Abstract
Treatment of the bis(chelate) complexes trans-[M(κ2-2-C6F4PPh2)2] (trans-1M; M = Ni, Pt) and cis-[Pt(κ2-2-C6F4PPh2)2] (cis-1Pt) with equimolar amounts or excess of PMe3 solution gave complexes of the type [(Me3P)xM(2-C6F4PPh2)2] (x = 2: 2Ma, 2Mbx = 1: 3Ma, 3Mb; M = Ni, Pt). The reactivity of complexes of the type 2M and 3M toward monovalent coinage metal ions (M′ = Cu, Ag, Au) was investigated next to the reaction of 1M toward [AuCl(PMe3)]. Four different complex types [(Me3P)2M(μ-2-C6F4PPh2)2M′Cl] (5MM′; M = Ni, Pt; M′ = Cu, Ag, Au), [(Me3P)M(κ2-2-C6F4PPh2)(μ-2-C6F4PPh2)M′Cl]x (x = 1: 6MM′; M = Pt; M′ = Cu, Au; x = 2: 6PtAg), head-to-tail-[(Me3P)ClM(μ-2-C6F4PPh2)2M′] (7MM′; M = Ni, Pt; M′ = Au), and head-to-head-[(Me3P)ClM(μ-2-C6F4PPh2)2M′] (8MM′; M = Ni, Pt; M′ = Cu, Ag, Au) were observed. Single-crystal X-ray analyses of complexes 5–8 revealed short metal–metal separations (2.7124(3)–3.3287(7) Å), suggestive of attractive metal–metal interactions. Quantum chemical calculations (atoms in molecules (AIM), electron localization function (ELF), non-covalent interaction (NCI), and natural bond orbital (NBO)) gave theoretical support that the interaction characteristics reach from a pure attractive non-covalent to an electron-shared (covalent) character.
Short abstract
A series of related mono and double bridged heterobimetallic d8−d10 complexes with a [MM′]3+ core (M = Ni, Pt; M′ = Cu, Ag, Au) have been synthesized. Dependent on the synthesis strategy, a variety of isomers of the composition [(Me3P)xM(2-C6F4PPh2)2M′Cl] (x = 1, 2) was isolated. All synthesized compounds feature short metal−metal separations (2.7124(3)−3.3287(7) Å). Metallophilic bonding trends were discussed along the isomeric series using DFT analysis (NCI, ELF, NBO).
Introduction
Heterobimetallic compounds are of great interest due to their tuneable metal–metal bonds or interactions.1 Hence, the chemistry of heterobinuclear complexes featuring two transition metals give rise to various intriguing redox, spectroscopic, and photophysical properties, which lead to a wide range of applications.2 These features extend the scope of their monometallic counterparts. Such binuclear complexes bearing d8–d8, d8–d10, or d10–d10 metal pairs (d8: IrI, NiII, PdII, PtII, AuIII; d10: CuI, AgI, AuI, HgII) have been generated unsupported or supported by bridging ligands.3 Heterobimetallic systems featuring groups 10 and 11 metals are of current interest due to their key role in cooperative catalysis.4 Recent success in trapping a reactive intermediate was reported for a Ni-catalyzed cross-coupling reaction, where a Ni–Cu complex could be isolated as a representative snapshot of the catalytic cycle (Chart 1, A).5 Similarly, transmetalation from methyl groups from a PtII toward a AuI center was reported to proceed via direct PtII–AuI bond formation (supported by mass spectrometric data).6 Structural information of such reactive intermediates is given for the homologue PtII–CuI complex (Chart 1, B) and analogue PtII–AgI and PtII–AuI complexes (Chart 1, C) where in solution a dynamic coinage metal–carbon bond behavior was observed.7
Chart 1. Examples of Complexes Featuring d8–d10-Metal Pairs.
Discrete synthetically prepared d8–d10 heterobimetallic compounds are important for the fundamental understanding of the relation between the nature of the metal–metal interactions and the complex structure.8 Various M–M′ interactions with late transition metals (M, M′) have been studied during the past decades, and they were found to span between long range dispersion (London) energy9 and ionic contribution10 to metal-to-metal charge transfer11 with significant covalency.12
The complexes trans-[M(κ2-2-C6F4PPh2)2] (M = Ni, Pd, Pt; trans-1M)13 were previously used as a d8-metal source to generate such bimetallic complexes.14,15 The four-membered rings of the ortho-metalated C,P-ligand can open by treatment of trans-1M with neutral alkyl phosphines like PMe3 or dppe ((diphenylphosphino)ethane), leading to a cis- or trans-orientation of the dangling κC-2-C6F4PPh2 ligands about the transition-metal center (Scheme 1).
Scheme 1. Synthesis of Complexes of Type trans-[(Me3P)2Pd(μ-2-C6F4PPh2)2M′Cl], [(dppe)M(κC-2-C6F4PPh2)(μ-2-C6F4PPh2)M′Cl] (IIdppe), and [(dppe)M(μ-2-C6F4PPh2)2M′Cl] (IIIdppe).
These dangling C,P-ligands show the ability to coordinate coinage metal ions in a mono bridged (IIdppe) or double bridge (trans-[(Me3P)2Pd(μ-2-C6F4PPh2)2M′Cl], IIIdppe) manner. Caused by steric crowding, the M···M′ separations are found to be longer for the dppe-containing complexes (2.9104(3)–3.8715(5) Å) in comparison to the PMe3-containing compounds (2.7707(11)–2.9423(3) Å). The M···M′ interaction types were reported to be either attractive non-covalent (dispersion) or donor–acceptor interactions. In order to obtain periodic trends for the interaction types among the d8-metals of the nickel triads and the d10-metal of coinage metal ions, we investigated the hitherto unknown nickel and platinum homologues, using the sterically less demanding PMe3 ligand, toward coinage metal ions. Due to platinum’s rich cis/trans-isomeric chemistry, we were also interested in a synthetic strategy to obtain the heavier homologue cis-isomer of trans-[(Me3P)2M(μ-2-C6F4PPh2)2M′Cl] and the influence of isomerization toward the M···M′ interaction.
Results and Discussion
Complexes of the Type [(Me3P)xM(2-C6F4PPh2)2]
The compounds trans-[M(κ2-2-C6F4PPh2)2] (M = Ni, Pd, Pt; trans-1M) were synthesized following the literature methods.13 For M = Pt, the analogous complex cis-[Pt(κ2-2-C6F4PPh2)2] (cis-1Pt) was formed during the synthesis and could be separated from the trans-isomer by fractional crystallization. The reaction of trans-1M with more than 2 equiv PMe3 resulted in the formation of trans-[(Me3P)2M(κC-2-C6F4PPh2)2] (2Ma, Scheme 2).14 The 31P NMR spectrum (Table 1) in C6D6 of 2Nia is similar to the palladium homologue. 2Nia potentially appears as a mixture of anti-2Nia and syn-2Nia-isomer in a 1:8 ratio, similar to that for 2Pda (1:6 ratio) as previously reported.14 Only one isomer was observed for 2Pta in C6D6 (Figure S6), whereas in CDCl3 a second signal set was visible in the 31P NMR spectrum (Figure S7). Our quantum chemical calculations at DFT level of theory have shown that in general the syn-isomer is thermodynamically more stable by 3.4–4.3 kcal mol–1 (Ni: 4.21, Pd: 3.42, Pt: 4.34 kcal mol–1). The magnitude is in agreement with the co-existence of both isomers in solution. Therefore, we assume that the major product detected by NMR spectroscopy could be assigned to the syn-isomer, where the isomeric shifts of the dangling PPh2 groups are systematically shifted to a higher field by approximately 6 ppm, presumably caused by the proximity of both PPh2 groups.
Scheme 2. Formation of trans-[(Me3P)2M(κC-2-C6F4PPh2)2] (2Ma).
Table 1. 31P NMR Chemical Shifts (ppm) of Complexes of the Type 2Ma (M = Ni, Pd,14 Pt) in C6D6a.
| M |
anti |
syn |
||
|---|---|---|---|---|
| PMe3 | μ-2-C6F4PPh2 | PMe3 | μ-2-C6F4PPh2 | |
| Ni | –15.0 | –3.6 | –15.0 | –9.4 |
| Pd | –19.1 | –2.9 | –18.7 | –8.4 |
| Pt | [−24.4 (2700)b] | [−5.4 (160)b] | –24.6 (2700) | –11.1 (90) |
JPt,P (Hz) coupling constants are given in brackets.
31P NMR shifts of the anti-isomer were observed in CDCl3 solution.
The 31P NMR spectrum of the crude reaction mixture of cis-1Pt with excess PMe3 in CH2Cl2 solution (with C6D6-insert) shows two multiplet resonances at −1.8 ppm (3JPt,P ≈ 240 Hz) and −2.8 ppm (3JPt,P ≈ 240 Hz) and one resonance at −31.3 ppm (1JPt,P = 2170 Hz) in a 1:1:2 ratio. This observation can be assigned to cis-[(Me3P)2Pt(κC-2-C6F4PPh2)2] (2Ptb), which occurs also as anti- and syn-isomer in a 1:1 ratio (ratio is estimated from integrals of 31P signals, Scheme 3). Quantum chemical calculations reveal that both cis isomers show similar thermodynamic stability (Δ|E| ∼2 kcal mol–1), thus indicating coexistence of both isomers in equilibrium. However, the cis-isomers anti/syn-2Ptb are thermodynamically less favored than the corresponding trans-isomers anti/syn-2Pta [syn-2Ptb (8.11) > anti-2Ptb (5.54) > anti-2Pta (4.34) > syn-2Pta (0.00 kcal mol–1)]. Attempts to isolate 2Ptb failed due to loss of PMe3 and formation of cis-[(Me3P)Pt(κ2-2-C6F4PPh2)(κC-2-C6F4PPh2)] (3Ptb). 3Ptb can also be synthesized by addition of 1 equiv PMe3 to cis-1Pt. The same behavior has been observed in the reaction of cis-[Pt(κ2-2-C6H4PPh2)2] with excess P(OMe)3 or ButNC as reported in the literature.16
Scheme 3. Formation of Intermediate Compounds cis-[(Me3P)2Pt(κC-2-C6F4PPh2)2] (2Ptb) and cis-[(Me3P)Pt(κ2-2-C6F4PPh2)(κC-2-C6F4PPh2)] (3Ptb).
Interestingly, dissolving a pure sample of trans-[(Me3P)2Ni(κC-2-C6F4PPh2)2] (2Nia) in C6D6 shows a slight loss of PMe3. According to the 31P and 19F NMR spectra, the characteristic signal pattern of traces (≈5%) of trans-[(Me3P)Ni(κ2-2-C6F4PPh2)2] (3Ma) could be detected (vide infra).
