Abstract
Background:
Age of asthma onset has emerged as an important determinant of asthma phenotypes; however, the comorbidities that predominate in either childhood- or adult-onset asthma are not known.
Objective:
To identify comorbidities associated with adult-onset asthma vs childhood-onset asthma and with age of asthma diagnosis.
Methods:
We analyzed data on 27,437 adult participants in the National Health and Nutrition Examination Surveys conducted from 2001 to 2018. Logistic regression adjusted for covariates was used to identify comorbidities associated with the asthma phenotypes and age of asthma diagnosis.
Results:
Approximately 12.6% of participants were ever diagnosed with asthma; the prevalence of childhood-onset (before 18 years old) and adult-onset (≥ 18 years old) current asthma was 2.7% and 5.5%, respectively. After adjustment for covariates including age, adult-onset asthma was associated with higher odds of obesity (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.09–1.96), hypercholesterolemia (OR, 1.67; 95% CI, 1.08–2.56), borderline high serum triglycerides (OR, 1.78; 95% CI, 1.17–2.71), and osteoarthritis (OR, 1.52; 95% CI, 1.042.20) than was childhood-onset asthma. Older age of asthma diagnosis (per 5-year increase) was also associated with higher odds of diabetes (OR, 1.04; 95% CI, 1.00–1.07) and hypertension (OR, 1.05; 95% CI, 1.02–1.07), whereas younger age of asthma diagnosis was associated with higher odds of chronic obstructive pulmonary disease (OR, 1.12; 95% CI, 1.04–1.19).
Conclusion:
Age- and covariates-adjusted prevalence of obesity, dyslipidemia, arthritis, diabetes, and hypertension is higher in adult-onset asthma than in childhood-onset asthma, and with older age of asthma diagnosis. Conversely, the prevalence of chronic obstructive pulmonary disease increases with younger age of asthma diagnosis.
Introduction
Asthma is a condition characterized by airway inflammation and bronchial hyperresponsiveness, causing reversible airflow obstruction and airway wall remodeling.1 Currently, asthma affects approximately 300 million people around the world, is the most common chronic disease in children, and accounted for 461,000 deaths in 2019.2 In the United States, the annual economic cost of the disease is estimated to be more than $56 billion.3 Asthma is a heterogenous disease in which age of onset has emerged as an important determinant of different phenotypes.4 Childhood-onset asthma is approximately 70% atopic, with T-helper-2 (Th2) type of airway inflammation, corticosteroid treatment responsiveness, and a good prognosis, whereas adult-onset asthma is 79 to 88% nonatopic, occurs mainly in women, and is less responsive to corticosteroids, leading to persistent airflow limitation.4 Risk factors for childhood-onset asthma include genetic predisposition and viral respiratory infections, whereas the determinants of adult-onset asthma consist of obesity, occupational exposures, female sex hormones, allergic rhinitis, exposure to cigarette smoke, and stressful life events.5
Comorbidities such as cardiovascular, metabolic, endocrine, respiratory, and psychiatric diseases are prevalent in asthma, owing to chronic inflammation, systemic corticosteroid use, reduced physical activity, and poor sleep.6 These comorbidities may obscure asthma diagnosis by mimicking the symptoms of the disease and can influence management, owing to the effects of the treatments of the comorbidities on asthma or of asthma therapy on the comorbid conditions.6 Despite reports that childhood-onset and adult-onset asthma differ in severity and risk factors, most studies on asthma comorbidities have not considered the age of asthma onset.7 The few existing studies investigated comorbidities in childhood- and adult-onset asthma in comparison with no asthma, and to the best of our knowledge, none examined comorbidities in adult-onset compared with childhood-onset asthma.8–10 Therefore, we proposed to identify comorbidities (1) in childhood- and adult-onset asthma compared with no asthma, (2) in adult-onset asthma compared with childhood-onset asthma, and (3) associated with the age at asthma diagnosis in a large sample representative of the US population.
Methods
Data Source
We used data from the National Health and Nutrition Examination Survey (NHANES) conducted from 2001 to 2018. The NHANES is a continuous survey done by the National Center for Health Statistics of the Centers for Disease Control and Prevention to evaluate the health status of the US noninstitutionalized civilian population.11 It derives a sample representative of the US population, using a complex multistage sampling design. The NHANES protocols were approved by the Institutional Review Boards of the Centers for Disease Control and Prevention and the National Center for Health Statistics, and informed consent was obtained from all participants (details at http://www.cdc.gov/nchs/nhanes/irba98.htm).11
For this analysis, we included adults aged 40 years or older who had data on asthma, comorbidities, and the covariates that were adjusted for. Of the 36,252 NHANES participants aged 40 years or older, 35,742 had data on asthma. After the exclusion of participants with missing data on smoking (N = 5422), family income (N = 2865), and health insurance (N = 18), our final sample size was 27,437.
Asthma Definitions
No asthma was defined by the answer “No” to the question, “Has a doctor or other health professional ever told you that you have asthma?”1 Those who answered “Yes” to the first question and provided the answer “No” to the question, “Do you still have asthma?” were classified as having had past asthma. Participants who responded “Yes” to the second question (“Do you still have asthma?”) and reported an age of asthma diagnosis at 18 years or less were classified as having childhood-onset current asthma. Those who reported an age of asthma diagnosis at 18 years or older were classified as having adult-onset current asthma.
Comorbidities
The comorbidities included in our analysis were obesity, dyslipidemia, diabetes, hypertension, cardiovascular disease (CVD), chronic kidney disease (CKD), arthritis, cancer or cancer history, anemia, depression, and chronic obstructive pulmonary disease (COPD). All of these have previously been associated with asthma because of chronic inflammation, systemic corticosteroid use, reduced physical activity, or frequent exacerbations.
