Abstract
Background:
Despite being a preventable and treatable disease, tuberculosis (TB) is a leading cause of death among young people globally. Each year, an estimated 1.8 million adolescents and young adults (AYAs; 10–24 years old) develop TB. In 2019, an estimated 161,000 AYAs died of the disease. AYAs have unique developmental, psychosocial, and healthcare needs, but these needs have been neglected in both TB care and research agendas. In order to improve outcomes in this age group, the specific needs of AYAs must be considered and addressed.
Methods:
Through a consensus process, an international panel of 34 clinicians, researchers, TB survivors, and advocates with expertise in child/adolescent TB and/or adolescent health proposed interventions for optimizing AYA engagement in TB care. The process consisted of reviewing the literature on TB in AYAs; identifying and discussing priority areas; and drafting and revising proposed interventions until consensus, defined a priori, was reached.
Results:
The panel acknowledged the dearth of evidence on best practices for identifying and managing AYAs with TB. The final consensus statement, based on expert opinion, proposes nine interventions to reform current practices that may harm AYA health and well-being, and nine interventions to establish high-quality AYA-centered TB services.
Conclusion:
AYA-specific interventions for TB care and research are critical for improving outcomes in this age group. In the absence of evidence on best practices, this consensus statement from an international group of experts can help address the needs of AYA with TB or at risk for TB.
INTRODUCTION
Each year, an estimated 1.8 million adolescents (aged 10–19 years) and young adults (aged 20–24 years) become sick with tuberculosis (TB), representing approximately 18% of the annual global TB incidence.1,2 Although it is preventable and treatable, TB is a leading cause of death among adolescents and young adults (AYAs) globally.3,4 The World Health Organization (WHO) estimates that in 2019, 71,000 adolescents (11,000 between the ages of 10–14 years and 60,000 between the ages of 15–19 years) and 90,000 young adults died of TB (Figure 1).5,6 TB is a leading cause of hospitalization and mortality among people with HIV, including AYAs.7–9
Figure 1: Tuberculosis Mortality among Adolescents by Sex and World Health Organization Regions, 2019*5.
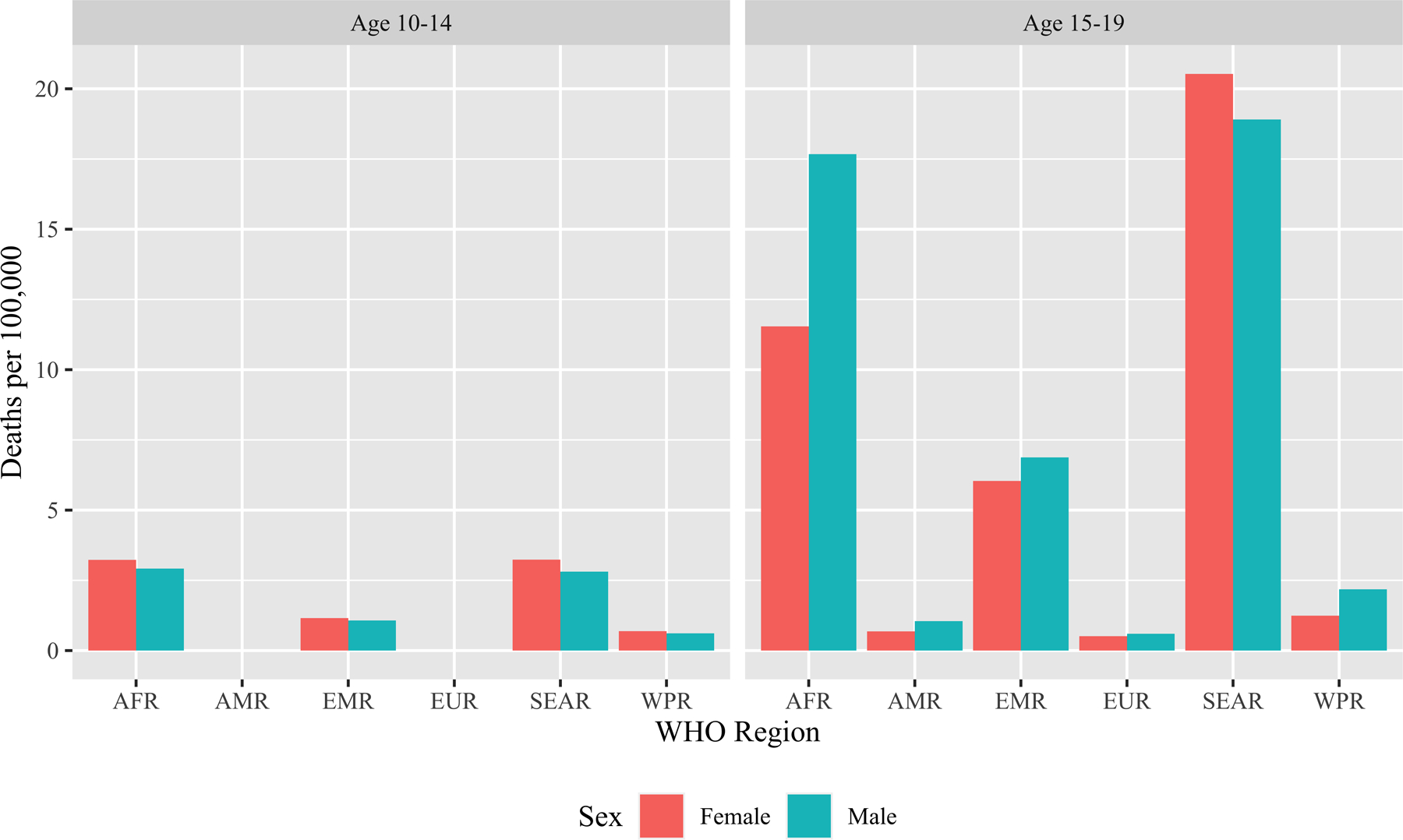
*Data not available for adolescents 10–14 years old in the Region of the Americas or for the European Region.
