Abstract
Aims
Atrial remodelling, defined as a change in atrial structure, promotes atrial fibrillation (AF). Bone morphogenetic protein 10 (BMP10) is an atrial-specific biomarker released to blood during atrial development and structural changes. We aimed to validate whether BMP10 is associated with AF recurrence after catheter ablation (CA) in a large cohort of patients.
Methods and results
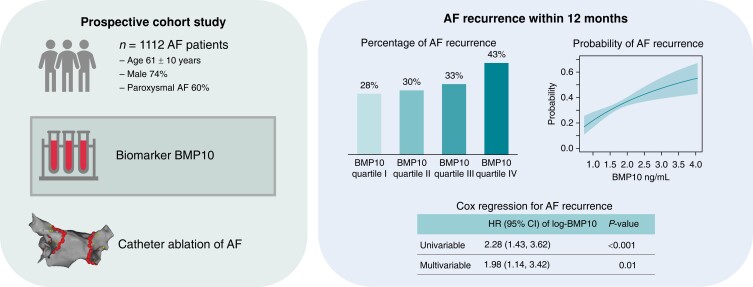
We measured baseline BMP10 plasma concentrations in AF patients who underwent a first elective CA in the prospective Swiss-AF-PVI cohort study. The primary outcome was AF recurrence lasting longer than 30 s during a follow-up of 12 months. We constructed multivariable Cox proportional hazard models to determine the association of BMP10 and AF recurrence. A total of 1112 patients with AF (age 61 ± 10 years, 74% male, 60% paroxysmal AF) was included in our analysis. During 12 months of follow-up, 374 patients (34%) experienced AF recurrence. The probability for AF recurrence increased with increasing BMP10 concentration. In an unadjusted Cox proportional hazard model, a per-unit increase in log-transformed BMP10 was associated with a hazard ratio (HR) of 2.28 (95% CI 1.43; 3.62, P < 0.001) for AF recurrence. After multivariable adjustment, the HR of BMP10 for AF recurrence was 1.98 (95% CI 1.14; 3.42, P = 0.01), and there was a linear trend across BMP10 quartiles (P = 0.02 for linear trend).
Conclusion
The novel atrial-specific biomarker BMP10 was strongly associated with AF recurrence in patients undergoing CA for AF.
ClinicalTrials.gov Identifier
NCT03718364; https://clinicaltrials.gov/ct2/show/NCT03718364
Keywords: Atrial fibrillation, Biomarker, Bone morphogenetic protein 10, BMP10, Catheter ablation, Pulmonary vein isolation
Graphical Abstract
Graphical Abstract.
AF, atrial fibrillation; BMP10, bone morphogenetic protein 10; HR, hazard ratio
What’s New?
This validation study in a large cohort of patients with atrial fibrillation (AF) showed that elevated plasma concentrations of the atrial-specific biomarker bone morphogenetic protein 10 (BMP10) measured prior to catheter ablation (CA) are associated with recurrent atrial fibrillation (AF) within 12 months.
Plasma BMP10 concentrations can assist physicians in predicting the success of CA.
Future studies are necessary to determine whether BMP10-guided patient selection for CA improves outcomes.
Introduction
Among the different methods of rhythm control for atrial fibrillation (AF), catheter ablation (CA) represents a safe and effective alternative to antiarrhythmic drugs for maintaining sinus rhythm.1,2 However, AF recurs in about one-third of patients after CA, which poses a major clinical challenge.3
Atrial remodelling, defined as change in atrial structure, promotes atrial arrhythmias.4–6 Bone morphogenetic protein 10 (BMP10), a member of the transforming growth factor β (TGF-β) superfamily, is an atrial-specific biomarker released to blood during atrial development and structural changes.7–9 It is known to maintain the contractile state of vascular smooth muscle cells.10 BMP10 was associated with AF recurrence in a small cohort of patients undergoing CA.11 Moreover, BMP10 has recently been shown to be independently associated with ischaemic stroke and cardiovascular events in AF patients with and without oral anticoagulation.12
Our aim was to validate the association of plasma BMP10 concentration with AF recurrence after CA in a large cohort of AF patients.
Methods
Patients and study procedures
We analysed data of patients from the ongoing prospective cohort study, Swiss Atrial Fibrillation Pulmonary Vein Isolation Registry (Swiss-AF-PVI; ClinicalTrials.gov identifier: NCT03718364). Atrial fibrillation patients aged ≥18 years undergoing first elective CA were included. Exclusion criteria were inability or unwillingness to sign informed consent.
The study complied with the Declaration of Helsinki, and the protocol was approved by the locally appointed ethics committee. Written informed consent was obtained from each participant.
