Abstract
Trained immunity is a long-term increase in responsiveness of innate immune cells, induced by certain infections and vaccines. During the last 3 years of the COVID-19 pandemic, vaccines that induce trained immunity, such as BCG, MMR, OPV, and others, have been investigated for their capacity to protect against COVID-19. Further, trained immunity-inducing vaccines have been shown to improve B and T cell responsiveness to both mRNA- and adenovirus-based anti-COVID-19 vaccines. Moreover, SARS-CoV-2 infection itself induces inappropriately strong programs of trained immunity in some individuals, which may contribute to the long-term inflammatory sequelae. In this review, we detail these and other aspects of the role of trained immunity in SARS-CoV-2 infection and COVID-19. We also examine the learnings from the trained immunity studies conducted in the context of this pandemic and discuss how they may help us in preparing for future infectious outbreaks.
Keywords: trained immunity, COVID-19, vaccines, BCG, clinical trials
Trained immunity-inducing vaccines have been suggested to protect against heterologous infections, including COVID-19. This review presents the studies performed during the pandemic that have investigated the effects of vaccines such as BCG, OPV, MMR, and others against COVID-19. In addition, data are reviewed that suggest that COVID-19 itself and the novel COVID-19 vaccines can also induce trained immunity programs.
Background
At the end of 2019, a new form of pneumonia was identified in China, and in January 2020, a new viral pathogen from the coronaviruses family was discovered and termed SARS-CoV-2. The infection caused by the new coronavirus SARS-CoV-2 was named coronavirus disease-19 (or COVID-19) and caused a major pandemic. In the 3 years since the start of the pandemic, hundreds of millions of people were infected with the new virus, and more than 5 million people lost their lives (https://covid19.who.int).
Soon after the beginning of the pandemic, it became clear that dysregulation of immune responses played a very important role for the pathophysiology of COVID-19. Initially, most of the attention was centered on the clear hyperinflammatory profile that characterized many of the patients with severe forms of the disease.1 , 2 An exaggerated production and release of proinflammatory cytokines, especially interleukin-1 (IL-1) and -6 (IL-6), has been hypothesized to induce systemic and local inflammation, local increase in immune cell recruitment in the lung, followed by endothelial cell activation, fluid extravasation, and impaired gas exchanges.3 This chain of events can subsequently lead to respiratory insufficiency, the need for oxygen supplementation, sometimes artificial ventilation, and unfortunately, in a small but significant number of patients, to death. This hyperinflammation-centric view of the pathogenesis of COVID-19 led to large clinical trials in severely ill patients, which resulted in the successful identification of several important approaches for immunomodulatory treatments, such as the use of steroids, as well as blockers of IL-1 or IL-6 bioactivity.4
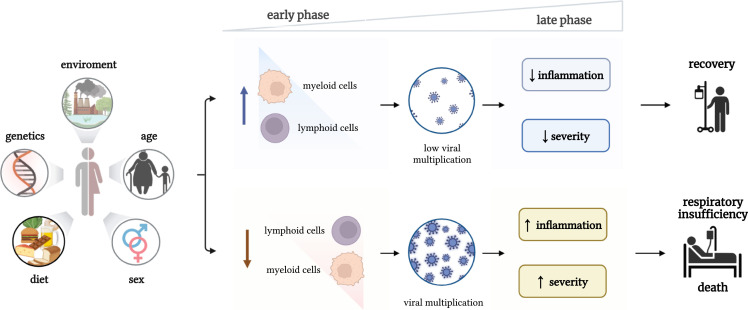
While hyperinflammation in the late stages of the disease clearly plays an important role in determining the patient’s outcome, more in-depth studies during the pandemic showed that the immune dysregulation in COVID-19 is more complex and presents both defective and overreactive features, depending on the patient and especially the phase of the disease. In this respect, it has been shown that the overproduction of IL-6 can also have immunosuppressive effects on the antigen-presenting features of myeloid cells with decrease of HLA-DR expression,2 while many patients with severe forms of the disease display defective T cell numbers and function.5 Comprehensive transcriptomic studies of various immune cell populations have shown varying degrees of defective immune activation in patients with severe disease, both in lymphoid and myeloid cells.6 These data argued therefore that immune defects are complex and variable in COVID-19 patients: on the one hand, if the immune responses are effective in the beginning of the disease, they would inhibit multiplication of the virus, resulting in low viremia, low systemic inflammation, and survival; on the other hand, if the host defense is defective in the first stages of the infection (when the patient is still asymptomatic), that would allow the virus to multiply, spread systemically, induce ineffective hyperinflammation, and have a poor prognosis (Figure 1 ). It has been therefore hypothesized that approaches that would boost innate immune responses from the beginning of the infection, even before antigen-specific T and B cell responses are activated, would likely improve the outcome of the patients.7
Figure 1.
Immune system dysregulation in the pathophysiology of COVID-19
When the immune responses are effective in the beginning of the disease, they will inhibit multiplication of the virus, resulting in low viremia, low systemic inflammation, and survival. In case the host defense response is defective in the first stages of the infection (when the patient is still asymptomatic), this would allow the virus to multiply, spread systemically, and to induce ineffective hyperinflammation and a poor prognosis.
Heterologous effects of vaccines: Activation of trained immunity responses
A large number of studies in the last century have shown that certain vaccines, especially with live attenuated microorganisms, are able to induce heterologous protection beyond the target disease.8 The immunological mechanisms mediating these effects are likely multiple, including induction of cross-reactive T cell responses, as well as long-term increase in the function of innate immune cells, a process termed trained immunity.9 Induction of trained immunity is antigen independent and can explain the broad protective effects induced by certain vaccines. The underlying molecular substrate is represented by epigenetic and metabolic rewiring of the cells: these processes lead to an increase in chromatin accessibility and an enhanced gene transcription for proteins that are necessary for host defense.9 Among the vaccines shown to induce heterologous protection against infections and trained immunity responses are bacillus Calmette-Guerin (BCG), measles-containing vaccines such as measles-mumps-Rubella (MMR), oral polio vaccine (OPV), and lately also influenza vaccines.10 , 11 , 12 Importantly, these vaccines do not all induce an identical transcriptional and functional trained immunity program. For example, BCG induces a trained immunity program biased toward myelopoiesis activation and enhancement of myeloid cell function,13 , 14 whereas ASO3-adjuvanted influenza vaccination induces a more potent antiviral interferon response.15
Extensive studies investigating the mechanisms through which certain vaccines such as BCG induce heterologous protection have recently described monocyte-specific trained immunity transcriptional programs as an important component of this protection.16 Importantly, single-cell sequencing technologies have shown that severe COVID-19 is characterized by defective trained immunity programs.16 In addition, a number of recent randomized trials have shown that BCG vaccination protects against experimental infection models in humans,17 , 18 as well as against respiratory tract infections in randomized trials in children19 , 20 or adults.2 These observations have led to the hypothesis that vaccines that can induce trained immunity may also protect against COVID-19.21 , 22 As a result, a relatively large number of experimental studies, as well as epidemiological studies and clinical (phase III randomized) trials have been initiated to explore the capacity of trained immunity-inducing vaccines (especially BCG) to protect against susceptibility and severity of COVID-19. Thus, 3 years into the pandemic, much has been learned on the importance of trained immunity for COVID-19, and a number of important conclusions can be drawn that can be used for a better preparedness against future pandemics. In this review, we present a summary of the studies performed during the pandemic on the trained immunity-inducing vaccines and their effects against COVID-19, as well as an overview of the long-term effects of COVID-19 itself and the new COVID-19 vaccines on trained immunity. We examine what we have learned and discuss how this knowledge on trained immunity may help us in preparing for future infectious outbreaks.
