Abstract
Ferroptosis is an iron-driven cell death modality characterized by iron accumulation and excessive lipid peroxidation. Ferroptosis is closely related to mitochondrial function, as indicated by studies showing that mitochondrial dysfunction and damage promote oxidative stress, which in turn induces ferroptosis. Mitochondria play crucial roles in cellular homeostasis, and abnormalities in their morphology and function are closely associated with the development of many diseases. Mitochondria are highly dynamic organelles, and their stability is maintained through a series of regulatory pathways. Mitochondrial homeostasis is dynamically regulated, mainly via key processes such as mitochondrial fission, mitochondrial fusion and mitophagy; however, mitochondrial processes are prone to dysregulation. Mitochondrial fission and fusion and mitophagy are intimately related to ferroptosis. Therefore, investigations into the dynamic regulation of mitochondrial processes during ferroptosis are important to provide a better understanding of the development of disease. In this paper, we systematically summarized changes in ferroptosis, mitochondrial fission and fusion and mitophagy to promote an in-depth understanding of the mechanism underlying ferroptosis and provide a corresponding reference for the treatment of related diseases.
Keywords: ferroptosis, reactive oxygen species, mitochondrial homeostasis, mitochondrial fission, mitochondrial fusion, mitophagy
Introduction
Ferroptosis is a recently discovered mode of iron-dependent cell death that is usually accompanied by the accumulation of a large number of iron ions and excessive lipid peroxidation 1. The main morphological features are membrane shrinkage with increased membrane density and a reduced number of or loss of mitochondrial cristae 2. During ferroptosis, intracellular redox homeostasis is imbalanced, and the levels of antioxidants such as glutathione (GSH) and glutathione peroxidase 4 (GPX4) decrease, while the levels of oxidizing substances (e.g., divalent iron ions and lipid reactive oxygen species [ROS]) start to increase 3, 4. Mitochondria, as the main sources of intracellular ROS, are closely related to ferroptosis 5. Mitochondria are important organelles in cellular energy metabolism; their biological functions include the production of ATP through oxidative phosphorylation and the maintenance of redox homeostasis and calcium homeostasis 6, 7. Since the production of heme and iron-sulfur clusters occurs mainly in mitochondria, these organelles contain much mitochondrial iron and integrate iron metabolism within the cytosol. Iron binding to mitochondrial ferritin prevents ROS production, while the mutation or degradation of mitochondrial ferritin leads to mitochondrial iron overload 8, 9. Therefore, the maintenance of normal mitochondrial morphology and function is essential to ensure that cells carry out various physiological activities. Mitochondrial quality control is an important regulatory mechanism in mitochondrial homeostasis that is realized mainly through mitochondrial fusion, mitochondrial fission and mitophagy. Mitochondrial quality control processes protect mitochondria from stress-induced damage, including through the selective elimination of damaged mitochondrial proteins or dysfunctional mitochondria, thereby maintaining mitochondrial morphology, structure and function and promoting cell survival 10. Under the influence of various stress factors, mitochondria are highly susceptible to damage, which results in mitochondrial dysfunction, reduced ATP production, and increased ROS production, which have been shown to be associated with the pathology of numerous diseases. In view of the close relationship between mitochondrial homeostasis and ferroptosis in cells and the importance of this link in the development and treatment of diseases, in this review, we focus on the relationship between ferroptosis and mitochondrial function. The aim of the review is to provide a reference for better understanding this relationship and for establishing a theoretical basis for in-depth study of the relationship in the context of disease.
Mechanisms underlying ferroptosis
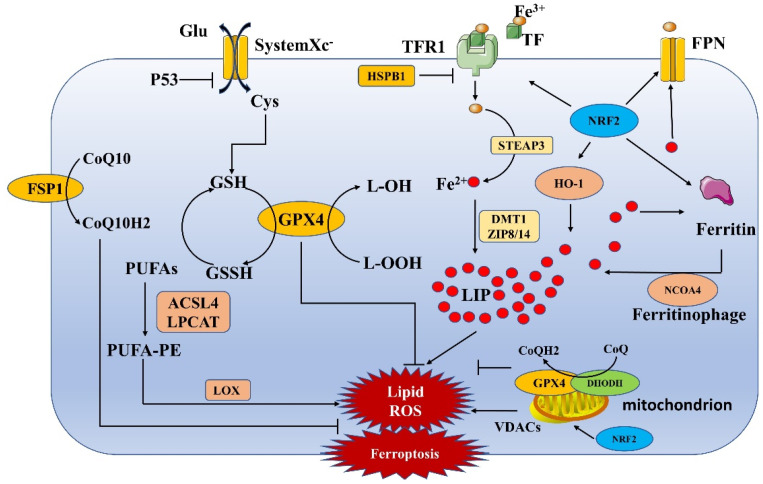
Ferroptosis is a form of cell death induced by iron-dependent accumulation of a large number of lipid peroxides (Figure 1). Both exogenous and endogenous pathways are involved in ferroptosis. The exogenous pathway induces ferroptosis mainly through the inhibition of system Xc-. The endogenous pathway network comprises various regulatory pathways, mainly lipid metabolism, iron metabolism and mitochondria-related pathways (Table 1) 4, 11.
Figure 1.
Mechanisms underlying ferroptosis. The process of ferroptosis is mainly accompanied by the accumulation of iron ions and the formation of lipid peroxides, which eventually lead to cell death. This process involves several pathways, such as the iron metabolism, System Xc-, lipid metabolism and mitochondria-related pathways.
Table 1.
Mechanisms underlying ferroptosis
| Group | Pathways | Key targets | Mechanisms |
|---|---|---|---|
| The exogenous pathway | Inhibition of system Xc- induced ferroptosis | system Xc-, GPX4, p53 | system Xc-/GSH/GPX4/ROS/Lipid ROS |
| p53/ system Xc- | |||
| The endogenous pathway | Lipid metabolism-induced ferroptosis | ACSL4, LPCAT3, LOX | PUFAs/ ACSL4 and LPCAT3/PUFA-PE/LOX/ Lipid ROS |
| Iron metabolism-regulated ferroptosis | TRF1, Ferritin, LIP, Fpn, HO-1, HSPB1, NCOA4, NRF2 | Fe3+/ TFR1/ STEAP3/ Fe2+/ DMT1 or ZIP8/14/LIP/ROS | |
| Fe2+/ Fpn/ Fe3+ | |||
| HO-1/ Fe2+/LIP | |||
| HSPB1/ TRF1 | |||
| NCOA4/ Ferritin/ Fe2+/LIP | |||
| NRF2/ TRF1, Ferritin, HO-1, Fpn/ Fe2+/LIP | |||
| Ferroptosis with mitochondrial involvement | GPX4, DHODH, NRF2, VDACs | ATP/AMPK/acetyl-CoA carboxylase | |
| TCA cycle/ electron transport and fatty acid biosynthesis | |||
| GPX4/ CoQ/CoQH2 | |||
| DHODH/ CoQ/CoQH2 | |||
| NRF2/ mitochondrial biogenesis | |||
| NRF2/ mitophagy (P62-dependent, PINK1/Parkin-independent) | |||
| NRF2/ proteasomal genes/ mitochondrial fission | |||
| NRF2/ MFN2/ mitochondrial fusion | |||
| Erastin/VDACs/ Lipid ROS | |||
| Others | FSP1, GPX4, P53 | FSP1-COQ10-NAD(P)H | |
| P53/STAT1/ALOX15/Lipid ROS | |||
| Sulfur transfer pathway |
ACSL4 Acyl-CoA synthetase long-chain family member 4; AMPK: AMP-activated protein kinase; DHODH: dihydroorotate dehydrogenase; DMT1: divalent metal transporter 1; GPX4: glutathione peroxidase 4; GSH: glutathione; HO-1: Heme oxygenase-1; HSPB1: heat shock protein B1; LIP: unstable iron pool; LOX: lipoxygenase; LPCAT3: lysophosphatidylcholine acyltransferase 3; MFN2: mitochondrial fusion protein 2; NCOA4: nuclear receptor coactivator 4; NRF2: nuclear factor red lineage 2 related factor 2; PINK1: PTEN-induced kinase 1; PUFAs: polyunsaturated fatty acids; ROS: reactive oxygen species; STEAP3: six-transmembrane epithelial antigen of prostate 3; TFR1: transferrin receptor protein 1; VDACs : mitochondrial voltage-dependent anion channels;ZIP8/14 zinc-iron regulatory protein family 8/14
Inhibition of System Xc-induced ferroptosis
System Xc-, which consists of two subunits, SLC7A11 and SLC3A2, is a reverse transporter of amino acids and an important antioxidant complex. Cystine and glutamate are exchanged intra- and extracellularly at a 1:1 ratio through system Xc-[1]. Inhibition of system Xc- affects GSH synthesis by blocking cystine uptake, which in turn leads to a decrease in GPX4 activity. This alteration ultimately leads to a reduction in cellular antioxidant capacity and the accumulation of lipid ROS, causing the onset of oxidative damage and ferroptosis. In addition, p53 can directly inhibit System Xc-induced ferroptosis 12, 13.
Lipid metabolism-induced ferroptosis
Ferroptosis cannot occur without lipids, and polyunsaturated fatty acids (PUFAs) are among the essential elements. Free PUFAs are esterified into membrane phospholipids and oxidized to further promote ferroptosis 14. Studies have shown that phosphatidylethanolamine (PE) containing arachidonic acid (AA) or its derivative epinephrine is the key phospholipid in ferroptosis. Acyl-CoA synthetase long-chain family member 4 (ACSL4) is important for the synthesis of phospholipids, while lysophosphatidylcholine acyltransferase 3 (LPCAT3) is important for the insertion of PUFAs into membrane phospholipids 15. ACSL4 and LPCAT3 together influence lipid peroxidation and are key nodes in the regulation of ferroptosis. Ultimately, PUFA-PEs contribute to further oxidation catalyzed by lipoxygenase (LOX), eventually inducing ferroptosis 16. Nuclear factor erythroid 2-related factor 2 (NRF2) can also regulate lipid metabolism, for example, through the ligand-mediated regulation of the transcription factor peroxisome proliferator-activated receptor γ (PPARγ)17.
