Abstract
Background
We report updated data for avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma from the third interim analysis of the phase III JAVELIN Renal 101 trial.
Patients and Methods
Progression-free survival (PFS), objective response rate (ORR), and duration of response per investigator assessment (RECIST version 1.1) and overall survival (OS) were evaluated in the overall population and in International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk groups; safety was also assessed.
Results
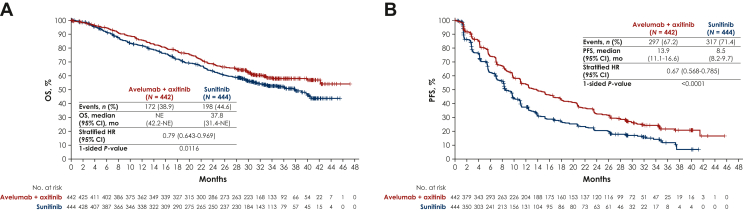
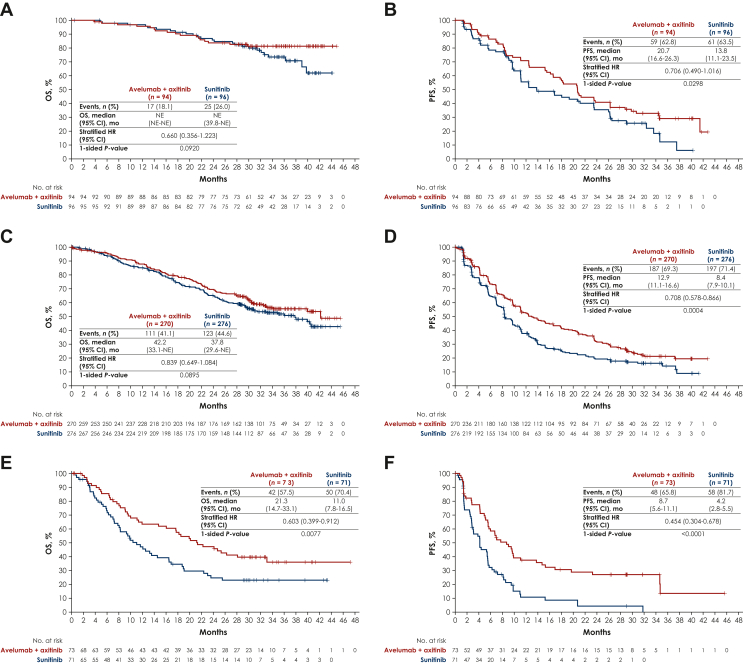
Overall, median OS [95% confidence interval (CI)] was not reached [42.2 months-not estimable (NE)] with avelumab plus axitinib versus 37.8 months (31.4-NE) with sunitinib [hazard ratio (HR) 0.79, 95% CI 0.643-0.969; one-sided P = 0.0116], and median PFS (95% CI) was 13.9 months (11.1-16.6 months) versus 8.5 months (8.2-9.7 months), respectively (HR 0.67, 95% CI 0.568-0.785; one-sided P < 0.0001). In patients with IMDC favorable-, intermediate-, poor-, or intermediate plus poor-risk disease, respectively, HRs (95% CI) for OS with avelumab plus axitinib versus sunitinib were 0.66 (0.356-1.223), 0.84 (0.649-1.084), 0.60 (0.399-0.912), and 0.79 (0.636-0.983), and HRs (95% CIs) for PFS were 0.71 (0.490-1.016), 0.71 (0.578-0.866), 0.45 (0.304-0.678), and 0.66 (0.550-0.787), respectively. ORRs, complete response rates, and durations of response favored avelumab plus axitinib overall and across all risk groups. In the avelumab plus axitinib arm, 81.1% had a grade ≥3 treatment-emergent adverse event (TEAE), and incidences of TEAEs and immune-related AEs were highest <6 months after randomization.
Conclusions
Avelumab plus axitinib continues to show improved efficacy versus sunitinib and a tolerable safety profile overall and across IMDC risk groups. The OS trend favors avelumab plus axitinib versus sunitinib, but data remain immature; follow-up is ongoing.
Trial registration
ClinicalTrials.govNCT02684006; https://clinicaltrials.gov/ct2/show/NCT02684006
Key words: renal cell carcinoma, risk factor, avelumab, immunotherapy, phase III
Highlights
-
•
Immunotherapy-based regimens can have varying efficacy benefits in patients with advanced renal cell carcinoma.
-
•
Patient outcomes can vary based on IMDC risk.
-
•
Follow-up from the JAVELIN Renal 101 trial confirms the efficacy and safety of first-line avelumab + axitinib versus sunitinib.
-
•
Avelumab + axitinib had a positive benefit-to-risk ratio versus sunitinib across all IMDC risk groups.
-
•
Long-term treatment with avelumab + axitinib did not result in new safety signals, and AEs decreased over time.