Abbreviations: AFR, African Region; AMR, Region of the Americas; EMR, Eastern Mediterranean Region; EUR, European Region; SEAR, South-East Asian Region; WHO, World Health Organization; WPR, Western Pacific Region.
AYAs face unique challenges with respect to TB and TB care. The risks of Mycobacterium tuberculosis infection and progression to TB disease increase during this period.10,11 Females are at greater risk for TB than males during early adolescence; risk among males increases during late adolescence.10 Risk for TB progression is exacerbated by HIV infection, which is a substantial concern in this age group. In 2021, approximately 28% of new HIV infections worldwide occurred in individuals between 15–24 years of age; moreover, AYAs experience worse outcomes in the HIV care cascade as compared to other age groups.12–14 Among AYAs with TB, those who are living with HIV, living in conditions of extreme poverty and/or violence, and/or were previously treated for TB disease are at risk for poor adherence to TB treatment and loss to follow-up.15–20
Between the ages of 10–24 years, individuals undergo rapid growth and development; acquire the physical, cognitive, emotional, and social resources required for achieving health and well-being in adulthood; and become more autonomous and independent of caregivers.21,22 TB illness and treatment impact these transitions, and these transitions, in turn, shape how AYAs experience TB illness and treatment.
The WHO and other institutions have highlighted the need for healthcare services and research to address the specific needs of AYAs.22–26 However, policies and practices by most national TB programs (NTPs) do not currently account for AYA-specific needs and considerations.27–29 In 2021, to inform the update of the WHO guidelines and operational handbook for the management of TB in children and adolescents,30,31 the WHO commissioned an evidence review to answer the following background question: How can adolescents with TB or eligible for TB preventive treatment (TPT) be optimally engaged in care? Given the dearth of evidence on best practices in this area,2,27,29 we convened an international expert panel to generate a consensus statement regarding needed interventions to optimize TB care for this age group.
METHODS
Background Review
To inform the consensus process, we conducted a background review evaluating published and unpublished data on the impact of TB and its treatment on five domains of adolescent well-being: (1) good health; (2) connectedness and contribution to society; (3) safety and a supportive environment; (4) learning, competence, education, skills, and employability; and (5) agency and resilience.29,32
Composition of the International Expert Panel
Authors SSC, PMW, LAE, and staff from the WHO’s Global TB Programme invited individuals from the following groups to participate in an international expert panel: clinicians who treat adolescents with TB; researchers with expertise in adolescent TB and/or adolescent health; adolescent/youth advocates; and survivors of TB illness during adolescence. We aimed to include panelists from diverse settings, including all six WHO regions. Invitations were issued via e-mail.
All invitees agreed to participate with the exception of two individuals from the Eastern Mediterranean Region, who did not respond to the invitations. The thirty-four participants identified themselves as one or more of the following: researchers (n=26), clinicians (n=19), advocates (n=10), and/or TB survivors (n=4). Panelists were from 16 countries, and reported working in adolescent TB, working in adolescent health, and/or receiving TB treatment in 36 countries (Figure 2). Those working as researchers, clinicians, and/or advocates reported a median of 10 (interquartile range: 7–14) years’ experience.
Figure 2: Countries Where Panelists Reside, Have Worked, and/or Have Received Tuberculosis Treatment*.
*Many participants have worked in more than one country.
Consensus Process
We convened two meetings of the international expert panel; the first occurred on May 17, 2021, and the second, on June 3, 2021. Both meetings were held virtually using the Zoom (Zoom Video Communications, San Jose, USA) platform. Meetings were conducted in English with simultaneous interpretation in Spanish and in Russian.
Ahead of the first meeting, we emailed a draft of the background review to all panelists.29 In addition, using a structured, open-ended survey on Google Forms (Google LLC, Mountain View, USA), we asked panelists to propose interventions to improve the screening, diagnosis, and treatment of M. tuberculosis infection and TB disease in adolescents. Interventions were specifically sought for implementation at each of the levels of the health facility, the community, or in national policy.
During the first meeting, we summarized findings from the background review. We then divided into two groups to discuss and begin to prioritize proposed interventions for (1) screening and diagnosis, and (2) treatment. Based on these discussions, SSC, PMW, and LAE drafted a set of proposed interventions, which were shared with all panelists by e-mail. Through a second Google Forms survey, panelists were asked to provide feedback on each proposed intervention. SSC, PMW, and LAE further revised the interventions based on this feedback.
