Hematopoiesis is the process of blood cell formation which defines the immune system of an organism. The hematopoietic stem cells (HSCs) form the precursor cells which undergo a yin yang mode of self-renewal and differentiation to form mature blood cells (1). These decisions of self-renewal or differentiation by the HSCs are guided by signals primarily derived from the bone marrow niche. The bone marrow niche is a shelter home for diverse hematopoietic and nonhematopoietic cell populations, whereby the latter supports the sustenance, differentiation, and migration of HSCs using secreted signaling factors (2). It is now increasingly evident that the biophysical environment of the bone marrow niche also contributes to shaping the function of HSCs. In order to sense the physical features of the external environment like the hydrostatic pressure generated by blood flow and extracellular matrix stiffness, the bone marrow niche cells need to rely upon mechanosensors (3, 4). The identification of these mechanoreceptors in the bone marrow niche and how it regulates the fate of HSCs is an area of active investigation (4, 5).
The physical properties of the niche influencing HSC behavior and the underlying mode of mechanotransduction form the central area of investigation undertaken in the current study in PNAS by Tian et al., 2023. Specifically, the authors delineate the mechanistic underpinnings of force generated by blood flow, its sensing, and subsequent signals relayed by the vascular niche to coordinate maintenance and differentiation of the stem-like multipotent blood progenitor cells during Drosophila larval hematopoiesis (6). The implications of disruption in this pathway on altered hematopoiesis are strikingly evident.
Drosophila hematopoiesis has provided important insights into the conserved molecular mechanisms that regulate blood cell development in both insects and vertebrates (7). It has also been used as a tool for studying various aspects of immunity, including the host response to infections and the mechanisms of immune evasion by pathogens (8). During the larval stages, the definitive wave of hematopoiesis gives rise to the development of the hematopoietic organ of Drosophila called the lymph gland. It harbors the blood progenitor cells in the medullary zone, and the differentiated blood cells or hemocytes comprising plasmatocytes, crystal cells, and lamellocytes are found in the cortical zone (CZ). The posterior signaling center (PSC) forms an in-house signaling niche that regulates blood progenitor development versus differentiation by emanating signaling factors, similar to the vertebrate bone marrow niche (7, 9). Interestingly, a second hematopoietic niche formed by the cardiac cells was previously identified by this group (10). The rationale for this identification came from the architecture of the lymph gland wherein the lobes of the tissue are seen to be attached along the cardiac tube. It was found that the cardiac cells express the Fibroblast Growth Fact (FGF) pathway ligand, branchless (bnl), which is sensed by the blood progenitors of the lymph gland to support its maintenance non-cell autonomously (10). The current study by Tian et.al. is an extension of their previous finding (10), wherein the authors pursue their understanding of cardiac cells as the hematopoietic niche and investigate the relevance of blood flow pumped by these cardiac cells toward blood progenitor maintenance. Using a combination of genetic and imaging-based approaches, the authors unravel the molecular mechanism by which the cardiac cells of the vascular niche sense the hydrostatic pressure of blood flow to relay bnl as the non autonomous signal to moderate progenitor maintenance. They found that cardiac cells express Piezo channel which senses mechanical forces resulting from blood flow through the cardiac tube (Fig. 1). Downstream of Piezo sensing, upregulation of intracellular Ca2+ signaling leading to Notch activation, and transcriptional repression of bnl expression in cardiac cells, the mechanical information is transduced into moderating FGF signaling in blood progenitor cells of the lymph gland. Finally, the authors also highlight the physiological relevance of this mechanism wherein a temporal regulation on blood progenitor differentiation is achieved by calibrating bnl expression in cardiac cells, under the modulation of heartbeat rate (6). Overall, through a series of carefully done genetic perturbations and rescue experiments, Tian et.al. carve out a unique pathway that bridges hydrostatic pressure of blood flow sensing via Piezo and its non autonomous regulation on blood progenitor maintenance, which constitutes the central and key message from this study (Fig. 1).
Fig. 1.