Introduction
Combination treatment with immune checkpoint inhibitors and vascular endothelial growth factor (VEGF) receptor (VEGFR) inhibitors has changed the treatment paradigm for patients with advanced renal cell carcinoma (aRCC).1,2 Avelumab is a human immunoglobulin G1 anti-programmed death-ligand 1 (PD-L1) monoclonal antibody that is approved in combination with axitinib for first-line (1L) treatment of patients with aRCC and as monotherapy for treatment of metastatic Merkel cell carcinoma, 1L maintenance treatment of locally advanced or metastatic urothelial carcinoma that has not progressed with 1L platinum-containing chemotherapy, and second-line (2L) treatment of locally advanced or metastatic urothelial carcinoma (in the United States and some other countries).3, 4, 5, 6, 7, 8, 9 Axitinib is a highly selective VEGFR tyrosine kinase inhibitor and is approved as monotherapy for 2L treatment of aRCC.10,11 The approval of the avelumab and axitinib combination treatment for aRCC was based on the results of JAVELIN Renal 101, a randomized phase III trial.8
At the first interim analysis (minimum follow-up, 6 months), avelumab plus axitinib treatment resulted in significantly improved progression-free survival (PFS) and objective response rate (ORR) compared with sunitinib treatment, both in the overall trial population and in the PD-L1+ population.8 Findings were confirmed in the second interim analysis (minimum follow-up, 13 months).9 The median PFS with avelumab plus axitinib versus sunitinib in the overall trial population was 13.3 months [95% confidence interval (CI) 11.1-15.3 months] versus 8.0 months (95% CI 6.7-9.8 months), respectively [hazard ratio (HR) 0.69, 95% CI 0.574-0.825; one-sided P < 0.0001] and in the PD-L1+ population was 13.8 months (95% CI 10.1-20.7 months) versus 7.0 months (95% CI 5.7-9.6 months), respectively (HR 0.62, 95% CI 0.490-0.777; one-sided P < 0.0001).9 The ORR with avelumab plus axitinib versus sunitinib in the overall population was 52.5% (95% CI 47.7%-57.2%) versus 27.3% (95% CI 23.2%-31.6%), and the complete response (CR) rate was 3.8% versus 2.0%, respectively.9
The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) classification is a commonly used prognostic model for patients with aRCC.12 The IMDC uses six factors (time interval from diagnosis to treatment, Karnofsky performance status, hemoglobin level, platelet count, neutrophil count, and serum calcium concentration) to categorize patients into favorable- (no risk factors), intermediate- (one or two risk factors), and poor- (three or more risk factors) risk groups. Recent phase III trials assessing different immune checkpoint inhibitor-based combination regimens as 1L treatment for aRCC have varied in terms of whether their primary analysis populations included patients with any IMDC risk score13, 14, 15 or only patients with intermediate- or high-risk scores.16,17 In the JAVELIN Renal 101 trial, the primary analysis population included patients with any IMDC risk score.8 In initial analyses from the trial, HRs for PFS and overall survival (OS) favored avelumab plus axitinib versus sunitinib in patients with favorable-, intermediate-, and poor-risk disease, and a higher proportion of patients in the combination arm achieved an objective response across all risk groups.9 Here we report updated efficacy and safety results for avelumab plus axitinib versus sunitinib from the third interim analysis of JAVELIN Renal 101 (minimum follow-up, 28 months), including efficacy analyses in the overall population and IMDC risk groups (favorable, intermediate, poor, or intermediate plus poor).
Methods
Study design and participants
JAVELIN Renal 101 is a phase III, multicenter, randomized, open-label trial evaluating the efficacy and safety of avelumab plus axitinib versus sunitinib in treatment-naïve patients with aRCC. The study design has been reported previously.8 In brief, the trial enrolled adults who had previously untreated aRCC with a clear-cell component, one or more measurable lesions according to RECIST version 1.1, and an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1. The primary endpoints are PFS and OS in patients with PD-L1+ tumors; analyses of PFS and OS in the overall population are key secondary endpoints. The trial was conducted in accordance with the ethics principles of the Declaration of Helsinki and the Good Clinical Practice guidelines, defined by the International Council for Harmonisation. All participating patients provided written informed consent.
Study treatment and assessments
Patients were randomized 1:1 to receive either avelumab (10 mg/kg intravenously every 2 weeks) plus axitinib (5 mg orally twice daily) or sunitinib (50 mg orally once daily for 4 weeks; 6-week cycle), stratified by ECOG PS (0 versus 1) and geographic region (United States versus Canada and Western Europe versus rest of the world). All patients continued treatment until confirmed disease progression, unacceptable toxicity, refusal to participate further, or loss to follow-up. If patients in the avelumab plus axitinib arm discontinued one of the study drugs for reasons other than confirmed disease progression, they could continue receiving the other drug. Efficacy endpoints assessed in this analysis were PFS, objective response, CR, and duration of response per investigator assessment (RECIST version 1.1), in addition to OS. Patients were categorized per IMDC risk group into favorable, intermediate, or poor subgroups, and outcomes were assessed in all three IMDC risk groups individually, as part of prespecified exploratory subgroup analyses, in addition to in patients with intermediate- or poor-risk scores as a combined subgroup of interest, which has been evaluated in other trials.
Statistical analyses
Statistical analyses for the trial were described previously.8 As prespecified in the statistical analysis plan for the study, the third interim analysis was performed 15 months after the final analysis of PFS.9 Efficacy endpoints were assessed in all randomized patients. Time-to-event analyses were performed using the Kaplan–Meier method, and CIs for median values were calculated using the Brookmeyer and Crowley method. HRs between treatment arms were calculated using the Cox proportional hazards model, stratified by the stratification factors stated in the previous section, and one-sided P values were calculated using the log-rank test. CIs for ORRs were calculated using the Clopper–Pearson method. Safety was evaluated in all patients who received one or more doses of a trial drug (avelumab, axitinib, or sunitinib).