Iron metabolism-regulated ferroptosis
Ferroptosis is primarily driven by iron-dependent lipid peroxidation. Many aspects of iron metabolism, such as iron uptake, storage and utilization, play important roles in the regulation of ferroptosis. Nonheme iron (Fe3+) binds to transferrin (TF), and then TF receptor protein 1 (TFR1) mediates the uptake of the TF-Fe3+ complex into the cell. Fe3+ is reduced to Fe2+ by six-transmembrane epithelial antigen of prostate 3 (STEAP3) and is then stored by divalent metal transporter 1 (DMT1) or zinc-iron regulatory protein family 8/14 (ZIP8/14) as part of the cytoplasmic labile iron pool (LIP) or as ferritin; the excess Fe2+ is oxidized to become Fe3+ outside the cell by the membrane iron transport export protein (Fpn)18. Heme oxygenase-1 (HO-1) accelerates ferroptosis by increasing the number of iron ions, while heat shock protein B1 (HSPB1) inhibits ferroptosis by reducing iron ion uptake via inhibition of TRF1 expression 19, 20. In addition, heme degradation and nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy increase the size of the LIP, sensitizing cells to ferroptosis via the Fenton reaction 21, 22. In addition, the metabolism, storage, and transport of many iron-related proteins, including ferritin, TFR, Fpn, and HO-1, are regulated by NRF2. NRF2 regulation thus alters intracellular iron levels, which in turn affects ferroptosis in cells 23.
Regulation of ferroptosis with mitochondrial involvement
Mitochondria are the main sources of cellular ROS, and mitochondrial ROS production may contribute to ferroptosis by promoting lipid peroxidation 24. Mitochondria are also the main organelles for ATP production, and electron transport and proton pumping in mitochondria play important roles in the induction of ferroptosis. When ATP is deficient, the energy sensor AMP-activated protein kinase (AMPK) is activated, which affects the activity of acetyl-CoA carboxylase (the rate-limiting enzyme for fatty acid synthesis) and inhibits ferroptosis 25, 26. Mitochondria also have additional functions in biosynthesis and cellular metabolism that are mediated by the TCA cycle and various anaplerotic reactions that complement the TCA cycle 27. The potential mechanism by which the TCA cycle regulates ferroptosis may be related to the function of this cycle in electron transport and fatty acid biosynthesis 5.
GPX4 and dihydroorotate dehydrogenase (DHODH) in mitochondria are key members of the two systems that protect cells from ferroptosis. Disabling one system forces cells to become more dependent on the other, while simultaneous inhibition of mitochondrial GPX4 and DHODH triggers ferroptosis mainly via mitochondrial lipid peroxidation 5, 28. Mechanistically, DHODH acts in parallel with mitochondrial GPX4 (independent of cytoplasmic GPX4 or FSP1) to inhibit ferroptosis in the mitochondrial inner membrane (MIM) by reducing CoQ to CoQH2 (a radical-trapping antioxidant with antiferroptotic activity) 28.
The antioxidant transcription factor NRF2 is also an important regulator of mitochondrial function and has important effects on ferroptosis. NRF2 can bind to mitochondria and thus can indicate and influence changes in mitochondrial function 29. Knockdown of NRF2 in mice impairs mitochondrial function, while activation of NRF2 enhances mitochondrial function and enhances stress resistance 30, 31. NRF2 regulates mitochondrial function in multiple ways and affects the dynamic homeostasis of mitochondria. NRF2 can affect mitochondrial biogenesis by regulating the expression of PGC-1α, NResF1, NResF2, TFAM and mitochondrial genes 32; it can also regulate mitophagy through a P62 dependent, PTEN-induced kinase 1 (PINK1)/Parkin-independent mechanism 33. Furthermore, NRF2 can regulate proteasomal genes and MFN2, thereby regulating mitochondrial fission and mitochondrial fusion, respectively 34. Overall, NRF2 regulates mitochondrial function, and alterations in mitochondrial function further influence ferroptosis.
Other mechanisms
Other pathways, such as the mitochondrial voltage-dependent anion channel pathway, p53 pathway, FSP1-COQ10-NAD(P)H pathway, NRF2-regulated iron and lipid metabolism pathways and sulfur transfer pathway, can also influence the course of ferroptosis 13, 17, 23, 35-38. Abnormalities in these pathways directly or indirectly lead to an imbalance in the intracellular oxidative response, contributing to ferroptosis.
Dynamic regulation of mitochondria
Mitochondria are constantly undergoing dynamic changes within a cell. Mitochondrial self-regulation occurs through constant fission and fusion, providing energy for cellular homeostasis and regulating macroautophagy, calcium homeostasis, innate immunity, signaling and apoptosis. The relative balance of mitochondrial fission and fusion is essential to maintain the quality and function of mitochondria and is an important basis for normal cellular activity 7, 39. In contrast, mitophagy involves the sequestration and removal of damaged mitochondria from a cell through a specific autophagic pathway, thereby maintaining intracellular mitochondrial homeostasis 40. The interaction and coregulation of mitophagy and mitochondrial fission and fusion are mechanisms important for the maintenance of mitochondrial homeostasis and mitochondrial quality (Table 2) 41, 42.
Table 2.
Dynamic regulation of mitochondria
| Pathways | Key genes | Mechanisms | Roles | ||
|---|---|---|---|---|---|
| Mitochondrial fission | DRP1-mediated pathway | DRP1, Mff, Fis1, MiD49, MiD51 | The recruited DRP1 binds to the protein receptor (Mff, Fis1, MiD49 and MiD51), which breaks the inner and MOM, leading to mitochondrial fission. | Splitting of damaged mitochondria into two types of mitochondria with normal and abnormal function. | |
| Mitochondrial fusion | MFN1, MFN2 and OPA1-mediated pathways | MFN1, MFN2, OPA1 | The fusion of the MOM is mainly mediated by MFN1 and MFN2, while the fusion of the MIM is mediated by OPA1. | Maintains mitochondrial homeostasis by fusing damaged mitochondria with normal mitochondria to perform relatively normal functions. | |
| Mitophagy | PINK1/Parkin pathways | PINK1/Parkin | PINK1 aggregates at the MOM and recruits and activates Parkin, which in turn activates Parkin to polyubiquitinate a variety of mitochondrial protein substrates. Ultimately, mitophagy is induced by the action of LC3. | Mitophagy can wrap and remove damaged mitochondria from the cell through the autophagic pathway, thereby maintaining intracellular mitochondrial homeostasis. | |
| PINK1/Parkin-independent pathways | OPTN, NDP52, ULK1, DFCP1, WIPI1, synphilin-1 | PINK1 directly recruits OPTN and NDP52 to damaged mitochondria and subsequently recruits and activates ULK1, DFCP1 and WIPI1, which in turn induce mitophagy. PINK1-synphilin-1 complex induces mitophagy by recruiting SIAH-1 to accelerate the ubiquitination of proteins in damaged mitochondria. |
|||
| Receptor-mediated pathways | Receptors in the MOM | NIX/BNIP3L, BNIP3, FUNDC1, NLRX1, FKBP8, Bcl2L13 | The receptors located in the MOM all contain a binding site for the LC3 receptor, which can directly bind LC3 to induce mitophagy. | ||
| Receptors in the MIM | PHB2 and CL | Mitochondrial damage leads to the exposure of PHB2, which in turn binds to LC3; moreover, PHB2 induces mitophagy by stabilizing PINK1 and promoting PRKN/Parkin recruitment, protein ubiquitination and OPTN recruitment to mitochondria. CL transfer from MIM to MOM directly interacts with LC3 to induce mitophagy |
|||
DRP1: dynamin-related protein 1; Fis1: mitochondrial fission protein 1; Mff: mitochondrial fission factor; MFN1: mitochondrial fusion protein 1; MFN2: mitochondrial fusion protein 2; MiD49: mitochondrial dynamics protein 49; MiD51: mitochondrial dynamics protein 51; MIM: mitochondrial inner membrane; MOM: mitochondrial outer membrane; OPA1: optic atrophy 1 protein; OPTN: Optineurin; PINK1: PTEN-induced kinase 1; ULK1: unc-51 like kinase 1; WIPI1: WD repeat domain phosphoinositide interacting 1
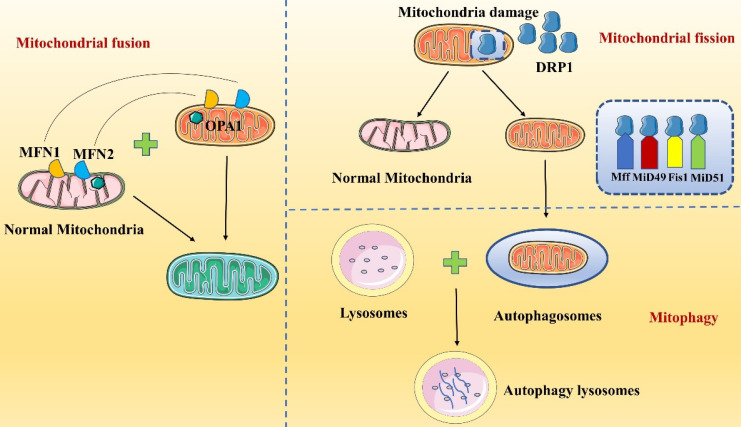
Mitochondrial fission
Mitochondria are double-membrane organelles with a mitochondrial outer membrane (MOM) and a MIM, which are separated by the intermembrane space (IMS). Mitochondrial fission contributes to quality control because it causes damaged mitochondria to split into two mitochondria: one mitochondrion with normal function and one with abnormal function (Figure 2). Then, abnormally functioning mitochondria are eliminated by mitophagy to maintain mitochondrial homeostasis in vivo 43. During mitochondrial fission, GTPase dynamin-related protein 1 (DRP1), also known as dynamin-1-like protein (DNM1L), is a key mediating factor 44-47. DRP1 interacts with a variety of protein receptors in the MIM or MOM; these receptors include mitochondrial fission factor (Mff), mitochondrial fission protein 1 (Fis1), mitochondrial dynamics protein 49 (MiD49) and mitochondrial dynamics protein 51 (MiD51) 48. Multiple DRP1 molecules are recruited to a single mitochondrion and bind to receptors, closely surrounding the mitochondrion to form a ring finger structure. Then, via their GTPase activity, DRP1 molecules hydrolyze GTP, which leads to MIM and MOM permeabilization and causes mitochondrial fission. After mitochondrial fission, DRP1 is relocalized to the cytosol 46, 47. Notably, DRP1 action is regulated by posttranslational modifications, such as ubiquitination and phosphorylation, and by metabolic signaling 49.
