Abstract
BACKGROUND:
Black race is associated with worse outcome in patients with breast cancer. The distant relapse-free survival (DRFS) between Black and White women with localized breast cancer who participated in National Cancer Institute–sponsored clinical trial was evaluated.
METHODS:
Pooled data were analyzed from 8 National Surgical Adjuvant Breast and Bowel Project (NSABP) trials including 9702 women with localized breast cancer treated with adjuvant chemotherapy (AC, n = 7485) or neoadjuvant chemotherapy (NAC, n = 2217), who self-reported as Black (n = 1070) or White (n = 8632) race. The association between race and DRFS was analyzed using log-rank tests and multivariate Cox regression.
RESULTS:
After adjustment for covariates including age, tumor size, nodal status, body mass index and taxane use, and treatment (AC vs NAC), Black race was associated with an inferior DRFS in estrogen receptor–positive (ER+; hazard ratio [HR], 1.24; 95% CI, 1.05– 1.46; P = .01), but not in ER–disease (HR, 0.97; 95% CI, 0.83– 1.14; P = .73), and significant interaction between race and ER status was observed (P = .03). There was no racial disparity in DRFS among patients with pathologic complete response (pCR) (log-rank P = .8). For patients without pCR, Black race was associated with worse DRFS in ER+ (HR, 1.67; 95% CI, 1.14– 2.45; P = .01), but not in ER– disease (HR, 0.91; 95% CI, 0.65– 1.28; P = .59).
CONCLUSIONS:
Black race was associated with significantly inferior DRFS in ER+ localized breast cancer treated with AC or NAC, but not in ER– disease. In the NAC group, racial disparity was also observed in patients with residual ER+ breast cancer at surgery, but not in those who had pCR.
Keywords: African Americans, breast neoplasms, humans, National Cancer Institute (US), neoadjuvant therapy, neoplasm, residual, retrospective study
INTRODUCTION
Breast cancer is the second most commonly diagnosed cancer and the second leading cause of cancer death among US women.1,2 Black race has been associated with worse outcome in both women and men with localized and advanced breast cancer.3–5 This disparity has been attributed to patient- and tumor-specific factors such as more advanced disease at presentation,6 higher rates of obesity,7 and higher rates of unacceptable side effects to chemotherapy,8,9 as well as differences in tumor microenvironment and systemic inflammatory signatures.10 Other factors contributing to racial disparities include differences in socioeconomic status and limitations in access to care.11 Studying race- and ethnicity-dependent outcome has been challenging because of heterogeneity within racial and ethnic groups and difficulty in controlling for social determinants of health.
Clinical trials provide an opportunity to control for some of the social determinants of health, such as access to care, medical comorbidities, and variations in treatment and adherence to therapy. However, Black and Hispanic patients are consistently underrepresented in phase 3 oncologic clinical trials, including trials sponsored by the US National Cancer Institute (NCI).12,13
Efforts have been made to overcome this limitation by pooling data from NCI-sponsored adjuvant trials in localized breast cancer. For example, a pooled analysis of 4 trials (B-13, B-14, B-19, and B-20) coordinated by the National Surgical Adjuvant Breast and Bowel Project (NSABP) including 5444 patients with localized breast cancer that completed accrual before 1988 found that there was no racial disparity in cancer-specific outcomes.14 On the other hand, in an analysis of 35 phase 3 trials coordinated by the Southwestern Oncology Group, including 19,547 patients with a variety of solid tumors and hematologic malignancies, there was racial disparity, even after controlling for clinical prognostic factors, income, and education, among those with breast, ovarian, or prostate cancer.15 Similarly, Black race was associated with a worse outcome in those with hormone receptor–positive (HR+), HER2–disease after controlling for covariates including obesity in a trial including 4817 women coordinated by the Eastern Cooperative Oncology Group.7 Moreover, a recent analysis of another NCI-sponsored trial (TAILORx) that included 10,273 women demonstrated a higher distant recurrence rate in Black compared with White participants with HR+ HER2–disease despite similar 21-gene assay recurrence scores.16
Neoadjuvant chemotherapy (NAC) has emerged as an effective means to downstage locally advanced disease and to allow for breast conservation and less axillary surgery.17 Although single-institution, retrospective studies have demonstrated a worse distant recurrence-free survival (DRFS) in Black compared with White patients treated with NAC,18,19 this has been previously investigated in only one prospective clinical trial that showed a trend toward worse DRFS in Black compared with White patients, though this was not statistically significant.20
Given the discrepant results of adjuvant chemotherapy (AC) trial analyses and lack of NAC studies, we aimed to evaluate racial disparity in DRFS using pooled analysis of 8 NSABP trials including 9702 women with localized breast cancer receiving NAC or AC regimens including doxorubicin and cyclophosphamide with or without a taxane; this analysis did not include 4 trials reported in a prior NSABP analysis.14 NSABP is an NCI-sponsored clinical trials cooperative group that has research sites at nearly 1000 cancer treatment centers in the United States, Canada, Puerto Rico, Australia, and Ireland.
