H2O, 60 °C, 4-8 h, 33-45%.
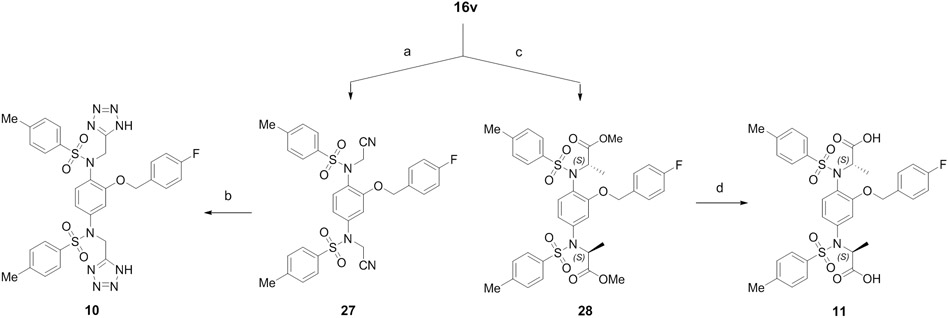
The tetrazole 10 and (S)-methylated 11 were prepared from intermediate 16v by following the synthetic route shown in Scheme 4. Alkylation with bromoacetonitrile and cyclization of the nitrile 27 with NaN3 yielded 10, while another compound 11 was synthesized stereospecifically through alkylation with methyl (R)-2-bromopropanoate followed by hydrolysis of the esters under basic conditions.
Scheme 4. Synthesis of analogs 10 and 11 including tetrazole and α-methyl acetatea.
aReagents and conditions: (a) bromoacetonitrile, K2CO3, DMF, rt, overnight, 86%; (b) NaN3, AcOH, DMF, 120 °C, 6 h, 30%; (c) Methyl (R)-2-bromopropanoate, K2CO3, DMF, rt, overnight, 84%; (d) NaOH, MeOH, H2O, 60 °C, 4 h, 44%.
The synthesis of additional derivatives with benzene core (12a-c) was achieved using the synthetic route shown in Scheme 5. For the preparation of the 4-nitroaniline 31a-c, 5 or 6-methoxybenzene analogs 29a-b were first synthesized from either the methylation of 2-bromo-4-fluoro-5-nitrophenol or selective nucleophilic aromatic substitution of 5-bromo-1,3-difluoro-2-nitrobenzene in methanol. Addition of 4-fluorobenzyl alcohol fragment yielded 30a-c, which were converted to the corresponding aniline analogs 31a-c by Buchwald-Hartwig amination and subsequent imine hydrolysis.[55] After nitro reduction, diamine intermediates were reacted with 4-toluenesulfonyl chloride to afford 32a-c. Alkylation with ethyl bromoacetate and base-promoted hydrolysis of the esters 33a-c finally yielded the target compounds 12a-c.
Scheme 5. Synthesis of 5 or 6-substituted benzene ring (12a-c) analogsa.
aReagents and conditions: (a) MeI, K2CO3, DMF, rt, overnight, 94% (for 29a); (b) KOH, MeOH, reflux, overnight, 74% (for 29b); (c) 4-fluorobenzyl alcohol, NaH, THF, 0 °C to rt, 5 h-overnight, 48-90%; (d) benzophenone imine, Pd2(dba)3, BINAP, NaOtBu, toluene, 100-120 °C, 3 h-overnight; (e) NaOAc, HONH2 HCl, MeOH, rt, overnight, 36-56% (over 2 steps); (f) Fe, AcOH, H2O, 60 °C, 30 min; (g) 4-toluenesulfonyl chloride, pyridine, DCM, rt, overnight, 9-73% (over 2 steps); (h) ethyl bromoacetate, K2CO3, DMF, rt, overnight, 77-96%; (i) NaOH, MeOH, H2O, 60 °C, 3-4 h, 37-79%.
Compound 12d with a naphthalene core was prepared as seen in Scheme 6. First, 4-nitro-1-naphthylamine analog 35 was generated by selective bromination of 4-nitronaphthalen-1-amine with NBS[56] and copper-catalyzed O-benzylation of 34.[57] Nitro reduction of the resulting intermediates 35 followed by sulfonamide formation gave 36, which was further reacted with ethyl bromoacetate and hydrolyzed under basic conditions to obtain the final products 12d.
Scheme 6. Synthesis of naphthalene (12d) analoga.
aReagents and conditions: (a) NBS, NH4OAc, ACN, rt, 10 min, 96%; (b) 4-fluorobenzyl alcohol, CuI, 8-hydroxyquinoline, K3PO4, 130 °C, overnight, 6%; (c) Fe, AcOH, H2O, 60 °C, 30 min; (d) 4-toluenesulfonyl chloride, pyridine, DCM, rt, overnight, 15% (over 2 steps); (e) ethyl bromoacetate, K2CO3, DMF, rt, overnight, 82%; (f) NaOH, MeOH, H2O, 60 °C, 3-4 h, 77%.
Inhibition of Keap1-Nrf2 PPI and structure-activity relationships (SAR).
To evaluate the inhibitory activity of small molecules synthesized above against Keap1-Nrf2 PPI, an FP assay was carried out using the procedure reported previously by our group.[58] Initially, the percent inhibition of a test compound was measured at three concentrations of 50, 5 and 0.5 μM. If a compound showed more than 50% inhibition at 5 μM, its IC50 value was determined by performing the full dose-response curve for SAR analysis. We first focused our studies on exploring R1 fragment on the benzene core, as shown in Table 1. The phenyl 7a showed moderate activity with an IC50 value of 3.47 μM, while the piperidine 7b reached only about 50% inhibition at 5 μM, indicating a preference for the aromatic phenyl over the saturated heterocyclic ring. To increase flexibility of the C2-substituent, benzylic analogs were prepared with increasing linker length. Interestingly, the N-benzylmethylamine 7c did not afford a gain in potency, whereas the C2-benzyloxy analog 7d exhibited improved inhibitory activity by 4-fold (IC50 = 0.79 μM) as compared to 7a. However, the reversed O-benzylic linker (8) resulted in 3-fold decrease in activity relative to 7d. We then prepared analogs of 7d with varying oxygen-containing linkers, and its length was changed from 0- to 3-carbon atoms. 7d-g exhibited comparable potency with IC50 values ranging from 0.79 to 1.37 μM suggesting the existence of sufficient space at the back of P3 subpocket. On the other hand, varying the substitutions (7h-j) at 4-position of the benzyl fragment and its replacement with pyridine (9a) were well tolerated with similar inhibitory activity (IC50 of 0.62-0.91 μM) as compared to 7d.
Table 1.
Inhibitory activity of 1,4-diamino substituted phenyl ring analogs 7a-o, 8, and 9a-ba

| |||||
|---|---|---|---|---|---|
| compd | R1 | % inhibition | IC50 (μM)b | ||
| 50 μM | 5 μM | 0.5 μM | |||
| 2a | - | ND | ND | ND | 0.63±0.08 |
| 7a |
|
98 | 75 | 30 | 3.47±1.24 |
| 7b |
|
89 | 51 | ND | ND |
| 7c |
|
101 | 74 | 14 | 6.17±2.70 |
| 7d |

|
96 | 97 | 56 | 0.79±0.08 |
| 8 |
|
97 | 96 | 35 | 2.62±0.55 |
| 7e |
|
95 | 93 | 31 | 1.37±0.19 |
| 7f |
|
109 | 90 | 41 | 1.02±0.09 |
| 7g |

|
103 | 99 | 48 | 0.95±0.16 |
| 7h |

|
91 | 93 | 55 | 0.77±0.12 |
| 7i |

|
105 | 88 | 57 | 0.91±0.10 |
| 7j |

|
98 | 92 | 40 | 0.62±0.09 |
| 9a |

|
98 | 96 | 48 | 0.76±0.15 |
| 7k |
|
99 | 101 | 63 | 0.48±0.06 |
| 7l |

|
98 | 92 | 51 | 0.39±0.06 |
| 7m |
|
102 | 92 | 39 | 0.89±0.12 |
| 7n |

|
98 | 79 | 41 | 0.79±0.12 |
| 7o |

|
94 | 76 | 40 | 1.19±0.15 |
| 9b |
|
99 | 98 | 40 | 0.83±0.20 |
Inhibitory potency was determined by FP assay, and all experiments were performed in triplicates. ND: not determined.
IC50 values are reported as an average of three replicates ± standard error of the mean (SEM).
We also replaced the benzyl group with two ring systems such as biphenyl or naphthalene rings, as outlined in Table 1. Compared to 7d with the same linker length, the naphthalene 7k and the biphenyl 7l displayed a slight improvement in inhibitory potency with similar IC50 values of 0.48 and 0.39 μM, respectively. In a series of naphthalene derivatives, both 2-substituted analog 7m with a shorter linker and 1-substituted analog 7n did not provide notable changes in activity (IC50 = 0.89 and 0.79 μM, respectively). However, for the biphenyl series, the shorter linker (7o) and the introduction of an oxygen between two phenyl rings (9b) led to a 3- and 2-fold reduction in activity (IC50 = 1.19 and 0.83 μM, respectively) relative to 7l (IC50 = 0.39 μM). Consequently, in the case of 1,4-bis(arylsulfonamido)benzene-N,N'-diacetic acids, C2-substituents generally led to improved potencies in the submicromolar range, as seen in Table 1.
Next, we turned our attention toward R2 modification on the benzenesulfonyl moieties of 7d, as shown in Table 2. Electron-donating groups, such as 4-methyl and 4-phenyl substituents (7p and 7q), exhibited comparable or better inhibitory activities (IC50 = 0.45 and 0.75 μM, respectively), along with 4-methoxy group (7d) (IC50 = 0.79 μM). In contrast, electron-withdrawing groups, including 4-fluoro and 4-trifluoromethyl substituents (7r and 7s), were 3- and 2-fold less potent than 7d, except for 4-bromo substituent (7t, IC50 = 0.59 μM). Additionally, we observed that the para position (7p) was preferred for PPI inhibition than the meta position (7u). Among the O-linked analogs involving a benzyl fragment (7d and 7p-u), 7p with 4-methyl substituents was the most active, which could be translated to O-linked benzene ring derivatives containing 4-fluorobenzyl (7i), naphthalene (7k), and biphenyl (7l) fragments that showed good affinities for Keap1 in Table 1. In the case of the 4-fluorobenzyl derivatives, the replacement of the methoxy groups (7i) with methyl substituents (7v) resulted in a 2-fold improvement in activity (IC50 = 0.91 vs 0.45 μM). However, unlike the 4-methyl group (7v), an unfavorable impact on potency was observed with the 2,4,6-trimethylphenyl group (7w, IC50 = 3.73 μM).[46] Potencies of compounds containing the naphthalene (7k and 7x) and biphenyl (7l and 7y) moieties remained unaffected by the change, with less than 2-fold difference in IC50 ranging from 0.38 to 0.44 μM.
Table 2.
Inhibitory activity of O-linked benzene ring derivatives 7p-y with various substituentsa

| ||||||
|---|---|---|---|---|---|---|
| compd | R1 | R2 | % inhibition | IC50 (μM)b | ||
| 50 μM | 5 μM | 0.5 μM | ||||
| 2a | - | - | ND | ND | ND | 0.63±0.08 |
| 7d |

|
4-OMe | 96 | 97 | 56 | 0.79±0.08 |
| 7p | 4-Me | 97 | 102 | 58 | 0.45±0.05 | |
| 7q | 4-phenyl | 99 | 97 | 47 | 0.75±0.07 | |
| 7r | 4-F | 103 | 87 | 41 | 2.41±0.46 | |
| 7s | 4-CF3 | 90 | 89 | 32 | 1.76±0.30 | |
| 7t | 4-Br | 87 | 91 | 54 | 0.59±0.10 | |
| 7u | 3-Me | 93 | 89 | 43 | 1.00±0.16 | |
| 7i |

|
4-OMe | 105 | 88 | 57 | 0.91±0.10 |
| 7v | 4-Me | 106 | 109 | 76 | 0.45±0.04 | |
| 7w | 2,4,6-Me | 104 | 74 | 29 | 3.73±0.96 | |
| 7k |

|
4-OMe | 99 | 101 | 63 | 0.48±0.06 |
| 7x | 4-Me | 109 | 107 | 68 | 0.38±0.06 | |
| 7l |

