Abstract
Ethnic or racial differences in breast cancer (BC) survival outcomes have been reported, but current data are largely restricted to comparisons between African Americans and non-Hispanic whites. Most analyses have traditionally been based on self-reported race which may not always be accurate, or are oversimplified in their classification. With increasing globalization, quantification of the genetic ancestry from genomic data may offer a solution to infer the complex makeup from admixture of races. Focusing on the larger and the latest studies, we will discuss recent findings on the differing host and tumor biology that may be driving these disparities, in addition to the extrinsic environmental or lifestyle factors. Socioeconomic disparities with lower cancer literacy may lead to late presentation, poorer adherence to treatment, and other lifestyle factors such as unhealthy diet, obesity, and inadequate physical activity. These hardships may also result in greater allostatic load, which is in turn associated with aggressive BC features in disadvantaged populations. Epigenetic reprogramming may mediate the effects of the environment or lifestyle factors on gene expression, with ensuing differences in BC characteristics and outcome. There is increasing evidence that germline genetics can influence somatic gene alterations or expression, as well as modulate the tumor or immune microenvironment. Although the precise mechanisms remain elusive, this may account for the varying distribution of different BC subtypes across ethnicities. These gaps in our knowledge highlight the need to interrogate the multiomics landscape of BC in diverse populations, ideally in large-scale collaborative settings with standardized methodology for the comparisons to be statistically robust. Together with improving BC awareness and access to good quality health care, a holistic approach with insights of the biological underpinnings is much needed to eradicate ethnic disparities in BC outcomes.
Key words: breast cancer, ethnicity, race, outcomes, disparities, biology
Highlights
-
•
There are ethnic differences in BC outcomes, though data are limited.
-
•
Drivers of these disparities include socioeconomic determinants as well as differing host and tumor biology.
-
•
Dissection of intrinsic drivers from extrinsic drivers can be challenging due to the complex interplay.
-
•
Greater diversity in clinical and translational research is much needed to address these gaps in our knowledge.
Introduction
Breast cancer (BC) is a major global health problem. In 2020, >2.3 million new cases were diagnosed, and ∼685,000 BC-related deaths occurred.1 The incidence of BC has been rising progressively worldwide over the past few decades, attributed to changing lifestyle factors such as increasing body mass index (BMI) and decreasing number of births, in addition to greater detection with improved awareness, screening, and data capture.2, 3, 4 In 2020, BC was the most diagnosed cancer among women in 157 out of 185 countries, and the leading cause of cancer death in 110 countries.1 Hence it is not just a Western disease, though our understanding and management of BC have largely been based on data and research from developed countries in the West.
While there is emerging evidence of ethnic differences in BC, many questions remain unanswered. Majority of this research have been conducted in the United States, with most comparing non-Hispanic white (NHW) and black or African American (AA) women. Although the higher BC mortality rates in many regions of the world1 and even among the underserved populations in developed countries may reflect suboptimal access to appropriate management, ethnicity-related biological differences may contribute to these disparities.
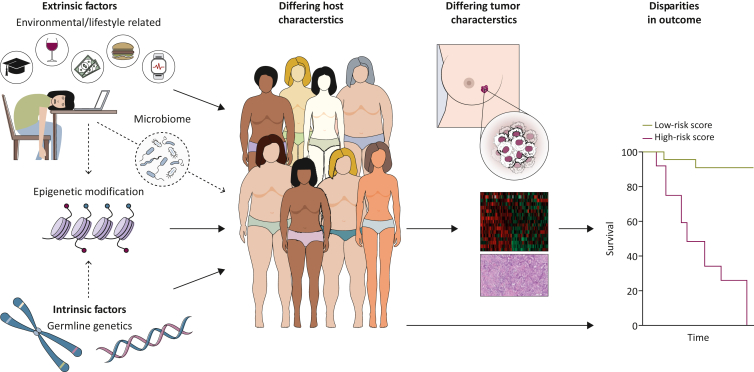
Given the complex interplay between intrinsic and extrinsic factors (Figure 1), the effects of underlying germline genetics and environmental or lifestyle factors cannot always be clearly delineated. There is paucity of large-scale molecular studies that are adequately powered to overcome confounding factors and selection bias for interethnic comparisons. The suboptimal classification of ethnicities also limits our interpretation of the existing data. Self-reported race or ethnicity, if captured, may not always be accurate and is often oversimplified in terms of its categorization in most studies, including clinical trials. As an example, despite the distinct phenotypic and genotypic differences between South Asian and East Asian populations,5 all Asian ethnicities are usually aggregated, and often with Pacific Islanders as the Asian/Pacific Islander (API) category, or combined with all non-white patients in the analyses on ethnic differences. Considerable heterogeneity also exists within each region, which may be further complicated by the impact of migration, acculturation, and intermarriage.5, 6, 7 Recent studies using single-nucleotide polymorphism (SNP) markers from genome-wide or targeted sequencing rather than self-reported race offer more granular details of the genetic ancestry footprint, with the potential to infer admixture estimates as well.6, 7, 8, 9, 10
Figure 1.
Interplay between intrinsic and extrinsic factors in shaping host and tumor characteristics, which result in different outcomes. Intrinsic factors include germline genetics which are generally not modifiable. Extrinsic factors refer to environmental or lifestyle issues, including exercise, diet (which may affect BMI, microbiome, and other features), and socioeconomic status, which may in turn be related to education level and stress (allostatic load). Germline genetics can influence somatic gene alterations or expression, and modulate the tumor or immune microenvironment, as well as affect treatment response and tolerability. Extrinsic factors may potentially influence not only the host phenotype, but also the tumor stage at presentation, biology, and outcomes. Epigenetic reprogramming may mediate the effects of the environment or lifestyle factors on gene expression, with ensuing differences in patient and BC biology that can affect outcomes.
In this review, we will summarize the BC outcomes among different ethnicities or populations reported in the literature, as well as the drivers of these disparities (Table 1), with focus on the latest and the larger studies. Significant gaps in our knowledge remain, especially with respect to the host and tumor biology underpinning the heterogeneity of BC across different ethnicities and regions globally.
Table 1.
Possible drivers of ethnic disparities in breast cancer outcomes
Socioeconomic determinants of health
|
Outcomes and Standard Clinicopathological Characteristics
The earliest studies dating back to the 1970s described the inferior survival observed in AA and Hispanic women with BC compared to NHW women, despite the lower incidence of BC among non-white women.11,12 While this can be accounted for by the more advanced stage at presentation and the higher frequency of grade 3 and estrogen receptor (ER)-negative tumors with fewer screen-detected cancers, black race remained an independent predictor of adverse outcomes even after adjustment for other variables,10,13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 including BMI,13, 14, 15,19 income, or socioeconomic status13,16, 17, 18 (Table 2). Although black race is typically associated with increased frequency of the aggressive triple-negative BC (TNBC) subtype, in adjuvant/neoadjuvant chemotherapy clinical trials, the disparities were observed in hormone receptor-positive human epidermal growth factor receptor 2 (HER2)-negative disease.10,15,19
Table 2.