During the second meeting of the international expert panel, using the anonymous polling feature on Zoom, each panelist voted to approve, approve with modifications, or reject each proposed intervention. We had established an a priori requirement of ≥80% approval to include each intervention in the consensus statement. We discussed all suggested modifications until we reached consensus. The final consensus statement was emailed to all panelists for endorsement.
RESULTS
Target Age Group
Although the WHO defines adolescents as individuals between the ages of 10–19 years,23 panelists overwhelmingly voted to expand the age range for this consensus statement to include young adults (20–24 years old). As detailed elsewhere,21 the physical and social transitions of adolescence – including brain development, completion of education, and independence from caregivers – often extend beyond 19 years of age; therefore, this expanded age range better reflects the group that would benefit from the proposed interventions.
Current Practices in Need of Reform
Part 1 of the consensus statement (Table 1) proposes nine interventions to reform current practices that are detrimental to the well-being of AYAs. A key obstacle to addressing TB in AYAs is that TB programs have traditionally reported data for children <15 years of age and adults ≥15 years of age. Although a modeling approach has been used to estimate the global incidence of TB in AYAs,1 the lack of epidemiologic data makes it challenging to measure the setting-specific public health impact of TB in this age group. Moreover, grouping younger AYAs with children and older AYAs with adults prevents examination of the unique clinical, developmental, and psychosocial needs of AYAs with TB. Given the age-related differences in TB presentation, diagnosis, and treatment across pediatric and AYA age groups,10 and building on the 2019 WHO request that NTPs with electronic case-based reporting systems stratify case notifications by five-year age bands for individuals <25 years old,33 the expert panel emphasized the critical need for data disaggregated by age and, ideally, further disaggregated by sex.
Table 1:
Proposed interventions to address needs of adolescents and young adults (AYAs) with or at risk of tuberculosis (TB), Part I
| Reforming current practices to improve AYA well-being. |
|
NTPs generally do not recognize AYAs as a priority group for active TB case-finding, contact tracing, provision of TPT, or treatment of TB disease.27 Yet, as consistently demonstrated by data from the last century, the risk of progression from TB infection to disease increases throughout adolescence and young adulthood.10,34–36 Moreover, the risk of primary infection and/or reinfection rises, likely due to AYAs’ increased social contacts and higher risk of transmitting M. tuberculosis, in comparison to both younger and older age groups.37–43 Further, AYAs are often parents or caregivers to young children, who are vulnerable to rapidly developing life-threatening forms of TB when they become infected in their households.44 Because of these increased risks to both individual and public health, AYAs should be prioritized in TB diagnosis, treatment, and prevention.
In some settings, TB treatment is delivered via facility-based treatment support (historically referred to as directly observed treatment, or DOT).45 However, schedule conflicts between facility hours and school, vocational training, and work can result in AYAs missing classes, work, and/or treatment doses. Facility-based treatment support creates additional treatment barriers, including transportation costs and wait times, that further contribute to inadequate treatment and/or loss to follow-up. This treatment delivery approach also adds to the burden of caregivers, who may face further barriers to accompany younger AYAs to the health facility, such as missed work and/or loss of income. Furthermore, facilities’ separate, labeled entrances or treatment areas for TB services can result in disclosure of TB status. Not only does this facility layout violate patients’ right to privacy, it also contributes to both anticipated and enacted stigma, as AYAs fear being seen engaging in TB services and can suffer discrimination from this disclosure.15,28,46 For these reasons, the expert panel states that family-oriented, community-based models of care should replace facility-based treatment support for AYAs. Within developmentally appropriate treatment models, treatment support may be delivered in a context-specific manner by community health workers, peer treatment supporters, and/or using digital adherence technologies such as video-supported treatment. Alternatively, medication administration by a family member or another trusted adult who is trained and supported by health providers may be considered for select AYAs.