|
4-OMe | 98 | 92 | 51 | 0.39±0.06 |
| 7y | 4-Me | 104 | 102 | 70 | 0.44±0.04 | |
Inhibitory potency was determined by FP assay, and all experiments were performed in triplicates.
IC50 values are reported as an average of three replicates ± standard error of the mean (SEM).
Based on the known metabolic stability of fluorine substitution and the chemical structure of 7v, one of the most potent inhibitors, we sought to explore the core moiety and the acetate group (X). As shown in Table 3, bioisosteric replacement of acetates with tetrazoles (10)[59] and the addition of (S)-methyl groups to α-carbon of acetates (11)[47] resulted in a significant reduction in inhibitory potency by 8- and 3-fold, respectively. Interestingly, the presence of an extra methoxy (12a-b) or 4-fluorobenzyloxy (12c) group at the 5- or 6-position of the 1,4-diamino substituted phenyl ring bearing 2-(4-fluorobenzyloxy) group led to a dramatic drop-off in potency, whereas the O-linked naphthalene analog 12d exhibited a remarkable improvement on the inhibitory activity against Keap1-Nrf2 PPI with an IC50 value of 64.5 nM. Overall, our SAR studies demonstrated that 12d was the most active direct inhibitor among the 1,4-diamino substituted aromatic ring analogs with O-linked fragments.
Table 3.
Activity of 5 or 6-substituted benzene ring (12a-c) and naphthalene (12d) analogsa

| ||||||
|---|---|---|---|---|---|---|
| compd | Core | X | % inhibition | IC50 (μM)b | ||
| 50 μM | 5 μM | 0.5 μM | ||||
| 2a |