Figure 2.
Regulation of mitochondrial homeostasis. Mitochondrial homeostasis is maintained mainly by mitochondrial fission, mitochondrial fusion and mitophagy.
Mitochondrial fusion
Mitochondrial fusion requires both the MOM and MIM. Fusion involves multiple proteins and is initiated by the tethering of mitochondria followed by sequential fusion of the MOMs and MIMs (Figure 2)50. Three main proteins participate in mitochondrial fusion in mammalian cells: mitochondrial fusion protein 1 (MFN1), mitochondrial fusion protein 2 (MFN2) and optic atrophy 1 protein (OPA1) 51, 52. MOM fusion is mediated primarily by MFN1 and MFN2, while MIM fusion is mediated by OPA1. MFN1 and MFN2 are in the MOM and form homodimeric or heterodimeric structures to connect two adjacent mitochondria, contributing to mitochondrial transmembrane protein interactions 53, 54. Next, the MFN GTPase structural domain hydrolyzes GTP, causing membrane conformational changes that initiate mitochondrial fusion. Hence, MFN1 is considered the major GTP-dependent membrane-bound protein for mitochondrial fusion; mitochondria cannot fuse in cells lacking MFN1, while MFN2 is thought to act at a later stage of the fusion process 55. MOM fusion is followed by MIM fusion, which is mediated mainly by OPA1, a kinesin-related GTP enzyme with two isoforms, long-OPA1 (L-OPA1) and short-OPA1 (S-OPA1), that synergistically regulate mitochondrial fusion. When the transmembrane potential of the MIMs is intact, the L-OPA1 isoform mediates mitochondrial fusion by binding to cardiolipin (CL)52. When the MIM potential is lost, such as in a stress state, L-OPA1 is rapidly hydrolyzed to S-OPA1, leading to mitochondrial fusion. During mitochondrial fusion, information and material are transferred between the MIMs and MOMs. In summary, MFN1 may interact with the MIM protein OPA1, and OPA1 induces MIM fusion by relying on MFN1 but not on MFN256.
Mitophagy
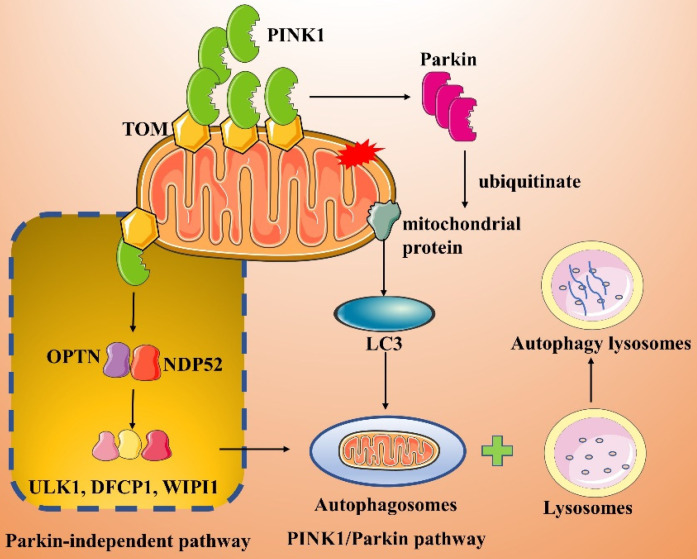
PINK1/Parkin and PINK1/Parkin-independent pathways
The PINK1/Parkin pathway is the classical and most extensively studied pathway in mitophagy (Figure 3)57-59. PINK1 is a mitochondrial serine/threonine kinase containing a mitochondrial targeting sequence (MTS), while Parkin is an E3 ubiquitin ligase present in the cytosol. Both proteins play important roles in mitochondrial autophagic degradation 60. Under normal cellular physiological conditions, PINK1 is sequentially translocated into the MOM by translocases. The MTS sequence of PINK1 is then excised, leading to its degradation. When a mitochondrion is damaged, its membrane potential rapidly decreases, and the import of PINK1 into the mitochondrion is inhibited; therefore, PINK1 accumulates on the MOM 60. The accumulated PINK1 recruits and activates Parkin, which in turn polyubiquitinates a variety of mitochondrial protein substrates 61, 62. Ultimately, in the presence of LC3 splice proteins, autophagosomes are targeted to the mitochondrion, inducing mitophagy.
Figure 3.
Parkin-dependent and -independent pathways of mitophagy activated by PINK1.
Lazarou et al. 63 showed that PINK1 directly recruits optineurin (OPTN) and NDP52 to damaged mitochondria in a Parkin-independent manner and subsequently recruits and activates the autophagy initiator unc-51-like kinase 1 (ULK1), double FYVE-containing protein 1 (DFCP1) and WD repeat domain phosphoinositide interacting 1 (WIPI1), which in turn induce mitophagy (Figure 3). In addition, synphilin-1 overexpression mediates the accumulation of large amounts of PINK1 on the MOM, and the PINK1-synphilin-1 complex induces mitophagy by recruiting SIAH-1 to accelerate the ubiquitination of proteins in damaged mitochondria 64.
Receptor-mediated pathways
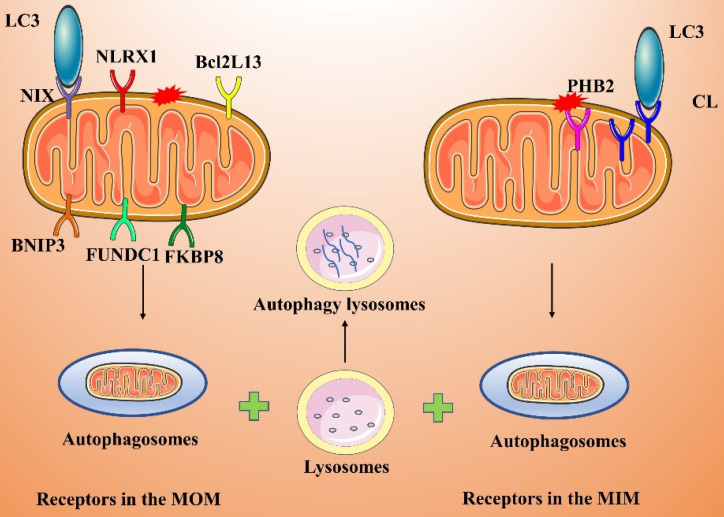
Some mitochondrial membrane proteins are LC3 receptors that directly recognize and bind LC3, thereby inducing mitophagy. LC3 receptors include Nip3-like protein X (NIX/BNIP3L), BNIP3, FUNDC1, NLRX1, FKBP8, Bcl2L13, PHB2, and CL (Figure 4).
Figure 4.
Receptor-mediated activation pathway of mitophagy.
Receptors in the MOM
NIX and BNIP3 are in the MOM, and both contain a BH3 structural domain that directly binds LC3 to induce mitophagy 65, 66. These two receptors mediate mitophagy primarily under hypoxic or postinjury conditions; in particular, hypoxia leads to effective inhibition of mitophagy by inhibiting the actions of NIX and BNIP3. Under hypoxic conditions, intracellular ROS production increases, and hypoxia-inducible factor 1 (HIF-1) can bind to NIX/BNIP3, thereby increasing the expression level of NIX/BNIP3 and promoting mitophagy 67.
FUNDC1 is an MOM protein that, under hypoxic conditions, induces mitophagy by binding directly to LC368, 69. FUNDC1 is in a phosphorylated form in the physiological state, but under hypoxia, FUNDC1 undergoes dephosphorylation, which enhances its interaction with LC3 and promotes mitophagy 70, 71. In addition, FUNDC1-induced mitophagy activity is regulated by ubiquitination. Under hypoxic conditions, membrane-associated ring finger protein 5 (MARCH5) promotes the ubiquitination of FUNDC1 at Lys119, which leads to the degradation of FUNDC1. In contrast, knocking down MARCH5 impairs the ubiquitination and degradation of FUNDC1, thereby enhancing hypoxia-induced mitophagy 72.
NLRX1 is an important cytoplasmic pattern recognition receptor and a member of the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family. NLRX1 has been identified as a novel mitophagy-related receptor 73, 74. For example, NLRX1 is essential for the induction of mitophagy by Listeria monocytogenes. Specifically, the virulence factor LLO in L. monocytogenes induces the oligomerization of NLRX1, which in turn promotes the intercalation of the LC3-interacting region (LIR) of NLRX1 into LC3 to induce mitophagy, maintain cellular homeostasis, and promote the survival of L. monocytogenes 73.
FKBP8 is in the MOM and interacts directly with LC3A to induce mitophagy in a Parkin-independent manner 75, 76. When damaged mitochondria are engulfed by autophagosomes, FKBP8 is translocated from mitochondria to the endoplasmic reticulum (ER) to prevent its own degradation.
BCL2L13 is a homolog of yeast Atg32 and is known to induce mitophagy in cells lacking Atg32. BCL2L13 localizes to the MOM and, similar to other LC3 receptors, binds directly to LC3 through its LIR to induce mitophagy 77. The ability of BCL2L13 to induce mitophagy is regulated by its phosphorylation. Phosphorylation of BCL2L13 Ser272 promotes BCL2L13 binding to LC3 and enhances its mitophagy-inducing action 78.
Receptors in the MIM
PHB2 and CL both localize to the MIM 79, 80. Parkin-mediated ubiquitination-related degradation of MOM proteins leads to MOM damage, resulting in exposure of the MIM protein PHB2, which in turn binds to LC3. Moreover, PHB2 induces mitophagy by stabilizing PINK1 and promoting the recruitment of PRKN/Parkin, which ubiquitinates the PHB2 protein, and by recruiting OPTN to mitochondria 81. Ultimately, autophagosomes are compensatorily recognized by different receptor proteins localized to the MOM and MIM of damaged mitochondria, perhaps improving the efficiency of mitophagy.