In contrast to observations along the nickel triad of the reaction of trans-1M with excess PMe3, the reaction of trans-1M with 1 equiv PMe3 shows a different behavior depending on the transition metal. The 31P NMR spectrum of the crude reaction mixture of trans-1Pt with 1 equiv PMe3 shows the characteristic signal pattern of 2Pta and unreacted trans-1Pt beside traces of unknown impurities. The 31P NMR spectrum of the crude reaction mixture of trans-1Pd with 1 equiv PMe3 shows a 1:1:1 ratio of trans-1Pd (−55.4 ppm), syn/anti-2Pda (−18.7, −8.4, −19.2, −2.9 ppm), and 3Pda (−35.5 ppm, 2JP,P = 228.7 Hz, doublet, 2P; −13.8 ppm, 2JP,P = 228.7 Hz, triplet, 1P). The presence of the symmetric isomer 3Pda could be confirmed by 19F NMR spectroscopy (−117.2, −128.6, −152.1, −159.5; Scheme 4). Attempts to isolate 3Pda from the reaction mixture failed. The crude reaction mixture of trans-1Ni with 1 equiv PMe3 shows the formation of 3Nia with a small amount of 3Nib (confirmed by 19F and 31P NMR spectroscopy). Rocamora et al. reported a similar nickel complex trans-[(Et3P)Ni(κ2-2-C6Cl4PPh2)2] to 3Nia as the final product.17 Stirring a dichloromethane (DCM) solution of 3Nia for 4 days at ambient temperature resulted in ring opening and slow formation of 3Nib. The obtained 1:3 mixture of the 3Nia and 3Nib does show eight additional signals in the 19F and two signals in the 31P NMR spectra. Through fractional crystallization, it was possible to separate and characterize the byproduct as [Ni2(κ2-2-C6F4PPh2)2(μ-2-C6F4PPh2)2] (4Ni). It was only possible to isolate an isomeric mixture of 3Nia and 3Nib as a second fraction. Counter-intuitively, the homologous platinum compound [Pt2(κ2-2-C6F4PPh2)2(μ-2-C6F4PPh2)2] (4Pt) was accessible by quickly treating trans-1Pt with an excess of PMe3 (ca. 2.8 equiv: 11% 4Pt, ca. 5.5 equiv: 17% 4Pt, addition of PMe3 within 1 s). Compound 4Pd was not accessible by either of the described synthesis methods used for 4Ni or 4Pt. In spite of the formation of [Pt2(κ2-2-C6H4PPh2)2(μ-2-C6H4PPh2)2] by refluxing a toluene solution of cis-[Pt(κ2-2-C6H4PPh2)2], similar treatment of cis-1Pt or trans-1M in toluene does not form compound 4M.18 The energy difference between trans-1M and 4M was calculated to be approximately −20 to −30 kcal mol–1 [M = Ni (−22.6 kcal mol–1), Pd (−29.3 kcal mol–1), Pt (−31.3 kcal mol–1)], which leads to the conclusion that PMe3 can act as a dimerization promoter. Starting from trans-1Ni or cis-1Pt and reacting it with 1 equiv of PMe3 resulted in compounds of the type 3Mb with cis-C-M-C arrangement. The same cis-C-M-C configuration is visible in both compounds of the type 4M. Our quantum chemical calculations reveal that the relative energies of 3Ma and 3Mb for M = Ni and Pt are similar (3Mb is slightly more stable by <1.6 kcal mol–1, respectively). The molecular structures of syn-2Ma, 3Mb, and 4M (M = Ni, Pt) were confirmed by single-crystal X-ray diffraction (Figure 1 and Table S1). A detailed structural description is given in the Supporting Information.
Scheme 4. Reaction of trans-1M with 1 equiv of PMe3.
Figure 1.
Molecular structures of syn-2Pta, 3Ptb, and 4Pt (left to right). Ellipsoids are shown at 50% probability level. Hydrogen atoms and solvent molecules are omitted, and only the ipso-carbons of the PPh2 groups are depicted for clarity.
Complexes of the Type [(Me3P)2M(μ-2-C6F4PPh2)2M′Cl]
Reacting cis-1Pt with excess PMe3 resulted in the formation of syn/anti-2Ptb (Scheme 3), which was impossible to isolate. An in situ reaction of syn/anti-2Ptb with M′Cl (M′ = Cu, Ag, Au(tht); tht = tetrahydrothiophene) was not suitable to form the homologue cis-5PtM′ due to the presence of unreacted PMe3. The excess PMe3 can easily react with the coinage metal chlorides to form various species of the form [M′Cl(PMe3)x]n (x = 1–4, n = 4–1).19 To prevent byproduct formation, the synthesis was started from 3Ptb, which was reacted with 1 equiv M′Cl (M′ = Cu, Ag) to form complexes of type cis-6PtM′ (vide infra). The isolated compounds cis-6PtM′ were treated with 1 equiv PMe3 in order to obtain complexes of type cis-5PtM′ (Scheme 5). Compound cis-6PtAu was accessible by reacting cis-1Pt with [AuCl(PMe3)] (Scheme 6).
Scheme 5. Formation of Heterobimetallic Complexes of the Type [(Me3P)2M(μ-2-C6F4PPh2)2M′Cl].
Scheme 6. Synthesis of Various Isomers of Compounds of the Composition [(Me3P)Pt(2-C6F4PPh2)2M′Cl].
Compound cis-5PtCu was accessible in a pure state by the mentioned synthesis route. Because of difficulties in handling the PMe3-toluene solution (air sensitivity and slow oxidation during storage), the addition of exactly 1 equiv PMe3 to form complex cis-5PtAg in a pure state was not possible. Isolated samples of cis-5PtAg showed impurities of either cis-6PtAg-dimer by adding small amounts of PMe3 or 3Ptb by adding excess PMe3 (presumably removing AgCl as [AgCl(PMe3)x]n (x = 1–4, n = 4–1)).19 The impurities could not be separated by crystallization. The presence of cis-5PtAg could be confirmed by 19F and 31P NMR spectroscopy in CDCl3 solution [19F: −106.8, −116.4, −150.8, −159.3; 31P: 14.3 (1J(107/109)Ag,P = 380 Hz, 1P, AgPPh2), −31.4 (1JPt,P = 2140 Hz, 1P, PMe3)]. The reaction of cis-6PtAu with 1 equiv PMe3 only led to product mixtures. It was possible to obtain crystals suitable for single-crystal X-ray diffraction of cis-5PtCu and cis-5PtAg from dichloromethane/n-hexane solution (Figure 2, Table S2).
Figure 2.
Molecular structures of trans-5NiCu, trans-5PtCu, and cis-5PtCu (left to right). Ellipsoids are shown at 50% probability level. Hydrogen atoms are omitted, and only the ipso-carbons of the PPh2 groups are depicted for clarity.
Cis-5PtCu and cis-5PtAg show the expected cis-configuration of the bridging ligands μ-2-C6F4PPh2. The same mutual cis-configuration was found in the corresponding complexes [(dppe)M(μ-2-C6F4PPh2)2M′Cl] (M = Pd, M′ = Cu, Ag; M = Pt, M′ = Cu; dppe = 1,2-bis(diphenylphosphino)ethane).15 We attribute the significant longer Pt···Cu separation in [(dppe)Pt(μ-2-C6F4PPh2)2CuCl] (d(Pt···Cu): 2.9466(3) Å) in comparison to cis-5PtCu to the steric crowding between the Cu–Cl unit and the phenyl groups of the dppe ligand. The longer Pt···Ag separation is also observed in [(dppe)Pt(μ-2-C6F4PPh2)2AgCl] (d(Pt···Ag): 3.0964(4) Å) in comparison to cis-5PtAg.
Following the reported synthesis strategy of the formation of trans-5PdM′ (M′ = Cu, Ag, Au),14 a respective mixture of anti/syn-2Ma (M = Ni, Pt) was reacted with CuCl, AgCl, or [AuCl(tht)] (tht = tetrahydrothiophen) in dichloromethane to form complexes of the type trans-5MM′ (M = Ni, Pt; M′ = Cu, Ag, Au (Scheme 5). The 31P NMR chemical shifts of the 9 complexes of the type trans-5MM′ are given in Table 2.
Table 2. 31P NMR Chemical Shifts of Complexes of the Type trans-5MM′ (M = Ni, Pd,14 Pt; M′ = Cu, Ag, Au) in CDCl3.
| M/M′ | Cu |
Ag |
Au |
|||
|---|---|---|---|---|---|---|
| PMe3 | μ-2-C6F4PPh2 | PMe3 | μ-2-C6F4PPh2 | PMe3 | μ-2-C6F4PPh2 | |
| Ni | –14.4 | 7.2 | –15.1 | 15.9 | –15.9 | 41.8 |
| Pda | –16.6 | 3.1 | –17.7 | 11.8 | –17.7 | 37.9 |
| Pt | –21.7 | –0.9 | –24.1 | 6.4 | –26.0 | 35.2 |
Measured in CD2Cl2.
By changing the coinage metal and keeping the d8-metal equal, the chemical shift of the 31P NMR signal of the PMe3 group is changing in a narrow range (ΔδP = 1.1–4.3 ppm) as expected. In contrast, by changing the d8-metal of the nickel triad and keeping the coinage metal equal, the chemical shift of the 31P NMR signal of the bridging μ-2-C6F4PPh2 shows a high field shift along the series Ni > Pd > Pt in a range of ΔδP = 8.1 (Cu), 9.5 (Ag), and 6.6 ppm (Au). For the series trans-5MCu (M = Ni, Pd,14 Pt), it was possible to determine all three molecular structures, which are isomorphous (orthorhombic, space group Pnma). The molecular structures of trans-5NiCu (left) and trans-5PtCu (middle) are shown in Figure 2, and selected interatomic distances and angles are given in Table S2.
The structure overlay (Figure S65) of complexes of the type trans-5MCu (M = Ni, Pd,14 Pt) shows that the three complexes are almost identical (root-mean-square deviation of atomic positions of M1, M′1, Cl1, P1, and P1*: rmsd < 0.04 Å). The M–C1 bond length is increasing in the order Ni < Pd < Pt by approximately 0.13 Å, and the M–P2/3 bond length are increasing in the order Ni < Pt < Pd by approximately 0.17 Å (see Supporting Information for details). The Cu1–P1 bond lengths show a minor elongation by approximately 0.03 Å and the Cu1–Cl1 bond lengths are slightly elongated by less than 0.02 Å, which is in contrast to the observations of the formal large ΔδP in the 31P NMR spectra. The M···Cu distances in the complexes of the type trans-5MCu [M = Ni: 2.82(1); Pd: 2.84(1); Pt: 2.82(1) Å] are similar. The sum of the covalent radii [Ni–Cu: 2.56(6); Pd–Cu: 2.71(8); Pt–Cu: 2.68(8) Å]20 in the series are shorter than the observed M–Cu distances by approximately 0.13–0.26 Å. The M···PPh2 distances in the series follow the order 3.38(1) (Ni) < 3.43(1) (Pd) < 3.45(1) Å (Pt), which would suggest an increasing diamagnetic shielding contribution of heavier metal atoms in the 31P NMR shifts of the PPh2 group as observed for PMe3. However, in the monometallic complexes of type syn-2Ma, the M···PPh2 distances display a higher deviation [3.33(1) (Ni) < 3.40(1) (Pd) < 3.44(1) Å (Pt)] and are closer to the d8-metal with a smaller magnitude in ΔδP in the 31P NMR spectra [1.7 ppm (syn-2Ma) vs 8.1 ppm (trans-5MCu)]. This would suggest that the diamagnetic shielding contribution of heavier metal atoms only plays a minor role in the 31P NMR shifts of the bridging μ-2-C6F4PPh2. Calculation of the 31P NMR shift difference of syn-2Ma (2.6 ppm) vs trans-5MCu (6.6 ppm) supports this claim (see Supporting Information for details).
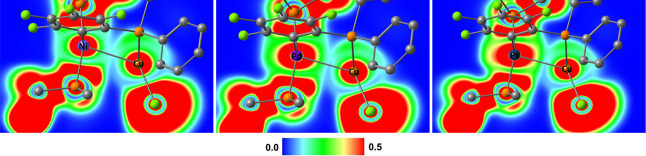
Due to the absence of major structural differences among the series of complexes of type trans-5MCu, we were interested if the electronic structure has an impact on the formal large ΔδP in the 31P NMR spectra. The non-covalent interaction (NCI) descriptor21 shows clearly an increasing non-covalent attractive interaction along the series Ni < Pd < Pt (Figure 3).
Figure 3.
NCI descriptor of trans-5NiCu (left), trans-5PdCu (middle), and trans-5PtCu (right). Isovalue is set to 0.45, and color range is from −0.05 to 0.05 au; color code: blue—attractive interactions; green—van der Waals interactions; red—non-attractive interactions.
Natural localized molecular orbital (NLMO) calculations22 of the series trans-5MCu exhibit a decrease Cu contribution in both Cu–P bonds in the order Ni < Pd < Pt by about 6% in total. In the same order, Cu contributions to the dz2-orbital of Ni (0.06%), Pd (0.14%), and Pt (0.24%) increase similarly the (main) M→Cu interaction energy (second-order perturbation theory: Ni: 1.12; Pd: 7.67; Pt: 38.73 kcal mol–1; Table 3 and Figure 4). In the electron localization function (ELF),23 an increase localized electron density is visible in the order Pd (0.121 au) < Ni (0.128 au) < Pt (0.140 au.) (Figure 5), which is especially interesting because of similar covalent radii of Pt (1.36(5) Å) and Pd (1.39(6) Å).20 The natural charge (NC) of Cu was found to be 0.67 in all three complexes, whereas for the nickel triad the value decreases in the order Ni (0.42) > Pd (0.26) > Pt (0.23). The possible intermetallic coulombic repulsions between M and M′ therefore drop in intensity along the series. The Wiberg bond order (WBO) supports the increasing M···Cu interaction in the same order [Ni (0.27) < Pd (0.32) < Pt (0.37)].