|
|
ND | ND | ND | 0.63±0.08 |
| 7v |

|
|
106 | 109 | 76 | 0.45±0.04 |
| 10 |
|
95 | 60 | 16 | 3.92±0.58 | |
| 11 |

|
98 | 78 | ND | 1.27±0.22 | |
| 12a |

|
74 | 41 | 26 | ND | |
| 12b |

|
|
75 | 45 | 7 | ND |
| 12c |

|
58 | 19 | 10 | ND | |
| 12d |

|
101 | 103 | ND | 0.065±0.004 | |
Inhibitory potency was determined by FP assay, and all experiments were performed in triplicates. ND: not determined.
IC50 values are reported as an average of three replicates ± standard error of the mean (SEM).
Biological evaluation of compound 12d in the TR-FRET assay.
To gain a better understanding of the inhibitory activity of 12d against Keap1-Nrf2 PPI, we further evaluated 12d using a time-resolved fluorescence energy transfer (TR-FRET) assay,[60] In the FP assay, the lower limit for IC50 is 50 nM, which is half of the Keap1 Kelch domain protein concentration used (100 nM). Accordingly, we developed a more sensitive TR-FRET assay with a lower limit of detection (IC50 = 2.5 nM), as a tool to differentiate potent direct inhibitors with nanomolar activity, like 12d. Briefly, His-tagged Keap1 Kelch domain, terbium-labeled anti-His antibody, and a fluorescein-labeled 9-mer Nrf2 peptide amide derived from Nrf2 ETGE motif (FITC-LDEETGEFL-NH2) were used together in the presence of varying concentrations of 12d or a positive control (2a). The distance-dependent transfer of excited state energy from the donor Tb to acceptor fluorescein was detected by measuring the time-resolved fluorescence intensities at 520 nm and 495 nm, providing a measure of competitive inhibitory activity (IC50). Compound 12d was found to have 2-fold greater potency with an IC50 value of 14.2 nM than the reference compound 2a (IC50 = 28.5 nM), demonstrating the 2-(4-fluorobenzyloxy) fragment of 12d was involved in binding to Keap1 Kelch domain and could more effectively disrupt the Keap1-Nrf2 PPI (Figure 3). In addition to improving the binding affinity as measured in our TR-FRET assay, the 4-fluorobenzyl substituent at the C2-position of the naphthalene core can also improve the lipophilicity of 12d as indicated by a significant increase in its cLogP from 3.03 for 2a to 6.08 for 12d. More relevant to cellular permeability and bioavailability is the significant improvement in the LogD at physiologically relevant pH: 0.33 for 12d vs −2.93 for 2a at pH 7.4 and 1.94 for 12d vs −1.38 for 2a at pH 6.5.
Figure 3.
Concentration-response curves of 2a and 12d; (A) FP assay, (B) TR-FRET assay. (C) IC50 values of 2a and 12d in FP and TR-FRET assays. The IC50 values are reported as an average of three replicates ± standard error of the mean (SEM).
Effects of compounds 7v and 12d on the transcription of Nrf2-ARE regulated genes.
Additionally, we examined the ability of the potent Keap1-Nrf2 PPI inhibitors 7v and 12d to induce the expression of Nrf2-ARE-controled genes in NCM460D cells. As shown in Figure 4, the naphthalene analog 12d significantly increased the transcription of Nrf2 target genes, GSTM3, HMOX1 and NQO1, while compound 7v with the benzene ring scaffold has no effects on the upregulation of the Nrf2-mediated genes. Particularly, the naphthalene 12d possessing the O-linked benzyl fragment was found to enhance the mRNA levels of NQO1, in comparison to the potent direct inhibitor 2a, reaching a 15-fold change in NQO1 expression at 100 μM.
Figure 4.
Expression of Nrf2 target genes by the potent Keap1-Nrf2 PPI inhibitors in NCM460D cells. Cells were treated with test compounds 7v and 12d (100, 10 and 1 μM), 0.1 % DMSO as a negative control, and two positive controls 2a (100, 10 and 1 μM) and CDDO (100 and 10 nM). After 24 h of incubation, the mRNA levels of GSTM3, HMOX1 or NQO1 were determined by RT-PCR analysis. GAPDH and HPRT were used as endogenous controls to normalize the target gene expression. The data are expressed as the fold change, relative to untreated DMSO control.
Molecular docking analysis.
In order to identify the role of O-linked fragments connected to core structure in the Keap1 Kelch domain binding site, we selected active compounds 2a, 7v and 12d, and performed molecular docking studies based on the co-crystal structure of 2b bound to the Kelch domain of Keap1 protein (PDB code: 4XMB).[61] Similar to the reference 2a (Figure 5A), the other O-linked derivatives occupy all of the binding subpockets on the Keap1 Kelch domain. As expected, the binding mode of 7v reveals that the 4-fluorobenzyl alcohol is inserted deeply into the central solvent-exposed channel (Figure 5B). Introduction of the fragment indeed appears to make hydrophobic contacts at the back of P3 subpocket, given that 7v shows enhanced activity relative to known compound 6. However, the benzene ring core (7v) is found to be less potent than the naphthalene scaffold (2a), probably due to the reduced hydrophobic interactions and the loss of π-cation interaction with Arg415. In addition, one carboxylic group of 7v displays no hydrogen bonds and electrostatic interactions with Arg483 in the P1 subpocket. Interestingly, the docked binding pose of O-linked naphthalene scaffold 12d indicates a different spatial orientation in the P3 central pocket (Figure 5C). The 4-fluorobenzyl alcohol moiety is located toward hydrophobic residue of Tyr334 in the P5 subpocket, while the naphthalene core is positioned toward the pore of P3 subpocket. It is likely that the strong preference for hydrophobic contact and π-cation interactions of the naphthalene core with Arg415 in the P3 pocket makes the O-linked fragment oriented forward. Since compound 12d is 2-fold more potent than 2a in our TR-FRET assay, the 2-(4-fluorobenzyl) group is expected to form additional hydrophobic interactions with Keap1 Kelch domain. According to our docking studies, the 2-(4-fluorobenzyl) fragment is positioned near the P5 region occupied by Tyr334 and Phe557 to provide the additional boost in binding affinity.
Figure 5.
Binding modes of compounds (represented in cyan sticks) bound to the Keap1 Kelch domain using molecular docking (derived from PDB code: 4XMB); (A) 2a, (B) 7v, (C) 12d. The surface of Keap1 was colored as the partial charge (represented in red for the most lipophilic areas, and blue for polar areas). Red dashed lines represent hydrogen bonds, and yellow lines represent π-π stacking or π-cation interactions.
CONCLUSION
In conclusion, a series of 1,4-bis(arylsulfonamido)benzene or naphthalene-N,N'-diacetic acid derivatives including 2-alkoxy or aryloxy fragments was designed based on the analysis of co-crystal structure of the Keap1 Kelch domain with the non-electrophilic inhibitor 2b. First, structure-activity relationship (SAR) studies around the 2-O-linked fragments (R1) exhibited a two-fold boost to the binding affinity as shown in our TR-FRET assay as well as enhanced inhibition of Keap1-Nrf2 protein-protein interaction in cellular assays resulting in increased expression of Nrf2-ARE-controled genes. In the case of the benzene core, the addition of 2-O-linked moieties such phenyl (7d and 7h-j), pyridinyl (9a), naphthalenyl (7k and 7m-n), and biphenyl (7l) resulted in improved inhibitory activity with IC50 values in the submicromolar range (0.39-0.91 μM) in our FP assay in comparison to lead 6 without 2-subsituents. In the case of the naphthalene scaffold, 2a and 12d exhibit a similar trend in the TR-FRET assay with 2-fold increase in inhibitory potency with the 2-(4-fluorobenzyloxy) group (IC50 = 28.5 vs 14.2 nM). However, potencies of the benzene core with an oxygen-containing linker remained limited due to its relatively weaker inhibition as compared to potent naphthalene analog 2a without a linker. Generally, the small molecule direct inhibitors such as those found in this paper interrupted the interactions between Keap1 and Nrf2 proteins with the following order of potency: naphthalene core with 2-O-linked fragment (12d) > naphthalene core without 2-O-linked fragment (2a) > benzene core with 2-O-linked fragment > benzene core without 2-O-linked fragment (6). These results were supported by our molecular docking studies. Different from 6, the 2-O-linked benzyl group of 7v occupied additional binding sites behind the P3 subpocket, leading to increased binding affinity via more hydrophobic interactions. When compared to the benzene ring scaffold, the naphthalene core structure played a more critical role in tightly binding to the Keap1 Kelch domain through strong hydrophobic and π-cation interactions with Arg415, enabling the formation of a more stable complex. Therefore, 1,4-diaminonaphthalene derivatives 2a and 12d displayed potent inhibitory activity of Keap1-Nrf2 PPI in our FP and TR-FRET assays. In particular, compound 12d with 2-(4-fluorobenzyloxy) group was shown to be the most potent inhibitor against Keap1-Nrf2 PPI, probably as a result of hydrophobic interactions of the 4-fluorobenzyl fragment toward the nonpolar P5 subpocket. Based on our docking studies, the 4-fluorobenzyl group of 7v and that of 12d occupied completely the opposite sides of the corresponding core moiety, as the 2-substituent of 12d is forced to project toward the P5 subpocket by the strong interactions of its naphthalene core in the P3 pocket. Surprisingly, the introduction of extra fragments such as methoxy (12a-b) and 4-fluorobenzyloxy (12c) substituents into the benzene core resulted in significantly reduced inhibitory activities. The additional moieties appeared to have a negative effect on forming a stable complex with regards to their proper occupation of all five pockets of the Keap1 Kelch domain. The most potent inhibitor 12d was further evaluated in a cell-based assay. The cellular activity data revealed that the naphthalene scaffold 12d is a promising Nrf2 activator, exhibiting strong induction of Nrf2-dependent gene NQO1 at 100 μM than 2a. Although further studies are needed for the development of Nrf2 activators through direct inhibition of the Keap1-Nrf2 PPI, the new 2-O-linked naphthalene core scaffold offers an additional site for structural diversity and optimization.
EXPERIMENTAL
General Chemistry.
All solvents and reagents were purchased from commercial sources and used as received unless specified otherwise. All moisture sensitive reactions were carried out using dry solvents under nitrogen atmosphere. Analytical thin layer chromatography was carried out using aluminum-backed Silica G TLC plates coated with DC Kieselgel 60 F254 (Merk or Sigma-Aldrich). Plates were visualized using ultraviolet light (254 nm) and/or by staining with potassium permanganate followed by heating. Flash chromatography was carried out on a Teledyne ISCO CombiFlash Companion system with prepacked Teledyne ISCO RediSep normal phase silica cartridges (230-400 mesh), eluting with a 0–100% ethyl acetate/ hexane or 0-20% methanol/dichloromethane gradient. 1H and 13C NMR spectra were recorded at 400 MHz and 100 MHz, respectively, at ambient temperature, using a Bruker Avance III 400 MHz Multinuclear NMR spectrometer. Chemical shifts (δ) are expressed in parts per million (ppm) relative to a solvent signal as an internal reference (CDCl3, MeOH-d4, or DMSO-d6). In the NMR tabulation, spin multiplicities are indicated as s (singlet), d (doublet), dd (doublet of doublets), t (triplet), q (quartet), m (multiplet), and brs (broad singlet). Coupling constants (J) are reported in hertz (Hz). Analytical liquid chromatography/ mass spectrometry (LC/MS) were performed on an Agilent 1200 Infinity high-performance LC (HPLC) system coupled to an Agilent 6410 single quadrupole mass spectrometer operating a multimode source, with an Inertsil ODS-3 C18 column (3 mm × 33 mm, 3 μM) at a temperature of 40 °C. The mobile phases A and B were water with 0.1% formic acid and methanol with 0.1% formic acid, respectively. The ratio of mobile phase B was increased linearly from 5% to 90% over 5 minutes, at a flow rate of 0.8 mL/min, monitoring UV absorbance at 280 nm. High-resolution mass spectra (HRMS) experiments were conducted by Center for Integrative Proteomics Research (CIPR) at Rutgers University. 1 μg/mL solutions were directly infused using the Thermo LTQ Orbitrap Velos ETD with Dionex Ultimate 3000 nano-flow 2D LC. Purities of all final compounds were determined to be ≥95% by UPLC analysis on a Waters Acquity UPLC™ system.