For this analysis, we used data available from patients enrolled between April 2010 and January 2020. We excluded 311 (21.86%) patients of 1423 patients, mainly due to missing baseline values of BMP10 concentration or prior CA.
Blood sampling
Venous blood samples from fasting study participants were obtained immediately prior to CA. We centrifuged the blood samples, aliquoted them into cryotubes, and stored them at –80°C in a centralized biobank. BMP10 concentration of EDTA plasma was measured centrally (Roche Diagnostics, Penzberg, Germany), under constant quality control and calibration by a cobas e601 analyser and a non-commercial robust prototype electro-chemiluminescence immunoassay (ECLIA). This assay involved the use of monoclonal antibodies against BMP10 that were specifically developed. The assays are based on the Elecsys® electro-chemiluminescence technology. The measuring range of BMP10 was 0.01–10 ng/mL. The coefficient of variation (CV) across the measurement period was assessed for two independent internal control samples, resulting in 2.43 and 1.75% (for mean, 1.33 and 2.52 ng/mL). The laboratory personnel were blinded to all clinical information.
Catheter ablation
All antiarrhythmic drugs were stopped the day before the procedure. We performed CA under conscious sedation using midazolam, fentanyl, and propofol. Using ultrasound, we obtained vascular access via the right femoral vein. Transseptal puncture was guided by fluoroscopy and continuous pressure recordings from the transseptal needle tip. We used intravenous heparin to keep the activated clotting time at a target of 350 s. The intracardiac and surface electrograms were shown on an oscilloscope recorded at a speed of 100 mm/s. Catheter ablation was performed using irrigated-tip radiofrequency catheters in combination with electroanatomic mapping systems or cryoballoon catheters. The procedural endpoint was the electric isolation of the pulmonary veins. No additional linear ablation or ablation of complex fractionated electrograms was performed in addition to pulmonary vein isolation. Physicians were not aware of the baseline BMP10 concentration.
Other study variables
We used standardized questionnaires to collect data on patient medical history prior to the scheduled CA. All patients underwent standard transthoracic and transoesophageal echocardiography the day before the procedure. Atrial fibrillation type was classified according to the AF guidelines of the European Society of Cardiology available at the time of data collection.13
Follow-up and outcome assessments
Follow-up was performed at 3, 6, and 12 months and included 12-lead electrocardiogram (ECG) and 7-day Holter monitoring. Moreover, patients were instructed to report to clinicians if they felt any symptoms potentially associated with AF recurrence. After collecting all available information, the outcome events were adjudicated by trained study personnel. For the present analysis, the primary outcome was AF or atrial tachycardia recurrence after CA during a follow-up of 12 months defined as episodes lasting ≥30 s. We did not apply a blanking period.
Statistical analysis
Baseline characteristics were stratified by AF recurrence. We presented categorical variables as numbers (percentages) and continuous variables as mean ± standard deviation (SD) or median (interquartile range [IQR]) if strongly skewed.
We fitted Cox proportional hazard models to determine the association of BMP10 concentration (continuously and as quartiles) with AF recurrence. The first Cox proportional hazard model was unadjusted while the second was adjusted for patient age and sex. In a third model, we adjusted for age, sex, BMI, and history of coronary artery disease, hypertension, heart failure, diabetes, stroke, and renal failure. We present the results as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). To assess the relevance of BMP10, we compared the models with and without BMP10 using a likelihood ratio test. Since the association between BMP10 and risk of AF recurrence may be non-linear, we explored different possible shapes for the association and sought the shape providing the best model fit, assessed by Akaike’s information criterion (AIC). We compared a linear fit BMP10, a model including the second, and one including the third degree polynomial of BMP10, as well as log-transformed BMP10 and a natural spline of log-BMP10. A linear association of AF recurrence and log-BMP10 provided the best model fit. Consequently, we used log-BMP10 as a predictor in all models. Similar to previous studies, we fitted models once with BMP10 divided into quartiles.11,12 Additionally, we used bootstrapping methods with 999 rounds to examine the potential bias of the Cox regression coefficients.
We constructed Kaplan–Meier curves for the time without AF recurrence according to BMP10 quartiles and performed a log-rank test. In a supplementary analysis, we tested interacting effects of different variables [age, sex, BMI, AF type, history of heart failure, history of hypertension, left ventricular ejection fraction (LVEF), and left atrial (LA) dimension in the parasternal long-axis (PLAX)] by including the respective interaction terms in the multivariable Cox proportional hazard model (model 3) for AF recurrence followed by a subgroup analysis.