Selected studies comparing BC outcomes across ethnicities in the United States, with the number of patients by ethnicity and hazard ratio (HR) from multivariable analyses (versus NHW unless indicated otherwise)
| Cohort, study | Stages Endpoints |
NHW | AA | Hispanic (H) | AS/API | Oth/UnK |
|---|---|---|---|---|---|---|
| 1970-1991, University of Texas Health Science Center12 | Stages I-IV OS |
4885 5-year OS 75% |
1016 5-year OS 65% HR 1.2 versus NHW and H, P = 0.004 |
777 5-year OS 70% HR versus NHW + AA not given, P = 0.14 |
— | |
| 1993-1998, Women’s Health Initiative13 | Stages I-IV Risk of death |
3455 | 242 HR 1.79 (1.05-3.05) |
103 | API 88 | UnK 39; AI/AN 11 |
| 1996-2000, Life After Cancer Epidemiology (LACE) and Pathways cohorts14 | Stages I-IV BC death |
1176 | 128 HR 1.71 (1.02-2.86) |
138 HR 0.61 (0.32-1.14) |
API 149 HR 0.69 (0.34-1.40) |
Oth 44 HR 1.68 (0.68-4.17) |
| 1999-2002, ECOG1199 Trial HR+, HER2– shown here (no outcome differences for TNBC and HER2+ categories)15 |
Stages I-III BC death |
2646 non-black | 161 HR 1.65 (1.11-2.46), P = 0.013 |
— | — | — |
| 2000-2013, California Cancer Registry16 | Stages I-IV 264 681 total BCSS |
HR 1.15 (1.10-1.20) | HR 0.89 (0.85-0.92) | API HR 0.78 (0.74-0.82) Ch HR 0.79 (0.72-0.87) Jp HR 0.69 (0.59-0.80) Fp HR 0.78 (0.73-0.85) |
— | |
| 2004-2011, SEER 18 registries database17 | Stage I for BCSS multivariable analysis shown here | 136 558 | 14 302 HR 1.57 (1.40-1.75), P < 0.001 |
13 992 HR 1.13 (0.98-1.30), P = 0.10 |
AS 11 948 HR 0.60 (0.49-0.73), P < 0.001 Ch HR 0.55 (0.35-0.88), P = 0.01 Jp HR 0.69 (0.45-1.08), P = 0.10 SAS HR 0.48 (0.20-1.15), P = 0.10 Oth HR 0.61 (0.46-0.80), P < 0.001 |
Oth 2614 HR 1.11 (0.80-1.54), P = 0.54 |
| 2007-2011, ECOG-ACRIN-510310 | Stages I-III DFS |
2473 | 386 HR 1.39 (1.07-1.81), P = 0.013 |
— | — | — |
| 2010-2017, National Cancer Database, neoadjuvant chemotherapy18 | Stages I-III aOR for pCR aHR OS for RD pCR |
31 779 HR+HER2– 10 220 HR–HER2+ 7630 HR+HER2+ 23 002 TNBC 56 192 16 439 |
7060 HR+HER2– HR 1.13 (1.03-1.24), P = 0.009 2350 HR–HER2+ HR 0.81 (0.73-0.89), P < 0.001 1503 HR+HER2+ HR 0.91 (0.79-1.04), P = 0.17 8592 TNBC HR 0.82 (0.77-0.87), P < 0.001 15 140; HR 1.16 (1.11-1.21), P < 0.001 4365; HR 1.02 (0.87-1.19), P = 0.83 |
3398 HR+HER2– HR 1.10 (0.96-1.25), P = 0.17 1049 HR–HER2+ HR 1.18 (1.02-1.35), P = 0.02 747 HR+HER2+ HR 1.29 (1.08-1.53), P = 0.005 2438 TNBC HR 1.08 (0.97-1.19), P = 0.15 5779; HR 0.78 (0.72-0.84), P < 0.001 1853; HR 0.82 (0.63-1.06), P = 0.13 |
API 1870 HR+HER2– HR 0.96 (0.80-1.13), P = 0.61 853 HR–HER2+ HR 1.17 (1.01-1.36), P = 0.03 505 HR+HER2+ HR 0.93 (0.76-1.15), P = 0.53 1165 TNBC HR 0.96 (0.83-1.10), P = 0.52 3288; HR 0.75 (0.67-0.84), P < 0.001 1105; HR 0.52 (0.33-0.82). P = 0.005 |
— |
| 1984-2010, pooled analysis of eight NSABP adjuvant/neoadjuvant chemotherapy trials19 | Stages I-III DRFS |
8632 | 1070 ER+HR 1.24 (1.05-1.46), P = 0.01 ER–HR 0.97 (0.83-1.14), P = 0.73 |
— | — | — |
| 1990-2016, SEER 18 registries database20 | Stages I-IV BCSS |
670 333 | 77 622 HR 1.305 (1.277-1.334), P < 0.001 |
73 365 HR 1.057 (1.031-1.085), P < 0.001 |
AS 55 193 Ch 11 023; HR 0.814 (0.756-0.877), P < 0.001 Fp 14 743; HR 0.825 (0.778-0.876), P < 0.001 Jp 9406; HR 0.762 (0.695-0.835), P < 0.001 Kr 3733; HR 0.838 (0.744-0.943), P = 0.003 SAS 4,975; HR 0.796 (0.716-0.884), P < 0.001 SEAS 1028; HR 1.045 (0.867-1.260), P = 0.65 Viet 3327; HR 0.810 (0.715-0.916), P < 0.001 Oth 6958; HR 0.610 (0.545-0.682), P < 0.001 |
|
| 2004-2015, SEER Oncotype Dx21 | ER+HER2– Node negative only for BCSS multivariable analysis in all patients shown here |
54 945 | 5697 HR 1.66 (1.37-2.02) |
6688 HR 0.94 (0.73-1.19) |
API 6033 HR 0.67 (0.50-0.90) |
AI/AN 295 HR 1.51 (0.63-3.65) |
| 2006-2010, TAILORx22,94 | Node negative IDFS all arms (RS 0-100) shown here |
7300 9-year IDFS 83.9% |
693 9-year IDFS 78.9% HR 1.24 (1.01-1.52) versus white (no difference for years 6-12) |
889 9-year IDFS 86.9% |
AS 405 9-year IDFS 87.1% |
PI 30 NA 39 |
| 2011-2017, RxPONDER23 | N1 (RS 0-25) IDFS IDFS with BMI adjustment DRFS DRFS with BMI adjustment |
2833 5-year IDFS 91.5% |
248 5-year IDFS 87.2% HR 1.37 (1.00-1.90), P = 0.05 HR 1.21 (0.81-1.82), P = 0.35 HR 1.71 (1.19-2.45), P = 0.004 HR 1.31 (0.81-2.10), P = 0.27 |
610 5-year IDFS 91.4% HR 0.92 (0.71-1.19), P = 0.55 HR 0.98 (0.74-1.29), P = 0.87 |
AS 324 5-year IDFS 93.9% HR 0.67 (0.45-1.00), P = 0.05 HR 0.74 (0.48-1.13), P = 0.17 |
NAPI 33 |
Year of diagnosis is indicated for each cohort.
95% confidence interval (CI) indicated in brackets.
AA, African American; AI/AN, American Indian/Alaskan native; aOR, adjusted odds ratio; API, Asian/Pacific Islander; AS, Asian; BC, breast cancer; BCSS, breast cancer-specific survival; Ch, Chinese; DFS, disease-free survival; DRFS, distant relapse-free survival; ER, estrogen receptor; Fp, Filipino; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; HR+, hormone receptor+; IDFS, invasive disease-free survival; Jp, Japanese; Kr, Korean; NAPI, Native American/Pacific Islander; NHW, non-Hispanic white; OS, overall survival; Oth, others; pCR, pathologic complete response; RD, residual disease; RS, recurrence score; SAS, South Asian; SEAS, Southeast Asian; SEER, Surveillance, Epidemiology, and End Results; TAILORx, Trial Assigning Individualized Options for Treatment; TNBC, triple-negative breast cancer; UnK, unknown; Viet, Vietnamese.
In the United States, BC mortality rates dropped by 43% during 1989-2020, declining at a rate of 1.9% annually from 2002 to 2011, then at a slower pace of 1.3% annually from 2011 to 2020.3 However, the lowest 5-year relative survival was observed in blacks in 2016-2020, with 27.6 deaths per 100 000 for black women versus 19.7 deaths per 100 000 for white women. BC mortality rate was the lowest in APIs at 11.7 per 100 000, followed by Hispanics at 13.7 per 100 000. The mortality rate in American Indian/Alaska Native (AI/AN) women was 20.5 per 100 000.3
Although a recent study using the National Cancer Database (NCDB) from 2004 to 2017 reported that Hispanic women were more likely to present at a later stage compared with NHW women [adjusted odds ratio (aOR) 1.19, 95% confidence interval (CI) 1.18-1.21; P < 0.01], particularly for Mexican women (aOR 1.55, 95% CI 1.51-1.60; P < 0.01),24 Hispanic ethnicity generally predicted similar or better outcomes than NHW women in multivariable analyses of most studies. Asian or API ethnicity was associated with better survival in multivariable analyses (Table 2), even when subclassified according to specific country of origin, with the exception of South East Asian women.20 Notably, the survival rates for API and Hispanic patients may be overestimated due to incomplete information or follow-up in the cancer registries for foreign-born individuals.25
Poorer BC outcomes have also been reported in Australian Aboriginal communities, Malay ethnicity, and minority immigrant populations within various countries.26, 27, 28, 29, 30 Globally, the geographical variation in BC mortality is vast—a disproportionate burden of BC deaths occurred in low to medium Human Development Index countries relative to those classified as having high or very high Human Development Index.1 While socioeconomic status and treatment barriers are major drivers of these disparities, underlying host and tumor differences merit further investigation (Table 1).
Hormone receptor-positive HER2-negative BC is the most common subtype across various ethnic groups. Its incidence is highest among NHW women in the United States compared with other ethnic groups for age ≥50 years. In women <50 years, the incidence rate per 100 000 is similar between NHW and API at 53 per 100 000 and 50 per 100 000, respectively.3 The rising BC incidence in younger cohorts of Asian women within and outside the United States is likely due to environmental factors such as adoption of a Westernized lifestyle, although exposure to industrial and environmental pollutants with estrogenic effects has also been postulated.4 Interestingly, invasive lobular carcinoma is less common in Asians, while mucinous cancer is more common, exceeding lobular cancers in younger women.31,32 Mucinous cancer is associated with better prognosis while lobular cancer has similar or worse prognosis than the most common ductal carcinoma.32,33 The frequency of HER2-positive tumors is generally similar across ethnicities, comprising ∼15%-20% of all BCs,3 though it may be higher relative to hormone receptor-positive HER2-negative tumors in ethnic groups who undergo less screening or have proportionately more younger women with BC. The frequency of TNBC in AA women, comprising >20% of all BCs, is strikingly higher compared with all other ethnic groups—about two times as high as NHW, AI/AN, and Hispanic, and nearly three times higher than in API women across all age groups.3 The underlying predisposing factors for the frequency of BC subtypes will be discussed in the ‘Molecular Subtypes, Germline, and Somatic Genomic Profiles’ section.