CL is a membrane lipid and LC3 receptor on the MIM that induces mitophagy 80, 82. CL, a characteristic phospholipid of the MIM, is involved in lipid-protein interactions and is a raw material necessary for mitochondrial functions (e.g., cristae formation and membrane fusion). After treating cells with CCCP or the mitophagy inducer rotenone, Chu et al. observed that CL was transferred from the MIM to the MOM and was highly expressed on the MOM 80. Inhibiting molecular interactions between CL and proteins, interfering with CL synthase or restricting CL transport to the MOM blocks mitophagy.
Ferroptosis and the dynamic regulation of mitochondria
Mitochondria are essential for the process of ferroptosis. Mitochondria are semiautonomous organelles that play key roles in the cellular stress response, energy metabolism and cell survival. Mitochondrial quality control is accomplished primarily through the dynamic regulation of mitochondrial fission, mitochondrial fusion and mitophagy. Maintaining mitochondrial quality control is important for mitochondrial homeostasis in vivo. Pathological conditions, such as mtDNA mutations, mitochondrial fusion and fission imbalance, and dysregulated mitophagy, can lead to abnormal mitochondrial morphology, oxidative phosphorylation dysfunction, reduced ATP synthesis, cytochrome C release, and activation of mitochondria-dependent cell signaling, which in turn can cause mitochondrial dysfunction. Changes in mitochondrial dynamics regulation can influence mitochondrial function and thus regulate the development of ferroptosis.
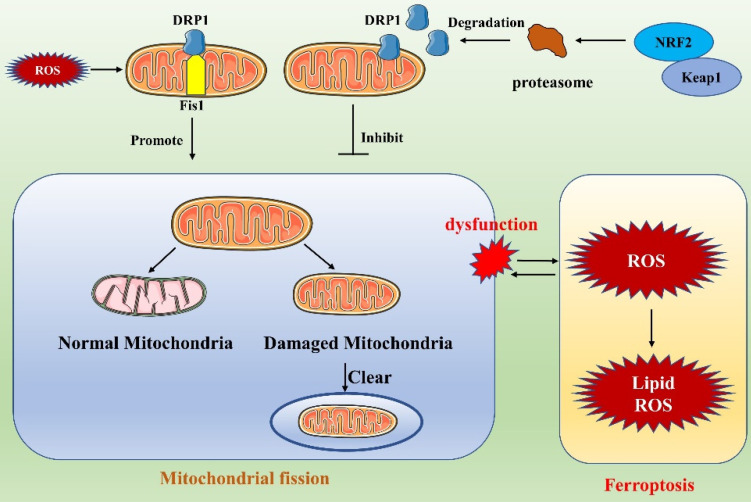
Ferroptosis and mitochondrial fission
Ferroptosis, which is characterized by iron dependence and lipid peroxide accumulation, is a regulated cell death modality that depends on ROS 4. Mitochondrial fission is an important means of cellular self-regulation and plays a key role in maintaining mitochondrial function. Ferroptosis and mitochondrial fission intersect (Figure 5). For example, NRF2, an important factor in the regulation of ferroptosis, regulates proteasomal genes that contribute to the degradation of DRP1, thereby inhibiting mitochondrial fission 83. The interaction of mitochondrial fission with ferroptosis is complex and diverse. Liu et al. 84 found that cisplatin-induced intestinal injury involves ferroptosis and that the protein and mRNA expression levels of the mitochondrial fission-related proteins DRP1 and Fis1 are increased during ferroptosis in injured intestines. They found that vitamin D3 treatment alleviates the accumulation of ROS by inhibiting ROS production, thereby attenuating excessive mitochondrial fission and increasing mitochondrial ATPase activity. This treatment also increases cellular antioxidant capacity, inhibits the accumulation of ROS and malondialdehyde, and suppresses ferroptosis. These outcomes strongly suggest an interaction between ferroptosis and mitochondrial fission 84. Other recent studies have shown that the relationship between mitochondrial fission and ferroptosis is profoundly involved in the development of cancer. Notably, Liu et al. found that the ferroptosis inducer erastin sensitizes non-small cell lung cancer (NSCLC) cells to celastrol by activating the ROS-mitochondrial fission-mitochondrial autophagy pathway, enhancing the anticancer effects of celastrol 85. Malignant mesothelioma (MM) is an aggressive tumor with a poor prognosis. Mitochondrial fission is enhanced after inhibition of carbonic anhydrase 9, a membrane-associated α-CA that has been targeted by drugs in a variety of cancers, and ferroptosis has been shown to participate in this process 86. Similarly, when ZENG et al. inhibited SHARPIN gene expression in cholangiocarcinoma, they found that ROS accumulation and mitochondrial fission were suppressed and that ferroptosis in cells was promoted through the p53/SLC7A11/GPX4 signaling pathway 87. These findings illustrate that in the treatment of tumors, targeting of a single gene may regulate mitochondrial fission and ferroptosis simultaneously, enhancing the anticancer effect. This may provide new ideas not only for drug development but also for a new level of tumor treatment.
Figure 5.
Link between ferroptosis and mitochondrial fission.
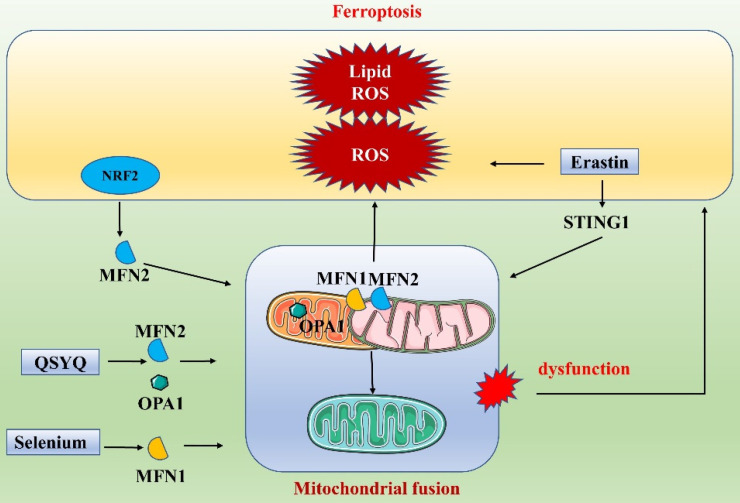
Ferroptosis and mitochondrial fusion
Under normal conditions, mitochondria are altered as fission and fusion processes are balanced. However, when this dynamic balance is disrupted, mitochondria may become functionally altered or defective. NRF2, a key factor in ferroptosis, also exerts an important regulatory function in mitochondrial fusion. NRF2 activation can promote the expression of MFN2, further promoting mitochondrial fusion. This suggests a close link between mitochondrial fusion and ferroptosis and a network of coregulated gene interactions 88. In recent years, many researchers have gradually explored the specific processes of ferroptosis and mitochondrial fusion (Figure 6). Li et al. found that targeting mitochondrial fusion-mediated ferroptosis may be a promising strategy for future cancer therapy 89. Specifically, they found that the ER protein STING1 (also known as STING or TMEM173) promoted ferroptosis in human pancreatic cancer cell lines by increasing mitochondrial fusion protein MFN1/2-dependent mitochondrial fusion. The classical ferroptosis inducer erastin induced STING1 translocation from the ER to mitochondria, where it accumulated, and the accumulated STING1 bound to MFN1/2, triggering mitochondrial fusion. The increases in ROS and fatty acid levels during mitochondrial fusion led to subsequent ROS production and lipid peroxidation, promoting ferroptosis. In contrast, deletion of the STING1 or MFN1/2 genes reduced the sensitivity of pancreatic cancer cells to ferroptosis. In addition, in a study on cerebral ischemia/reperfusion (I/R) injury, Shi et al. elaborated on the link between mitochondrial fusion and ferroptosis 90. They found that treatment with selenium attenuated cerebral I/R injury and increased mouse survival. Furthermore, in a mouse model of middle cerebral artery occlusion (MCAO) and in N2a cells subjected to oxygen/glucose deprivation/reoxygenation, selenium treatment significantly attenuated oxidative stress and iron ion accumulation. Mechanistically, selenium promoted mitochondrial fusion through upregulation of MFN1 expression, which in turn attenuated oxidative stress and ferroptosis. An increasing number of drugs have been found to regulate both mitochondrial fusion and ferroptosis. For example, QiShenYiQi dripping pill (QSYQ) reduces myocardial ischemia-induced mitochondrial prolapse by improving mitochondrial homeostasis and biosynthesis. Specifically, QSYQ promotes mitochondrial biogenesis (PGC-1α, Nrf1 and TFAM) and mitochondrial fusion (MFN-2 and OPA1) and inhibits excessive mitochondrial fission (phosphorylation of DRP1 at Ser616) 91. These results suggest that dysfunction of mitochondrial fusion, which is part of the dynamic regulation of mitochondria, affects overall mitochondrial function and consequently affects ferroptosis. In conclusion, dysregulation of mitochondrial fusion leads to an energy crisis, coordinates ferroptosis, and affects the redox state of the cell.
Figure 6.
Link between ferroptosis and mitochondrial fusion.
