Abstract
Background
The clinical relevance of promoter mutations and single nucleotide polymorphism rs2853669 of telomerase reverse transcriptase (TERT) and telomere length in patients with isocitrate dehydrogenase (IDH) wild-type glioblastoma (GBM) patients remains unclear. Moreover, some studies speculated that TERT promoter status might influence the prognostic role of O6-methylguanine DNA methyltransferase (MGMT) promoter methylation in newly diagnosed GBM. We carried out a large study to investigate their clinical impact and their interaction in newly diagnosed GBM patients.
Patients and methods
We included 273 newly diagnosed IDH wild-type GBM patients who started treatment at Veneto Institute of Oncology IOV – IRCCS (Padua, Italy) from December 2016 to January 2020. TERT promoter mutations (−124 C>T and −146 C>T) and SNP rs2853669 (−245 T>C), relative telomere length (RTL) and MGMT methylation status were retrospectively assessed in this prospective cohort of patients.
Results
Median overall survival (OS) of 273 newly diagnosed IDH wild-type GBM patients was 15 months. TERT promoter was mutated in 80.2% of patients, and most had the rs2853669 single nucleotide polymorphism as T/T genotype (46.2%). Median RTL was 1.57 (interquartile range 1.13-2.32). MGMT promoter was methylated in 53.4% of cases. At multivariable analysis, RTL and TERT promoter mutations were not associated with OS or progression-free survival (PFS). Notably, patients C carrier of rs2853669 (C/C+C/T genotypes) showed a better PFS compared with those with the T/T genotype (hazard ratio 0.69, P = 0.007). In terms of OS and PFS, all interactions between MGMT, TERT and RTL and between TERT and rs2853669 genotype were not statistically significant.
Conclusions
Our findings suggest the presence of the C variant allele at the rs2853669 of the TERT promoter as an attractive independent prognostic biomarker of disease progression in IDH wild-type GBM patients. RTL and TERT promoter mutational status were not correlated to survival regardless of MGMT methylation status.
Key words: glioblastoma, TERT, MGMT, telomere length
Highlights
-
•
TERT promoter status and telomere length were not associated to OS and PFS in newly diagnosed IDH wild-type glioblastoma.
-
•
No interaction between TERT promoter status, relative telomere length and MGMT methylation status in terms of OS and PFS.
-
•
The C variant allele at the rs2853669 of the TERT promoter resulted in an independent biomarker of disease progression.
Introduction
Glioblastoma (GBM) is the most common and highly aggressive type of glioma, practically relapsing after first-line standard therapy, including surgery, radiotherapy (RT) and temozolomide (TMZ) chemotherapy. The presence of promoter methylation of the O6-methylguanine DNA methyltransferase (MGMT) gene represents an important predictive factor of alkylating agent efficacy such as TMZ and nitrosoureas. In the last years, however, new molecular mechanisms and specific genes involved in the growth of GBM have been better evaluated. Among these, telomerase reverse transcriptase (TERT) promoter mutations are frequently found in isocitrate dehydrogenase (IDH) wild-type GBM and may have a potential clinical impact and an important role in tumorigenesis.1 TERT is the catalytic component of the telomerase enzyme, a specialized reverse transcriptase that uses an internal RNA template to maintain telomere length by adding hexamer repeats to telomeres. Following embryogenesis, telomerase expression is suppressed in somatic cells, resulting in progressive telomere shortening, which is regarded as one of the main tumor suppressive mechanisms. Telomerase reactivation and up-regulation is a universal event in most human tumors.2,3 Overexpression of TERT/telomerase leads to cellular immortality preventing cellular replicative senescence and crisis induced by telomere erosion, thereby promoting tumor formation and progression.4 Moreover, besides its canonical role in telomere length maintenance, TERT may also promote carcinogenesis through telomere length-independent functions, including enhancement of cell proliferation and resistance to apoptosis.5,6 In cancer, TERT gene expression can be up-regulated by genetic and epigenetic factors, such as TERT amplifications, variants, rearrangements, promoter methylation and promoter mutations.7,8 Mutually exclusive recurrent C-to-T transitions at nucleotides 1,295,228 (C228T; −124 C>T) and 1,295,250 (C250T; −146 C>T) within the core promoter of TERT gene are quite common in solid tumors, including gliomas.9, 10, 11, 12 Both mutations create de novo binding sites for E-twenty-six (ETS) transcription factors, leading to increased TERT gene expression and telomerase activity.13,14 It has been suggested that the effect of TERT promoter (TERTp) mutations may be affected by the presence of the rs2853669 single nucleotide polymorphism (SNP) at −245 bp within the TERT core promoter. The −245T>C variant allele disrupts an ETS2 binding site, thus resulting in decreased TERT expression.15,16
Since the acquisition of unlimited proliferation capacity represents a critical hallmark required for cell malignant transformation, the telomere/telomerase complex represents an important indicator of tumor formation/progression. On this ground, mutations in the TERT promoter can potentially be used as prognostic biomarkers, as emerging data suggest that these alterations are associated with worse prognoses in different cancer types.12 Despite many studies have evaluated the association between TERT promoter mutations and pathological features in GBM, however, the results were often controversial.10,17, 18, 19, 20 In particular, some studies have highlighted a negative prognostic impact of TERTp mutations,10,18,21, 22, 23 whereas others have suggested that the adverse impact of the TERTp mutations may be related to clinical confounding factors such as age, initial surgical procedure and molecular factors such as IDH mutations or MGMT methylation status.20,24, 25, 26, 27, 28 Studies on large cohorts of homogeneous patients can be useful to evaluate the independent prognostic value of the TERTp mutations. Here, we focused our research on a large series of newly diagnosed IDH wild-type GBM, investigating the potential clinical impact of TERT promoter mutations, rs2853669 genotype, telomere length and their interaction with MGMT methylation status.