Studies outside the United States have indicated significant heterogeneity between Indian/South Asian and Chinese/East Asian ethnicities, highlighting the need to disaggregate Asian ethnicity groups for such studies. The frequency of TNBC in Indian and Malay women has been reported in the range of ∼20%-30%, with older studies using earlier versions of American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) criteria for hormone receptor and HER2 testing. Conversely, TNBC is less common in Chinese/East Asians, with similar or lower frequency than NHW.34, 35, 36, 37 These discrepancies were also seen in women <40 years of age who do not routinely undergo BC screening, and hence not subjected to selection bias with overdiagnosis of hormone receptor-positive HER2-negative BCs.36,37
Socioeconomic Disparities and Adherence To Therapy
Lower socioeconomic status and education levels, together with poorer access to health care, are recognized as key drivers of inferior BC survival outcomes in disadvantaged populations or communities. According to the 2022 American Cancer Society report, the largest black–white disparities in 5-year relative survival were seen in hormone receptor-positive HER2-negative BC (88% versus 96%), hormone receptor-negative HER2-positive BC (78% versus 86%), and stage III BC (64% versus 77%). This may be related to reduced access to effective systemic treatments such as endocrine and HER2-targeted therapy, and lower rates of early detection from screening.3 In a prospective study on women with abnormal screening mammogram in 109 imaging facilities across the United States from 2009 to 2019, the risk of no biopsy within 90 days remained higher for black women compared with NHW after adjustment for selected individual-, neighborhood-, health care-level factors and screening facility (relative risk 1.20, 95% CI 1.08-1.34). This suggests that unmeasured factors such as systemic bias and other health care system factors may have contributed to delayed diagnosis.38 Black women were also more likely to decline the recommended breast surgery compared with NHW women (aOR 2.12, 95% CI 1.82-2.47) according to Surveillance, Epidemiology, and End Results (SEER) data.39
In a study on patients with stage I to III BC in the NCDB, insurance accounted for 37.0% of the total excess risk of death in black patients compared with NHW, followed by tumor characteristics (23.2%), comorbidities (11.3%), and treatment (4.8%).40 The remaining 23.7% not accounted for are likely related to factors not evaluated, such as differences in host and tumor biology with varying treatment response and other barriers (details in the paragraph below). Similar findings have been reported with other ethnicities. For example, Malay ethnicity remained an independent predictor of both lower BC-specific survival and overall survival (OS) in Singapore even after accounting for clinicopathological characteristics, treatment, socioeconomic status and the receipt of medical subsidies.28
Adherence to treatment is an important issue that is not consistently evaluated. Black race was significantly associated with nonadherence even after adjustment for net worth or socioeconomic status in multivariable analyses. Black women also reported more toxicities such as hot flashes, night sweats, and joint pain; were more likely to lack understanding of the importance of treatment compliance; and experienced cost-related barriers.41,42 Health-seeking behavior may be related to literacy, cultural perceptions, and other concerns, affecting adherence to treatment as well as surveillance. These findings underscore the importance of education, modifying health-seeking behavior, and shared decision making with a culturally sensitive approach. Pharmacogenomics or certain SNPs may affect the tolerability of certain drugs. For example, AAs experienced significantly more grade 3 to 4 peripheral neuropathy in the ECOG-ACRIN-5103 trial compared with European Americans (OR 2.9; P = 2.4 × 10−11), leading to more dose reductions that adversely affected the disease-free survival (DFS).10
By contrast, being Asian, compared with being NHW, was significantly associated with higher adherence to adjuvant endocrine therapy (OR 1.48, 95% CI 1.15-1.89) in a cohort study linking SEER database to Medicare Claims,43 as well as in the TAILORx study.22 The rates of discontinuation of endocrine therapy or abemaciclib were also lower among patients from Asia in a preliminary report from the MONARCH-E adjuvant abemaciclib trial for both the control and experimental arms. While cultural or psychosocial factors may account for the differences, Asian patients appeared to have better tolerability of endocrine therapy, with lower frequency of clinically significant arthralgia, hot flashes, and fatigue, despite a higher frequency of neutropenia from the cyclin-dependent kinase inhibitor.44 Although docetaxel dose reductions were more frequent in Asian patients in the CLEOPATRA trial testing the addition of pertuzumab to docetaxel and trastuzumab in patients with HER2-positive advanced BC, efficacy was similar to non-Asians.45
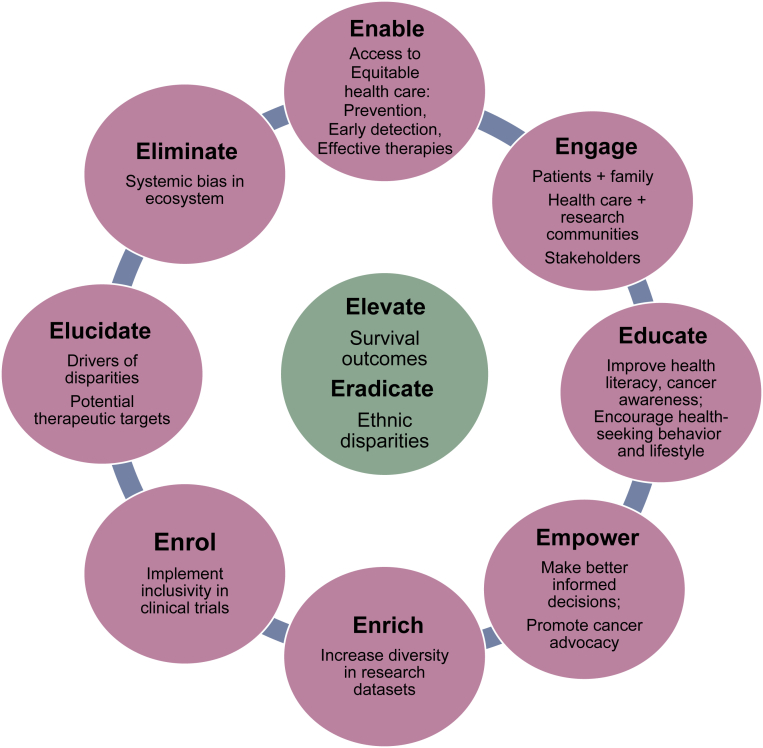
In a recent review of studies on oral anticancer medication adherence among women with BC in Africa, the nonadherence rates ranged from 4.3% to 65.4% for endocrine medications, 80.9% for cytotoxic chemotherapies, and 32.7% for combined medications. The significant barriers identified were Islamic religion, comorbidities, mastectomy, anastrozole treatment, side-effects, unawareness of medical insurance coverage, and seeking treatment from traditional healers.46 The African Breast Cancer–Disparities in Outcomes prospective cohort study conducted in sub-Saharan Africa reported that surgery with multimodality treatment was initiated in only a minority of patients with localized BC, and a majority of women did not complete local or systemic therapy as indicated clinically.47 Such important statistics are unfortunately not available from many areas of the world. Efforts to improve survival outcomes among underserved populations require a multifaceted strategy with adequate resources to address these systemic inequalities (Figure 2).
Figure 2.
A multifaceted approach to eradicate ethnic disparities in breast cancer outcomes.
BMI and Comorbidities, Lifestyle/Environmental Factors, and Allostatic Overload
Obesity has been associated with an increased risk of developing BC, and inferior survival in nonmetastatic, but not metastatic BC.48,49 A meta-analysis found that obesity predicted modestly worse DFS and OS in all subtypes.49 Comorbidities associated with obesity can increase the risk of non-BC deaths and affect the tolerability of cancer treatments.50 Other explanations include the widespread practice of capping the chemotherapy dose for individuals with obesity, and the differential distribution of lipophilic drugs such as taxanes in the fatty tissue.51 While obesity is often defined by BMI, other measures of adiposity may be more predictive. Excessive estradiol production in the adipose tissue of individuals with obesity, insulin resistance with hyperinsulinemia, and altered adipokines with higher leptin and inflammation have been implicated in mediating a protumorigenic environment.52 Obesity may promote an immunosuppressive tumor microenvironment (TME) with M2 macrophages53; loss of beneficial effect from high stromal tumor-infiltrating lymphocytes (TILs) was also observed in patients with overweight receiving neoadjuvant chemotherapy for TNBC.54
Ethnic groups with worse cancer survival outcomes are often the groups for whom obesity and related comorbidities are more prevalent.13,15,23,35 The preliminary analysis of race and outcomes in the RxPONDER trial provided insight into this important issue. The percentage of patients with BMI 35 kg/m2 and above ranged from 2% in Asians, 16% in Hispanics, 18% in NHW, 27% in Native Americans/Pacific Islander (NAPI), to 35% in blacks. The distribution of recurrence scores (RSs) was generally well balanced across racial groups. The 5-year invasive DFS was the lowest in black women and highest in Asians (Table 2), with corresponding hazard ratios (HRs) as shown after adjustment for age, menopausal status, treatment arm, grade, and RS. When BMI was adjusted for in the multivariable Cox regression analysis, the differences diminished, suggesting that BMI contributes partially to the adverse outcomes. There was no evidence of lower treatment adherence among black patients in the trial; investigation of other biological factors driving these disparities is ongoing.23
Diet varies between different ethnicities and may be modified to reduce the risk of cancer recurrence. However, a recent systematic review and meta-analysis by the Global Cancer Update Programme concluded that the evidence of potential associations between diet, use of supplements, and BC outcomes remains limited currently.55 Alcohol has been implicated in carcinogenesis via various mechanisms, including increase in estradiol and other metabolic effects.56 Daily alcohol intake was associated with late recurrence in a pooled analysis on ER-positive BCs with an adjusted HR 1.28 (95% CI 1.01-1.62),57 but other studies have reported mixed results.55 This may merit further investigation given the racial differences in alcohol intake.58
With the current research interest in microbiome, a metagenomic analysis of The Cancer Genome Atlas (TCGA) uncovered distinct microbiome profiles in white, black, and Asian BCs. Associations with genes involved in angiogenesis, metastasis, treatment resistance, and oncogenic signaling pathways were found.59 Another study reported that infecting BC cells with a high-fat-diet-derived microbiome from mice increased BC cell proliferation, and administering fish oil supplements before BC resection modulated the microbiota in tumors and normal breast tissue.60 In a different trial, 36 healthy adults were randomized to high-fiber or fermented-food (e.g. kimchi, sauerkraut, kombucha, yogurt) diet for 17 weeks. Differential effects on microbiome diversity were demonstrated, with decrease in inflammatory markers and modulation of immune responses in the fermented-food diet arm.61 While these studies suggest the ability of diet to alter the microbiome and the TME, whether such dietary interventions can improve BC outcomes in humans remains to be explored.