Ferroptosis and mitophagy
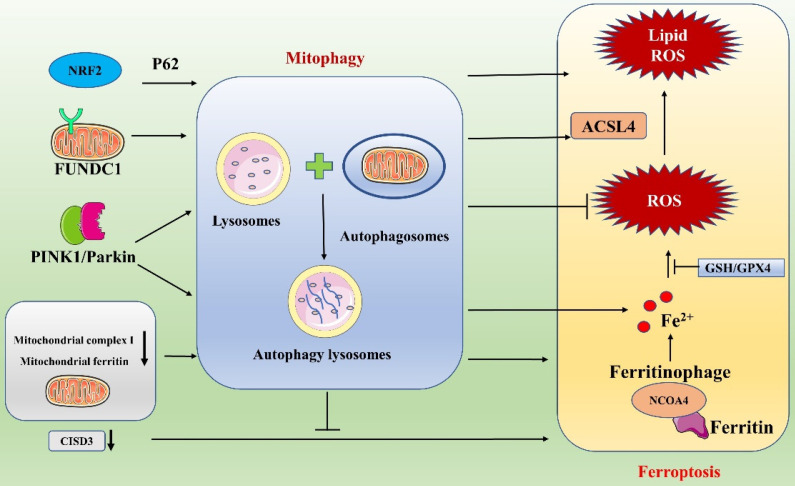
Macroautophagy (hereafter referred to as autophagy) is a conserved cellular process capable of maintaining homeostasis in vivo by degrading various biomolecules and organelles via the lysosomal pathway 92. Recent studies have progressively revealed that there is an important interaction between ferroptosis and autophagy: ferroptosis requires the autophagic mechanism for its execution 93, 94. Among the discovered autophagic processes, selective types of autophagy (e.g., ferritinophagy, lipophagy, mitophagy, clockophagy, etc.) play important roles in driving cells toward ferroptosis. The specific processes include NCOA4-promoted ferritinophagy, BECN1-mediated systemic Xc- inhibition, RAB7A-dependent lipophagy, STAT3-induced lysosomal membrane permeabilization, SQSTM1-dependent clockophagy, HSP90-associated chaperone protein-mediated autophagy and PINK1-associated mitophagy, all of which regulate the cellular ferroptosis process 93, 94.
As a type of selective autophagy, mitophagy plays a pivotal role in maintaining mitochondrial homeostasis by removing damaged mitochondria. Mitophagy is a mechanism for selective degradation of damaged mitochondria through autophagic flux and occurs via ubiquitin-dependent and ubiquitin-independent pathways 95. When cells are pathologically altered, damaged or dysfunctional mitochondria cause further damage to cells by producing large amounts of ROS and releasing proapoptotic factors. Therefore, timely removal of damaged mitochondria is essential for cellular homeostasis and viability. Ferroptosis may lead to cell damage, and mitophagy might play a protective role by suppressing the release of ROS from dysfunctional mitochondria (Figure 7) 96. Interestingly, mitophagy can either enhance or suppress ferroptosis. In response to mild stress or in the early stages of iron overload, mitophagy may sequester iron in mitophagosomes and thus reduce the amounts of source materials for ROS in ferroptosis 97. However, extensive mitophagy may ultimately provide additional iron, which amplifies lipid peroxidation and ferroptosis. Therefore, the degree of autophagic flux during mitophagy may vary, and the effects of mitophagy on ferroptosis may differ. As research has advanced, some inhibitors of ferroptosis (e.g., some antioxidants) have been shown to affect mitophagy; similarly, certain drugs related to the regulation of mitophagy also exert important effects on ferroptosis 96, 98. The synergistic effects of these drugs are significant in the treatment of diseases. For example, the compound WJ460, which targets the oncoprotein Myoferlin, synergistically promotes mitophagy and ROS accumulation in pancreatic cancer cells with the ferroptosis inducers erastin and RSL3, thereby leading to ferroptosis. In contrast, the mitochondrial inhibitor Mdivi1 and an iron chelator inhibit ROS production and restore cell proliferation 98. These findings suggest new research ideas for sensitivity studies with certain anticancer drugs.
Figure 7.
Link between ferroptosis and mitophagy.
In recent years, an increasing number of researchers have linked mitophagy with ferroptosis and have explored their associations and mechanisms of action in depth. FUNDC1, one of the receptor proteins that mediates mitophagy, has a key link with ferroptosis. The relationship between FUNDC1-mediated mitophagy and ferroptosis has been studied in detail in the context of cardiovascular disease by Peng and Pei et al 99, 100. In a study on paraquat toxicity to the heart, Peng et al. found that FUNDC1/JNK-mediated ferroptosis plays an important role in cardiac and mitochondrial damage induced by paraquat exposure: it prevented paraquat-induced mitophagy and ferroptosis when FUNDC1 was absent and effectively reduced cardiac injury 99. Pei et al. found that FUNDC1 deficiency caused metabolic and cardiac remodeling/contraction dysfunction in mice on a short-term high-fat diet. Specifically, FUNDC1 regulates ferroptosis via ACSL4 and influences the course of the disease 100. These findings support an interaction between FUNDC1 and ferroptosis and suggest that FUNDC1 may serve as a target for the diagnosis and treatment of cardiovascular disease. In addition, Granata et al. analyzed ferroptosis and mitophagy in the context of renal transplantation 96. They concluded that during I/R injury in kidney transplantation, oxidative stress is a key activator of several pathways, including the ferroptosis and mitophagy pathways. Ferroptosis may lead to renal damage, whereas mitophagy may play a protective role by reducing the release of ROS via elimination of dysfunctional mitochondria. A deeper understanding of these two pathways may lead to the identification of a new noninvasive biomarker for early detection of delayed graft function or to the development of clinically translatable pharmacological strategies. In our in-depth study, we found that ferroptosis and mitophagy are mediated through many interacting network nodes that can influence each other and play unique roles. For example, ferritinophagy plays a critical role in ferroptosis, and interactions between mitophagy and ferritinophagy pathways can promote ferroptosis 97. Fan et al. found that a major trophic sensor of glucose flux (protein O-GlcNAcylation) plays an important coordinating role in ferroptosis and mitophagy. Specifically, they found that protein O-GlcNAcylation regulates ferritin uptake and mitochondrial behavior, thereby controlling ferroptosis. When ferroptosis is induced, O-GlcNAc transferase is inactivated, leading to de-O-GlcNAcylation of ferritin, which activates NCOA4-mediated ferritinophagy and mitophagy; together, ferroptosis and ferritinophagy provide sources of unstable iron necessary for the Fenton reaction, leading to accelerated ROS production and lipid peroxidation 97. Moreover, simultaneous inhibition of ferritinophagy and mitophagy (e.g., double knockdown of NCOA4 and PINK1) almost completely blocks ferroptosis. Similarly, Singh et al. suggested that mitochondrial phagocytosis, ferritin phagocytosis, and lysosomal destabilization may play key roles in ferroptosis 101. In their study of diabetic retinopathy (DR), Singh et al. found that high levels of glucose induced TXNIP upregulation and that the associated redox stress caused mitochondrial dysfunction, mitophagy, ferritinophagy, and lysosomal destabilization. Then, unstable ions reacted with hydrogen peroxide to generate hydroxyl radicals and cause membrane phospholipid peroxidation due to reduced GSH levels and GPX4 activity, triggering ferroptosis.
Interestingly, abnormalities in certain mitochondrial molecules and functions are known to affect mitophagy and ferroptosis. For example, inhibition of mitochondrial complex I can trigger an increase in mitophagy-dependent ROS production, leading to ferroptosis in melanoma cells 102. Li et al. found that knockdown of the mitochondria-localized protein CISD3 significantly accelerates lipid peroxidation and exacerbates iron aggregation triggered by System Xc- suppression or cystine deprivation, which in turn promotes cellular ferroptosis. In contrast, activation of mitophagy alleviates CISD3 knockdown-induced ferroptosis by eliminating damaged mitochondria 103. Furthermore, maintenance of mitochondrial iron homeostasis plays an important role in the prevention of ferroptosis. Mitochondrial ferritin deficiency promotes ferroptosis mediated via mitophagy in osteoblasts of type 2 diabetic osteoporosis patients 104. In conclusion, a close association between mitochondrial autophagy and ferroptosis has been reported, and an in-depth exploration of the mechanisms involved in this association will provide new insights for future studies on certain diseases.
Discussion
With a deepening understanding of ferroptosis, an increasing number of researchers have found that the maintenance of normal mitochondrial function plays an important role in ferroptosis (Table 3)5. Mitochondrial maintenance depends on a series of processes, such as mitochondrial fission, mitochondrial fusion and mitophagy, that are regulated by mitochondria themselves. Mitochondria undergo self-regulation through continuous fission and fusion, thereby maintaining the stability of mitochondria and cells. Through mitophagy, damaged mitochondria are eliminated from cells, thereby maintaining intracellular mitochondrial homeostasis. Mitophagy and mitochondrial fission/fusion are critical pathways that interact with and regulate one another to maintain mitochondrial homeostasis and normal cellular biological functions 105. In contrast, ferroptosis is an iron-dependent cell death modality induced by excessive ROS and lipid peroxide production. During the onset of ferroptosis, the main pathway of ROS production is in mitochondria. Dysregulation of mitochondrial function leads to an energy crisis, coordinates ferroptosis, and affects the cellular redox state. Thus, mitochondrial dynamics regulatory processes are an important means of maintaining mitochondrial quality control. Numerous mitochondria-regulated molecules, such as the mitochondrial fission genes DRP1 and FIS1, the mitochondrial fusion-related genes MFN1/2 and OPA1, and the mitophagy-related genes PINK1 and FUNDC1, are involved in ferroptosis. Changes in these genes profoundly affect mitochondrial function through different pathways, thus affecting intracellular oxidative and antioxidant systems and ultimately inhibiting or promoting ferroptosis. In addition, ferroptosis and mitochondrial dynamics are synergistically regulated, and an imbalance between the two disrupts intracellular homeostasis. For example, simultaneous inhibition of ferritinophagy and mitophagy (through double knockdown of NCOA4 and PINK1) almost blocks ferroptosis induced by O-GlcNAcylation 97. Mitochondria thus play an important role in the initiation of ferroptosis; for example, the ferroptosis agonist erastin directly targets the mitochondrial anion channel to induce ferroptosis 35. Given the evidence, the role of mitochondria in ferroptosis is unquestionable.
Table 3.