To prevent TB transmission, AYAs with pulmonary TB are required to isolate at the beginning of treatment. The criteria for ending isolation vary between settings. According to the widely accepted “two-week rule,” individuals with TB are released from isolation after two weeks if they are clinically improving and adherent to and tolerating treatment.30 The “two-week rule” is not based in scientific evidence; in fact, multiple human-to-guinea pig studies suggest that in most cases, infectiousness ceases within a few days of effective therapy.47–49 However, in other settings, individuals with TB may be required to isolate for longer periods. For instance, in Lima, Peru, health providers instruct AYAs to isolate at home for two months, and in former Soviet countries, AYAs are routinely hospitalized for the full treatment course.50–52 Additionally, general isolation guidelines may differ from school policies with respect to when students with TB are permitted to return to class. For example, while China’s national TB guidelines are consistent with the “two-week rule” for isolation, many schools will not permit students with TB to return until they complete their entire course of treatment.53 Similarly, only 17 of 28 (61%) European NTPs that participated in a survey about adolescent TB allowed adolescents with non-infectious TB to return to school.27 Adolescence and young adulthood are critical periods for cognitive, social, and psychological development. As shown from data across international settings, prolonged school absence and social isolation lead to the loss of interpersonal relationships and educational setbacks, reinforce stigma, and contribute to mental health challenges. These problems hinder AYAs’ ability to accomplish the developmental tasks, educational attainment, and skills needed for their future health and livelihoods.29,50–52,54–56 Therefore, the expert panel states that isolation policies should be implemented only on the basis of evidence for infectiousness, and that AYAs should be encouraged to return to school, vocational training, or work as soon as they are no longer infectious, have demonstrated adherence, and have appropriate support and treatment adherence structures are in place.30
When prescribing TB regimens, clinicians do not consistently consider or address adverse drug effects that are particularly harmful to AYAs. Because rifampicin and other rifamycins interfere with hormonal contraception, clinicians should explore with AYAs the need for alternative forms of contraception. This issue is particularly important given that TB disease in pregnancy is associated with increased risks of obstetric complications, low birthweight, and infant death.57,58 Skin discoloration associated with clofazimine—a frequent and effective component of regimens for multidrug-resistant TB (MDR-TB)—is distressing for AYAs. In settings with high MDR-TB prevalence, clofazimine-related skin discoloration is commonly recognized as a sign of MDR-TB treatment; as a result, it may be stigmatizing and may have implications for social relationships, such as described impacts on potential marriage for young women in India.54 Healthcare providers should counsel AYAs and their family members about this adverse effect, and provide reassurance that skin discoloration resolves soon after clofazimine discontinuation. The irreversible sensorineural hearing loss associated with injectable agents (amikacin, streptomycin, kanamycin, and capreomycin), which are still used to treat MDR-TB, is devastating for AYAs, many of whom are in school or vocational training or entering the workforce and can be expected to have decades of healthy life ahead of them. Moreover, facility-based administration of injectable agents interrupts schooling, vocational training, and work. Therefore, injectable agents should be particularly avoided for AYAs, unless absolutely needed as part of a salvage regimen when there are no alternative options.
Finally, younger AYAs are often excluded from TB research, either given ethical concerns, logistical barriers for obtaining consent from the parent/guardian and assent from the adolescent, and/or preference among some researchers for enrolling only participants with microbiologically confirmed TB (as few pre-pubescent adolescents have microbiologically confirmed TB).59 Some studies do not enroll individuals <18 years because they cannot consent to participation themselves, while others restrict enrollment to “adults” and exclude individuals <15 years. Even when studies that are primarily focused on adults include AYAs <18 years, they often enroll few participants in this age group, resulting in limited power for any age-stratified analysis; consequently, clinicians may feel uncomfortable applying study findings to the management of individual adolescents.60 Exclusion from research hinders or, at a minimum, delays the ability of younger AYAs to access and benefit from advances in TB diagnostics and treatment regimens. For example, the BPaLM (bedaquiline, pretomanid, linezolid, moxifloxacin) and BPaL (bedaquiline, pretomanid, linezolid) regimens for MDR-TB are not recommended for individuals <15 years old.61 Yet, because these regimens are shorter, all-oral, and consist of fewer drugs, they enable AYAs to complete treatment with fewer missed doses and return more promptly to their education, work, and social lives. The expert panel concludes that AYAs—especially those under the age of 18 years—should be prioritized in TB research, including clinical trials and observational studies of new regimens. The panel also proposes that AYAs aged 10–24 years receive the shortest effective TB treatment recommended by WHO, given their particular challenges for treatment adherence and the negative impact of TB treatment on their education and psychosocial development.29
Development and Implementation of High-Quality AYA-Centered TB Services
AYAs experience many barriers to accessing health services, including conflicts between clinic hours and classes/work; concerns about privacy and confidentiality; anticipation of being judged and/or treated disrespectfully by providers; lack of services tailored to their specific clinical and developmental needs; limited health literacy; and challenges navigating care independently. To address these and other challenges, the WHO—informed by analyses of existing studies and policies, as well as by surveys of 735 primary care providers in 81 countries and 1143 adolescents in 104 countries—has established five principles and eight standards for quality health services for adolescents (Table 2).23,24
Table 2:
Summary of the World Health Organization’s Principles and Standards of Adolescent-Friendly Health Care
| Principles23 |
|---|
| 1. Accessibility: adolescents are able to obtain health services (e.g., convenient operating hours, transportation costs and time not prohibitive) |
| 2. Acceptability: adolescents are willing to obtain health services (e.g., confidentiality, privacy, non-judgmental providers, appealing care environment) |
| 3. Equity: all adolescents, regardless of social standing, are able to obtain the available health services |
| 4. Appropriateness: the health services that adolescents need are the ones that are provided, either directly or through referral |