BCG vaccination: Effects on COVID-19
Experimental animal models of SARS-CoV-2 infections developed early during the pandemic have proven useful in investigating the capacity of the BCG vaccine to protect against SARS-CoV-2 infection and mortality. In one study, it was reported that intravenous (i.v.) administration of BCG in mice induced protection of human-ACE2 transgenic mice against mortality in a model of SARS-CoV-2 infection, which was mediated by reduced viral loads, tissue pathology, inflammatory cell recruitment, and cytokine production.23 This important observation showed the possibility to obtain heterologous protection against SARS-CoV-2. Importantly, however, subcutaneous (s.c.) administration of BCG did not show protective effects in this model, an observation that was also validated by a subsequent investigation.24 These initial reports have been corroborated by two subsequent studies showing protective effects of i.v. BCG in either the K18-hACE225 or a hamster model26 of SARS-CoV-2 infection, which was associated with induction of trained immunity responses such as myeloid cell differentiation, a transcriptional program in myeloid cells of antigen presentation and repair and activation of glycolysis. Not all studies were able to show protection against SARS-CoV-2 by BCG vaccination in rodent models, however, despite robust protection induced against influenza infection.27 Interestingly, murine studies also suggested induction of cross-reactive antibodies against SARS-CoV-2 infection by BCG vaccination.28 , 29 Finally, a non-human primate (NHP) model in rhesus macaques demonstrated rapid induction of innate immune cells such as monocytes and γδ-T cells by aerosol-administered BCG, but this did not result in overall protection of the animals.30 All in all, these animal studies provide compelling arguments that trained immunity-mediated protection against SARS-CoV-2 infection can be induced (Figure 2 ), but the route of administration is very important—with i.v. administration of BCG being effective (in three of the four studies currently published), while s.c. administration fails to provide protection. Such a more potent protection induced by i.v. administration of BCG has been demonstrated in other infectious models as well.31 The mechanisms behind the enhanced effective protection induced by i.v. as compared with s.c. administration need to be investigated in more detail. It can be hypothesized that i.v. BCG provides a more direct engagement of the bone marrow compartment and at the level required to induce trained immunity in immune cell progenitors, which may explain this stronger effect.
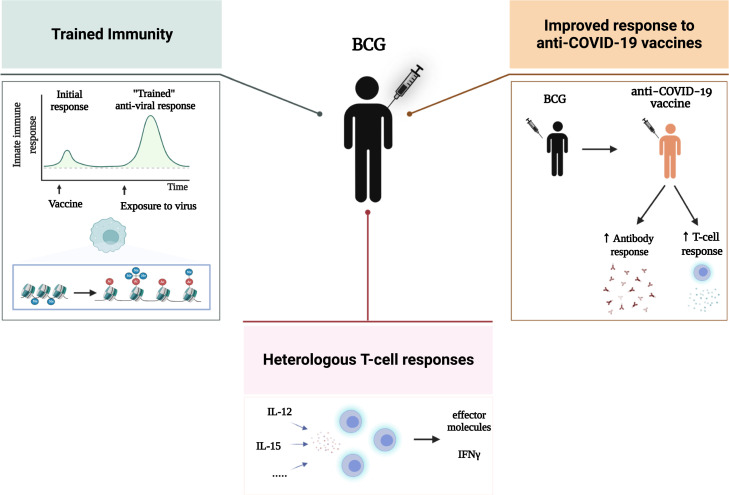
Figure 2.
Immunological effects of BCG vaccination in SARS-CoV-2 infection: Direct antiviral effects through activation of trained immunity and T cell heterologous immunity, as well as improvement of the serological and T cell response of specific vaccines
In parallel with the experimental studies, and due to the severity of the pandemic in 2020 and at the beginning of 2021, epidemiological studies and randomized trials with trained immunity-inducing vaccines were also initiated. Two hypotheses were under investigation: one that BCG given in childhood might protect against COVID-19 decades later and one that a recent BCG vaccination might protect against COVID-19. A number of initial epidemiological studies suggested that a program of BCG vaccination during childhood in particular geographical regions is associated with a low prevalence of COVID-19 and lower risk of severe COVID-19 in various countries.32 , 33 , 34 However, such studies often suffer from inevitable biases. Specifically, most African and South American countries use BCG in childhood and also had a later start of the pandemic. Hence, later studies when the pandemic had picked up in these continents could not replicate the initial associations.35 , 36 , 37 Moreover, the immunological effects during induction of trained immunity are believed to have a duration of several months and from 1 up to 2 years,13 , 38 rather than the decades needed for the protection induced by neonatal BCG vaccination, although some epidemiological studies have found BCG in childhood to be associated with a decrease in all-cause mortality.39 Interest therefore gathered around the hypothesis that a recent BCG would protect against COVID-19, and this hypothesis could be tested in randomized clinical trials providing solid advice regarding the capacity of BCG (and other vaccines) to protect against COVID-19.
Several randomized clinical trials investigating BCG vaccination effect on COVID-19 susceptibility have been initiated in 2020 and 2021. As severe COVID-19 patients are often characterized by hyperinflammation, a first important question was the safety of BCG vaccination (which can enhance innate immune responses). A retrospective study of recent BCG vaccination in healthy individuals demonstrated that BCG is safe with regard to COVID-19 severity,40 which supported the decision to perform large clinical trials. Subsequently, a relatively large number of studies have been initiated in countries around the world, including Europe (the Netherlands, Greece, Denmark, Hungary, and Poland), the Americas (the United States and Brazil), Asia (India), Africa (Guinea-Bissau, Mozambique, and South Africa), and Australia (see Table 1 ). The results of the trials published so far were heterogeneous, but a number of broad patterns can be discerned.
Table 1.
Randomized clinical trials with BCG vaccination and derivative vaccines during the COVID-19 pandemic
| Trial | Country | Number and type volunteersa | Strain of BCG | Revaccination | Effect on susceptibility | Mortality placebo vs. BCG | Reference |
|---|---|---|---|---|---|---|---|
| BCG-CORONA | the Netherlands | 1,600 HC | BCG-Denmark | no | no | 0 vs. 1 | Ten Doesschate et al.41 |
| BCG-Elderly | the Netherlands | 2,000 EL | BCG-Denmark | no | no | 3 vs. 2 | Moorlag et al.42 |
| BCG-CORONA | South Africa | 1,000 HC | BCG-Denmark | yes | no | 4 vs. 0 | Upton et al.43 |
| ACTIVATE-2 | Greece | 300 EL | BCG-Moscow | yes | yes | 3 vs. 0 | Tsilika et al.44 |
| BCG-PRIME | the Netherlands | 6,000 EL | BCG-Denmark | no | no | 18 vs. 13 | Koekenbier et al.45 |
| BCG-Brazil | Brazil | 400 HC | BCG-Moscow | yes | yes | no mortality | Dos Anjos et al.46 |
| BRACE | International | 4,000 HC | BCG-Denmark | no/yes | no | no mortality | Pittet et al.47 |
| BCG-Danish | Denmark | 1,300 HC | BCG-Denmark | no/yes | no | no mortality | unpublished data |
| BCG-Poland | Poland | 695 HC | BCG-10 vaccine (Biomed Lublin, Poland) | yes | no | no mortality | Faustman et al.48 |
| BCG-India | India | 1,450 EL | – | yes | unpublished | unpublished | unpublished data |
| Multiple BCG | USA | 144 DM1 | BCG-Japan | yes | yes | no mortality | Faustman et al.49 |
| BCG-COVID | Brazil | 300 COVID patients | – | yes | yes: effect on symptoms | no mortality | Jalalizadeh et al.50 |
| BRIC | India | 495 HRA | BCG-Moscow | yes | yes | 1 vs. 0 | Sinha et al.51 |
| VPM1002 | Germany | 2,000 EL | VPM1002 | no | yes | no mortality | Blossey et al.52 |
| Mycobact-w | India | 100 HC | – | yes | yes | no mortality | Jaiswal et al.53 |
Approximate numbers of: HC, health-care providers; EL, elderly volunteers; DM1, type 1 diabetes; COVID, convalescent COVID-19 individuals; HRA, high-risk adults.
First, the protection against susceptibility to COVID-19 differs in various populations. The majority of the larger studies performed in Europe, South Africa, or internationally do not show a protective effect of BCG vaccination against the susceptibility to COVID-19, both when the studies were conducted in healthcare workers41 , 43 , 47 , 48 and in individuals of older age with an increased susceptibility to severe infections.42 However, a number of smaller studies from Greece,44 Brazil,46 India,51 and the US49 suggested beneficial effects of BCG.