2-Phenyl-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7a).
To a solution of 17a (34 mg, 0.05 mmol) in methanol (0.3 mL) was added sodium hydroxide (10 mg, 0.24 mmol) in water (0.1 mL) at room temperature. The reaction mixture was stirred at 60 °C overnight. After the reaction was completed as monitored by TLC or LCMS, the crude mixture was concentrated under reduced pressure and the resulting residue dissolved in water and then washed with dichloromethane. The aqueous phase was acidified with 6 N HCl to pH around 1 and extracted with dichloromethane. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The resulting solid was dissolved with the minimum amount of dichloromethane and triturated with hexane. The product was collected by filtration as a white solid (21 mg, 67%); 1H NMR (400 MHz, MeOH-d4) δ 7.60 (d, J = 8.8 Hz, 2H), 7.58 (d, J = 8.8 Hz, 2H), 7.40-7.32 (m, 4H), 7.22-7.16 (m, 3H), 7.06-6.99 (m, 5H), 4.43 (s, 2H), 3.90 (s, 2H), 3.89 (s, 3H), 3.87 (s, 3H); 13C NMR (100 MHz, MeOH-d4) δ 172.1, 171.7, 165.0, 164.9, 143.6, 141.4, 139.6, 138.0, 133.0, 132.7, 132.2, 131.23, 131.19, 130.0, 129.3, 129.1, 128.9, 128.6, 115.3, 115.2, 115.0, 56.30, 56.27, 53.12, 53.06; LC/MS (ESI−) m/z 639.0 [M − H]−; HRMS (ESI−) m/z calcd for C30H28N2O10S2 [M − H]− 639.1113; found 639.1118. Rt: 4.11 min, HPLC purity: 99.0%.
2-(Piperidin-1-yl)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7b).
Prepared as described in the experimental procedure of 7a from intermediate 17b to obtain the title compound as a white solid (19 mg, 25%); 1H NMR (400 MHz, DMSO-d6) δ 7.74 (d, J = 8.8 Hz, 2H), 7.56 (d, J = 8.8 Hz, 2H), 7.22 (d, J = 8.4 Hz, 1H), 7.11 (d, J = 9.2 Hz, 2H), 7.05 (d, J = 9.2 Hz, 2H), 6.85 (dd, J = 8.4, 2.4 Hz, 1H), 6.71 (d, J = 2.4 Hz, 1H), 4.53 (s, 2H), 4.35 (s, 2H), 3.84 (s, 3H), 3.82 (s, 3H), 2.58-2.54 (m, 4H), 1.48-1.41 (m, 6H); 13C NMR (100 MHz, DMSO-d6) δ 170.1, 169.9, 162.8, 162.6, 149.7, 139.7, 132.2, 132.1, 131.1, 130.1, 129.6, 121.9, 120.3, 114.32, 114.27, 55.7, 52.5, 52.0, 49.3, 25.9, 23.6; LC/MS (ESI−) m/z 646.1 [M − H]−; HRMS (ESI−) m/z calcd for C29H33N3O10S2 [M − H]− 646.1535; found 646.1541. Rt: 3.61 min, HPLC purity: 99.6%.
2-(Benzyl(methyl)amino)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7c).
Prepared as described in the experimental procedure of 7a from intermediate 17c to obtain the title compound as a white solid (53 mg, 66%); 1H NMR (400 MHz, DMSO-d6) δ 12.76 (brs, 2H, CO2H), 7.73 (d, J = 8.8 Hz, 2H), 7.54 (d, J = 8.8 Hz, 2H), 7.34-7.28 (m, 3H), 7.12 (d, J = 7.6 Hz, 2H), 7.15 (d, J = 8.4 Hz, 1H), 7.07 (d, J = 8.8 Hz, 2H), 7.01 (d, J = 8.8 Hz, 2H), 6.82 (d, J = 8.4 Hz, 1H), 6.72 (s, 1H), 4.53 (s, 2H), 4.33 (s, 2H), 4.02 (s, 2H), 3.83 (s, 3H), 3.81 (s, 3H), 2.40 (s, 3H); 13C NMR (100 MHz, MeOH-d4) δ 173.4, 171.9, 165.3, 165.0, 147.3, 142.8, 134.9, 134.1, 131.9, 131.5, 131.3, 131.1, 131.0, 129.8, 127.0, 123.8, 115.5, 115.3, 114.4, 62.8, 56.33, 56.31, 53.2, 52.8, 42.1; LC/MS (ESI−) m/z 682.1 [M − H]−; HRMS (ESI−) m/z calcd for C32H33N3O10S2 [M − H]− 682.1535; found 682.1525. Rt: 3.88 min, HPLC purity: 98.1%.
2-Benzyloxy-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7d).
Prepared as described in the experimental procedure of 7a from intermediate 17d to obtain the title compound as a white solid (21 mg, 46%); 1H NMR (400 MHz, MeOH-d4) δ 7.60 (d, J = 9.2 Hz, 2H), 7.50 (d, J = 8.8 Hz, 1H), 7.42 (d, J = 8.8 Hz, 2H), 7.33-7.31 (m, 3H), 7.10-7.07 (m, 2H), 7.03 (d, J = 9.2 Hz, 2H), 6.87 (d, J = 2.4 Hz, 1H), 6.82-6.78 (m, 3H), 4.59 (s, 2H), 4.41 (s, 2H), 4.31 (s, 2H), 3.87 (s, 3H), 3.80 (s, 3H); 13C NMR (100 MHz, MeOH-d4) δ 172.7, 172.3, 165.0, 164.6, 156.3, 142.9, 137.3, 135.2, 132.8, 131.3, 130.5, 129.5, 129.1, 128.3, 127.7, 120.9, 115.2, 115.0, 114.4, 71.3, 56.3, 56.1, 30.7; LC/MS (ESI−) m/z 669.1 [M − H]−; HRMS (ESI−) m/z calcd for C31H30N2O11S2 [M − H]− 669.1218; found 669.1224. Rt: 4.17 min, HPLC purity: 98.2%.
2-Phenoxy-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7e).
Prepared as described in the experimental procedure of 7a from intermediate 17e to obtain the title compound as a white solid (61 mg, 55%); 1H NMR (400 MHz, MeOH-d4) δ 7.61 (d, J = 8.4 Hz, 1H), 7.58 (d, J = 8.8 Hz, 2H), 7.52 (d, J = 8.8 Hz, 2H), 7.25 (dd, J = 8.0, 8.0 Hz, 2H), 7.12 (t, J = 8.0 Hz, 1H), 6.99-6.96 (m, 3H), 6.91 (d, J = 8.8 Hz, 2H), 6.51 (d, J = 8.8 Hz, 2H), 6.44 (d, J = 2.0 Hz, 1H), 4.43 (s, 2H), 4.31 (s, 2H), 3.90 (s, 3H), 3.82 (s, 3H); 13C NMR (100 MHz, MeOH-d4) δ 172.5, 172.0, 164.9, 164.8, 155.8, 155.5, 142.6, 135.5, 132.7, 131.2, 131.0, 130.9, 130.8, 128.9, 125.6, 122.9, 120.5, 117.5, 115.3, 115.2, 56.3, 56.2, 52.9, 52.4; LC/MS (ESI−) m/z 655.1 [M − H]−; HRMS (ESI−) m/z calcd for C30H28N2O11S2 [M − H]− 655.1062; found 655.1068. Rt: 4.10 min, HPLC purity: 99.1%.
2-Phenethoxy-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7f).
Prepared as described in the experimental procedure of 7a from intermediate 17f to obtain the title compound as a white solid (60 mg, 68%); 1H NMR (400 MHz, MeOH-d4) δ 7.58 (d, J = 8.8 Hz, 2H), 7.48 (d, J = 8.8 Hz, 2H), 7.30-7.26 (m, 3H), 7.21-7.20 (m, 1H), 7.09 (d, J = 8.0 Hz, 2H), 6.99 (d, J = 9.2 Hz, 2H), 6.96 (d, J = 9.2 Hz, 2H), 6.78 (dd, J = 8.8, 2.0 Hz, 1H), 6.68 (d, J = 2.0 Hz, 1H), 4.41 (s, 2H), 4.05 (s, 2H), 3.84 (s, 3H), 3.78 (s, 3H), 3.62 (t, J = 6.8 Hz, 2H), 2.54 (t, J = 6.8 Hz, 2H); 13C NMR (100 MHz, MeOH-d4) δ 172.7, 172.2, 164.9, 164.6, 156.1, 142.7, 138.9, 135.1, 133.0, 131.2, 130.7, 129.9, 129.6, 127.7, 127.4, 120.9, 115.2, 115.0, 113.9, 69.8, 56.31, 56.28, 53.2, 51.7, 35.9; LC/MS (ESI−) m/z 683.1 [M − H]−; HRMS (ESI−) m/z calcd for C32H32N2O11S2 [M − H]− 683.1375; found 683.1382. Rt: 4.27 min, HPLC purity: 97.0%.
2-(3-Phenylpropoxy)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7g).
Prepared as described in the experimental procedure of 7a from intermediate 17g to obtain the title compound as a white solid (15 mg, 52%); 1H NMR (400 MHz, DMSO-d6) δ 12.92 (brs, 2H, CO2H), 7.61 (d, J = 9.2 Hz, 2H), 7.49 (d, J = 9.2 Hz, 2H), 7.35 (d, J = 8.4 Hz, 1H), 7.32-7.28 (m, 2H), 7.22-7.18 (m, 1H), 7.12 (d, J = 8.0 Hz, 2H), 7.06 (d, J = 9.2 Hz, 2H), 7.00 (d, J = 9.2 Hz, 2H), 6.81 (dd, J = 8.4, 2.0 Hz, 1H), 6.58 (d, J = 2.0 Hz, 1H), 4.40 (s, 2H), 4.22 (s, 2H), 3.82 (s, 3H), 3.73 (s, 3H), 3.42-3.40 (m, 2H), 2.41 (t, J = 7.8 Hz, 2H), 1.52-1.47 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 170.2, 170.0, 162.8, 162.5, 154.5, 141.2, 140.9, 133.4, 131.5, 129.9, 129.6, 129.1, 128.3, 128.2, 125.8, 125.3, 118.9, 114.3, 114.0, 111.3, 66.9, 55.7, 55.5, 51.8, 50.5, 31.3, 29.9; LC/MS (ESI−) m/z 697.2 [M − H]−; HRMS (ESI−) m/z calcd for C33H34N2O11S2 [M − H]− 697.1531; found 697.1525. Rt: 4.39 min, HPLC purity: 97.6%.
2-(4-Methoxybenzyloxy)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7h).
Prepared as described in the experimental procedure of 7a from intermediate 17h to obtain the title compound as a white solid (66 mg, 67%); 1H NMR (400 MHz, DMSO-d6) δ 12.78 (brs, 2H, CO2H), 7.62 (d, J = 8.8 Hz, 2H), 7.41 (d, J = 8.8 Hz, 2H), 7.35 (d, J = 8.4 Hz, 1H), 7.09 (d, J = 9.2 Hz, 2H), 6.99 (d, J = 8.8 Hz, 2H), 6.87 (d, J = 8.8 Hz, 2H), 6.87 (d, J = 9.2 Hz, 2H), 6.83 (dd, J = 8.4, 2.4 Hz, 2H), 6.77 (d, J = 2.4 Hz, 1H), 4.51 (s, 2H), 4.41 (s, 2H), 4.16 (s, 2H), 3.84 (s, 3H), 3.80 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ 170.2, 169.9, 162.8, 162.4, 158.9, 154.4, 140.9, 133.2, 131.2, 129.9, 129.6, 129.0, 128.7, 127.7, 125.6, 119.1, 114.3, 113.9, 113.6, 111.9, 69.3, 55.7, 55.5, 55.0, 51.8, 50.4; LC/MS (ESI−) m/z 699.1 [M − H]−; HRMS (ESI−) m/z calcd for C32H32N2O12S2 [M − H]− 699.1324; found 699.1329. Rt: 4.13 min, HPLC purity: 96.6%.
2-(4-Fluorobenzyloxy)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7i).
Prepared as described in the experimental procedure of 7a from intermediate 17i to obtain the title compound as a white solid (56 mg, 44%); 1H NMR (400 MHz, DMSO-d6) δ 12.82 (brs, 2H, CO2H), 7.62 (d, J = 8.4 Hz, 2H), 7.41 (d, J = 8.4 Hz, 2H), 7.36 (d, J = 8.4 Hz, 1H), 7.17-7.08 (m, 6H), 6.86-6.83 (m, 3H), 6.78 (d, J = 2.0 Hz, 1H), 4.58 (s, 2H), 4.41 (s, 2H), 4.19 (s, 2H), 3.84 (s, 3H), 3.75 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.3, 170.0, 162.8, 162.4, 154.3, 141.0, 133.4, 132.1, 131.2, 129.9, 129.7, 129.1 (JC,F = 8 Hz), 129.0, 125.7, 119.3, 115.0 (JC,F = 21 Hz), 114.3, 113.9, 111.9, 68.8, 55.7, 55.5, 51.8, 50.6; LC/MS (ESI+) m/z 689.2 [M + H]+; HRMS (ESI+) m/z calcd for C31H29FN2O11S2 [M + H]+ 689.1270; found 689.1281. Rt: 4.19 min, HPLC purity: 95.1%.
2-(4-Methylbenzyloxy)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7j).
Prepared as described in the experimental procedure of 7a from intermediate 17j to obtain the title compound as a white solid (29 mg, 87%); 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 9.2 Hz, 2H), 7.47-7.43 (m, 3H), 7.11 (d, J = 7.6 Hz, 2H), 6.98-6.95 (m, 3H), 6.90 (d, J = 8.8 Hz, 2H), 6.70 (d, J = 8.8 Hz, 2H), 6.59 (dd, J = 8.4, 2.4 Hz, 2H), 4.62 (s, 2H), 4.33 (s, 2H), 4.28 (s, 2H), 3.84 (s, 3H), 3.76 (s, 3H), 2.35 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.2, 163.4, 163.1, 155.1, 141.4, 138.1, 134.0, 132.5, 131.3, 130.2, 129.7, 129.3, 127.5, 127.4, 126.6, 119.3, 114.2, 113.9, 113.8, 70.4, 55.8, 55.6, 52.2, 51.0, 21.3; LC/MS (ESI−) m/z 683.1 [M − H]−; HRMS (ESI−) m/z calcd for C32H32N2O11S2 [M − H]− 683.1375; found 683.1381. Rt: 4.31 min, HPLC purity: 95.6%.
2-(Naphthalen-2-ylmethoxy)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7k).
Prepared as described in the experimental procedure of 7a from intermediate 17k to obtain the title compound as a white solid (59 mg, 67%); 1H NMR (400 MHz, DMSO-d6) δ 7.95-7.92 (m, 1H), 7.88 (d, J = 8.0 Hz, 2H), 7.61 (d, J = 8.8 Hz, 2H), 7.57-7.53 (m, 3H), 7.43 (d, J = 8.8 Hz, 2H), 7.39 (d, J = 9.2 Hz, 1H), 7.21 (dd, J = 8.4, 1.2 Hz, 1H), 7.05 (d, J = 9.2 Hz, 2H), 6.87-6.85 (m, 2H), 6.76 (d, J = 9.2 Hz, 2H), 4.77 (s, 2H), 4.43 (s, 2H), 4.26 (s, 2H), 3.82 (s, 3H), 3.54 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.3, 170.0, 162.8, 162.3, 154.5, 141.0, 133.5, 132.6, 132.5, 131.3, 129.9, 129.7, 129.0, 127.9, 127.6, 126.3, 126.2, 125.7, 125.5, 124.9, 114.3, 113.9, 111.9, 69.6, 55.8, 55.3, 51.8, 50.6; LC/MS (ESI−) m/z 719.1 [M − H]−; HRMS (ESI−) m/z calcd for C35H32N2O11S2 [M − H]− 719.1375; found 719.1382. Rt: 4.46 min, HPLC purity: 97.6%.