Physical activity of moderate intensity for at least 150 min each week (or equivalent) is recommended in various guidelines for cancer survivors and healthy individuals to minimize the risk of chronic diseases including cancer, and to overcome the detrimental effects from weight gain.62 Although a systematic review found consistent evidence from observational studies associating physical activity with reduced all-cause and BC-specific mortality, this may have been confounded in some studies by variables that were not adjusted for, such as BMI.63 A window-of-opportunity clinical trial showed that the physical exercise intervention altered tumor expression of genes involved in immunity and inflammation, suggesting additional benefit from physical activity.64 The Carolina BC Study reported that only 35% of study participants met current national physical activity guidelines post-BC diagnosis, and after adjustment for other variables including BMI, AA BC survivors were less likely to meet these activity recommendations (aOR 1.38, 95% CI 1.01-1.88).65 Such sedentary lifestyle may be exacerbated by financial hardship and lack of time and resources for partaking in regular exercise, but is less likely in developing countries where daily activities involve considerably more physical activity. Exercise intervention programs enrolling survivors from diverse backgrounds may be useful in ameliorating functional and health outcomes.62
Allostatic load refers to the sequelae of chronic exposure to environmental challenges such as systemic racism, neighborhood deprivation, financial hardship, and social isolation, leading to physiologic dysregulation and possibly culminating in diseases such as cancer. While there are no specific biomarkers for quantifying allostatic load, studies have evaluated markers of the neuroendocrine (e.g. cortisol), cardiovascular (e.g. blood pressure), metabolic (e.g. BMI, lipids, glucose), and immune system (white blood cell count, C-reactive protein) to score the biologic impact.66 Elevated allostatic load was associated with higher grade and larger size of tumors in 409 AA nonmetastatic patients with BC from the Women’s Circle of Health Follow-Up Study.67 In another study on women with newly diagnosed BC, allostatic load was higher in black and Hispanic populations than white women who experienced marital dissolution, lacked college education, currently smoked, and were less physically active. Higher load was associated with increased probability of poorly differentiated and ER-negative tumors. Intriguingly, a significant correlation with leukocyte mitochondrial DNA copy number variation was found, reflecting the compensatory production of more copies of mitochondria for maintaining normal cellular function in response to oxidative stress.68 Neighborhood deprivation was also associated with black race, young age, higher allostatic load, higher grade tumors, and lower levels of global DNA methylation in leukocytes.69 While the precise mechanisms remain unclear, epigenetic reprogramming may play a role in shaping the unfavorable biology in certain ethnicities (further details in the ‘Epigenetics and Methylation’ section).
Molecular Subtypes, Germline, and Somatic Genomic Profiles
The distribution of PAM50 intrinsic subtypes across different ethnicities generally mirrors the distribution of hormone receptor and HER2 immunohistochemical subtypes, though each immunohistochemical subtype may be classified differently based on the gene expression profile. In a diverse cohort of 1319 women with BC from the Life After Cancer Epidemiology and Pathways studies, compared with NHW women, AA women were more likely to have basal-like tumors (age-adjusted OR 4.4, 95% CI 2.3-8.4), whereas API women had reduced odds of basal-like subtype (OR 0.5, 95% CI 0.3-0.9). Further ethnicity breakdown for the API category was not provided.70 Similar findings of increased frequency of basal subtype in AA women were reported from an analysis of TCGA. Among the TNBC subtypes, the prevalence of basal-like 1 and mesenchymal stem-like tumors was higher in AA compared with NHW (26% versus 17% and 18% versus 5% respectively; P < 0.05 for aOR).71 In the Carolina BC Study, the OR for basal-like subtype was 3.11 (95% CI 2.22-4.37) for black women compared with whites. Among the hormone receptor-positive HER2-negative cases, luminal A subtype was less common and recurrence scores were higher.72 More recently, using BluePrint molecular subtyping, the frequency of basal subtype among hormone receptor-positive BCs in black women was higher at 14.5% compared with 7.2% in whites (P < 0.001).73 While immunohistochemical details were not presented with these preliminary results, AA women were more likely to have tumors with lower ER staining levels than European American women in another study.74
Studies have shown that the molecular portrait of BCs from Chinese patients in China, Taiwan, and Singapore was similar transcriptomically to white BCs, but luminal B subtype was more common than luminal A, while in series of BCs from white patients, luminal A was more prevalent than luminal B.4,75 This is possibly influenced by the higher proportion of premenopausal patients in the Asian series, where there may also be lower uptake of screening mammogram detecting indolent cancers. A more recent multiomics study in China revealed that 23% of their 360 TNBCs were of the luminal androgen receptor (LAR) subtype, compared with 12% of white and 9% of AA TNBCs from TCGA, while 33% of the 9 Asian TNBCs in TCGA (detailed ethnicity not available) were of the LAR subtype. The LAR subtype has a better prognosis than the basal-like immunosuppressed and mesenchymal-like subtype, and frequently harbors mutations in the phosphoinositide 3-kinase–protein kinase B (PI3K–Akt) pathway (∼70%).76 Few germline variants from whole-exome sequencing were associated with the LAR subtype in this Chinese cohort77; the effect of other predisposing factors or the environment warrants further research.
Pathogenic variants of certain BC-predisposing genes and even SNPs may be associated with odds of developing specific subtypes of BC.78, 79, 80 Although there is now increasing information of germline pathogenic variants with increased testing in many parts of the world,81, 82, 83, 84, 85 direct comparison of the prevalence of BRCA1/BRCA2 pathogenic variants in different populations or ethnicities is challenging with varying selection criteria and testing methods used in different studies. Overall, the prevalence of BRCA1/BRCA2 pathogenic variants appears similar, with the exception of Ashkenazi Jew ancestry, where it may occur in at least 2.5% of the general population, compared with <0.5% in other ethnicities.4,6 However, the spectrum of germline variants varies across different ethnic groups and populations, consequent from founder effects in specific geographic regions.4,84 The frequency of variants of unknown significance is also higher in non-white populations, with the relative paucity of data on the spectra of mutations.4,6
Despite the higher frequency of basal-like or TNBC in black women, a large population-based case-control study by the Cancer Risk Estimates Related to Susceptibility (CARRIERS) consortium did not reveal any meaningful differences in the prevalence of pathogenic variants in 12 BC susceptibility genes between black and NHW women with BC.85 However, a different study discovered that the Duffy antigen receptor for chemokines (DARC) [also known as the atypical chemokine receptor 1 (ACKR1)] genotype status was associated with TNBC risk in AAs, especially women of West African ancestry.86 DARC/ACKR1 has a variant allele (rs2814778) known as the Duffy-null allele, that is highly conserved among individuals from western sub-Saharan African region where malaria is endemic. The absence of the Duffy antigen on erythrocytes offered protection against malaria pathogens. This Duffy-null variant is also responsible for ‘benign ethnic neutropenia’ that is usually harmless, but may lead to unnecessary chemotherapy dose reduction in black patients, potentially compromising the efficacy.87 DARC was previously found to be a negative regulator of BC cell growth in preclinical studies, via sequestration of angiogenic chemokines and inhibition of tumor neovascularity.88 TCGA analyses showed that DARC/ACKR1 expression is lower in BCs of AAs compared with NHW, and in basal compared with luminal A tumors. Low DARC/ACKR1 expression was associated with more infiltrating M0 macrophages, fewer B cells, and worse survival in AAs.89 Levels of potentially proinflammatory cytokines related to cancer progression and immunosuppression, such as CXCL8, are higher in AA women compared with NHW women, driven by this Duffy-null variant.89
There is increasing evidence that germline genetic makeup can regulate genomic alterations or expression in the tumor as well as the microenvironment or immune system,7,90, 91, 92 and the germline genome varies considerably across ethnicities. Although the precise mechanisms remain elusive, the Carolina BC Study identified germline genes whose genetically regulated tumor expression was associated with continuous risk of recurrence scores (CRS). Tumors from black women had higher CRS than whites, but the germline genes involved differ with race.90
In a SEER database study, black women were more likely than NHW women to have an RS >25 (17.7% versus 13.7%; P < 0.001); this association remained significant after adjustment in multivariable models. The risk of BC-specific mortality among node-negative tumors was the highest in black women, while APIs had the lowest mortality (Table 2).21 Black women were also less likely to receive chemotherapy in the group with RS >30, while there were no differences of chemotherapy use between other ethnic groups.93 By contrast, in the TAILORx study, no differences in the RS, ESR1, PGR, or HER2 expression by race or ethnicity were found.22 This may be related to the smaller sample size or trial patient selection different from the real world. However, black women had worse clinical outcomes despite similar 21-gene assay RS results and comparable systemic therapy in the TAILORx trial (Table 2), while Hispanic and Asian women had better outcomes.22 In a recent update, there was no difference in DFS, distant relapse free-interval, relapse free-interval, or OS anymore between blacks and whites for years 6-12, suggesting that the disparities for black women were associated with early but not late recurrence.94
Differential expression of several other genes or pathways has been demonstrated in BCs from AA women. Differential activation of insulin-like growth factor 1 and signature of BRCA1 deficiency, with upregulation of several transcriptional signatures of proliferation, has been described.95 A 38-gene hepatocyte growth factor expression signature was associated with a higher risk of recurrence in black women from the Carolina BC Study.96 Systematic analysis of the gene expression data from several datasets revealed a unique DNA damage repair signature enriched in black patients. Characterized by upregulation of homologous recombination gene expression and downregulation of single-strand break repair genes, this gene signature was associated with cell cycle dysregulation (P < 0.001) and inferior survival.97 In TCGA dataset, resistin, a gene linked to insulin resistance, obesity, BC, and induction of interleukin-6 proinflammatory cytokine, was expressed more than four times higher in AA BCs compared with white tumors.98 Analysis of TCGA by genetic ancestry rather than self-identified race and ethnicity also showed a significantly greater risk of mortality for patients with African ancestry compared with European ancestry (HR 1.84, 95% CI 1.1-3.08; P = 0.021); every 10% increase in the African ancestry proportion was associated with an 8% increase in the relative risk of mortality. The gene set upregulated in African ancestry patients was enriched for early and late estrogen response, apical junction, and KRAS signaling pathways, with associated methylation differences.9
In terms of somatic genomic alterations, an earlier comparison of TCGA data (105 AA versus 663 whites) reported more TP53 mutations (42.9% versus 27.6%; P = 0.003) and fewer PIK3CA mutations (20.0% versus 33.9%; P = 0.008) in addition to greater intratumor genetic heterogeneity in AAs.71 In a subsequent analysis, after adjusting for intrinsic subtype frequency differences, most molecular differences were no longer significant.99 More recently, whole-genome analysis of 97 BCs from women in Nigeria, in comparison with 76 BCs from AA and white women in TCGA, revealed a higher rate of genomic instability, increased intratumoral heterogeneity, and a distinct subtype characterized by early clonal GATA3 mutations with much younger age at diagnosis.100 These differences highlight the pressing need to uncover the diversity of BCs from different regions in the world.