Obesity was defined as a body mass index (weight in kilograms divided by height in meters squared) of 30 kg/m2 or more. Dyslipidemia was determined using well-established cutoffs. Serum total cholesterol was classified into levels less than 200 mg/dL (normal), 200 mg/dL to 239 mg/dL (borderline high), and greater than or equal to 240 mg/dL (high).12 Serum low density lipoprotein (LDL) cholesterol was categorized into levels less than 130 mg/dL (normal), 130 mg/dL to 159 mg/dL (borderline high), and greater than or equal to 160 mg/dL (high).12 Serum triglycerides were classified into levels < 150 mg/dL (normal), 150 mg/dL to 199 mg/dL (borderline high), and ≥ 200 mg/dL (high).12 Diabetes was defined as taking antidiabetic drugs, or hemoglobin A1C ≥6.5%, or fasting plasma glucose ≥ 126 mg/ dL.13 Hypertension was defined by the use of antihypertensive medication, mean systolic blood pressure (of 4 measurements on 2 separate occasions) ≥ 140 mm Hg, or mean diastolic blood pressure ≥ 90 mm Hg.14 CVD was defined by self-reported diagnosis of congestive heart failure, coronary heart disease, angina or angina pectoris, or having had a heart attack or stroke. CKD was defined as glomerular filtration rate < 60; glomerular filtration rate was estimated from the Chronic Kidney Disease Epidemiology Collaboration equation using serum creatinine.15,16 Arthritis and cancer or cancer history were defined by self-reported diagnosis. Anemia was defined as treatment for anemia in the past 3 months or hemoglobin concentration < 12 g/dL in women and < 13 g/dL in men.17 Depressive symptoms were assessed using the Patient Health Questionnaire 9 (PHQ-9), and a score ≥ 10 was suggestive of depression.18 COPD was defined as postbronchodilator forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) ratio < 0.70.17
Spirometry and Fractioned Exhaled Nitric Oxide
Spirometry was performed on participants in NHANES 2007–2012 by trained technicians after a pretest screening questionnaire to determine medical safety. After explanation and demonstration of spirometry procedures, participants performed 5 to 8 FVC maneuvers that were considered acceptable and reproducible based on the American Thoracic Society criteria.11 Our analysis for spirometry included FEV1/FVC ratio, peak expiratory flow (PEF), and forced expiratory flow rate 25%–75% of the FVC (FEF25%–75%). Fractioned exhaled Nitric Oxide (FeNO) was measured using a Food and Drug Administration- approved, hand-held analyzer to detect nitric oxide in exhaled breath that also followed American Thoracic Society and European Respiratory Society equipment recommendations.11
Covariates
The NHANES collected data on age, sex, race and ethnicity, annual household income, cigarette smoking, and health insurance coverage, using questionnaires. Poverty income ratio, which served as a proxy for socioeconomic status, was estimated using guidelines and adjustment for family size, year, and state.19 Past and current smokers were asked about the intensity of smoking (average daily packs of cigarettes smoked) and the duration of smoking (number of years of smoking). Pack-years of smoking were calculated as the number of packs of cigarettes smoked per day multiplied by the number of years.20
Statistical Analysis
Descriptive analyses were performed, and P values for differences in characteristics were calculated using a chi-square test for categorical variables and t tests for the continuous ones. Logistic regression was used to calculate the odds ratio (OR) and 95% confidence interval (CI) for the association with comorbidities, and linear regression was used to estimate regression coefficients (β) and 95% CI for the association with lung function and FeNO. These analyses compared past and current asthma with no asthma and adult-onset current asthma with childhood-onset current asthma, and estimated the comorbidities, lung function impairment, and FeNO-associated age of asthma diagnosis. All models were adjusted for age, poverty income ratio, NHANES survey cycle, and pack-years of cigarette smoking used as continuous variables; and sex, race and ethnicity, and health insurance were used as categorical variables. The analyses were performed in SAS (version 9.4; SAS Institute, Cary, NC), accounting for the NHANES sampling weights and complex survey design to generate nationally representative estimates. P values < .05 were considered statistically significant in all analyses.
Results
Descriptive Results
Our sample consisted of 27,437 participants with a median age of 55 years whose characteristics are shown in Table 1. Asthma prevalence was 4.4% for past asthma, 2.7 for current asthma with childhood onset, and 5.5% for current asthma with adult onset. Participants with past asthma disproportionately lacked health insurance compared with the rest of them. Participants with childhood onset of current asthma were mostly male, non-Hispanic Black, and current smokers, and had the highest prevalence of airflow obstruction, hypertriglyceridemia, rheumatoid arthritis, and COPD of all participants. Those with adult onset of current asthma were older, and mostly non-Hispanic White or former smokers. They had the highest prevalence of obesity, borderline high serum triglycerides, diabetes, hypertension, CVD, CKD, osteoarthritis, other or unknown arthritis, cancer or history of cancer, anemia, and a PHQ-9 score suggestive of depression among all participants (Table 1).
Table 1.
Characteristics of Study Participants Overall and by Asthma Subtypes (N = 27,437)
| Characteristics | All | No asthma | Past asthma | Current asthma |
P value | |
|---|---|---|---|---|---|---|
| Childhood onset | Adult onset | |||||
|
| ||||||
| All participants, % | 100 | 87.4 | 4.4 | 2.7 | 5.5 | |
| Median age (y) | 55 | 55 | 54 | 52 | 57 | |
| Male sex, % | 46.7 | 48.0 | 42.2 | 44.7 | 30.0 | <.001 |
| Race/ethnicity, % | <.001 | |||||
| Non-Hispanic White | 73.6 | 73.5 | 74.0 | 73.3 | 75.9 | |
| Non-Hispanic Black | 10.2 | 10.0 | 11.4 | 14.5 | 10.7 | |
| Mexican American | 9.8 | 10.1 | 9.5 | 7.2 | 7.1 | |
| Other | 6.3 | 6.4 | 5.1 | 5.0 | 6.3 | |
| Median PIR | 3.3 | 3.4 | 3.5 | 3.0 | 2.7 | <.001 |
| Uninsured | 11.7 | 11.9 | 12.0 | 11.6 | 8.1 | .011 |
| Smoking, % | <.001 | |||||
| Current | 17.7 | 17.4 | 17.2 | 23.0 | 21.1 | |
| Former | 27.4 | 27.0 | 30.8 | 26.3 | 31.3 | |
| Obesity, % | 38.2 | 36.8 | 40.8 | 46.0 | 53.4 | <.001 |
| Airflow obstruction | 20.4 | 18.6 | 26.0 | 38.2 | 35.7 | <.001 |
| Blood total cholesterol | .06 | |||||
| Borderline high | 32.8 | 17.2 | 18.9 | 13.4 | 20.4 | |
| High | 17.4 | 33.0 | 31.3 | 30.0 | 32.1 | |
| Serum LDL cholesterol | .38 | |||||
| Borderline high | 23.8 | 23.9 | 26.0 | 23.0 | 22.1 | |
| High | 12.1 | 12.1 | 12.2 | 7.5 | 14.3 | |
| Blood triglycerides | .01 | |||||
| Borderline high | 16.4 | 16.5 | 15.3 | 12.3 | 18.8 | |
| High | 23.3 | 23.0 | 22.6 | 26.9 | 26.3 | |
| Diabetes | 16.3 | 15.8 | 17.4 | 17.9 | 22.7 | <.001 |
| Hypertension | 48.6 | 47.7 | 49.3 | 51.9 | 60.6 | <.001 |
| CVD | 12.8 | 11.9 | 12.5 | 18.4 | 25.0 | <.001 |
| CKD | 13.0 | 12.9 | 11.0 | 11.9 | 16.0 | .03 |
| Arthritis | <.001 | |||||
| Rheumatoid arthritis | 5.4 | 5.1 | 5.2 | 12.1 | 9.1 | |
| Osteoarthritis | 15.7 | 15.0 | 18.6 | 15.5 | 25.9 | |
| Other/Unknown | 14.1 | 13.4 | 14.9 | 17.0 | 22.7 | |
| Cancer | 14.1 | 13.7 | 15.1 | 14.9 | 19.2 | <.001 |
| Anemia | 10.2 | 9.7 | 10.8 | 12.4 | 15.8 | <.001 |
| Depression | 7.7 | 6.8 | 8.8 | 15.4 | 17.1 | <.001 |
| COPD | 1.7 | 1.6 | 2.9 | 2.6 | 1.5 | .02 |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; LDL, low density lipoprotein; PIR, poverty income ratio.