| 5. Effectiveness: the appropriate services are provided in a way as to make a positive contribution to adolescents’ health (e.g., providers trained to provide care for AYAs, evidence-based protocols and guidelines) |
| Standards24 |
1. Health literacy
|
2. Community support
|
3. Appropriate package of services
|
4. Providers’ competencies
|
5. Facility characteristics
|
6. Equity and non-discrimination:
|
7. Data and quality improvement:
|
8. Adolescents’ participation:
|
To our knowledge, no published studies have evaluated the impact of AYA-specific interventions on TB outcomes. Multiple studies, however, highlight needs for AYA-centered TB care and key sources of support for AYA. In qualitative studies from the Russian Federation and Ukraine, adolescents hospitalized for TB treatment described the emotional and psychological benefit of friendships with each other during an otherwise profoundly isolating and distressing experience.51,52 The study from the Russian Federation further emphasized the importance of providers’ attentiveness to both the medical and emotional challenges faced by AYAs receiving inpatient treatment for TB.51 Similarly, adolescents with drug-resistant TB in India recommended that TB care include peer support platforms and improved provider-patient communication.54 In another qualitative study, health providers in Botswana identified unmet needs for achieving AYA-friendly TB services.28,46 Potential interventions included establishing peer support programs; providing community-based (rather than facility-based) treatment support; offering clinic hours that do not conflict with school or work; shortening clinic wait times; addressing TB-related stigma; providing more support for adherence and psychosocial needs, particularly for AYAs from socially vulnerable families; and training healthcare workers on how to provide high-quality care to AYAs.28 Current gaps in the integration of TB services with other health services for AYAs may also contribute to inadequate care, as AYA health needs—including those related to mental health and sexual and reproductive health—may not be fully managed within current non-differentiated models of TB care.29
Despite the absence of data on AYA-targeted interventions to improve TB outcomes, evidence-based interventions for other AYA health priorities serve as a guide for the development of AYA-friendly TB services. For example, peer-support interventions have been associated with improved AYA well-being, including decreased internalized stigma, HIV care retention, and adherence to antiretroviral therapy among AYAs living with HIV.62–66 Other adolescent-centered HIV care interventions—including adolescent-friendly HIV treatment clinics and family-based economic empowerment interventions—positively impact AYA retention in HIV services and HIV viral suppression.13,67–69 While efforts to scale-up and evaluate support for AYAs in HIV programs are in progress, the benefits for AYA outcomes are becoming clear.13,62–69
Consistent with current evidence and the principles and standards for quality health services for AYAs outlined by the WHO, the expert panel proposed that NTPs develop plans to provide setting-specific AYA-friendly services (Table 3). The development of these plans should be overseen by a committee of AYAs who have been treated for TB and their families, youth advocates, experts in AYA health services, and experts in TB care. The committee should be actively involved in assessing current gaps and barriers to delivering quality AYA health services within TB programs. Plans should be informed by AYA-disaggregated TB data and indicators, as well as by existing evidence and frameworks for AYA-friendly models of care for HIV, sexual and reproductive health, and other health conditions. Furthermore, the implementation of AYA-centered services should be monitored and disclosed as part of national reporting.
Table 3:
Proposed interventions to address needs of adolescents and young adults (AYAs) with or at risk of tuberculosis (TB), Part II
| The following setting-specific components can be included in national plans to develop and provide AYA-centered TB services |
|---|
|
CONCLUSION
Despite being a preventable and treatable disease, TB kills approximately 161,000 AYAs each year.5,6 To improve these outcomes—and to minimize the negative consequences of TB and its treatment on AYA well-being29—NTPs and clinicians who provide TB care must consider and address the specific needs of AYAs. This consensus statement on improving care for AYAs with TB or at risk of TB outlines the steps needed to reform current practices that are harmful to AYAs, and to develop high-quality AYA-friendly TB services across the globe. This work represents a milestone for addressing AYA needs in TB care and mapping the ways forward to optimize AYA treatment outcomes and well-being. As such, this work contributed to the WHO’s operational handbook on the management of TB in children and adolescents.30
Informed by this expert consensus statement, the global TB community can ensure that TB prevention, diagnosis, and treatment are optimized for this age group, with full consideration of AYAs’ development and well-being. These reforms are needed to address the needs of individuals in this vulnerable age group in order to achieve improved TB outcomes—and ultimately, to help end the global TB epidemic.
ACKNOWLEDGEMENTS
We thank Kerri Viney, Sabine Verkuijl, Annemieke Brands, Tiziana Masini, and Farai Mavhunga of the Global Tuberculosis Programme of the World Health Organization; without their support, this work would not have been possible. We are grateful to Lily Meyersohn for organizing and supporting the consensus process and to our translators, Jean Pierre Seminario, Alexandra Nigay, and Nataliya Petrova.
FUNDING and DISCLAIMERS
This work was funded by the Global Tuberculosis Programme, World Health Organization, Geneva, Switzerland. Dr. Chiang is supported by the Fogarty International Center at the U.S. National Institutes of Health (NIH) (5K01TW010829). Dr. Enane is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the NIH (5K23HD095778) and by the East Africa International Epidemiology Databases to Evaluate AIDS (IeDEA) regional consortium (U01AI069911). The authors alone are responsible for the views expressed in this article, and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
REFERENCES
- 1.Snow KJ, Sismanidis C, Denholm J, Sawyer SM, Graham SM The incidence of tuberculosis among adolescents and young adults: a global estimate. Eur Respir J 2018;51(2):1702352. DOI: 10.1183/13993003.02352-2017. [DOI] [PubMed] [Google Scholar]
- 2.Snow KJ, Cruz AT, Seddon JA, et al. Adolescent tuberculosis. Lancet Child Adolesc Health 2020;4(1):1:68–79. DOI: 10.1016/S2352-4642(19)30337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guthold R, Baltag V, Katwan E, Lopez G, Diaz T, Ross DA The top global causes of adolescent mortality and morbidity by age and sex, 2019. J Adolesc Health 2021;69(4):540. DOI: 10.1016/j.jadohealth.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 4.UNICEF. Adolescent health dashboard: regional dashboard. Available at: https://data.unicef.org/resources/adolescent-health-dashboard-regional-profiles/. Accessed Aug. 19, 2022.