Table 3. NC and NBO Characteristics of the Main Donor–Acceptor Interaction [LP—Valence Lone Pair, LV—Lone Vacant Orbital, NBO Energy Level (ENBO, au), Donor–Acceptor Energy (kcal mol–1)] and WBO of trans- and cis-5MM′ (M = Ni, Pd, Pt; M′ = Cu, Ag, Au).
| trans-5NiCu | trans-5PdCu | trans-5PtCu | trans-5PdAg | trans-5PtAg | trans-5PdAu | trans-5PtAu | cis-5PtCu | cis-5PtAg | |
|---|---|---|---|---|---|---|---|---|---|
| NC(M) | 0.42 | 0.26 | 0.23 | 0.26 | 0.23 | 0.27 | 0.24 | 0.23 | 0.23 |
| NC(M′) | 0.67 | 0.67 | 0.67 | 0.64 | 0.65 | 0.47 | 0.47 | 0.71 | 0.69 |
| ENBO(LP(M)) | –0.26 | –0.28 | –0.22 | –0.27 | –0.22 | –0.27 | –0.21 | –0.22 | –0.22 |
| LP(M) | 100% d | 99% d | 97% d | 99% d | 97% d | 99% d | 97% d | 97% d | 97% d |
| ENBO(LV(M′)) | 0.09 | 0.10 | 0.17 | 0.35 | 0.39 | 0.17 | 0.20 | 0.18 | 0.34 |
| LV(M′) | 99% s | 99% s | 99% s | 99% s | 99% s | 95% s | 95% s | 99% s | 99% s |
| EM→M′ | 1.12 | 7.67 | 38.73 | 7.34 | 26.38 | 9.62 | 30.41 | 39.86 | 27.28 |
| WBO | 0.27 | 0.32 | 0.37 | 0.32 | 0.35 | 0.39 | 0.39 | 0.36 | 0.33 |
Figure 4.
LP(Pt)→LV(M′) main donor–acceptor interaction derived by NBO analyses of trans-5PtCu (left, M′ = Cu), trans-5PtAg (middle left, M′ = Ag), cis-5PtCu (middle right, M′ = Cu), and cis-5PtAg (right, M′ = Ag). NBOs are displayed with an isosurface value of 0.05.
Figure 5.
ELF of trans-5NiCu (left), trans-5PdCu (middle), and trans-5PtCu (right). Color range is from ELF = 0.0 to 0.5 au; color code: blue—strong delocalization, red—electron-gas-like pair probability. Hydrogen atoms are omitted for clarity.
Topological analyses (atoms in molecules, AIM) have been performed to get a better understanding of the covalency of the M···M′ interactions.24 The results of the topological parameters of compounds of the type 5MM′ are listed in Table 4. For all complexes of type 5MM′, critical points with (3, −1) characteristic (bond critical point, bcp) were found along the bond path between M and M′. The definition of a covalent bond “is based on two conditions, namely (i) the existence of a critical point rb and its associated maximum electron density path linking the nuclei in question (necessary condition) and (ii) H(rb) < 0 which indicates that the accumulation of electron charge in the internuclear region is stabilizing (sufficient condition)”25 (H(rb) = electron energy density), which is the case for all complexes of type 5MM′. Dinda and Samuelson gave further classification of the bond characteristic depending on the |V(rb)|/G(rb) ratio (with V(rb) = potential energy density, G(rb) = Lagrangian kinetic energy).12a Typical covalent interactions feature |V(rb)|/G(rb) > 2, whereas ionic character is characterized with |V(rb)|/G(rb) < 1. For complexes of type 5MM′, the bcps are in the range 1 < |V(rb)|/G(rb) < 2, which is characteristic for bonds with intermediate character and does show that the M···M′ interactions are not purely ionic in nature but have some electron-shared (covalent) character. Also, the relatively low electron density ρ(rb) at the bcps together with a positive Laplacian of electron density (∇2ρ(rb) > 0) and similar modulus of Lagrangian kinetic energy density and potential energy density (G(rb) ≙ |V(rb)|) are in support of the presence of a closed shell bonding with donor–acceptor characteristics.26,27 The electron density at the bcp ρ(rb) in the series trans-5MCu does increase in the expected order (M = Ni < Pd < Pt), which is in good agreement with the observations of ELF (Figure 5).
Table 4. Results of the Theoretical Topological Analysis of the Bond Critical Points (3, −1) between M and M′ in Complexes trans- and cis-5MM′ (M = Ni, Pd, Pt; M′ = Cu, Ag, Au)a.
| trans-5NiCu | trans-5PdCu | trans-5PtCu | trans-5PdAg | trans-5PtAg | trans-5PdAu | trans-5PtAu | cis-5PtCu | cis-5PtAg | |
|---|---|---|---|---|---|---|---|---|---|
| ρ(rb) | 0.02208 | 0.02620 | 0.03132 | 0.02879 | 0.03348 | 0.03607 | 0.04117 | 0.03090 | 0.03178 |
| ∇2ρ(rb) | 0.03699 | 0.05862 | 0.07437 | 0.07135 | 0.08161 | 0.08916 | 0.09973 | 0.07583 | 0.07717 |
| G(rb) | 0.01299 | 0.01785 | 0.02215 | 0.02193 | 0.02516 | 0.02773 | 0.03093 | 0.02235 | 0.02358 |
| V(rb) | –0.01672 | –0.02105 | –0.02572 | –0.02601 | –0.02994 | –0.03319 | –0.03698 | –0.02575 | –0.02788 |
| |V(rb)|/G(rb) | 1.288 | 1.179 | 1.161 | 1.186 | 1.190 | 1.197 | 1.196 | 1.152 | 1.182 |
| G(rb)/ρ(rb) | 0.588 | 0.681 | 0.707 | 0.762 | 0.752 | 0.769 | 0.751 | 0.723 | 0.742 |
| H(rb) | –0.00374 | –0.00320 | –0.00357 | –0.00409 | –0.00478 | –0.00546 | –0.00605 | –0.00340 | –0.00430 |
| sign(λ2(rb))ρ(rb) | –0.022 | –0.026 | –0.031 | –0.029 | –0.033 | –0.036 | –0.041 | –0.031 | –0.032 |
Electron density (ρ(rb) in au), Laplacian of electron density (∇2ρ(rb) in au), Lagrangian kinetic energy density (G(rb) in au), potential energy density (V(rb) in au), ratio |V(rb)|/G(rb), ratio G(rb)/ρ(rb) in au, electron energy density (H(rb) in au), the product of sign of second largest eigenvalue of Hessian matrix of electron density (λ2(rb)), and ρ(rb) in au.
Closed shell interactions were previously categorized by Macchi et al. as metallic (shared) bonding and donor–acceptor interactions.27 We will use these terms as described in the literature. To differentiate between metallic (shared) bonding behavior and donor–acceptor interactions, Macchi et al. suggested for heavy metal interactions that the G(rb)/ρ(rb) ratio can be used as a classification criterion (G(rb)/ρ(rb) < 1: metallic (shared) bonding vs G(rb)/ρ(rb) ∼ 1: closed shell donor–acceptor interaction).27 In the series trans-5MCu, the magnitude of G(rb)/ρ(rb) is in the expected range and does increase (closer to 1) in the order trans-5NiCu < trans-5PdCu < trans-5PtCu. The increase closed shell donor–acceptor interaction down the nickel triad is supported by NBO analysis. Besides the main donor–acceptor interactions listed in Table 3, a detailed list of the second-order perturbation theory between M and M′ is given in the Supporting Information. The Σ[LP(M)→LV(Cu)] interactions are M = Ni: 1.23, Pd: 8.19, and Pt: 39.76 kcal mol–1 (see Supporting Information for details), whereas the Σ[BD*(M–C/P)←LP(Cu)] interactions are M = Ni: 1.06, Pd: 4.13, and Pt: 10.27 kcal mol–1. This proves the better donor ability of Pt toward Cu over Ni and Pd, as well as that Cu is a weaker (or similar) donor toward the d8-metal. Within the respective series trans-5PdM′ or trans-5PtM′, the intensity of the metal–metal interactions is increasing in the order Cu < Ag < Au (WBO, NC, AIM). However, the sum of M→M′ donor–acceptor interaction intensity appears to follow the trend Ag ≈ Cu < Au (NBO) for M = Pd and Ag < Au < Cu (NBO) for M = Pt (see the Supporting Information for details). This change in trend is originated in the differences of how the metal–metal interactions are analyzed. The AIM part is electron density based and describes the situation in whole, whereas with NBO only the orbital part of the interaction can be extracted.
Due to a lack in structural difference, we assume that the 31P NMR shift of the phosphorous atom of the bridging ligand μ-2-C6F4PPh2 toward higher field (Table 2) is originated in electronic differences within the complex series caused by metal–metal interactions. For the series trans-5MAg and trans-5MAu (M = Ni, Pd, Pt), the same high field shift of the 31P NMR resonance of the bridging ligand μ-2-C6F4PPh2 down the nickel triad was detected. The trends found by AIM and NBO calculations (M = Pd, Pt; M′ = Ag, Au) are in accord with the results found for the series trans-5MCu (M = Ni, Pd, Pt). Within the respective series trans-5MCu, trans-5MAg, and trans-5MAu, increasing metal–metal interaction energies correlate with a high field shift at the 31P nuclei of the bridging ligands μ-2-C6F4PPh2 in the order Ni < Pd < Pt.
Compound trans-5PdAu shows similar BD*(Pd–C/P)←LP(Au) (10.17 kcal mol–1) and LP(Pd)→LV(Au) interactions (10.62 kcal mol–1). Therefore, the main character of the interaction should be described as predominantly metallic (shared) bonding. The complex cis-5PtCu and cis-5PtAg show similar metal–metal interactions as observed in the corresponding compounds trans-5PtCu and trans-5PtAg, respectively. The magnitude of |V(rb)|/G(rb) is slightly lower for cis-5PtM′ as found in the respective trans-5PtM′ complex, which is indicative of a more pronounced ionic contribution in the cis configuration over trans. This is reflected in a higher NC at Cu over Ag in complexes with cis arrangement over trans leading to a higher coulomb repulsion. In conclusion, the metallophilic interactions can be described as predominantly metallic (shared) bonding (Ni–Cu, Pd–Au) with additional donor–acceptor bonding characteristic (Pd→Cu, Pd→Ag, Pt→Cu, Pt→Ag, Pt→Au).
Complexes of the Type [(Me3P)M(2-C6F4PPh2)2M′Cl]
As described above, trans-1Ni and cis-1Pt show the ability to form complexes of the form 3Mb (M = Ni, Pt), where one 2-C6F4PPh2 ligand remains chelating and the other is dangling. Treatment of 3Ptb with 1 equiv CuCl or AgCl gave the expected complex cis-6PtCu and cis-6PtAg (Scheme 6), that proves the ability of the dangling PPh2 unit to coordinate a coinage metal chloride.
The 31P NMR spectra of both complexes show the expected three equally intense resonances, corresponding to the three inequivalent phosphorus nuclei. In both complexes, a respective signal appears at ca. −30 ppm, flanked by 195Pt satellites of ca. 2200 Hz, and can be assigned to the PMe3 ligand. The signals at −60 ppm, flanked by 195Pt satellites of ca. 1600 Hz, can be assigned to the chelating κ2-2-C6F4PPh2 ligand. The third resonance appears at 16.5 ppm for cis-6PtCu (flanked by 195Pt satellites of ca. 340 Hz) and at 21.6 ppm for cis-6PtAg (split into a doublet of doublets; 600 Hz due to coupling with 107Ag and 690 Hz due to coupling with 109Ag, flanked by 195Pt satellites of ca. 395 Hz) can be assigned to the bridging μ-2-C6F4PPh2 ligand. In both cases, the 19F NMR spectra show eight equally intense signals, confirming two different 2-C6F4PPh2 ligand environments. The spectroscopic results show the same pattern as observed for the starting material 3Ptb (−6.8, 3JPt,P = 207 Hz, κC-PPh2; −29.3, 1JPt,P = 2350 Hz, PMe3; −59.9, 1JPt,P = 1540 Hz, κ2-PPh2), supporting that the reaction only takes place at the dangling κC-2-C6F4PPh2 ligand and no isomerization takes place.