MATERIALS AND METHODS
Study/Patient Selection
A pooled analysis of 8 NSABP trials was performed (Appendix 1), including 5 trials of adjuvant chemotherapy only (B-15, B-22, B-23, B-28, B-30), 1 trial including both adjuvant and neoadjuvant chemotherapy arms (B-18 arm 1 and arm 2), and 2 trials including neoadjuvant chemotherapy only (B-27 arm 1 and arm 2, B-40 arm 1A); patients enrolled in the B-27 third arm including both AC and NAC were not included in this analysis. The accrual dates for the trials are included in Supporting Table 1. These 8 trials were selected by reviewing treatment with doxorubicin and cyclophosphamide with or without a taxane for localized invasive cancer. Of the trials included in our analyses, only B-40 had information regarding HER2 expression available. Therefore, we could not perform subgroup analysis according to HER2 expression status. Additionally, B-36 was excluded because patients were treated with trastuzumab at the discretion of the treating physician. Of the remaining trials, there were 14 involving doxorubicin and cyclophosphamide. To minimize the effects of treatment variations, we included only the trials in which patients received doxorubicin 60 mg/m2 intravenously and cyclophosphamide 600 mg/m2 intravenously with or without a taxane every 3 weeks (n = 9). B-16 was excluded because of its limited eligibility criteria. Lastly, B-2 9 (arm 3) was excluded because it included <8% Black participants. The AC treatment group was defined as patients who received surgery first, followed by chemotherapy. The NAC treatment group was defined as those who received all chemotherapy before surgery (B-27 arm 3 was excluded because of receipt of taxane after surgery). Of the 10,522 patients in these trials, only patients of Black (n = 1070, 11.0%) or White (n = 8632, 89.0%) race were included in the analyses. Race was self-reported, and ethnicity was not considered (ie, White race encompasses both Hispanic and non-Hispanic White patients).
Statistical Analysis
Patient and tumor characteristics such as age (years), ER status (positive vs negative), tumor size (≥2 cm vs <2 cm), nodal status (positive vs negative), obesity status (obese vs nonobese), and taxane use (yes vs no) were compared using Wilcoxon rank-s um tests for continuous variables and Pearson χ2 test for categorical variables. Obesity was defined as body mass index ≥30 kg/m2. Pathological tumor size and node status were used for the AC group, whereas clinical tumor size and node status were used for the NAC group. Pathologic complete response (pCR) was defined by NSABP as no residual invasive disease in the breast only and was also compared between Black and White patients in the NAC cohort. Notably, the information about the residual ductal carcinoma in situ in the pCR group was not provided.
The outcome evaluated was DRFS, defined as time to first distant relapse or a second primary cancer. Death before a distant recurrence or second primary cancer was censored. The median follow-up was 115.2 months, and we censored the follow-up beyond 210 months. Kaplan-Meier survival curves were estimated for each racial group (adjusted Kaplan-Meier curves and log-rank tests21 were also estimated; see Appendix 2) and log-rank tests were used to compare survival between Black and White patients. Multivariate Cox proportional hazard models stratified by the trials were used to compare survival between Black and White patients while adjusting for age, ER status, tumor size, nodal status, obesity status, and taxane use and treatment. Because prior studies indicated racial disparities were evident primarily in ER+ breast cancer,7,16 separate Cox models were used for ER+ and ER– disease. A formal comparison of racial disparity between ER+ and ER– disease was made by testing the interaction between race and ER status. Within the NAC cohort, separate analyses were also performed by pCR, because pCR concerns good long-term prognosis. Because prior studies indicated racial disparity in ER+ breast cancer,7,16 separate analyses for patients with ER+ and ER– disease were performed in patients with residual cancer after NAC.
The proportionality of the Cox models was examined based on Schoenfeld residuals,22,23 and no violation was identified. Statistical significance was specified a priori as P < .05, and the P values reported are 2-sided. All analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, North Carolina).
This study was reviewed by the Albert Einstein College of Medicine institutional review board and was deemed exempt status (institutional review board #2018– 8959).