- 5.World Health Organization. Maternal, newborn, child and adolescent health and ageing: Data portal. WHO. Available at: https://platform.who.int/data/maternal-newborn-child-adolescent-ageing/adolescent-data/adolescent---mortality-causes-of-death. Accessed Aug. 19, 2022.
- 6.World Health Organization. The Global Health Observatory. Global health estimates: leading causes of death. Available at: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death. Accessed Oct. 11, 2022.
- 7.Ferrand RA, Bandason T, Musvaire P, et al. Causes of acute hospitalization in adolescence: burden and spectrum of HIV-related morbidity in a country with an early-onset and severe HIV epidemic: a prospective survey. PLoS Med 2010;7(2):e1000178. DOI: 10.1371/journal.pmed.1000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford N, Matteelli A, Shubber Z, et al. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc 2016;19(1):20714. DOI: 10.7448/IAS.19.1.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamada Y, Getahun H, Tadesse BT, Ford N HIV-associated tuberculosis. Int J STD AIDS 2021;32(9):780–790. DOI: 10.1177/0956462421992257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seddon JA, Chiang SS, Esmail H, Coussens AK The wonder years: What can primary school children teach us about immunity to Mycobacterium tuberculosis? Front Immunol 2018;9(9):2946. DOI: 10.3389/fimmu.2018.02946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baguma R, Mbandi SK, Rodo MJ, et al. Inflammatory determinants of differential tuberculosis risk in pre-adolescent children and young adults. Front Immunol 2021;12:639965. DOI: 10.3389/fimmu.2021.639965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IN DANGER: UNAIDS Global AIDS Update 2022. Available at: https://www.unaids.org/sites/default/files/media_asset/2022-global-aids-update_en.pdf. Accessed Sept. 20, 2022.
- 13.Enane LA, Davies MA, Leroy V, et al. Traversing the cascade: urgent research priorities for implementing the ‘treat all’ strategy for children and adolescents living with HIV in sub-Saharan Africa. J Virus Erad 2018;15(4):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enane LA, Vreeman RC, Foster C Retention and adherence: global challenges for the long-term care of adolescents and young adults living with HIV. Curr Opin HIV AIDS 2018;13(3):212–219. DOI: 10.1097/COH.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 15.Enane LA, Lowenthal ED, Arscott-Mills T, et al. Loss to follow-up among adolescents with tuberculosis in Gaborone, Botswana. Int J Tuberc Lung Dis 2016;20(10):1320–1325. [DOI] [PubMed] [Google Scholar]
- 16.Reif LK, Rivera V, Bertrand R, et al. Outcomes across the tuberculosis care continuum among adolescents in Haiti. Public Health Action 2018;8(3):103–109. DOI: 10.5588/pha.18.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulongeni P, Hermans S, Caldwell J, Bekker LG, Wood R, Kaplan R HIV prevalence and determinants of loss-to-follow-up in adolescents and young adults with tuberculosis in Cape Town. PLoS One 2019;14(2):2:e0210937. DOI: 10.1371/journal.pone.0210937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang SS, Beckhorn CB, Wong M, Lecca L, Franke MF Patterns of suboptimal adherence among adolescents treated for tuberculosis. Int J Tuberc Lung Dis 2020;24(7):723–725. DOI: 10.5588/ijtld.20.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Oliveira MCB, Sant’Anna CC, Raggio Luiz R, Kristki AL Unfavorable outcomes in tuberculosis: Multidimensional factors among adolescents in Rio de Janeiro, Brazil. Am J Trop Med Hyg 2020;103:6:2492–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohlenberg A, Kodmon C, van den Boom M, van der Werf MJ Tuberculosis surveillance in adolescents: What to learn from European Union/European Economic Area data? Int J Tuberc Lung Dis 2020;24(3):347–352. DOI: 10.5588/ijtld.19.0547. [DOI] [PubMed] [Google Scholar]
- 21.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC The age of adolescence. Lancet Child Adolesc Health 2018;2(3):223–228. DOI: 10.1016/s2352-4642(18)30022-1. [DOI] [PubMed] [Google Scholar]
- 22.Patton GC, Sawyer SM, Santelli JS, et al. Our future: A Lancet commission on adolescent health and wellbeing. Lancet 2016;387(10036):2423–78. DOI: 10.1016/S0140-6736(16)00579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Making health services adolescent friendly: Developing national quality standards for adolescent-friendly health services. Available at: https://apps.who.int/iris/bitstream/handle/10665/75217/9789241503594_eng.pdf?sequence=1. Accessed Feb. 21, 2022.
- 24.World Health Organization. Global standards for quality health care services for adolescents. Available at: https://www.who.int/maternal_child_adolescent/documents/global-standards-adolescent-care/en/. Accessed Feb. 21, 2022.
- 25.World Health Organization. Global accelerated action for the health of adolescents (AA-HA!): Guidance to support country implementation. Available at: https://apps.who.int/iris/bitstream/handle/10665/255415/9789241512343-eng.pdf?sequence=1. Accessed Feb. 21, 2022.