For the purpose of illustration, we calculated the predicted probabilities of AF recurrence according to BMP10 concentration (continuously) based on logistic regression models (unadjusted model 1 and multivariable model 3). For model 3, the predicted probability was plotted for an ‘average patient’ setting the continuous variables to the mean and the categorical variables to the most frequent category.
The presented P-values are two-sided. Due to the exploratory nature of the analysis, we performed no correction for multiple testing and interpreted P-values as a continuous variable adding to the evidence against the relevant null hypothesis. All analyses were performed using the statistical software R version 4.2.1 (2022-06-23, R Core Team).
Results
We included 1112 AF patients (74% male) with a mean (±SD) age of 61 ± 10 years. Baseline plasma BMP10 concentrations ranged from 0.76 to 3.75 ng/mL [median 1.72 ng/mL (IQR 1.50, 1.99)]. Of the included patients, 666 (60%) had paroxysmal AF, and the mean (±SD) CHA2DS2-VASc score was 1.8 ± 1.4. The mean LA dimension (PLAX) was 41 ± 7 mm, the mean indexed LA volume (LAVI) was 38 ± 12 mL/m2, and the median LVEF was 60% (IQR 53–64%). Table 1 shows the baseline characteristics overall and stratified by AF recurrence.
Table 1.
Baseline characteristics overall and stratified by AF recurrence
| Overall | AF recurrence | ||
|---|---|---|---|
| No recurrent AF | Recurrent AF | ||
| Number of patients | 1112 | 738 (66.4) | 374 (33.6) |
| Age (years) | 61.3 ± 9.8 | 60.8 ± 9.7 | 62.3 ± 9.7 |
| Male sex | 818 (73.6) | 551 (74.7) | 267 (71.4) |
| Body mass index (kg/m2) | 27.3 ± 4.8 | 27.4 ± 4.8 | 27.1 ± 4.8 |
| AF type | |||
| Paroxysmal | 666 (59.9) | 456 (61.8) | 210 (56.1) |
| Persistent | 446 (40.1) | 282 (38.2) | 164 (43.9) |
| Echocardiography | |||
| LVEF (%) | 60 (53; 64) | 60 (53; 65) | 60 (52; 63) |
| LA dimension (PLAX) (mm) | 41.1 ± 6.8 | 40.8 ± 6.7 | 41.8 ± 6.9 |
| LAVI (mL/m2) | 38.1 ± 12.4 | 36.9 ± 11.8 | 40.7 ± 13.3 |
| CHA2DS2-VASc score | 1.8 ± 1.4 | 1.7 ± 1.4 | 1.9 ± 1.4 |
| EHRA score | 2.4 ± 0.7 | 2.4 ± 0.7 | 2.4 ± 0.7 |
| History of CAD | 61 (5.5) | 43 (5.8) | 18 (4.8) |
| History of hypertension | 625 (56.5) | 411 (56.0) | 214 (57.5) |
| History of heart failure | 79 (7.1) | 49 (6.7) | 30 (8.1) |
| History of diabetes | 89 (8.0) | 66 (9.0) | 23 (6.2) |
| History of stroke | 73 (6.6) | 47 (6.4) | 26 (7.0) |
| History of renal failure | 69 (6.2) | 48 (6.5) | 21 (5.7) |
Values are given as mean ± standard deviation, median (interquartile range), or numbers (percentage). Missing values: body mass index (n = 3), LVEF (n = 42), LA (n = 114), LAVI (n = 442), EHRA score (n = 415), history of hypertension (n = 6), history of heart failure (n = 6), history of diabetes (n = 6), history of stroke (n = 6), and history of renal failure (n = 8).
AF, atrial fibrillation; CAD, coronary artery disease; CHA2DS2-VASc score: congestive heart failure, hypertension, age ≥75 years (2 points), diabetes mellitus, prior stroke/transient ischaemic attack/thromboembolism (2 points), vascular disease, age 65–74 years, female sex; EHRA score (European Heart Rhythm Association score): 1 = no symptoms, 2 = mild symptoms, 3 = severe symptoms, 4 = disabling symptoms; LA dimension (PLAX), left atrial dimension in the parasternal long-axis; LAVI, indexed left atrial volume; LVEF, left ventricular ejection fraction.