The prevalence of TP53 somatic mutations was higher in BCs from a number of studies in Asia (largely East Asian), compared with Caucasian series such as TCGA and Molecular Taxonomy of Breast Cancer International Consortium (METABRIC).101, 102, 103 A higher proportion of HER2-enriched and luminal B subtypes was also reported.102,103 However, the younger patient population with fewer screen-detected BCs in Asia makes direct comparison with TCGA more challenging, compounded by the lower proportion of premenopausal BCs in TCGA at ∼20%. Although a higher immune score was found in Asian BCs, a naive combined cluster analysis did not find any exclusive clusters unique to Asian tumors.102,103
A real-world clinical cancer sequencing cohort study profiled 2448 BCs from patients with diverse genetic ancestry: 1618 European, 394 Ashkenazi Jew, 219 African, 153 East Asian, and 64 South Asian. When analyzed according to immunohistochemical subtype, the most striking findings were the higher prevalence of TP53 mutations and a lower frequency of PIK3CA mutations in hormone receptor-positive HER2-negative BCs from women of African ancestry. Although limited by small numbers in the other subtypes, the frequency of TP53 or PIK3CA mutations was similar in East Asian and South Asian tumors, apart from the high frequency of PIK3CA mutations in East Asian TNBCs exceeding 40%,6 likely contributed by the LAR subtype.
Other Differences
Epigenetics and methylation
Epigenetic changes such as DNA methylation, histone modification, expression of noncoding RNA, or micro-RNA can modulate gene expression independently of the DNA sequence. Environmental factors, social exposures, stress, and allostatic load have the potential to alter the epigenome in a heritable as well as dynamic fashion. Serving as the link between the genome and the extrinsic factors, the plasticity of the epigenetic landscape may contribute toward disparities in cancer outcomes.104
Methylation reduces the transcription of genes primarily at the promoter and enhancer regions, and is one of the best studied epigenetic mechanisms in cancer.104 One of the earliest studies on ethnic differences in methylation of BCs described a significantly higher frequency of hypermethylation in hormone receptor-negative BCs from AA women <50 years old compared with white women, using methylation-specific PCR on a panel of five genes (HIN-1, Twist, Cyclin D2, RAR-β, and RASSF1A).105 Subsequently, the first genome-wide methylation study to address racial disparities identified 157 CpG loci that were differentially methylated, indicating that different pathways may be implicated in BCs in AA women.106 In another study, using array technology, DNA methylation was evaluated at 1287 CpGs in the promoters of cancer-related genes in BCs from the Carolina BC Study, and found that the differential methylation of genes such as DSC2, KCNK4, GSTM1, AXL, DNAJC15, HBII-52, TUSC3, and TES was linked to worse survival in AAs. This was further validated by TCGA dataset, supporting the hypothesis that epigenetic variation contributes to ethnic disparities in outcomes.107
While there are still limited data comparing BCs in other ethnicities, this is a rapidly evolving of research, especially with the availability of next-generation assays that can capture additional enhancer and intergenic regions of the genome for comprehensive genome-wide coverage.104 In the latest report from the AURORA US Metastasis Project, DNA hypermethylation and/or focal deletions near HLA-A were identified, typically in basal-like brain and liver metastases. The ensuing lower expression of HLA-A resulted in lower immune cell infiltrates.108 Such epigenetic differences may potentially account for some of the ethnic disparities observed in BC (details in the following section).
Immune/tumor microenvironment
There is increasing recognition of the differences between human populations in their transcriptional responses to immune challenges, which have evolved with natural selection and genetic adaptation.109 While natural variation in the parameters of innate immune cells has been shown to be preferentially controlled by genetic factors, environmental exposure appears to be the main driver of adaptive immune cells.110 The extensive DNA methylation differences in primary monocytes between individuals of African and European descent support their role in ethnicity/ancestry-related immune gene regulation.111
Although interrogation of bulk RNA-sequencing expression data from TCGA did not reveal any immune differences overall between 162 AA and 697 white BCs of all subtypes,112 differences were demonstrated comparing BCs from 920 black patients and 395 white patients in the Women’s Circle of Health Study.113 Using the Nanostring PanCancer Immune Panel, a stronger CD4+ and B-cell response was observed in black tumors, but with a more exhausted CD8+ T-cell profile. The ‘ExCD8-r’ signature, defined as the ratio of the aggregated expression of programmed cell death protein 1 (PD-1), LAG-3, and Eomes to the absolute CD8+ T-cell fraction, was identified. It was associated with inferior survival, especially for hormone receptor-positive BCs. The CD8lowExCD8-rhigh subgroup comprising the absolute fraction of CD8+ T cells and ExCD8-r signature was most prevalent among black TNBCs and had the worst survival.113 Most recently, RNA sequencing with ancestry quantification was performed on TNBCs from European American, AA, West African/Ghanaian, and East African/Ethiopian women, in addition to digital spatial profiling and immunohistochemistry. Ancestry-associated gene expression profiles were uncovered, with extensive heterogeneity among the different cohorts. Basal-like 1 TNBC was the predominant subtype for Ghanaians while mesenchymal subtype was the most common among AAs and Ethiopians. Interestingly, pathways related to cardiac disease, obesity, diabetes, and insulin signaling were activated among AA patients, but not in Ghanaian and Ethiopian patients, reflecting the impact of differential environmental exposures. The most significant differences in immune cell infiltration were detected upon comparison by ancestry rather than by self-reported race. West African ancestry that is enriched for the Duff-null variant, but not East African or European ancestry, was associated with an immune-suppressive TME.7
‘Tumor Microenvironment of Metastasis’ (TMEM), the tripartite arrangement of an invasive carcinoma cell, a macrophage, and an endothelial cell, is a metastasis biomarker that may serve as intravasation sites for tumor cells into the circulation. TMEM doorway density, evaluated with triple immunohistochemical staining for Pan-Mena (tumor cell marker), CD31 (endothelial cell marker), and CD68 (macrophage marker), was an independent predictor for relapse free-interval in 600 patients with Stage I-III BC from the E2197 adjuvant chemotherapy trial.114 In the preliminary report of an observational study, the TMEM doorway score was higher in residual BCs after neoadjuvant chemotherapy from black patients compared with white patients, especially for ER-positive HER2-negative subtype.115 This may explain the worse prognosis in black patients and possibly other ethnicities, but will require further validation.
By contrast, Asian BCs appear to harbor a more immune-active microenvironment than Western BCs. In a multiomics study with virtual microdissection on 187 Korean BCs, compared with BCs from TCGA, Korean BC status was independently associated with increased TIL and decreased transforming growth factor-β signaling expression signatures, after adjustment for confounders including age, menopausal status, and ER status.102 Similar findings were made in a study that profiled 560 Malaysian BCs (89% Chinese), and combined with other datasets to compare Asian and Caucasian tumors.103 In an analysis of eight Gene Expression Omnibus datasets and TCGA, a higher ESTIMATE immune score was found in Asian tumors, which may account for the more favorable prognosis observed.116 A recent study from Hawaii on 183 BCs treated with neoadjuvant chemotherapy also found a higher percentage of TILs in Asian and Pacific Islander patients compared with white patients on multivariate analysis, but survival outcomes were not reported.117 The underlying causes of these observations, whether it is related to BMI, diet, microbiome, or genetic or epigenetic factors, will be worth exploring in future research.
Conclusions and Future Directions
In summary, ethnicity matters in determining BC outcomes. Firstly, it is also evident from this comprehensive overview that there are major deficits in our knowledge of BC characteristics and outcomes in non-white populations, especially in regions of the world facing majority of the global BC burden. Secondly, cancer is not simply a disorder of the genome. While our understanding remains limited, other hallmarks of cancer, such as immune evasion, protumorigenic inflammation, dysregulation of cellular metabolism, epigenetic reprogramming, and polymorphic microbiomes,118 may be instrumental in driving these disparities. Given the intersection of socioeconomic determinants of health with the host and tumor biology, it is often challenging to dissect the contributions of the intrinsic and extrinsic factors discussed.
Finally, there is a pressing need to overcome these survival disparities with a multifaceted approach (Figure 2), engaging not only patients and the health care, scientific communities, but also stakeholders including policy makers, funding agencies, and health authorities. Education and access to optimal health care at affordable costs will be integral in circumventing the adverse BC trajectory of underserved populations. The underrepresentation of non-white populations in epidemiological studies, clinical trials, and translational research119,120 is also hampering our progress to promote equitable BC care for all. Current treatment paradigms may need to be refined further in view of ethnic differences in host and tumor biology which are yet to be fully elucidated. Following on the success of the human genome projects sequencing diverse populations of the world and the international consortia studying BC predisposition, it may be timely for a global BC multiomics initiative to unravel the ethnic and regional heterogeneity of BC. It is hoped that greater appreciation of the intricacies of ethnic differences in BC will lead to concerted efforts at various levels to collaborate and mitigate or overcome these disparities.
Acknowledgments
Funding
None declared.
Disclosure
YSY is supported by Singapore National Medical Research Council (NMRC) Clinician Scientist Award (MOH-CSAINV18nov-0009) and reports honoraria/personal fees from Novartis, Pfizer, Lilly/DKSH, Astra Zeneca, Eisai, MSD, Specialised Therapeutics, and Roche; travel support from DKSH and Astra Zeneca; and research support from MSD, outside the submitted work.
