Abstract
Background
Up to 30% of metastatic breast cancer (BC) patients develop brain metastases (BM). Prognosis of patients with BM is poor and long-term survival is rare. Identification of factors associated with long-term survival is important for improving treatment modalities.
Patients and methods
A total of 2889 patients of the national registry for BM in BC (BMBC) were available for this analysis. Long-term survival was defined as overall survival (OS) in the upper third of the failure curve resulting in a cut-off of 15 months. A total of 887 patients were categorized as long-term survivors.
Results
Long-term survivors compared to other patients were younger at BC and BM diagnosis (median 48 versus 54 years and 53 versus 59 years), more often had HER2-positive tumors (59.1% versus 36.3%), less frequently luminal-like (29.1% versus 35.7%) or triple-negative breast cancer (TNBC) (11.9% versus 28.1%), showed better Eastern Cooperative Oncology Group (ECOG) performance status (PS) at the time of BM diagnosis (ECOG 0-1, 76.9% versus 51.0%), higher pathological complete remission rates after neoadjuvant chemotherapy (21.6% versus 13.7%) and lower number of BM (n = 1, BM 40.9% versus 25.4%; n = 2-3, BM 26.5% versus 26.7%; n ≥4, BM 32.6% versus 47.9%) (P < 0.001). Long-term survivors had leptomeningeal metastases (10.4% versus 17.5%) and extracranial metastases (ECM, 73.6% versus 82.5%) less frequently, and asymptomatic BM more often at the time of BM diagnosis (26.5% versus 20.1%), (P < 0.001). Median OS in long-term survivors was about two times higher than the cut-off of 15 months: 30.9 months [interquartile range (IQR) 30.3] overall, 33.9 months (IQR 37.1) in HER2-positive, 26.9 months (IQR 22.0) in luminal-like and 26.5 months (IQR 18.2) in TNBC patients.
Conclusions
In our analysis, long-term survival of BC patients with BM was associated with better ECOG PS, younger age, HER2-positive subtype, lower number of BM and less extended visceral metastases. Patients with these clinical features might be more eligible for extended local brain and systemic treatment.
Key words: brain metastases, breast cancer, long-term survivors
Highlights
-
•
Median OS in long-term survivors was about two times higher than the cut-off of 15 months (IQR 30.3 months).
-
•
Median OS according to subtypes were 33.9 in HER2-positive, 26.9 in luminal-like and 26.5 months in TNBC patients
-
•
Long-term prognostic factors were age, ECOG, number of BM, pos. HR/HER2 status, no ECM and syst. treatment after BM.
Introduction
The incidence of brain metastases (BM) from breast cancer (BC) is increasing, treatment options are limited and the management of those patients is a major challenge in clinical daily routine.1 Furthermore, the patients’ prognoses are poor and could not be significantly improved over the past decades.2
Many prognostic factors are known to be associated with a higher risk of developing BM such as young age, higher tumor stage at diagnosis, histological grade, tumor size, tumor biology and nodal status.3
Triple-negative and HER2-positive subtype are predictive factors for developing BM.4 BM rates of 25%-46% for patients with metastatic triple-negative breast cancer (TNBC) and 30%-50% in patients with metastatic HER2-positive BC have been published.5
Despite commonly known short median survival times of BC patients with BM differing between 7 and 8 months, long-term survivors with survival rates longer than 2 years represent around 25% of the whole cohort as shown in a former analysis of the BMBC registry in 2018 with a total of 1712 patients.2 Nonetheless, therapeutic options for systemic therapy of BM and clinical management of BMBC patients remain unsatisfactory and the overall survival (OS) rates are still poor.
Until now only fewer data have been published concerning long-term survival among BMBC patients. Altundag et al.6 described a cohort of 82 BC patients with BM with long-term survival defined as survival of >18 months. Murthy et al. reported nine patients with long-term survival longer than 5 years among 66 BMBC patients.7
Identifying the factors associated with long-term survival could help optimize diagnostic and therapeutic options for those patients who are more eligible for extended treatment modalities.
In the large BMBC registry, a German multicenter registry, the clinical data of BMBC patients are documented and, simultaneously, translational projects on available tumor samples of BMBC patients are carried out.
The aim of this retrospective analysis was to characterize the cohort of long-term survivors in the BMBC registry defined as survival in the upper third of the failure curve due to a lack of a commonly accepted definition in published data and to identify the clinical factors associated with long-term survival among those patients.
Patients and methods
Patients in the national multicenter BMBC registry registered before the 5 December 2020 were included in this analysis. The aims of the evaluation were as follows:
-
1.
To assess the OS and progression-free survival (PFS) after diagnosis of BM in the updated number of patients of the BMBC registry.
-
2.
To characterize the OS, and short-term and long-term survival after diagnosis of BM in an explorative analysis regarding different clinical parameters: Age at first diagnosis of BC and of BM, number of BM, localization of BM, tumor grading, histological tumor type, Eastern Cooperative Oncology Group (ECOG)/Karnofsky performance status (PS) at the time of BM diagnosis, existence of leptomeningeal metastases, maximum diameter of BM at first BM diagnosis, neurological symptoms at first diagnosis of BM, extracranial metastases (ECM) at the time of BM diagnosis, localization of ECM, year of diagnosis, therapies of BM (first therapy of BM).