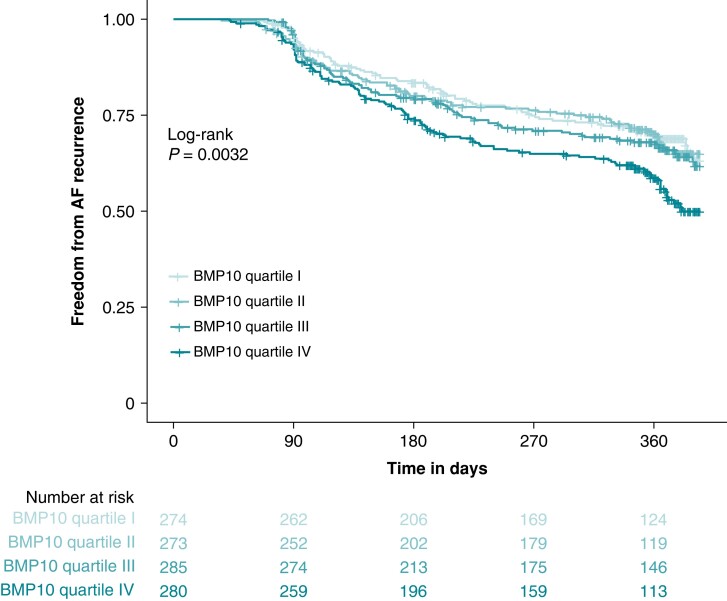
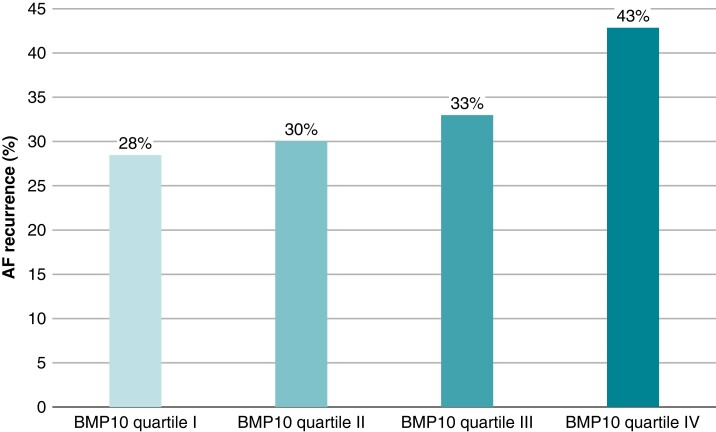
After a follow-up of 12 months, 374 (33.6%) patients had AF recurrence. Figure 1 shows the cumulative incidence of AF recurrence stratified by BMP10 quartiles. Patients in BMP10 quartile IV had the highest observed AF recurrence rate (Figure 2). BMP10 quartiles were the following: quartile I 0.76–1.50 ng/mL, quartile II 1.50–1.72 ng/mL, quartile III 1.72–1.99 ng/mL, and quartile IV 1.99–3.75 ng/mL.
Figure 1.
Kaplan–Meier curve for freedom from AF recurrence. Freedom from AF recurrence per follow-up days after catheter ablation according to BMP10 quartiles. P-value was calculated by log-rank test. AF, atrial fibrillation; BMP10, bone morphogenetic protein 10.
Figure 2.
Observed AF recurrence rate in the BMP10 quartiles. Percentage of patients with documented AF recurrence in the respective BMP10 quartiles. AF, atrial fibrillation; BMP10, bone morphogenetic protein 10.
In unadjusted Cox proportional hazard model 1 (Table 2), a per-unit increase in log-transformed BMP10 was associated with a HR of 2.28 (95% CI 1.43; 3.62, P < 0.001) for AF recurrence. There was a stepwise increase across BMP10 quartiles (P = 0.002 for linear trend). After adjustment for age and sex (model 2), the HR of BMP10 was 2.08 (95% CI 1.23; 3.52, P = 0.006) with evidence for a linear trend across BMP10 quartiles (P = 0.01). After further multivariable adjustment (model 3), the HR of BMP10 was 1.98 (95% CI 1.14; 3.42, P = 0.01). Using patients in quartile I as a reference, the HR of BMP10 in quartile II was 1.00 (95% CI 0.73; 1.37), in quartile III was 1.07 (95% CI 0.78; 1.46), and in quartile IV was 1.46 (95% CI 1.06; 2.02). Evidence of a linear trend across BMP10 quartiles remained (P = 0.02 for linear trend). Internal validation using bootstrapping revealed a mean bias on a coefficient basis of 0.01 (95% percentile CI −0.46; 0.44) for model 1, 0.01 (95% percentile CI −0.53; 0.51) for model 2, and 0.01 (95% percentile CI −0.56; 0.54) for model 3. Compared to the estimated coefficients for BMP10 of 0.82, 0.73, and 0.68, this bias has a small impact. To assess the relevance of BMP10, we compared model 2 and model 3 with and without BMP10, obtaining a P-value of 0.006 and 0.015, respectively. The HRs of the covariables of model 3 are shown in Table S1.