References
- 1.Arnold M., Morgan E., Rumgay H., et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23. doi: 10.1016/j.breast.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeiffer R.M., Webb-Vargas Y., Wheeler W., Gail M.H. Proportion of U.S. trends in breast cancer incidence attributable to long-term changes in risk factor distributions. Cancer Epidemiol Biomarkers Prev. 2018;27(10):1214–1222. doi: 10.1158/1055-9965.EPI-18-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giaquinto A.N., Sung H., Miller K.D., et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524–541. doi: 10.3322/caac.21754. [DOI] [PubMed] [Google Scholar]
- 4.Yap Y.S., Lu Y.S., Tamura K., et al. Insights into breast cancer in the east vs the west: a review. JAMA Oncol. 2019;5(10):1489–1496. doi: 10.1001/jamaoncol.2019.0620. [DOI] [PubMed] [Google Scholar]
- 5.Auton A., Brooks L.D., Durbin R.M., et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arora K., Tran T.N., Kemel Y., et al. Genetic ancestry correlates with somatic differences in a real-world clinical cancer sequencing cohort. Cancer Discov. 2022;12(11):2552–2565. doi: 10.1158/2159-8290.CD-22-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martini R., Delpe P., Chu T.R., et al. African ancestry-associated gene expression profiles in triple-negative breast cancer underlie altered tumor biology and clinical outcome in women of African descent. Cancer Discov. 2022;12(11):2530–2551. doi: 10.1158/2159-8290.CD-22-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belleau P., Deschênes A., Chambwe N., Tuveson D.A., Krasnitz A. Genetic ancestry inference from cancer-derived molecular data across genomic and transcriptomic platforms. Cancer Res. 2022;83:49–58. doi: 10.1158/0008-5472.CAN-22-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee K.K., Rishishwar L., Ban D., et al. Association of genetic ancestry and molecular signatures with cancer survival disparities: a pan-cancer analysis. Cancer Res. 2022;82(7):1222–1233. doi: 10.1158/0008-5472.CAN-21-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider B.P., Shen F., Jiang G., et al. Impact of genetic ancestry on outcomes in ECOG-ACRIN-E5103. JCO Precis Oncol. 2017;2017 doi: 10.1200/PO.17.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westbrook K.C., Brown B.W., McBride C.M. Breast cancer: a critical review of a patient sample with a ten-year follow-up. South Med J. 1975;68(5):543–548. [PubMed] [Google Scholar]
- 12.Elledge R.M., Clark G.M., Chamness G.C., Osborne C.K. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86(9):705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 13.Chlebowski R.T., Chen Z., Anderson G.L., et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 14.Kroenke C.H., Sweeney C., Kwan M.L., et al. Race and breast cancer survival by intrinsic subtype based on PAM50 gene expression. Breast Cancer Res Treat. 2014;144(3):689–699. doi: 10.1007/s10549-014-2899-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparano J.A., Wang M., Zhao F., et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst. 2012;104(5):406–414. doi: 10.1093/jnci/djr543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis L., Canchola A.J., Spiegel D., Ladabaum U., Haile R., Gomez S.L. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36(1):25–33. doi: 10.1200/JCO.2017.74.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iqbal J., Ginsburg O., Rochon P.A., Sun P., Narod S.A. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313(2):165–173. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 18.Ma S.J., Serra L.M., Yu B., et al. Racial/ethnic differences and trends in pathologic complete response following neoadjuvant chemotherapy for breast cancer. Cancers. 2022;14(3):534. doi: 10.3390/cancers14030534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim G., Pastoriza J.M., Qin J., et al. Racial disparity in distant recurrence-free survival in patients with localized breast cancer: a pooled analysis of National Surgical Adjuvant Breast and Bowel Project trials. Cancer. 2022;128(14):2728–2735. doi: 10.1002/cncr.34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu A.Y.L., Thomas S.M., DiLalla G.D., et al. Disease characteristics and mortality among Asian women with breast cancer. Cancer. 2022;128(5):1024–1037. doi: 10.1002/cncr.34015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoskins K.F., Danciu O.C., Ko N.Y., Calip G.S. Association of race/ethnicity and the 21-gene recurrence score with breast cancer-specific mortality among US women. JAMA Oncol. 2021;7(3):370–378. doi: 10.1001/jamaoncol.2020.7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albain K.S., Gray R.J., Makower D.F., et al. Race, ethnicity, and clinical outcomes in hormone receptor-positive, HER2-negative, node-negative breast cancer in the randomized TAILORx trial. J Natl Cancer Inst. 2021;113(4):390–399. doi: 10.1093/jnci/djaa148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdou Y, Barlow WE, Gralow JR, et al. Race and clinical outcomes in the RxPONDER Trial (SWOG S1007). Paper presented at San Antonio Breast Cancer Symposium. December 12-16, 2022; San Antonio, TX. Abstract GS1-01.
- 24.Swami N., Nguyen T., Dee E.C., et al. Disparities in primary breast cancer stage at presentation among Hispanic subgroups. Ann Surg Oncol. 2022;29(13):7977–7987. doi: 10.1245/s10434-022-12302-9. [DOI] [PubMed] [Google Scholar]
- 25.Pinheiro P.S., Morris C.R., Liu L., Bungum T.J., Altekruse S.F. The impact of follow-up type and missed deaths on population-based cancer survival studies for Hispanics and Asians. J Natl Cancer Inst Monogr. 2014;2014(49):210–217. doi: 10.1093/jncimonographs/lgu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dasgupta P., Harris V.M., Garvey G., Aitken J.F., Baade P.D. Factors associated with cancer survival disparities among Aboriginal and Torres Strait Islander peoples compared with other Australians: a systematic review. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.968400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhoo-Pathy N., Hartman M., Yip C.H., et al. Ethnic differences in survival after breast cancer in South East Asia. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong F.Y., Wong R.X., Zhou S., et al. Effects of housing value and medical subsidy on treatment and outcomes of breast cancer patients in Singapore: a retrospective cohort study. Lancet Reg Health West Pac. 2021;6 doi: 10.1016/j.lanwpc.2020.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lofters A.K., McBride M.L., Li D., et al. Disparities in breast cancer diagnosis for immigrant women in Ontario and BC: results from the CanIMPACT study. BMC Cancer. 2019;19(1):42. doi: 10.1186/s12885-018-5201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Hemelrijck W.M.J., De Schutter H., de Valk H.A.G., Silversmit G., Rosskamp M., Vandenheede H. Breast cancer by migrant background in Belgium: lower risk, but worse survival in women of non-European origin. Int J Cancer. 2020;147(2):350–360. doi: 10.1002/ijc.32726. [DOI] [PubMed] [Google Scholar]
- 31.Kong X., Liu Z., Cheng R., et al. Variation in breast cancer subtype incidence and distribution by race/ethnicity in the United States from 2010 to 2015. JAMA Netw Open. 2020;3(10) doi: 10.1001/jamanetworkopen.2020.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J., Kim J.Y., Lee H.B., et al. Characteristics and prognosis of 17 special histologic subtypes of invasive breast cancers according to World Health Organization classification: comparative analysis to invasive carcinoma of no special type. Breast Cancer Res Treat. 2020;184(2):527–542. doi: 10.1007/s10549-020-05861-6. [DOI] [PubMed] [Google Scholar]
- 33.Dalenc F., Lusque A., De La Motte Rouge T., et al. Impact of lobular versus ductal histology on overall survival in metastatic breast cancer: a French retrospective multicentre cohort study. Eur J Cancer. 2022;164:70–79. doi: 10.1016/j.ejca.2021.12.031. [DOI] [PubMed] [Google Scholar]
- 34.Kulkarni A., Kelkar D.A., Parikh N., Shashidhara L.S., Koppiker C.B., Kulkarni M. Meta-analysis of prevalence of triple-negative breast cancer and its clinical features at incidence in Indian patients with breast cancer. JCO Glob Oncol. 2020;6:1052–1062. doi: 10.1200/GO.20.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yap Y.-S., Lo S.-K., Ng R., et al. P2-14-07: Ethnic differences in breast cancer molecular subtypes and survival outcomes in a multi-ethnic Singaporean population. Cancer Res. 2011;71(suppl 24) P2-14-07-P12-14-07. [Google Scholar]
- 36.Singh M., Ding Y., Zhang L.Y., et al. Distinct breast cancer subtypes in women with early-onset disease across races. Am J Cancer Res. 2014;4(4):337–352. [PMC free article] [PubMed] [Google Scholar]
- 37.Lin C.H., Yap Y.S., Lee K.H., et al. Contrasting epidemiology and clinicopathology of female breast cancer in Asians vs the US population. J Natl Cancer Inst. 2019;111(12):1298–1306. doi: 10.1093/jnci/djz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawson M.B., Bissell M.C.S., Miglioretti D.L., et al. Multilevel factors associated with time to biopsy after abnormal screening mammography results by race and ethnicity. JAMA Oncol. 2022;8(8):1115–1126. doi: 10.1001/jamaoncol.2022.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fwelo P., Yusuf Z.I., Adjei A., Huynh G., Du X.L. Racial and ethnic disparities in the refusal of surgical treatment in women 40 years and older with breast cancer in the USA between 2010 and 2017. Breast Cancer Res Treat. 2022;194(3):643–661. doi: 10.1007/s10549-022-06653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jemal A., Robbins A.S., Lin C.C., et al. Factors that contributed to black-white disparities in survival among nonelderly women with breast cancer between 2004 and 2013. J Clin Oncol. 2018;36(1):14–24. doi: 10.1200/JCO.2017.73.7932. [DOI] [PubMed] [Google Scholar]