Results
Patients
The study population included 886 patients with aRCC who were randomized to receive either avelumab plus axitinib (N = 442) or sunitinib (N = 444).8 Baseline characteristics were reported previously and were generally well balanced between both arms. At the data cutoff (28 April 2020), median follow-up was 34.1 months in the avelumab plus axitinib arm and 33.6 months in the sunitinib arm (≥28 months in all patients). In the avelumab plus axitinib arm, 114 patients (25.8%) were still receiving treatment; 86 (19.5%) were still receiving both avelumab and axitinib, 10 (2.3%) were receiving avelumab alone, and 18 (4.1%) were receiving axitinib alone; in the sunitinib arm, 52 (11.7%) remained on study treatment.
Efficacy
The analysis of OS remained immature. In the overall population, median OS was not reached [95% CI 42.2 months-not estimable (NE)] in the avelumab plus axitinib arm versus 37.8 months (95% CI 31.4 months-NE) in the sunitinib arm (HR 0.79, 95% CI 0.643-0.969; one-sided P = 0.0116; Figure 1A). In the PD-L1+ population, median OS was not reached (95% CI 40.0 months-NE) with avelumab plus axitinib versus 36.2 months (95% CI 30.0 months-NE) with sunitinib (HR 0.81, 95% CI 0.623-1.042; one-sided P = 0.0498; Supplementary Figure S1A, available at https://doi.org/10.1016/j.esmoop.2023.101210). Consistent with previous analyses, avelumab plus axitinib significantly prolonged PFS compared with sunitinib.8,9 In the overall population, median PFS was 13.9 months (95% CI 11.1-16.6 months) with avelumab plus axitinib versus 8.5 months (95% CI 8.2-9.7 months) with sunitinib (HR 0.67, 95% CI 0.568-0.785; one-sided P < 0.0001; Figure 1B). In the PD-L1+ population, median PFS was 13.9 months (95% CI 11.0-17.8 months) with avelumab plus axitinib versus 8.2 months (95% CI 6.9-9.4 months) with sunitinib (HR 0.58, 95% CI 0.473-0.715; one-sided P < 0.0001; Supplementary Figure S1B, available at https://doi.org/10.1016/j.esmoop.2023.101210). Avelumab plus axitinib also improved ORR, CR rate, and duration of response compared with sunitinib in the overall population. ORR with avelumab plus axitinib versus sunitinib was 59.3% (95% CI 54.5-63.9) versus 31.8% (95% CI 27.4-36.3), with a CR rate of 4.8% versus 3.2%, respectively. Among responding patients, the median duration of response with avelumab plus axitinib versus sunitinib was 19.4 months (95% CI 15.2-22.3 months) versus 14.5 months (95% CI 8.8-17.1 months), respectively.
Figure 1.
Kaplan–Meier analysis of (A) overall survival (OS) and (B) progression-free survival (PFS) in the overall population.
HR, hazard ratio; NE, not estimable.
Although not powered to assess statistical significance, efficacy analyses favored avelumab plus axitinib versus sunitinib across IMDC risk subgroups (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101210, Figure 2). In the favorable-, intermediate-, poor-, and intermediate plus poor-risk subgroups, respectively, HRs (95% CIs) for OS with avelumab plus axitinib versus sunitinib were 0.66 (0.356-1.223), 0.84 (0.649-1.084), 0.60 (0.399-0.912), and 0.79 (0.636-0.983), and HRs for PFS were 0.71 (0.490-1.016), 0.71 (0.578-0.866), 0.45 (0.304-0.678), and 0.66 (0.550-0.787). Analyses of ORR, CR rate, and duration of response also favored avelumab plus axitinib versus sunitinib across IMDC risk subgroups (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101210). OS and PFS for avelumab plus axitinib compared with sunitinib across other prespecified subgroups, including those defined by ECOG PS, PD-L1 status, and other characteristics, are shown in Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2023.101210.
Figure 2.
Kaplan–Meier analysis of overall survival (OS) and progression-free survival (PFS) by IMDC subgroups. (A) OS in the favorable-risk subgroup. (B) PFS in the favorable-risk subgroup. (C) OS in the intermediate-risk subgroup. (D) PFS in the intermediate-risk subgroup. (E) OS in the poor-risk subgroup. (F) PFS in the poor-risk subgroup.
CI, confidence interval; HR, hazard ratio; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; NE, not estimable.
Post-study therapy
Following treatment discontinuation in the avelumab plus axitinib and sunitinib arms, respectively, 204 (46.2%) and 269 (60.6%) patients received one or more subsequent anticancer drug therapies (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.101210). The most common categories of drugs given as 2L treatment were VEGF or VEGFR inhibitors in the avelumab plus axitinib arm and programmed cell death protein 1 or PD-L1 inhibitors in the sunitinib arm. In patients who discontinued avelumab plus axitinib, longer OS was observed in those who received subsequent anticancer drug therapy versus those who did not (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2023.101210).