Materials and methods
Study design
This study evaluated all consecutive IDH wild-type GBM patients who started treatment at Veneto Institute of Oncology IOV – IRCCS (Padua, Italy) from December 2016 to January 2020. Although TERT promoter mutations, rs2853669 genotype and relative telomere length (RTL) were retrospectively assessed, all patient data were retrieved from prospectively maintained computerized medical records. The study was approved by the local institutional review board (Veneto Institute of Oncology Ethics Committee n. 919) and complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, with the good clinical practice guidelines and with the Declaration of Helsinki. Written informed consent was obtained from the patients involved in the study.
Patients
Patients with histologically confirmed newly diagnosed IDH wild-type GBM (according to WHO 2016 classification)29 receiving oncological treatment and formalin-fixed paraffin-embedded (FFPE) tumor tissue sample available for TERT analyses were eligible for inclusion in the study. As for internal protocol, neuroradiological assessment was carried out before starting oncological treatment, 3-4 weeks after RT if available and every 2-3 months or when clinically indicated. Neuroradiological assessment was based on RANO criteria. MGMT methylation status was also collected.
Endpoints
The primary endpoint was to assess the prognostic role of TERTp mutational status, rs2853669 genotype and RTL in terms of overall survival (OS) and progression-free survival (PFS). The secondary endpoints included: (i) assessing the potential prognostic role of the interactions among TERTp mutations, rs2853669, RTL and MGMT methylation status; (ii) evaluating the associations among TERTp mutations, rs2853669, RTL and MGMT methylation status; and (iii) assessing the impact of TERTp mutational status, rs2853669 and RTL with neuroradiological response.
Tumor samples and DNA analyses
All GBM specimens were FFPE. DNA was extracted using the QIAmp DNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Genomic DNA amplification for TERT promoter region (260 bp) containing −124 C>T and −146 C>T mutation sites, as well as the SNP rs2853669 (−245 T>C) was carride out as previously described.30 The amplified products were purified with the Illustra ExoProStar (GE Healthcare, Amersham, UK) and sequenced on a 3730xl DNA analyzer (Applied Biosystems, Foster City, CA). All samples were analyzed in forward and reverse directions. Telomere length was determined by multiplex PCR assay.31 RTL values were calculated as telomere/single-copy gene ratio, according to a previous report.32 MGMT promoter methylation was assessed by pyrosequencing as described in Indraccolo et al.30 IDH1/2 mutational status was assessed by immunohistochemistry or DNA sequencing.30
Statistical analysis
Categorical data were summarized as number and percentage, and continuous data as median and interquartile range (IQR). Comparisons between groups were carried out using chi-square test or Fisher’s exact test (categorical data), and Mann–Whitney test or Kruskal–Wallis test (continuous data). Correlation between continuous data was assessed with Pearson’s correlation coefficient. Survival curves were estimated using the Kaplan–Meier method and compared between the groups using the log-rank test. Cox regression models were estimated to assess the effect of gene characteristics (TERTp mutations, RTL and MGMT methylation status) on OS and PFS, adjusting for major clinical confounding factors [demographics, Eastern Cooperative Oncology Group performance status (ECOG PS) and treatment features]. TERTp mutation was included as 3-group variable (mutated −124 C>T versus mutated −146 C>T versus wild-type) in model A and as 2-group variable (mutated −124 C>T or −146 C>T versus wild-type) in model B. Effect sizes were reported as hazard ratio with a 95% confidence interval (CI). All tests were two-sided and a P value <0.05 was considered statistically significant. Statistical analysis was carried out using R 4.1 (R Foundation for Statistical Computing, Vienna, Austria).33
Results
Newly diagnosed IDH wild-type GBM patients
The analysis included 273 newly diagnosed IDH wild-type GBM patients (159 males and 114 females; median age 63 years) with a median follow-up of 12 months (IQR 6-21 months). Patient characteristics are reported in Table 1. About surgery, 105 patients (38.5%) underwent radical resection and 168 (61.5%) partial resection/biopsy. ECOG PS was 0-1 in 183 patients (70.9%). Most patients, 204 (78.5%) received the standard treatment with RT and TMZ, the others were treated with RT or TMZ alone. MGMT methylation status was available for analysis in 266 cases (97.4%) and was methylated in 142 patients (53.4%).
Table 1.