2-([1,1'-Biphenyl]-4-ylmethoxy)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7l).
Prepared as described in the experimental procedure of 7a from intermediate 17l to obtain the title compound as a white solid (65 mg, 81%); 1H NMR (400 MHz, DMSO-d6) δ 7.70 (d, J = 7.6 Hz, 2H), 7.63-7.61 (m, 4H), 7.51-7.37 (m, 6H), 7.12 (d, J = 8.0 Hz, 2H), 7.09 (d, J = 8.8 Hz, 2H), 6.87-6.84 (m, 3H), 6.81 (d, J = 2.4 Hz, 1H), 4.65 (s, 2H), 4.42 (s, 2H), 4.23 (s, 2H), 3.82 (s, 3H), 3.69 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.3, 170.0, 162.8, 162.4, 154.4, 141.0, 139.7, 139.5, 135.0, 133.4, 131.2, 129.9, 129.7, 129.0, 127.5, 127.4, 126.6, 126.5, 125.6, 119.3, 114.3, 114.0, 111.9, 69.2, 55.7, 55.4, 51.8, 50.6; LC/MS (ESI−) m/z 745.1 [M − H]−; HRMS (ESI−) m/z calcd for C37H34N2O11S2 [M − H]− 745.1531; found 745.1538. Rt: 4.61 min, HPLC purity: 96.8%.
2-(Naphthalen-2-yloxy)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7m).
Prepared as described in the experimental procedure of 7a from intermediate 17m to obtain the title compound as a white solid (32 mg, 27%); 1H NMR (400 MHz, DMSO-d6) δ 12.87 (brs, 2H, CO2H), 7.94 (d, J = 8.0 Hz, 1H), 7.86 (d, J = 9.2 Hz, 1H), 7.75 (d, J = 8.0 Hz, 1H), 7.59 (d, J = 8.8 Hz, 2H), 7.57-7.48 (m, 3H), 7.45 (d, J = 8.8 Hz, 2H), 7.04 (d, J = 2.4 Hz, 1H), 7.00 (dd, J = 8.8, 2.4 Hz, 1H), 6.93 (d, J = 8.8 Hz, 2H), 6.85 (d, J = 8.8 Hz, 2H), 6.76 (dd, J = 8.8, 2.4 Hz, 1H), 6.49 (d, J = 2.4 Hz, 1H), 4.38 (s, 2H), 4.31 (s, 2H), 3.75 (s, 3H), 3.66 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.3, 169.9, 162.64, 162.57, 153.4, 151.9, 140.8, 133.9, 133.5, 131.1, 130.2, 129.9, 129.8, 129.3, 127.7, 127.1, 126.9, 126.8, 125.3, 120.9, 119.5, 115.3, 115.0, 114.2, 114.1, 55.7, 55.5, 51.6, 51.3; LC/MS (ESI−) m/z 705.1 [M − H]−; HRMS (ESI−) m/z calcd for C34H30N2O11S2 [M − H]− 705.1218; found 705.1224. Rt: 4.39 min, HPLC purity: 97.5%.
2-(Naphthalen-1-ylmethoxy)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7n).
Prepared as described in the experimental procedure of 7a from intermediate 17n to obtain the title compound as a white solid (84 mg, 82%); 1H NMR (400 MHz, DMSO-d6) δ 12.74 (brs, 2H, CO2H), 7.99-7.97 (m, 1H), 7.91 (d, J = 8.4 Hz, 1H), 7.79-7.77 (m, 1H), 7.63 (d, J = 8.8 Hz, 2H), 7.58-7.55 (m, 2H), 7.47-7.43 (m, 2H), 7.39 (d, J = 8.8 Hz, 2H), 7.14 (d, J = 7.2 Hz, 1H), 7.08 (d, J = 8.8 Hz, 2H), 6.95-6.93 (m, 2H), 6.72 (d, J = 8.8 Hz, 2H), 5.03 (s, 2H), 4.46 (s, 2H), 4.15 (s, 2H), 3.80 (s, 3H), 3.59 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.2, 169.9, 162.8, 162.3, 154.5, 141.0, 133.3, 133.0, 131.22, 131.15, 130.1, 129.9, 129.7, 128.9, 128.5, 128.2, 126.3, 125.9, 125.8, 125.2, 125.0, 123.1, 120.1, 114.3, 113.8, 112.1, 67.8, 55.7, 55.3, 51.8, 50.6; LC/MS (ESI−) m/z 719.1 [M − H]−; HRMS (ESI−) m/z calcd for C35H32N2O11S2 [M − H]− 719.1375; found 719.1382. Rt: 4.39 min, HPLC purity: 97.8%.
2-([1,1'-Biphenyl]-4-yloxy)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (7o).
Prepared as described in the experimental procedure of 7a from intermediate 17o to obtain the title compound as a white solid (68 mg, 74%); 1H NMR (400 MHz, DMSO-d6) δ 12.91 (brs, 2H, CO2H), 7.66 (d, J = 7.6 Hz, 2H), 7.58-7.47 (m, 9H), 7.37 (t, J = 7.2 Hz, 1H), 7.03-7.01 (m, 3H), 6.96 (d, J = 8.8 Hz, 2H), 6.59 (d, J = 8.8 Hz, 2H), 6.45 (d, J = 2.0 Hz, 1H), 4.34 (s, 2H), 4.33 (s, 2H), 3.75 (s, 3H), 3.73 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.2, 169.9, 162.7, 162.6, 153.8, 153.2, 140.7, 139.2, 136.0, 133.8, 131.1, 129.8, 129.4, 129.2, 129.0, 127.9, 127.3, 127.2, 126.4, 121.7, 119.2, 115.6, 114.4, 114.2, 55.60, 55.57, 51.7, 51.3; LC/MS (ESI−) m/z m/z 731.1 [M − H]−; HRMS (ESI−) m/z calcd for C36H32N2O11S2 [M − H]− 731.1375; found 731.1381. Rt: 4.55 min, HPLC purity: 99.5%.
2-Benzyloxy-1,4-bis(4-methylphenylsulfonamido)benzene-N,N'-diacetic acid (7p).
Prepared as described in the experimental procedure of 7a from intermediate 17p to obtain the title compound as a white solid (20 mg, 70%); 1H NMR (400 MHz, DMSO-d6) δ 12.82 (brs, 2H, CO2H), 7.56 (d, J = 8.0 Hz, 2H), 7.39-7.36 (m, 5H), 7.33-7.32 (m, 3H), 7.14 (d, J = 8.0 Hz, 2H), 7.02-7.00 (m, 2H), 6.84 (d, J = 8.4 Hz, 1H), 6.77 (s, 1H), 4.54 (s, 2H), 4.42 (s, 2H), 4.22 (s, 2H), 2.40 (s, 3H), 2.28 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.1, 169.8, 154.3, 143.6, 143.0, 140.8, 136.7, 135.8, 135.5, 133.2, 129.6, 129.2, 128.1, 127.7, 127.3, 126.8, 125.5, 119.3, 112.0, 69.4, 51.8, 50.6, 21.0, 20.9; LC/MS (ESI−) m/z 637.1 [M − H]−; HRMS (ESI−) m/z calcd for C31H30N2O9S2 [M − H]− 637.1320; found 637.1326. Rt: 4.44 min, HPLC purity: 98.3%.
2-Benzyloxy-1,4-bis(4-phenylphenylsulfonamido)benzene-N,N'-diacetic acid (7q).
Prepared as described in the experimental procedure of 7a from intermediate 17q to obtain the title compound as a white solid (25 mg, 87%); 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 8.4 Hz, 2H), 7.63 (d, J = 8.4 Hz, 2H), 7.56-7.53 (m, 5H), 7.44-7.35 (m, 10H), 7.19-7.11 (m, 3H), 6.94 (d, J = 2.0 Hz, 1H), 6.92 (d, J = 6.4 Hz, 2H), 6.69 (dd, J = 8.4, 2.0 Hz, 1H), 4.56 (s, 2H), 4.34 (s, 2H), 4.32 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 171.3, 170.6, 155.0, 146.1, 145.4, 141.3, 139.1, 139.0, 138.3, 137.1, 135.4, 134.4, 129.2, 129.0, 128.7, 128.50, 128.46, 128.4, 128.1, 127.9, 127.5, 127.3, 127.2, 127.10, 127.06, 126.5, 119.6, 113.8, 70.2, 52.4, 50.9; LC/MS (ESI−) m/z 761.1 [M − H]−; HRMS (ESI−) m/z calcd for C41H34N2O9S2 [M − H]− 761.1633; found 761.1628. Rt: 5.05 min, HPLC purity: 95.0%.
2-Benzyloxy-1,4-bis(4-fluorophenylsulfonamido)benzene-N,N'-diacetic acid (7r).
Prepared as described in the experimental procedure of 7a from intermediate 17r to obtain the title compound as a white solid (32 mg, 54%); 1H NMR (400 MHz, DMSO-d6) δ 12.79 (brs, 2H, CO2H), 7.76 (dd, J = 8.4, 5.2 Hz, 2H), 7.62 (d, J = 8.4 Hz, 1H), 7.56 (dd, J = 8.4, 5.2 Hz, 2H), 7.45-7.37 (m, 3H), 7.34-7.32 (m, 2H), 7.15-7.08 (m, 2H), 7.06-7.04 (m, 2H), 6.88 (dd, J = 8.4, 1.6 Hz, 1H), 6.82 (d, J = 1.6 Hz, 1H), 4.61 (s, 2H), 4.46 (s, 2H), 4.25 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 170.1, 169.8, 163.3 (JC,F = 30 Hz), 154.4, 140.8, 135.9, 135.7, 134.7, 133.4, 130.5 (JC,F = 10 Hz), 129.8 (JC,F = 10 Hz), 129.6, 128.2, 127.8, 126.9, 126.0, 119.7, 116.4 (JC,F = 22 Hz), 115.9 (JC,F = 23 Hz), 114.3, 112.1, 69.6, 52.0, 50.7; LC/MS (ESI−) m/z 645.1 [M − H]−; HRMS (ESI−) m/z calcd for C29H24F2N2O9S2 [M − H]− 645.0819; found 645.0826. Rt: 4.32 min, HPLC purity: 98.7%.
2-Benzyloxy-1,4-bis(4-(trifluoromethyl)phenylsulfonamido)benzene-N,N'-diacetic acid (7s).
Prepared as described in the experimental procedure of 7a from intermediate 17s to obtain the title compound as a white solid (25 mg, 64%); 1H NMR (400 MHz, MeOH-d4) δ 7.87-7.85 (m, 4H), 7.70 (d, J = 8.4 Hz, 2H), 7.59 (d, J = 8.0 Hz, 2H), 7.55 (d, J = 8.0 Hz, 1H), 7.31-7.29 (m, 3H), 7.04-7.01 (m, 2H), 6.91-6.87 (m, 2H), 4.54 (s, 2H), 4.49 (s, 2H), 4.37 (s, 2H); 13C NMR (100 MHz, MeOH-d4) δ 172.2, 171.9, 156.3, 145.0, 143.9, 142.6, 136.9, 135.7, 135.4, 129.8, 129.5, 129.23, 129.17, 128.4, 127.7, 127.2, 127.0, 121.6, 114.7, 71.3, 53.5, 52.2; LC/MS (ESI−) m/z 745.1 [M − H]−; HRMS (ESI−) m/z calcd for C31H24F6N2O9S2 [M − H]− 745.0755; found 745.0764. Rt: 4.78 min, HPLC purity: 97.9%.
2-Benzyloxy-1,4-bis(4-bromophenylsulfonamido)benzene-N,N'-diacetic acid (7t).
Prepared as described in the experimental procedure of 7a from intermediate 17t to obtain the title compound as a white solid (31 mg, 56%); 1H NMR (400 MHz, DMSO-d6) δ 12.88 (brs, 2H, CO2H), 7.81 (d, J = 7.6 Hz, 2H), 7.60 (d, J = 7.6 Hz, 2H), 7.52 (d, J = 7.6 Hz, 2H), 7.43-7.39 (m, 3H), 7.36-7.34 (m, 3H), 7.07-7.02 (m, 2H), 6.92 (d, J = 8.4 Hz, 1H), 6.76 (s, 1H), 4.54 (s, 2H), 4.46 (s, 2H), 4.25 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 170.1, 169.8, 154.4, 140.7, 138.7, 137.5, 135.6, 135.5, 132.3, 131.9, 129.4, 128.7, 128.3, 127.8, 127.3, 126.94, 126.85, 125.6, 120.1, 112.2, 69.6, 52.0, 50.7; LC/MS (ESI+) m/z 767.0 [M(279Br,) + H]+, 769.0 [M(79Br, 81Br) + H]+, 771.0 [M(281Br) + H]+ ; HRMS (ESI+) m/z calcd for C29H24Br2N2O9S2 [M(279Br,) + H]+ 766.9324, [M(79Br, 81Br) + H]+ 768.9303 and [M(281Br) + H]+ 770.9283; found 766.9380, 768.9356 and 770.9332. Rt: 4.79 min, HPLC purity: 97.0%.
2-Benzyloxy-1,4-bis(3-methylphenylsulfonamido)benzene-N,N'-diacetic acid (7u).
Prepared as described in the experimental procedure of 7a from intermediate 17u to obtain the title compound as a white solid (29 mg, 81%); 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 8.8 Hz, 1H), 7.43 (d, J = 8.8 Hz, 2H), 7.37-7.27 (m, 7H), 7.17 (d, J = 7.2 Hz, 1H), 7.12 (d, J = 7.6 Hz, 1H), 7.00-6.98 (m, 3H), 6.55 (dd, J = 8.4, 2.4 Hz, 1H), 4.59 (s, 2H), 4.31 (s, 2H), 4.29 (s, 2H), 2.35 (s, 3H), 2.18 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.3, 170.5, 155.0, 141.3, 139.7, 139.3, 139.0, 138.3, 135.6, 134.3, 134.0, 133.5, 128.9, 128.54, 128.48, 128.13, 128.10, 127.6, 127.0, 126.6, 124.9, 124.5, 119.3, 114.1, 70.2, 52.4, 50.9, 21.3, 21.2; LC/MS (ESI−) 637.1 [M − H]−; HRMS (ESI−) m/z calcd for C31H30N2O9S2 [M − H]− 637.1320; found 637.1311. Rt: 4.47 min, HPLC purity: 96.0%.
2-(4-Fluorobenzyloxy)-1,4-bis(4-methylphenylsulfonamido)benzene-N,N'-diacetic acid (7v).
Prepared as described in the experimental procedure of 7a from intermediate 17v to obtain the title compound as a white solid (66 mg, 70%); 1H NMR (400 MHz, DMSO-d6) δ 12.83 (brs, 2H, CO2H), 7.56 (d, J = 7.6 Hz, 2H), 7.40-7.35 (m, 5H), 7.18-7.14 (m, 4H), 7.08-7.05 (m, 2H), 6.84 (d, J = 8.4 Hz, 1H), 6.78 (s, 1H), 4.55 (s, 2H), 4.43 (s, 2H), 4.20 (s, 2H), 2.40 (s, 3H), 2.29 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.3, 169.9, 160.5, 154.3, 143.7, 143.1, 140.9, 136.7, 135.5, 133.3, 132.1 (JC,F = 3 Hz), 129.6, 129.3, 129.1 (JC,F = 8 Hz), 127.4, 126.8, 125.6, 119.3, 115.0 (JC,F = 22 Hz), 112.0, 68.8, 51.8, 50.6, 21.0, 20.9; LC/MS (ESI−) m/z 655.1 [M − H]−; HRMS (ESI−) m/z calcd for C31H29FN2O9S2 [M − H]− 655.1226; found 655.1221. Rt: 4.45 min, HPLC purity: 97.2%.
2-(4-Fluorobenzyloxy)-1,4-bis(2,4,6-trimethylphenylsulfonamido)benzene-N,N'-diacetic acid (7w).
To a solution of 17w (102 mg, 0.12 mmol) in dichloromethane (2 mL) was added trifluoroacetic acid (0.5 mL) at room temperature. The reaction mixture was stirred at room temperature for overnight. After the reaction was completed, the crude mixture was added to water, and extracted with dichloromethane. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatograph (0-10% methanol/ dichloromethane) to give the product as a pale yellow solid (79 mg, 89%); 1H NMR (400 MHz, CDCl3) δ 11.22 (brs, 2H, CO2H), 7.45 (d, J = 8.4 Hz, 1H), 7.08-7.00 (m, 4H), 6.87 (s, 2H), 6.72 (s, 2H), 6.64-6.61 (m, 2H), 4.56 (s, 6H), 2.36 (s, 6H), 2.30 (s, 3H), 2.28 (s, 3H), 2.18 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 176.6, 175.6, 162.8 (JC,F = 246 Hz) 155.4, 143.4, 142.3, 140.7, 140.4, 134.5, 133.6, 132.1, 131.9, 131.8, 131.3, 129.5 (JC,F = 8 Hz), 125.8, 121.4, 115.7 (JC,F = 21 Hz), 112.6, 69.9, 50.8, 50.2, 23.3, 23.0, 21.2, 21.1; LC/MS (ESI−) m/z 711.2 [M − H]−; HRMS (ESI−) m/z calcd for C35H37FN2O9S2 [M − H]− 711.1852; found 711.1847. Rt: 5.07 min, HPLC purity: 97.2%.