- 26.Zeitvogel K Fogarty’s adolescent research key to future good health. Available at: https://www.fic.nih.gov/News/GlobalHealthMatters/march-april-2018/Pages/adolescent-health-research.aspx. Accessed Feb. 21, 2022.
- 27.Blok N, van den Boom M, Erkens C, et al. Variation in policy and practice of adolescent tuberculosis management in the WHO European Region. Eur Respir J 2016;48(3):943–6. DOI: 10.1183/13993003.00704-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laycock KM, Eby J, Arscott-Mills T, et al. Towards quality adolescent-friendly services in TB care. Int J Tuberc Lung Dis 2021;25(7):579–583. DOI: 10.5588/ijtld.21.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moscibrodzki P, Enane LA, Hoddinott G, et al. The impact of tuberculosis on the well-being of adolescents and young adults. Pathogens 2021;10(12). DOI: 10.3390/pathogens10121591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. WHO Operational Handbook on Tuberculosis, Module 5: Management of Tuberculosis in Children and Adolescents. Available at: https://www.who.int/publications/i/item/9789240046832. Accessed Jun. 29, 2022. [PubMed]
- 31.World Health Organization. WHO Consolidated Guidelines on Tuberculosis, Module 5: Management of Tuberculosis in Children and Adolescents. Available at: https://www.who.int/publications/i/item/9789240046764. Accessed Jun. 29, 2022. [PubMed]
- 32.Ross DA, Hinton R, Melles-Brewer M, et al. Adolescent well-being: A definition and conceptual framework. J Adolesc Health 2020;67(4):472–476. DOI: 10.1016/j.jadohealth.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Global tuberculosis report 2020. World Health Organization. Available at: https://www.who.int/publications/i/item/9789240013131. Accessed Feb. 12, 2021.
- 34.Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. New Engl J Med 2004;350(20):2060–7. DOI: 10.1056/NEJMsa031667. [DOI] [PubMed] [Google Scholar]
- 35.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004;8(4):4:392–402. [PubMed] [Google Scholar]
- 36.Martinez L, Cords O, Horsburgh CR, et al. The risk of tuberculosis in children after close exposure: A systematic review and individual-participant meta-analysis. Lancet 2020;395(10228):973–984. DOI: 10.1016/S0140-6736(20)30166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Middelkoop K, Bekker LG, Liang H, et al. Force of tuberculosis infection among adolescents in a high HIV and TB prevalence community: A cross-sectional observation study. BMC Infect Dis 2011;11:156. DOI: 10.1186/1471-2334-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 2008;5(3):e74. DOI: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnstone-Robertson SP, Mark D, Morrow C, et al. Social mixing patterns within a South African township community: implications for respiratory disease transmission and control. Am J Epidemiol 2011;174(11):1246–55. DOI: 10.1093/aje/kwr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood R, Racow K, Bekker LG, et al. Indoor social networks in a South African township: potential contribution of location to tuberculosis transmission. PLoS One 2012;7(6):e39246. DOI: 10.1371/journal.pone.0039246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grijalva CG, Goeyvaerts N, Verastegui H, et al. A household-based study of contact networks relevant for the spread of infectious diseases in the highlands of Peru. PLoS One 2015;10(3):e0118457. DOI: 10.1371/journal.pone.0118457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ajelli M, Litvinova M Estimating contact patterns relevant to the spread of infectious diseases in Russia. J Theor Biol 2017;419:1–7. DOI: 10.1016/j.jtbi.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 43.Zurcher K, Riou J, Morrow C, et al. Estimating tuberculosis transmission risks in a primary care clinic in South Africa: Modeling of environmental and clinical data. J Infect Dis 2022. DOI: 10.1093/infdis/jiab534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez-Velez CM, Marais BJ. Tuberculosis in children. New Engl J Med 2012;367(4):348–61. DOI: 10.1056/NEJMra1008049. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization. WHO Consolidated Guidelines on Tuberculosis, Module 4: Treatment: Tuberculosis Care and Support. In: Organization WH, ed. Geneva, Switzerland: World Health Organization; 2022. [Google Scholar]
- 46.Enane LA, Eby J, Arscott-Mills T, et al. TB and TB-HIV care for adolescents and young adults. Int J Tuberc Lung Dis 2020;24(2):240–249. DOI: 10.5588/ijtld.19.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen E, Khamis F, Migliori GB, et al. De-isolation of patients with pulmonary tuberculosis after start of treatment - clear, unequivocal guidelines are missing. Int J Infect Dis 2017;56:34–38. DOI: 10.1016/j.ijid.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 48.Migliori GB, Nardell E, Yedilbayev A, et al. Reducing tuberculosis transmission: a consensus document from the World Health Organization Regional Office for Europe. Eur Respir J 2019;53(6). DOI: 10.1183/13993003.00391-2019. [DOI] [PubMed] [Google Scholar]