Characteristics of newly diagnosed IDH wild-type GBM patients
| N of newly diagnosed GBM patients | 273 |
|---|---|
| Age (years)a | 63 (54-70) |
| Sex | |
| Males | 159 (58.2) |
| Females | 114 (41.8) |
| ECOG PSb | |
| 0 | 80 (31.0) |
| 1 | 103 (39.9) |
| 2 | 51 (19.8) |
| 3 | 24 (9.3) |
| First surgery | |
| Complete | 105 (38.5) |
| Non-complete | 168 (61.5) |
| RT + TMZc | |
| No | 56 (21.5) |
| Yes | 204 (78.5) |
| Maintenance TMZ cycles (number)a | 4 (1-7) |
| Second surgery | 60 (21.9) |
| Second-line treatment | 135 (49.4) |
| TERT promoter | |
| −124 C>T | 163 (59.7) |
| −146 C>T | 56 (20.5) |
| Wild-type | 54 (19.8) |
| rs2853669 genotype | |
| CC | 32 (11.7) |
| TC | 115 (42.1) |
| TT | 126 (46.2) |
| RTLa | 1.57 (1.13-2.32) |
| MGMTd | |
| Unmethylated | 124 (46.6) |
| Methylated | 142 (53.4) |
ECOG PS, Eastern Cooperative Oncology Group performance status; GBM, glioblastoma; IDH, isocitrate dehydrogenase; MGMT, O6-methylguanine DNA methyltransferase; RT, radiotherapy; RTL, relative telomere length; TMZ, temozolomide.
Data expressed as n (%) or amedian (interquartile range). Data not available in b15, c13 and d7 patients.
All patients were analyzed for TERT promoter mutational status and RTL. TERT promoter was mutated in 219 (80.2%) patients (163 with −124 C>T mutation and 56 with −146 C>T mutation). We also genotyped patients for the rs2853669 SNP at −245 bp. A total of 147 patients (53.8%) carried the minor C-variant allele, for which 32 patients were homozygous and 115 were heterozygous. One hundred and twenty-six patients (46.2%) had the T/T genotype. Median RTL was 1.57 (IQR 1.13-2.32) and telomere length showed an inverse correlation with age (r = −0.21, P = 0.0004).
Overall survival
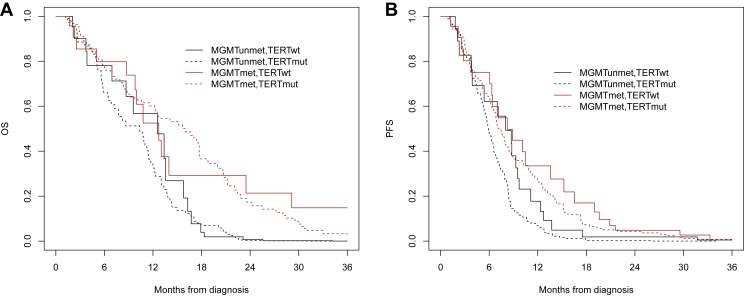
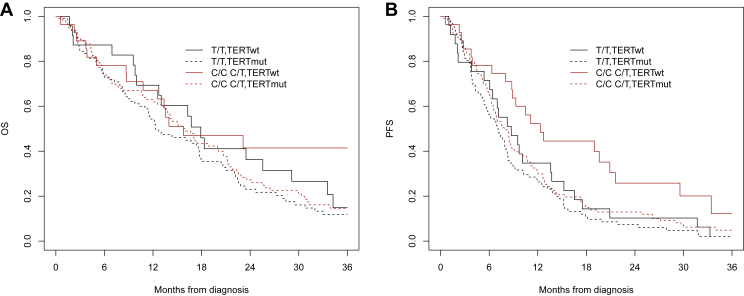
Overall, at the analysis cut-off date of 5 February 2021, 211 patients died (77.2%). Median OS was 15 months (95% CI 13-18 months). OS was 59.9% at 1 year, 27.3% at 2 years and 14.8% at 3 years (Supplementary Figure S1, available at https://doi.org/10.1016/10.1016/j.esmoop.2023.101570). At multivariable analysis, RTL, TERTp mutations and rs2853669 genotype were not associated with OS, whereas younger age, ECOG PS 0-1, complete surgery, RT + TMZ therapy, second surgery at recurrence, second-line treatment and methylated MGMT were associated with improved OS (full results in Table 2). In the models, all interactions between MGMT, TERTp and RTL and between TERT and rs2853669 were not statistically significant (Table 2). In particular, OS was not statistically different between TERT-mutated and TERT wild-type patients in the subgroup with methylated MGMT (P = 0.92) and unmethylated MGMT (P = 0.77) (Figure 1A). In the methylated MGMT subgroup, median OS was 20 months (95% CI 17-22 months) in TERT-mutated patients and 14 months (95% CI 10 months to not reached) in TERT wild-type patients. In the unmethylated MGMT subgroup, median OS was 12 months (95% CI 10-14 months) in TERT-mutated patients and 13 months (95% CI 12-18 months) in TERT wild-type patients. Kaplan–Meier curve analysis stratifying patients by TERTp mutational status and rs2853669 genotype also showed no significant difference in OS between groups (P = 0.50, Figure 2A).
Table 2.