One potential difference between the studies showing a beneficial effect of BCG vaccination and the other studies is the prior vaccination with BCG as infants in the individuals from the studies showing a protective effect: these individuals underwent revaccination during the trial, while the majority of participants in the European trials were BCG-naive at the beginning of the trials (Table 1). Indeed, BCG revaccination has been previously shown to be associated with reduced all-cause mortality12 and enhanced protection also against tuberculosis (TB).54 However, sub-group analysis of the BCG-PRIME participants with previous exposure to BCG, together with the negative results of the BCG-CORONA studies from South Africa and Poland, does not support the hypothesis of a beneficial effect of BCG revaccination over a first dose of BCG.43 , 48 Genetic or environmental differences between the populations cannot be excluded, as geographical influence on BCG effects against TB has been documented in children,55 , 56 but this remains to be further investigated. Another potential difference is the underlying vulnerability of participants. Three trials found a protective effect of BCG against COVID-19 incidence; these trials from Greece,44 India,51 and the US49 were conducted in multi-morbid patients and in vulnerable type 1 diabetes patients, respectively. It may be that BCG can improve a weak immune system but cannot improve on a well-functioning immune system as that of most healthcare workers. However, two studies in individuals of older age with co-morbidities but without hospitalizations from the Netherlands did not show protective effects (Table 1).
Second, none of the published trials to date had enough power to be able to draw conclusions regarding the effect of BCG on the severity of the disease. While all of the studies in healthcare workers did not observe any effect on severity, due to the low number of severe events in these relatively young populations, the incidence rate of hospitalization in BCG-PRIME study in elderly individuals with co-morbidities was 14% lower, although this was not statistically significant either.45 Similarly, none of the studies had enough power to identify an effect of BCG vaccination on mortality. Interestingly however, in all four BCG-COVID19 studies in which mortality had been recorded (as well as the earlier published ACTIVATE trial)2 mortality was smaller in the BCG-vaccinated group compared with the placebo group: summary statistics of these trials reported a 39% lower mortality in the BCG-vaccinated groups.57 More studies as well as a broad meta-analysis of all studies currently reported or being completed is needed in order to be able to draw a firm conclusion regarding the effects of BCG vaccination on COVID-19 severity.
Third, the potential beneficial effects of BCG vaccination on the severity of COVID-19 may be explained by improvement of the immune responses in the vaccinated individuals. In this respect, both cellular and humoral immune responses were higher in BCG-vaccinated elderly who developed COVID-19, compared with unvaccinated individuals who were infected with SARS-CoV-2.42 Furthermore, BCG vaccination reduced the concentration of inflammatory mediators in the circulation,58 diminished the production of cytokines associated with severe COVID-19,59 and enhanced the frequency of memory T cells60 (Figure 2).
Finally, in addition to these studies that employed one standard dose of BCG vaccine, additional studies using different schedules or variants of BCG/mycobacterial vaccines also showed suggestive protective effects. In this respect, a randomized trial has been recently reported in which patients with type 1 diabetes received recent repeated BCG administrations. Interestingly, such multi-dose approach showed a very strong protection against COVID-19, with 92% less infections in the vaccinated individuals.49 Although this is a small study, the magnitude of the effect warrants future investigations. Another interesting approach has investigated the effect of BCG administration in convalescent patients with COVID-19: improvements in the anosmia and ageusia in the BCG-vaccinated individuals has been reported.50 A recent trial has also investigated the impact of VPM1002, a vaccine variant containing BCG expressing listeriolysin, and suggested consistent trends toward lower severity of the disease: a lower number of hospital and ICU admissions and decrease of disease duration from 14 to 9 days.52 The total number of infections was not impacted by VPM1002 vaccination in this study. Another mycobacterial vaccine stimulant, Mycobacterium-w, activated NK cell responses with a gene expression profile that favors antibody-dependent cellular cytotoxicity (ADCC) and subsequently induced a very strong reduction in COVID-19 susceptibility by 85% in a small study.53 More studies are needed to confirm the promising results of these alternative vaccination strategies.
Other vaccines inducing trained immunity effects
Although BCG was by far the most in-depth studied vaccine in the context of heterologous effects on COVID-19, other vaccines have also been previously reported to induce non-specific protection against infections. One of the most consistent inducers of protective effects against all-cause mortality in children are the measles-containing vaccines.61 Though fewer studies have been performed with measles-containing vaccines, one randomized trial with MMR revaccination in Brazil reported a significant decrease in the severity (but not susceptibility) to COVID-19,62 mirroring the effects reported for BCG. The results of a larger international study (CROWN-CORONATION) remain to be reported (ClinicalTrials.gov Identifier: NCT04333732).
Epidemiological studies have also earlier reported beneficial heterologous pan-viral effect of the OPV,63 , 64 and observational studies suggest an effect on COVID-19 as well. In this respect, mothers of children vaccinated with OPV also displayed decreased susceptibility to SARS-CoV-2 infection, compared with age-matched women.65 In this case, the protection of the mothers would likely be provided by their exposure to the OPV through fecal viral shedding from the infants. The hypothesis of a beneficial effect of OPV on COVID-19 was subsequently strengthened by a randomized trial in 1,000 individuals that reported a significant reduction of COVID-19 incidence in the OPV-vaccinated individuals compared with controls,66 and a randomized trial in 3,700 individuals aged 50 or above in Guinea-Bissau recently showed that OPV was associated with a reduced risk of all-cause morbidity in males (but not females) during the pandemic.67 These data are complemented by experimental studies showing that OPV-defective viral genomes can induce non-specific protection against a number of infections, including with SARS-CoV-2.68 This effect of the defective OPV genomes may be induced either through interference with viral replication or through activation of innate immune responses and potential induction of an interferon activation program. These data warrant increased efforts to determine the beneficial heterologous effects of OPV and the mechanisms that mediate them in more detail.
Recent immunological studies have also reported induction of a strong antiviral trained immunity program by ASO3 adjuvanted influenza vaccination.15 This has been independently validated in immunological studies showing a more regulated immune response against SARS-CoV-2 in humans,69 which was accompanied by lower incidence and severity of COVID-19 in influenza-vaccinated individuals.70 , 71 , 72 Randomized trials are necessary to confirm these beneficial effects. The same is true for the anti-zoster vaccine Shingrix, which was also reported to induce a significant reduction of both COVID-19 incidence (16% lower) and hospitalization (32% lower) in a large epidemiological study.73
Finally, one of the most intriguing possibilities regarding the role of trained immunity in the vaccination against COVID-19 is that the novel specific vaccines currently in use may also exert trained immunity effects that could contribute to their efficacy. While the mRNA-based platform, which is at the basis of one of the most successful anti-COVID-19 vaccines, is known to induce strong inflammation,74 very recent studies have shown that the mRNA vaccines also induce long-term transcriptional reprogramming of myeloid cells.75 This results in functional changes of both innate and adaptive immune cells, and the former can be considered a de facto induction of trained immunity.76 Whether the mRNA vaccines can thus induce also cross-protection against other infections and whether these properties affect their effects against COVID-19 remain to be investigated. Interestingly, a very recent study reported that vaccination of Hong Kong residents with either the BNT162b2 mRNA vaccine or the inactivated virus vaccine CoronaVac indeed may have enhanced resistance to TB.77 On the other hand, the role of such effects in mediating some of the rare but severe inflammatory complications of vaccination (such as myocarditis and pericarditis)78 needs to be investigated.
The impact of BCG on the specific immune responses induced by the novel anti-COVID-19 vaccines
As mentioned earlier, BCG enhanced cellular and humoral immune responses in individuals infected with SARS-CoV-2,42 which raises the possibility that it may improve the specific responses induced by the novel anti-COVID-19 vaccines as well. Indeed, BCG has been previously shown to improve vaccination responses in children,79 , 80 while in adults it potentiates the responses to influenza and Salmonella vaccines.81 , 82 Moreover, approaches of using BCG as the “prime” in prime-boost strategies for TB vaccination have also been tested.83 , 84 A number of proof-of-principle studies have subsequently assessed whether BCG can also improve immune responses after vaccination with the novel mRNA and adenovirus-based anti-COVID-19 vaccines. Indeed, BCG revaccination 30 days before the Pfizer-BioNTech anti-COVID-19 vaccines induced significantly higher titers of neutralizing anti-SARS-CoV2 antibodies, compared with individuals who received placebo before the COVID-19 vaccine.85 Similarly, BCG revaccination qualitatively and quantitatively enhanced SARS-CoV-2 neutralizing antibodies and T cell responses induced by the Oxford/AstraZeneca adenovirus-based vaccine in SARS-CoV-2 seronegative young Indian adults.86
Altogether, mounting evidence suggests that BCG vaccination can act as an amplifier of specific immune responses induced by the novel COVID-19 vaccines and could thus improve the quality and durability of their effects (Figure 2). Whether this can lead to an improved clinical efficacy remains to be investigated.