2-(Naphthalen-2-ylmethoxy)-1,4-bis(4-methylphenylsulfonamido)benzene-N,N'-diacetic acid (7x).
Prepared as described in the experimental procedure of 7a from intermediate 17x to obtain the title compound as a white solid (59 mg, 46%); 1H NMR (400 MHz, DMSO-d6) δ 12.83 (brs, 2H, CO2H), 7.96-7.93 (m, 1H), 7.88 (d, J = 8.0 Hz, 2H), 7.56-7.54 (m, 5H), 7.41-7.37 (m, 3H), 7.33 (d, J = 8.0 Hz, 2H), 7.18 (d, J = 8.4 Hz, 1H), 7.05 (d, J = 8.4 Hz, 2H), 6.87-6.85 (m, 2H), 4.74(s, 2H), 4.44 (s, 2H), 4.27 (s, 2H), 2.37 (s, 3H), 2.09 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.2, 169.9, 154.4, 143.7, 143.0, 140.9, 136.7, 135.5, 133.5, 133.4, 132.6, 132.5, 129.6, 129.2, 127.84, 127.81, 127.6, 127.4, 126.8, 126.3, 126.1, 125.7, 125.5, 124.9, 119.3, 112.0, 69.6, 51.9, 50.6, 21.0, 20.8; LC/MS (ESI−) m/z 687.2 [M − H]−; HRMS (ESI−) m/z calcd for C35H32N2O9S2 [M − H]− 687.1476; found 687.1473. Rt: 4.72 min, HPLC purity: 99.9%.
2-([1,1'-Biphenyl]-4-ylmethoxy)-1,4-bis(4-methylphenylsulfonamido)benzene-N,N'-diacetic acid (7y).
Prepared as described in the experimental procedure of 7a from intermediate 17y to obtain the title compound as a white solid (52 mg, 58%); 1H NMR (400 MHz, DMSO-d6) δ 12.82 (brs, 2H, CO2H), 7.71 (d, J = 7.6 Hz, 2H), 7.63 (d, J = 8.0 Hz, 2H), 7.57 (d, J = 8.0 Hz, 2H), 7.50 (dd, J = 7.6, 7.6 Hz, 2H), 7.41-7.38 (m, 6H), 7.17 (d, J = 8.4 Hz, 2H), 7.11 (d, J = 8.4 Hz, 2H), 6.85 (dd, J = 8.4, 2.4 Hz, 1H), 6.82 (d, J = 2.4 Hz, 1H), 4.61 (s, 2H), 4.44 (s, 2H), 4.25 (s, 2H), 2.38 (s, 3H), 2.26 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.2, 169.9, 154.4, 143.7, 143.1, 140.9, 139.7, 139.5, 136.7, 135.5, 135.0, 133.3, 129.6, 129.3, 129.0, 127.5, 127.42, 127.35, 126.8, 126.6, 126.4, 125.6, 119.3, 112.0, 69.2, 51.8, 50.6, 21.0; LC/MS (ESI−) m/z 713.1 [M − H]−; HRMS (ESI−) m/z calcd for C37H34N2O9S2 [M − H]− 713.1633; found 713.1630. Rt: 4.86 min, HPLC purity: 99.5%.
2-(Phenoxymethyl)-1,4-bis(4-methyoxyphenylsulfonamido)benzene-N,N'-diacetic acid (8).
Prepared as described in the experimental procedure of 7a from intermediate 21 to obtain the title compound as a white solid (44 mg, 72%); 1H NMR (400 MHz, MeOH-d4) δ 7.53 (d, J = 8.8 Hz, 4H), 7.40 (d, J = 2.4 Hz, 1H), 7.26 (dd, J = 8.0, 8.0 Hz, 2H), 7.09 (dd, J = 8.8, 2.4 Hz, 1H), 7.01 (d, J = 8.8 Hz, 2H), 6.95-6.91 (m, 5H), 6.77 (d, J = 8.8 Hz, 1H), 5.51-5.48 (m, 1H), 5.16-5.13 (m, 1H), 4.44-4.40 (m, 1H), 4.37 (s, 2H), 4.16-4.12 (m, 1H), 3.85 (s, 3H), 3.82 (s, 3H); 13C NMR (100 MHz, MeOH-d4) δ 172.1, 171.7, 165.1, 164.8, 160.0, 142.1, 141.6, 138.1, 131.4, 131.2, 131.1, 130.5, 130.4, 128.6, 128.2, 122.0, 116.0, 115.9, 115.4, 115.2, 67.1, 56.3, 56.3, 54.1, 53.1; LC/MS (ESI−) m/z 699.1 [M − H]−; HRMS (ESI−) m/z calcd for C31H30N2O11S2 [M − H]− 669.1218; found 669.1225. Rt: 4.25 min, HPLC purity: 98.2%.
2-(Pyridin-3-ylmethoxy)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (9a).
Prepared as described in the experimental procedure of 7a from intermediate 26a to obtain the title compound as a white solid (7 mg, 45%); 1H NMR (400 MHz, DMSO-d6) δ 12.71 (brs, 2H, CO2H), 8.54 (d, J = 4.4 Hz, 1H), 8.33 (s, 1H), 7.63 (d, J = 8.8 Hz, 2H), 7.47 (d, J = 8.0 Hz, 1H), 7.40-7.35 (m, 4H), 7.10 (d, J = 8.8 Hz, 2H), 6.87-6.80 (m, 4H), 4.67 (s, 2H), 4.43 (s, 2H), 4.21 (s, 2H), 3.85 (s, 3H), 3.76 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.2, 169.8, 162.8, 162.3, 154.3, 149.0, 148.2, 141.0, 134.7, 133.3, 131.4, 131.1, 129.9, 129.6, 128.9, 125.7, 123.3, 119.3, 114.3, 113.8, 111.9, 67.2, 55.7, 55.4, 51.7, 50.6; LC/MS (ESI−) m/z 670.0 [M − H]−; HRMS (ESI−) m/z calcd for C30H29N3O11S2 [M − H]− 670.1171; found 670.1167. Rt: 3.15 min, HPLC purity: 99.0%.
2-(4-Phenoxybenzyloxy)-1,4-bis(4-methoxyphenylsulfonamido)benzene-N,N'-diacetic acid (9b).
Prepared as described in the experimental procedure of 7a from intermediate 26b to obtain the title compound as a white solid (15 mg, 33%); 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 8.8 Hz, 2H), 7.50 (d, J = 8.4 Hz, 2H), 7.40-7.34 (m, 3H), 7.14 (t, J = 7.2 Hz, 1H), 7.03 (d, J = 8.4 Hz, 2H), 7.02-7.00 (m, 2H), 6.94-6.92(m, 5H), 6.75 (d, J = 8.8 Hz, 2H), 6.52 (d, J = 7.6 Hz, 1H), 4.65 (s, 2H), 4.42 (s, 2H), 4.39 (s, 2H), 3.86 (s, 3H), 3.77 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 165.0, 164.5, 158.7, 158.5, 156.3, 142.9, 135.2, 132.8, 132.1, 131.3, 131.0, 130.6, 130.1, 127.8, 124.6, 120.7, 120.1, 120.0, 119.5, 115.2, 115.0, 114.5, 70.8, 56.3, 56.1, 53.2, 51.9; LC/MS (ESI−) m/z 761.1 [M − H]−; HRMS (ESI−) m/z calcd for C38H36N2O12S2 [M − H]− 761.1480; found 761.1476. Rt: 4.61 min, HPLC purity: 97.0%.
N,N'-bis((1H-tetrazol-5-yl)methyl)-2-(4-fluorobenzyloxy)-1,4-bis(4-methylphenylsulfonamido)benzene (10).
To a solution of 27 (40 mg, 0.06 mmol) in N,N-dimethylformamide (1 mL) were added sodium azide (25 mg, 0.39 mmol) and acetic acid (3 drops) at room temperature. The reaction mixture was stirred at 120 °C for 6 hours. After the reaction was completed, the crude mixture was added to water, and extracted with ethyl acetate. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatograph (0-10% methanol/dichloromethane) to give the product as a white solid (14 mg, 30%); 1H NMR (400 MHz, MeOH-d4) δ 7.40 (d, J = 8.4 Hz, 2H), 7.36-7.33 (m, 4H), 7.21 (d, J = 8.4 Hz, 1H), 7.15 (d, J = 8.4 Hz, 2H), 7.13-7.03 (m, 4H), 6.76 (d, J = 2.4 Hz, 1H), 6.67 (dd, J = 8.4, 2.4 Hz, 1H), 5.12 (s, 2H), 5.10 (s, 2H), 4.50 (s, 2H), 2.43 (s, 3H), 2.36 (s, 3H); 13C NMR (100 MHz, MeOH-d4) δ 162.8, 156.6, 155.6, 146.1, 145.4, 142.1, 137.3, 135.2, 134.7, 133.1, 130.9, 130.7 (JC,F = 4 Hz), 130.6, 129.1, 128.6, 127.2, 121.4, 116.2 (JC,F = 22 Hz), 114.8, 70.8, 45.5, 44.2, 21.62, 21.58; LC/MS (ESI+) m/z 705.2 [M + H]+; HRMS (ESI+) m/z calcd for C31H29FN10O5S2 [M + H]+ 705.1821; found 705.1822. Rt: 4.38 min, HPLC purity: 95.1%.
(2S,2'S)-2,2'-((2-((4-Fluorobenzyl)oxy)-1,4-phenylene)bis(tosylazanediyl))dipropionic acid (11).
Prepared as described in the experimental procedure of 7a from intermediate 28 to obtain the title compound as a white solid (28 mg, 44%); 1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 7.6 Hz, 2H), 7.47-7.45 (m, 1H), 7.42 (d, J = 7.6 Hz, 2H), 7.32 (d, J = 7.2 Hz, 2H), 7.16-7.12 (m, 6H), 6.90-6.80 (m, 2H), 4.77-4.74 (m, 1H), 4.57-4.46 (m, 3H), 2.36 (s, 3H), 2.30 (s, 3H), 1.10 (d, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 174.8, 163.7, 161.2, 156.7, 143.7, 143.2, 138.2, 137.3, 136.8, 134.6, 131.6, 129.2, 129.1, 129.0, 127.9, 127.7, 123.9, 116.6, 115.4, 115.1, 69.5, 57.8, 57.7, 21.4, 21.3, 16.9, 15.9; LC/MS (ESI−) m/z 683.2 [M − H]−; HRMS (ESI−) m/z calcd for C33H33FN2O9S2 [M − H]− 683.1539; found 683.1545. Rt: 4.55 min, HPLC purity: 100.0%.
2-(4-Fluorobenzyloxy)-5-methoxy-1,4-bis(4-methylphenylsulfonamido)benzene-N,N'-diacetic acid (12a).
Prepared as described in the experimental procedure of 7a from intermediate 35a to obtain the title compound as a beige solid (16 mg, 79%); 1H NMR (400 MHz, CDCl3) δ 7.51 (d, J = 8.0 Hz, 2H), 7.46 (d, J = 8.0 Hz, 2H), 7.28-7.24 (m, 2H), 7.15 (s, 1H), 7.12-7.08 (m, 2H), 7.01 (s, 1H), 6.97 (d, J = 7.2 Hz, 4H), 4.62 (s, 2H), 4.41 (s, 2H), 4.38 (s, 2H), 3.29 (s, 3H), 2.42 (s, 3H), 2.33 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.19, 170.15, 161.7 (JC,F = 241 Hz), 149.1, 147.8, 143.34, 143.25, 136.5, 132.4 (JC,F = 3 Hz), 129.30, 129.25, 129.2 (JC,F = 9 Hz), 127.7, 127.2, 127.0, 117.7, 116.8, 114.9 (JC,F = 21 Hz), 69.2, 55.6, 51.0, 21.0, 20.9; LC/MS (ESI−) m/z 685.1 [M − H]−; HRMS (ESI−) m/z calcd for C32H31FN2O10S2 [M − H]− 685.1331; found 685.1338. Rt: 4.47 min, HPLC purity: 97.2%.
2-(4-Fluorobenzyloxy)-6-methoxy-1,4-bis(4-methylphenylsulfonamido)benzene-N,N'-diacetic acid (12b).
Prepared as described in the experimental procedure of 7a from intermediate 35b to obtain the title compound as a white solid (35 mg, 37%); 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 8.0 Hz, 2H), 7.56 (d, J = 8.0 Hz, 2H), 7.29 (d, J = 8.0 Hz, 2H), 7.229-7.19 (m, 2H), 7.15 (d, J = 8.0 Hz, 2H), 7.04-7.00 (m, 2H), 6.56 (s, 1H), 6.41 (s, 1H), 4.85-4.76 (m, 2H), 4.43 (s, 2H), 4.21-4.10 (m, 2H), 3.51 (s, 3H), 2.44 (s, 3H), 2.36 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 172.6, 170.4, 162.8 (JC,F = 247 Hz), 157.7, 156.7, 144.6, 144.4, 142.3, 135.9, 135.5, 130.9 (JC,F = 2 Hz), 129.8, 129.6, 129.5, 128.1, 128.0, 116.2, 115.7 (JC,F = 21 Hz), 106.5, 105.4, 70.7, 56.4, 53.6, 52.1, 21.71, 21.66; LC/MS (ESI−) m/z 685.1 [M − H]−; HRMS (ESI−) m/z calcd for C32H31FN2O10S2 [M − H]− 685.1331; found 685.1327. Rt: 4.47 min, HPLC purity: 96.0%.
2,6-Bis(4-fluorobenzyloxy)-1,4-bis(4-methylphenylsulfonamido)benzene-N,N'-diacetic acid (12c).
Prepared as described in the experimental procedure of 7a from intermediate 35c to obtain the title compound as a white solid (25 mg, 45%); 1H NMR (400 MHz, CDCl3) δ 7.58 (d, J = 8.0 Hz, 2H), 7.52 (d, J = 8.4 Hz, 2H), 7.29-7.26 (m, 2H), 7.21-7.18 (m, 4H), 7.04-6.99 (m, 6H), 6.56 (s, 2H), 4.84-4.75 (m, 4H), 4.41 (s, 2H), 4.15 (s, 2H), 2.43 (s, 3H), 2.28 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.1, 162.9 (JC,F = 246 Hz), 156.8, 144.6, 144.5, 142.3, 135.7, 135.5, 130.7, 129.8, 129.6 (JC,F = 8 Hz), 128.1, 116.5, 115.8 (JC,F = 21 Hz), 106.5, 70.8, 52.2, 52.0, 21.8, 21.7; LC/MS (ESI−) m/z 779.2 [M − H]−; HRMS (ESI−) m/z calcd for C38H34F2N2O10S2 [M − H]− 779.1550; found 779.1545. Rt: 4.82 min, HPLC purity: 95.4%.
2-(4-Fluorobenzyloxy)-1,4-bis(4-methylphenylsulfonamido)naphthalene-N,N'-diacetic acid (12d).
Prepared as described in the experimental procedure of 7a from intermediate 35d to obtain the title compound as a white solid (38 mg, 77%); 1H NMR (400 MHz, DMSO-d6) δ 12.82 (brs, 2H, CO2H), 8.74-8.66 (m, 1H), 8.31-8.21 (m, 1H), 7.63 (d, J = 8.4 Hz, 1H), 7.57 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.0 Hz, 1H), 7.47-7.41 (m, 1H), 7.37-7.33 (m, 3H), 7.20-7.14 (m, 4H), 7.10 (d, J = 8.0 Hz, 1H), 7.03-6.99 (m, 1H), 6.97-6.69 (m, 1H), 6.96-6.93 (m, 1H), 4.56-3.79 (m, 4H), 4.40-4.35 (m, 1H), 4.19-4.08 (m, 1H), 2.33-2.25 (m, 6H); 13C NMR (100 MHz, MeOH-d4) δ 172.5, 172.0, 163.9 (JC,F = 243 Hz), 153.2, 153.0, 145.6, 145.5, 145.0, 139.4, 139.3, 137.8, 137.6, 137.2, 137.2, 136.7, 136.6, 133.2 (JC,F = 4 Hz), 130.6, 130.5 (JC,F = 10 Hz), 130.3 (JC,F = 9 Hz), 129.5, 129.2, 128.9, 127.9, 127.50, 127.45, 126.0, 125.9, 124.3, 118.5, 118.4, 116.2 (JC,F = 22 Hz), 116.1 (JC,F = 22 Hz), 70.9, 70.8, 54.1, 53.7, 21.7, 21.64, 21.57, 21.5; LC/MS (ESI+) m/z 707.3 [M + H]+; HRMS (ESI+) m/z calcd for C35H31FN2O9S2 [M + H]+ 707.1528; found 707.1540. Rt: 4.51 min, HPLC purity: 95.4%.
Fluorescence Polarization Competition Assay.