- 41.Hershman D.L., Tsui J., Wright J.D., Coromilas E.J., Tsai W.Y., Neugut A.I. Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. J Clin Oncol. 2015;33(9):1053–1059. doi: 10.1200/JCO.2014.58.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheeler S.B., Spencer J., Pinheiro L.C., et al. Endocrine therapy nonadherence and discontinuation in black and white women. J Natl Cancer Inst. 2019;111(5):498–508. doi: 10.1093/jnci/djy136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farias A.J., Du X.L. Association between out-of-pocket costs, race/ethnicity, and adjuvant endocrine therapy adherence among Medicare patients with breast cancer. J Clin Oncol. 2017;35(1):86–95. doi: 10.1200/JCO.2016.68.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yap Y.S., Kim S.B., Chiu J.W.Y., et al. 48P abemaciclib combined with adjuvant endocrine therapy in patients from Asia with high risk early breast cancer: monarchE. Ann Oncol. 2021;32:S41–S42. [Google Scholar]
- 45.Swain S.M., Im Y.H., Im S.A., et al. Safety profile of pertuzumab with trastuzumab and docetaxel in patients from Asia with human epidermal growth factor receptor 2-positive metastatic breast cancer: results from the phase III trial CLEOPATRA. Oncologist. 2014;19(7):693–701. doi: 10.1634/theoncologist.2014-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onwusah D.O., Ojewole E.B., Chimbari M.J. Adherence to oral anticancer medications among women with breast cancer in Africa: a scoping review. JCO Glob Oncol. 2023;9 doi: 10.1200/GO.21.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foerster M., McCormack V., Anderson B.O., et al. Treatment guideline concordance, initiation, and abandonment in patients with non-metastatic breast cancer from the African breast cancer-disparities in outcomes (ABC-DO) cohort in sub-Saharan Africa: a prospective cohort study. Lancet Oncol. 2022;23(6):729–738. doi: 10.1016/S1470-2045(22)00198-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saleh K., Carton M., Dieras V., et al. Impact of body mass index on overall survival in patients with metastatic breast cancer. Breast. 2021;55:16–24. doi: 10.1016/j.breast.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lohmann A.E., Soldera S.V., Pimentel I., et al. Association of obesity with breast cancer outcome in relation to cancer subtypes: a meta-analysis. J Natl Cancer Inst. 2021;113(11):1465–1475. doi: 10.1093/jnci/djab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Sadawi M., Hussain Y., Copeland-Halperin R.S., et al. Racial and socioeconomic disparities in cardiotoxicity among women with HER2-positive breast cancer. Am J Cardiol. 2021;147:116–121. doi: 10.1016/j.amjcard.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desmedt C., Fornili M., Clatot F., et al. Differential benefit of adjuvant docetaxel-based chemotherapy in patients with early breast cancer according to baseline body mass index. J Clin Oncol. 2020;38(25):2883–2891. doi: 10.1200/JCO.19.01771. [DOI] [PubMed] [Google Scholar]
- 52.Lohmann A.E., Goodwin P.J., Chlebowski R.T., Pan K., Stambolic V., Dowling R.J.O. Association of obesity-related metabolic disruptions with cancer risk and outcome. J Clin Oncol. 2016;34(35):4249–4255. doi: 10.1200/JCO.2016.69.6187. [DOI] [PubMed] [Google Scholar]
- 53.Singh A., Mayengbam S.S., Yaduvanshi H., Wani M.R., Bhat M.K. Obesity programs macrophages to support cancer progression. Cancer Res. 2022;82(23):4303–4312. doi: 10.1158/0008-5472.CAN-22-1257. [DOI] [PubMed] [Google Scholar]
- 54.Floris G., Richard F., Hamy A.-S., et al. Body mass index and tumor-infiltrating lymphocytes in triple-negative breast cancer. J Natl Cancer Inst. 2020;113(2):146–153. doi: 10.1093/jnci/djaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Becerra-Tomás N., Balducci K., Abar L., et al. Postdiagnosis dietary factors, supplement use and breast cancer prognosis: Global Cancer Update Programme (CUP Global) systematic literature review and meta-analysis. Int J Cancer. 2023;152(4):616–634. doi: 10.1002/ijc.34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seitz H.K., Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7(8):599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 57.Nechuta S., Chen W.Y., Cai H., et al. A pooled analysis of post-diagnosis lifestyle factors in association with late estrogen-receptor-positive breast cancer prognosis. Int J Cancer. 2016;138(9):2088–2097. doi: 10.1002/ijc.29940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chartier K., Caetano R. Ethnicity and health disparities in alcohol research. Alcohol Res Health. 2010;33(1-2):152–160. [PMC free article] [PubMed] [Google Scholar]
- 59.Parida S., Siddharth S., Xia Y., Sharma D. Concomitant analyses of intratumoral microbiota and genomic features reveal distinct racial differences in breast cancer. NPJ Breast Cancer. 2023;9(1):4. doi: 10.1038/s41523-023-00505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soto-Pantoja D.R., Gaber M., Arnone A.A., et al. Diet alters entero-mammary signaling to regulate the breast microbiome and tumorigenesis. Cancer Research. 2021;81(14):3890–3904. doi: 10.1158/0008-5472.CAN-20-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wastyk H.C., Fragiadakis G.K., Perelman D., et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184(16):4137–4153.e4114. doi: 10.1016/j.cell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bandera E.V., Alfano C.M., Qin B., Kang D.W., Friel C.P., Dieli-Conwright C.M. Harnessing nutrition and physical activity for breast cancer prevention and control to reduce racial/ethnic cancer health disparities. Am Soc Clin Oncol Educ Book. 2021;41:1–17. doi: 10.1200/EDBK_321315. [DOI] [PubMed] [Google Scholar]
- 63.Ballard-Barbash R., Friedenreich C.M., Courneya K.S., Siddiqi S.M., McTiernan A., Alfano C.M. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(11):815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ligibel J.A., Dillon D., Giobbie-Hurder A., et al. Impact of a pre-operative exercise intervention on breast cancer proliferation and gene expression: results from the pre-operative health and body (PreHAB) study. Clin Cancer Res. 2019;25(17):5398–5406. doi: 10.1158/1078-0432.CCR-18-3143. [DOI] [PubMed] [Google Scholar]
- 65.Hair B.Y., Hayes S., Tse C.-K., Bell M.B., Olshan A.F. Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer. 2014;120(14):2174–2182. doi: 10.1002/cncr.28630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Obeng-Gyasi S., Tarver W., Carlos R.C., Andersen B.L. Allostatic load: a framework to understand breast cancer outcomes in black women. NPJ Breast Cancer. 2021;7(1):100. doi: 10.1038/s41523-021-00309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xing C.Y., Doose M., Qin B., et al. Prediagnostic allostatic load as a predictor of poorly differentiated and larger sized breast cancers among black women in the Women’s Circle of Health Follow-Up Study. Cancer Epidemiol Biomarkers Prev. 2020;29(1):216–224. doi: 10.1158/1055-9965.EPI-19-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao H., Song R., Ye Y., Chow W.H., Shen J. Allostatic score and its associations with demographics, healthy behaviors, tumor characteristics, and mitochondrial DNA among breast cancer patients. Breast Cancer Res Treat. 2021;187(2):587–596. doi: 10.1007/s10549-021-06102-0. [DOI] [PubMed] [Google Scholar]
- 69.Shen J., Fuemmeler B.F., Sheppard V.B., et al. Neighborhood disadvantage and biological aging biomarkers among breast cancer patients. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-15260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sweeney C., Bernard P.S., Factor R.E., et al. Intrinsic subtypes from PAM50 gene expression assay in a population-based breast cancer cohort: differences by age, race, and tumor characteristics. Cancer Epidemiol Biomarkers Prev. 2014;23(5):714–724. doi: 10.1158/1055-9965.EPI-13-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keenan T., Moy B., Mroz E.A., et al. Comparison of the genomic landscape between primary breast cancer in African American versus white women and the association of racial differences with tumor recurrence. J Clin Oncol. 2015;33(31):3621–3627. doi: 10.1200/JCO.2015.62.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Troester M.A., Sun X., Allott E.H., et al. Racial differences in PAM50 subtypes in the Carolina Breast Cancer Study. J Natl Cancer Inst. 2018;110(2):176–182. doi: 10.1093/jnci/djx135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reid S.A., Pal T., Shu X.-O., et al. Whole transcriptomic analysis of HR+ breast cancer in black women classified as basal-type by BluePrint. J Clini Oncol. 2022;40(suppl 16):517. [Google Scholar]
- 74.Purrington K.S., Gorski D., Simon M.S., et al. Racial differences in estrogen receptor staining levels and implications for treatment and survival among estrogen receptor positive, HER2-negative invasive breast cancers. Breast Cancer Res Treat. 2020;181(1):145–154. doi: 10.1007/s10549-020-05607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang X., Dugo M., Callari M., et al. Molecular portrait of breast cancer in China reveals comprehensive transcriptomic likeness to Caucasian breast cancer and low prevalence of luminal A subtype. Cancer Med. 2015;4(7):1016–1030. doi: 10.1002/cam4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang Y.Z., Ma D., Suo C., et al. Genomic and transcriptomic landscape of triple-negative breast cancers: subtypes and treatment strategies. Cancer Cell. 2019;35(3):428–440.e425. doi: 10.1016/j.ccell.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Ma D., Chen S.Y., Ren J.X., et al. Molecular features and functional implications of germline variants in triple-negative breast cancer. J Natl Cancer Inst. 2021;113(7):884–892. doi: 10.1093/jnci/djaa175. [DOI] [PubMed] [Google Scholar]
- 78.Hu C., Hart S.N., Gnanaolivu R., et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384(5):440–451. doi: 10.1056/NEJMoa2005936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan C.H.T., Munusamy P., Loke S.Y., et al. Identification of novel breast cancer risk loci. Cancer Res. 2017;77(19):5428–5437. doi: 10.1158/0008-5472.CAN-17-0992. [DOI] [PubMed] [Google Scholar]