COVID-19 induces long-term changes in myeloid cell compartment
The consequences of trained immunity as an immunological process for COVID-19 have been most intensively studied from the point of view of vaccination (Figure 3 ). However, SARS-CoV-2 infection itself is also accompanied by strong and complex immunological effects, and an increasing number of studies have investigated the long-term effects of the infection on innate immune cells. In line with this, a recent study using single-cell sequence technologies has shown the establishment of trained immunity in a newly described population of T-bet-enriched CD16+ and IRF1-enriched CD14+ monocytes with sequential trained and activated epigenomic states.87 Moreover, epigenetic memory was induced not only in the peripheral innate immune cell populations but in their progenitors as well,88 a hallmark of the induction of central trained immunity in the bone marrow.9
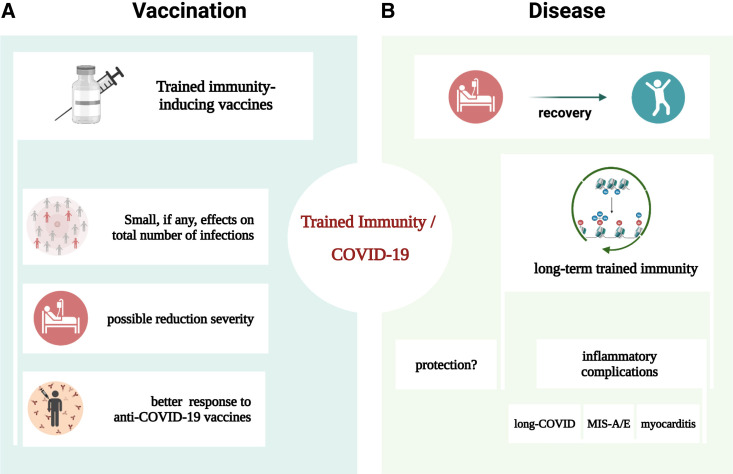
Figure 3.
Trained immunity impacts on COVID-19
(A) Vaccines with trained immunity-inducing capacity: effects of single-dose administrations are very limited against total number of infections, with possible exceptions in some populations. Multiple dose administrations may be better, but more trials are needed.
(B) COVID-19 itself inappropriately induces long-term trained immunity programs that may play a role in the pathophysiology of long-COVID.
An important question relates to the clinical consequences of such trained immunity programs induced by COVID-19 (Figure 3). One possibility would be that such a program may contribute to the protection against re-infection or the severity of a secondary viral infection, although this remains to be formally demonstrated. It is interesting to observe that patients with COVID-19 are generally accompanied by less co-infections compared with other viral infections such as influenza. Another possibility is that an inappropriately strong induction of trained immunity may contribute to the long-term inflammatory complications of COVID-19. Indeed, long-COVID-19 patients display transcriptional dysregulation in their innate immune cells,89 as well as immunological dysfunction characterized by highly activated innate immune cells with high expression of type I and III interferons that persists for more than half a year following COVID-19.90 Interestingly, single-cell profiling identified a population of CD9+ monocytes persisting for at least 3–4 months after COVID-19, which showed trained immunity characteristics with enhanced production of chemokines (IL-8 and MCP-1).91 Aberrant innate immune cell populations, but also cytotoxic T cells, were observed in the airways of patients with ongoing respiratory complications after COVID-19.92
Post-COVID-19 long-term dysregulation of the innate immune cell function could also play a role in the severely exaggerated inflammation in pediatric or adult patients with multisystem inflammatory syndrome (MIS-C and MIS-A). Severe forms of MIS-C are characterized by a monocyte/dendritic cell signature with higher production of both cytokines and chemokines.93 , 94 Other important pathophysiological components are likely to involve superantigen-induced immune activation.95 In addition, the pathophysiology of MIS-A is also characterized by inappropriate activation of the innate immune cells and inflammation and has been shown to respond to anti-cytokine therapies in small studies.96 , 97 More studies are needed to understand the involvement of trained immunity in these long-term complications of COVID-19.
Conclusions from the pandemic: The impact of trained immunity for COVID-19
Trained immunity has emerged as an important immunological process in which innate immune cells undergo long-term epigenetic and functional changes after infections or vaccinations. Because of the capacity of certain vaccines, especially those consisting of live attenuated microorganisms, to induce broad heterologous protection against infections, an increased interest had emerged at the beginning of the COVID-19 pandemic to assess the impact of these vaccines and trained immunity induction on SARS-CoV-2 infection.21 After experimental, epidemiological, and clinical studies performed in the last 3 years, a number of important conclusions can be drawn regarding the role of trained immunity (Box 1 ). Briefly, most of the studies in healthy individuals do not demonstrate an overall protection against total number of COVID-19 infections, whereas some smaller studies in vulnerable groups found protective effects. A decrease of disease severity was suggested, but not formally demonstrated, to be induced by several of these vaccines.
Box 1. Conclusions on the role of trained immunity in COVID-19.
-
1.
Experimental studies in animals showed protection induced by i.v. administration of BCG against SARS-CoV-2 infection.
-
2.
Trained immunity-inducing vaccines (BCG, MMR, Shingrix) do not protect against total number of SARS-CoV-2 infections, with the possible exception of OPV, influenza, and multiple BCG vaccinations in some populations.
-
3.
Trained immunity-inducing vaccines are likely to decrease the clinical severity of COVID-19 and overall mortality, but large randomized trials with enough statistical power are needed to be able to draw definitive conclusions.
-
4.
BCG vaccination improves B and T cell responsiveness to both mRNA- and adenovirus-based anti-COVID-19 vaccines.
-
5.
The novel anti-COVID-19 vaccines induce long-term trained immunity programs.
-
6.
SARS-CoV-2 infection can induce inappropriately strong induction of trained immunity in some individuals, which can contribute to the long-term inflammatory complications.
What could be the lessons for future pandemics with new pathogens? While the data till now mainly suggest an impact of trained immunity-inducing vaccines on disease severity, this could still have important beneficial effects in reducing the effects of a pandemic. Indeed, mathematical modeling of the use of trained immunity-inducing vaccines shows an important impact on mortality and morbidity during a pandemic, even at very low efficacy of 5%–15%.98 , 99 The rapid development of specific and effective vaccines against SARS-CoV-2 represented one of the major successes of biomedical research during this pandemic. Moreover, the protection offered by these vaccines against severe COVID-19 makes the direct deployment of trained immunity-inducing vaccines unnecessary for the current pandemic. Nevertheless, the research done on trained immunity during COVID-19 has been extremely valuable at several levels.
First, these studies provide the proof-of-principle for the potential deployment of trained immunity-inducing vaccines against novel pathogens in the future. Such deployment may well become extremely important in a future pandemic, until specific vaccines can be developed and tested. Trained immunity-inducing vaccines that are already approved and shown to be safe can thus become a tool for “bridge vaccination” to mitigate the consequences for health care and the economy in the beginning of a future pandemic (Figure 4 ). Second, they suggest that induction of trained immunity may improve the quality of current specific COVID-19 vaccines for which durability of the response is a major weakness. Third, the novel platforms used for the design of the novel anti-COVID-19 vaccines (mRNA, adenovirus-based) are strongly proinflammatory and are likely to induce trained immunity programs. It is important to study their non-specific effects on other infections and on overall health and to harness their potential capacity for inducing both trained immunity as well as specific immune responses.