NOTE. Bold indicates significant difference.
Comorbidities in Past and Current Asthma vs No Asthma
Past Asthma vs No Asthma
After adjustment for all covariates and compared with no asthma, past asthma was associated with 20 to 45% higher odds of hypertension (OR, 1.20; 95% CI, 1.03–1.41), CVD (OR, 1.33; 95% CI, 1.06–1.68), osteoarthritis (OR, 1.45; 95% CI, 1.13–1.85), and other or unknown arthritis (OR, 1.31; 95% CI, 1.04–1.66). Past asthma was also associated with higher prevalence of airflow obstruction (OR, 1.74; 95% CI, 1.25–2.43) and COPD (OR, 1.92; 95% CI, 1.22–3.01) (Table 2). We observed reductions in FEV1/FVC ratio (β, −1.75; 95% CI, −2.79 to −0.70), PEF (β, −0.32; 95% CI, −0.53 to −0.11) FEF25%−75% (β, −0.28; 95% CI, −0.43 to −0.12), and higher FeNO (β, 1.88; 95% CI, 0.26–3.50) in participants with past asthma than in those with no asthma (Table 3).
Table 2.
Adjusted Odds Ratios and 95% CI for Association of Past Asthma and Current Childhood and Adult Onsets of Asthma vs no Asthma With Comorbidities, NHANES 2001–2018 (N = 27,437)
| Comorbidities | Past and current asthma vs no asthma | Current asthma vs past asthma | |||
|---|---|---|---|---|---|
|
|
|
||||
| Past asthma | Current asthma | ||||
|
|
|||||
| Childhood-onset | Adult-onset | Childhood-onset | Adult-onset | ||
|
| |||||
| Obesity | 1.14 (0.95–1.37) | 1.35 (1.09–1.67) a | 1.93 (1.65–2.26) b | 1.16 (0.88–1.53) | 1.63 (1.31–2.04) b |
| Blood total cholesterol | |||||
| Borderline high | 0.92 (0.76–1.12) | 0.79 (0.62–1.02) | 0.99 (0.84–1.17) | 0.65 (0.42–1.01) | 1.10 (0.80–1.52) |
| High | 1.07 (0.84–1.36) | 0.68 (0.47–1.00) | 1.16 (0.95–1.42) | 0.85 (0.60–1.19) | 1.11 (0.88–1.41) |
| Serum LDL cholesterol | |||||
| Borderline high | 1.13 (0.88–1.46) | 0.89 (0.58–1.37) | 0.97 (0.76–1.24) | 0.56 (0.27–1.16) | 1.14 (0.66–1.97) |
| High | 1.04 (0.67–1.61) | 0.55 (0.32–0.95) c | 1.20 (0.89–1.61) | 0.77 (0.46–1.29) | 0.88 (0.60–1.28) |
| Blood triglycerides | |||||
| Borderline high | 0.93 (0.74–1.19) | 0.77 (0.57–1.05) | 1.28 (1.01–1.63) c | 0.81 (0.57–1.17) | 1.38 (0.99–1.93) |
| High | 1.00 (0.80–1.25) | 1.18 (0.94–1.48) | 1.37 (1.14–1.65) b | 1.15 (0.84–1.58) | 1.38 (1.02–1.85) c |
| Diabetes | 1.24 (0.98–1.56) | 1.36 (1.03–1.78) c | 1.62 (1.37–1.91) b | 0.99 (0.71–1.37) | 1.20 (0.94–1.54) |
| Hypertension | 1.20 (1.03–1.41) c | 1.48 (1.18–1.85) a | 1.59 (1.36–1.87) b | 1.21 (0.90–1.61) | 1.32 (1.06–1.65) c |
| CVD | 1.33 (1.06–1.68) c | 2.42 (1.81–3.24) b | 2.55 (2.14–3.03) b | 1.63 (1.18–2.26) a | 1.86 (1.40–2.48) b |
| CKD | 1.01 (0.75–1.37) | 1.37 (0.94–2.01) | 1.17 (0.94–1.45) | 1.35 (0.82–2.22) | 1.20 (0.86–1.66) |
| Arthritis | |||||
| Rheumatoid arthritis | 1.18 (0.86–1.62) | 3.11 (2.24–4.31) b | 2.38 (1.90–2.99) b | 2.40 (1.49–3.86) b | 1.93 (1.36–2.73) b |
| Osteoarthritis | 1.45 (1.13–1.85) a | 1.58 (1.14–2.19) a | 2.44 (2.00–2.98) b | 1.04 (0.67–1.60) | 1.57 (1.17–2.11) a |
| Other/unknown | 1.31 (1.04–1.66) c | 1.76 (1.34–2.31) b | 2.40 (1.93–3.00) b | 1.29 (0.89–1.88) | 1.84 (1.36–2.48) b |
| Cancer/history of cancer | 1.24 (0.97–1.57) | 1.48 (1.04–2.10) c | 1.44 (1.20–1.73) b | 1.16 (0.78–1.70) | 1.14 (0.85–1.53) |
| Anemia | 1.15 (0.89–1.50) | 1.29 (1.01–1.64) c | 1.48 (1.22–1.80) b | 1.11 (0.76–1.61) | 1.29 (0.94–1.79) |
| Depression | 1.29 (0.94–1.76) | 1.98 (1.33–2.96) a | 2.19 (1.73–2.77) b | 1.53 (0.96–2.44) | 1.74 (1.24–2.43) a |
| Airflow obstruction | 1.74 (1.25–2.43) a | 2.94 (1.64–5.26) b | 2.51 (1.82–3.45) b | 1.67 (0.95–2.93) | 1.56 (1.01–2.41) c |
| COPD | 1.92 (1.22–3.01) a | 1.77 (0.92–3.41) | 0.99 (0.61–1.63) | 0.96 (0.48–1.92) | 0.57 (0.30–1.09) |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; LDL, low density lipoprotein; NHANES, National Health and Nutrition Examination Survey; PIR, poverty income ratio.
NOTE. All models were adjusted for age, sex, race and ethnicity, poverty income ratio, pack-years of cigarette smoking, health insurance, and NHANES survey cycle.