Factors associated with OS in newly diagnosed IDH wild-type GBM patients
| Univariate analysis |
Multivariable analysis (model A) |
Multivariable analysis (model B) |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, yearsa | 1.03 (1.02-1.04) | <0.0001 | 1.02 (1.01-1.03) | 0.003 | 1.02 (1.01-1.03) | 0.001 |
| Male sex | 1.03 (0.78-1.36) | 0.80 | 0.94 (0.69-1.27) | 0.69 | 0.94 (0.69-1.28) | 0.72 |
| ECOG PS | <0.0001 | 0.0003 | 0.0006 | |||
| 0-1 | Reference | Reference | Reference | |||
| 2-3 | 3.55 (2.58-4.87) | 1.99 (1.36-2.91) | 1.92 (1.32-2.80) | |||
| First surgery | <0.0001 | 0.01 | 0.01 | |||
| Complete | Reference | Reference | Reference | |||
| Non-complete | 2.26 (1.68-3.05) | 1.60 (1.11-2.29) | 1.57 (1.10-2.25) | |||
| RT + TMZ | <0.0001 | 0.01 | 0.02 | |||
| No | Reference | Reference | Reference | |||
| Yes | 0.41 (0.30-0.57) | 0.62 (0.42-0.92) | 0.64 (0.43-0.95) | |||
| Second surgery | <0.0001 | 0.0065 | 0.008 | |||
| No | Reference | Reference | Reference | |||
| Yes | 0.28 (0.19-0.41) | 0.52 (0.33-0.83) | 0.54 (0.34-0.85) | |||
| Second-line treatment | <0.0001 | 0.003 | 0.002 | |||
| No | Reference | Reference | Reference | |||
| Yes | 0.29 (0.22-0.40) | 0.57 (0.39-0.82) | 0.57 (0.39-0.82) | |||
| MGMT | 0.002 | <0.0001 | <0.0001 | |||
| Unmethylated | Reference | Reference | Reference | |||
| Methylated | 0.65 (0.49-0.86) | 0.46 (0.34-0.63) | 0.46 (0.33-0.62) | |||
| TERT promoter |
0.25 0.13 |
0.69 0.51 |
— | — | ||
| Mutated −124 C>T | 1.31 (0.76-1.88) | 1.08 (0.73-1.58) | ||||
| Mutated −146 C>T | 1.29 (0.83-1.99) | 0.84 (0.51-1.38) | ||||
| Wild-type | Reference | Reference | ||||
| TERT promoter | 0.13 | — | — | 0.89 | ||
| Mutated (−124 C>T or −146 C>T) | 1.30 (0.92-1.85) | 1.02 (0.70-1.48) | ||||
| Wild-type | Reference | Reference | ||||
| rs2853669 genotype | 0.68 | 0.29 | 0.25 | |||
| CC/TC | 0.94 (0.71-1.24) | 0.85 (0.63-1.14) | 0.84 (0.62-1.13) | |||
| TT | Reference | Reference | Reference | |||
| RTLa | 0.94 (0.86-1.03) | 0.18 | 0.97 (0.88-1.08) | 0.69 | 0.98 (0.89-1.09) | 0.79 |
All interactions between MGMT, TERT and RTL (MGMT∗TERT P = 0.08; MGMT∗RTL P = 0.47; TERT∗RTL P = 0.27; MGMT∗TERT∗RTL P = 0.10) and the interaction between TERT and rs2853669 genotype (P = 0.86) were not statistically significant.
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; GBM, glioblastoma; HR, hazard ratio; IDH, isocitrate dehydrogenase; MGMT, O6-methylguanine DNA methyltransferase; OS, overall survival; RT, radiotherapy; RTL, relative telomere length; TERT, telomerase reverse transcriptase; TMZ, temozolomide.
Included as continuous variable.
Figure 1.
(A) Overall survival and (B) progression-free survival according to MGMT (methylated, unmethylated) and TERTp (mutated, wild-type) stratification in IDH wild-type newly diagnosed GBM patients.
GBM, glioblastoma; IDH, isocitrate dehydrogenase; MGMT, O6-methylguanine DNA methyltransferase; mut, mutated; OS, overall survival; PFS, progression-free survival; TERT, telomerase reverse transcriptase; wt, wild-type.
Figure 2.
Overall survival (A) and progression-free survival (B) according to TERT promoter status (mutated, wild-type) and rs2853669 genotype (C/C+C/T, T/T) stratification in IDH wild-type newly diagnosed GBM patients.
GBM, glioblastoma; IDH, isocitrate dehydrogenase; mut, mutated; OS, overall survival; PFS, progression-free survival; TERT, telomerase reverse transcriptase; wt, wild-type.
Progression-free survival
Overall, 247 patients (90.5%) had a disease progression. Median PFS was 7.0 months (95% CI 6.0-8.0 months). PFS was 31.2% at 1 year, 11.2% at 2 years and 3.2% at 3 years (Supplementary Figure S1, available at https://doi.org/10.1016/10.1016/j.esmoop.2023.101570). At multivariable analysis, younger age, ECOG PS 0-1, complete surgery, RT + TMZ therapy, second surgery at recurrence and methylated MGMT were associated with improved PFS (full results in Table 3). Notably, whereas RTL and TERTp mutations were not associated with PFS, C variant of rs2853669 (C/C+C/T genotypes) of TERT promoter is an independent prognostic marker of PFS (P = 0.007) (Table 3). In the models, all interactions between MGMT, TERTp and RTL and between TERT and rs2853669 were not statistically significant (Table 3). In particular, PFS was not statistically different between TERT-mutated and TERT wild-type patients in the subgroup with methylated MGMT (P = 0.59) and unmethylated MGMT (P = 0.11) (Figure 1B). In the methylated MGMT subgroup, median PFS was 8 months (95% CI 9-11 months) and 10 months (95% CI 9-19 months) in TERT-mutated and TERT wild-type cases, respectively. In the unmethylated MGMT subgroup, median PFS was 6 months (95% CI 5-8 months) and 8 months (95% CI 7-12 months) in TERT-mutated and TERT wild-type cases, respectively. Interestingly, Kaplan–Meier survival analysis stratifying patients by TERT promoter mutational status and rs2853669 genotype showed that patients without TERT promoter mutations and C carriers of rs2853669 had the better PFS (P = 0.03, Figure 2B).