-
3.
Comparison of the OS (categorized in long-term and short-term survival) after diagnosis of BM between the groups of BC subtypes (HER2-positive, luminal-like, TNBC).
Biological subtypes were defined as HER2-positive, TNBC [estrogen (ER)- and progesterone (PR)-negative, and HER2-negative] and luminal-like (ER- and/or PR-positive, HER2-negative; luminal A- and B-like subtypes were evaluated together because of the missing values of tumor grading causing an issue in the strict distinction of luminal A-like and luminal B-like patients).
Continuous data were summarized using the number of available data, mean, standard deviation, median, minimum and maximum for each group. Categorical and ordinal data were summarized using the number and percentage of patients in each group. OS was defined as the time interval from first diagnosis of BM to death due to any reason.
PFS was defined as the time interval from first diagnosis of BM to first progress of BM or ECM or death due to any cause. Further, Kaplan–Meier curves, the median survival times and the survival rates after 2, 3 and 4 years with the corresponding 95% confidence intervals (CIs) were determined. Differences in the survival curves were tested by the log-rank test. Univariate and multivariate Cox proportional hazards models for OS were carried out to report hazard ratios with the corresponding 95% CIs and to adjust for the covariates. For the analysis of the time intervals from first diagnosis of BC to diagnosis of BM, cumulative incidence rates were determined with the diagnosis of ECM as competing risk. Differences in the cumulative incidence curves were tested by Gray’s test.
Long-term survival was defined as OS in the upper third of the failure curve resulting in a cut-off of 15 months. Short-term survival, therefore, was defined as OS <15 months. Patients censored at a time point lower than 15 months were automatically assigned to the group of short-term survivors.
Univariate and multivariate logistic regression analyses were carried out to report odds ratios with the corresponding 95% CIs.
All reported P values were two-sided, and the significance level was set at 0.05. CIs symmetrically cover 95%. Adjustment for multiple testing was not planned. The data were analyzed using SAS® (Statistical Analysis Software, SAS Institute, Cary, NC) version 9.4 with SAS Enterprise Guide Version 7.1 and 8.3 on Microsoft Windows 10 Enterprise. Ethical approval: FF42/2013, Ethikkommission at the Landesärztekammer Hessen.
Results
Patients’ characteristics
Clinical data of 2889 patients from the BMBC registry were available for the analysis. Survival in the upper third of the survival curve resulted in an OS >15 months. A total of 887 patients could be categorized as long-term survivors. Accordingly, all patients living <15 months were included in the group of short-term survivors (n = 2002).
In the overall cohort (n = 2889), the median age at BC and BM diagnosis was 52 and 57 years, respectively. A total of 1667 (60.1%) of the patients were hormone receptor (HR)-positive (ER- and/or PR-positive), 1105 (39.9%) were HR-negative (ER- and PR-negative), 1091 (41.8%) patients were HER2-positive and 1522 (58.2%) were HER2-negative. A total of 442 (15.3%) of the patients showed a leptomeningeal disease at the time of BM diagnosis. A total of 1484 (56.9%) of the patients had 1-3 BM and 1122 (43.1%) had >4 BM at first diagnosis. ECM at the time of BM diagnosis could be detected in 2304 (79.8%) of the patients. The majority of the patients (58.9%; n = 763) had a good PS (ECOG 0-1) at the time of BM diagnosis.