Bold indicates significant results.
P < .01.
P < .001.
P < .05.
Table 3.
Adjusted Regression Coefficient (β [95% CI]) for the Association of Past Asthma and Current Childhood and Adult Onsets of Asthma With Lung Function and Exhaled Nitric Oxide, NHANES 2007–2012 (N = 27,437)
| Asthma phenotypes | FEV1/FVC (%) | PEF (L/s) | FEF25%–75% (L/s) | FeNO |
|---|---|---|---|---|
|
| ||||
| Past and current asthma vs no asthma | ||||
| No asthma | Reference | Reference | Reference | Reference |
| Past asthma | −1.75 (−2.79 to −0.70) a | −0.32 (−0.53 to −0.11) a | −0.28 (−0.43 to −0.12) b | 1.88 (0.26−3.50) c |
| Childhood-onset asthma | −4.59 (−6.63 to −2.56) b | −0.76 (−1.28 to −0.23) a | −0.57 (−0.81 to −0.33) b | 5.36 (1.88−8.83) a |
| Adult-onset asthma | −4.07 (−5.60 to −2.54) b | −0.69 (−1.04 to −0.34) b | −0.43 (−0.58 to −0.28) b | 6.69 (3.26−10.12) b |
| Current vs past asthma | ||||
| Past asthma | Reference | Reference | Reference | Reference |
| Childhood-onset asthma | −2.65 (−4.44 to −0.87) a | −0.36 (−0.89 to 0.16) | −0.26 (−0.50 to −0.03) c | 4.09 (0.12−8.07) c |
| Adult-onset asthma | −2.43 (−4.43 to −0.43) c | −0.39 (−0.83 to 0.05) | −0.19 (−0.42 to 0.03) | 5.45 (1.06−9.84) c |
| Adult- vs childhood-onset asthma | ||||
| Childhood-onset asthma | Reference | Reference | Reference | Reference |
| Adult-onset asthma | 0.20 (−2.37 to 2.77) | −0.07 (−0.69 to 0.56) | 0.05 (−0.23 to 0.33) | 1.17 (−4.55 to 6.90) |
| Age of asthma diagnosis (per 5-y) | 0.15 (−0.13 to 0.44) | −9.63 (−60.79 to 41.54) | 12.86 (−10.84 to 36.55) | 0.40 (0.34–1.15) |
Abbreviations: FEF25%–75%, forced expiratory flow rate 25%–75%; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; NHANES, National Health and Nutrition Examination Survey; PEF, peak expiratory.
NOTE. All models were adjusted for age, sex, race and ethnicity, PIR, pack-years of cigarette smoking, health insurance, and NHANES survey cycle.
Bold indicates significant results.
P < .01.
P < .001.
P < .05.
Childhood-onset Asthma Compared With No Asthma
In the analysis adjusted for covariates and compared with no asthma, childhood-onset asthma was associated with 35% higher odds of obesity (OR, 1.35; 95% CI, 1.09–1.67) and 45% lower odds of hypercholesterolemia (OR, 0.55; 95% CI, 0.32–0.95). It was associated with 30% to 3.1-fold higher odds of diabetes (OR, 1.36; 95% CI, 1.03–1.78), hypertension (OR, 1.48; 95% CI, 1.18–1.85), CVD (OR, 2.42; 95% CI, 1.81–3.24), rheumatoid arthritis (3.11; 95% CI, 2.24–4.31), osteoarthritis (OR, 1.58; 95% CI. 1.14–2.19), and other or unknown arthritis (OR, 1.76; 95% CI, 1.34–2.31). It was associated with 29% to 98% higher odds of cancer or cancer history (OR, 1.48; 95% CI, 1.04–2.10), anemia (OR, 1.29; 95% CI, 1.01–1.64), and a PHQ-9 score suggestive of depression (OR, 1.98; 95% CI, 1.33–2.96) than was no asthma. The odds of airflow obstruction increased by almost 3-fold with childhood-onset asthma (OR, 2.94; 95% CI, 1.64–5.26) compared with no asthma (Table 2). Childhood-onset asthma was also associated with lower FEV1/FVC ratio (β, −4.59; 95% CI, −6.63 to −2.56), PEF (β, −0.76; 95% CI, −1.28 to −0.23), FEF25%−75% (β, −0.57; 95% CI, −0.81 to −0.33), and higher FeNO (β, 5.36; 95% CI, 1.88–8.83) than was no asthma (Table 3).
Adult-onset Asthma Compared With No Asthma
In the adjusted analysis, adult-onset asthma was associated with 28 to 93% higher odds of obesity (OR, 1.93; 95% CI, 1.65–2.26), borderline hypertriglyceridemia (OR, 1.28; 95% CI, 1.01–1.63), and hypertriglyceridemia (OR, 1.37; 95% CI, 1.14–1.65). It was associated with 53% to 2.55-fold higher odds of diabetes (OR, 1.62; 95% CI, 1.371.91), hypertension (OR, 1.59; 95% CI, 1.36–1.87), CVD (OR, 2.55; 95% CI, 2.14–3.03), rheumatoid arthritis (2.38; 95% CI, 1.90–2.99), osteoarthritis (OR, 2.44; 95% CI, 2.00–2.98), and other or unknown arthritis (OR, 2.40; 95% CI, 1.93–3.00). It was associated with 44% to 2.19-fold higher odds of cancer or cancer history (OR, 1.44; 95% CI, 1.20–1.73), anemia (OR, 1.48; 95% CI, 1.22–1.80), and a PHQ-9 score suggestive of depression (OR, 2.19; 95% CI, 1.73–2.77) than was no asthma. The odds of airflow obstruction increased by 2.5-fold with adult-onset asthma (OR, 2.51; 95% CI, 1.82–3.45) (Table 2). Adult-onset asthma was also associated with lower FEV1/FVC ratio (β, −4.07; 95% CI, −5.60 to −2.54), PEF (β, −0.69; 95% CI, −1.04 to −0.34), FEF25%−75% (β, −0.43; 95% CI, −0.58 to −0.28), and higher FeNO (β, 6.69; 95% CI, 3.26–10.12) than was no asthma (Table 3).
Comorbidities in Current vs Past Asthma
After adjustment for all covariates, current asthma of childhood onset was associated with 63% to 2.40-fold higher odds compared with past asthma of CVD (OR, 1.63; 95% CI, 1.18–2.26) and rheumatoid arthritis (OR, 2.40; 95% CI, 1.49–3.86), lower FEV1/FVC (β, −2.65; 95% CI, −4.44 to −0.87), FEF25%−75% (β, −0.26; 95% CI, −0.50 to −0.03), and higher FeNO (β, 4.09; 95% CI, 0.12–8.07) (Tables 2 and 3).