Table 3.
Factors associated with PFS in newly diagnosed IDH wild-type GBM patients
| Univariate analysis |
Multivariable analysis (model A) |
Multivariable analysis (model B) |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, yearsa | 1.01 (1.01-1.02) | 0.01 | 1.01 (1.01-1.02) | 0.008 | 1.01 (1.01-1.02) | 0.008 |
| Male sex | 1.06 (0.82-1.37) | 0.62 | 1.03 (0.78-1.35) | 0.82 | 1.03 (0.78-1.35) | 0.81 |
| ECOG PS | <0.0001 | 0.0002 | 0.0001 | |||
| 0-1 | Reference | Reference | Reference | |||
| 2-3 | 2.79 (2.09-3.72) | 1.88 (1.34-2.62) | 1.89 (1.35-2.63) | |||
| First surgery | <0.0001 | <0.0001 | <0.0001 | |||
| Complete | Reference | Reference | Reference | |||
| Non-complete | 1.89 (1.44-2.46) | 1.83 (1.36-2.46) | 1.83 (1.36-2.47) | |||
| RT + TMZ | <0.0001 | 0.0004 | 0.0004 | |||
| No | Reference | Reference | Reference | |||
| Yes | 0.45 (0.32-0.61) | 0.52 (0.36-0.73) | 0.52 (0.36-0.74) | |||
| MGMT | 0.0002 | <0.0001 | <0.0001 | |||
| Unmethylated | Reference | Reference | Reference | |||
| Methylated | 0.61 (0.47-0.79) | 0.55 (0.42-0.73) | 0.55 (0.42-0.73) | |||
| TERT promoter | — | — | ||||
| Mutated −124 C>T | 1.35 (0.97-1.88) | 0.07 | 1.19 (0.83-1.69) | 0.33 | ||
| Mutated −146 C>T | 1.54 (1.03-2.32) | 0.03 | 1.26 (0.81-1.95) | 0.28 | ||
| Wild-type | Reference | Reference | ||||
| TERT promoter | 0.04 | — | — | 0.28 | ||
| Mutated (−124 C>T or −146 C>T) | 1.39 (1.01-1.92) | 1.20 (0.85-1.70) | ||||
| Wild-type | Reference | Reference | ||||
| rs2853669 genotype | 0.03 | 0.007 | 0.007 | |||
| CC/TC | 0.76 (0.59-0.97) | 0.69 (0.52-0.90) | 0.69 (0.52-0.90) | |||
| TT | Reference | Reference | Reference | |||
| RTLa | 0.99 (0.92-1.07) | 0.82 | 0.99 (0.91-1.08) | 0.91 | 0.99 (0.91-1.08) | 0.87 |
All interactions between MGMT, TERT and RTL (MGMT∗TERT P = 0.16; MGMT∗RTL P = 0.41; TERT∗RTL P = 0.70; MGMT∗TERT∗RTL P = 0.21) and the interaction between TERT and rs2853669 genotype (P = 0.69) were not statistically significant.
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; GBM, glioblastoma; HR, hazard ratio; IDH, isocitrate dehydrogenase; MGMT, O6-methylguanine DNA methyltransferase; PFS, progression-free survival; RT, radiotherapy; RTL, relative telomere length; TERT, telomerase reverse transcriptase; TMZ, temozolomide.
Included as continuous variable.
Neuroradiological assessment
All but 11 patients were available for neuroradiological assessment. A total of 7 patients (2.7%) had a complete response (CR) as assessed by local investigators, whereas 31 patients (11.8%) had a partial response (PR), 193 patients (73.7%) had stable disease (SD) and 31 patients (11.8%) reported a progression of the disease. Objective response rate (ORR; CR or PR) was achieved in 38/262 patients (14.5%) and disease control rate (DCR; CR, PR, or SD) in 231/262 patients (88.2%). Factors associated with ORR and DCR are reported in Supplementary Tables S1 and S2, available at https://doi.org/10.1016/10.1016/j.esmoop.2023.101570, respectively. ORR was more frequent in patients with ECOG PS 0-1 (P = 0.0003), whereas DCR was more frequent in patients with ECOG PS 0-1 (P = 0.02) and those who received RT + TMZ (P = 0.0005). No statistically significant associations were found between best response and molecular characteristics; however, ORR tends to be more frequent in patients C carriers of rs2853669 (P = 0.07).