Safety
With extended follow-up, the safety profile for avelumab plus axitinib remained consistent with the safety profile reported previously,8 and no new safety signals were identified (Table 1). Among patients treated with avelumab plus axitinib (n = 434) or sunitinib (n = 439), treatment-emergent adverse events (TEAEs) of any grade occurred in 434 (100%) and 436 (99.3%), including grade ≥3 TEAEs in 352 (81.1%) and 340 (77.4%), respectively. Overall, 138 patients (31.8%) in the avelumab plus axitinib arm discontinued one or more study drugs due to a TEAE [avelumab, 116 (26.7%); axitinib, 88 (20.3%)], and 53 (12.2%) discontinued both study drugs; 71 patients (16.2%) discontinued sunitinib due to a TEAE. In the avelumab plus axitinib arm, the most common TEAEs that led to discontinuation of avelumab or axitinib were increase in the levels of alanine aminotransferase (n = 19, 4.4%) and aspartate aminotransferase (n = 13, 3.0%) and infusion-related reaction (n = 8, 1.8%).
Table 1.
Summary of the most common TEAEs (any grade in ≥20%) occurring at any time during treatment with avelumab plus axitinib or sunitinib
| Avelumab + axitinib (n = 434) |
Sunitinib (n = 439) |
|||
|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Any TEAE, n (%) | 434 (100) | 352 (81.1) | 436 (99.3) | 340 (77.4) |
| Diarrhea | 304 (70.0) | 45 (10.4) | 228 (51.9) | 14 (3.2) |
| Hypertension | 228 (52.5) | 124 (28.6) | 162 (36.9) | 83 (18.9) |
| Fatigue | 201 (46.3) | 18 (4.1) | 192 (43.7) | 17 (3.9) |
| Nausea | 177 (40.8) | 8 (1.8) | 183 (41.7) | 8 (1.8) |
| PPE | 158 (36.4) | 28 (6.5) | 161 (36.7) | 19 (4.3) |
| Dysphonia | 148 (34.1) | 2 (0.5) | 20 (4.6) | 1 (0.2) |
| Cough | 142 (32.7) | 1 (0.2) | 104 (23.7) | 0 (0) |
| Decreased appetite | 137 (31.6) | 10 (2.3) | 141 (32.1) | 5 (1.1) |
| Hypothyroidism | 135 (31.1) | 3 (0.7) | 82 (18.7) | 2 (0.5) |
| Headache | 115 (26.5) | 1 (0.2) | 82 (18.7) | 2 (0.5) |
| Arthralgia | 113 (26.0) | 5 (1.2) | 67 (15.3) | 3 (0.7) |
| Stomatitis | 112 (25.8) | 8 (1.8) | 112 (25.5) | 4 (0.9) |
| Back pain | 111 (25.6) | 5 (1.2) | 84 (19.1) | 8 (1.8) |
| Dyspnea | 102 (23.5) | 12 (2.8) | 64 (14.6) | 7 (1.6) |
| Weight decreased | 101 (23.3) | 17 (3.9) | 42 (9.6) | 5 (1.1) |
| Vomiting | 97 (22.4) | 6 (1.4) | 98 (22.3) | 8 (1.8) |
| Constipation | 94 (21.7) | 0 (0) | 73 (16.6) | 0 (0) |
| Pruritus | 91 (21.0) | 0 (0) | 28 (6.4) | 0 (0) |
| ALT increased | 89 (20.5) | 30 (6.9) | 48 (10.9) | 12 (2.7) |
The table shows TEAEs regardless of treatment duration from first patient first dose to data cutoff (April 2020).
ALT, alanine aminotransferase; PPE, palmar plantar erythrodysesthesia; TEAE, treatment-emergent adverse event.
Incidences of TEAEs were also assessed in patients who remained on treatment in the avelumab plus axitinib arm at different time intervals from randomization: <6 months (n = 434), 6 months to <1 year (n = 293), 1 to <2 years (n = 214), and ≥2 years (n = 133). The incidence of the most common TEAEs was highest during the <6-month interval and decreased in later intervals, except for diarrhea, which had a similar frequency throughout the different durations of treatment (Table 2); however, diarrhea rarely led to permanent discontinuation of avelumab plus axitinib (n = 3; 1 patient each after 0 to <6 months, 6 months to <1 year, and 1 to <2 years). The incidence of immune-related AEs (irAEs) was also highest during the <6-month interval and decreased over time. The most common category of irAEs was thyroid disorders, which were most frequent during the <6-month interval (24.4%; Table 3). High-dose glucocorticoids (≥40 mg total daily dose of prednisone or equivalent) were administered because of an irAE in 63 patients (14.5%) treated with avelumab plus axitinib.
Table 2.