Detailed patients’ characteristics of the overall cohort as well as in short-term and long-term survivors are shown in Table 1.
Table 1.
Comparison of patients' characteristics between short-term and long-term survival categorical parameters
| Parameter | Category | Short-term survivors N = 2002 (%) | Long-term survivors N = 887 (%) | Overall N = 2889 (%) | P valuea |
|---|---|---|---|---|---|
| Age at first diagnosis of BC, years | Median | 54.0 | 48.0 | 52.0 | <0.001 |
| Age at diagnosis of BM, years | Median | 59.0 | 53.0 | 57.0 | <0.001 |
| HER2-status at diagnosis of BC | Negative | 1180 (65.1) | 342 (42.8) | 1522 (58.2) | <0.001 |
| Positive | 633 (34.9) | 458 (57.3) | 1091 (41.8) | ||
| Missing | 189 | 87 | 276 | ||
| Hormone receptor status | Negative | 826 (42.8) | 279 (33.1) | 1105 (39.9) | <0.001 |
| Positive | 1104 (57.2) | 563 (66.9) | 1667 (60.1) | ||
| Missing | 72 | 45 | 117 | ||
| Tumor subtypeb | HER2+ | 668 (36.3) | 492 (59.1) | 1160 (43.4) | <0.001 |
| Luminal-like | 657 (35.7) | 242 (29.1) | 899 (33.6) | ||
| TNBC | 517 (28.1) | 99 (11.9) | 616 (23.0) | ||
| Missing | 160 | 54 | 214 | ||
| Pathological tumor stage after neoadjuvant therapy | ypT0 | 83 (13.7) | 60 (21.6) | 143 (16.2) | <0.001 |
| ypTis | 33 (5.4) | 22 (7.9) | 55 (6.2) | ||
| ypT1 | 174 (28.7) | 95 (34.2) | 269 (30.4) | ||
| ypT2 | 180 (29.7) | 58 (20.9) | 238 (26.9) | ||
| ypT3 | 71 (11.7) | 22 (7.9) | 93 (10.5) | ||
| ypT4a-c | 52 (8.6) | 14 (5.0) | 66 (7.5) | ||
| ypT4d | 13 (2.1) | 7 (2.5) | 20 (2.3) | ||
| Missingc | 1396 | 609 | 2005 | ||
| Tumor grading | G1 | 34 (1.9) | 12 (1.5) | 46 (0.8) | 0.019 |
| G2 | 723 (39.5) | 362 (45.4) | 1085 (41.3) | ||
| G3 | 1073 (58.6) | 424 (53.1) | 1497 (57.0) | ||
| Missing | 172 | 89 | 261 | ||
| ECOG (Karnofsky performance status) | ECOG 0 (100%) | 95 (10.6) | 90 (22.6) | 185 (14.3) | <0.001 |
| ECOG 1 (80%-90%) | 362 (40.4) | 216 (54.3) | 578 (44.6) | ||
| ECOG 2 (60%-70%) | 296 (33.0) | 76 (19.1) | 372 (28.7) | ||
| ECOG 3 (40%-50%) | 113 (12.6) | 15 (3.8) | 128 (9.9) | ||
| ECOG 4 (10%-30%) | 31 (3.5) | 1 (0.3) | 32 (2.5) | ||
| Missing | 1105 | 489 | 1594 | ||
| Number of BM | 1 | 453 (25.4) | 337 (40.9) | 790 (30.3) | <0.001 |
| 2-3 | 476 (26.7) | 218 (26.5) | 694 (26.6) | ||
| ≥4 | 853 (47.9) | 269 (32.6) | 1122 (43.1) | ||
| Missing | 220 | 63 | 283 | ||
| Leptomeningeal disease (clinical diagnosis) | No | 1652 (82.5) | 795 (89.6) | 2447 (84.7) | <0.001 |
| Yes | 350 (17.5) | 92 (10.4) | 442 (15.3) | ||
| Cytological confirmed meningiosis at diagnosis of BM | No | 1743 (88.6) | 816 (93.3) | 2559 (90.0) | <0.001 |
| Yes | 224 (11.4) | 59 (6.7) | 283 (10.0) | ||
| Missing | 35 | 12 | 47 | ||
| Local treatment of BM | Surgery only | 88 (5.7) | 47 (5.7) | 135 (5.7) | <0.001 |
| RT only | 1184 (77.3) | 462 (55.8) | 1646 (69.8) | ||
| Surgery and RT | 259 (16.9) | 319 (38.5) | 578 (24.5) | ||
| Missing | 471 | 59 | 530 | ||
| ECM at diagnosis of BMd | No | 351 (17.5) | 234 (26.4) | 585 (20.2) | <0.001 |
| Yes | 1651 (82.5) | 653 (73.6) | 2304 (79.8) | ||
| Missing | 0 | 0 | 0 |
BM, brain metastases; ECM, extracranial metastases; ECOG, Eastern Cooperative Oncology Group; NCTX, neoadjuvant chemotherapy; pCR, pathological complete remission; RT, radiotherapy; TNBC, triple-negative breast cancer.
Fisher’s exact test resp. Chi-square test between short-term and long-term survivors.
If HER2-status at diagnosis of BC was unknown, but anti-HER2-targeted therapy was given, the subtype was set to HER2-positive.
Missing data include patients without NCTX and patients with NCTX and missing information about pCR.
Diagnosis of ECM not later than 60 days after diagnosis of BM.