Associations between MGMT methylation, TERTp mutations and rs2853669 and RTL
MGMT status resulted methylated in 112/214 (52.3%) patients with TERTp-mutated and 30/52 (57.7%) patients with TERTp wild-type (P = 0.58). The proportion of patients with methylated MGMT was 82/160 (51.2%) in patients with −124 C>T mutation, 30/54 (55.5%) in those with −146 C>T mutation and 30/52 (57.7%) in those TERT wild-type (P = 0.67). RTL was not statistically different between patients with TERT-mutated (median 1.54, IQR 1.11-2.22) and those with TERT wild-type (median 1.86, IQR 1.17-2.70) (P = 0.10), or among patients with CC genotype (median 1.61, IQR 1.21-2.35), those with TC genotype (median 1.52, IQR 1.12-2.31) and those with TT genotype (median 1.57, IQR 1.09-2.31) (P = 0.85). RLT, however, was lower in patients with methylated MGMT (median 1.49, IQR 1.02-2.28) with respect to those with unmethylated MGMT (median 1.72, IQR 1.24-2.47) (P = 0.04).
Discussion
In IDH wild-type GBM, the prevalence of TERT promoter mutation is relatively high with a range of 44%-100%12 although its potential prognostic role remains controversial.1 It has been suggested that the prognostic role of TERT promoter mutations could be influenced by other molecular factors, including MGMT promoter methylation,1 but such findings are still under debate.
In the present study, carried out in a large mono-institutional cohort, we identified TERT promoter mutations in ∼80% of newly diagnosed IDH wild-type GBM patients and we investigated the interaction of MGMT methylation status, TERT promoter mutations, telomere length and rs2853669 genotype in terms of OS and PFS. We identify the C variant allele at the rs2853669 SNP as an independent prognostic marker of improved disease progression, whereas both TERT promoter mutational status and RTL did not impact on clinical outcome of GBM patients. Moreover, TERT promoter mutational status did not influence the prognostic role of MGMT methylation status.
Even if no biological mechanism of interaction between TERT promoter mutation and MGMT methylation in GBM patients has yet been proposed,34 three large studies analyzed their impact on prognosis reporting conflicting results.20,27,28 In agreement with our findings, Gramatzki et al.28 recently showed no interaction between TERT promoter mutations and MGMT methylation status in two independent cohorts of IDH wild-type GBM patients. In addition, in line with our results, the authors found that TERT promoter mutational status was not associated with survival in patients with methylated MGMT promoter.28 Two other previous studies, however, showed a significant interaction between TERT promoter mutations and MGMT promoter methylation, with TERT mutant promoter impairing survival in MGMT unmethylated patients.20,27 Likely, these conflicting results among the studies might be due to the clinical heterogeneity of the enrolled patients, different type of studies (retrospective versus prospective), different type of technical methods for analyzing MGMT methylation status (PCR versus pyrosequencing) and different cut-off for MGMT methylation in case of pyrosequencing.
The important finding emerging from our study is that the C variant allele of rs2853669 was associated with improved disease progression in newly diagnosed IDH1/2 wild-type GBM patients. The lack of association between the C variant and survival is unclear, but the heterogeneity of second-line therapies and re-surgery at relapse might likely have impacted on this correlation. Our association of the C variant of rs2853669 with a favorable patient outcome is in agreement with prognostic data from other cancer types35,36 and likely depends on the destroying effect of the C allele on a pre-existing binding site for ETS transcription factors on TERT promoter, resulting in reduced TERT expression.15 Nevertheless, even opposite results, with the C allele associated with a worse prognosis, have been reported.22,24,37,38 A possible reason for these conflicting results could be the genetic or the epigenetic context of rs2853669: other SNPs in the TERT gene or different methylation levels at specific regions of the TERT promoter could differently contribute to TERT expression and, in turn, clinical outcome.38, 39, 40, 41, 42 In addition, we should underline that the genetic variants and mutations at the TERT promoter are surrogate markers of sustained telomerase expression that drives cancer cell immortalization. Although these genetic markers are considered reliable indicators of TERT expression, many altered pathways affecting cancer cell transcription factors might contribute differently to the final activation of TERT promoter. The results from our study on the prognostic role of rs2853669, however, suggest that greater attention to this SNP might substantially improve the GBM patient risk stratification, allowing the identification of patients with poorer disease progression. The validation of this result in an independent cohort of newly diagnosed IDH wild-type GBM patients will be mandatory to sustain the prognostic role of the C allele of the SNP rs2853669 in the disease progression. The germline nature of the SNP allows simple and minimally invasive assessment of the genotype; thus, larger studies might easily be planned to evaluate the relevance of screening for this polymorphism for prognostic purposes.
In addition to MGMT methylation status and TERT promoter mutation interaction, we also analyzed tumor telomere length as a potential molecular marker for prognosis. Maintenance of telomere length is an important process by which cancer cells escape replicative senescence. In literature, there is no agreement concerning the role of telomere length in tumor cells as markers of disease progression of most investigated tumors, including glioma.43,44 In agreement with a previous study,44 we did not find any association of tumor telomere length with prognosis in newly diagnosed GBM patients.
The strengths of our study rely in the large cohort of newly diagnosed GBMs, and the selection of IDH wild-type GBM to avoid any bias due to IDH mutation. The study, however, has also some limitations that should be considered, including the retrospective design, the single-center data collection and the heterogeneity of the first line and/or subsequent treatments; notably, we did not include patients with ‘molecular GBM’ as defined in the 2021 WHO classification and so, the role of TERT promoter mutation was not assessed for these patients.