Ferroptosis and Mitochondrial Fission, Fusion, Mitophagy
| Group | Mitochondrion | Ferroptosis | Mechanism | Related articles | Ref. |
|---|---|---|---|---|---|
| Ferroptosis and mitochondrial fission | DRP1 Fis1 Mff |
NRF2, ROS, lipid peroxidation | Vitamin D3/ DRP1, Fis1/ROS/ lipid peroxidation | Vitamin D3 attenuates cisplatin-induced intestinal injury by inhibiting ferroptosis, oxidative stress, and ROS-mediated excessive mitochondrial fission. | 84 |
| Erastin/ROS/DRP1, Fis1, MFF/ mitophagy (PINK1, Parkin) | Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS-mitochondrial fission-mitophagy axis. | 85 | |||
| NRF2/ proteasomal genes /DRP1 | The Keap1-Nrf2 Stress Response Pathway Promotes Mitochondrial Hyperfusion Through Degradation of the Mitochondrial Fission Protein Drp1 | 83 | |||
| CA9/ Mitochondrial fission | Carbonic anhydrase 9 confers resistance to ferroptosis/apoptosis in malignant mesothelioma under hypoxia. | 86 | |||
| Mitochondrial fission/p53/SLC7A11/GPX4 | SHARPIN promotes cell proliferation of cholangiocarcinoma and inhibits ferroptosis via p53/SLC7A11/GPX4 signaling. | 87 | |||
| Ferroptosis and mitochondrial fusion | MFN1 MFN2 OPA1 |
NRF2, ROS, lipid peroxidation | NRF2/MFN2 | Sulforaphane enriched transcriptome of lung mitochondrial energy metabolism and provided pulmonary injury protection via Nrf2 in mice | 88 |
| Erastin /STING1/ MFN1/2/ROS/ lipid peroxidation/ferroptosis | STING1 Promotes Ferroptosis Through MFN1/2-Dependent Mitochondrial Fusion. | 89 | |||
| Selenium/ MFN1/ROS/ferroptosis | Selenium Alleviates Cerebral Ischemia/Reperfusion Injury by Regulating Oxidative Stress, Mitochondrial Fusion and Ferroptosis. | 90 | |||
| QSYQ/ MFN2, OPA1 | QiShenYiQi dripping pill alleviates myocardial ischemia-induced ferroptosis via improving mitochondrial dynamical homeostasis and biogenesis. | 91 | |||
| Ferroptosis and mitophagy | PINK1 Parkin FUNDC1 |
System Xc-, GPX4, ACSL4, NRF2, ROS, Fe2+, NCOA4, lipid peroxidation |
Mitophagy/dysfunctional mitochondria/ROS/ferroptosis | Oxidative Stress and Ischemia/Reperfusion Injury in Kidney Transplantation: Focus on Ferroptosis, Mitophagy and New Antioxidants. | 96 |
| Myoferlin/ mitophagy/ System Xc-/GPX4/ROS | Myoferlin targeting triggers mitophagy and primes ferroptosis in pancreatic cancer cells. | 98 | |||
| Dynamic O-GlcNAcylation/ ferritinophagy (NCOA4), mitophagy/ Fe2+/ROS/ lipid peroxidation/ ferroptosis | Dynamic O-GlcNAcylation coordinates ferritinophagy and mitophagy to activate ferroptosis. | 97 | |||
| NRF2/ mitophagy (P62-dependent, PINK1/Parkin-independent) | PMI: a DeltaPsim independent pharmacological regulator of mitophagy. | 33 | |||
| High Glucose/TXNIP/ ferritinophagy (NCOA4), mitophagy/ Fe2+, GSH, GPX4/ROS/ lipid peroxidation/ ferroptosis | Mitophagy, Ferritinophagy and Ferroptosis in Retinal Pigment Epithelial Cells Under High Glucose Conditions: Implications for Diabetic Retinopathy and Age-Related Retinal Diseases. | 101 | |||
| FUNDC1/JNK/ lipid peroxidation/ferroptosis | Ablation of FUNDC1-dependent mitophagy renders myocardium resistant to paraquat-induced ferroptosis and contractile dysfunction. | 99 | |||
| FUNDC1/ACSL4 | FUNDC1 insufficiency sensitizes high fat diet intake-induced cardiac remodeling and contractile anomaly through ACSL4-mediated ferroptosis. | 100 | |||
| Erastin/ROS/DRP1, Fis1, MFF/ mitophagy (PINK1/Parkin) | Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS-mitochondrial fission-mitophagy axis. | 85 | |||
| Mitochondrial complex I/ mitophagy (PINK1)/ROS/ferropsosis | Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. | 102 | |||
| Mitophagy/CISD3/ lipid peroxidation | CISD3 inhibition drives cystine-deprivation induced ferroptosis. Cell Death Dis | 103 | |||
| Mitochondrial Ferritin Deficiency / Fe2+/ROS / mitophagy (PINK1/Parkin)/ferroptosis | Mitochondrial Ferritin Deficiency Promotes Osteoblastic Ferroptosis Via Mitophagy in Type 2 Diabetic Osteoporosis. | 104 |
ACSL4 Acyl-CoA synthetase long-chain family member 4; DRP1: dynamin-related protein 1; Fis1: mitochondrial fission protein 1; GPX4: glutathione peroxidase 4; GSH: glutathione; HIF-1 hypoxia-inducible factor 1; Mff: mitochondrial fission factor; MFN1: mitochondrial fusion protein 1; MFN2: mitochondrial fusion protein 2; NCOA4: nuclear receptor coactivator 4; NRF2: nuclear factor red lineage 2 related factor 2; NSCLC: non-small cell lung cancer; OPA1: optic atrophy 1 protein; PINK1: PTEN-induced kinase 1; PUFAs: polyunsaturated fatty acids; ROS: reactive oxygen species
However, the specific mechanism of ferroptosis and mitochondrial dynamics regulation has not been fully explored, and the specific molecular network needs to be further constructed. Many issues remain to be addressed. (1) Interestingly, the relationship between ferroptosis and mitochondrial fission/fusion and mitophagy does not point to a single outcome; i.e., mitochondrial regulation alone can either promote or inhibit ferroptosis 96, 97. For example, in a study on mitochondrial fusion and ferroptosis, Li et al. found that an increased supply of ROS and fatty acids during mitochondrial fusion promotes ferroptosis 89; however, Shi et al. found that promoting mitochondrial fusion via treatment of disease with selenium attenuates oxidative stress and inhibits ferroptosis 90. Similarly, a study on ferroptosis and mitophagy revealed that normal mitophagy contributes to ferroptosis inhibition, while excessive mitophagy pathway activation promotes ferroptosis. These findings suggest that intracellular mitochondrial fission and fusion and mitophagy are dynamic regulatory processes that may exert opposite effects on ferroptosis depending on the degree of their regulatory impact. Therefore, strategies to promote a regulatory balance in mitochondria are becoming important directions for future research. (2) A complex network of genes regulates ferroptosis, mitochondrial fission, mitochondrial fusion and mitophagy. For example, NRF2 is a key hub gene in the regulation of ferroptosis and mitochondrial dynamics, and it has multiple regulatory effects on mitochondrial biogenesis, mitochondrial fission and fusion, and mitophagy 33, 34. Precise identification of intersecting nodes in the NRF2 interaction network is key to understanding its role in the regulation of these biological processes. There are still many key genes whose roles remain undetermined; these need to be explored. (3) The dynamic regulation of ferroptosis and mitochondria has a significant role in the development and treatment of tumors 85, 89, 98. Coordinated bidirectional regulation of ferroptosis and mitochondria can enhance tumor cell death and thus induce therapeutic effects. This may provide research possibilities for the development of new antitumor drugs and for combination treatments. Studying the interaction networks between the two and finding their focused intersection targets will provide important references to direct the development and application of oncology drugs. However, finding precise regulatory targets is difficult. (4) There is a close link between ferroptosis and mitochondrial biogenesis. For example, NRF2, a key factor in ferroptosis, can affect mitochondrial biogenesis by regulating the expression of PGC-1α, NResF1, NResF2, TFAM and mitochondrial genes 32, 34. In addition, NRF2 has been shown to represent an important link between mitochondrial fission, mitochondrial fusion and mitophagy. Thus, there is a complex regulatory network among the three processes, which interact with and influence each other. However, the exact regulatory network is still unclear.
Although many questions remain to be addressed, mitochondrial fission, mitochondrial fusion and mitophagy clearly exert significant effects on ferroptosis. Studying these biological mechanisms may lead to new ideas for the prevention and treatment of diseases in the future.
Summary
In conclusion, mitochondrial fission and fusion and mitophagy maintain mitochondrial homeostasis and have important effects on ferroptosis. A deeper understanding of the relationships among these regulatory processes may lead to the development of new preventive and therapeutic strategies for future disease research.
Acknowledgments
This work was supported by the Beijing Municipal Science & Technology Commission (No. Z171100001017077), Beijing Municipal Science and Technology Commission Clinical Diagnosis and Treatment Technology Research and Demonstration Application Project (No. Z191100006619038), Capital Medical Development and Research Special Project (No. 2020-1-2012), Capital Medical University Clinical Medicine Advanced Sophisticated Subject Construction Project (No. 1192070312), and National Natural Science Foundation of China (No. 82103553).
Author contributions
F.L. and F.C. designed the project and performed revisions; J.L. wrote the paper, generated the figures and tables and revised the manuscript; and Y.J., J.B., and Y.D. provided valuable advice during the writing process and the generation of figures and tables. All authors read and approved the final paper.