Survival analysis
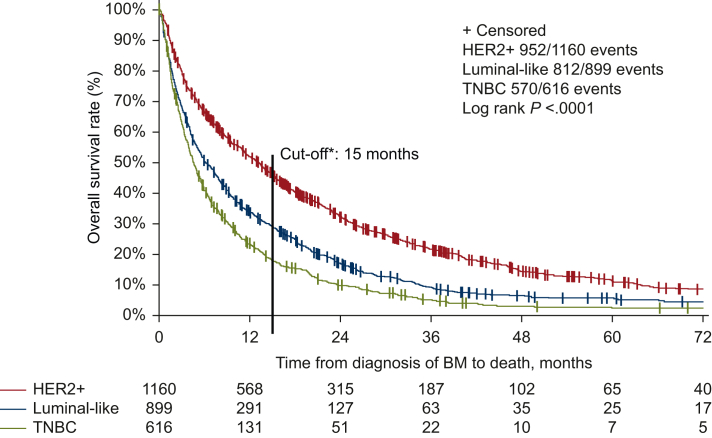
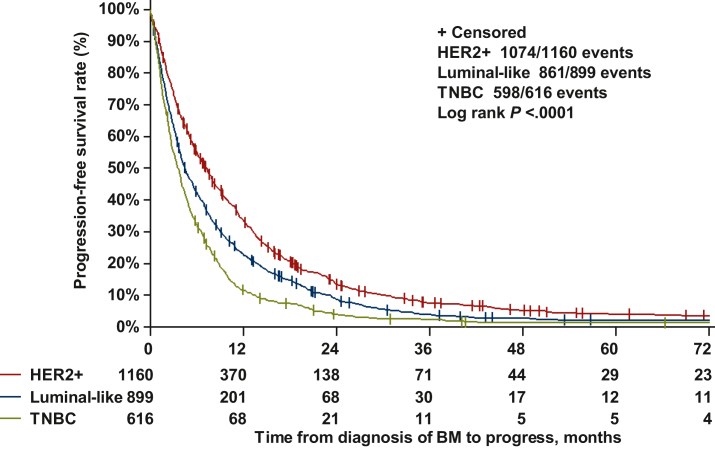
Median survival in the overall cohort was 7.5 months (95% CI 6.9-8.0 months) overall, 13.2 months (95% CI 11.4-14.4 months) in HER2-positive, 6.1 months (95% CI 5.4-7.3 months) in luminal-like and 4.5 months (95% CI 4.0-5.1 months) in TNBC patients (P < 0.0001, Figure 1). The association between OS and other clinicopathological parameters is shown in Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2023.101213 (univariate and multivariate Cox regression analysis). Median PFS was 5.0 months overall, 7.3 months in HER2-positive (95% CI 6.5-7.9 months), 4.5 months (95% CI 4.0-5.1 months) months in luminal-like and 3.5 months (95% CI 3.2-4.0 months) in TNBC patients (P < 0.0001, Figure 2). Time from BC diagnosis until diagnosis of BC according to tumor subtype is shown in Supplementary Figure 1, available at https://doi.org/10.1016/j.esmoop.2023.101213.
Figure 1.
OS in the overall cohort according to tumor subtypes.
∗Previously published figure25 was updated by adding a line with the cut-off of 15 months dividing the patients into groups of long-term versus short-term survivors which corresponds to an OS in the upper third versus the two lower thirds of the failure curve. BM, brain metastases; OS, overall survival; TNBC, triple-negative breast cancer.
Figure 2.
Progression-free survival (time from diagnosis of BM to progress of BM, 25 ECM or death) in the overall cohort according to tumor subtypes.
BM, brain metastases; TNBC, triple-negative breast cancer.
Median OS in long-term survivors was about two times higher than the cut-off of 15 months, 30.9 months [interquartile range (IQR) 30.3 months] overall, 33.9 months (IQR 37.1 months) in HER2-positive, 26.9 months (IQR 22.0 months) in luminal-like and 26.5 months (IQR 18.2 months) in TNBC patients. Within the group of long-term survivors, estimated 2-year survival rates depending on different subtypes were 70.2% (95% CI 65.8% to 74.1%) for HER2-positive, 58.6% (95% CI 51.9% to 64.7%) for luminal-like and 55.3% (95% CI 44.8% to 64.6%) for TNBC patients. Looking at the probability of a 4-year survival the following results were estimated: 31.7% (95% CI 27.1% to 36.4%) for HER2-positive, 23.1% (95% CI 17.4% to 29.2%) for luminal-like and 17.2% (95% CI 9.6% to 26.7%) for TNBC patients (table not shown, see Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.101213).
Comparison of patients’ characteristics between long-term and short-term survival
Long-term survivors compared to other patients were younger at BM diagnosis (median 53 years versus 59 years, P < 0.001). 59.1% (n = 492) of the long-term survivors had a HER2-positive, 29.1% (n = 242) had a luminal-like and 11.9% (n = 99) had a TNBC subtype. More than half of the long-term survivors (53.1%) had a poorly differentiated primary tumor (G3). Compared to other patients, long-term survivors showed better ECOG PS at the time of BM diagnosis (ECOG PS 0-1, 76.9% versus 51.0%), higher pathological complete remission (pCR) rate after neoadjuvant chemotherapy (21.6% versus 13.7%) and lower number of BM (n = 1 BM, 40.9% versus 25.4%; n = 2-3, BM 26.5% versus 26.7%; n ≥4, BM 32.6% versus 47.9%) (P < 0.001). Moreover, long-term survivors had leptomeningeal metastases (10.4% versus 17.5%) and ECM (73.6% versus 82.5%) less often, asymptomatic BM more often at the time of BM diagnosis (26.5% versus 20.1%) and more often received radiotherapy and surgery for the BM than radiotherapy alone (38.5% versus 16.9%) (P < 0.001, Table 1). In univariate analysis we carried out a logistic regression analysis showing the same prognostic factors being statistically significant for a long-term survival above 15 months (for details see Table 2).
Table 2.