Occurrence of the TEAEs of any grade in patients who continued receiving treatment with avelumab plus axitinib after different time intervals. Individual AEs occurring at any grade in ≥20% of patients in the avelumab plus axitinib arm are shown
| Preferred term | Avelumab + axitinib |
|||
|---|---|---|---|---|
| Time interval ≥0 to <6 months (n = 434) |
Time interval ≥6 months to <1 year (n = 293) |
Time interval ≥1 to <2 years (n = 214) |
Time interval ≥2 years (n = 133) |
|
| Patients with any grade TEAE, n (%) | 432 (99.5) | 277 (94.5) | 202 (94.4) | 122 (91.7) |
| Diarrhea | 212 (48.8) | 146 (49.8) | 122 (57.0) | 55 (41.4) |
| Hypertension | 213 (49.1) | 25 (8.5) | 22 (10.3) | 10 (7.5) |
| Fatigue | 163 (37.6) | 40 (13.7) | 37 (17.3) | 26 (19.5) |
| Nausea | 116 (26.7) | 46 (15.7) | 36 (16.8) | 16 (12.0) |
| PPE | 133 (30.6) | 45 (15.4) | 45 (21.0) | 9 (6.8) |
| Dysphonia | 133 (30.6) | 18 (6.1) | 15 (7.0) | 9 (6.8) |
| Cough | 76 (17.5) | 30 (10.2) | 45 (21.0) | 23 (17.3) |
| Decreased appetite | 93 (21.4) | 26 (8.9) | 28 (13.1) | 12 (9.0) |
| Hypothyroidism | 106 (24.4) | 16 (5.5) | 15 (7.0) | 3 (2.3) |
| Headache | 82 (18.9) | 9 (3.1) | 19 (8.9) | 13 (9.8) |
| Arthralgia | 67 (15.4) | 25 (8.5) | 27 (12.6) | 18 (13.5) |
| Stomatitis | 95 (21.9) | 20 (6.8) | 18 (8.4) | 11 (8.3) |
| Back pain | 63 (14.5) | 20 (6.8) | 29 (13.6) | 15 (11.3) |
| Dyspnea | 75 (17.3) | 16 (5.5) | 19 (8.9) | 8 (6.0) |
| Weight decreased | 62 (14.3) | 38 (13.0) | 18 (8.4) | 5 (3.8) |
| Vomiting | 60 (13.8) | 22 (7.5) | 19 (8.9) | 8 (6.0) |
| Constipation | 59 (13.6) | 24 (8.2) | 15 (7.0) | 6 (4.5) |
| Pruritus | 56 (12.9) | 23 (7.8) | 19 (8.9) | 10 (7.5) |
| ALT increased | 58 (13.4) | 25 (8.5) | 13 (6.1) | 7 (5.3) |
AE, adverse event; ALT, alanine aminotransferase; PPE, palmar plantar erythrodysesthesia; TEAE, treatment-emergent adverse event.
Table 3.
Occurrence of individual irAEs of any grade per category in patients who remained on treatment with avelumab plus axitinib after different time intervals
| Cluster and preferred term | Avelumab + axitinib |
|||
|---|---|---|---|---|
| Time interval ≥0 to <6 months (n = 434) |
Time interval ≥6 months to <1 year (n = 293) |
Time interval ≥1 to <2 years (n = 214) |
Time interval ≥2 years (n = 133) |
|
| Patients with any grade irAE, n (%) | 155 (35.7) | 46 (15.7) | 34 (15.9) | 13 (9.8) |
| Immune-related endocrinopathies: thyroid disorders Hypothyroidism Hyperthyroidism Blood TSH increased Thyroiditis Autoimmune thyroiditis Blood TSH Primary hypothyroidism Thyroxine free decreased |
106 (24.4) 91 (21.0) 14 (3.2) 10 (2.3) 5 (1.2) 3 (0.7) 2 (0.5) 0 (0) 0 (0) |
18 (6.1) 14 (4.8) 2.0 (0.7) 3 (1.0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) |
17 (7.9) 12 (5.6) 1 (0.5) 3 (1.4) 0 (0) 1 (0.5) 0 (0) 1 (0.5) 3 (1.4) |
8 (6.0) 4 (3.0) 0 (0) 4 (3.0) 0 (0) 1 (0.8) 0 (0) 1 (0.8) 0 (0) |
| Immune-related rash Rash Pruritus Rash maculopapular Rash pruritic Rash macular Dermatitis bullous |
24 (5.5) 10 (2.3) 7 (1.6) 3 (0.7) 3 (0.7) 2 (0.5) 1 (0.2) |
9 (3.1) 1 (0.3) 6 (2.0) 4 (1.4) 1 (0.3) 0 (0) 0 (0) |
6 (2.8) 3 (1.4) 2 (0.9) 0 (0) 0 (0) 1 (0.5) 0 (0) |
2 (1.5) 1 (0.8) 0 (0) 1 (0.8) 0 (0) 0 (0) 0 (0) |
| Immune-related hepatitis ALT increased AST increased Transaminases increased Hepatic function abnormal Hepatotoxicity Immune-mediated hepatitis Liver disorder Hepatitis |
25 (5.3) 19 (4.4) 13 (3.0) 2 (0.5) 1 (0.2) 1 (0.2) 1 (0.2) 1 (0.2) 0 (0) |
7 (2.4) 4 (1.4) 3 (1.0) 0 (0) 1 (0.3) 1 (0.3) 0 (0) 0 (0) 1 (0.3) |
2 (0.9) 2 (0.9) 2 (0.9) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) |
0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) |
| Immune-related endocrinopathies: adrenal insufficiency Adrenal insufficiency |
10 (2.3) 10 (2.3) |
4 (1.4) 4 (1.4) |
1 (0.5) 1 (0.5) |
0 (0) 0 (0) |
| Immune-related colitis Diarrhea Colitis Autoimmune colitis Enteritis |
8 (1.8) 8 (1.8) 2 (0.5) 1 (0.2) 0 (0) |
9 (3.1) 8 (2.7) 2 (0.7) 1 (0.3) 0 (0) |
6 (2.8) 5 (2.3) 2 (0.9) 0 (0) 1 (0.5) |
0 (0) 0 (0) 0 (0) 0 (0) 0 (0) |