Adult onset of current asthma was associated with higher prevalence of obesity (OR, 1.63; 95% CI, 1.31–2.04), hypertriglyceridemia (OR, 1.38; 95% CI, 1.02–1.85), hypertension (OR, 1.32; 95% CI, 1.06–1.65), and CVD (OR, 1.86; 95% CI, 1.40–2.48). Adult onset of current asthma was also associated with higher prevalence of rheumatoid arthritis (OR, 1.93; 95% CI, 1.36–2.73), osteoarthritis (OR, 1.57; 95% CI, 1.17–2.11), other or unknown arthritis (OR, 1.84; 95% CI, 1.36–2.48), a PHQ-9 score suggestive of depression (OR, 1.74; 95% CI, 1.24–2.43), and airflow obstruction (OR, 1.56; 95% CI, 1.01–2.41) than was past asthma (Table 2). FEV1/FVC ratio (β, −2.43; 95% CI, −4.43 to −0.43) was lower and FeNO (β, 5.45; 95% CI, 1.06–9.84) was higher in adult onset of current asthma than in past asthma after adjustment for covariates (Table 3).
Comorbidities in Adult vs Childhood Onset of Current Asthma
After adjustment for age and all other covariates, adult-onset asthma was associated with higher odds of obesity (OR, 1.46; 95% CI, 1.09–1.96), hypercholesterolemia (OR, 1.67; 95% CI, 1.08–2.56), high serum LDL cholesterol (OR, 2.10; 95% CI, 1.20–3.67), and borderline high serum triglycerides (OR, 1.78; 95% CI, 1.17–2.71) than in childhood-onset asthma. It was also associated with higher odds of osteoarthritis (OR, 1.52; 95% CI, 1.04–2.20) and other or unknown arthritis (OR, 1.44; 95% CI, 1.03–2.00) (Fig 1). Lung function impairment and airway inflammation in adult-onset current asthma were not different from childhood-onset current asthma (Table 3).
Figure 1.
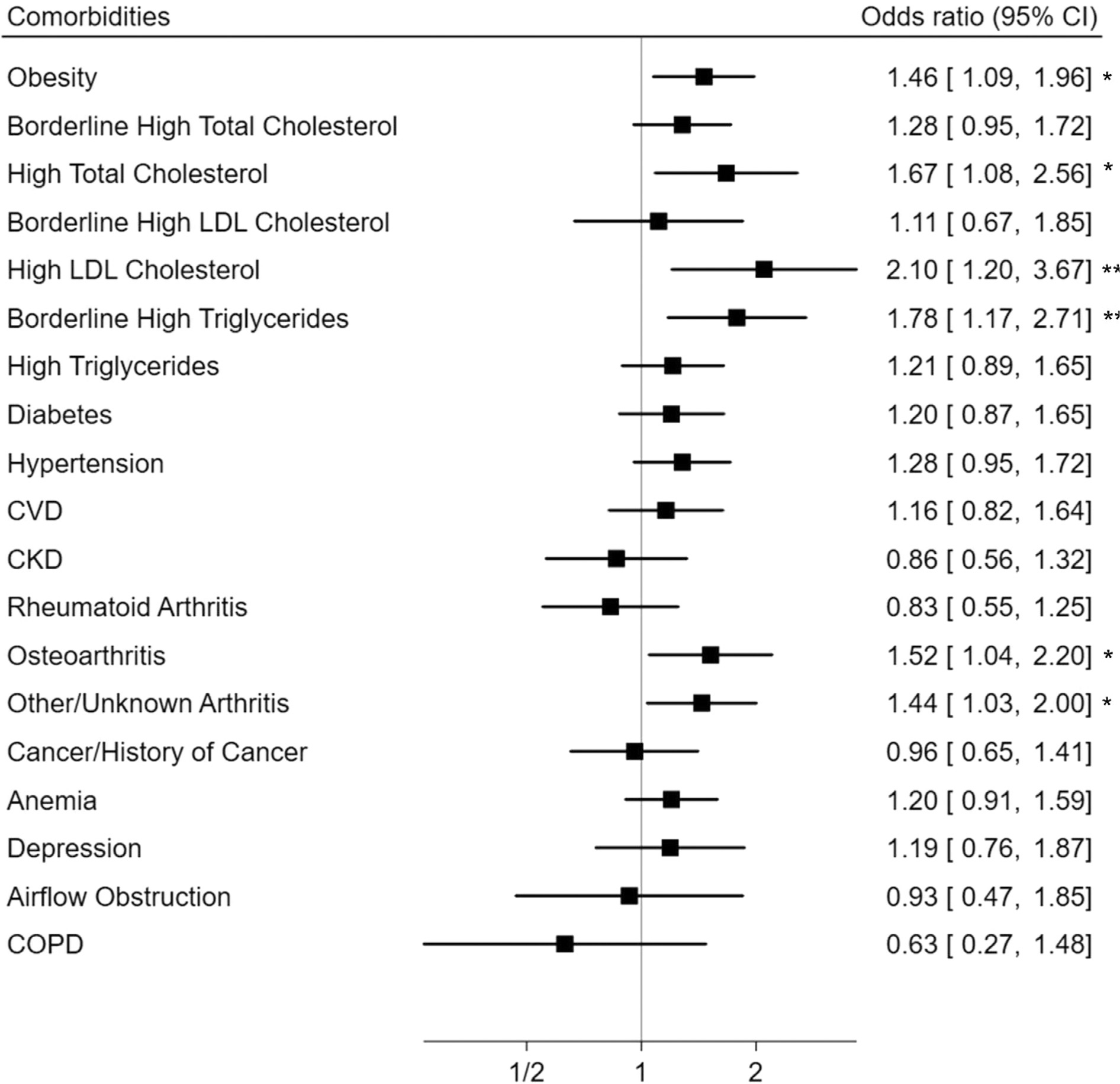
Comorbidities associated with adult vs childhood asthma onset among adults with current asthma. Models adjusted for age, sex, race and ethnicity, poverty income ratio, pack-years of cigarette smoking, health insurance, and NHANES survey cycle. CI: confidence interval, CKD: chronic kidney disease, COPD: chronic obstructive pulmonary disease, CVD: cardiovascular disease, LDL: low density lipoprotein, NHANES: National Health and Nutrition Examination Survey. * P < 0.05, ** P < 0.01.
Age of Asthma Diagnosis and Comorbidities
After adjusting for all covariates, older age of asthma diagnosis was associated with increased odds of obesity (OR, 1.06; 95% CI, 1.03–1.10), borderline high triglycerides and hypertriglyceridemia (OR, 1.05; 95% CI, 1.01–1.08), diabetes (OR, 1.00–1.07), hypertension (OR, 1.05; 95% CI, 1.02–1.07), osteoarthritis (OR, 1.05; 95% CI, 1.01–1.09), and other or unknown arthritis (OR, 1.06; 95% CI, 1.03–1.10). We observed an inverse relationship between the age of asthma diagnosis and COPD (OR, 0.90; 95% CI, 0.84–0.96) (Fig 2). Each 5-year decrease in age of asthma diagnosis was associated with 12% higher odds of COPD (OR, 1.12; 95% CI, 1.04–1.19). We found no association between age of asthma diagnosis and impaired lung function or airway inflammation (Table 3).