RESULTS
Patient Characteristics
There were 9702 patients included in our analysis, all women, of whom 1070 (11.0%) were of Black race and 8632 (89.0%) were of White race. Notably, patients characterized as “other” and Hispanics were not included in the analysis. It was not clear from the data provided which race was included in the “other.” Likewise, patients in the Hispanic group were not categorized as Black or White. Of these, 7485 (77.1%) were in the AC treatment group and 2217 (22.9%) were in the NAC treatment group (Table 1). Compared with White patients, Black patients were younger (47.6 vs 49.7, P < .001) and more likely to be obese (48.4% vs 27.1%, P < .001), to receive NAC (26% vs 22.5%, P = .01), to have ER– disease (47.1% vs 34%, P < .001), to be node negative (32.1% vs 27.3%, P < .001), and to have a tumor size ≥2 cm (68.1% vs 63.4%, P < .001) (Table 1).
TABLE 1.
Patient Characteristics, Entire Cohort
| Full Sample (N = 9702) | White (N = 8632) | Black (N = 1070) | P Value | |
|---|---|---|---|---|
|
| ||||
| Age, Mean (SD) | 49.4 (10) | 49.7 (10) | 47.6 (10) | <.0001 |
| AC/NAC, Count (%) | 0.0097 | |||
| AC | 7485 (77.1) | 6693 (77.5) | 792 (74) | |
| NAC | 2217 (22.9) | 1939 (22.5) | 278 (26) | |
| ER Status, Count (%) | <.0001 | |||
| Negative | 3436 (35.4) | 2932 (34) | 504 (47.1) | |
| Positive | 4942 (50.9) | 4551 (52.7) | 391 (36.5) | |
| Unknown | 1324 (13.6) | 1149 (13.3) | 175 (16.4) | |
| Node Statusa, Count (%) | 0.0007 | |||
| Negative | 2703 (27.9) | 2359 (27.3) | 344 (32.1) | |
| Positive | 6930 (71.4) | 6216 (72) | 714 (66.7) | |
| Unknown | 69 (0.7) | 57 (0.7) | 12 (1.1) | |
| Tumor Size, Count (%) | 0.0003 | |||
| <2 cm | 3265 (33.7) | 2959 (34.3) | 306 (28.6) | |
| >=2 cm | 6205 (64) | 5476 (63.4) | 729 (68.1) | |
| Unknown | 232 (2.4) | 197 (2.3) | 35 (3.3) | |
| Obese, Count (%) | <.0001 | |||
| Non-obese | 6845 (70.6) | 6293 (72.9) | 552 (51.6) | |
| Obeseb | 2855 (29.4) | 2337 (27.1) | 518 (48.4) | |
| Unknown | 2 (0) | 2 (0) | ||
| Taxane, Count (%) | 0.3212 | |||
| No Taxane | 5803 (59.8) | 5148 (59.6) | 655 (61.2) | |
| Taxane | 3899 (40.2) | 3484 (40.4) | 415 (38.8) | |
| BMI Group, Count (%) | <.0001 | |||
| <20 | 495 (5.1) | 473 (5.5) | 22 (2.1) | |
| [20, 25) | 3318 (34.2) | 3132 (36.3) | 186 (17.4) | |
| [25, 30) | 3032 (31.3) | 2688 (31.1) | 344 (32.1) | |
| >=30 | 2855 (29.4) | 2337 (27.1) | 518 (48.4) | |
| Unknown | 2 (0) | 2 (0) | ||
Pathologic tumor size and node status for adjuvant group; clinical (pre-treatment) tumor size and node status for neoadjuvant group.
Obesity defined as BMI ≥ 30kg/m2.