Probability of long-term survival according to different clinical parameters (univariate logistic regression analysis)
| Parameter at diagnosis | Category | Number of events (%) | Odds ratioa | 95% CI | P value |
|---|---|---|---|---|---|
| Ageb | <60 years | 608 (37.0) | |||
| ≥60 years | 279 (22.4) | 0.49 | 0.42-0.58 | <0.001 | |
| ECOGb | 0-1 | 306 (40.1) | |||
| 2-4 | 92 (17.3) | 0.31 | 0.24-0.41 | <0.001 | |
| Hormone receptor | Negative | 279 (25.2) | |||
| Positive | 563 (33.8) | 1.51 | 1.27-1.79 | <0.001 | |
| HER2 receptor | Negative | 342 (22.5) | |||
| Positive | 458 (42.0) | 2.50 | 2.11-2.96 | <0.001 | |
| Biological Subtype | HER2+ | 492 (42.2) | |||
| Luminal-like | 242 (26.9) | 0.50 | 0.41-0.60 | <0.001 | |
| TNBC | 99 (16.1) | 0.26 | 0.24-0.33 | <0.001 | |
| Number of BM | 1 | 337 (42.7) | <0.001 | ||
| 2-3 | 218 (31.4) | 0.62 | 0.50-0.76 | <0.001 | |
| ≥4 | 269 (24.0) | 0.42 | 0.35-0.52 | <0.001 | |
| pCR (ypT0) | No | 218 (29.4) | |||
| Yes | 60 (42.0) | 1.73 | 1.20-2.51 | 0.003 | |
| Leptomeningeal metastases | No | 795 (32.5) | |||
| Yes | 92 (20.8) | 0.55 | 0.43-0.70 | <0.001 | |
| Clinical symptomsb | No | 235 (36.9) | |||
| Yes | 652 (29.0) | 0.70 | 0.58-0.84 | <0.001 | |
| ECMb | No | 234 (40.0) | |||
| Yes | 653 (28.3) | 0.59 | 0.49-0.72 | <0.001 | |
| Chemotherapy after diagnosis of BM | No | 388 (22.0) | |||
| Yes | 499 (44.2) | 2.8 | 2.38-3.30 | <0.001 | |
| Endocrine therapy | No | 649 (26.0) | |||
| Yes | 238 (60.7) | 4.4 | 3.53-5.49 | <0.001 | |
| HER2-targeted therapy | No | 429 (20.4) | |||
| Yes | 458 (58.0) | 5.37 | 4.50-6.41 | <0.001 |
BM, brain metastases; CI, confidence interval; ECM, extracranial metastases; ECOG, Eastern Cooperative Oncology Group; pCR, pathological complete remission; TNBC, triple-negative breast cancer.
An odds ratio ≥1 means to have a higher probability to be assigned to the group of long-term survivors.
At diagnosis of BM.
Multivariate analysis
In multivariate analysis (Table 3), the following factors were confirmed to be associated with longer survival (P < 0.001): younger age [<60 versus ≥60, odds ratio (OR) 0.59], better PS (ECOG 0-1 versus ECOG 2-4, OR 0.45), lower number of BM (1 versus 2-3 versus ≥4, OR 0.79, 0.46), positive HR status (positive versus negative OR 1.87), positive HER2 status (positive versus negative OR 2.74), no ECM at the time of BM diagnosis (yes versus no OR 0.65) as well as chemotherapy application after the BM diagnosis (yes versus no OR 2.19). In contrast, pCR rates after neoadjuvant therapy of primary BC (yes versus no, OR 1.8, P = 0.069), leptomeningeal disease (yes versus No, OR 0.99, P = 0.949) and clinical symptoms at BM diagnosis (yes versus no, OR 0.94, P = 0.726) were not associated with a categorization in the group of long-term survivors.
Table 3.
Probability of long-term survival according to different clinical parameters (multivariate logistic regression analysis)
| Parameter at diagnosis | Category | Odds ratioa | 95% CI | P value |
|---|---|---|---|---|
| Ageb | <60 years | 1 | ||
| ≥60 years | 0.59 | 0.44-0.79 | <0.001 | |
| ECOGb | 0-1 | 1 | ||
| 2-4 | 0.45 | 0.33-0.61 | <0.001 | |
| Hormone receptor status | Negative | 1 | ||
| Positive | 1.87 | 1.39-2.50 | <0.001 | |
| HER2 status | Negative | 1 | ||
| Positive | 2.74 | 2.06-3.64 | <0.001 | |
| Number of BM | 1 | 1 | <0.001 | |
| 2-3 | 0.79 | 0.55-1.13 | 0.191 | |
| ≥4 | 0.46 | 0.33-0.65 | <0.001 | |
| pCR | No | 1 | ||
| Yes | 1.8 | 0.995-3.38 | 0.069 | |
| Leptomeningeal metastases | No | 1 | ||
| Yes | 0.99 | 0.64-1.53 | 0.949 | |
| Clinical symptomsb | No | 1 | ||
| Yes | 0.94 | 0.66-1.34 | 0.726 | |
| ECMb | No | 1 | ||
| Yes | 0.65 | 0.46-0.93 | 0.017 | |
| Chemotherapy after diagnosis of BM | No | 1 | ||
| Yes | 2.19 | 1.64-2.92 | <0.001 |
BM, brain metastases; CI, confidence interval; ECM, extracranial metastases; ECOG, Eastern Cooperative Oncology Group; pCR, pathological complete remission.
An odds ratio ≥1 means to have a higher probability to be assigned to the group of long-term survivors.
At diagnosis of BM.