In conclusion, our findings suggest that TERT promoter mutational status and telomere length do not impact on prognosis in newly histologically defined IDH wild-type GBM patients. Moreover, no interaction was found between MGMT methylation status and TERT promoter mutational status. The presence of the C allele of the SNP rs2853669, however, demonstrated to be an attractive independent prognostic biomarker of disease progression that can be easily evaluated also at the germinal level. Hence, even if the analysis of TERT promoter mutations and telomere length might not include additional prognostic information in these patients, the genotyping of rs2853669 might be a useful additional biomarker for risk progression. Due to the relatively small population analyzed in our work, however, further studies in independent cohorts of newly diagnosed IDH wild-type GBM patients should be undertaken to extend and validate this finding.
Acknowledgements
We thank ‘Fondazione Giovanni Celeghin ONLUS’ (Pernumia, Italy) and ‘Fondazione Luca Ometto ONLUS’ (Padua, Italy) for partially supporting this work.
Funding
This work was supported by ‘Ricerca Corrente 2023’ funding from the Italian Ministry of Health (CDC 099183- L03P03) to cover publication costs.
Disclosure
The authors have declared no conflicts of interest.
Footnotes
Supplementary data
References
- 1.Olympios N., Gilard V., Marguet F., Clatot F., Di Fiore F., Fontanilles M. TERT promoter alterations in glioblastoma: a systematic review. Cancers. 2021;13(5):1147. doi: 10.3390/cancers13051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackburn E.H., Greider C.W., Szostak J.W. Telomeres and telomerase: the path from maize, tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12(10):1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 3.Shay J.W., Wright W.E. Role of telomeres and telomerase in cancer. Semin Cancer Biol. 2011;21(6):349–353. doi: 10.1016/j.semcancer.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Ségal-Bendirdjian E., Geli V. Non-canonical roles of telomerase: unraveling the imbroglio. Front Cell Dev Biol. 2019;7:332. doi: 10.3389/fcell.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martínez P., Blasco M.A. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat Rev Cancer. 2011;11(3):161–176. doi: 10.1038/nrc3025. [DOI] [PubMed] [Google Scholar]
- 7.Akincilar S.C., Unal B., Tergaonkar V. Reactivation of telomerase in cancer. Cell Mol Life Sci. 2016;73(8):1659–1670. doi: 10.1007/s00018-016-2146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leão R., Apolónio J.D., Lee D., Figueiredo A., Tabori U., Castelo-Branco P. Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: clinical impacts in cancer. J Biomed Sci. 2018;25(1):22. doi: 10.1186/s12929-018-0422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinagre J., Almeida A., Pópulo H., et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 10.Killela P.J., Pirozzi C.J., Healy P., et al. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014;5(6):1515–1525. doi: 10.18632/oncotarget.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell R.J.A., Rube H.T., Xavier-Magalhães A., et al. Understanding TERT promoter mutations: a common path to immortality. Mol Cancer Res. 2016;14(4):315–323. doi: 10.1158/1541-7786.MCR-16-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hafezi F., Perez Bercoff D. The Solo play of TERT promoter mutations. Cells. 2020;9(3):749. doi: 10.3390/cells9030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn S., Figl A., Rachakonda P.S., et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 14.Huang F.W., Hodis E., Xu M.J., Kryukov G.V., Chin L., Garraway L.A. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339(6122):957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu C.P., Hsu N.Y., Lee L.W., Ko J.L. Ets2 binding site single nucleotide polymorphism at the hTERT gene promoter--effect on telomerase expression and telomere length maintenance in non-small cell lung cancer. Eur J Cancer. 2006;42(10):1466–1474. doi: 10.1016/j.ejca.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Rachakonda P.S., Hosen I., de Verdier P.J., et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci U S A. 2013;110(43):17426–17431. doi: 10.1073/pnas.1310522110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidenreich B., Rachakonda P.S., Hosen I., et al. TERT promoter mutations and telomere length in adult malignant gliomas and recurrences. Oncotarget. 2015;6(12):10617–10633. doi: 10.18632/oncotarget.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labussière M., Di Stefano A.L., Gleize V., et al. TERT promoter mutations in gliomas, genetic associations and clinico-pathological correlations. Br J Cancer. 2014;111(10):2024–2032. doi: 10.1038/bjc.2014.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park C.K., Lee S.H., Kim J.Y., et al. Expression level of hTERT is regulated by somatic mutation and common single nucleotide polymorphism at promoter region in glioblastoma. Oncotarget. 2014;5(10):3399–3407. doi: 10.18632/oncotarget.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arita H., Yamasaki K., Matsushita Y., et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun. 2016;4(1):79. doi: 10.1186/s40478-016-0351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nencha U., Rahimian A., Giry M., et al. TERT promoter mutations and rs2853669 polymorphism: prognostic impact and interactions with common alterations in glioblastomas. J Neurooncol. 2016;126(3):441–446. doi: 10.1007/s11060-015-1999-3. [DOI] [PubMed] [Google Scholar]