Abbreviations
- AA
arachidonic acid
- ACSL4
acyl-CoA synthetase long-chain family member 4
- AMPK
AMP-activated protein kinase
- DFCP1
double FYVE-containing protein 1
- DHODH
dihydroorotate dehydrogenase
- DMT1
divalent metal transporter 1
- DNM1L
dynamin-1-like protein
- DRP1
dynamin-related protein 1
- Fis1
mitochondrial fission protein 1
- GPX4
glutathione peroxidase 4
- GSH
glutathione
- HIF-1
hypoxia-inducible factor 1
- HO-1
heme oxygenase-1
- HSPB1
heat shock protein B1
- IMS
intermembrane space
- LIP
labile iron pool
- LOX
lipoxygenase
- LPCAT3
lysophosphatidylcholine acyltransferase 3
- MARCH5
membrane-associated ring finger protein 5
- MCAO
middle cerebral artery occlusion
- Mff
mitochondrial fission factor
- MFN1
mitochondrial fusion protein 1
- MFN2
mitochondrial fusion protein 2
- MiD49
mitochondrial dynamics protein 49
- MiD51
mitochondrial dynamics protein 51
- MIM
mitochondrial inner membrane
- MOM
mitochondrial outer membrane
- MTS
mitochondrial targeting sequence
- NCOA4
nuclear receptor coactivator 4
- NLR
NOD-like receptor
- NRF2
nuclear factor red lineage 2 related factor 2
- NSCLC
non-small cell lung cancer
- OPA1
optic atrophy 1 protein
- OPTN
Optineurin
- PE
phosphatidylethanolamine
- PGC-1α
proliferator-activated receptor gamma coactivator 1α
- PINK1
PTEN-induced kinase 1
- PUFAs
polyunsaturated fatty acids
- ROS
reactive oxygen species
- STEAP3
six-transmembrane epithelial antigen of prostate 3
- TFR1
transferrin receptor protein 1
- ULK1
unc-51 like kinase 1
- WIPI1
WD repeat domain phosphoinositide interacting 1
- ZIP8/14
zinc-iron regulatory protein family 8/14
References
- 1.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234–45. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N. et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gan B. Mitochondrial regulation of ferroptosis. J Cell Biol. 2021. 220. [DOI] [PMC free article] [PubMed]
- 6.Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 7.Yapa NMB, Lisnyak V, Reljic B, Ryan MT. Mitochondrial dynamics in health and disease. FEBS Lett. 2021;595:1184–204. doi: 10.1002/1873-3468.14077. [DOI] [PubMed] [Google Scholar]
- 8.Richardson DR, Lane DJ, Becker EM, Huang ML, Whitnall M, Suryo Rahmanto Y. et al. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci U S A. 2010;107:10775–82. doi: 10.1073/pnas.0912925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corsi B, Cozzi A, Arosio P, Drysdale J, Santambrogio P, Campanella A. et al. Human mitochondrial ferritin expressed in HeLa cells incorporates iron and affects cellular iron metabolism. J Biol Chem. 2002;277:22430–7. doi: 10.1074/jbc.M105372200. [DOI] [PubMed] [Google Scholar]
- 10.Ng MYW, Wai T, Simonsen A. Quality control of the mitochondrion. Dev Cell. 2021;56:881–905. doi: 10.1016/j.devcel.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, Zhou X, Xie F, Zhang L, Yan H, Huang J. et al. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun (Lond) 2022;42:88–116. doi: 10.1002/cac2.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang L, Hickman JH, Wang SJ, Gu W. Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle. 2015;14:2881–5. doi: 10.1080/15384101.2015.1068479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Gu W. p53 in ferroptosis regulation: the new weapon for the old guardian. Cell Death Differ. 2022;29:895–910. doi: 10.1038/s41418-022-00943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang WS, Stockwell BR. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016;26:165–76. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayir H, Anthonymuthu TS, Tyurina YY, Patel SJ, Amoscato AA, Lamade AM. et al. Achieving Life through Death: Redox Biology of Lipid Peroxidation in Ferroptosis. Cell Chem Biol. 2020;27:387–408. doi: 10.1016/j.chembiol.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee C. Collaborative Power of Nrf2 and PPARgamma Activators against Metabolic and Drug-Induced Oxidative Injury. Oxid Med Cell Longev. 2017;2017:1378175. doi: 10.1155/2017/1378175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frazer DM, Anderson GJ. The regulation of iron transport. Biofactors. 2014;40:206–14. doi: 10.1002/biof.1148. [DOI] [PubMed] [Google Scholar]
- 19.Kwon MY, Park E, Lee SJ, Chung SW. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget. 2015;6:24393–403. doi: 10.18632/oncotarget.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X. et al. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34:5617–25. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santana-Codina N, Gikandi A, Mancias JD. The Role of NCOA4-Mediated Ferritinophagy in Ferroptosis. Adv Exp Med Biol. 2021;1301:41–57. doi: 10.1007/978-3-030-62026-4_4. [DOI] [PubMed] [Google Scholar]
- 22.Santana-Codina N, Mancias JD. The Role of NCOA4-Mediated Ferritinophagy in Health and Disease. Pharmaceuticals (Basel) 2018. 11. [DOI] [PMC free article] [PubMed]
- 23.Kerins MJ, Ooi A. The Roles of NRF2 in Modulating Cellular Iron Homeostasis. Antioxid Redox Signal. 2018;29:1756–73. doi: 10.1089/ars.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng J, Conrad M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020;32:920–37. doi: 10.1016/j.cmet.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L. et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol. 2020;22:225–34. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Dong X, Du W, Shi X, Chen K, Zhang W. et al. LKB1-AMPK axis negatively regulates ferroptosis by inhibiting fatty acid synthesis. Signal Transduct Target Ther. 2020;5:187. doi: 10.1038/s41392-020-00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–43. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–90. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo SC, Hannink M. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp Cell Res. 2008;314:1789–803. doi: 10.1016/j.yexcr.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greco T, Fiskum G. Brain mitochondria from rats treated with sulforaphane are resistant to redox-regulated permeability transition. J Bioenerg Biomembr. 2010;42:491–7. doi: 10.1007/s10863-010-9312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neymotin A, Calingasan NY, Wille E, Naseri N, Petri S, Damiano M. et al. Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis. Free Radic Biol Med. 2011;51:88–96. doi: 10.1016/j.freeradbiomed.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarro E, Gonzalez-Lafuente L, Perez-Liebana I, Buendia I, Lopez-Bernardo E, Sanchez-Ramos C. et al. Heme-Oxygenase I and PCG-1alpha Regulate Mitochondrial Biogenesis via Microglial Activation of Alpha7 Nicotinic Acetylcholine Receptors Using PNU282987. Antioxid Redox Signal. 2017;27:93–105. doi: 10.1089/ars.2016.6698. [DOI] [PubMed] [Google Scholar]
- 33.East DA, Fagiani F, Crosby J, Georgakopoulos ND, Bertrand H, Schaap M. et al. PMI: a DeltaPsim independent pharmacological regulator of mitophagy. Chem Biol. 2014;21:1585–96. doi: 10.1016/j.chembiol.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen QM. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic Biol Med. 2022;179:133–43. doi: 10.1016/j.freeradbiomed.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Skonieczna M, Cieslar-Pobuda A, Saenko Y, Foksinski M, Olinski R, Rzeszowska-Wolny J. et al. The Impact of DIDS-Induced Inhibition of Voltage-Dependent Anion Channels (VDAC) on Cellular Response of Lymphoblastoid Cells to Ionizing Radiation. Med Chem. 2017;13:477–83. doi: 10.2174/1573406413666170421102353. [DOI] [PubMed] [Google Scholar]
- 36.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–92. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–8. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 38.McBean GJ. The transsulfuration pathway: a source of cysteine for glutathione in astrocytes. Amino Acids. 2012;42:199–205. doi: 10.1007/s00726-011-0864-8. [DOI] [PubMed] [Google Scholar]
- 39.Al Ojaimi M, Salah A, El-Hattab AW. Mitochondrial Fission and Fusion: Molecular Mechanisms, Biological Functions, and Related Disorders. Membranes (Basel) 2022. 12. [DOI] [PMC free article] [PubMed]
- 40.Onishi M, Yamano K, Sato M, Matsuda N, Okamoto K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021;40:e104705. doi: 10.15252/embj.2020104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheffer DDL, Garcia AA, Lee L, Mochly-Rosen D, Ferreira JCB. Mitochondrial Fusion, Fission, and Mitophagy in Cardiac Diseases: Challenges and Therapeutic Opportunities. Antioxid Redox Signal. 2022;36:844–63. doi: 10.1089/ars.2021.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu W, Liu Y, Yin H. Mitochondrial Dynamics: Biogenesis, Fission, Fusion, and Mitophagy in the Regulation of Stem Cell Behaviors. Stem Cells Int. 2019;2019:9757201. doi: 10.1155/2019/9757201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–5. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bleazard W, McCaffery JM, King EJ, Bale S, Mozdy A, Tieu Q. et al. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frohlich C, Grabiger S, Schwefel D, Faelber K, Rosenbaum E, Mears J. et al. Structural insights into oligomerization and mitochondrial remodelling of dynamin 1-like protein. EMBO J. 2013;32:1280–92. doi: 10.1038/emboj.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE. et al. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170:1021–7. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji WK, Hatch AL, Merrill RA, Strack S, Higgs HN. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. Elife. 2015;4:e11553. doi: 10.7554/eLife.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loson OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24:659–67. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Bliek AM, Shen Q, Kawajiri S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol. 2013. 5. [DOI] [PMC free article] [PubMed]
- 50.Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62:341–60. doi: 10.1042/EBC20170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ban T, Ishihara T, Kohno H, Saita S, Ichimura A, Maenaka K. et al. Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat Cell Biol. 2017;19:856–63. doi: 10.1038/ncb3560. [DOI] [PubMed] [Google Scholar]
- 53.Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–62. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- 54.Yan L, Qi Y, Huang X, Yu C, Lan L, Guo X. et al. Structural basis for GTP hydrolysis and conformational change of MFN1 in mediating membrane fusion. Nat Struct Mol Biol. 2018;25:233–43. doi: 10.1038/s41594-018-0034-8. [DOI] [PubMed] [Google Scholar]