Table 2.
Association of BMP10 concentration and AF recurrence
| BMP10 | Model 1: unadjusted | Model 2: age- and sex-adjusted | Model 3: multivariable adjusteda |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Continuousb | 2.28 (1.43; 3.62), P < 0.001 | 2.08 (1.23; 3.52), P = 0.006 | 1.98 (1.14; 3.42), P = 0.01 |
| Quartile I | Reference | Reference | Reference |
| Quartile II | 1.05 (0.77; 1.43) | 1.02 (0.75; 1.40) | 1.00 (0.73; 1.37) |
| Quartile III | 1.13 (0.84; 1.53) | 1.10 (0.81; 1.49) | 1.07 (0.78; 1.46) |
| Quartile IV | 1.58 (1.19; 2.10) | 1.50 (1.10; 2.06) | 1.46 (1.06; 2.02) |
| P for linear trend | 0.002 | 0.01 | 0.02 |
| P for quadratic trend | 0.17 | 0.17 | 0.14 |
AF, atrial fibrillation; BMP10, bone morphogenetic protein 10; CI, confidence interval; HR, hazard ratio.
Model 3 adjusted for age, sex, body mass index, history of coronary artery disease, hypertension, heart failure, diabetes, stroke, and renal failure.
BMP10 was log-transformed. Model 1 & model 2: n = 1112; model 3: n = 1101.
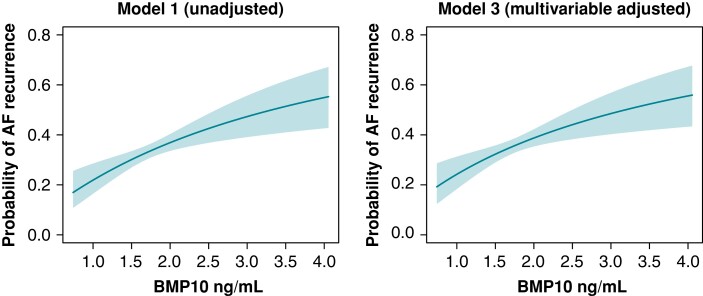
The probability for AF recurrence increased with increasing BMP10 concentration in unadjusted logistic regression model 1 as well as in multivariable adjusted model 3 (Figure 3). Model 3 was illustrated for an average patient in our cohort: male, age 61 years, body mass index 27 kg/m2, history of hypertension, no history of coronary artery disease, no history of heart failure, no history of diabetes, no history of stroke, and no history of renal failure.
Figure 3.
Predicted probabilities of AF recurrence according to BMP10 concentration. The probability for AF recurrence was calculated using logistic regression for unadjusted model 1 and multivariable adjusted model 3. Model 3 was calculated for an ‘average patient’: male, age 61 years, body mass index 27 kg/m2, history of hypertension, no history of coronary artery disease, no history of heart failure, no history of diabetes, no history of stroke, and no history of renal failure. AF, atrial fibrillation; BMP10, bone morphogenetic protein 10.
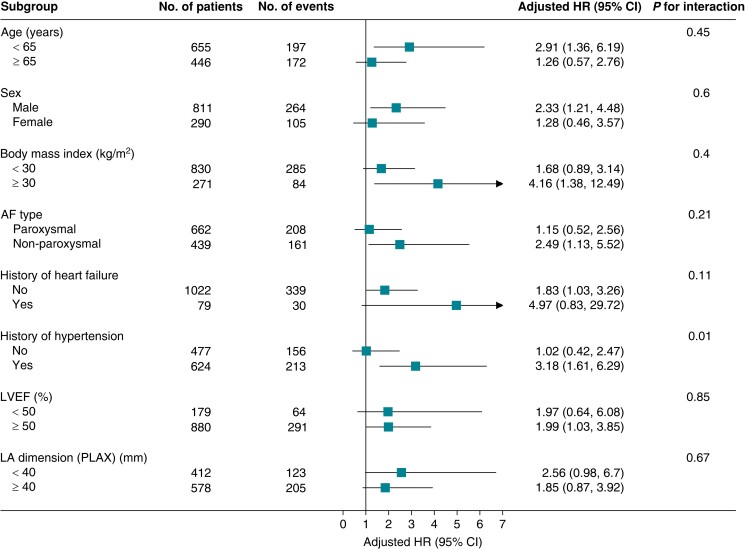
In the supplementary analysis (Figure 4), there was some evidence of a potential interaction between history of hypertension (yes/no) and BMP10 with regard to AF recurrence (P = 0.01 for interaction). For patients with a history of hypertension, an increase in BMP10 was associated with a larger hazard of recurrence. This was not the case for patients with no history of hypertension. We found no evidence for interaction in the other subgroups [age, sex, BMI, AF type, history of heart failure, LVEF, and LA dimension (PLAX)].