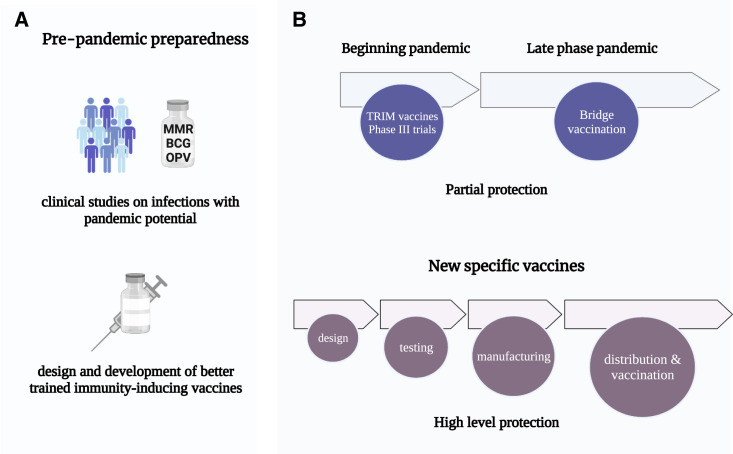
Figure 4.
A framework for using trained immunity-based vaccines in future pandemics
(A) Development of improved vaccines with trained immunity-inducing capacity for pandemic preparedness.
(B) Rapid phase III trials using vaccines with trained immunity-inducing capacity in the beginning of a pandemic with a new pathogen will identify those that can partially protect against infections and/or severity. Such vaccines can be quickly used to diminish the impact of the new pathogen, in parallel with the design, testing, manufacturing, and distribution of specific vaccines that will induce higher levels of protection.
Finally, the current trained immunity-inducing vaccines are not ideal: in fact, only approximately 50% individuals are good responders after BCG vaccination.17 Efforts should be made for the development of better trained immunity-inducing vaccines as a component of pandemic preparedness for the future. Designing novel anti-COVID-19 vaccines that induce both adaptive immunity and trained immunity responses and improved efficacy and safety should be encouraged, as recently shown in experimental studies.100 Only then the entire potential of the immune system would be truly harnessed to protect us against new and dangerous pathogens.
Acknowledgments
E.J.G.-B. has received honoraria from Abbott CH, bioMérieux, Brahms GmbH, GSK, InflaRx GmbH, Sobi, and XBiotech Inc.; independent educational grants from Abbott CH, AbbVie, bioMérieux Inc., InflaRx GmbH, Johnson & Johnson, MSD, Novartis, Sobi, UCB, and XBiotech Inc.; and funding from the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), the Horizon 2020 European Grants ImmunoSep and RISKinCOVID (granted to the Hellenic Institute for the Study of Sepsis), and from the Horizon Europe Project EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis). M.G.N. was partly supported by an ERC Advanced Grant (833247) and a Spinoza Grant of the Netherlands Organization for Scientific Research. M.G.N. and L.A.B.J. are scientific founders of TTxD and Lemba.
Declaration of interests
The authors declare no competing interests.
References
- 1.Jouan Y., Baranek T., Si-Tahar M., Paget C., Guillon A. Lung compartmentalization of inflammatory biomarkers in COVID-19-related ARDS. Crit. Care. 2021;25:120. doi: 10.1186/s13054-021-03513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.E., Katsaounou P., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Veerdonk F.L., Giamarellos-Bourboulis E., Pickkers P., Derde L., Leavis H., van Crevel R., Engel J.J., Wiersinga W.J., Vlaar A.P.J., Shankar-Hari M., et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022;28:39–50. doi: 10.1038/s41591-021-01643-9. [DOI] [PubMed] [Google Scholar]
- 5.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J. Intensive Care. 2020;8 doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulte-Schrepping J., Reusch N., Paclik D., Baßler K., Schlickeiser S., Zhang B., Krämer B., Krammer T., Brumhard S., Bonaguro L., et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440.e23. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Netea M.G., Giamarellos-Bourboulis E.J., Domínguez-Andrés J., Curtis N., van Crevel R., van de Veerdonk F.L., Bonten M. Trained immunity: a tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell. 2020;181:969–977. doi: 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34:431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L.A.B., van der Meer J.W.M., Mhlanga M.M., Mulder W.J.M., et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziogas A., Netea M.G. Trained immunity-related vaccines: innate immune memory and heterologous protection against infections. Trends Mol. Med. 2022;28:497–512. doi: 10.1016/j.molmed.2022.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Domínguez-Andrés J., van Crevel R., Divangahi M., Netea M.G. Designing the next generation of vaccines: relevance for future pandemics. mBio. 2020;11:e02616–e02620. doi: 10.1128/mBio.02616-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benn C.S., Fisker A.B., Whittle H.C., Aaby P. Revaccination with live attenuated vaccines confer additional beneficial nonspecific effects on overall survival: a review. EBioMedicine. 2016;10:312–317. doi: 10.1016/j.ebiom.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleinnijenhuis J., Quintin J., Preijers F., Joosten L.A., Ifrim D.C., Saeed S., Jacobs C., van Loenhout J., de Jong D., Stunnenberg H.G., et al. Bacille calmette-guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cirovic B., de Bree L.C.J., Groh L., Blok B.A., Chan J., van der Velden W.J.F.M., Bremmers M.E.J., van Crevel R., Händler K., Picelli S., et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe. 2020;28:322–334.e5. doi: 10.1016/j.chom.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wimmers F., Donato M., Kuo A., Ashuach T., Gupta S., Li C., Dvorak M., Foecke M.H., Chang S.E., Hagan T., et al. The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell. 2021;184:3915–3935.e21. doi: 10.1016/j.cell.2021.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B., Moorlag S.J., Dominguez-Andres J., Bulut Ö., Kilic G., Liu Z., van Crevel R., Xu C.J., Joosten L.A., Netea M.G., Li Y. Single-cell RNA sequencing reveals induction of distinct trained-immunity programs in human monocytes. J. Clin. Invest. 2022;132 doi: 10.1172/JCI147719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100.e5. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Walk J., de Bree L.C.J., Graumans W., Stoter R., van Gemert G.J., van de Vegte-Bolmer M., Teelen K., Hermsen C.C., Arts R.J.W., Behet M.C., et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-08659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aaby P., Roth A., Ravn H., Napirna B.M., Rodrigues A., Lisse I.M., Stensballe L., Diness B.R., Lausch K.R., Lund N., et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011;204:245–252. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 20.Prentice S., Nassanga B., Webb E.L., Akello F., Kiwudhu F., Akurut H., Elliott A.M., Arts R.J.W., Netea M.G., Dockrell H.M., et al. BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: an investigator-blind randomised controlled trial. Lancet Infect. Dis. 2021;21:993–1003. doi: 10.1016/S1473-3099(20)30653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Neill L.A.J., Netea M.G. BCG-induced trained immunity: can it offer protection against COVID-19? Nat. Rev. Immunol. 2020;20:335–337. doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chumakov K., Avidan M.S., Benn C.S., Bertozzi S.M., Blatt L., Chang A.Y., Jamison D.T., Khader S.A., Kottilil S., Netea M.G., et al. Old vaccines for new infections: exploiting innate immunity to control COVID-19 and prevent future pandemics. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2101718118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilligan K.L., Namasivayam S., Clancy C.S., O'Mard D., Oland S.D., Robertson S.J., Baker P.J., Castro E., Garza N.L., Lafont B.A.P., et al. Intravenous administration of BCG protects mice against lethal SARS-CoV-2 challenge. J. Exp. Med. 2022;219 doi: 10.1084/jem.20211862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Counoupas C., Johansen M.D., Stella A.O., Nguyen D.H., Ferguson A.L., Aggarwal A., Bhattacharyya N.D., Grey A., Hutchings O., Patel K., et al. A single dose, BCG-adjuvanted COVID-19 vaccine provides sterilising immunity against SARS-CoV-2 infection. npj Vaccines. 2021;6 doi: 10.1038/s41541-021-00406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B.Z., Shuai H., Gong H.R., Hu J.C., Yan B., Yuen T.T., Hu Y.F., Yoon C., Wang X.L., Hou Y., et al. Bacillus Calmette-Guerin-induced trained immunity protects against SARS-CoV-2 challenge in K18-hACE2 mice. JCI Insight. 2022;7 doi: 10.1172/jci.insight.157393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh A.K., Wang R., Lombardo K.A., Praharaj M., Bullen C.K., Um P., Davis S., Komm O., Illei P.B., Ordonez A.A., et al. Dynamic single-cell RNA sequencing reveals BCG vaccination curtails SARS-CoV-2 induced disease severity and lung inflammation. bioRxiv. 2022 doi: 10.1101/2022.03.15.484018. Preprint at. [DOI] [Google Scholar]