Figure 2.
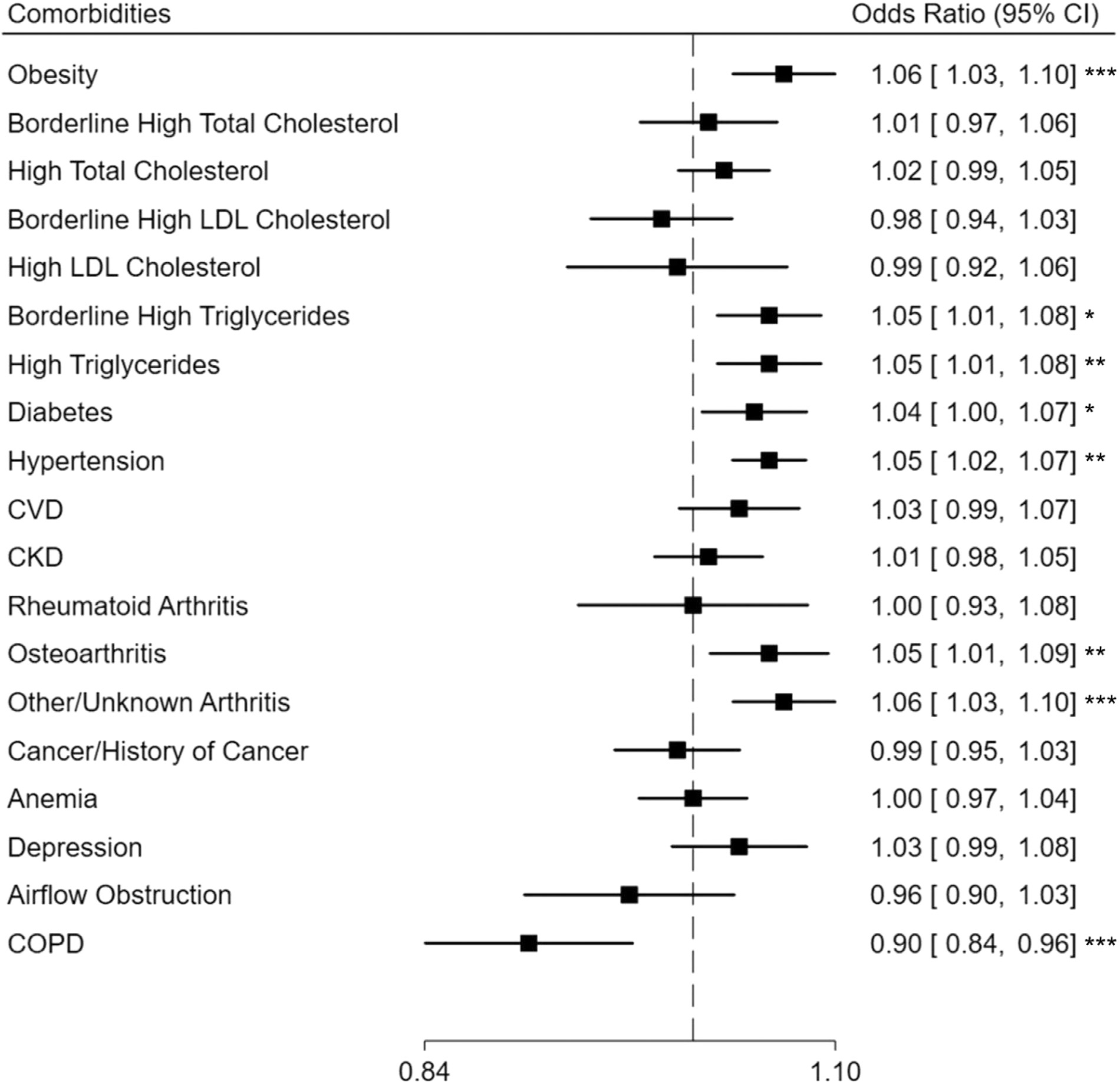
Association between age of asthma diagnosis and comorbidities among adults with current asthma. Odds ratios reported for each 5 years increase in age of asthma diagnosis and adjusted for age, sex, race and ethnicity, poverty income ratio, pack-years of cigarette smoking, health insurance, and NHANES survey cycle. CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; LDL, low density lipoprotein; NHANES, National Health and Nutrition Examination Survey. * P < 0.05, ** P < 0.01, *** P < 0.001.
Discussion
In this nationally representative sample, and after adjusting for all covariates, participants with past asthma and/or current asthma had higher prevalence of obesity, dyslipidemia, diabetes, hypertension, CVD, arthritis, cancer or cancer history, anemia, depression, and/or airflow obstruction than those without asthma. Among participants with current asthma, adult-onset asthma was associated with higher prevalence of obesity, dyslipidemia, osteoarthritis, and other or unknown arthritis than childhood-onset asthma. Older age of asthma diagnosis was additionally associated with diabetes and hypertension, whereas younger age of asthma diagnosis was associated with higher odds of COPD.