Discussion
In our analysis of 2889 BC patients with BM, 887 patients showed a long-term survival, defined as survival in the upper third of the failure curve, resulting in survival above 15 months in the present analysis. There is no generally accepted definition for long-term survival with BM irrespective of primary tumor type in published data. Long-term survival times between 12 months,8 24 months9 and up to 5 years10 can be found in the literature. A median OS of BC patients with BM ranging between 6 months for the triple-negative subtype and 21 months for the triple-positive subtype was reported in the SEER database.11 A survival of >15 months can be therefore regarded as long in this group of real-world patients with poor prognosis. Creating a dichotomic system for survival times could prove helpful for the daily clinical routine in order to help choose eligible patients for certain therapy strategies and predictions of estimated survival prognosis. Nonetheless, there are certain limitations to such a categorization system including censored patients in the survival curve being automatically assigned to the group of short-term survivors due to missing data. But an additionally carried-out Cox regression analysis for the whole cohort supported the results regarding prognostic factors (data not shown, see Supplementary data Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2023.101213). This should be taken into consideration while interpreting categorized survival times.
Regarding our analysis, several factors were associated with long-term survival, including younger age (below 60), a good ECOG PS, asymptomatic BM, HER2-positive subtype, achieving a pCR after neoadjuvant chemotherapy, a limited number of BM, the absence of leptomeningeal metastases and no ECM at the time of BM diagnosis.
Our findings are in line with results of descriptive analyses of other groups.12 Altundag et al.6,13 reported in a retrospective study of 420 BM BC patients with a survival longer than 18 months that younger age, ER-positive subtype and a limited number of BM were associated with longer survival. Lee et al. showed that PS, number of BM, treatment modalities and systemic chemotherapy after BM were associated with better prognosis and therefore longer survival but reported that only 23.1% of the patients survived longer than 12 months.12
Sperduto et al.14 also reported a median survival time in BC patients with BM differing between 3.4 and 25.3 months depending on different factors such as PS, age and subtype and therefore established the first diagnosis-specific graded prognostic assessment (GPA) score in order to identify BC patients with longer survival being eligible for treatment. There are various prognostic scores that have been developed trying to categorize patients in different prognostic groups including many of those clinical parameters named. The GPA breast score in its updated version,15 for example, includes ECOG status, BC subtype, age, number of BM and ECM in the calculation. However, our group has already shown that the sensitivity and specificity of all published scores are limited.16,17 The probability of classifying patients with survival above 12 months in the best prognostic group differed between a specificity of 68.7% for the breast GPA compared with 48.1% for the updated breast GPA and 21.8% for the original GPA.16 Thus, patients might be also categorized in groups that do not reflect their prognosis by calculating scores with clinical parameters. Therefore, we aimed to further investigate a cohort of long-term survivors with BM in the BMBC registry. Indeed, in our analysis, we found that in addition to already published parameters, a pCR rate after neoadjuvant chemotherapy was associated with longer survival rates in case of BM disease in univariate analysis but not in multivariate analysis. This could be explained by pCR depending on different covariates such as subtype, grading and lymph node status. In two large neoadjuvant chemotherapy trials (n >3000 patients), we reported that a non-pCR was associated with a higher probability of developing BM as the first site of metastatic disease.18
Interestingly, we could not confirm leptomeningeal metastases and asymptomatic BM as independent prognostic variables for survival in multivariate analysis after having shown an association with longer survival in univariate analysis. Other reports also show that there are a few patients with leptomenigeal metastases with a reported 1-year survival rate of 20%.19 In line with those results, 6.4% of our patients among the long-term survivors had cytologically confirmed leptomeningeal disease, showing that even in the group of a leptomeningeal disease with poor prognosis with a reported median survival time of 3-4 months a subgroup with a better prognosis exists.11
In the univariate analysis, we could confirm that patients with asymptomatic BM had a higher chance of achieving a long-term survival above 15 months. One of the possible reasons for no significant association in the multivariate analysis could be a small number of patients with asymptomatic BM at first diagnosis in the BMBC registry. Furthermore, we could show that a lower number of BM was associated with a higher probability of achieving a long-term survival. In our previous analysis of patients with asymptomatic BM, a significant correlation between asymptomatic BM and lower number of BM could be shown.17 Therefore, our analysis provides a rationale for modern study concepts investigating early detection approaches of BM. Until now, it is unclear whether there is a gain in survival time or a lead-time bias due to the earlier detection of BM.
In addition, we observed that receiving systemic therapy, namely chemotherapy, endocrine or targeted therapy, was associated with long-term survival in univariate and/or multivariate analysis. This could be explained by a better visceral disease control and therefore longer survival.20
Several targeted therapeutic options and novel agents especially in the HER2-positive subgroup such as trastuzumab deruxtecan21,22 and tucatinib23 have also shown an intracranial effect of systemic therapies. The application of this treatment options could result in a relevant further improvement of survival of patients with BM of BC.
Concerning tumor biology, our analysis indicates that patients with HER2-positive and HR-positive tumors were more often represented in the group of long-term survivors in our analysis in contrast to patients with TNBC tumors who had a lower probability of long-term survival which is in line with published data.20,24 A different mechanism of tumor cell dissemination in the brain depending on tumor subtype can be assumed. The value of clinical data might be limited to further understand the nature of long-term survival in BC patients with BM.