- 27.Kaufmann E., Khan N., Tran K.A., Ulndreaj A., Pernet E., Fontes G., Lupien A., Desmeules P., McIntosh F., Abow A., et al. BCG vaccination provides protection against IAV but not SARS-CoV-2. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahali N., Bahloul C. Induction of cross-reacting antibodies against the COVID-19 by BCG vaccination in the mouse model. Curr. Microbiol. 2022;79 doi: 10.1007/s00284-022-02971-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Specht A.G., Kurtz S.L., Elkins K.L., Specht H., Beamer G. BCG vaccination of Diversity Outbred mice induces cross-reactive antibodies to SARS-CoV-2 spike protein. bioRxiv. 2022 doi: 10.1101/2022.04.18.488640. Preprint at. [DOI] [Google Scholar]
- 30.White A.D., Sibley L., Sarfas C., Morrison A.L., Bewley K., Churchward C., Fotheringham S., Gkolfinos K., Gooch K., Handley A., et al. Influence of aerosol delivered BCG vaccination on immunological and disease parameters following SARS-CoV-2 challenge in rhesus macaques. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.801799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darrah P.A., Zeppa J.J., Maiello P., Hackney J.A., Wadsworth M.H., 2nd, Hughes T.K., Pokkali S., Swanson P.A., 2nd, Grant N.L., Rodgers M.A., et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577:95–102. doi: 10.1038/s41586-019-1817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg M.K., Yu Q., Salvador C.E., Melani I., Kitayama S. Mandated Bacillus Calmette-Guerin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abc1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivas M.N., Ebinger J.E., Wu M., Sun N., Braun J., Sobhani K., Van Eyk J.E., Cheng S., Arditi M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J. Clin. Invest. 2021;131 doi: 10.1172/JCI145157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escobar L.E., Molina-Cruz A., Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19) Proc. Natl. Acad. Sci. USA. 2020;117:17720–17726. doi: 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindestam Arlehamn C.S., Sette A., Peters B. Lack of evidence for BCG vaccine protection from severe COVID-19. Proc. Natl. Acad. Sci. USA. 2020;117:25203–25204. doi: 10.1073/pnas.2016733117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamiel U., Kozer E., Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA. 2020;323:2340–2341. doi: 10.1001/jama.2020.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ledesma J.R., Lurie P., Yorlets R.R., Daly G., Chrysanthopoulou S., Lurie M.N. Spurious early ecological association suggesting BCG vaccination effectiveness for COVID-19. PLoS One. 2022;17 doi: 10.1371/journal.pone.0274900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleinnijenhuis J., Quintin J., Preijers F., Benn C.S., Joosten L.A., Jacobs C., van Loenhout J., Xavier R.J., Aaby P., van der Meer J.W., et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014;6:152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rieckmann A., Villumsen M., Sørup S., Haugaard L.K., Ravn H., Roth A., Baker J.L., Benn C.S., Aaby P. Vaccinations against smallpox and tuberculosis are associated with better long-term survival: a Danish case-cohort study 1971–2010. Int. J. Epidemiol. 2017;46:695–705. doi: 10.1093/ije/dyw120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giamarellos-Bourboulis E.J., Tsilika M., Moorlag S., Antonakos N., Kotsaki A., Domínguez-Andrés J., Kyriazopoulou E., Gkavogianni T., Adami M.E., Damoraki G., et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183:315–323.e9. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ten Doesschate T., van der Vaart T.W., Debisarun P.A., Taks E., Moorlag S.J.C.F.M., Paternotte N., Boersma W.G., Kuiper V.P., Roukens A.H.E., Rijnders B.J.A., et al. Bacillus Calmette-Guerin vaccine to reduce healthcare worker absenteeism in COVID-19 pandemic, a randomized controlled trial. Clin. Microbiol. Infect. 2022;28:1278–1285. doi: 10.1016/j.cmi.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moorlag S.J.C.F.M., Taks E., Ten Doesschate T., van der Vaart T.W., Janssen A.B., Müller L., Ostermann P., Dijkstra H., Lemmers H., Simonetti E., et al. Efficacy of BCG vaccination against respiratory tract infections in older adults during the coronavirus disease 2019 pandemic. Clin. Infect. Dis. 2022;75:e938–e946. doi: 10.1093/cid/ciac182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Upton C.M., van Wijk R.C., Mockeliunas L., Simonsson U.S.H., McHarry K., van den Hoogen G., Muller C., von Delft A., van der Westhuizen H.M., van Crevel R., et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. EClinicalmedicine. 2022;48 doi: 10.1016/j.eclinm.2022.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsilika M., Taks E., Dolianitis K., Kotsaki A., Leventogiannis K., Damoulari C., Kostoula M., Paneta M., Adamis G., Papanikolaou I., et al. ACTIVATE-2: A double-blind randomized trial of BCG vaccination against COVID-19 in individuals at risk. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.873067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koekenbier E.L., Fohse K., van de Maat J.S., Oosterheert J.J., van Nieuwkoop C., Hoogerwerf J.J., Grobusch M.P., van den Bosch M.A.A.J., van de Wijgert J.H.H., Netea M.G., et al. BCG-PRIME study group Bacillus Calmette-Guerin vaccine for prevention of COVID-19 and other respiratory tract infections in older adults with comorbidities: a randomized controlled trial. Clin. Microbiol. Infect. 2023 doi: 10.1016/j.cmi.2023.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dos Anjos L.R.B., da Costa A.C., Cardoso A.D.R.O., Guimarães R.A., Rodrigues R.L., Ribeiro K.M., Borges K.C.M., Carvalho A.C.O., Dias C.I.S., Rezende A.O., et al. Efficacy and safety of BCG revaccination with M. bovis BCG Moscow to prevent COVID-19 infection in health care workers: A randomized Phase II clinical trial. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.841868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pittet L.F., Messina N.L., Orsini F., Moore C.L., Abruzzo V., Barry S., Bonnici R., Bonten M., Campbell J., Croda J., et al. Randomized trial of BCG vaccine to protect against Covid-19 in health care workers. N. Engl. J. Med. 2023;388:1582–1596. doi: 10.1056/NEJMoa2212616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czajka H., Zapolnik P., Krzych Ł., Kmiecik W., Stopyra L., Nowakowska A., Jackowska T., Darmochwał-Kolarz D., Szymański H., Radziewicz-Winnicki I., et al. A multi-center, randomised, double-blind, placebo-controlled Phase III clinical trial evaluating the impact of BCG re-vaccination on the incidence and severity of SARS-CoV-2 infections among symptomatic healthcare professionals during the COVID-19 pandemic in Poland-First results. Vaccines (Basel) 2022;10 doi: 10.3390/vaccines10020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faustman D.L., Lee A., Hostetter E.R., Aristarkhova A., Ng N.C., Shpilsky G.F., Tran L., Wolfe G., Takahashi H., Dias H.F., et al. Multiple BCG vaccinations for the prevention of COVID-19 and other infectious diseases in type 1 diabetes. Cell Rep. Med. 2022;3 doi: 10.1016/j.xcrm.2022.100728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jalalizadeh M., Buosi K., Dionato F.A.V., Dal Col L.S.B., Giacomelli C.F., Ferrari K.L., Pagliarone A.C., Leme P.A.F., Maia C.L., Yadollahvandmiandoab R., et al. Randomized clinical trial of BCG vaccine in patients with convalescent COVID-19: clinical evolution, adverse events, and humoral immune response. J. Intern. Med. 2022;292:654–666. doi: 10.1111/joim.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinha S., Ajayababu A., Thukral H., Gupta S., Guha S.K., Basu A., Gupta G., Thakur P., Lingaiah R., Das B.K., et al. Efficacy of Bacillus Calmette-Guerin (BCG) vaccination in reducing the incidence and severity of COVID-19 in high-risk population (BRIC): a Phase III, multi-centre, quadruple-blind randomised control trial. Infect. Dis. Ther. 2022;11:2205–2217. doi: 10.1007/s40121-022-00703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blossey A.M., Bruckner S., May M., Parzmair G.P., Sharma H., Shaligram U., Grode L., Kaufmann S.H.E., Netea M.G., Schindler C. VPM1002 as prophylaxis against Severe respiratory tract Infections Including COVID-19 in the Elderly: a phase III randomised, double-blind, placebo-controlled, multicenter clinical study. Clin. Infect. Dis. 2022;76:1304–1310. doi: 10.1093/cid/ciac881. [DOI] [PubMed] [Google Scholar]