Figure 4.
Association of BMP10 and AF recurrence across various subgroups. Adjusted HRs (95% CI) of BMP10 for AF recurrence in the different subgroups were calculated using multivariable adjusted Cox proportional hazard models (model 3). AF, atrial fibrillation; BMP10, bone morphogenetic protein 10; CI, confidence intervals; HR, hazard ratio; LA dimension (PLAX), left atrial dimension in the parasternal long-axis; LVEF, left ventricular ejection fraction; No., number.
Discussion
In our large cohort of AF patients undergoing first elective CA, plasma concentration of the atrial-specific biomarker BMP10 measured immediately prior to CA was strongly associated with AF recurrence within 1 year. This finding was robust after multivariable adjustment and remained consistent across different subgroups.
Atrial fibrillation recurrence after CA constitutes a major clinical challenge. A recent meta-analysis of six randomized controlled studies including 1212 patients with paroxysmal AF revealed that AF recurred in 53% of patients treated with antiarrhythmic drugs and 32% of patients who underwent CA.3 In our cohort, a similar AF recurrence rate of 33% after CA was observed. Notably, we also included patients with non-paroxysmal AF (40% of the total study population).
Owing to its atrial expression and association with structural changes, BMP10 may be an appealing biomarker to predict AF-related outcomes.7,9,11 BMP10 is regulated by the paired-like homeodomain transcription factor 2 (PITX2).11,14 PITX2 is located in a region of the chromosome 4q25 where gene variants associated with an increased risk of AF and AF recurrence were identified in genome-wide association studies.15–17 In contrast to PITX2, which is measured in cardiac specimens obtained by an invasive procedure, BMP10 concentrations are conveniently determined in peripheral blood samples.11 BMP10 concentrations were shown to be associated with change in rhythm status in AF patients who underwent electrical cardioversion, with higher BMP10 concentrations measured during AF compared to sinus rhythm.18 Also, a higher BMP10 concentration was determined in cryptogenic stroke patients who were detected to have AF compared to those without AF and in AF patients with silent brain infarcts.19,20 Moreover, BMP10 was recently identified to be strongly associated with the risk of ischaemic stroke in AF patients irrespective of oral anticoagulation therapy.12 BMP10 had an added value on top of clinical variables and NT-proBNP and also increased the performance of established risk scores.12 A proposed explanation for the role of BMP10 in AF is that a higher concentration of BMP10 might reflect an advanced stage of atrial cardiomyopathy, which is related to a higher risk of AF and a higher risk of stroke, but the exact underlying pathophysiology of BMP10 remains unclear.21
In a study by Reyat et al.11 in 359 patients, BMP10 outperformed 11 other cardiovascular biomarkers in predicting AF recurrence after CA. In another study with 382 patients, BMP10 concentration was associated with ongoing AF but not with AF recurrence after cardioversion or after ≥2 symptomatic ECG-documented AF episodes in the previous 6 months.22 However, these patients were not treated using CA.22 In our cohort of 1112 patients, BMP10 was strongly associated with AF recurrence during a follow-up of 12 months after CA. Even after multivariable adjustment, the hazard remained strongly elevated in our analysis. When we compared the models with and without BMP10, we found that BMP10 was highly relevant and an important contributor to the models. Compared to the patients in the study of Reyat et al.,11 our patients were about 6 years younger on average and had fewer comorbidities. When grouping our patients according to BMP10 quartiles, the patients in quartile IV had a disproportionately higher risk of AF recurrence, whereas differences in quartiles I–III were small. Similarly, in the study by Reyat et al.,11 the lower two BMP10 quartiles appeared to show no relevant difference.
Our subgroup analysis did not reveal a difference between men and women in the association of BMP10 and AF recurrence (P for interaction = 0.6), which is important as biomarkers can differ according to sex.23 Moreover, BMP10 was fairly robust with respect to patient age, BMI, AF type, echocardiographic parameters, and comorbidities with the exception of a history of hypertension. Hijazi et al.12 found that hypertension in a multivariable model does not explain the variance in BMP10 when analysed as outcome (partial R2 close to zero). However, in the study by Hodgson et al.,24 ProBMP10 was negatively associated with hypertension in logistic regression models after adjusting for sex and BMI. Nakano et al.25 found BMP10 to be elevated during hypertension-induced cardiac hypertrophy in a rat model. At this point, it is too early to say whether there is a biological explanation for the interaction with hypertension or whether this is merely a chance finding due to multiple testing.