To determine the inhibitory potency of the Keap1-Nrf2 interaction, the FP assay was performed in a 384-well non-bonding surface (NBS) black plate (Corning, 3575). All FP assay data were recorded using a PerkinElmer Wallac Victor 3V microplate reader equipped with excitation (485 nm) and emission (535 nm) filters appropriate for the label detected. The assay buffer solution was 10 mM HEPES pH 7.4 buffer containing 3.4 mM EDTA, 150 mM NaCl, and 0.005% Tween-20. The fluorescent probe used for the FP assay was 9-mer Nrf2 ETGE motif derived peptide, FITC-LDEETGEFL-NH2. In each well, 10 μL of test compounds in 20 % DMSO/HEPES buffer were incubated with 10 μL of 400 nM Keap1 Kelch domain (final concentration, 100 nM) in HEPES assay buffer for 30 minutes at room temperature. Then 20 μL of 20 nM FITC- 9mer Nrf2 amide (final concentration, 10 nM) in the assay buffer was dispensed to each well and incubated again for 1 hour at room temperature. For an evaluation of % inhibition at a single concentration of 50, 5, 0.5 μM, 200, 20, 2 μM of samples in 20 % DMSO/HEPES buffer were prepared, respectively. For determination of IC50 value, compounds were prepared in a ten-point dilution with 20 % DMSO/HEPES buffer. All experiments were performed in triplicates, and all plates were centrifuged at 2500 rpm for 2 minutes at room temperature before and after incubation. Subsequently, the fluorescence polarization was determined by measuring the parallel and perpendicular fluorescence intensity (F║ and F┴) with respect to the linearly polarized excitation light. The % inhibition at each concentration of compounds was calculated according to the method previously reported by our group.58 The IC50 values were determined by fitting the % inhibition data to a four-parameter logistic fit using Sigma Plot 12.3 software.
Time-Resolved Fluorescence Resonance Energy Transfer Assay.
TR-FRET assay was performed in a 384-well non-bonding surface (NBS) white plate (Corning, 3574). All TR-FRET assay data were recorded on a PerkinElmer Wallac Victor 3V microplate reader using an excitation wavelength of 340 nm with filters detecting the fluorescent emission signals of terbium at 495 nm and fluorescein at 520 nm. The assay buffer solution was 10 mM HEPES pH 7.4 buffer containing 0.5 mM EDTA, 150 mM NaCl, and 0.005% Tween-20. Terbium labeled-anti-His antibody was purchased from Thermo Fisher Scientific. The fluorescent probe used for the TR-FRET assay was 9-mer Nrf2 ETGE motif derived peptide, FITC-LDEETGEFL-NH2. Test compounds were serially diluted in neat DMSO by a semi-log dilution (100 μM to 1 nM), and added to the assay well plate. His-tagged Keap1 Kelch domain (20 nM) and Terbium-anti-His antibody (2 nM) diluted with the assay buffer were mixed with a ratio of 1:1, and then pre-incubated for 30 minutes at room temperature. Ten microliters of a mixture of the protein (final, 5 nM) and the antibody (final, 0.5 nM) were dispensed to each well where 0.2 μL of compound (final, 1 μM to 0.01 nM) in DMSO was added in advance. After incubation for 30 minutes at room temperature, 9.8 μL of 51 nM FITC-9mer Nrf2 peptide amide (final, 25 nM) in the assay buffer was added and incubated again for 1 hour at room temperature. All experiments were performed in triplicates, and all plates were centrifuged at 2500 rpm for 2 minutes at room temperature before and after incubation. Subsequently, the TR-FRET fluorescence emission intensities at two different nanometers were measured using the microplate reader, and then their ratio was calculated according to the following equation: (intensity of 520nm)/(intensity of 495 nm) x 10,000. The IC50 values were determined by fitting the 520 nm/495 nm ratio data to a four-parameter logistic fit using Sigma Plot 12.3 software.
Cell culture and treatment
NCM460D cells were cultured in 300 μL RPMI supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were seeded at 75% confluency in three 48-well plates for ~90% confluency on the day of treatment. On the day of treatment, each well was treated with test compounds (100, 10, and 1 μM), 0.1% DMSO control a negative control, and positive controls 2a (100, 10, and 1 μM) and CDDO (100 and 10 nM), except for one plate with 1% DMSO replacing 1 μM compound 2. The well plate was swirled and mixed for 2-3 minutes, and then incubated for 24 hours at 37°C. Media was aspirated prior to collecting the treated NCM460D cells in 500 μL of Trizol.
RNA quantification, cDNA synthesis, and RT-qPCR
RNA was extracted from NCM460D cells in Trizol using chloroform-based methods and following the instructions from the RNeasy Micro Kit (Qiagen, catalog number 74004). For cDNA, 500 ng of RNA was measured by nanodrop and processed per the SuperScript III first-strand synthesis protocol provided by Invitrogen (Thermo Fisher Scientific, catalog number 18080051). RT-qPCR was conducted with the ABI PRISM 7900HT Sequence Detection System for SYBR green (Thermo Fisher Scientific, catalog number 4309155) and reaction mix containing a 0.5 μM final primer concentration.
Molecular docking simulation.
To analyze the interactions of designed compounds with the active site of Keap1 Kelch domain, molecular docking simulation was performed using the Autodock4.0 software.[62] The co-crystal structure of compound 2b bound to Keap1 protein was obtained from the RCSB protein data bank (4XMB).[61] For protein preparation, all water molecules were removed from the structure file, missing hydrogen atoms were added, and Kollman charges were calculated using Autodock tool. The ligand structures were built in MOE and prepared for docking using Autodock. The docking grid was centered on the observed binding pocket of the ligand, and the number of grid points in each direction was set to X, Y, Z = 44, 40, 40 with spacing of 0.375 Å resolution. The prepared ligands were docked into the binding sites of the Keap1 kelch domain. For a docking parameter file, Lamarckian genetic algorithm was used,[63] the number of GA runs was 100, and all other parameters were set to default values. The results of the docking experiments were reported in kcal/mol, and the docked conformations were ranked according to their binding energy. To validate the docking method, the prepared compound 2b was re-docked using the same method, and then compared with the corresponding X-ray crystal structure. As a result, the top-scoring docked pose was similar to the crystal structure of the ligand with a low RMSD value of 0.94 Å. This demonstrated that the docking method was reliable protocol that could be used for docking simulation of other compounds with the Keap1 Kelch domain.
Supplementary Material
ACKNOWLEDGMENT
We thank the Center for Integrative Proteomics Research (CIPR), Rutgers University for performing the HRMS experiments.
Funding Sources
This work was supported in part by the National Cancer Institute [R01CA133791] and the Rutgers TechAdvance Grant [TA2019-0300].
ABBREVIATIONS
- ACN
acetonitrile
- AcOH
acetic acid
- ARE
antioxidant response element
- BINAP
2,2′-bis (diphenylphosphino)-1,1′-binaphthalene
- BTB
broad-complex, Tramtrack and Bric-a-Brac
- CDDO-Me
bardoxolone methyl
- COPD
chronic obstructive pulmonary disease
- Cul3
cullin3
- DCM
dichloromethane
- DGR
double glycine repeat
- DMF
N,N-dimethylformamide
- DMSO
dimethyl sulfoxide
- FITC
fluorescein isothiocyanate
- FP
fluorescence polarization
- GCL
glutamate-cysteine ligase
- GCLM
glutamate-cysteine ligase modifier
- GPx
glutathione peroxidase
- GST
glutathione S-transferase
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HO-1
heme-oxygenase-1
- HRMS
high resolution mass spectrometry
- HTS
high throughput screening
- IVR
intervening region
- Keap1
Kelch-like ECH-associated protein 1
- LC-MS
liquid chromatography mass spectrometry
- Maf
musculoaponeurotic fibrosarcoma
- MD
molecular dynamics
- MM-GBSA
molecular mechanics generalized Born surface area
- MS
multiple sclerosis
- MsCl
mesyl chloride
- MW
molecular weight
- NBS
N-bromosuccinimide
- Neh
Nrf2-ECH homology
- NQO1
NAD(P)H:quinone oxidoreductase 1
- Nrf2
nuclear factor erythroid 2-related factor 2
- PDB
Protein Data Bank
- PK
pharmacokinetics
- PPI
protein–protein interaction
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SAR
structure–activity relationship
- SOD
superoxide dismutase
- TEA
triethylamine
- THF
tetrahydrofuran
- THIQ
tetrahydroisoquinoline
- TR-FRET
time-resolved fluorescence resonance energy transfer
- TRX
thioredoxin
Footnotes
- Synthetic procedure and characterization of intermediates
- Table S1 original data of activity in FP assay for final compounds
- 1H and 13C NMR spectra of final compounds
- UPLC analysis and purity spectra of final compounds
REFERENCES
- [1].Finkel T, Holbrook NJ, Oxidants, oxidative stress and the biology of ageing, Nature, 408 (2000) 239–247. [DOI] [PubMed] [Google Scholar]
- [2].He L, He T, Farrar S, Ji L, Liu T, Ma X, Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species, Cell. Physiol. Biochem, 44 (2017) 532–553. [DOI] [PubMed] [Google Scholar]
- [3].Sies H, Berndt C, Jones DP, Oxidative stress, Annu. Rev. Biochem, 86 (2017) 715–748. [DOI] [PubMed] [Google Scholar]
- [4].Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y.-i., An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements, Biochem. Biophys. Res. Commun, 236 (1997) 313–322. [DOI] [PubMed] [Google Scholar]
- [5].Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang M-I, Kobayashi A, Yamamoto M, Kensler TW, Talalay P, Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers, Proc. Natl. Acad. Sci. U. S. A, 101 (2004) 2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Venugopal R, Jaiswal AK, Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene, Proc. Natl. Acad. Sci. U. S. A, 93 (1996) 14960–14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, de Galarreta CMR, Cuadrado A, Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol, J. Biol. Chem, 279 (2004) 8919–8929. [DOI] [PubMed] [Google Scholar]
- [8].Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, Wolf CR, Yamamoto M, The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin, Biochem. Soc. Trans, 28 (2000) 33–41. [DOI] [PubMed] [Google Scholar]
- [9].Lee S, Hu L, Nrf2 activation through the inhibition of Keap1–Nrf2 protein–protein interaction, Med. Chem. Res, 29 (2020) 846–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nguyen T, Nioi P, Pickett CB, The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress, J. Biol. Chem, 284 (2009) 13291–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, Cadenas S, Lamas S, Antioxidant responses and cellular adjustments to oxidative stress, Redox Biol., 6 (2015) 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Canning P, Sorrell FJ, Bullock AN, Structural basis of Keap1 interactions with Nrf2, Free Radical Biol. Med, 88 (2015) 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA, The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase, Mol. Cell. Biol, 24 (2004) 8477–8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Canning P, Cooper CDO, Krojer T, Murray JW, Pike ACW, Chaikuad A, Keates T, Thangaratnarajah C, Hojzan V, Marsden BD, Gileadi O, Knapp S, von Delft F, Bullock AN, Structural basis for Cul3 protein assembly with the BTB-Kelch family of E3 ubiquitin ligases, J. Biol. Chem, 288 (2013) 7803–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang DD, Hannink M, Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress, Mol. Cell. Biol, 23 (2003) 8137–8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P, Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants, Proc. Natl. Acad. Sci. U. S. A, 99 (2002) 11908–11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang DD, Lo S-C, Cross JV, Templeton DJ, Hannink M, Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex, Mol. Cell. Biol, 24 (2004) 10941–10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Baird L, Dinkova-Kostova AT, Diffusion dynamics of the Keap1–Cullin3 interaction in single live cells, Biochem. Biophys. Res. Commun, 433 (2013) 58–65. [DOI] [PubMed] [Google Scholar]