Clinical Outcomes in the Entire Cohort
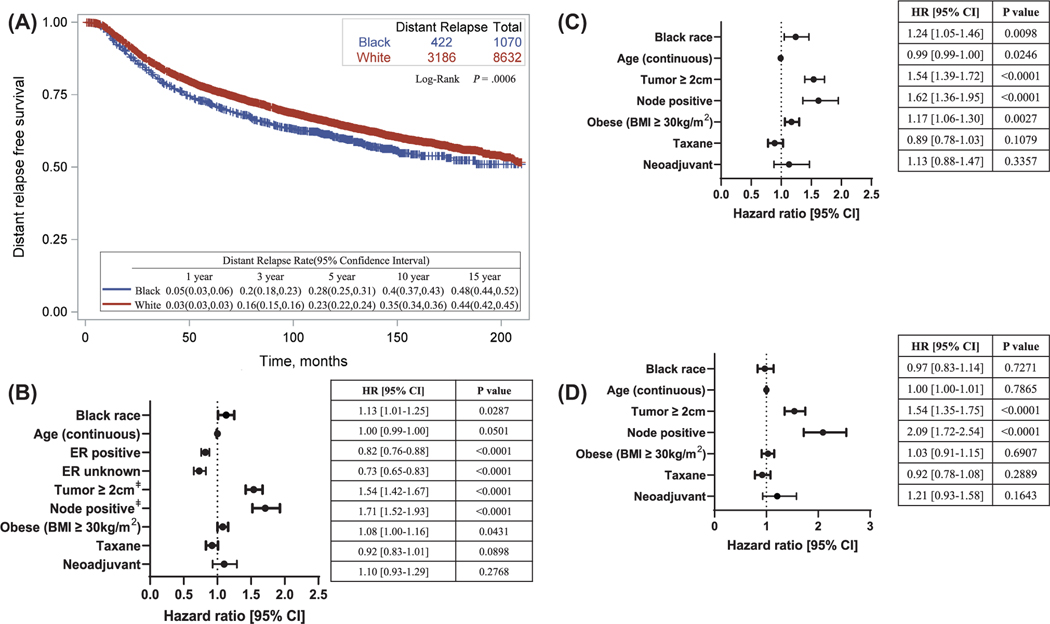
In the entire cohort, Black patients had a worse DRFS compared with White patients (log-rank P < .001) (Fig. 1A), and Black race remained significantly associated with a worse DRFS after adjusting for age, ER status, tumor size, node status, obesity, receipt of taxane, and treatment (AC vs NAC) (hazard ratio, 1.13; 95% CI, 1.01– 1.25; P = .03) (Fig. 1B). However, multivariate analysis stratifying patients by ER status showed that Black race was significantly associated with a worse DRFS in ER+ disease (hazard ratio, 1.24; 95% CI, 1.05–1 .46; P = .01) (Fig. 1C), but not in ER– disease (hazard ratio, 0.97; 95% CI, 0.83–1 .14; P = .73) (Fig. 1D). The Cox model including the interaction term revealed significant interaction between race and ER status (P = .03) (Supporting Table 2).
Figure 1.
Association between race and distant relapse-free survival, entire cohort. (A) Kaplan-Meier curve describing distant relapse-free survival in the entire cohort. Log-rank test was used to compare survival in White versus Black race patients. (B) Cox regression model adjusting for covariates and stratified by protocol. Comparison groups: White race, ER–, node negative, tumor <2 cm, nonobese (BMI <30 kg/m2), no taxane, adjuvant chemotherapy. (C) Cox-regression model in ER+ disease only. (D) Cox regression model in ER– disease only. ‡Pathologic tumor size and node status for the adjuvant group; clinical (pretreatment) tumor size and node status for the neoadjuvant group. BMI indicates body mass index; ER, estrogen receptor.
Given the differences in tumor characteristics between AC (Supporting Table 3) and NAC (Supporting Table 4) treatment groups, we repeated the analyses stratified by use of AC or NAC. Although Black patients had worse DRFS compared with White patients, the association was not statistically significant after adjustment for covariates including obesity (Supporting Figs. 1 and 2).
Clinical Outcomes in the NAC Treatment Group
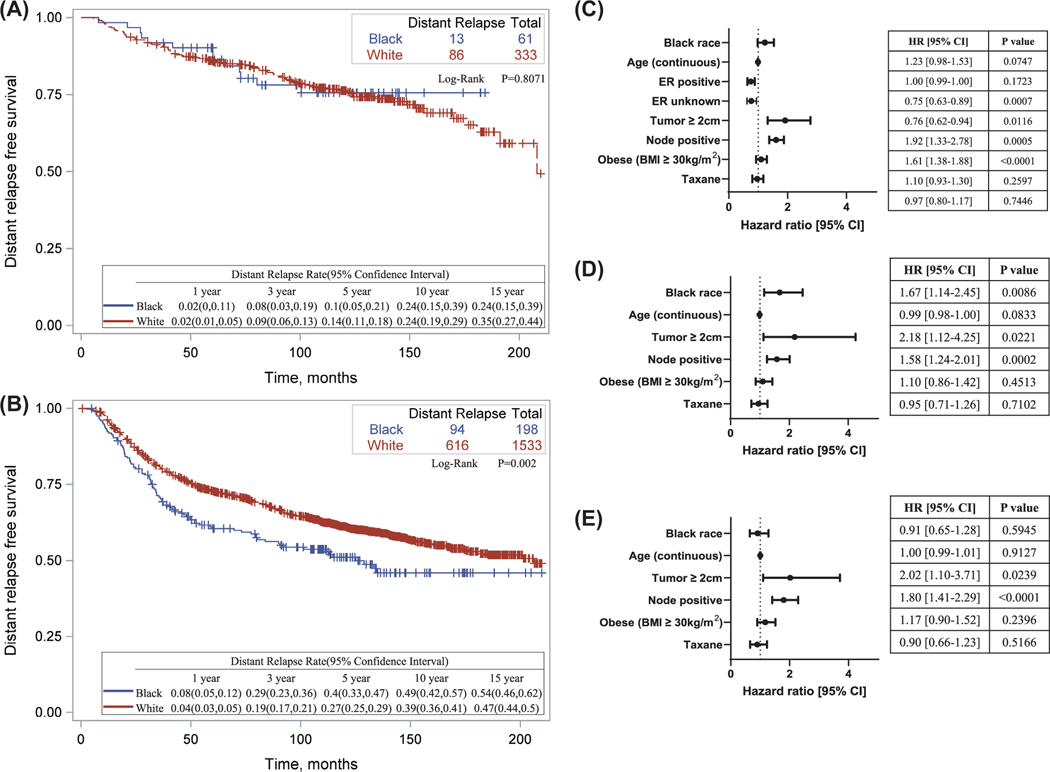
Because pCR portends to better long-term outcome, the NAC treatment group was further stratified by pCR status. There were 394 patients (17.8%) who achieved pCR, of whom 61 (15.5%) were Black and 333 (84.5%) were White. There were 1731 (81.5%) patients who did not achieve pCR, of whom 198 (11.4%) were Black and 1533 (88.6%) were White. pCR status was unknown in 92 (4.3%) of the patients. Among patients who achieved pCR, there was no racial disparity in DRFS (log-rank P = .81) (Fig. 2A). Among those who did not achieve pCR, however, Black patients had a significantly worse DRFS (log-rank P = .002) (Fig. 2B). However, after adjusting for covariates, Black race was not significantly associated with a worse DRFS (hazard ratio, 1.23; 95% CI, 0.98– 1.53; P = .08) (Fig. 2C).
Figure 2.