The molecular structure was confirmed by single-crystal X-ray diffraction analysis for cis-6PtCu and cis-6PtAg (Figure 6 and Table S3; detailed structure description is given in the Supporting Information). The homologue gold compound cis-6PtAu was accessible from the reaction of cis-1Pt with 1 equiv [AuCl(PMe3)], in a similar manner to its PPh3 analogue cis-[(Ph3P)Pt(κ2-2-C6F4PPh2)(μ-2-C6F4PPh2)AuCl].18b The 31P NMR spectrum in C6D6 of cis-6PtAu reveals the expected three equally intense resonances at 37.6, −31.9 (195Pt satellites of 2285 Hz), and −62.6 ppm (195Pt satellites of 1700 Hz), corresponding to three inequivalent phosphorus nuclei. After leaving cis-6PtAu in solution for several hours, a new set of signals appear at 33.9 (195Pt satellites of 205 Hz), 12.5 (195Pt satellites of 2285 Hz), and −35.5 ppm (2JP,P = 16 Hz, 195Pt satellites of 3655 Hz). The signal at 33.9 ppm can be assigned to the bridging ligand μ-2-C6F4PPh2 with a P–Au functionality, and the signal at −35.5 ppm to the PMe3 ligand bonded at the Pt center. The third signal is low field shifted by about 74 ppm, indicating a bridging functionality of the μ-2-C6F4PPh2 ligand, with the Pt–P bond remaining intact. The same signal pattern can be observed after the reaction of 3Ptb with 1 equiv [AuCl(tht)] and can be assigned to compound trans-7PtAu (Scheme 6). The bridging μ-2-C6F4PPh2 ligands do show a head-to-tail arrangement at both metal centers with a trans orientation about the Pt atom. Complex trans-7PtAu also undergoes isomerization to cis-6PtAu in solution, indicating the presence of an equilibrium between both isomers. This observation stands in contrast to the behavior of cis-[(Ph3P)Pt(κ2-2-C6F4PPh2)(μ-2-C6F4PPh2)AuCl], which did not show any isomerization product even after stirring the solution for 5 days.18b This can be explained by PMe3 being less sterically demanding as PPh3, which appears to favor isomerization.
Figure 6.

Molecular structures of cis-6PtAg-dimer. Ellipsoids are shown at a 50% probability level. Hydrogen atoms are omitted, and only the ipso-carbons of the PPh2 groups are depicted for clarity.
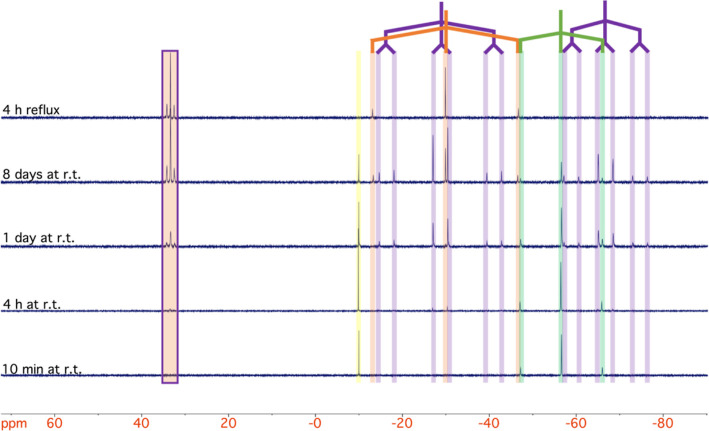
Also, upon mixing trans-1Pt with [AuCl(PPh3)], no reaction was observed.18b In contrast, trans-1Pt with 1 equiv [AuCl(PMe3)] in CH2Cl2 does show slow reaction to trans-6PtAu, which immediately reacts further to trans-8PtAu. The reaction was followed by 31P NMR spectroscopy. The 31P NMR spectrum of the crude reaction mixture (Figure 7) shows the characteristic signals of the starting materials ([AuCl(PMe3)], trans-1Pt) besides the signal sets for trans-6PtAu and trans-8PtAu. By addition of n-hexane to the crude reaction mixture, it was possible to grow crystals of trans-6PtAu and trans-8PtAu suitable for single-crystal X-ray diffraction (vide infra). A pure sample of trans-8PtAu was accessible by refluxing a mixture of trans-1Pt with 1 equiv [AuCl(PMe3)] in CH2Cl2 for 4 days. The formation of trans-8PtAu from the reaction of trans-1Pt with 1 equiv [AuCl(PMe3)] is in agreement with the reaction behavior found for the palladium homologue; however, an intermediate species trans-6PdAu was not observed.14 It was possible to determine molecular structures using single-crystal X-ray diffraction analysis for all four isomers cis-6PtAu, trans-6PtAu, trans-7PtAu, and trans-8PtAu (Figure 8 and Table S3; detailed structure description is given in the Supporting Information).
Figure 7.
31P NMR spectra of the reaction mixture of trans-1Pt with [AuCl(PMe3)] in DCM (from bottom to top: 10 min, 4 h, 1 day, and 8 days at room temperature and fresh prepared reaction mixture refluxed for 4 h). Signal assignments have been highlighted: green (trans-1Pt: −56.58 ppm (1JPt,P = 2280 Hz)), yellow ([AuCl(PMe3)]: 9.97 ppm), purple (trans-6PtAu: 33.35 ppm (3JPt,P = 230 Hz), −28.75 ppm (2JP,P = 410 Hz, 1JPt,P = 3010 Hz), −66.76 ppm (2JP,P = 410 Hz, 1JPt,P = 1920 Hz)), and orange (trans-8PtAu: 33.35 ppm (1JPt,P = 200 Hz), −29.93 (1JPt,P = 4080 Hz)).
Figure 8.
Molecular structures of cis-6PtAu, trans-6PtAu, trans-7PtAu, and trans-8PtAu (from left to right). Ellipsoids are shown at 50% probability level. Hydrogen atoms and solvent molecules are omitted, and only the ipso-carbons of the PPh2 groups are depicted for clarity. Selected interatomic separations (Å) and angles (deg) of cis-6PtAu and trans-6PtAu are given in Table S3: trans-7PtAu: Pt1–Au1 2.848(1), Pt1–P2 2.333(4), Pt1–C1 2.05(1), Pt1–Cl1 2.369(3), Pt1–P3 2.236(3), Au1–P1 2.279(4), Au1–C7 2.07(1), P2–Pt1–C1 172.6(4), P3–Pt1–Cl1 173.7(1), P1–Au1–C7 178.0(4); trans-8PtAu: Pt1–Au1 2.8008(2), Pt1–C1 2.074(3), Pt1–C7 2.065(3), Pt1–Cl1 2.3890(7), Pt1–P3 2.2060(7), Au1–P1 2.2996(7), Au1–P2 2.3044(7), C1–Pt1–C7 173.42(10), P3–Pt1–Cl1 177.94(3), P1–Au1–P2 174.16(2).
The 31P NMR spectra of the corresponding reactions of 3Nib with either CuCl or AgCl show two signals in a 2:1 ratio (Cu: 4.2, −4.7 ppm; Ag: 14.6 (1J(109)AgP = 566 Hz, 1J(107)AgP = 490 Hz), −6.1 ppm). For both complexes, the 19F NMR spectra show four equally intense signals, which indicates a symmetric μ-2-C6F4PPh2 environment. Therefore, the reaction led to the formation of the nickel homologous trans-8NiM′ (M′ = Cu, Ag, Scheme 7), which stands in contrast to the reaction behavior of 3Ptb with either CuCl or AgCl. The 31P NMR spectra of the reaction of 3Nib with [AuCl(tht)] or trans-1Ni with [AuCl(PMe3)] show three signals at 45.3 (AuPPh2), 21.2 (2JP,P = 330 Hz, NiPPh2), and −14.8 ppm (2JP,P = 330 Hz, NiPMe3) together with the characteristic signal of gold dimer (42 ppm)8c and some additional unknown impurities. The ratio of the main product to the gold dimer does increase from 2:1 in the reaction of trans-1Ni with [AuCl(PMe3)] to 10:1 in the reaction of 3Nib with [AuCl(tht)] in favor of the desired Ni–Au complex. The 19F NMR spectrum of the isolated complex clearly reveals two unsymmetrical 2-C6F4PPh2 ligands (−106.3, −116.4, −121.0, −129.9, −152.8, −153.0, −159.4, −160.1 ppm). The 2JP,P coupling constant of 330 Hz stays in good agreement with the trans Me3P–Ni-PPh2 arrangement, indicating the main complex to be cis–trans-7NiAu, which was separated from the byproducts by fractional crystallization. It was possible to analyze the molecular structure of trans-8NiCu·acetone and cis–trans-7NiAu with single-crystal X-ray diffraction (Figure 9; detailed structure description is given in the Supporting Information).
Scheme 7. Synthesis of Compounds of the Composition [(Me3P)NiCl(2-C6F4PPh2)2M′].
Figure 9.

Molecular structures of trans-8NiCu (left) and cis–trans-7NiAu (right). Ellipsoids are shown at 50% probability level. Hydrogen atoms and solvent molecules are omitted, and only the ipso-carbons of the PPh2 groups are depicted for clarity. Selected interatomic separations (Å) and angles (deg): trans-8NiCu: Ni1–Cu1 2.7124(3), Ni1–C1 1.939(2), Ni1–C7 1.925(2), Ni1–Cl1 2.2609(6), Ni1–P3 2.1420(6), Cu1–P1 2.2582(5), Cu1–P2 2.2593(5), Cu1–Cl1 2.4589(5), Cu1–O1 2.200(2), C1–Ni1–C7 172.12(8), Cl1–Ni1–P3 176.27(2), P1–Cu1–P2 143.26(2), O1–Ni1–Cl1 107.53(5), Ni1–Cl1–Cu1 70.01(2); cis–trans-7NiAu: Ni1–Au1 2.853(1), Ni1–C1 1.895(8), Ni1–Cl1 2.204(2), Ni1–P2 2.258(2), Ni1–P3 2.211(2), Au1–P1 2.277(2), Au1–C7 2.070(8), C1–Ni1–Cl1 173.9(3), P2–Ni1–P3 167.7(1), P1–Au1–C7 168.0(3).
Quantum chemical calculations were carried out to shed some light into the relative energy levels of the seven possible monomeric isomers of complexes of the type 6, 7, and 8 (Chart 2 and Table 5). For M = Ni, the isomers trans-8NiCu, trans-8NiAg, and cis–trans-7NiAu were experimentally observed and calculated to be thermodynamically stable isomers. For M′ = Cu and Ag, isomers 6 and cis–trans-7NiM′ were predicted to have similar energy levels in comparison to the experimentally observed isomer trans-8NiM′. For M = Pt and M′ = Cu or Ag, the isomers cis-6PtM′ and trans-6PtM′ revealed similar energy levels, with the former being lower. The monomeric or dimeric form of cis-6PtM′ were experimentally accessible. The respective experimentally observed interconversion of cis-6PtAu and trans-7PtAu stayed slightly in contrast with the higher energy level of the latter (ΔE = 4.81 kcal mol–1). The energy level of trans-8PtAu against trans-6PtAu was by about 3.97 kcal mol–1 and stayed in contrast to the experimentally observed isomerization of trans-6PtAu into trans-8PtAu. Nonetheless, the magnitude of energy difference is in the range that both isomers can coexist in solution. Alternatively, more complex scenarios (e.g., by coordination-dissociation effects of discrete solvent molecules or dimerization with the μ-Cl bridging mode) might also play reasonable roles and could potentially further lower the energy levels of the experimentally observed isomers. The isomer cis–trans-7MAu was predicted to be the thermodynamically most stable isomer for M = Ni, Pd,14 and Pt. However, attempts to isolate the complex cis–trans-7PtAu by refluxing a toluene solution of cis-6PtAu or trans-7PtAu failed because of decomposition (Figure S66).
Chart 2. Isomers of Compounds of the Composition [(Me3P)M(2-C6F4PPh2)2M′Cl].
Table 5. Energy Difference (ΔE, kcal mol–1) between the Coordination Isomers of Complexes of the Type 6MM′, 7MM′, and 8MM′ (M = Ni, Pt; M′ = Cu, Ag, Au)a.
| M | M′ | cis-6MM′ | trans-6MM′ | cis–trans-7MM′ | cis-cis-7MM′ | trans-7MM′ | cis-8MM′ | trans-8MM′ |
|---|---|---|---|---|---|---|---|---|
| Ni | Cu | 2.17 | 2.04 | 2.11 | 5.97 | 6.89 | 12.03 | 0.00 |
| Pt | 0.00 | 2.32 | 6.64 | 7.79 | 11.79 | 14.83 | 11.09 | |
| Ni | Ag | 0.37 | 0.00 | 1.89 | 6.22 | 7.43 | 11.75 | 1.53 |
| Pt | 0.00 | 2.00 | 8.08 | 9.92 | 13.23 | 16.89 | 10.76 | |
| Ni | Au | 8.05 | 8.51 | 0.00 | 4.60 | 6.57 | 15.06 | 2.58 |
| Pt | 1.17 | 3.37 | 0.00 | 2.28 | 5.99 | 13.44 | 7.34 |
Energy values of the crystallographically characterized isomers are written in italics and bold style.