- 22.Mosrati M.A., Malmström A., Lysiak M., et al. TERT promoter mutations and polymorphisms as prognostic factors in primary glioblastoma. Oncotarget. 2015;6(18):16663–16673. doi: 10.18632/oncotarget.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batista R., Cruvinel-Carloni A., Vinagre J., et al. The prognostic impact of TERT promoter mutations in glioblastomas is modified by the rs2853669 single nucleotide polymorphism. Int J Cancer. 2016;139(2):414–423. doi: 10.1002/ijc.30057. [DOI] [PubMed] [Google Scholar]
- 24.Spiegl-Kreinecker S., Lötsch D., Ghanim B., et al. Prognostic quality of activating TERT promoter mutations in glioblastoma: interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro Oncol. 2015;17(9):1231–1240. doi: 10.1093/neuonc/nov010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nonoguchi N., Ohta T., Oh J.E., Kim Y.H., Kleihues P., Ohgaki H. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013;126(6):931–937. doi: 10.1007/s00401-013-1163-0. [DOI] [PubMed] [Google Scholar]
- 26.Simon M., Hosen I., Gousias K., et al. TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015;17(1):45–52. doi: 10.1093/neuonc/nou158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen H.N., Lie A., Li T., et al. Human TERT promoter mutation enables survival advantage from MGMT promoter methylation in IDH1 wild-type primary glioblastoma treated by standard chemoradiotherapy. Neuro Oncol. 2017;19(3):394–404. doi: 10.1093/neuonc/now189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gramatzki D., Felsberg J., Hentschel B., et al. Telomerase reverse transcriptase promoter mutation- and O6-methylguanine DNA methyltransferase promoter methylation-mediated sensitivity to temozolomide in isocitrate dehydrogenase-wild-type glioblastoma: is there a link? Eur J Cancer. 2021;147:84–94. doi: 10.1016/j.ejca.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Louis D.N., Perry A., Wesseling P., et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Indraccolo S., Lombardi G., Fassan M., et al. Genetic, epigenetic, and immunologic profiling of MMR-deficient relapsed glioblastoma. Clin Cancer Res. 2019;25(6):1828–1837. doi: 10.1158/1078-0432.CCR-18-1892. [DOI] [PubMed] [Google Scholar]
- 31.Gianesin K., Noguera-Julian A., Zanchetta M., et al. Premature aging and immune senescence in HIV-infected children. AIDS. 2016;30(9):1363–1373. doi: 10.1097/QAD.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rampazzo E., Bertorelle R., Serra L., et al. Relationship between telomere shortening, genetic instability, and site of tumour origin in colorectal cancers. Br J Cancer. 2010;102(8):1300–1305. doi: 10.1038/sj.bjc.6605644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dessau R.B., Pipper C.B. ["R"--project for statistical computing] Ugeskr Laeger. 2008;170(5):328–330. [PubMed] [Google Scholar]
- 34.Vuong H.G., Nguyen T.Q., Ngo T.N.M., Nguyen H.C., Fung K.M., Dunn I.F. The interaction between TERT promoter mutation and MGMT promoter methylation on overall survival of glioma patients: a meta-analysis. BMC Cancer. 2020;20(1):897. doi: 10.1186/s12885-020-07364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giunco S., Boscolo-Rizzo P., Rampazzo E., et al. TERT promoter mutations and rs2853669 polymorphism: useful markers for clinical outcome stratification of patients with oral cavity squamous cell carcinoma. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.782658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambrozkiewicz F., Trailin A., Červenková L., et al. CTNNB1 mutations, TERT polymorphism and CD8+ cell densities in resected hepatocellular carcinoma are associated with longer time to recurrence. BMC Cancer. 2022;22(1):884. doi: 10.1186/s12885-022-09989-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dratwa M., Wysoczańska B., Butrym A., Łacina P., Mazur G., Bogunia-Kubik K. TERT genetic variability and telomere length as factors affecting survival and risk in acute myeloid leukaemia. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-02767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko E., Seo H.W., Jung E.S., Kim B hui, Jung G. The TERT promoter SNP rs2853669 decreases E2F1 transcription factor binding and increases mortality and recurrence risks in liver cancer. Oncotarget. 2016;7(1):684–699. doi: 10.18632/oncotarget.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei R., Cao L., Pu H., et al. TERT polymorphism rs2736100-C is associated with EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res. 2015;21(22):5173–5180. doi: 10.1158/1078-0432.CCR-15-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma R., Liu C., Lu M., et al. The TERT locus genotypes of rs2736100-CC/CA and rs2736098-AA predict shorter survival in renal cell carcinoma. Urol Oncol. 2019;37(5):301.e1–301.e10. doi: 10.1016/j.urolonc.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Lu Y., Yan C., Du J., et al. Genetic variants affecting telomere length are associated with the prognosis of esophageal squamous cell carcinoma in a Chinese population. Mol Carcinog. 2017;56(3):1021–1029. doi: 10.1002/mc.22567. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z., Wang J., Bai Y., et al. The associations of TERT-CLPTM1L variants and TERT mRNA expression with the prognosis of early stage non-small cell lung cancer. Cancer Gene Ther. 2017;24(1):20–27. doi: 10.1038/cgt.2016.74. [DOI] [PubMed] [Google Scholar]
- 43.Giunco S., Rampazzo E., Celeghin A., Petrara M.R., De Rossi A. Telomere and telomerase in carcinogenesis: their role as prognostic biomarkers. Curr Pathobiol Rep. 2015;3(4):315–328. [Google Scholar]
- 44.Lötsch D., Ghanim B., Laaber M., et al. Prognostic significance of telomerase-associated parameters in glioblastoma: effect of patient age. Neuro Oncol. 2013;15(4):423–432. doi: 10.1093/neuonc/nos329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.