Association between race and DRFS in neoadjuvant treatment cohort, stratified by pCR. (A) Kaplan-Meier curve describing distant-relapse free survival in patients who achieved pathologic complete response following neoadjuvant chemotherapy. Log-rank and Wilcoxon tests were used to compare survival in White with Black race patients. (B) Kaplan- Meier curve describing distant- relapse free survival in patients who did not achieve pathologic complete response following neoadjuvant chemotherapy. Log-rank and Wilcoxon tests were used to compare survival in White versus Black race patients. (C) Cox regression model adjusting for covariates in no pCR only. (D) Cox regression model in ER+ disease with no pCR. (E) Cox regression model in ER– disease with no pCR. Comparison groups: White race, ER– , node negative, tumor <2 cm, nonobese (BMI <30 kg/m2), no taxane. BMI indicates body mass index; ER, estrogen receptor; HR, hazard ratio; pCR, pathologic complete response.
Because prior studies show racial disparity in patients with ER+ disease, the NAC treatment group without pCR was stratified by ER status (Fig. 2D,E). In ER+ disease, Black race was significantly associated with a worse DRFS even after adjusting for all covariates (hazard ratio, 1.67; 95% CI, 1.14– 2.45; P = .009), whereas no significant association with DRFS was found in the ER– group (hazard ratio, 0.91; 95% CI, 0.65– 1.28; P = .59). Although the estimates of racial disparity were very different between the ER groups, the interaction between race and ER status was not significant (P = .13) (Supporting Table 5).
In summary, there was no racial disparity in DRFS among patients who achieved pCR. For patients who did not achieve pCR, Black race was associated with worse DRFS in ER+ disease, but not in ER– disease.
DISCUSSION
In this pooled analysis of data from 8 prospective NCI-sponsored clinical trials, we found that after adjusting for covariates including age, tumor size, node status, obesity, receipt of taxane, and treatment (AC vs NAC), Black race was associated with a worse DRFS in ER+ disease but not in ER– disease. Formal testing in the entire cohort demonstrated a significant interaction between race and ER status. Within the NAC cohort, there was no racial disparity in DRFS among patients who achieved pCR. For patients who did not achieve pCR, Black race was associated with worse DRFS in ER+ disease, but not in ER– disease. However, formal testing did not reveal a significant interaction between race and ER status, likely because of a limited sample size.
Using data from patients who qualify for participation in national NCI-sponsored clinical trials minimizes some of the potential factors contributing to racial disparities noted in population-based studies, including access to care and significant comorbidities. Furthermore, using clinical trial data ensured administration of and adherence to a predetermined treatment regimen. However, social determinants of health were not considered in these trials. The patient accrual took place more than 20 years ago, when there was less known about how these parameters may affect the long-term outcomes of some patients.