The metal–metal interactions in complexes of type 6, 7, and 8 (M = Ni, Pt; M′ = Cu, Au) were investigated in an analogous manner to the complexes of type 5 (vide supra). According to the NBO/NLMO calculations, the energy levels of the main donor-orbital of Pt with one PMe3 ligand in their coordination sphere are ranging between −0.22 and −0.24 au (Table 6, Figure 10) and are very similar to the energy levels of the complexes of type 5 (−0.21 to −0.22 au, Table 3).
Table 6. NC and NBO Characteristics of the Main Donor–Acceptor Interaction [LP—Valence Lone Pair, LV—Lone Vacant Orbital, BD*—Valence Antibond, NBO Energy Level (ENBO, au), Donor–Acceptor Energy (kcal mol–1)] and WBO of Complexes of the Type 6MM′, 7MM′, and 8MM′ (M = Ni, Pt; M′ = Cu, Au).
| cis-6PtCu | cis-6PtAu | trans-6PtAu | cis–trans-7NiAu | trans-7PtAu | trans-8NiCu·acetone | trans-8PtAu | |
|---|---|---|---|---|---|---|---|
| NC(M) | 0.24 | 0.26 | 0.25 | 0.47 | 0.24 | 0.54 | 0.34 |
| NC(M′) | 0.61 | 0.37 | 0.38 | 0.36 | 0.35 | 0.72 | 0.30 |
| ENBO(LP(M)) | –0.23 | –0.22 | –0.22 | –0.31Au | –0.24 | –0.25 | –0.23 |
| LP(M) | 96% d | 96% d | 97% d | 89% dAu | 97% d | 99% d | 97% d |
| ENBO(LV(M′)) | 0.05 | 0.14 | |||||
| LV(M′) | 95% s | 99% s | |||||
| ENBO(BD*(M′–P/C)) | 0.39 | 0.38 | 0.09Ni–P | 0.22 | 0.20 | ||
| BD*(M′–P/C) | 74% Au (88% s, 12% d) | 74% Au (88% s, 12% d) | 75% Ni (57% s, 43% d) | 74% Au (86% s, 13% d) | 74% Au (90% s, 10% d) | ||
| ED→A | 26.44 | 6.62 | 13.94 | 4.82 | 14.10 | 1.71 | 19.20 |
| WBO | 0.36 | 0.30 | 0.37 | 0.32 | 0.41 | 0.30 | 0.44 |
Figure 10.
LP(Pt)→BD*(Au′–C/P) main donor–acceptor interaction derived by NBO analyses of cis-6PtAu (left), trans-6PtAu (middle left), trans-7PtAu (middle right), and trans-8PtAu (right). NBOs are displayed with an isosurface value of 0.05.
The Pt···Cu separation in cis-6PtCu is significant longer, by approximately 0.06 Å, in comparison to trans-5PtCu and cis-5PtCu, caused by steric repulsion of the chloride atom toward the ligand backbone. The topologic analysis (Table 7) of cis-6PtCu reveals a bcp between Pt and Cu with the same characteristics found in trans-5PtCu and cis-5PtCu (1 < |V(rb)|/G(rb) < 2, ∇2ρ(rb) > 0, G(rb) ≙ |V(rb)|, H(rb) < 0, WBO = 0.36–0.37), categorizing the Pt···Cu interaction in cis-6PtCu as predominantly metallic (shared) with an additional donor–acceptor bonding characteristic. However, the copper contribution in the main donor lone pair located at Pt in cis-6PtCu is about 0.29% and is similar to trans-5PtCu (0.24%) and cis-5PtCu (0.34%). Also, the lower acceptor orbital energy at copper (linear Cl–Cu–P arrangement) in cis-6PtCu (by about 0.1 au) in comparison to trans-5PtCu and cis-5PtCu (trigonal planar Cl–Cu–P2 arrangement) leads to a less intense interaction energy (ΔE2 ≈ 13 kcal mol–1). Therefore, a formal loss of Pt···Cu interaction intensity is present going from cis-6PtCu to either trans-5PtCu or cis-5PtCu, which is also reflected in lower ρ(rb) in cis-6PtCu. This behavior of Pt···Cu interaction intensity is visible by a shortening of the Pt···Cu separation in complexes of type 5 over 6 due to the absence of steric repulsion. In contrast, loss of interaction intensity between Pt···Cu caused by an increase in the coordination number of copper was observed between [(dppe)Pt(κC-2-C6F4PPh2)(μ-2-C6F4PPh2)CuCl] (linear Cl–Cu–P arrangement with significant donor–acceptor interactions) and [(dppe)Pt(μ-2-C6F4PPh2)2CuCl] (trigonal planar Cl–Cu–P2 arrangement absence of significant metallophilic interaction), where steric repulsion is present in both configurations.15 Interestingly, in complexes with a trigonal planar ClM′(PR3)2 unit (type 5) and complexes with a linear ClM′PR3 unit (type 6) a trend of stronger Pt→M′ donor–acceptor interaction with M′ = Au < Cu is observed.
Table 7. Results of the Theoretical Topological Analysis of the Bond Critical Points (3, −1) between M and M′ in Complexes 6MM′, 7MM′, and 8MM′ (M = Ni, Pt; M′ = Cu, Au)a.
| cis-6PtCu | cis-6PtAu | trans-6PtAu | cis–trans-7NiAu | trans-7PtAu | trans-8NiCu·acetone | trans-8PtAu | |
|---|---|---|---|---|---|---|---|
| ρ(rb) | 0.02865 | 0.02747 | 0.03683 | 0.02971 | 0.04399 | 0.04910 | |
| ∇2ρ(rb) | 0.06417 | 0.06334 | 0.08831 | 0.06891 | 0.10923 | 0.11946 | |
| G(rb) | 0.01908 | 0.01815 | 0.02673 | 0.02119 | 0.03430 | 0.03867 | |
| V(rb) | –0.02213 | –0.02048 | –0.03143 | –0.02516 | –0.04137 | –0.04757 | |
| |V(rb)|/G(rb) | 1.160 | 1.129 | 1.176 | 1.187 | 1.206 | 1.230 | |
| G(rb)/ρ(rb) | 0.666 | 0.661 | 0.726 | 0.713 | 0.780 | 0.788 | |
| H(rb) | –0.00305 | –0.00233 | –0.00470 | –0.00397 | –0.00707 | –0.00890 | |
| sign(λ2(rb))ρ(rb) | –0.029 | –0.027 | –0.037 | –0.030 | –0.044 | –0.049 |
Electron density (ρ(rb) in au), Laplacian of electron density (∇2ρ(rb) in au), Lagrangian kinetic energy density (G(rb) in au), potential energy density (V(rb) in au), ratio |V(rb)|/G(rb), ratio G(rb)/ρ(rb) in au, electron energy density (H(rb) in au), ratio H(rb)/ρ(rb) in au, the product of sign of the second largest eigenvalue of Hessian matrix of electron density (λ2(rb)), and ρ(rb) in au.
In the series of Pt–Au complexes, the Pt···Au distances are decreasing in the order cis-6PtAu > trans-6PtAu > trans-5PtAu > trans-7PtAu > trans-8PtAu. Topologic analyses of the 5 complexes reveal the expected characterization at the bcps (1 < |V(rb)|/G(rb) < 2, ∇2ρ(rb) > 0, G(rb) ≙ |V(rb)|, H(rb) < 0, WBO = 0.30–0.44), categorizing the Pt···Au interactions in PtAu complexes as predominantly metallic (shared) with an additional donor–acceptor bonding characteristic. The NCI descriptor and ELF increases in the expected trend from cis-6PtAu < trans-6PtAu < trans-5PtAu < trans-7PtAu < trans-8PtAu (Figures S67–S70) and is in agreement with the trend of the Pt···Au distances. The magnitudes of ρ(rb) and ∇2ρ(rb) and WBO are increasing in the same order, which is indicative for an increase of strength of the metallophilic interactions. The G(rb)/ρ(rb) ratio is getting closer to 1, which suggests more intense donor–acceptor type interaction going from cis-6PtAu to trans-8PtAu. The magnitude of ENBO main LP→LV/BD* interaction does follow the expected trend as observed by AIM analyses for complexes with a linear coordination sphere at Au (complexes type 6PtAu, 7PtAu, and 8PtAu, Figure 10).
Complex cis–trans-7NiAu shows similar characteristics at the bcp as observed in the series of Pt–Au-complexes (1 < |V(rb)|/G(rb) < 2, ∇2ρ(rb) > 0, G(rb) ≙ |V(rb)|, H(rb) < 0, WBO = 0.32). NBO analysis reveals a stronger Ni←Au donor–acceptor interaction over Ni→Au by 6.27 kcal mol–1, which is also the case for trans-7PtAu (ΣENBO(Pt→Au) < ΣENBO(Pt←Au) by 1.18 kcal mol–1). In complexes of type 7, the gold atom is a stronger donor over the d8-metal (see the Supporting Information for details).
NBO calculations at trans-8NiCu reveal that the Cl atom acts as a lone pair donor toward the copper center (∑ENBO/LP(Cl)→LV(Cu) = 30.78 kcal mol–1). The NC at Cl (−0.61) and Cu (0.72) supports the presence of attractive coulomb interaction. We find the Cl atom to be covalently bound to nickel and showing attractive dative bonding modes toward copper (WBOCu–Cl = 0.77 vs WBONi–Cl = 1.01), which is in agreement with the interatomic distances. The Cu1···O1 distance is approximately 2.20(1) Å and significantly longer than the sum of the covalent radii (1.98(4) Å).20 A similar donor–acceptor behavior between Cu and O (∑ENBO/LP(O)→LV(Cu) = 19.98 kcal mol–1, NCO: −0.61, WBOCu–O = 0.44) is observed for Cu and Cl but with less intensity. The presence of formal weak Cu1···O1 interactions was also observed in complexes with O=PPh(C6H4PPh2)2 as the donor in the copper coordination sphere.28 The calculation of the NCI descriptor for trans-8NiCu·acetone underlines the presence of attractive NCI between Cu1···Cl1 and Cu1···O1 (Figure 11).
Figure 11.

NCI descriptor of trans-8NiCu·acetone. Isovalue is set to 0.45, and color range is from −0.05 to 0.05 au; color code: blue—attractive interactions; green—van der Waals interactions; red—non-attractive interactions.
The Ni···Cu separation in trans-8NiCu is significantly shorter than in compound trans-5NiCu (by 0.1 Å), which can be explained by an additional chloride bridge in trans-8NiCu. However, AIM calculations did not support a bcp between Ni and Cu in trans-8NiCu. We address this absence of a bcp with three parameters: (i) Cu is distorted tetrahedral coordinated by P, Cl, and O donor atoms and is therefore sterically saturated for weak covalent interactions, (ii) the NCs for Ni and Cu are significantly higher in magnitude and therefore the Ni and Cu are more positively charged in comparison to other complexes presented in this study, and (iii) the donor–acceptor possibility is reduced by a comparative large gap between donor and acceptor orbital energy levels. According to the NCI descriptor, the Ni···Cu interaction should be described as very weak attractive non-covalent intermetallic (dispersion) interaction.