Therefore, the translational research project of the BMBC registry aims at setting up a scientific base for research projects to explore factors associated with BM development and prognosis depending on BC subtypes.
Conclusions
In conclusion, our analysis showed that several clinical factors such as younger age, better PS, lower number of BM, positive HR status, positive HER2 status, no ECM at the time of BM diagnosis as well as application of the systemic treatment after the BM diagnosis were associated with long-term survival in BC patients with BM.
Further research should be carried out to better understand those factors associated with long-term survivor with BM. Our results provide a rationale for the design of studies to prove intensified treatment strategies in this patient cohort.
Acknowledgments
Funding
Financial support for the management of BMBC registry was provided by an unrestricted research grant from Daiichi-Sankyo, Tokio, Japan to GBG (grant number PO 4500316539).
Disclosure
IW received speaker’s honoraria from Astra Zeneca, Merck, Pfizer, Roche, Daiichi-Sankyo, Seagen, Gilead and Novartis. KL received speaker’s honoraria from Roche, Novartis, Pfizer, Exact Sciences, MSD, Eisai, Lilly, Seagen, AstraZeneca and Daiichy Sankyo. MS has received personal fees from AstraZeneca, BioNTech, Daiichi-Sankyo, Eisai, Lilly, MSD, Novartis, Pantarhei Bioscience, Pfizer, Pierre Fabre, Roche and Seagen. His institution has received research funding from AstraZeneca, BioNTech, Eisai, Genentech, German Breast Group, Novartis, Palleos, Pantarhei Bioscience, Pierre Fabre and Seagen. In addition, he has a patent for EP 2390370 B1 and a patent for EP 2951317 B1 issued. SL reports grants, non-financial support and other from Daiichi-Sankyo, during the conduct of the study; grants and other from Abbvie, other from Amgen, grants and other from AstraZeneca, other from BMS, grants and other from Celgene, other from Eirgenix, other from Eisai Europe Ltd, other from GSK, grants, non-financial support and other from Immunomedics/Gilead, other from Lilly, other from Merck, grants from Molecular Health, grants, non-financial support and other from Novartis, grants, non-financial support and other from Pfizer, other from Pierre Fabre, other from Relay Therapeutics, grants, non-financial support and other from Roche, other from Sanofi, non-financial support and other from Seagen, other from Olema Pharmaceutics, outside the submitted work; In addition, SL has a patent EP14153692.0 pending, a patent EP21152186.9 pending, a patent EP15702464.7 issued, a patent EP19808852.8 pending and a patent Digital Ki67 Evaluator with royalties paid. CD reports advisory role for MSD Oncology, Daiichi-Sankyo, Molecular Health, AstraZeneca, Roche and Lilly; CD received research funding from Myriad Genetics, Roche and German Breast Group; CD owns following patents: VMScope digital pathology software, Patent WO2020109570A1, Patent WO2015114146A1, Patent WO2010076322A1. MT received personal fees from Agendia, Amgen, Art tempi, AstraZeneca, Aurikamed, Celgene, Daiichi-Sankyo, Eisai, Exact Sciences, Gilead Science, Grünenthal, GSK, Hexal, Lilly, MSD, Novartis, Onkowissen, Organon, Pfizer, Pierre Fabre, Roche, Seagen, Servier, Streamd Up!, Viatris, Vifor, trial funding from Exact Science and institutional trial honoraria from AstraZeneca, Celgene, Novartis, Roche. VM received speaker honoraria from Amgen, Astra Zeneca, Daiichi-Sankyo, Eisai, GSK, Pfizer, MSD, Medac, Novartis, Roche, Teva, Seagen, Onkowissen, high5 Oncology, Medscape, Gilead and Pierre Fabre; consultancy honoraria from Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi-Sankyo, Eisai, Lilly, Sanofi, Seagen and Gilead; Institutional research support from Novartis, Roche, Seagen, Genentech; travel grants: Roche, Pfizer, Daiichi-Sankyo, Gilead. RW received support for advisory, consultancy, speaker and travel grants from Agendia, Amgen, Aristo, AstraZeneca, Boeringer Ingelheim, Carl Zeiss, Celgene, Clinsol, Clovis Oncology, Daiichi-Sankyo, Eisai, Exact Sciences, Genomic Health, Gilead, Glaxo Smith Kline, Hexal, Lilly, Medstrom Medical, MSD, Mundipharma, Mylan, Nanostring, Novartis, Odonate, Paxman, Palleos, Pfizer, Pierre Fabre, PINK, PumaBiotechnolgogy, Riemser, Roche, Sandoz/Hexal, Sanofi Genzyme, Seattle Genetics /Seagen, Stemline, Tesaro Bio, Teva, Veracyte and Viatris. TD received Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, IOMEDICO and participated on a Data Safety Monitoring Board or Advisory Board for Novartis, IOMEDICO. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Smedby K.E., Brandt L., Backlund M.L., Blomqvist P. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer. 2009;101(11):1919–1924. doi: 10.1038/sj.bjc.6605373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witzel I., Laakmann E., Weide R., et al. Treatment and outcomes of patients in the brain metastases in breast cancer network registry. Eur J Cancer. 2018;102:1–9. doi: 10.1016/j.ejca.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Koniali L., Hadjisavvas A., Constantinidou A., et al. Risk factors for breast cancer brain metastases: a systematic review. Oncotarget. 2020;11(6):650–669. doi: 10.18632/oncotarget.27453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cacho-Díaz B., Salmerón-Moreno K., Alvarez-Alvarez A., et al. Identification of risk factors for central nervous system metastasis in patients with breast cancer with neurologic symptoms. Cancer. 2020;126(15):3456–3463. doi: 10.1002/cncr.32928. [DOI] [PubMed] [Google Scholar]