Comorbidities in Childhood and Adult Onset of Current Asthma vs No Asthma
Despite overwhelming evidence that comorbidities are prevalent in asthma, few studies have examined the prevalence of comorbidities in asthma vs no asthma by differentiating between childhood onset and adult onset of the disease. However, these studies focused on cardiovascular outcomes mostly in participants with adult-onset asthma and only to a lesser extent in participants with childhood-onset asthma. Consistent with our finding of an association between childhood-onset asthma and CVD, the Childhood Origins of Asthma Cohort study observed that adolescents with asthma developed more subclinical arterial injury than those without asthma, in a sample of 89 participants.21 However, the studies on childhood-onset or adult-onset asthma and CVD have produced conflicting results. The Wisconsin Sleep cohort study suggested that adult-onset asthma was a predictor of CVD, and in the Atherosclerosis Risk in Communities, adult-onset but not childhood-onset asthma was associated with carotid atherosclerosis in women but not in men.8,22 This preponderance of women in the association of adult-onset asthma with CVD was confirmed in a large meta-analysis of 666,355 participants.23 The mechanism for CVD prevalence in asthma is purportedly due primarily to chronic inflammation, and the stronger effect in women may be attributed to the effect of estrogen in enhancing proinflammatory cytokines released from macrophages, monocytes, and vascular cells.23
Besides cardiovascular diseases, obesity has been a widely reported comorbidity in both childhood- and adult-onset asthma.24 However, the temporality between the 2 conditions is not clear; there is evidence suggesting that childhood-onset asthma may be a risk factor for obesity, whereas adult-onset asthma could be a consequence of obesity.4,24,25 The proposed mechanisms for obesity among individuals with asthma include systemic inflammation, dysregulation of adipokines, and gut microbiome changes, and some shared genetic characteristics or epigenetic alterations between the 2 conditions.24 Both asthma and obesity may also have common biological pathways; for instance, chitinase 3-like protein 1 contributes to visceral adiposity and also plays a role in Th2 pulmonary inflammation.24 Besides obesity, asthma has been linked to dyslipidemia and metabolic abnormalities, and these disorders may contribute to asthma severity through the production of proinflammatory cytokines, the reduction of endogenous anti-inflammatory activity, and the increased bronchomotor tone.26 Likewise, airway inflammation can, in turn, lead to subsequent hypertriglyceridemia and insulin resistance.26 We found an increase in arthritis prevalence among participants with either childhood- or adult-onset asthma compared with participants with no asthma. Well-conducted prospective studies have reported an increased risk of rheumatoid arthritis associated with asthma and vice versa.27,28 Common factors that may predispose individuals to asthma and arthritis are genetic predisposition (eg, HLA-DRB1) and common immunologic mechanisms. For instance, the immunologic natural killer group 2D (NKG2D), a transmembrane protein expressed on several immune cells, including TH17 cells and leukotriene B4, plays a role in both asthma and rheumatoid arthritis.27 Moreover, there are disease-modifying drugs, immunosuppressant medications, and nonsteroidal anti-inflammatory drugs used for rheumatoid arthritis that may have pulmonary toxicity and increase the risk of asthma diagnosis.28 Consistent with our findings, other studies have also reported associations between asthma and cancer, anemia, or depression, although the mechanisms and molecular basis for these associations are not fully understood.29,30
Comorbidities in Adult vs Childhood Onsets of Current Asthma
Few studies have investigated comorbidities in adult-onset asthma compared with childhood-onset asthma. Typically, adult-onset asthma has been associated with female sex, cigarette smoking, low socioeconomic status, and impaired lung function.31,32 However, we found no differences in lung function impairment and airway inflammation measured by FeNO between adult- and childhood-onset asthma or with age of asthma diagnosis. Consistent with our findings on the relationship of adult-onset asthma with obesity and dyslipidemia compared with childhood-onset asthma, a cross-sectional study conducted in 81 participants found that adult-onset asthma and older age of asthma diagnosis were associated with metabolic syndrome.9 Otherwise, no other published studies have, to the best of our knowledge, compared obesity and dyslipidemia prevalence between childhood-onset and adult-onset asthma. Obesity has been reported to be a risk factor for late-onset but not early-onset asthma; rather, early-onset asthma seems to be a complication of obesity.4,33 Nevertheless, the reasons for a higher prevalence of obesity in adult-onset asthma than in childhood-onset asthma are not clear, and obesity could also explain the higher prevalence of diabetes, hypertension, and osteoarthritis in adult-onset asthma than in childhood-onset asthma.34–36 Consistent with previous reports, we found that a younger age of asthma diagnosis was associated with a higher prevalence of COPD.37,38 It has been hypothesized that early-onset asthma may cause impaired lung growth and development into adulthood that could lead to COPD.39 An additional potential mechanism could be airway remodeling owing to chronic airway inflammation from childhood into adulthood.
Our study has limitations. Owing to the cross-sectional design of the NHANES, the temporality between asthma and comorbidities could not be established. Our analysis classified current asthma into childhood onset and adult onset; however, among adults with childhood-onset asthma, we could not differentiate between those who had asthma persisting from childhood to adulthood and those with childhood asthma that relapsed after remission. According to a recent Japanese study, asthma persisting from childhood to adulthood represents 30% of childhood-onset asthma, and this phenotype may be associated with worse lung function and asthma severity than adult-onset asthma.40,41 Asthma and age of asthma diagnosis were defined by self-report, which may have led to a missed diagnosis of asthma, especially in those with mild forms of the disease. Moreover, the analysis could not assess the effect of early treatment interventions on the studied outcomes. Nonetheless, our study has major strengths. It included a large sample representative of the US population, which makes the results generalizable to American adults. We included an extensive list of common comorbidities; we included lung function assessment and FeNO, and we used rigorous case definitions based on combinations of questionnaires and also laboratory and examination results. All models were adjusted for covariates and potential confounders, including age and sociodemographic characteristics, pack-years of cigarette smoking, and other covariates, which minimized residual confounding.
In conclusion, obesity, diabetes, hypertension, CVD, arthritis, cancer or history of asthma, anemia, depression, and airflow obstruction are prevalent in both childhood-onset and adult-onset asthma. Adult-onset asthma was associated with higher prevalence of obesity, dyslipidemia, osteoarthritis, and other or unknown arthritis than was childhood-onset asthma. In addition, increased age of asthma diagnosis was associated with diabetes and hypertension, whereas younger age of asthma diagnosis was associated with higher odds of COPD. Future studies should include a longitudinal assessment of asthma to determine temporality between the disease and the comorbidities.
Funding:
Dr Mendy’s contribution was partly funded by grant P30 ES006096 from the US National Institutes of Health. Dr Mersha acknowledges partial support from the National Human Genome Research Institute, grant R01 HG011411.
Footnotes
Disclosures: The authors have no conflicts of interest to report.
References
- 1.Mendy A, Forno E, Niyonsenga T, Carnahan R, Gasana J. Prevalence and features of asthma-COPD overlap in the United States 2007–2012. Clin Respir J. 2018;12 (8):2369–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396 (10258):1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ilmarinen P, Tuomisto LE, Kankaanranta H. Phenotypes, risk factors, and mechanisms of adult-onset asthma. Mediators Inflamm. 2015;2015: 514868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Nijs SB, Venekamp LN, Bel EH. Adult-onset asthma: is it really different? Eur Respir Rev. 2013;22(127):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su X, Ren Y, Li M, Zhao X, Kong L, Kang J. Prevalence of comorbidities in asthma and nonasthma patients: a meta-analysis. Medicine. 2016;95(22):e3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pividori M, Schoettler N, Nicolae DL, Ober C, Im HK. Shared and distinct genetic risk factors for childhood-onset and adult-onset asthma: genome-wide and transcriptome-wide studies. Lancet Respir Med. 2019;7(6):509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onufrak SJ, Abramson JL, Austin HD, Holguin F, McClellan WM, Vaccarino LV. Relation of adult-onset asthma to coronary heart disease and stroke. Am J Cardiol. 2008;101(9):1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Boer GM, Tramper-Stranders GA, Houweling L, van Zelst CM, Pouw N, Verhoeven GT, et al. Adult but not childhood onset asthma is associated with the metabolic syndrome, independent from body mass index. Respir Med 2021;188:106603. [DOI] [PubMed] [Google Scholar]
- 10.Ilmarinen P, Tuomisto LE, Niemelä O, Danielsson J, Haanpää J, Kankaanranta T, et al. Comorbidities and elevated IL-6 associate with negative outcome in adult-onset asthma. Eur Respir J. 2016;48(4):1052–1062. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services (DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). The National Health and Nutrition Examination Survey (NHANES). Available at: https://www.cdc.gov/nchs/nhanes/index.htm. Accessed April 27, 2022.