Conclusions
It has been shown that the homologous compounds trans-[(Me3P)2M(κC-2-C6F4PPh2)2] (M = Ni (2Nia), Pt (2Pta)) are accessible by the addition of excess PMe3 to trans-[M(κ2-2-C6F4PPh2)2] (M = Ni (1Ni), Pt (1Pt)). Complexes of the type [(Me3P)M(κ2-2-C6F4PPh2)(κC-2-C6F4PPh2)] (M = Ni (3Nib), Pt (3Ptb)) were accessible by the stoichiometric addition of PMe3 to either trans-1Ni or cis-1Pt. As byproducts, complexes of the type [M2(κ2-2-C6F4PPh2)2(μ-2-C6F4PPh2)2] (M = Ni (4Ni), Pt (4Pt)) could be isolated in pure state. Treatment of 2Nia, 2Pta, 3Nib, or 3Ptb with coinage metal chlorides gave bimetallic complexes of type trans-[(Me3P)2M(μ-2-C6F4PPh2)2M′Cl] (trans-5MM′; M = Ni, Pt; M′ = Cu, Ag, Au), [(Me3P)M(κ2-2-C6F4PPh2)(μ-2-C6F4PPh2)M′Cl]x (x = 1: cis-6PtCu; x = 2: cis-6PtAg-dimer), or [(Me3P)ClM(μ-2-C6F4PPh2)M′] (cis–trans-7NiAu, trans-7PtAu, trans-8NiCu, trans-8NiAg). Treatment of trans-1Pt with [AuCl(PMe3)] gave trans-6PtAu as an intermediate, which isomerized to trans-8PtAu, whereas treatment of cis-1Pt with [AuCl(PMe3)] resulted in the formation of cis-6PtAu, which stayed in equilibrium with trans-7PtAu. From the reaction of cis-6PtCu or cis-6PtAg-dimer with 1 equiv PMe3, the homologous complexes cis-[(Me3P)2Pt(μ-2-C6F4PPh2)2M′Cl] (cis-5PtM′; M′ = Cu, Ag) could be observed. Quantum chemical calculations (AIM, ELF, NCI, and NBO) gave insight into the M···M′ interaction characteristics. The interaction type reaches from pure attractive non-covalent (trans-8NiCu·acetone) to bonds with intermediate character (not purely ionic in nature but have some electron-shared (covalent) character). This intermediate character could be further divided into interaction types with predominantly metallic (shared) bonding (Ni–Cu, Pd–Cu, Pd–Ag, Pt–Au) and additional with donor–acceptor bonding characteristic (Ni←Au, Pd←Au, Pt←Au, Pt→Cu, Pt→Ag, Pt→Au). The interaction strength is increasing in the order Ni < Pd < Pt (at the same coinage metal and in the same complex type). Depending on the coinage metal coordination sphere, the interaction strength along group 11 is either increasing (e.g., trans-5MM′, trigonal-planar coordination at M′) or decreasing (e.g., trans-5MM′, linear coordination at M′). Along a series of Pt–Au complexes, it could be demonstrated that with the following changes, (1) decreasing steric hindrance (from cis-6PtAu to trans-6PtAu), (2) double over single bridged Pt–Au (from trans-6PtAu to trans-7PtAu), and (3) accumulated negative charged ligands at Pt and neutral ligands at Au (from trans-7PtAu to trans-8PtAu), the Pt···Au interaction can be intensified.
Experimental Section
General Comments
Dichloromethane, diethyl ether, and n-hexane were dried by the use of a standard column drying system, and tetrahydrofuran was distilled from Na/benzophenone. [M(κ2-2-C6F4PPh2)2] (M = Ni, Pd, Pt),13 [AuCl(tht)]29 (tht = tetrahydrothiophene), and [AuCl(PMe3)]30 (from [AuCl(tht)] with PMe3 solution in CH2Cl2) were prepared by the literature methods. CuCl and AgCl were prepared by the literature methods.15 Other chemicals were commercially available and used as received. 1H (300, 500 MHz), 19F (282, 471 MHz), and 31P (121, 202 MHz) NMR spectra were measured as CDCl3 solutions, unless otherwise stated, on a Bruker Avance 300 or Bruker Avance 500 spectrometer at room temperature. Chemical shifts were initially referenced to residual solvent signals (1H), CFCl3 (19F), or external 85% H3PO4 (31P). Electrospray mass spectra were measured for cis-7NiAu, trans-8NiCu, and trans-8NiAg on an “expression CMS L” spectrometer (Advion, Ithaca, USA) or for all other compounds on an “HP 5970 MSD” spectrometer. Elemental analyses were carried out by the Microanalytical Unit at the Research School of Chemistry, ANU.
Single-Crystal X-ray Crystallography
Crystals suitable for single-crystal X-ray diffraction were obtained from dichloromethane (trans-5PtAg), benzene/MeOH (syn-2Nia), dichloromethane/methanol (syn-2Pta·0.88(CH2Cl2), 3Ptb, 4Ni·0.63(CH2Cl2), 4Pt·0.52(CH2Cl2), trans-5NiCu), diethyl ether/MeOH (3Nib), dichloromethane/n-hexane (3Ptb, trans-5PtCu, cis-5PtCu, cis-5PtAg, cis-6PtCu, cis-6PtAg-dimer, trans-6PtAu·0.72(CH2Cl2), cis–trans-7NiAu, trans-7PtAu, trans-8PtAu), dichloromethane/MeOH/n-hexane (trans-5PtAu), toluene/n-hexane (cis-6PtAu), or acetone/n-hexane (trans-8NiCu·2(C3H6O)). Using a drop of inert oil (Nujol), crystals were mounted on a nylon loop or glass capillary and transferred into a stream of cold nitrogen. For cis–trans-7NiAu and trans-8NiCu, the single-crystal diffraction data sets were collected with ω-scans at an “IPDS-2(T)” diffractometer (STOE, Darmstadt, Germany) using Mo Kα radiation (λ = 0.71073 Å). The absorption correction was performed with XShape using the integration correction type. For all other compounds, the reflections were collected on a D8 Bruker diffractometer equipped with an APEX-II area detector using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) from a 1 μS micro source. The computer programs SMART31 and SAINT32 were used for data collection in φ- and ω-scan modes and data processing, respectively, and absorption corrections were done using SADABS.33 All structures were solved using direct methods and refined with full-matrix least-squares methods on F2 using the SHELX-TL package.34,35 X-ABS2 was used for syn-2Pta·0.88(CH2Cl2), trans-7PtAu, trans-5PtAg, and cis-5PtAg to perform the absorption correction.36 Parameters of data collection and structure refinement of the crystal structures discussed in this paper are reported in the Supporting Information. CIF files have been deposited with the Cambridge Crystallographic Data Center (CCDC) and can be obtained free of charge (for inquiry contact: CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK, fax: +44-1223-336033, e-mail: deposit@ccdc.cam.ac.uk) quoting the following reference numbers: CCDC-2027717 (syn-2Nia), 2027716 (syn-2Pta·0.88(CH2Cl2)), 2027705 (3Nib), 2027707 (3Ptb (P21/c)), 2027712 (3Ptb (Pn)), 2027710 (4Ni·0.63(CH2Cl2)), 2027711 (4Pt·0.52(CH2Cl2)), 2027719 (trans-5NiCu), 2027720 (trans-5PtCu), 2027722 (trans-5PtAu), 2027709 (cis-5PtCu), 2027706 (cis-5PtAg), 2027715 (cis-6PtCu), 2027721 (cis-6PtAg-dimer), 2027708 (cis-6PtAu), 2027727 (trans-6PtAu·0.722(CH2Cl2)), 2027713 (cis–trans-7NiAu), 2027725 (trans-7PtAu), 2027724 (trans-8NiCu·2(C3H6O)), 2027726 (trans-8PtAu), 2027714 (A), 2027718 (B1), 2027723 (B2·CH2Cl2).
Quantum Chemical Calculations
The geometry optimizations were carried out with ORCA 5.0.2 or 5.0.337 using the restricted PBE0 functional with relativistically recontracted Karlsruhe basis sets ZORA-def2- TZVPP38 (for H, C, F, P, Cl, Ni, Cu) and SARC-ZORA-TZVPP (for Pd, Ag, Pt, Au),39 the scalar relativistic ZORA Hamiltonian,40 atom-pairwise dispersion correction with the Becke–Johnson damping scheme (D3BJ),41 and COSMO solvation (CH2Cl2, ε = 8.9, rsolv = 3.55, xfeps = 0.8333).42 Very-TightSCF and slowconv options were applied, and the DEFGRID3 was used with a radial integration accuracy of 10 for all transition-metal atoms for all calculations. Calculations were started from the molecular structures obtained by single-crystal X-ray diffraction analysis, and isomers were created by modifying these structures. Numerical frequency calculations were performed to prove convergence at the local minimum after geometry optimization and to obtain the Gibbs free energy (293.15 K). After optimization of the H-atom positions of the molecular structures obtained by single-crystal X-ray diffraction analyses, NBO and NLMO calculations were performed using Gaussian0943 with the NBO6 package22 using the restricted PBE0 functional with Karlsruhe basis sets def2-TZVPP (for all atoms).38 NBO and ELF graphics were generated using ChemCraft.44 NCI,21 ELF,23 AIM24 and WBO45 calculations were carried out by using MultiWFN46 at the same level of theory as used for NBO/NLMO calculations. The NCI results were depicted with VMD.47
Acknowledgments
The authors thank Dr. Erik Wächtler for crystallographic analysis of compound cis-6PtAu and Dr. Michael Patzschke for computational insight. The authors are grateful for computing time at the High-Performance Computing Cluster at TU Bergakademie Freiberg, which was funded by Deutsche Forschungsgemeinschaft (DFG)—397252409. This research work was funded in part by the German Federal Ministry of Environment, Nature Conservation, Nuclear Safety and Consumer Protection (BMUV), under Project 1501667 (Am-BALL).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.3c00311.