- [19].Rachakonda G, Xiong Y, Sekhar KR, Stamer SL, Liebler DC, Freeman ML, Covalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3, Chem. Res. Toxicol, 21 (2008) 705–710. [DOI] [PubMed] [Google Scholar]
- [20].Tong KI, Padmanabhan B, Kobayashi A, Shang C, Hirotsu Y, Yokoyama S, Yamamoto M, Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response, Mol. Cell. Biol, 27 (2007) 7511–7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tong KI, Kobayashi A, Katsuoka F, Yamamoto M, Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism, Biol. Chem, 387 (2006) 1311–1320. [DOI] [PubMed] [Google Scholar]
- [22].Baird L, Swift S, Llères D, Dinkova-Kostova AT, Monitoring Keap1–Nrf2 interactions in single live cells, Biotechnol. Adv, 32 (2014) 1133–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kensler TW, Wakabayashi N, Biswal S, Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway, Annu. Rev. Pharmacol. Toxicol, 47 (2007) 89–116. [DOI] [PubMed] [Google Scholar]
- [24].Kobayashi A, Kang M-I, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M, Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1, Mol. Cell. Biol , 26 (2006) 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li W, Yu S, Liu T, Kim J-H, Blank V, Li H, Kong ANT, Heterodimerization with small Maf proteins enhances nuclear retention of Nrf2 via masking the NESzip motif, Biochim. Biophys. Acta, 1783 (2008) 1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Niture SK, Kaspar JW, Shen J, Jaiswal AK, Nrf2 signaling and cell survival, Toxicol. Appl. Pharmacol , 244 (2010) 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K, Redox- and non-redox-metal-induced formation of free radicals and their role in human disease, Arch Toxicol., 90 (2016) 1–37. [DOI] [PubMed] [Google Scholar]
- [28].Magesh S, Chen Y, Hu L, Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents, Med. Res. Rev, 32 (2012) 687–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Boutten A, Goven D, Artaud-Macari E, Boczkowski J, Bonay M, NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease, Trends Mol. Med, 17 (2011) 363–371. [DOI] [PubMed] [Google Scholar]
- [30].Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, Williamson TP, Kraft AD, Lee J-M, Li J, Johnson JA, The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease, Antioxid. Redox Signaling, 11 (2008) 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, Yang M, Raghupathi K, Novas M, Sweetser MT, Viglietta V, Dawson KT, Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis, N. Engl. J. Med, 367 (2012) 1087–1097. [DOI] [PubMed] [Google Scholar]
- [32].Keum Y-S, Jeong W-S, Tony Kong AN, Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms, Mutat. Res, 555 (2004) 191–202. [DOI] [PubMed] [Google Scholar]
- [33].Abed DA, Goldstein M, Albanyan H, Jin H, Hu L, Discovery of direct inhibitors of Keap1–Nrf2 protein–protein interaction as potential therapeutic and preventive agents, Acta Pharm. Sin. B, 5 (2015) 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG, Bardoxolone methyl and kidney function in CKD with type 2 diabetes, N. Engl. J. Med, 365 (2011) 327–336. [DOI] [PubMed] [Google Scholar]
- [35].Linker RA, Lee D-H, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Brück W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, Gold R, Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway, Brain, 134 (2011) 678–692. [DOI] [PubMed] [Google Scholar]
- [36].Hu C, Eggler AL, Mesecar AD, van Breemen RB, Modification of Keap1 cysteine residues by sulforaphane, Chem. Res. Toxicol , 24 (2011) 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].De Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease, N. Engl. J. Med, 369 (2013) 2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M, Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model, Mol. Cell. Biol, 26 (2006) 2887–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pallesen JS, Tran KT, Bach A, Non-covalent small-molecule Kelch-like ECH-associated protein 1–nuclear factor erythroid 2-related factor 2 (Keap1–Nrf2) inhibitors and their potential for targeting central nervous system diseases, J. Med. Chem, 61 (2018) 8088–8103. [DOI] [PubMed] [Google Scholar]
- [40].Hu L, Magesh S, Chen L, Wang L, Lewis TA, Chen Y, Khodier C, Inoyama D, Beamer LJ, Emge TJ, Shen J, Kerrigan JE, Kong AN, Dandapani S, Palmer M, Schreiber SL, Munoz B, Discovery of a small-molecule inhibitor and cellular probe of Keap1-Nrf2 protein-protein interaction, Bioorg. Med. Chem. Lett, 23 (2013) 3039–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jiang ZY, Lu MC, Xu LL, Yang TT, Xi MY, Xu XL, Guo XK, Zhang XJ, You QD, Sun HP, Discovery of potent Keap1-Nrf2 protein-protein interaction inhibitor based on molecular binding determinants analysis, J. Med. Chem, 57 (2014) 2736–2745. [DOI] [PubMed] [Google Scholar]
- [42].Jiang Z-Y, Xu LL, Lu M-C, Chen Z-Y, Yuan Z-W, Xu X-L, Guo X-K, Zhang X-J, Sun H-P, You Q-D, Structure–activity and structure–property relationship and exploratory in vivo evaluation of the nanomolar Keap1–Nrf2 protein–protein interaction inhibitor, J. Med. Chem, 58 (2015) 6410–6421. [DOI] [PubMed] [Google Scholar]
- [43].Abed DA, Lee S, Wen X, Ali AR, Mangipudy V, Aleksunes LM, Hu L, Optimization of 1,4-bis(arylsulfonamido)naphthalene-N,N'-diacetic acids as inhibitors of Keap1-Nrf2 protein-protein interaction to suppress neuroinflammation, Bioorg. Med. Chem, 44 (2021) 116300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Davies TG, Wixted WE, Coyle JE, Griffiths-Jones C, Hearn K, McMenamin R, Norton D, Rich SJ, Richardson C, Saxty G, Willems HMG, Woolford AJA, Cottom JE, Kou J-P, Yonchuk JG, Feldser HG, Sanchez Y, Foley JP, Bolognese BJ, Logan G, Podolin PL, Yan H, Callahan JF, Heightman TD, Kerns JK, Monoacidic inhibitors of the Kelch-like ECH-associated protein 1: nuclear factor erythroid 2-related factor 2 (KEAP1:NRF2) protein–protein interaction with high cell potency identified by fragment-based discovery, J. Med. Chem, 59 (2016) 3991–4006. [DOI] [PubMed] [Google Scholar]
- [45].Zhuang C, Narayanapillai S, Zhang W, Sham YY, Xing C, Rapid identification of Keap1–Nrf2 small-molecule inhibitors through structure-based virtual screening and Hit-based substructure search, J. Med. Chem, 57 (2014) 1121–1126. [DOI] [PubMed] [Google Scholar]
- [46].Lu M-C, Shao H-L, Liu T, You Q-D, Jiang Z-Y, Discovery of 2-oxy-2-phenylacetic acid substituted naphthalene sulfonamide derivatives as potent KEAP1-NRF2 protein-protein interaction inhibitors for inflammatory conditions, Eur. J. Med. Chem, 207 (2020) 112734. [DOI] [PubMed] [Google Scholar]
- [47].Abed DA, Lee S, Hu L, Discovery of disubstituted xylylene derivatives as small molecule direct inhibitors of Keap1-Nrf2 protein-protein interaction, Bioorg. Med. Chem, 28 (2020) 115343. [DOI] [PubMed] [Google Scholar]
- [48].Jiang Z-Y, Xu L-L, Lu M-C, Pan Y, Huang H-Z, Zhang X-J, Sun H-P, You Q-D, Investigation of the intermolecular recognition mechanism between the E3 ubiquitin ligase Keap1 and substrate based on multiple substrates analysis, J. Comput. Aided Mol. Des, 28 (2014) 1233–1245. [DOI] [PubMed] [Google Scholar]
- [49].Lu M-C, Chen Z-Y, Wang Y-L, Jiang Y-L, Yuan Z-W, You Q-D, Jiang Z-Y, Binding thermodynamics and kinetics guided optimization of potent Keap1–Nrf2 peptide inhibitors, RSC Adv., 5 (2015) 85983–85987. [Google Scholar]
- [50].Zhuang C, Wu Z, Xing C, Miao Z, Small molecules inhibiting Keap1–Nrf2 protein–protein interactions: a novel approach to activate Nrf2 function, MedChemComm, 8 (2017) 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Meng N, Tang H, Zhang H, Jiang C, Su L, Min X, Zhang W, Zhang H, Miao Z, Zhang W, Zhuang C, Fragment-growing guided design of Keap1-Nrf2 protein-protein interaction inhibitors for targeting myocarditis, Free Radical Biol. Med, 117 (2018) 228–237. [DOI] [PubMed] [Google Scholar]
- [52].Zhong M, Lynch A, Muellers SN, Jehle S, Luo L, Hall DR, Iwase R, Carolan JP, Egbert M, Wakefield A, Streu K, Harvey CM, Ortet PC, Kozakov D, Vajda S, Allen KN, Whitty A, Interaction Energetics and Druggability of the Protein–Protein Interaction between Kelch-like ECH-Associated Protein 1 (KEAP1) and Nuclear Factor Erythroid 2 Like 2 (Nrf2), Biochemistry, 59 (2020) 563–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Suryadevara PK, Olepu S, Lockman JW, Ohkanda J, Karimi M, Verlinde CLMJ, Kraus JM, Schoepe J, Van Voorhis WC, Hamilton AD, Buckner FS, Gelb MH, Structurally simple inhibitors of lanosterol 14α-demethylase are efficacious in a rodent model of acute Chagas disease, J. Med. Chem, 52 (2009) 3703–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Seon Jung H, Yun T, Cho Y, Bae Jeon H, Simple and convenient copper-catalyzed amination of aryl halides to primary arylamines using NH4OH, Tetrahedron, 72 (2016) 5988–5993. [Google Scholar]
- [55].Wang X, Xu Y, Mo F, Ji G, Qiu D, Feng J, Ye Y, Zhang S, Zhang Y, Wang J, Silver-mediated trifluoromethylation of aryldiazonium salts: conversion of amino group into trifluoromethyl group, J. Am. Chem. Soc, 135 (2013) 10330–10333. [DOI] [PubMed] [Google Scholar]
- [56].Yasuda D, Nakajima M, Yuasa A, Obata R, Takahashi K, Ohe T, Ichimura Y, Komatsu M, Yamamoto M, Imamura R, Synthesis of Keap1-phosphorylated p62 and Keap1-Nrf2 protein-protein interaction inhibitors and their inhibitory activity, Bioorg. Med. Chem. Lett, 26 (2016) 5956–5959. [DOI] [PubMed] [Google Scholar]
- [57].Niu J, Guo P, Kang J, Li Z, Xu J, Hu S, Copper(I)-catalyzed aryl bromides to form intermolecular and intramolecular carbon–oxygen bonds, J. Org. Chem, 74 (2009) 5075–5078. [DOI] [PubMed] [Google Scholar]
- [58].Inoyama D, Chen Y, Huang X, Beamer LJ, Kong A-NT, Hu L, Optimization of fluorescently labeled Nrf2 peptide probes and the development of a fluorescence polarization assay for the discovery of inhibitors of Keap1-Nrf2 interaction, J. Biomol. Screen, 17 (2012) 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lu M-C, Tan S-J, Ji J-A, Chen Z-Y, Yuan Z-W, You Q-D, Jiang Z-Y, Polar recognition group study of Keap1-Nrf2 protein–protein interaction inhibitors, ACS Med. Chem. Lett, 7 (2016) 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lee S, Abed DA, Beamer LJ, Hu L, Development of a Homogeneous Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) Assay for the Inhibition of Keap1–Nrf2 Protein–Protein Interaction, SLAS Discov., 26 (2021) 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jain AD, Potteti H, Richardson BG, Kingsley L, Luciano JP, Ryuzoji AF, Lee H, Krunic A, Mesecar AD, Reddy SP, Moore TW, Probing the structural requirements of non-electrophilic naphthalene-based Nrf2 activators, Eur. J. Med. Chem, 103 (2015) 252–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ, AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility, J. Comput. Chem, 30 (2009) 2785–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ, Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function, J. Comput. Chem, 19 (1998) 1639–1662. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.