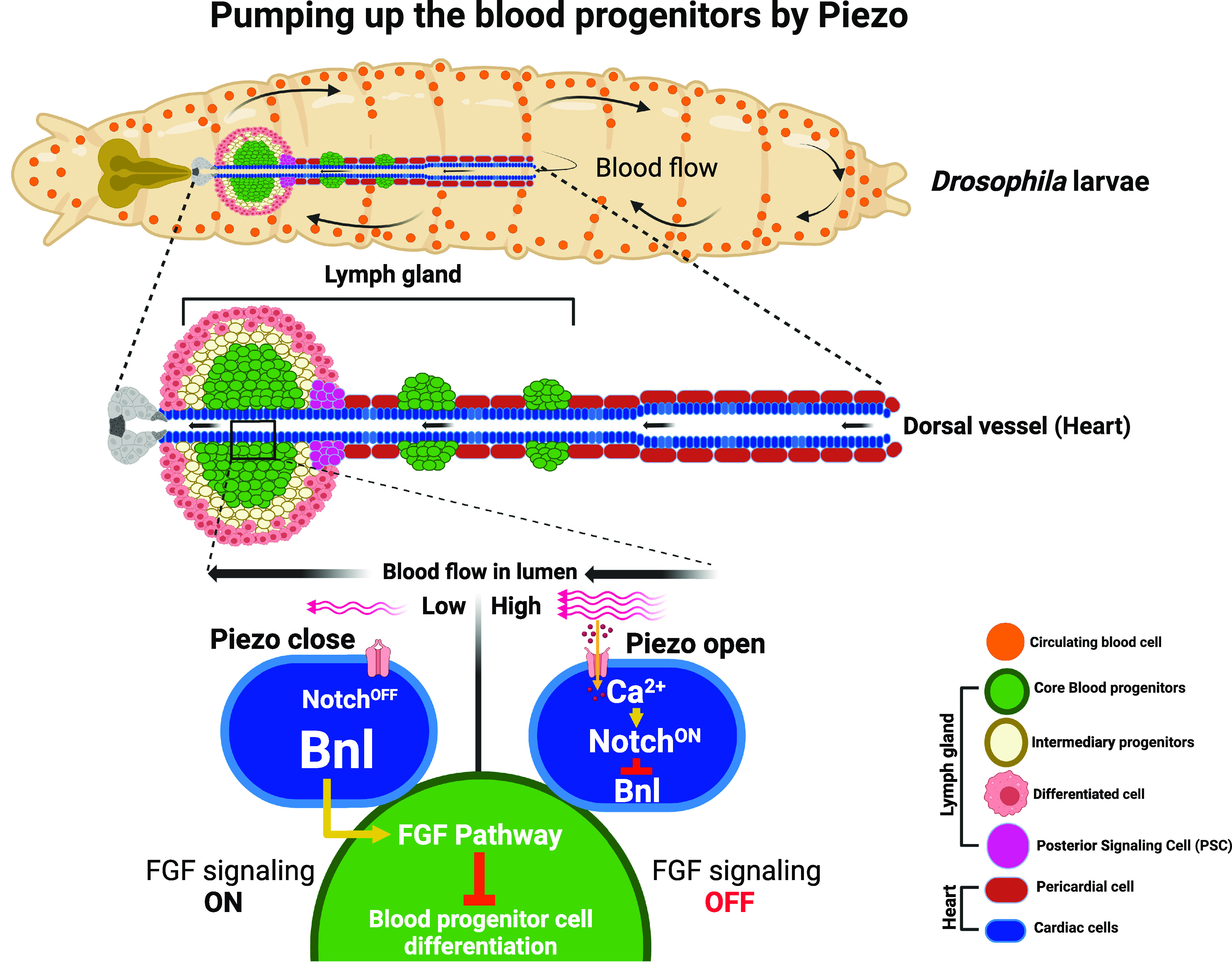
The model highlights the overall result presented by Tian et al., in which the authors show that during Drosophila larval development, in addition to signals emanating from the posterior signaling center (PSC, magenta), mechanosensing of blood flow dynamics by the cardiac cells (blue) of the hematopoietic vascular niche mediates a non autonomous control on the development of hematopoietic progenitor cells (green) of the lymph gland. Piezo-mediated sensing of hydrostatic pressure of blood flow by the vascular niche cells leads to moderation of branchless (bnl) expression in them. Consequently, it leads to non autonomous control of FGF signaling pathway in the blood progenitor cells, whose function sustains progenitor homeostasis.
Belonging to the family of mechanically activated ion channels, Piezo was identified by Ardem Patapoutian and his group and was named from the Greek word “píesi” meaning pressure (11). A lineage of studies thereafter emerged in probing the functions of Piezo channels using both vertebrate and invertebrate model systems. This led to the identification of various physiological processes employing Piezo, such as touch sensing, vascular development, proprioception, and blood pressure (12). The implications of Piezo in governing blood development or hematopoiesis have attracted researchers to investigate this avenue in detail (3). The findings present in this study underscore the importance of Piezo sensing of mechanical forces during animal development and its consequence on blood progenitor cells. The requirement of Piezo in this process is very timely, given the growing curiosity overall in blood research to comprehend mechanosensing and its implications on blood cell physiology. As Piezo is shown to respond to blood flow for downstream progenitor maintenance, it would be interesting to know the temporal pattern of Piezo expression. This would provide a developmental perspective of Piezo function and help tie age-related consequences on Piezo expression with blood progenitor function.