The findings of this study are in line with the existing literature that Black women receiving chemotherapy for localized breast cancer have an inferior DRFS.14,24–28 Consistent with prior reports on racial disparity in ER+ disease,7,16 Black race was significantly associated with higher distant recurrence rates in ER+ disease when adjusted for other prognostic covariates in the overall cohort. In addition, we show here that Black race was associated with worse DRFS in patients with ER+ residual tumors following neoadjuvant therapy. The decision to stratify according to ER status and pCR was also based on parameters affecting long-term outcomes in patients. ER+, compared with ER– disease, tends to have a weaker response to chemotherapy, and pCR is associated with a better long-term outcome. Further research is needed to better understand whether the observed disparity is due to adherence to endocrine therapy, and/or biological, environmental, and/or social determinants of health.
Consistent with prior reports, we did not observe substantial racial disparities in pCR rates after NAC.29– 32 In fact, Black women in our analysis had somewhat higher pCR rates than White women (21.9% vs 17.2%, P = .0051), which is likely because of a higher prevalence of basal-like or triple-negative cancer subtype in Black women33,34 that is more chemosensitive.35– 39 Because of limitations in the data set, we were not able to evaluate molecular subtypes in this study directly, although the higher prevalence of ER–disease among Black women would suggest that Black women in our group have a higher prevalence of basal-like subtype in line with the existing literature.40
Strengths of this study include the large number of patients included in the analysis, the long follow-u p duration, inclusion of subjects who had access to care and absence of comorbidities that would preclude optimal adjuvant systemic chemotherapy and endocrine therapy, and the use of standard state-of-the-art chemotherapy regimens in controlled clinical settings. Limitations include use of self-reported race and an absence of information regarding social determinants of health. Other significant limitations are lack of information on adherence to and duration of adjuvant endocrine therapy and lack of information regarding adjuvant chemotherapy adherence, including completion of planned chemotherapy, treatment delays, and dose reductions. Prior studies reporting similar racial disparities in which treatment information was available did not observe racial differences in adherence to endocrine therapy or chemotherapy administered in the context of clinical trials, suggesting that other factors may be contributing.16,41 Race is a social construct, and self-identified race has an imperfect correlation with geographical ancestry as determined by genetic studies.42–4 4 Furthermore, both Black and White races are heterogeneous populations, and there is emerging evidence that presentation and outcomes within Black race vary widely depending on additional ethnic variables such as country of origin.45–4 7 This intraracial heterogeneity merits further exploration in future analyses and prospective collection of genetic ancestry may be a step toward personalized prognosis and treatment options. Regarding social determinants of health, patient eligibility for enrollment to clinical trials controls for some but not all of these factors. One study found that after adjusting for poverty and Medicaid enrollment, race was not in and of itself a risk factor for worse survival.48 However, a pooled analysis of 2 Eastern Cooperative Oncology Group clinical trials found that insurance and neighborhood socioeconomic status did not affect outcomes.49
In conclusion, in this pooled analysis of 8 NSABP trials including 9702 women with localized breast cancer treated with AC or NAC systemic chemotherapy including cyclophosphamide and doxorubicin without taxane, Black women with ER+ disease had a worse DRFS compared with White women, especially in those with residual cancer following NAC. Further research is needed to fully understand the mechanisms behind this disparity.
Supplementary Material
LAY SUMMARY:
Black women with breast cancer have worse outcomes compared with White women.
We investigated if this held true in the context of clinical trials that provide controlled treatment setting.
Black women with cancer expressing estrogen receptors (ERs) had worse outcome than White women. If breast cancers did not express ERs, there was no racial disparity in outcome.
We also observed racial disparity in women who received chemotherapy before their cancer was removed, but only if they had cancer expressing ERs and residual disease on completion of treatment. If the cancer disappeared with presurgical chemotherapy, there was no racial disparity.
FUNDING SUPPORT
Supported in part by grants from the Department of Health and Human Services and the National Institutes of Health, National Cancer Institute (5P30CA013330, 1UG1CA189859, T32CA200561, U10CA180868, UG1CA189867, and U10CA180822), Peter T. Rowley (DOH01-ROWLEY-2019-00037), Breast Cancer Research Foundation (BCRF-21-140), and the Gruss Lipper Family Foundation.