Synthesis; XRD; 1H, 19F, 31P NMR spectra; parameters of data collection and structure refinement; discussion of molecular structures; NMR calculations; structural overlay of trans-5NiCu, trans-5PdCu, and trans-5PtCu; additional ELF and NCI; second-order perturbation theory analysis of Fock matrix in NBO basis; and graphical representations of optimized molecular structures, total energies, and atomic coordinates (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- a Wächtler E.; Gericke R.; Zhechkov L.; Heine T.; Langer T.; Gerke B.; Pöttgen R.; Wagler J. Pyridine-2-thiolate bridged tin–palladium complexes with Sn(PdN2Cl2), Sn(PdN2S2), Sn(PdN2C2) and Sn(Pd2N4) skeletons. Chem. Commun. 2014, 50, 5382–5384. 10.1039/c3cc47912a. [DOI] [PubMed] [Google Scholar]; b Wächtler E.; Wahlicht S.; Privér S. H.; Bennett M. A.; Gerke B.; Pöttgen R.; Brendler E.; Gericke R.; Wagler J.; Bhargava S. K. Tin(IV) Compounds with 2-C6F4PPh2 Substituents and Their Reactivity toward Palladium(0): Formation of Tin–Palladium Complexes via Oxidative Addition. Inorg. Chem. 2017, 56, 5316–5327. 10.1021/acs.inorgchem.7b00410. [DOI] [PubMed] [Google Scholar]; c Wächtler E.; Gericke R.; Brendler E.; Gerke B.; Langer T.; Pöttgen R.; Zhechkov L.; Heine T.; Wagler J. Group 10–group 14 metal complexes [E–TM]IV: the role of the group 14 site as an L, X and Z-type ligand. Dalton Trans. 2016, 45, 14252–14264. 10.1039/c6dt01621a. [DOI] [PubMed] [Google Scholar]; d Wächtler E.; Oro L. A.; Iglesias M.; Gerke B.; Pöttgen R.; Gericke R.; Wagler J. Synthesis and Oxidation of a Paddlewheel-Shaped Rhodium/Antimony Complex Featuring Pyridine-2-Thiolate Ligands. Chem.—Eur. J. 2017, 23, 3447–3454. 10.1002/chem.201605485. [DOI] [PubMed] [Google Scholar]; e Sircoglou M.; Saffon N.; Miqueu K.; Bouhadir G.; Bourissou D. Activation of M–Cl Bonds with Phosphine–Alanes: Preparation and Characterization of Zwitterionic Gold and Copper Complexes. Organometallics 2013, 32, 6780. 10.1021/om4005884. [DOI] [Google Scholar]; f Sircoglou M.; Mercy M.; Saffon N.; Coppel Y.; Bouhadir G.; Maron L.; Bourissou D. Gold(I) Complexes of Phosphanyl Gallanes: From Interconverting to Separable Coordination Isomers. Angew. Chem., Int. Ed. 2009, 48, 3454. 10.1002/anie.200900737. [DOI] [PubMed] [Google Scholar]; g Derrah E. J.; Sircoglou M.; Mercy M.; Ladeira S.; Bouhadir G.; Miqueu K.; Maron L.; Bourissou D. Original Transition Metal→Indium Interactions upon Coordination of a Triphosphine-Indane. Organometallics 2011, 30, 657–660. 10.1021/om1011769. [DOI] [Google Scholar]; h Bauer J.; Braunschweig H.; Dewhurst R. D. Metal-Only Lewis Pairs with Transition Metal Lewis Bases. Chem. Rev. 2012, 112, 4329–4346. 10.1021/cr3000048. [DOI] [PubMed] [Google Scholar]
- a Broere D. L. J.; Modder D. K.; Blokker E.; Siegler M. A.; van der Vlugt J. I. Metal–Metal Interactions in Heterobimetallic Complexes with Dinucleating Redox-Active Ligands. Angew. Chem., Int. Ed. 2016, 55, 2406. 10.1002/anie.201509412. [DOI] [PubMed] [Google Scholar]; b Abubekerov M.; Khan S. I.; Diaconescu P. L. Ferrocene-bis(phosphinimine) Nickel(II) and Palladium(II) Alkyl Complexes: Influence of the Fe–M (M = Ni and Pd) Interaction on Redox Activity and Olefin Coordination. Organometallics 2017, 36, 4394–4402. 10.1021/acs.organomet.7b00626. [DOI] [Google Scholar]; c Tereniak S. J.; Carlson R. K.; Clouston L. J.; Young V. G.; Bill E.; Maurice R.; Chen Y. S.; Kim H. J.; Gagliardi L.; Lu C. C. Role of the Metal in the Bonding and Properties of Bimetallic Complexes Involving Manganese, Iron, and Cobalt. J. Am. Chem. Soc. 2014, 136, 1842–1855. 10.1021/ja409016w. [DOI] [PubMed] [Google Scholar]; d Raju S.; Singh H. B.; Butcher R. J. Metallophilic interactions: observations of the shortest metallophilicinteractions between closed shell (d10···d10, d10···d8, d8···d8) metal ions [M···M’ M = Hg(II) and Pd(II) and M’ = Cu(I), Ag(I), Au(I), and Pd(II)]. Dalton Trans. 2020, 49, 9099–9117. 10.1039/d0dt01008a. [DOI] [PubMed] [Google Scholar]; e Navarro M.; Moreno J. J.; Pérez-Jiménez M.; Campos J. Small molecule activation with bimetallic systems: a landscape of cooperative reactivity. Chem. Commun. 2022, 58, 11220. 10.1039/d2cc04296g. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Berenguer J. R.; Lalinde E.; Moreno M. T. Luminescent cyclometalated-pentafluorophenyl PtII, PtIV and heteropolynuclear complexes. Coord. Chem. Rev. 2018, 366, 69–90. 10.1016/j.ccr.2018.04.002. [DOI] [Google Scholar]
- a Liu T.; Zhou X.; Zhang H. X.; Xia B. H. Theoretical studies on metal–metal interaction and spectroscopic properties of a series of hetero-binuclear d8–d10 complexes containing iridium(I) and gold(I). Dalton Trans. 2008, 0, 1065–1072. 10.1039/b716374f. [DOI] [PubMed] [Google Scholar]; b Cook T. R.; Esswein A. J.; Nocera D. G. Metal-Halide Bond Photoactivation from a PtIII-AuII Complex. J. Am. Chem. Soc. 2007, 129, 10094. 10.1021/ja073908z. [DOI] [PubMed] [Google Scholar]; c Yip H. K.; Che C. M.; Peng S. M. Luminescent and Coordinatively Unsaturated Heterobimetallic d10-d8 Complexes. Photoredox Properties and X-Ray Crystal Structure of [AuPt(μ-dppm)2(CN)2]+ [dppm = bis(diphenylphosphino)methane]. J. Chem. Soc., Chem. Commun. 1991, 1626–1628. 10.1039/c39910001626. [DOI] [Google Scholar]; d Yip H. K.; Lin H. M.; Cheung K. K.; Che C. M.; Wang Y. Luminescent Heterobimetallic Complexes. Electronic Structure, Spectroscopy, and Photochemistry of [AuPt(dppm)2(CN)2]ClO4 and the X-ray Crystal Structure of [AgPt(dppm)2(CN)2(CF3SO3)]. Inorg. Chem. 1994, 33, 1644. 10.1021/ic00086a014. [DOI] [Google Scholar]; e Xia B. H.; Zhang H. X.; Che C. M.; Leung K. H.; Phillips D. L.; Zhu N.; Zhou Z. Y. Metal-Metal Interactions in Heterobimetallic d8-d10 Complexes. Structures and Spectroscopic Investigation of [M′M″(μ-dcpm)2(CN)2]+ (M′ = Pt, Pd; M″ = Cu, Ag, Au) and Related Complexes by UV-vis Absorption and Resonance Raman Spectroscopy and ab Initio Calculations. J. Am. Chem. Soc. 2003, 125, 10362. 10.1021/ja0355325. [DOI] [PubMed] [Google Scholar]; f Balch A. L.; Catalano V. J. Ligation-Induced Changes in Metal-Metal Bonding in Luminescent Binuclear Complexes Containing Gold(I) and Iridium(I). Inorg. Chem. 1991, 30, 1302. 10.1021/ic00006a027. [DOI] [Google Scholar]; g Luo J.; Khusnutdinova J. R.; Rath N. P.; Mirica L. M. Unsupported d8–d8 interactions in cationic PdII and PtII complexes: evidence for a significant metal–metal bonding character. Chem. Commun. 2012, 48, 1532–1534. 10.1039/c1cc15420f. [DOI] [PubMed] [Google Scholar]; h Rozhkov A. V.; Katlenok E. A.; Zhmykhova M. V.; Kuznetsov M. L.; Khrustalev V. N.; Tugashov K. I.; Bokach N. A.; Kukushkin V. Y. Spodium bonding to anticrown-Hg3 boosts phosphorescence of cyclometalated-PtII complexes. Inorg. Chem. Front. 2023, 10, 493–510. 10.1039/d2qi02047e. [DOI] [Google Scholar]; i Fernandez-Cestau J.; Rama R. J.; Rocchigiani L.; Bertrand B.; Lalinde E.; Linnolahti M.; Bochmann M. Synthesis and Photophysical Properties of Au(III)–Ag(I) Aggregates. Inorg. Chem. 2019, 58, 2020–2030. 10.1021/acs.inorgchem.8b02987. [DOI] [PubMed] [Google Scholar]; j Kaub C.; Lebedkin S.; Li A.; Kruppa S. V.; Strebert P. H.; Kappes M. M.; Riehn C.; Roesky P. W. Bimetallic d10-Metal Complexes of a Bipyridine Substituted N-Heterocyclic Carbene. Chem.—Eur. J. 2018, 24, 6094–6104. 10.1002/chem.201705757. [DOI] [PubMed] [Google Scholar]; k Shahsavari H. R.; Giménez N.; Lalinde E.; Moreno M. T.; Fereidoonnezhad M.; Babadi Aghakhanpour R.; Khatami M.; Kalantari F.; Jamshidi Z.; Mohammadpour M. Heterobimetallic PtII-AuI Complexes Comprising Unsymmetrical 1,1-Bis(diphenylphosphanyl)methane Bridges: Synthesis, Photophysical, and Cytotoxic Studies. Eur. J. Inorg. Chem. 2019, 2019, 1360–1373. 10.1002/ejic.201801297. [DOI] [Google Scholar]
- Pérez-Temprano M. H.; Casares J. A.; Espinet P. Bimetallic Catalysis using Transition and Group 11 Metals: An Emerging Tool for C-C Coupling and Other Reactions. Chem.—Eur. J. 2012, 18, 1864–1884. 10.1002/chem.201102888. [DOI] [PubMed] [Google Scholar]
- Gallego D.; Brück A.; Irran E.; Meier F.; Kaupp M.; Driess M.; Hartwig J. F. From Bis(silylene) and Bis(germylene) Pincer-Type Nickel(II) Complexes to Isolable Intermediates of the Nickel-Catalyzed Sonogashira Cross-Coupling Reaction. J. Am. Chem. Soc. 2013, 135, 15617–15626. 10.1021/ja408137t. [DOI] [PubMed] [Google Scholar]
- a Serra D.; Moret M. E.; Chen P. Transmetalation of Methyl Groups Supported by PtII-AuI Bonds in the Gas Phase, in Silico, and in Solution. J. Am. Chem. Soc. 2011, 133, 8914–8926. 10.1021/ja110405q. [DOI] [PubMed] [Google Scholar]; b Arsenault G. J.; Anderson C. M.; Puddephatt R. J. Complexes with platinum-gold and -silver bonds: catalysis by silver(I) of alkyl exchange reactions between platinum centers. Organometallics 1988, 7, 2094–2097. 10.1021/om00100a002. [DOI] [Google Scholar]
- a Moret M.-E.; Serra D.; Bach A.; Chen P. Transmetalation Supported by a PtII-CuI Bond. Angew. Chem., Int. Ed. 2010, 49, 2873–2877. 10.1002/anie.200906480. [DOI] [PubMed] [Google Scholar]; b Baya M.; Belío Ú.; Campillo D.; Fernández I.; Fuertes S.; Martín A. Pt–M Complexes (M = Ag, Au) as Models for Intermediates in Transmetalation Processes. Chem.—Eur. J. 2018, 24, 13879–13889. 10.1002/chem.201802542. [DOI] [PubMed] [Google Scholar]; c Baya M.; Belío Ú.; Fernández I.; Fuertes S.; Martín A. Unusual Metal–Metal Bonding in a Dinuclear Pt–Au Complex: Snapshot of a Transmetalation Process. Angew. Chem., Int. Ed. 2016, 55, 6978–6982. 10.1002/anie.201602081. [DOI] [PubMed] [Google Scholar]
- a Bennett M. A.; Contel M.; Hockless D. C. R.; Welling L. L.; Willis A. C. Bis{(2-diphenylphosphino)phenyl}mercury: A P-Donor Ligand and Precursor to Mixed Metal–Mercury (d8–d10) Cyclometalated Complexes Containing 2-C6H4PPh2. Inorg. Chem. 2002, 41, 844–855. 10.1021/ic010890z. [DOI] [PubMed] [Google Scholar]; b Bennett M. A.; Bhargava S. K.; Griffiths K. D.; Robertson G. B.; Wickramasinghe W. A.; Willis A. C. Dinuclear Complexes of Gold(I) Containing Bridging Cyclometalated Arylphosphane or Arylarsane Ligands. Angew. Chem., Int. Ed. 1987, 26, 258–260. 10.1002/anie.198702581. [DOI] [Google Scholar]; c Bennett M. A.; Bhargava S. K.; Mirzadeh N.; Privér S. H.; Wagler J.; Willis A. C. Synthesis and interconversions of digold(I), tetragold(I), digold(II), gold(I)–gold(III) and digold(III) complexes of fluorine-substituted aryl carbanions. Dalton Trans. 2009, 7537. 10.1039/b906769h. [DOI] [PubMed] [Google Scholar]; d Modern Supramolecular Gold Chemistry; Laguna A., Ed.; Wiley VCH: Winheim, Germany, 2008. Chapter 2 & 4. [Google Scholar]; e Puddephatt R. J. Chemistry of Bis(diphenylphosphino)methane. Chem. Soc. Rev. 1983, 12, 99. 10.1039/cs9831200099. [DOI] [Google Scholar]