- 5.Lin N.U., Amiri-Kordestani L., Palmieri D., Liewehr D.J., Steeg P.S. CNS metastases in breast cancer: old challenge, new frontiers. Clin Cancer Res. 2013;19(23):6404–6418. doi: 10.1158/1078-0432.CCR-13-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altundag K., Bondy M.L., Mirza N.Q., et al. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis. Cancer. 2007;110(12):2640–2647. doi: 10.1002/cncr.23088. [DOI] [PubMed] [Google Scholar]
- 7.Murthy P., Kidwell K.M., Schott A.F., et al. Clinical predictors of long-term survival in HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2016;155(3):589–595. doi: 10.1007/s10549-016-3705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhaak E., Schimmel W.C.M., Gehring K., Hanssens P.E.J., Sitskoorn M.M. Cognitive functioning and health-related quality of life of long-term survivors with brain metastases up to 21 months after gamma knife radiosurgery. Neurosurgery. 2021;88(5):E396–E405. doi: 10.1093/neuros/nyaa586. [DOI] [PubMed] [Google Scholar]
- 9.Enders F., Geisenberger C., Jungk C., et al. Prognostic factors and long-term survival in surgically treated brain metastases from non-small cell lung cancer. Clin Neurol Neurosurg. 2016;142:72–80. doi: 10.1016/j.clineuro.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Chao S.T., Barnett G.H., Liu S.W., et al. Five-year survivors of brain metastases: a single-institution report of 32 patients. Int J Radiat Oncol Biol Phys. 2006;66(3):801–809. doi: 10.1016/j.ijrobp.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Lamba N., Wen P.Y., Aizer A.A. Epidemiology of brain metastases and leptomeningeal disease. Neuro Oncol. 2021;23(9):1447–1456. doi: 10.1093/neuonc/noab101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S.S., Ahn J.H., Kim M.K., et al. Brain metastases in breast cancer: prognostic factors and management. Breast Cancer Res Treat. 2008;111(3):523–530. doi: 10.1007/s10549-007-9806-2. [DOI] [PubMed] [Google Scholar]
- 13.Altundag K. Characteristics of breast cancer patients with brain metastases who live longer than 18 months. Breast. 2017;34:132–133. doi: 10.1016/j.breast.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Sperduto P.W., Kased N., Roberge D., et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperduto P.W., Mesko S., Li J., et al. Beyond an updated graded prognostic assessment (breast GPA): a prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int J Radiat Oncol Biol Phys. 2020;107(2):334–343. doi: 10.1016/j.ijrobp.2020.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riecke K., Müller V., Weide R., et al. Predicting prognosis of breast cancer patients with brain metastases in the BMBC registry-comparison of three different GPA prognostic scores. Cancers (Basel) 2021;13(4):844. doi: 10.3390/cancers13040844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laakmann E., Witzel I., Neunhöffer T., et al. Characteristics and clinical outcome of breast cancer patients with asymptomatic brain metastases. Cancers (Basel) 2020;12(10):2787. doi: 10.3390/cancers12102787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laakmann E., Witzel I., Fasching P.A., et al. Development of central nervous system metastases as a first site of metastatic disease in breast cancer patients treated in the neoadjuvant trials GeparQuinto and GeparSixto. Breast Cancer Res. 2019;21(1):60. doi: 10.1186/s13058-019-1144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morikawa A., Jordan L., Rozner R., et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer. 2017;17:23–28. doi: 10.1016/j.clbc.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anders C.K., Deal A.M., Miller C.R., et al. The prognostic contribution of clinical breast cancer subtype, age, and race among patients with breast cancer brain metastases. Cancer. 2011;117(8):1602–1611. doi: 10.1002/cncr.25746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortés J., Kim S.B., Chung W.P., et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 22.Bartsch R., Berghoff A.S., Furtner J., et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022;28(9):1840–1847. doi: 10.1038/s41591-022-01935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curigliano G., Mueller V., Borges V., et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33(3):321–329. doi: 10.1016/j.annonc.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Lin N.U., Claus E., Sohl J., Razzak A.R., Arnaout A., Winer E.P. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113:2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laakmann E., Witzel I., Neunhöffer T., et al. Characteristics of patients with brain metastases from human epidermal growth factor receptor 2-positive breast cancer: subanalysis of brain metastases in breast cancer registry. ESMO Open. 2022;7(3) doi: 10.1016/j.esmoop.2022.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.