| Immune-related endocrinopathies: type 1 diabetes mellitus Diabetes mellitus Hyperglycemia Diabetic ketoacidosis Type 1 diabetes mellitus |
4 (0.9) 2 (0.5) 2 (0.5) 0 (0) 0 (0) |
2 (0.7) 0 (0) 1 (0.3) 0 (0) 2 (0.7) |
1 (0.5) 0 (0) 1 (0.5) 0 (0) 0 (0) |
1 (0.8) 1 (0.8) 1 (0.8) 1 (0.8) 0 (0) |
| Immune-related myocarditis Autoimmune myocarditis Myocarditis |
2 (0.5) 1 (0.2) 1 (0.2) |
0 (0) 0 (0) 0 (0) |
0 (0) 0 (0) 0 (0) |
0 (0) 0 (0) 0 (0) |
| Immune-related nephritis and renal dysfunction Acute kidney injury |
2 (0.5) 2 (0.5) |
0 (0) 0 (0) |
0 (0) 0 (0) |
0 (0) 0 (0) |
| Immune-related pancreatitis Autoimmune pancreatitis Pancreatitis necrotizing Pancreatitis |
2 (0.5) 1 (0.2) 1 (0.2) 0 (0) |
0 (0) 0 (0) 0 (0) 0 (0) |
1 (0.5) 0 (0) 0 (0) 1 (0.5) |
0 (0) 0 (0) 0 (0) 0 (0) |
| Immune-related pneumonitis Pneumonitis |
2 (0.5) 2 (0.5) |
1 (0.3) 1 (0.3) |
0 (0) 0 (0) |
0 (0) 0 (0) |
| Immune-related endocrinopathies: pituitary dysfunction Hypophysitis |
1 (0.2) 1 (0.2) |
0 (0) 0 (0) |
0 (0) 0 (0) |
0 (0) 0 (0) |
| Other irAEs: myasthenic Myasthenia gravis |
0 (0) 0 (0) |
0 (0) 0 (0) |
0 (0) 0 (0) |
1 (0.8) 1 (0.8) |
| Other irAEs: myositis Myositis |
0 (0) 0 (0) |
0 (0) 0 (0) |
0 (0) 0 (0) |
1 (0.8) 1 (0.8) |
| Other irAEs Immune-mediated arthritis Psoriasis |
0 (0) 0 (0) 0 (0) |
0 (0) 0 (0) 0 (0) |
1 (0.5) 0 (0) 1 (0.5) |
2 (1.5) 1 (0.8) 1 (0.8) |
irAEs shown include clusters of MedDRA Preferred Terms classified as immune related based on medical review.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; irAE, immune-related adverse event; TSH, thyroid-stimulating hormone.
Discontinuation of avelumab or axitinib due to a TEAE occurred within 6 months in 19.4% (avelumab, 16.1%; axitinib, 10.1%) and in lower proportions of the patients who remained on treatment at subsequent time intervals [discontinuation of either drug (avelumab/axitinib) in 8.5% (6.8%/5.8%) at 6 months to <1 year, 7.0% (5.1%/5.1%) at 1 to <2 years, and 7.5% (6.8%/6.0%) at ≥2 years]. Similarly, discontinuation of both study drugs occurred within 6 months in 6.5% of patients and in lower proportions of the patients who remained on treatment at subsequent time intervals (2.7%, 3.3%, and 5.3% at 6 months to <1 year, 1 to <2 years, and ≥2 years, respectively). In an exploratory analysis of patients who discontinued from the study because of TEAEs in the avelumab plus axitinib arm (n = 94), median OS and PFS were 29.8 months (95% CI 19.4 months-NE) and 11.1 months (95% CI 8.2-13.9 months), respectively, and ORR was 50.0% (39.5-60.5), including CR in one patient (1.1%). In patients who discontinued from the study because of AEs in the sunitinib arm (n = 61), median OS and PFS were 37.8 months (95% CI 27.2-40.6 months) and 14.0 months (95% CI 6.9-30.9 months), respectively, and ORR was 27.9% (17.1-40.8), including CR in one patient (1.6%).
Discussion
Consistent with results reported from prior interim analyses,8,9 the current analysis with extended follow-up from the JAVELIN Renal 101 trial confirms the efficacy benefits of avelumab plus axitinib versus sunitinib in patients with aRCC in the overall and PD-L1+ populations, as well as across IMDC risk groups. In the Kaplan–Meier analysis of PFS in the overall population, curves for avelumab plus axitinib versus sunitinib continued to show separation at later time points, and the HR in the current analysis (0.67, 95% CI 0.568-0.785; one-sided P < 0.0001) remains similar to that reported in earlier analyses (first interim analysis, 0.69, 95% CI 0.56-0.84; one-sided P < 0.001; second interim analysis, 0.69, 95% CI 0.574-0.825; one-sided P < 0.001).8,9 The analysis of OS remains immature. While the HR for OS in the overall population favored avelumab plus axitinib versus sunitinib (0.79, 95% CI 0.643-0.969; one-sided P = 0.0116), similar to prior analyses (first interim analysis, 0.78, 95% CI 0.554-1.084; one-sided P = 0.14; second interim analysis, 0.80, 95% CI 0.616-1.027; one-sided P = 0.0392],8,9 it did not meet prespecified criteria for statistical significance at interim analysis. Follow-up will continue until the final analysis.