- 80.Ahearn T.U., Zhang H., Michailidou K., et al. Common variants in breast cancer risk loci predispose to distinct tumor subtypes. Breast Cancer Res. 2022;24(1):2. doi: 10.1186/s13058-021-01484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ang B.H., Ho W.K., Wijaya E., et al. Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation in Asian patients with breast cancer. J Clin Oncol. 2022;40(14):1542–1551. doi: 10.1200/JCO.21.01647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ossa Gomez C.A., Achatz M.I., Hurtado M., et al. Germline pathogenic variant prevalence among Latin American and US Hispanic individuals undergoing testing for hereditary breast and ovarian cancer: a cross-sectional study. JCO Glob Oncol. 2022;8 doi: 10.1200/GO.22.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Busheri L., Dixit S., Nare S., et al. Breast cancer biobank from a single institutional cohort in an urban setting in India: tumor characteristics and survival outcomes. Cancer Treat Res Commun. 2021;28 doi: 10.1016/j.ctarc.2021.100409. [DOI] [PubMed] [Google Scholar]
- 84.Bhaskaran S.P., Huang T., Rajendran B.K., et al. Ethnic-specific BRCA1/2 variation within Asia population: evidence from over 78 000 cancer and 40 000 non-cancer cases of Indian, Chinese, Korean and Japanese populations. J Med Genet. 2021;58(11):752–759. doi: 10.1136/jmedgenet-2020-107299. [DOI] [PubMed] [Google Scholar]
- 85.Domchek S.M., Yao S., Chen F., et al. Comparison of the prevalence of pathogenic variants in cancer susceptibility genes in black women and non-Hispanic white women with breast cancer in the United States. JAMA Oncol. 2021;7(7):1045–1050. doi: 10.1001/jamaoncol.2021.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Newman L.A., Jenkins B., Chen Y., et al. Hereditary susceptibility for triple negative breast cancer associated with Western Sub-Saharan African ancestry: results from an international surgical breast cancer collaborative. Ann Surg. 2019;270(3):484–492. doi: 10.1097/SLA.0000000000003459. [DOI] [PubMed] [Google Scholar]
- 87.Hershman D., Weinberg M., Rosner Z., et al. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. J Natl Cancer Inst. 2003;95(20):1545–1548. doi: 10.1093/jnci/djg073. [DOI] [PubMed] [Google Scholar]
- 88.Wang J., Ou Z.L., Hou Y.F., et al. Enhanced expression of Duffy antigen receptor for chemokines by breast cancer cells attenuates growth and metastasis potential. Oncogene. 2006;25(54):7201–7211. doi: 10.1038/sj.onc.1209703. [DOI] [PubMed] [Google Scholar]
- 89.Jenkins B.D., Martini R.N., Hire R., et al. Atypical chemokine receptor 1 (DARC/ACKR1) in breast tumors is associated with survival, circulating chemokines, tumor-infiltrating immune cells, and African ancestry. Cancer Epidemiol biomarkers Prev. 2019;28(4):690–700. doi: 10.1158/1055-9965.EPI-18-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patel A., García-Closas M., Olshan A.F., et al. Gene-level germline contributions to clinical risk of recurrence scores in black and white patients with breast cancer. Cancer Res. 2022;82(1):25–35. doi: 10.1158/0008-5472.CAN-21-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hammer C., Mellman I. Coming of age: human genomics and the cancer-immune set point. Cancer Immunol Res. 2022;10(6):674–679. doi: 10.1158/2326-6066.CIR-21-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Y., Gusev A., Kraft P. Germline cancer gene expression quantitative trait loci are associated with local and global tumor mutations. Cancer Res. 2023;83:1191–1202. doi: 10.1158/0008-5472.CAN-22-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han Y., Miao Z.F., Lian M., Peterson L.L., Colditz G.A., Liu Y. Racial and ethnic disparities in 21-gene recurrence scores, chemotherapy, and survival among women with hormone receptor-positive, node-negative breast cancer. Breast Cancer Res Treat. 2020;184(3):915–925. doi: 10.1007/s10549-020-05902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sparano J, Gray RJ, Makower D, et al. Trial assigning individualized options for treatment (TAILORx): an update including 12-year event rates. Paper presented at the San Antonio Breast Cancer Symposium. December 12-16, 2022; San Antonio, TX. Abstract GS1-05.
- 95.Lindner R., Sullivan C., Offor O., et al. Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0071915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jones G.S., Hoadley K.A., Benefield H., et al. Racial differences in breast cancer outcomes by hepatocyte growth factor pathway expression. Breast Cancer Res Treat. 2022;192(2):447–455. doi: 10.1007/s10549-021-06497-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mazumder A., Jimenez A., Ellsworth R.E., et al. The DNA damage repair landscape in black women with breast cancer. Ther Adv Med Oncol. 2022;14 doi: 10.1177/17588359221075458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stewart P.A., Luks J., Roycik M.D., Sang Q.X., Zhang J. Differentially expressed transcripts and dysregulated signaling pathways and networks in African American breast cancer. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huo D., Hu H., Rhie S.K., et al. Comparison of breast cancer molecular features and survival by African and European ancestry in The Cancer Genome Atlas. JAMA Oncol. 2017;3(12):1654–1662. doi: 10.1001/jamaoncol.2017.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ansari-Pour N., Zheng Y., Yoshimatsu T.F., et al. Whole-genome analysis of Nigerian patients with breast cancer reveals ethnic-driven somatic evolution and distinct genomic subtypes. Nat Commun. 2021;12(1):6946. doi: 10.1038/s41467-021-27079-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yap Y.S., Singh A.P., Lim J.H.C., et al. Elucidating therapeutic molecular targets in premenopausal Asian women with recurrent breast cancers. NPJ Breast Cancer. 2018;4:19. doi: 10.1038/s41523-018-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kan Z., Ding Y., Kim J., et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 2018;9(1):1725. doi: 10.1038/s41467-018-04129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pan J.W., Zabidi M.M.A., Ng P.S., et al. The molecular landscape of Asian breast cancers reveals clinically relevant population-specific differences. Nat Commun. 2020;11(1):6433. doi: 10.1038/s41467-020-20173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salas L.A., Peres L.C., Thayer Z.M., et al. A transdisciplinary approach to understand the epigenetic basis of race/ethnicity health disparities. Epigenomics. 2021;13(21):1761–1770. doi: 10.2217/epi-2020-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mehrotra J., Ganpat M.M., Kanaan Y., et al. Estrogen receptor/progesterone receptor-negative breast cancers of young African-American women have a higher frequency of methylation of multiple genes than those of Caucasian women. Clin Cancer Res. 2004;10(6):2052–2057. doi: 10.1158/1078-0432.ccr-03-0514. [DOI] [PubMed] [Google Scholar]
- 106.Ambrosone C.B., Young A.C., Sucheston L.E., et al. Genome-wide methylation patterns provide insight into differences in breast tumor biology between American women of African and European ancestry. Oncotarget. 2014;5(1):237–248. doi: 10.18632/oncotarget.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Conway K., Edmiston S.N., Tse C.K., et al. Racial variation in breast tumor promoter methylation in the Carolina Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2015;24(6):921–930. doi: 10.1158/1055-9965.EPI-14-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garcia-Recio S., Hinoue T., Wheeler G.L., et al. Multiomics in primary and metastatic breast tumors from the AURORA US network finds microenvironment and epigenetic drivers of metastasis. Nat Cancer. 2023;4(1):128–147. doi: 10.1038/s43018-022-00491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quach H., Rotival M., Pothlichet J., et al. Genetic adaptation and neandertal admixture shaped the immune system of human populations. Cell. 2016;167(3):643–656.e617. doi: 10.1016/j.cell.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patin E., Hasan M., Bergstedt J., et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat Immunol. 2018;19(3):302–314. doi: 10.1038/s41590-018-0049-7. [DOI] [PubMed] [Google Scholar]
- 111.Husquin L.T., Rotival M., Fagny M., et al. Exploring the genetic basis of human population differences in DNA methylation and their causal impact on immune gene regulation. Genome Biol. 2018;19(1):222. doi: 10.1186/s13059-018-1601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O'Meara T., Safonov A., Casadevall D., et al. Immune microenvironment of triple-negative breast cancer in African-American and Caucasian women. Breast Cancer Res Treat. 2019;175(1):247–259. doi: 10.1007/s10549-019-05156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yao S., Cheng T.D., Elkhanany A., et al. Breast tumor microenvironment in black women: a distinct signature of CD8+ T-cell exhaustion. J Natl Cancer Inst. 2021;113(8):1036–1043. doi: 10.1093/jnci/djaa215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sparano J.A., Gray R., Oktay M.H., et al. A metastasis biomarker (MetaSite Breast™ Score) is associated with distant recurrence in hormone receptor-positive, HER2-negative early-stage breast cancer. NPJ Breast Cancer. 2017;3:42. doi: 10.1038/s41523-017-0043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karadal B, Kim G, Sharma V, et al. Racial disparity in tumor microenvironment and outcomes in residual breast cancer treated with neoadjuvant chemotherapy. Paper presented at San Antonio Breast Cancer Symposium. December 12-16, 2022; San Antonio, TX. Abstract GS1-02.
- 116.Chen C.H., Lu Y.S., Cheng A.L., et al. Disparity in tumor immune microenvironment of breast cancer and prognostic impact: Asian versus Western populations. Oncologist. 2020;25(1):e16–e23. doi: 10.1634/theoncologist.2019-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bansil S., Silva A., Taniguchi A., et al. Racial/ethnic differences among tumor-infiltrating lymphocytes in breast cancer tumors. Oncologist. 2023;28:116–122. doi: 10.1093/oncolo/oyac239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 119.Loree J.M., Anand S., Dasari A., et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5(10) doi: 10.1001/jamaoncol.2019.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Halmai N.B., Carvajal-Carmona L.G. Diversifying preclinical research tools: expanding patient-derived models to address cancer health disparities. Trends Cancer. 2022;8(4):291–294. doi: 10.1016/j.trecan.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]