- 53.Jaiswal S.R., Arunachalam J., Saifullah A., Lakhchaura R., Tailor D., Mehta A., Bhagawati G., Aiyer H., Khamar B., Malhotra S.V., Chakrabarti S. Impact of an Immune Modulator Mycobacterium-w on Adaptive natural killer cells and Protection against COVID-19. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.887230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemes E., Geldenhuys H., Rozot V., Rutkowski K.T., Ratangee F., Bilek N., Mabwe S., Makhethe L., Erasmus M., Toefy A., et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N. Engl. J. Med. 2018;379:138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mangtani P., Abubakar I., Ariti C., Beynon R., Pimpin L., Fine P.E., Rodrigues L.C., Smith P.G., Lipman M., Whiting P.F., Sterne J.A. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin. Infect. Dis. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 56.Wilson M.E., Fineberg H.V., Colditz G.A. Geographic latitude and the efficacy of bacillus Calmette-Guerin vaccine. Clin. Infect. Dis. 1995;20:982–991. doi: 10.1093/clinids/20.4.982. [DOI] [PubMed] [Google Scholar]
- 57.Aaby P., Netea M.G., Benn C.S. Beneficial non-specific effects of live vaccines against COVID-19 and other unrelated infections. Lancet Infect. Dis. 2023;23:e34–e42. doi: 10.1016/S1473-3099(22)00498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pavan Kumar N., Padmapriyadarsini C., Rajamanickam A., Marinaik S.B., Nancy A., Padmanaban S., Akbar N., Murhekar M., Babu S. Effect of BCG vaccination on proinflammatory responses in elderly individuals. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abg7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Messina N.L., Germano S., McElroy R., Rudraraju R., Bonnici R., Pittet L.F., Neeland M.R., Nicholson S., Subbarao K., Curtis N., et al. Off-target effects of bacillus Calmette-Guerin vaccination on immune responses to SARS-CoV-2: implications for protection against severe COVID-19. Clin. Transl. Immunology. 2022;11 doi: 10.1002/cti2.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar N.P., Padmapriyadarsini C., Rajamanickam A., Bhavani P.K., Nancy A., Jayadeepa B., Selvaraj N., Asokan D., Renji R.M., Venkataramani V., et al. BCG vaccination induces enhanced frequencies of memory T cells and altered plasma levels of common gammac cytokines in elderly individuals. PLoS One. 2021;16 doi: 10.1371/journal.pone.0258743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Higgins J.P., Soares-Weiser K., López-López J.A., Kakourou A., Chaplin K., Christensen H., Martin N.K., Sterne J.A., Reingold A.L. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355 doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fedrizzi E.N., Girondi J.B.R., Sakae T.M., Steffens S.M., Silvestrin A.N.d.S., Claro G.S., Iskenderian H.A., Hillmann B., Gervasi L., Trapani A., et al. Efficacy of the measles-mumps-rubella (MMR) vaccine in the reducing the severity of Covid-19: an interim analysis of a randomised controlled clinical trial. medRxiv. 2022 doi: 10.1101/2021.09.14.21263598. Preprint at. [DOI] [Google Scholar]
- 63.Chumakov M.P., Voroshilova M.K., Antsupova A.S., Boĭko V.M., Blinova M.I., Priĭmiagi L.S., Rodin V.I., Seĭbil’ V.B., Siniak K.M., Smorodintsev A.A. Live enteroviral vaccines for the emergency nonspecific prevention of mass respiratory diseases during fall-winter epidemics of influenza and acute respiratory diseases. Zh. Mikrobiol. Epidemiol. Immunobiol. 1992;11–12:37–40. [PubMed] [Google Scholar]
- 64.Lund N., Andersen A., Hansen A.S., Jepsen F.S., Barbosa A., Biering-Sørensen S., Rodrigues A., Ravn H., Aaby P., Benn C.S. The effect of oral polio vaccine at birth on infant mortality: A randomized trial. Clin. Infect. Dis. 2015;61:1504–1511. doi: 10.1093/cid/civ617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Habibzadeh F., Sajadi M.M., Chumakov K., Yadollahie M., Kottilil S., Simi A., Stafford K., Saeidimehr S., Rafiei M., Gallo R.C. COVID-19 infection among women in Iran exposed vs unexposed to children who received attenuated poliovirus used in oral polio vaccine. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.35044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yagovkina N.V., Zheleznov L.M., Subbotina K.A., Tsaan A.A., Kozlovskaya L.I., Gordeychuk I.V., Korduban A.K., Ivin Y.Y., Kovpak A.A., Piniaeva A.N., et al. Vaccination with oral polio vaccine reduces COVID-19 incidence. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.907341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fisker A.B., Martins J.S.D., Nanque L.M., Jensen A.M., Ca E.J.C., Nielsen S., Martins C.L., Rodrigues A. Oral polio vaccine to mitigate the risk of illness and mortality during the coronavirus disease 2019 pandemic: A cluster-randomized trial in Guinea-Bissau. Open Forum Infect. Dis. 2022;9 doi: 10.1093/ofid/ofac470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao Y., Lidsky P.V., Shirogane Y., Aviner R., Wu C.T., Li W., Zheng W., Talbot D., Catching A., Doitsh G., et al. A defective viral genome strategy elicits broad protective immunity against respiratory viruses. Cell. 2021;184:6037–6051.e14. doi: 10.1016/j.cell.2021.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Debisarun P.A., Gössling K.L., Bulut O., Kilic G., Zoodsma M., Liu Z., Oldenburg M., Rüchel N., Zhang B., Xu C.J., et al. Induction of trained immunity by influenza vaccination - impact on COVID-19. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Almadhoon H.W., Hamdallah A., Elsayed S.M., Hagrass A.I., Hasan M.T., Fayoud A.M., Al-Kafarna M., Elbahnasawy M., Alqatati F., Ragab K.M., et al. The effect of influenza vaccine in reducing the severity of clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-18618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tayar E., Abdeen S., Alah M.A., Chemaitelly H., Bougmiza I., Ayoub H.H., Kaleeckal A.H., Latif A.N., Shaik R.M., Al-Romaihi H.E., et al. Effectiveness of influenza vaccination against SARS-CoV-2 infection among healthcare workers in Qatar. medRxiv. 2022 doi: 10.1101/2022.05.09.22274802. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fink G., Orlova-Fink N., Schindler T., Grisi S., Ferrer A.P.S., Daubenberger C., Brentani A. Inactivated trivalent influenza vaccination is associated with lower mortality among patients with COVID-19 in Brazil. BMJ Evid. Based Med. 2020 doi: 10.1136/bmjebm-2020-111549. [DOI] [PubMed] [Google Scholar]