We acknowledge NRG Oncology/NSABP for providing us the data used for this study.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–451. doi: 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69:211–233. doi: 10.3322/caac.21555 [DOI] [PubMed] [Google Scholar]
- 4.Shin JY, Kachnic LA, Hirsch AE. The impact of race in male breast cancer treatment and outcome in the United States: a population-based analysis of 4,279 patients. Int J Breast Cancer. 2014;2014:685842. doi: 10.1155/2014/685842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sineshaw HM, Freedman RA, Ward EM, Flanders WD, Jemal A. Black/White disparities in receipt of treatment and survival among men with early-stage breast cancer. J Clin Oncol. 2015;33:2337–2344. doi: 10.1200/JCO.2014.60.5584 [DOI] [PubMed] [Google Scholar]
- 6.Joslyn SA, West MM. Racial differences in breast carcinoma survival. Cancer. 2000;88:114–123. [DOI] [PubMed] [Google Scholar]
- 7.Sparano JA, Wang M, Zhao F, et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst. 2012;104:406–414. doi: 10.1093/jnci/djr543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633 [DOI] [PubMed] [Google Scholar]
- 9.Schneider BP, Li L, Radovich M, et al. Genome-wide association studies for taxane-induced peripheral neuropathy in ECOG-5 103 and ECOG-1199. Clin Cancer Res. 2015;21:5082–5091. doi: 10.1158/1078-0432.CCR-15-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim G, Pastoriza JM, Condeelis JS, et al. The contribution of race to breast tumor microenvironment composition and disease progression. Front Oncol. 2020;10:1022. doi: 10.3389/fonc.2020.01022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tammemagi CM, Nerenz D, Neslund-D udas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among Black and White patients with breast cancer. JAMA. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765 [DOI] [PubMed] [Google Scholar]
- 12.Gopishetty S, Kota V, Guddati AK. Age and race distribution in patients in phase III oncology clinical trials. Am J Transl Res. 2020;12: 5977–5983. [PMC free article] [PubMed] [Google Scholar]
- 13.Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5:e191870. doi: 10.1001/jamaoncol.2019.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. 2001;36–43. doi: 10.1093/oxfordjournals.jncimonographs.a003458 [DOI] [PubMed] [Google Scholar]
- 15.Albain KS, Unger JM, Crowley JJ, Coltman CA Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albain KS, Gray RJ, Makower DF, et al. Race, ethnicity, and clinical outcomes in hormone receptor-positive, HER2-negative, node-negative breast cancer in the randomized TAILORx trial. J Natl Cancer Inst. 2021;113:390–399. doi: 10.1093/jnci/djaa148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483 [DOI] [PubMed] [Google Scholar]
- 18.Howard-McNatt M, Lawrence J, Melin SA, Levine EA, Shen P, Stewart JH. Race and recurrence in women who undergo neoadjuvant chemotherapy for breast cancer. Am J Surg. 2013;205:397–401. doi: 10.1016/j.amjsurg.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 19.Pastoriza JM, Karagiannis GS, Lin J, et al. Black race and distant recurrence after neoadjuvant or adjuvant chemotherapy in breast cancer. Clin Exp Metastasis. 2018;35:613–623. doi: 10.1007/s10585-018-9932-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodward WA, Huang EH, McNeese MD, et al. African-A merican race is associated with a poorer overall survival rate for breast cancer patients treated with mastectomy and doxorubicin-based chemotherapy. Cancer. 2006;107:2662–2668. doi: 10.1002/cncr.22281 [DOI] [PubMed] [Google Scholar]
- 21.Xie J, Liu C. Adjusted Kaplan-M eier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24:3089–3110. doi: 10.1002/sim.2174 [DOI] [PubMed] [Google Scholar]
- 22.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 23.Schoenfeld D Partical residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 24.Jatoi I, Anderson WF, Rao SR, Devesa SS. Breast cancer trends among Black and White women in the United States. J Clin Oncol. 2005;23:7836–7841. doi: 10.1200/JCO.2004.01.0421 [DOI] [PubMed] [Google Scholar]
- 25.Kabat GC, Ginsberg M, Sparano JA, Rohan TE. risk of recurrence and mortality in a multi-e thnic breast cancer population. J Racial Ethn Health Disparities. 2017;4:1181–1 188. doi: 10.1007/s40615-016-0324-y [DOI] [PubMed] [Google Scholar]
- 26.Menashe I, Anderson WF, Jatoi I, Rosenberg PS. Underlying causes of the Black-White racial disparity in breast cancer mortality: a population-based analysis. J Natl Cancer Inst. 2009;101:993–1000. doi: 10.1093/jnci/djp176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485–493. doi: 10.1001/jamasurg.2017.0005 [DOI] [PubMed] [Google Scholar]