In prespecified exploratory analyses, avelumab plus axitinib showed efficacy benefits versus sunitinib across all IMDC risk groups. HRs for OS and PFS favored avelumab plus axitinib versus sunitinib not only in patients with intermediate [HRs (95% CIs) of 0.84 (0.649-1.084) and 0.71 (0.578-0.866), respectively] or poor [0.60 (0.399-0.912) and 0.45 (0.304-0.678), respectively] IMDC risk scores but also in patients with a favorable risk score [0.66 (0.356-1.223) and 0.71 (0.490-1.016), respectively]. In other phase III trials evaluating 1L treatment for patients with aRCC, OS and PFS benefits have been seen across IMDC risk groups for combinations of an immune checkpoint inhibitor with a VEGFR inhibitor,13, 14, 15 whereas in an exploratory analysis from the phase III CheckMate 214 trial of nivolumab plus ipilimumab versus sunitinib, no PFS or OS benefit was seen in patients with a favorable IMDC risk score.16 Analyses of OS and PFS also favored avelumab plus axitinib across other prespecified subgroups, including those defined by ECOG PS or PD-L1 status. Exploratory analyses found that patients received a range of subsequent anticancer drug therapies after discontinuing avelumab plus axitinib; further studies are needed to determine the optimal sequence of therapy.
Long-term treatment with avelumab plus axitinib did not result in any new safety signals, and safety findings in the current analysis are consistent with prior analyses.8 In general, frequencies of TEAEs and irAEs decreased over time, with the highest incidence seen within 6 months of treatment. Similarly, discontinuation of one or both study drugs was most frequent in patients treated for <6 months compared with later time intervals.
In conclusion, extended follow-up from the JAVELIN Renal 101 trial provides further evidence of the positive benefit-to-risk ratio for 1L avelumab plus axitinib treatment in patients with aRCC, both in the overall population and across IMDC risk groups.
Acknowledgements
The authors thank the patients and their families, investigators, coinvestigators, and the study teams at each of the participating centers. This trial was sponsored by Pfizer as part of an alliance between Pfizer and Merck (CrossRef Funder ID: 10.13039/100009945). Medical writing support was provided by Hiba Al-Ashtal of Clinical Thinking and was funded by Pfizer and Merck. Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant [grant number P30 CA008748].
Funding
This research was sponsored by Pfizer as part of an alliance between Pfizer and Merck (CrossRef Funder ID: 10.13039/100009945) (no grant number).
Disclosure
JBAGH has provided consulting or advisory roles for Achilles Therapeutics, AIMM Therapeutics, Bristol Myers Squibb, Immunocore, Ipsen, MSD, Neogene Therapeutics, Neon Therapeutics, Novartis, Pfizer, Roche/Genentech, Sanofi, Seattle Genetics, and Third Rock Ventures; and has received research funding from Amgen, Bristol Myers Squibb, MSD, Neon Therapeutics, and Novartis. JL has received honoraria from Bristol Myers Squibb, CRUK, Dynavax, Eisai, GSK, Incyte, iOnctura, Merck, Novartis, Pfizer, Roche, touchEXPERTS, and touchIME; has provided consulting or advisory roles for Apple Tree, Boston Biomedical, Bristol Myers Squibb, GSK, Immunocore, Incyte, iOnctura, Iovance, Novartis, Pfizer, and YKT Global; has received speaker fees from Aptitude, AstraZeneca, Bristol Myers Squibb, Calithera, Eisai, Ervaxx, EUSA Pharma, GSK, Incyte, Ipsen, Merck, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, and Ultimovacs; has received institutional research funding from Achilles Therapeutics, Aveo, Bristol Myers Squibb, Covance, Immunocore, MSD, Nektar Therapeutics, Novartis, Pfizer, Pharmacyclics, and Roche; and has received grants from Achilles Therapeutics, Aveo, Bristol Myers Squibb, Immunocore, MSD, Nektar Therapeutics, Novartis, Pfizer, Pharmacyclics, and Roche. TKC reports institutional and personal, paid and unpaid support for research, advisory boards, consultancy, and honoraria from AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers Squibb, Calithera, Circle Pharma, Eisai, Merck, Exelixis, GlaxoSmithKline, IQVA, Infinity, Ipsen, Jansen, Kanaph, Lilly, MSD, Nikang, Nuscan, Novartis, Pfizer, Roche, Sanofi/Aventis, Surface Oncology, Takeda, Tempest, UpToDate, and CME events (PeerView, OncLive, MJH, and others), outside the submitted work; reports institutional patents filed on molecular alterations and immunotherapy response/toxicity and ctDNA; reports equity for Osel, Pionyr, Precede Bio, and Tempest; has served in committees for NCCN, GU Steering Committee, ASCO/ESMO, ACCRU, and KidneyCAN; reports that medical writing and editorial assistance support may have been funded by communications companies in part; reports no speaker’s bureau; has mentored several non-US citizens on research projects with potential funding (in part) from non-US sources/foreign components; reports that the institution (Dana-Farber Cancer Institute) may have received additional independent funding of drug companies and/or royalties potentially involved in research around the subject matter; and is supported in part by the Dana-Farber/Harvard Cancer Center Kidney SPORE (2P50CA101942-16) and Program 5P30CA006516-56, the Kohlberg Chair at Harvard Medical School and the Trust Family, Michael Brigham, Pan Mass Challenge, and Loker Pinard Funds for Kidney