- 12.He J, Gu D, Reynolds K, Wu X, Muntner P, Zhao J, et al. Serum total and lipoprotein cholesterol levels and awareness, treatment, and control of hypercholesterolemia in China. Circulation. 2004;110(4):405–411. [DOI] [PubMed] [Google Scholar]
- 13.Mendy A, Gopal R, Alcorn JF, Forno E. Reduced mortality from lower respiratory tract disease in adult diabetic patients treated with metformin. Respirology. 2019;24(7):646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendy A Association of urinary nitrate with lower prevalence of hypertension and stroke and with reduced risk of cardiovascular mortality. Circulation. 2018;137(21):2295–2297. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendy A, Salo PM, Wilkerson J, Feinstein L, Ferguson KK, Fessler MB, et al. Association of urinary levels of bisphenols F and S used as bisphenol A substitutes with asthma and hay fever outcomes. Environ Res. 2020;183: 108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendy A, Forno E, Niyonsenga T, Gasana J. Blood biomarkers as predictors of longterm mortality in COPD. Clin Respir J. 2018;12(5):1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Lopez J, Bolge SC, Zhu VJ, Stang PE. Depression among people with type 2 diabetes mellitus, US National Health and Nutrition Examination Survey (NHANES), 2005–2012. BMC Psychiatry. 2016;16:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beckles GL, Truman BI, Centers for Disease Control and Prevention (CDC). Education and income - United States, 2005 and 2009. MMWRSuppl. 2011;60:13–17. [PubMed] [Google Scholar]
- 20.Mahabee-Gittens EM, Mendy A, Merianos AL. Assessment of severe COVID-19 outcomes using measures of smoking status and smoking intensity. Int J Environ Res Public Health. 2021;18(17):8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tattersall MC, Evans MD, Korcarz CE, Mitchell C, Anderson E, DaSilva DF, et al. Asthma is associated with carotid arterial injury in children: the Childhood Origins of Asthma (COAST) cohort. PLoS One. 2018;13:(9) e0204708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tattersall MC, Barnet JH, Korcarz CE, Hagen EW, Peppard PE, Stein JH. Late-Onset asthma predicts cardiovascular disease events: the Wisconsin sleep cohort. J Am Heart Assoc. 2016;5:(9) e003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Gao S, Yu M, Sheng Z, Tan W. Association of asthma with coronary heart disease: a meta-analysis of 11 trials. PLoS One. 2017;12:(6) e0179335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Contreras ZA, Chen Z, Roumeliotaki T, Annesi-Maesano I, Baiz N, von Berg A, et al. Does early onset asthma increase childhood obesity risk? A pooled analysis of 16 European cohorts. Eur Respir J. 2018;52:(3) 1800504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leone N, Courbon D, Berr C, Barberger-Gateau P, Tzourio C, Alpérovitch A, et al. Abdominal obesity and late-onset asthma: cross-sectional and longitudinal results: the 3c study. Obesity. 2012;20(3):628–635. [DOI] [PubMed] [Google Scholar]
- 26.Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic abnormalities in children with asthma. Am J Respir Crit Care Med. 2011;183(4):441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolfes MC, Juhn YJ, Wi C, Sheen YH. Asthma and the risk of rheumatoid arthritis: an insight into the heterogeneity and phenotypes of asthma. Tuberc Respir Dis. 2017;80(2):113–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SY, Min C, Oh DJ, Choi HG. Increased risk of asthma in patients with rheumatoid arthritis: a longitudinal follow-up study using a national sample cohort. Sci Rep. 2019;9(1):6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salameh L, Mahboub B, Khamis A, Alsharhan M, Tirmazy SH, Dairi Y, et al. Asthma severity as a contributing factor to cancer incidence: a cohort study. PLoS One. 2021;16:(5):e0250430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brigham EP, McCormack MC, Takemoto CM, Matsui EC. Iron status is associated with asthma and lung function in US women. PLoS One. 2015;10:(2) e0117545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trivedi M, Denton E. Asthma in children and adults-what are the differences and what can they tell us about asthma? Front Pediatr. 2019;7:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan DJ, Walters EH, Perret JL, Burgess JA, Johns DP, Lowe AJ, et al. Clinical and functional differences between early-onset and late-onset adult asthma: a populationbased Tasmanian Longitudinal Health Study. Thorax. 2016;71(11):981–987. [DOI] [PubMed] [Google Scholar]
- 33.Rönmark E, Andersson C, Nyström L, Forsberg B, Järvholm B, Lundbäck B. Obesity increases the risk of incident asthma among adults. Eur Respir J. 2005;25(2):282–288. [DOI] [PubMed] [Google Scholar]
- 34.Seravalle G, Grassi G. Sleep apnea and hypertension. High Blood Press Cardiovasc Prev. 2022;29(1):23–31. [DOI] [PubMed] [Google Scholar]
- 35.Bray GA. Obesity increases risk for diabetes. Int J Obes Relat Metab Disord. 1992;16 (suppl 4):S13–S17. [PubMed] [Google Scholar]
- 36.Lementowski PW, Zelicof SB. Obesity and osteoarthritis. Am J Orthop. 2008;37(3):148–151. [PubMed] [Google Scholar]
- 37.McGeachie MJ. Childhood asthma is a risk factor for the development of chronic obstructive pulmonary disease. Curr Opin Allergy Clin Immunol. 2017;17(2):104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tai A, Tran H, Roberts M, Clarke N, Wilson J, Robertson CF. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax. 2014;69(9):805–810. [DOI] [PubMed] [Google Scholar]
- 39.Hayden LP, Cho MH, Raby BA, Beaty TH, Silverman EK, Hersh CP, et al. Childhood asthma is associated with COPD and known asthma variants in COPDGene: a genome-wide association study. Respir Res. 2018;19(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.To M, Tsuzuki R, Katsube O, Yamawaki S, Soeda S, Kono Y, et al. Persistent asthma from childhood to adulthood presents a distinct phenotype of adult asthma. J Allergy Clin Immunol Pract. 2020;8(6):1921–1927. e2. [DOI] [PubMed] [Google Scholar]
- 41.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349(15):1414–1422. [DOI] [PubMed] [Google Scholar]