The measurement of atrial BMP10 in circulating blood holds promise for adding prognostic precision to the clinical evaluation of AF ablation and recurrence risk, as it can be readily assessed through peripheral blood samples. The incorporation of biomarkers has been shown to be useful for risk prediction in previous studies.26,27 Moreover, BMP10 offers an objective, quantifiable measure for atrial tissue alterations that is independent of the availability of echocardiography or the expertise of an echocardiographer. Although a commercial assay for BMP10 is not currently available, the prototype assay is running on central lab platforms and is of similar design as other blood tests (e.g. troponin), making a rapid and cost-efficient use feasible. Provided a formal device registration would be done, the BMP10 test could be implemented in any hospital with routine blood analysis, as it operates on high-throughput lab analysers, potentially making it an attractive option for further evidence generation and clinical use.
Further studies are necessary to improve the current understanding of the biology of BMP10, especially in the context of AF. In addition, clinically relevant decision rules for the use of BMP10 concentration in clinical routine should be defined in future studies.
Strengths and limitations
The key strengths of our study include the large sample size, standardized evaluation of the outcome events by trained study personnel, and the standardized measurement of BMP10 concentration.
There are some limitations that deserve mentioning. The recruitment period of 10 years was rather long, and technical standards for CA changed over time. Since we excluded patients with prior CA, our results apply exclusively to patients undergoing CA for the first time. Moreover, follow-up examination was performed using Holter monitoring, which is less reliable than continuous rhythm monitoring with implantable devices, and therefore, AF recurrence may have been missed in some patients. However, patients were instructed to report to clinicians if they felt any symptoms potentially associated with AF recurrence. Furthermore, BMP10 concentrations were measured only at study enrolment. Lastly, most study participants were of European origin thus limiting the generalizability of our results.
Conclusion
In AF patients undergoing CA for the first time, plasma concentrations of the atrial-specific biomarker BMP10 were independently associated with a higher risk of AF recurrence within a 12-month follow-up period after CA. Whether BMP10-guided patient selection for CA or adapted ablation approaches based on BMP10 levels improve outcomes needs to be determined in future studies.
Supplementary Material
Contributor Information
Elisa Hennings, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Stefanie Aeschbacher, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Michael Coslovsky, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland.
Rebecca E Paladini, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Pascal B Meyre, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Gian Voellmin, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Livia Blum, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Peter Kastner, Roche Diagnostics GmbH, Penzberg, Germany.
André Ziegler, Roche Diagnostics International AG, Rotkreuz, Switzerland.
David Conen, Population Health Research Institute, McMaster University, Hamilton, Canada.
Christine S Zuern, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Philipp Krisai, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Patrick Badertscher, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Christian Sticherling, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Stefan Osswald, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Sven Knecht, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Michael Kühne, Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Spitalstrasse 2, 4056 Basel, Switzerland; Cardiology, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Switzerland.
Supplementary material
Supplementary material is available at Europace online.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Bone morphogenetic protein 10 concentrations were measured free of charge by Roche Diagnostics.
Data availability
The consent forms, as approved by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz), do not allow the data to be made publicly available. The data will be shared on reasonable request to the corresponding author.
References
- 1. Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin Aet al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol 2009;2:349–61. [DOI] [PubMed] [Google Scholar]
- 2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 3. Turagam MK, Musikantow D, Whang W, Koruth JS, Miller MA, Langan M-Net al. Assessment of catheter ablation or antiarrhythmic drugs for first-line therapy of atrial fibrillation: a meta-analysis of randomized clinical trials. JAMA Cardiol 2021;6:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol 2014;63:2335–45. [DOI] [PubMed] [Google Scholar]