- 55.Allegra A, Innao V, Allegra AG, Musolino C. Relationship between mitofusin 2 and cancer. Adv Protein Chem Struct Biol. 2019;116:209–36. doi: 10.1016/bs.apcsb.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Mattie S, Riemer J, Wideman JG, McBride HM. A new mitofusin topology places the redox-regulated C terminus in the mitochondrial intermembrane space. J Cell Biol. 2018;217:507–15. doi: 10.1083/jcb.201611194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka K. The PINK1-Parkin axis: An Overview. Neurosci Res. 2020;159:9–15. doi: 10.1016/j.neures.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen TN, Padman BS, Lazarou M. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016;26:733–44. doi: 10.1016/j.tcb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Quinn PMJ, Moreira PI, Ambrosio AF, Alves CH. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol Commun. 2020;8:189. doi: 10.1186/s40478-020-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA. et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–21. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park S, Choi SG, Yoo SM, Nah J, Jeong E, Kim H. et al. Pyruvate stimulates mitophagy via PINK1 stabilization. Cell Signal. 2015;27:1824–30. doi: 10.1016/j.cellsig.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 62.Ordureau A, Sarraf SA, Duda DM, Heo JM, Jedrychowski MP, Sviderskiy VO. et al. Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell. 2014;56:360–75. doi: 10.1016/j.molcel.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL. et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–14. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szargel R, Shani V, Abd Elghani F, Mekies LN, Liani E, Rott R. et al. The PINK1, synphilin-1 and SIAH-1 complex constitutes a novel mitophagy pathway. Hum Mol Genet. 2016;25:3476–90. doi: 10.1093/hmg/ddw189. [DOI] [PubMed] [Google Scholar]
- 65.Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A. et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson AB. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem. 2012;287:19094–104. doi: 10.1074/jbc.M111.322933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Novak I, Dikic I. Autophagy receptors in developmental clearance of mitochondria. Autophagy. 2011;7:301–3. doi: 10.4161/auto.7.3.14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu W, Li W, Chen H, Jiang L, Zhu R, Feng D. FUNDC1 is a novel mitochondrial-associated-membrane (MAM) protein required for hypoxia-induced mitochondrial fission and mitophagy. Autophagy. 2016;12:1675–6. doi: 10.1080/15548627.2016.1193656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen M, Chen Z, Wang Y, Tan Z, Zhu C, Li Y. et al. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy. 2016;12:689–702. doi: 10.1080/15548627.2016.1151580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P. et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–85. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 71.Chen G, Han Z, Feng D, Chen Y, Chen L, Wu H. et al. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol Cell. 2014;54:362–77. doi: 10.1016/j.molcel.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 72.Chen Z, Liu L, Cheng Q, Li Y, Wu H, Zhang W. et al. Mitochondrial E3 ligase MARCH5 regulates FUNDC1 to fine-tune hypoxic mitophagy. EMBO Rep. 2017;18:495–509. doi: 10.15252/embr.201643309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Yao Y, Qiu X, Wang G, Hu Z, Chen S. et al. Listeria hijacks host mitophagy through a novel mitophagy receptor to evade killing. Nat Immunol. 2019;20:433–46. doi: 10.1038/s41590-019-0324-2. [DOI] [PubMed] [Google Scholar]
- 74.Li S, Zhou Y, Gu X, Zhang X, Jia Z. NLRX1/FUNDC1/NIPSNAP1-2 axis regulates mitophagy and alleviates intestinal ischaemia/reperfusion injury. Cell Prolif. 2021;54:e12986. doi: 10.1111/cpr.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lim GG, Lim KL. Parkin-independent mitophagy-FKBP8 takes the stage. EMBO Rep. 2017;18:864–5. doi: 10.15252/embr.201744313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saita S, Shirane M, Nakayama KI. Selective escape of proteins from the mitochondria during mitophagy. Nat Commun. 2013;4:1410. doi: 10.1038/ncomms2400. [DOI] [PubMed] [Google Scholar]
- 77.Li M, Jia J, Zhang X, Dai H. Selective binding of mitophagy receptor protein Bcl-rambo to LC3/GABARAP family proteins. Biochem Biophys Res Commun. 2020;530:292–300. doi: 10.1016/j.bbrc.2020.07.039. [DOI] [PubMed] [Google Scholar]
- 78.Murakawa T, Yamaguchi O, Hashimoto A, Hikoso S, Takeda T, Oka T. et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun. 2015;6:7527. doi: 10.1038/ncomms8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei Y, Chiang WC, Sumpter R Jr, Mishra P, Levine B. Prohibitin 2 Is an Inner Mitochondrial Membrane Mitophagy Receptor. Cell. 2017;168:224–38. doi: 10.1016/j.cell.2016.11.042. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA. et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yan C, Gong L, Chen L, Xu M, Abou-Hamdan H, Tang M. et al. PHB2 (prohibitin 2) promotes PINK1-PRKN/Parkin-dependent mitophagy by the PARL-PGAM5-PINK1 axis. Autophagy. 2020;16:419–34. doi: 10.1080/15548627.2019.1628520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kagan VE, Jiang J, Huang Z, Tyurina YY, Desbourdes C, Cottet-Rousselle C. et al. NDPK-D (NM23-H4)-mediated externalization of cardiolipin enables elimination of depolarized mitochondria by mitophagy. Cell Death Differ. 2016;23:1140–51. doi: 10.1038/cdd.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sabouny R, Fraunberger E, Geoffrion M, Ng AC, Baird SD, Screaton RA. et al. The Keap1-Nrf2 Stress Response Pathway Promotes Mitochondrial Hyperfusion Through Degradation of the Mitochondrial Fission Protein Drp1. Antioxid Redox Signal. 2017;27:1447–59. doi: 10.1089/ars.2016.6855. [DOI] [PubMed] [Google Scholar]
- 84.Liu X, Wang S, Jin S, Huang S, Liu Y. Vitamin D(3) attenuates cisplatin-induced intestinal injury by inhibiting ferroptosis, oxidative stress, and ROS-mediated excessive mitochondrial fission. Food Funct. 2022;13:10210–24. doi: 10.1039/d2fo01028c. [DOI] [PubMed] [Google Scholar]
- 85.Liu M, Fan Y, Li D, Han B, Meng Y, Chen F. et al. Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS-mitochondrial fission-mitophagy axis. Mol Oncol. 2021;15:2084–105. doi: 10.1002/1878-0261.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Z, Jiang L, Chew SH, Hirayama T, Sekido Y, Toyokuni S. Carbonic anhydrase 9 confers resistance to ferroptosis/apoptosis in malignant mesothelioma under hypoxia. Redox Biol. 2019;26:101297. doi: 10.1016/j.redox.2019.101297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng C, Lin J, Zhang K, Ou H, Shen K, Liu Q. et al. SHARPIN promotes cell proliferation of cholangiocarcinoma and inhibits ferroptosis via p53/SLC7A11/GPX4 signaling. Cancer Sci. 2022;113:3766–75. doi: 10.1111/cas.15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cho HY, Miller-DeGraff L, Blankenship-Paris T, Wang X, Bell DA, Lih F. et al. Sulforaphane enriched transcriptome of lung mitochondrial energy metabolism and provided pulmonary injury protection via Nrf2 in mice. Toxicol Appl Pharmacol. 2019;364:29–44. doi: 10.1016/j.taap.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li C, Liu J, Hou W, Kang R, Tang D. STING1 Promotes Ferroptosis Through MFN1/2-Dependent Mitochondrial Fusion. Front Cell Dev Biol. 2021;9:698679. doi: 10.3389/fcell.2021.698679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi Y, Han L, Zhang X, Xie L, Pan P, Chen F. Selenium Alleviates Cerebral Ischemia/Reperfusion Injury by Regulating Oxidative Stress, Mitochondrial Fusion and Ferroptosis. Neurochem Res. 2022;47:2992–3002. doi: 10.1007/s11064-022-03643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu L, Fan Z, Gu L, Liu J, Cui Z, Yu B. et al. QiShenYiQi dripping pill alleviates myocardial ischemia-induced ferroptosis via improving mitochondrial dynamical homeostasis and biogenesis. J Ethnopharmacol. 2023;308:116282. doi: 10.1016/j.jep.2023.116282. [DOI] [PubMed] [Google Scholar]
- 92.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–64. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 93.Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem Biol. 2020;27:420–35. doi: 10.1016/j.chembiol.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89–100. doi: 10.1016/j.semcancer.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 95.Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–21. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Granata S, Votrico V, Spadaccino F, Catalano V, Netti GS, Ranieri E, Oxidative Stress and Ischemia/Reperfusion Injury in Kidney Transplantation: Focus on Ferroptosis, Mitophagy and New Antioxidants. Antioxidants (Basel) 2022. 11. [DOI] [PMC free article] [PubMed]
- 97.Yu F, Zhang Q, Liu H, Liu J, Yang S, Luo X. et al. Dynamic O-GlcNAcylation coordinates ferritinophagy and mitophagy to activate ferroptosis. Cell Discov. 2022;8:40. doi: 10.1038/s41421-022-00390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rademaker G, Boumahd Y, Peiffer R, Anania S, Wissocq T, Liegeois M. et al. Myoferlin targeting triggers mitophagy and primes ferroptosis in pancreatic cancer cells. Redox Biol. 2022;53:102324. doi: 10.1016/j.redox.2022.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peng H, Fu S, Wang S, Xu H, Dhanasekaran M, Chen H. et al. Ablation of FUNDC1-dependent mitophagy renders myocardium resistant to paraquat-induced ferroptosis and contractile dysfunction. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166448. doi: 10.1016/j.bbadis.2022.166448. [DOI] [PubMed] [Google Scholar]
- 100.Pei Z, Liu Y, Liu S, Jin W, Luo Y, Sun M. et al. FUNDC1 insufficiency sensitizes high fat diet intake-induced cardiac remodeling and contractile anomaly through ACSL4-mediated ferroptosis. Metabolism. 2021;122:154840. doi: 10.1016/j.metabol.2021.154840. [DOI] [PubMed] [Google Scholar]
- 101.Singh LP, Yumnamcha T, Devi TS. Mitophagy, Ferritinophagy and Ferroptosis in Retinal Pigment Epithelial Cells Under High Glucose Conditions: Implications for Diabetic Retinopathy and Age-Related Retinal Diseases. JOJ Ophthalmol. 2021;8:77–85. [PMC free article] [PubMed] [Google Scholar]
- 102.Basit F, van Oppen LM, Schockel L, Bossenbroek HM, van Emst-de Vries SE, Hermeling JC. et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8:e2716. doi: 10.1038/cddis.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Wang X, Huang Z, Zhou Y, Xia J, Hu W. et al. CISD3 inhibition drives cystine-deprivation induced ferroptosis. Cell Death Dis. 2021;12:839. doi: 10.1038/s41419-021-04128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X, Ma H, Sun J, Zheng T, Zhao P, Li H. et al. Mitochondrial Ferritin Deficiency Promotes Osteoblastic Ferroptosis Via Mitophagy in Type 2 Diabetic Osteoporosis. Biol Trace Elem Res. 2022;200:298–307. doi: 10.1007/s12011-021-02627-z. [DOI] [PubMed] [Google Scholar]
- 105.Jiang Y, Krantz S, Qin X, Li S, Gunasekara H, Kim YM. et al. Caveolin-1 controls mitochondrial damage and ROS production by regulating fission - fusion dynamics and mitophagy. Redox Biol. 2022;52:102304. doi: 10.1016/j.redox.2022.102304. [DOI] [PMC free article] [PubMed] [Google Scholar]