- a Pyykko P.; Mendizabal F. Theory of the d10-d10 Closed-Shell Attraction: 2. Long-Distance Behaviour and Nonadditive Effects in Dimers and Trimers of Type [(X-Au-L),] (n = 2, 3; X = Cl, I, H; L = PH3, PMe3, -N≡CH). Chem.—Eur. J. 1997, 3, 1458–1465. 10.1002/chem.19970030912. [DOI] [Google Scholar]; b Pyykko P.; Runeberg N.; Mendizabal F. Theory of the d10-d10 Closed-Shell Attraction: 1. Dimers Near Equilibrium. Chem.—Eur. J. 1997, 3, 1451–1457. 10.1002/chem.19970030911. [DOI] [Google Scholar]; c Guajardo Maturana R.; Muñoz-Castro A. Insights into metal–ligand and metal–metal interaction in coinage metal triangles. Insights of d10-d10, d10-d8 and d8-d8 contacts from [Au3In(CH3N=COCH3)3] (n = 2, 4, 6) via relativistic DFT calculations. Chem. Phys. Lett. 2016, 651, 34. 10.1016/j.cplett.2016.03.013. [DOI] [Google Scholar]
- Crespo O.; Laguna A.; Fernández E. J.; López-de-Luzuriaga J. M.; Jones P. G.; Teichert M.; Monge M.; Pyykko P.; Runeberg N.; Schütz M.; Werner H. J. Experimental and Theoretical Studies of the d8-d10 Interaction between Pd(II) and Au(I): Bis(chloro[(phenylthiomethyl)diphenylphosphine]gold(I))-dichloropalladium(II) and Related Systems. Inorg. Chem. 2000, 39, 4786–4792. 10.1021/ic000420p. [DOI] [PubMed] [Google Scholar]
- Pan Q. J.; Guo Y. R.; Zhang H. X. Theoretical Study of Metallophilic Interactions and Excited States of Heterobimetallic d10-d8 Complexes with Bridging Ligands: The Tuning of Electronic Spectroscopy. Organometallics 2010, 29, 3261–3270. 10.1021/om100080u. [DOI] [Google Scholar]
- a Dinda S.; Samuelson A. G. The Nature of Bond Critical Points in Dinuclear Copper(I) Complexes. Chem.—Eur. J. 2012, 18, 3032–3042. 10.1002/chem.201101219. [DOI] [PubMed] [Google Scholar]; b Lepetit C.; Fau P.; Fajerwerg K.; Kahn M. L.; Silvi B. Topological analysis of the metal-metal bond: A tutorial review. Coord. Chem. Rev. 2017, 345, 150–181. 10.1016/j.ccr.2017.04.009. [DOI] [Google Scholar]
- Bennett M. A.; Bhargava S. K.; Keniry M. A.; Privér S. H.; Simmonds P. M.; Wagler J.; Willis A. C. A Triad of Bis(orthometalated) d8-Complexes Containing Four-Membered Rings. Organometallics 2008, 27, 5361–5370. 10.1021/om8004806. [DOI] [Google Scholar]
- Wächtler E.; Privér S. H.; Wagler J.; Heine T.; Zhechkov L.; Bennett M. A.; Bhargava S. K. Metallophilic Contacts in 2-C6F4PPh2 Bridged Heterobinuclear Complexes: A Crystallographic and Computational Study. Inorg. Chem. 2015, 54, 6947–6957. 10.1021/acs.inorgchem.5b00939. [DOI] [PubMed] [Google Scholar]
- Gericke R.; Bennett M. A.; Privér S. H.; Bhargava S. K. Formation of Heterobimetallic Complexes by Addition of d10-Metal Ions to cis-[(dppe)M(κC-2-C6F4PPh2)2] (M = Ni, Pd, and Pt). Organometallics 2017, 36, 3178–3188. 10.1021/acs.organomet.7b00145. [DOI] [Google Scholar]
- Bennett M. A.; Bhargava S. K.; Ke M.; Willis A. C. Complexes of platinum(II), platinum(IV), rhodium(III) and iridium(III) containing orthometallated triphenylphosphine. J. Chem. Soc., Dalton Trans. 2000, 3537–3545. 10.1039/b004908p. [DOI] [Google Scholar]
- Font-Bardia M.; González-Platas J.; Muller G.; Panyella D.; Rocamora M.; Solans X. Preparation of Four-membered Phosphonickelocycles. Unusual Facile Stabilization of Five-co-ordinate Complexes. J. Chem. Soc., Dalton Trans. 1994, 3075–3084. 10.1039/dt9940003075. [DOI] [Google Scholar]
- a Bennett M. A.; Bhargava S. K.; Messelhäuser J.; Privér S. H.; Welling L. L.; Willis A. C. ortho-Metallated complexes of platinum(II) and diplatinum(I) containing the carbanions (2-diphenylphosphino)phenyl and (2-diphenylphosphino)-n-tolyl (n = 5, 6). J. Chem. Soc., Dalton Trans. 2007, 3158–3169. 10.1039/b702808c. [DOI] [PubMed] [Google Scholar]; b Mirzadeh N.; Bennett M. A.; Wächtler E.; Zhechkov L.; Heine T.; Bhargava S. K. Formation of heterobinuclear Pt–Au complexes by chelate ring-opening of cis-[Pt(κ2-C6R4PPh2)2] (R = H, F). J. Organomet. Chem. 2015, 783, 130–134. 10.1016/j.jorganchem.2015.02.009. [DOI] [Google Scholar]
- a Schmidbaur H.; Adlkofer J.; Schwirten K. Trimethylphosphin-Komplexe von CuCl und AgCl : Darstellung, NMR- und NQR-Spektren. Chem. Ber. 1972, 105, 3382–3388. 10.1002/cber.19721051025. [DOI] [Google Scholar]; b Bowmaker G. A.; de Silva E. N.; Healy P. C.; Skelton B. W.; White A. H. Structural, vibrational and solid state CP MAS 31P NMR spectroscopic studies of complexes of trimethylphosphine with copper(I) and silver(I) halides. J. Chem. Soc., Dalton Trans. 1999, 901. 10.1039/A808778D. [DOI] [Google Scholar]; c Schmidbaur H.; Franke R. Organogold-Chemie. X. Methylgold(I) und Gold(I)-halogenide als komplexbildende Zentren für Trimethylphosphin und Trimethylphosphit. Chem. Ber. 1972, 105, 2985–2997. 10.1002/cber.19721050921. [DOI] [Google Scholar]; d de Silva E. N.; Bowmaker G. A.; Healy P. C. Vibrational and solid state (CP/MAS) 31P NMR spectroscopic studies of bis(trimethylphosphine)gold(I) halides31P NMR spectroscopic studies of bis(trimethylphosphine)gold(I) halides. J. Mol. Struct. 2000, 516, 263–272. 10.1016/s0022-2860(99)00204-5. [DOI] [Google Scholar]
- Cordero B.; Gómez V.; Platero-Prats A. E.; Revés M.; Echeverría J.; Cremades E.; Barragán F.; Alvarez S. Covalent radii revisited. J. Chem. Soc., Dalton Trans. 2008, 2832. 10.1039/b801115j. [DOI] [PubMed] [Google Scholar]
- Johnson E. R.; Keinan S.; Mori-Sánchez P.; Contreras-García J.; Cohen A. J.; Yang W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. 10.1021/ja100936w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendening E. D.; Badenhoop J. K.; Reed A. E.; Carpenter J. E.; Bohmann J. A.; Morales C. M.; Landis C. R.; Weinhold F.. NBO 6.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, 2013. http://nbo6.chem.wisc.edu/.
- Becke A. D.; Edgecombe K. E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. 10.1063/1.458517. [DOI] [Google Scholar]
- Bader R. F. W.Atoms in Molecules; Clarendon Press: Oxford, 1994. [Google Scholar]
- Koch W.; Frenking G.; Gauss J.; Cremer D.; Collins J. R. Helium Chemistry: Theoretical Predictions and Experimental Challenge. J. Am. Chem. Soc. 1987, 109, 5917–5934. 10.1021/ja00254a005. [DOI] [Google Scholar]
- a Bianchi R.; Gervasio G.; Marabello D. Experimental Electron Density Analysis of Mn2(CO)10: Metal-Metal and Metal-Ligand Bond Characterization. Inorg. Chem. 2000, 39, 2360–2366. 10.1021/ic991316e. [DOI] [PubMed] [Google Scholar]; b Bianchi R.; Gervasio G.; Marabello D. The experimental charge density in transition metal compounds. C. R. Chim. 2005, 8, 1392–1399. 10.1016/j.crci.2004.12.015. [DOI] [Google Scholar]
- Macchi P.; Proserpio D. M.; Sironi A. Experimental Electron Density in a Transition Metal Dimer: Metal-Metal and Metal-Ligand Bonds. J. Am. Chem. Soc. 1998, 120, 13429–13435. 10.1021/ja982903m. [DOI] [Google Scholar]
- Dau T. M.; Asamoah B. D.; Belyaev A.; Chakkaradhari G.; Hirva P.; Jänis J.; Grachova E. V.; Tunik S. P.; Koshevoy I. O. Adjustable coordination of a hybrid phosphine–phosphine oxide ligand in luminescent Cu, Ag and Au complexes. J. Chem. Soc., Dalton Trans. 2016, 45, 14160–14173. 10.1039/c6dt02435a. [DOI] [PubMed] [Google Scholar]
- Uson R.; Laguna A.; Laguna M.; Briggs D. A.; Murray H. H.; Fackler J. P. (Tetrahydrothiophene)gold (I) or gold (III) complexes. Inorg. Synth. 1989, 26, 85. 10.1002/9780470132579.ch17. [DOI] [Google Scholar]
- Müller T. E.; Green J. C.; Mingos D. P.; McPartlin C. M.; Whittingham C.; Williams D. J.; Woodroffe T. M. Complexes of gold(I) and platinum(II) with polyaromatic phosphine ligands. J. Organomet. Chem. 1998, 551, 313–330. 10.1016/s0022-328x(97)00522-6. [DOI] [Google Scholar]
- SMART Software Ver. 5.625 for the CCD Detector System; Bruker AXS Inc.: Madison, WI, 2001.
- SAINTPLUS Software Ver. 6.22 for the CCD Detector System; Bruker AXS Inc.: Madison, WI, 2001.
- Blessing R. H. An empirical correction for absorption anisotropy. Acta Crystallogr., Sect. A: Found. Adv. 1995, A51, 33–38. 10.1107/s0108767394005726. [DOI] [PubMed] [Google Scholar]
- Sheldrick G. M.SHELXTL, Ver. 2013/4; Universität Göttingen: Germany, 2013.
- Sheldrick G. M. A short history of SHELX. Acta Crystallogr., Sect. A: Found. Adv. 2008, 64, 112–122. 10.1107/s0108767307043930. [DOI] [PubMed] [Google Scholar]
- Parkin S.; Moezzi B.; Hope H. XABS2: an empirical absorption correction program. J. Appl. Crystallogr. 1995, 28, 53–56. 10.1107/s0021889894009428. [DOI] [Google Scholar]
- Neese F. Software update: The ORCA program system - Version 5.0. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2022, 12, e1606 10.1002/wcms.1606. [DOI] [Google Scholar]
- a Weigend F.; Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]; b Pantazis D. A.; Neese F. All-electron basis sets for heavy elements. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2014, 4, 363–374. 10.1002/wcms.1177. [DOI] [Google Scholar]
- Rolfes J. D.; Neese F.; Pantazis D. A. All-electron scalar relativistic basis sets for the elements Rb–Xe. J. Comput. Chem. 2020, 41, 1842–1849. 10.1002/jcc.26355. [DOI] [PubMed] [Google Scholar]
- a Lenthe E. V.; Baerends E. J.; Snijders J. G. Relativistic regular two-component Hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610. 10.1063/1.466059. [DOI] [Google Scholar]; b van Wüllen C. Molecular density functional calculations in the regular relativistic approximation: Method, application to coinage metal diatomics, hydrides, fluorides and chlorides, and comparison with first-order relativistic calculations. J. Chem. Phys. 1998, 109, 392–399. 10.1063/1.476576. [DOI] [Google Scholar]
- a Grimme S.; Ehrlich S.; Goerigk L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. 10.1002/jcc.21759. [DOI] [PubMed] [Google Scholar]; b Grimme S.; Antony J.; Ehrlich S.; Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- Bon R. S.; van Vliet B.; Sprenkels N. E.; Schmitz R. F.; de Kanter F. J. J.; Stevens C. V.; Swart M.; Bickelhaupt F. M.; Groen M. B.; Orru R. V. A. Multicomponent Synthesis of 2-Imidazolines. J. Org. Chem. 2005, 70, 3542–3553. 10.1021/jo050132g. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Keith T.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas O.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09, Revision B.01/E.01; Gaussian, Inc.: Wallingford CT, 2010/2013.
- Chemcraft ver. 1.8 (build 164), 2016. http://www.chemcraftprog.com.
- Wiberg K. B. Application Of The Pople-Santry-Segal CNDO Method To The Cyclopropylcarbinyl And Cyclobutyl Cation And To Bicyclobutane. Tetrahedron 1968, 24, 1083–1096. 10.1016/0040-4020(68)88057-3. [DOI] [Google Scholar]
- Lu T.; Chen F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Humphrey W.; Dalke A.; Schulten K. VMD: Visual molecular dynamics. J. Mol. Graphics 1996, 14, 33. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]; http://www.ks.uiuc.edu/Research/vmd/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.