- 5. Cunha PS, Laranjo S, Heijman J, Oliveira MM. The atrium in atrial fibrillation - a clinical review on how to manage atrial fibrotic substrates. Front Cardiovasc Med 2022;9:879984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jansen HJ, Bohne LJ, Gillis AM, Rose RA. Atrial remodeling and atrial fibrillation in acquired forms of cardiovascular disease. Hear Rhythm O2 2020;1:147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qu X, Liu Y, Cao D, Chen J, Liu Z, Ji Het al. BMP10 preserves cardiac function through its dual activation of SMAD-mediated and STAT3-mediated pathways. J Biol Chem 2019;294:19877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qu X, Harmelink C, Baldwin HS. Endocardial-myocardial interactions during early cardiac differentiation and trabeculation. Front Cardiovasc Med 2022;9:857581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Ouwerkerk AF, Bosada FM, van Duijvenboden K, Hill MC, Montefiori LE, Scholman KTet al. Identification of atrial fibrillation associated genes and functional non-coding variants. Nat Commun 2019;10:4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang L, Rice M, Swist S, Kubin T, Wu F, Wang Set al. BMP9 and BMP10 act directly on vascular smooth muscle cells for generation and maintenance of the contractile state. Circulation 2021;143:1394–410. [DOI] [PubMed] [Google Scholar]
- 11. Reyat JS, Chua W, Cardoso VR, Witten A, Kastner PM, Kabir SNet al. Reduced left atrial cardiomyocyte PITX2 and elevated circulating BMP10 predict atrial fibrillation after ablation. JCI Insight 2020;5:e139179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hijazi Z, Benz AP, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JWet al. Bone morphogenetic protein 10: a novel risk marker of ischaemic stroke in patients with atrial fibrillation. Eur Heart J 2023;44:208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei Bet al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 14. Tessari A, Pietrobon M, Notte A, Cifelli G, Gage PJ, Schneider MDet al. Myocardial Pitx2 differentially regulates the left atrial identity and ventricular asymmetric remodeling programs. Circ Res 2008;102:813–22. [DOI] [PubMed] [Google Scholar]
- 15. Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson Aet al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 2007;448:353–7. [DOI] [PubMed] [Google Scholar]
- 16. Kirchhof P, Kahr PC, Kaese S, Piccini I, Vokshi I, Scheld H-Het al. PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circ Cardiovasc Genet 2011;4:123–33. [DOI] [PubMed] [Google Scholar]
- 17. Shoemaker MB, Muhammad R, Parvez B, White BW, Streur M, Song Yet al. Common atrial fibrillation risk alleles at 4q25 predict recurrence after catheter-based atrial fibrillation ablation. HeartRhythm 2013;10:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meyre PB, Aeschbacher S, Blum S, Voellmin G, Kastner PM, Hennings Eet al. Biomarkers associated with rhythm status after cardioversion in patients with atrial fibrillation. Sci Rep 2022;12:1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palà E, Bustamante A, Pagola J, Juega J, Francisco-Pascual J, Penalba Aet al. Blood-based biomarkers to search for atrial fibrillation in high-risk asymptomatic individuals and cryptogenic stroke patients. Front Cardiovasc Med 2022;9:908053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palà E, Escudero-Martínez I, Penalba A, Bustamante A, Lamana-Vallverdú M, Mancha Fet al. Association of blood-based biomarkers with radiologic markers and cognitive decline in atrial fibrillation patients. J stroke Cerebrovasc Dis Off J Natl Stroke Assoc 2022;31:106833. [DOI] [PubMed] [Google Scholar]
- 21. Sinner MF, von Falkenhausen AS. A specific new biomarker for atrial fibrillation and its sequelae? Eur Heart J 2023;44:219–20. [DOI] [PubMed] [Google Scholar]
- 22. Staszewsky L, Meessen JMTA, Novelli D, Wienhues-Thelen U-H, Disertori M, Maggioni APet al. Total NT-proBNP, a novel biomarker related to recurrent atrial fibrillation. BMC Cardiovasc Disord 2021;21:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De With RR, Artola Arita V, Nguyen B-O, Linz D, Ten CH, Spronk Het al. Different circulating biomarkers in women and men with paroxysmal atrial fibrillation: results from the AF-RISK and RACE V studies. Europace 2022;24:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodgson J, Swietlik EM, Salmon RM, Hadinnapola C, Nikolic I, Wharton Jet al. Characterization of GDF2 mutations and levels of BMP9 and BMP10 in pulmonary arterial hypertension. Am J Respir Crit Care Med 2020;201:575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakano N, Hori H, Abe M, Shibata H, Arimura T, Sasaoka Tet al. Interaction of BMP10 with Tcap may modulate the course of hypertensive cardiac hypertrophy. Am J Physiol Heart Circ Physiol 2007;293:H3396–403. [DOI] [PubMed] [Google Scholar]
- 26. Börschel CS, Ohlrogge AH, Geelhoed B, Niiranen T, Havulinna AS, Palosaari Tet al. Risk prediction of atrial fibrillation in the community combining biomarkers and genetics. Europace 2021;23:674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toprak B, Brandt S, Brederecke J, Gianfagna F, Vishram-Nielsen JKK, Ojeda FMet al. Exploring the incremental utility of circulating biomarkers for robust risk prediction of incident atrial fibrillation in European cohorts using regressions and modern machine learning methods. Europace 2023;25:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The consent forms, as approved by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz), do not allow the data to be made publicly available. The data will be shared on reasonable request to the corresponding author.