The quest to identify factors guiding the stemness of HSCs is an ancient problem but a valuable one, as it helps understand the mechanisms underlying the sustenance of this precious precursor population. In this context, the importance of the niche in HSC maintenance is well established and significant efforts have been made to understand the nature and composition of the bone marrow niche that drive HSC development. While the chemical aspect of the niche which includes the signaling molecules released by the resident cells is well appreciated, it is becoming imperative to also understand the biomechanical mode of niche regulation for blood development. The intricate relationship between blood circulation and emergence of HSCs during early embryo development (13) and the hemodynamic forces that influence endothelial cells of the niche, lining the vasculature (14), are examples that showcase mechanical forces influencing HSC development. It is also noted that HSCs themselves have mechanosensors to sense the mechanical force, thereby autonomously impinging on its downstream function (15). Thus, probing into mechanisms and the functions that are regulated by this paradigm and the understanding of a mechanosensory-based regulation of HSC state and function will funnel researchers toward engineering functional HSCs in a dish (16). It would also not be overarching to predict that mechanosensation may represent a unique target for therapeutic intervention in blood-related disorders (3). Finally, cues of sensory, neuronal, and nutritional origin have been shown to impact hematopoietic progenitor homeostasis (17–20). The current study further extends sensory input of mechanical nature in hematopoietic homeostasis. Given the intricate relationship between animal physiology, metabolism, and neuronal signaling on cardiac physiology and blood flow, examining how changes in any of these parameters influence biomechanical properties of the hematopoietic microenvironment and thereby influence hematopoietic progenitor development will be important to address. This understanding will not only reveal insights into the development of a functional hematopoietic system but will also reveal how systemic changes and metabolic conditions such as diabetes, obesity, and neurodegeneration, to name a few, lead to hematologic dysfunction and pathologies.
Specifically, the authors delineate the mechanistic underpinnings of force generated by blood flow, its sensing and subsequent signals relayed by the vascular niche to coordinate maintenance and differentiation of the stem-like multipotent blood progenitor cells during Drosophila larval hematopoiesis.
While there is enough body of work that implicates mechanical forces in regulating hematopoietic stem and progenitor development, the underlying processes and physiology governing this phenomenon remain poorly understood. The current study in PNAS by Tian et al. is an endeavor in this direction. It fills a void in our understanding and provides mechanistic insight into mechanosensing to mechanotransduction by the vascular niche and its modulation on the development of hematopoietic progenitor cells in Drosophila.
Acknowledgments
Author contributions
A. Kapoor, A. Kumar, and T.M. performed research; and wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
See companion article, “A mechanosensitive vascular niche for Drosophila hematopoiesis,” 10.1073/pnas.2217862120.
References
- 1.Orkin S. H., Zon L. I., Hematopoiesis: An evolving paradigm for stem cell biology. Cell 132, 631–644 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiel M. J., Morrison S. J., Maintaining hematopoietic stem cells in the vascular Niche. Immunity 25, 862–864 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Horton P. D., Dumbali S., Wenzel P. L., Mechanoregulation in hematopoiesis and hematologic disorders. Curr. Stem. Cell Rep. 6, 86–95 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horton P. D., Dumbali S. P., Bhanu K. R., Diaz M. F., Wenzel P. L., Biomechanical regulation of hematopoietic stem cells in the developing embryo. Curr. Tissue Microenviron. Rep. 2, 1–15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang P., et al. , The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res. Ther. 10, 327 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian Y., Morin-Poulard I., Vanzo N., A mechanosensitive vascular niche for Drosophila hematopoiesis. Proc. Natl. Acad. Sci. U.S.A. 120, e2217862120 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee U., Girard J. R., Goins L. M., Spratford C. M., Drosophila as a genetic model for hematopoiesis. Genetics 211, 367–417 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letourneau M., et al. , Drosophila hematopoiesis under normal conditions and in response to immune stress. FEBS Lett. 590, 4034–4051 (2018), 10.1002/1873-3468.12327. [DOI] [PubMed] [Google Scholar]
- 9.Jung S. H., Evans C. J., Uemura C., Banerjee U., The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132, 2521–2533 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Destalminil-Letourneau M., Morin-Poulard I., Tian Y., Vanzo N., Crozatier M., The vascular niche controls drosophila hematopoiesis via fibroblast growth factor signaling. Elife 10, 1–21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coste B., et al. , Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo J., et al. , Trends in piezo channel research over the past decade: A bibliometric analysis. Front. Pharmacol. 12, 668714 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamo L., et al. , Biomechanical forces promote embryonic haematopoiesis. Nature 459, 1131–1135 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H. J., Li N., Evans S. M., Diaz M. F., Wenzel P. L., Biomechanical force in blood development: Extrinsic physical cues drive pro-hematopoietic signaling. Differentiation 86, 92–103 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramanathan G., Mannhalter C., Increased expression of transient receptor potential canonical 6 (TRPC6) in differentiating human megakaryocytes. Cell Biol. Int. 40, 223–231 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scapin G., Shah D. I., Pulsation activates mechanosensitive Piezo1 to form long-term hematopoietic stem cells. Blood 134, 445–445 (2019).31167801 [Google Scholar]
- 17.Shim J., Mukherjee T., Banerjee U., Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat. Cell Biol. 14, 394–400 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shim J., et al. , Olfactory control of blood progenitor maintenance. Cell 155, 1143–1153 (2018), 10.1016/j.cell.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benmimoun B., Polesello C., Waltzer L., Haenlin M., Dual role for insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development 139, 1713–1717 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Makhijani K., Alexander B., Tanaka T., Rulifson E., Brückner K., The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 138, 5379–5391 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]