- 28.Wojcik BE, Spinks MK, Optenberg SA. Breast carcinoma survival analysis for African American and White women in an equal-access health care system. Cancer. 1998;82:1310–1318. doi: [DOI] [PubMed] [Google Scholar]
- 29.Mancino AT, Rubio IT, Henry-Tillman R, et al. Racial differences in breast cancer survival: the effect of residual disease. J Surg Res. 2001;100:161–165. doi: 10.1006/jsre.2001.6232 [DOI] [PubMed] [Google Scholar]
- 30.Chavez-Macgregor M, Litton J, Chen H, et al. Pathologic complete response in breast cancer patients receiving anthracycline- and taxane-based neoadjuvant chemotherapy: evaluating the effect of race/ethnicity. Cancer. 2010;116:4168–4177. doi: 10.1002/cncr.25296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tichy JR, Deal AM, Anders CK, Reeder-Hayes K, Carey LA. Race, response to chemotherapy, and outcome within clinical breast cancer subtypes. Breast Cancer Res Treat. 2015;150:667–674. doi: 10.1007/s10549-015-3350-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warner ET, Ballman KV, Strand C, et al. Impact of race, ethnicity, and BMI on achievement of pathologic complete response following neoadjuvant chemotherapy for breast cancer: a pooled analysis of four prospective Alliance clinical trials (A151426). Breast Cancer Res Treat. 2016;159:109–118. doi: 10.1007/s10549-016-3918-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106:dju055. doi: 10.1093/jnci/dju055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warner ET, Tamimi RM, Hughes ME, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33:2254–2261. doi: 10.1200/JCO.2014.57.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biswas T, Efird JT, Prasad S, Jindal C, Walker PR. The survival benefit of neoadjuvant chemotherapy and pCR among patients with advanced stage triple negative breast cancer. Oncotarget. 2017;8:112712–112719. doi: 10.18632/oncotarget.22521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109 [DOI] [PubMed] [Google Scholar]
- 37.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-t erm survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147 [DOI] [PubMed] [Google Scholar]
- 38.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421 [DOI] [PubMed] [Google Scholar]
- 39.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595 [DOI] [PubMed] [Google Scholar]
- 40.Allott EH, Geradts J, Cohen SM, et al. Frequency of breast cancer subtypes among African American women in the AMBER consortium. Breast Cancer Res. 2018;20:12. doi: 10.1186/s13058-018-0939-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sparano JA, Zhao F, Martino S, et al. Long-term follow-up of the E1199 Phase III trial evaluating the role of taxane and schedule in operable breast cancer. J Clin Oncol. 2015;33:2353–2360. doi: 10.1200/JCO.2015.60.9271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaseniit KE, Haque IS, Goldberg JD, Shulman LP, Muzzey D. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694–1702. doi: 10.1038/s41436-020-0869-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee YL, Teitelbaum S, Wolff MS, Wetmur JG, Chen J. Comparing genetic ancestry and self-reported race/ethnicity in a multiethnic population in New York City. J Genet. 2010;89:417–423. doi: 10.1007/s12041-010-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yaeger R, Avila-Bront A, Abdul K, et al. Comparing genetic ancestry and self-described race in African Americans born in the United States and in Africa. Cancer Epidemiol Biomarkers Prev. 2008;17:1329–1338. doi: 10.1158/1055-9965.EPI-07-2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barreto-Coelho P, Cerbon D, Schlumbrecht M, Parra CM, Hurley J, George SHL. Differences in breast cancer outcomes amongst Black US-born and Caribbean-born immigrants. Breast Cancer Res Treat. 2019;178:433–440. doi: 10.1007/s10549-019-05403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camacho-Rivera M, Kalwar T, Sanmugarajah J, Shapira I, Taioli E. Heterogeneity of breast cancer clinical characteristics and outcome in US Black women- - effect of place of birth. Breast J. 2014;20:489–495. doi: 10.1111/tbj.12302 [DOI] [PubMed] [Google Scholar]
- 47.Sung H, DeSantis CE, Fedewa SA, Kantelhardt EJ, Jemal A. Breast cancer subtypes among Eastern-African-born Black women and other Black women in the United States. Cancer. 2019;125:3401–3411. doi: 10.1002/cncr.32293 [DOI] [PubMed] [Google Scholar]
- 48.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–496. doi: 10.1093/jnci/94.7.490 [DOI] [PubMed] [Google Scholar]
- 49.Obeng-Gyasi S, O’Neill A, Zhao F, et al. Impact of insurance and neighborhood socioeconomic status on clinical outcomes in therapeutic clinical trials for breast cancer. Cancer Med. 2021;10:45–52. doi: 10.1002/cam4.3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.