Cancer Research at DFCI. LA has provided consulting or advisory roles for Astellas Pharma, Bristol Myers Squibb, Eisai, Janssen, Ipsen, MSD, Novartis, Pfizer, and Roche; and has received travel, accommodations, and expenses from Bristol Myers Squibb, Ipsen, and MSD. BIR has provided consulting or advisory roles for 3D Medicines, Aravive, Arrowhead Pharmaceuticals, Aveo, Bristol Myers Squibb, Corvus Pharmaceuticals, Eisai, GlaxoSmithKline, Pfizer, Merck, Shionogi, Surface Oncology, and Synthorx; reports leadership with MJH Life Sciences; has received travel, accommodations, and expenses from Bristol Myers Squibb, Pfizer, and Merck; reports stock and other ownership interests from PTC Therapeutics; and has received research funding from Aravive, Arrowhead Pharmaceuticals, AstraZeneca/MedImmune, Bristol Myers Squibb, Dragonfly Therapeutics, Immunomedics, Incyte, Exelixis, Pfizer, Roche/Genentech, Seattle Genetics, Surface Oncology, Taris, and Merck. MBA has provided consulting or advisory roles for Genentech/Roche, Novartis, Bristol Myers Squibb, MSD, Exelixis, Eisai, Agenus, Arrowhead Pharmaceuticals, Werewolf Pharma, Surface Oncology, Iovance Biotherapeutics, Pyxis, Pneuma Respiratory, Leads Biolabs, Fathom Biotechnology, Aveo, Cota Healthcare, Neoleukin Therapeutics, Adagene, Idera, Ellipses Pharma, AstraZeneca, PACT Pharma, Asher Biotherapeutics, Seagen, Pfizer, Scholar Rock, Simcha Therapeutics, Takeda, Calithera Biosciences, and Sanofi; reports stock and other ownership interests in Werewolf Pharma and Pyxis; and has received institutional research funding from Bristol Myers Squibb. MS has provided consulting or advisory roles for Bristol Myers Squibb, Eisai, EUSA Pharma, Ipsen, MSD, and Roche; has received travel, accommodations, and expenses from Bristol Myers Squibb, Ipsen, and Roche; and has received honoraria from Alkermes, Bristol Myers Squibb, Eisai, EUSA Pharma, Ipsen, Janssen Oncology, and MSD. KP has received honoraria from Roche, Pfizer, Nektar, MSD, Regeneron, and AstraZeneca; and has provided consulting or advisory roles for Nektar. EM reports employment, stock, and other ownership interest with Pfizer. JW reports employment, stock, and other ownership interest with Pfizer. MM reports employment, stock, and other ownership interest with Pfizer. AdP reports employment, stock, and other ownership interest with Pfizer; and has received honoraria from Pfizer. RJM has provided consulting or advisory roles for AstraZeneca, Aveo, Calithera Biosciences, Eisai, Exelixis, Genentech/Roche, Incyte, Pfizer, and Merck; has received travel, accommodations, and expenses from Bristol Myers Squibb; and has received research funding from Aveo, Bristol Myers Squibb, Eisai, Exelixis, Genentech/Roche, Novartis, Pfizer, and Merck.
Data Sharing
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Supplementary data
References
- 1.Carlo M.I., Voss M.H., Motzer R.J. Checkpoint inhibitors and other novel immunotherapies for advanced renal cell carcinoma. Nat Rev Urol. 2016;13:420–431. doi: 10.1038/nrurol.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choueiri T.K., Motzer R.J. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354–366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 3.Bavencio (avelumab). Prescribing information. EMD Serono, Inc., an affiliate of Merck KGaA; Rockland, MA, USA: 2022. [Google Scholar]
- 4.Bavencio (avelumab) Merck Europe B.V., an affiliate of Merck KGaA; Amsterdam, The Netherlands: 2022. Summary of product characteristics. [Google Scholar]
- 5.Patel M.R., Ellerton J., Infante J.R., et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19:51–64. doi: 10.1016/S1470-2045(17)30900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman H.L., Russell J., Hamid O., et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powles T., Park S.H., Voog E., et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 8.Motzer R.J., Penkov K., Haanen J., et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choueiri T.K., Motzer R.J., Rini B.I., et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31:1030–1039. doi: 10.1016/j.annonc.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inlyta (axitinib) Pfizer Laboratories Div Pfizer Inc; 2022. Prescribing information. [Google Scholar]
- 11.Hu-Lowe D.D., Zou H.Y., Grazzini M.L., et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272–7283. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 12.Heng D.Y., Xie W., Regan M.M., et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 13.Powles T., Plimack E.R., Soulieres D., et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21:1563–1573. doi: 10.1016/S1470-2045(20)30436-8. [DOI] [PubMed] [Google Scholar]
- 14.Motzer R., Alekseev B., Rha S.Y., et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 15.Choueiri T.K., Powles T., Burotto M., et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384:829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer R.J., Tannir N.M., McDermott D.F., et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choueiri T.K., Powles T.B., Albiges L., et al. Phase III study of cabozantinib (C) in combination with nivolumab (N) and ipilimumab (I) in previously untreated advanced renal cell carcinoma (aRCC) of IMDC intermediate or poor risk (COSMIC-313) Ann Oncol. 2022;33(suppl 7):S808–S869. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.