- 73.Bruxvoort K.J., Ackerson B., Sy L.S., Bhavsar A., Tseng H.F., Florea A., Luo Y., Tian Y., Solano Z., Widenmaier R., et al. Recombinant adjuvanted zoster vaccine and reduced risk of coronavirus disease 2019 diagnosis and hospitalization in older adults. J. Infect. Dis. 2022;225:1915–1922. doi: 10.1093/infdis/jiab633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ndeupen S., Qin Z., Jacobsen S., Bouteau A., Estanbouli H., Igyártó B.Z. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24 doi: 10.1016/j.isci.2021.103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arunachalam P.S., Scott M.K.D., Hagan T., Li C., Feng Y., Wimmers F., Grigoryan L., Trisal M., Edara V.V., Lai L., et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature. 2021;596:410–416. doi: 10.1038/s41586-021-03791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li C., Lee A., Grigoryan L., Arunachalam P.S., Scott M.K.D., Trisal M., Wimmers F., Sanyal M., Weidenbacher P.A., Feng Y., et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 2022;23:543–555. doi: 10.1038/s41590-022-01163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X., Peng K., Cheng F.W.T., Lam D.C.L., Cheung C.L., Chui C.S.L., Lai F.T.T., Wan E.Y.F., Wong C.K.H., Ma T., et al. Tuberculosis following two-dose SARS-CoV-2 vaccination with messenger RNA vaccine (BNT162b2) and inactivated virus vaccine (CoronaVac) J. Infect. 2022;86:256–308. doi: 10.1016/j.jinf.2022.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Le Vu S., Bertrand M., Jabagi M.J., Botton J., Drouin J., Baricault B., Weill A., Dray-Spira R., Zureik M. Age and sex-specific risks of myocarditis and pericarditis following Covid-19 messenger RNA vaccines. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-31401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zimmermann P., Donath S., Perrett K.P., Messina N.L., Ritz N., Netea M.G., Flanagan K.L., van der Klis F.R.M., Curtis N., MIS BAIR group The influence of neonatal Bacille Calmette-Guérin (BCG) immunisation on heterologous vaccine responses in infants. Vaccine. 2019;37:3735–3744. doi: 10.1016/j.vaccine.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 80.Nissen T.N., Birk N.M., Smits G., Jeppesen D.L., Stensballe L.G., Netea M.G., van der Klis F., Benn C.S., Pryds O., Calmette Study Group Bacille calmette-guerin (BCG) vaccination at birth and antibody responses to childhood vaccines. A randomised clinical trial. Vaccine. 2017;35:2084–2091. doi: 10.1016/j.vaccine.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 81.Leentjens J., Kox M., Stokman R., Gerretsen J., Diavatopoulos D.A., van Crevel R., Rimmelzwaan G.F., Pickkers P., Netea M.G. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: A randomized, placebo-controlled pilot study. J. Infect. Dis. 2015;212:1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- 82.Blok B.A., Arts R.J.W., van Crevel R., Aaby P., Joosten L.A.B., Benn C.S., Netea M.G. Differential effects of BCG vaccine on immune responses induced by vi polysaccharide typhoid fever vaccination: an explorative randomized trial. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1177–1184. doi: 10.1007/s10096-020-03813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma J., Tian M., Fan X., Yu Q., Jing Y., Wang W., Li L., Zhou Z. Mycobacterium tuberculosis multistage antigens confer comprehensive protection against pre- and post-exposure infections by driving Th1-type T cell immunity. Oncotarget. 2016;7:63804–63815. doi: 10.18632/oncotarget.11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu Y., Cai M., Ma J., Teng X., Tian M., Bassuoney E.B.M.B., Fan X. Heterologous boost following Mycobacterium bovis BCG reduces the late persistent, rather than the early stage of intranasal tuberculosis challenge infection. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.02439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramos-Martinez E., Falfán-Valencia R., Pérez-Rubio G., Andrade W.A., Rojas-Serrano J., Ambrocio-Ortiz E., Galicia-Álvarez D.S., Bárcenas-Montiel I., Velasco-Medina A., Velázquez-Sámano G. Effect of BCG revaccination on occupationally exposed medical personnel vaccinated against SARS-CoV-2. Cells. 2021;10 doi: 10.3390/cells10113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rakshit S., Adiga V., Ahmed A., Parthiban C., Kumar N., Shivalingaiah S., Rao S., D'Souza G., Dwarkanath P., Dias M., et al. BCG revaccination qualitatively and quantitatively enhances SARS-CoV-2 spike-specific neutralizing antibody and T cell responses induced by the COVISHIELDTM vaccine in SARS-CoV-2 seronegative young Indian adults. Res Sq. 2022 doi: 10.21203/rs.3.rs-1395683/v1. [DOI] [Google Scholar]
- 87.You M., Chen L., Zhang D., Zhao P., Chen Z., Qin E.Q., Gao Y., Davis M.M., Yang P. Single-cell epigenomic landscape of peripheral immune cells reveals establishment of trained immunity in individuals convalescing from COVID-19. Nat. Cell Biol. 2021;23:620–630. doi: 10.1038/s41556-021-00690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheong J.-G., Ravishankar A., Sharma S., Parkhurst C.N., Nehar-Belaid D., Ma S., Paddock L., Fatou B., Karakaslar O., Thibodeau A., et al. Epigenetic memory of COVID-19 in innate immune cells and their progenitors. bioRxiv. 2022 doi: 10.1101/2022.02.09.479588. Preprint at. [DOI] [Google Scholar]
- 89.Ryan F.J., Hope C.M., Masavuli M.G., Lynn M.A., Mekonnen Z.A., Yeow A.E.L., Garcia-Valtanen P., Al-Delfi Z., Gummow J., Ferguson C., et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022;20:26. doi: 10.1186/s12916-021-02228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phetsouphanh C., Darley D.R., Wilson D.B., Howe A., Munier C.M.L., Patel S.K., Juno J.A., Burrell L.M., Kent S.J., Dore G.J., et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022;23:210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 91.Pandori W.J., Padgett L.E., Alimadadi A., Gutierrez N.A., Araujo D.J., Huh C.J., Olingy C.E., Dinh H.Q., Wu R., Vijayanand P., et al. Single-cell immune profiling reveals long-term changes in myeloid cells and identifies a novel subset of CD9(+) monocytes associated with COVID-19 hospitalization. J. Leukoc. Biol. 2022;112:1053–1063. doi: 10.1002/JLB.4COVA0122-076R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vijayakumar B., Boustani K., Ogger P.P., Papadaki A., Tonkin J., Orton C.M., Ghai P., Suveizdyte K., Hewitt R.J., Desai S.R., et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity. 2022;55:542–556.e5. doi: 10.1016/j.immuni.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peart Akindele N., Kouo T., Karaba A.H., Gordon O., Fenstermacher K.Z.J., Beaudry J., Rubens J.H., Atik C.C., Zhou W., Ji H., et al. Distinct cytokine and chemokine dysregulation in hospitalized children with acute coronavirus disease 2019 and multisystem inflammatory syndrome with similar levels of nasopharyngeal severe acute respiratory syndrome coronavirus 2 shedding. J. Infect. Dis. 2021;224:606–615. doi: 10.1093/infdis/jiab285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Cevins C., Luka M., Smith N., Meynier S., Magérus A., Carbone F., García-Paredes V., Barnabei L., Batignes M., Boullé A., et al. A monocyte/dendritic cell molecular signature of SARS-CoV-2-related multisystem inflammatory syndrome in children with severe myocarditis. Med. 2021;2:1072–1092.e7. doi: 10.1016/j.medj.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng M.H., Zhang S., Porritt R.A., Noval Rivas M., Paschold L., Willscher E., Binder M., Arditi M., Bahar I. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc. Natl. Acad. Sci. USA. 2020;117:25254–25262. doi: 10.1073/pnas.2010722117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chalasani V., Parameswaran P., Cherico A., Villgran V., Lowther H., Marco J. SARS-CoV-2 multisystem inflammatory syndrome in an adult presenting with polyarthritis treated with anakinra. Rheumatol. Oxf. Engl. 2022;61:e33–e34. doi: 10.1093/rheumatology/keab697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cattaneo P., Volpe A., Cardellino C.S., Riccardi N., Bertoli G., Ursini T., Ustalli V., Lodi G., Daroui I., Angheben A. Multisystem inflammatory syndrome in an adult (MIS-A) successfully treated with anakinra and glucocorticoids. Microorganisms. 2021;9 doi: 10.3390/microorganisms9071393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hupert N., Marín-Hernández D., Gao B., Águas R., Nixon D.F. Heterologous vaccination interventions to reduce pandemic morbidity and mortality: modeling the US winter 2020 COVID-19 wave. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2025448119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang A.Y., Aaby P., Avidan M.S., Benn C.S., Bertozzi S.M., Blatt L., Chumakov K., Khader S.A., Kottilil S., Nekkar M., et al. One vaccine to counter many diseases? Modeling the economics of oral polio vaccine against child mortality and COVID-19. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.967920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Afkhami S., D'Agostino M.R., Zhang A., Stacey H.D., Marzok A., Kang A., Singh R., Bavananthasivam J., Ye G., Luo X., et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell. 2022;185:896–915.e19. doi: 10.1016/j.cell.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]