- 49.Dharmadhikari AS, Mphahlele M, Venter K, et al. Rapid impact of effective treatment on transmission of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2014;18(9):9:1019–25. DOI: 10.5588/ijtld.13.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliva Rapoport VA E; Senador L; Wong M; Beckhorn CB; Coit J; Roche S; Galea JT; Lecca L; Chiang SS The impact of prolonged home isolation on adolescents with drug-susceptible tuberculosis in Lima, Peru: A qualitative study. BMJ Open 2022;12:e063287. DOI: 10.1136/bmjopen-2022-063287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zvonareva O, Witte S, Kabanets N, Filinyuk O Adolescents in a tuberculosis hospital: Qualitative study of how relationships with doctors, caregivers, and peers mediate their mental wellbeing. PLoS One 2021;16(10):e0257379. DOI: 10.1371/journal.pone.0257379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karayeva E The impact of hospitalization on ukrainian adolescents who have completed tuberculosis treatment in Kyiv City, Ukraine [Master’s Thesis]. Brown School of Public Health. Providence, U.S.A.; 2020. [Google Scholar]
- 53.Zhang S, Li X, Zhang T, Fan Y, Li Y The experiences of high school students with pulmonary tuberculosis in China: a qualitative study. BMC Infect Dis 2016;16(1):758. DOI: 10.1186/s12879-016-2077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das M, Mathur T, Ravi S, et al. Challenging drug-resistant TB treatment journey for children, adolescents and their care-givers: A qualitative study. PLoS One 2021;16:3:e0248408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franck C Assessing the importance of stigma in children’s experience of MDR-TB treatment in the Western Cape Province, South Africa [Master’s Thesis]. London School of Hygiene & Tropical Medicine. London, U.K.; 2012. [Google Scholar]
- 56.Franck C, Seddon JA, Hesseling AC, Schaaf HS, Skinner D, Reynolds L Assessing the impact of multidrug-resistant tuberculosis in children: an exploratory qualitative study. BMC Infect Dis 2014;14:426. DOI: 10.1186/1471-2334-14-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathad JS, Gupta A Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis 2012;55(11):1532–49. DOI: 10.1093/cid/cis732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salazar-Austin N, Hoffmann J, Cohn S, et al. Poor obstetric and infant outcomes in human immunodeficiency virus-infected pregnant women with tuberculosis in South Africa: The Tshepiso Study. Clin Infect Dis 2018;66(6):6:921–929. DOI: 10.1093/cid/cix851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nachman S, Ahmed A, Amanullah F, et al. Towards early inclusion of children in tuberculosis drugs trials: A consensus statement. Lancet Infect Dis 2015;15(6):711–20. DOI: 10.1016/S1473-3099(15)00007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dorman SE, Nahid P, Kurbatova EV, et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. New Engl J Med 2021;384(18):1705–1718. DOI: 10.1056/NEJMoa2033400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization. Rapid communication: Key changes to the treatment of drug-resistant tuberculosis. Available at: https://www.who.int/publications/i/item/WHO-UCN-TB-2022-2. Accessed Jun. 29, 2022.
- 62.Willis N, Milanzi A, Mawodzeke M, et al. Effectiveness of community adolescent treatment supporters (CATS) interventions in improving linkage and retention in care, adherence to ART and psychosocial well-being: a randomised trial among adolescents living with HIV in rural Zimbabwe. BMC Public Health 2019;19(1):117. DOI: 10.1186/s12889-019-6447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Denison JA, Burke VM, Miti S, et al. Project YES! Youth Engaging for Success: A randomized controlled trial assessing the impact of a clinic-based peer mentoring program on viral suppression, adherence and internalized stigma among HIV-positive youth (15–24 years) in Ndola, Zambia. PLoS One 2020;15(4):e0230703. DOI: 10.1371/journal.pone.0230703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Enane LA, Apondi E, Toromo J, et al. “A problem shared is half solved” - a qualitative assessment of barriers and facilitators to adolescent retention in HIV care in western Kenya. AIDS Care 2020;32(1):104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rencken CA, Harrison AD, Mtukushe B, et al. “Those people motivate and inspire me to take my treatment.” Peer support for adolescents living with HIV in Cape Town, South Africa. J Int Assoc Provid AIDS Care; 2021;20:23259582211000525. DOI: 10.1177/23259582211000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Enane LA, Apondi E, Omollo M, et al. “I just keep quiet about it and act as if everything is alright” - The cascade from trauma to disengagement among adolescents living with HIV in western Kenya. J Int AIDS Soc 2021;24(4):e25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ssewamala FM, Dvalishvili D, Mellins CA, et al. The long-term effects of a family based economic empowerment intervention (Suubi+Adherence) on suppression of HIV viral loads among adolescents living with HIV in southern Uganda: Findings from 5-year cluster randomized trial. PLoS One 2020;15(2):e0228370. DOI: 10.1371/journal.pone.0228370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tozan Y, Capasso A, Sun S, et al. The efficacy and cost-effectiveness of a family-based economic empowerment intervention (Suubi + Adherence) on suppression of HIV viral loads among adolescents living with HIV: results from a Cluster Randomized Controlled Trial in southern Uganda. J Int AIDS Soc 2021;24(6):e25752. DOI: 10.1002/jia2.25752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanoni BC, Sibaya T, Cairns C, et al. Higher retention and viral suppression with adolescent-focused HIV clinic in South Africa. PLoS One 2017;12(12):e0190260. DOI: 10.1371/journal.pone.0190260. [DOI] [PMC free article] [PubMed] [Google Scholar]


