Abstract
Background
Age‐related macular degeneration (AMD) is a common eye disease and leading cause of sight loss worldwide. Despite its high prevalence and increasing incidence as populations age, AMD remains incurable and there are no treatments for most patients. Mounting genetic and molecular evidence implicates complement system overactivity as a key driver of AMD development and progression. The last decade has seen the development of several novel therapeutics targeting complement in the eye for the treatment of AMD. This review update encompasses the results of the first randomised controlled trials in this field.
Objectives
To assess the effects and safety of complement inhibitors in the prevention or treatment of AMD.
Search methods
We searched CENTRAL on the Cochrane Library, MEDLINE, Embase, LILACS, Web of Science, ISRCTN registry, ClinicalTrials.gov, and the WHO ICTRP to 29 June 2022 with no language restrictions. We also contacted companies running clinical trials for unpublished data.
Selection criteria
We included randomised controlled trials (RCTs) with parallel groups and comparator arms that studied complement inhibition for advanced AMD prevention/treatment.
Data collection and analysis
Two authors independently assessed search results and resolved discrepancies through discussion. Outcome measures evaluated at one year included change in best‐corrected visual acuity (BCVA), untransformed and square root‐transformed geographic atrophy (GA) lesion size progression, development of macular neovascularisation (MNV) or exudative AMD, development of endophthalmitis, loss of ≥ 15 letters of BCVA, change in low luminance visual acuity, and change in quality of life. We assessed risk of bias and evidence certainty using Cochrane risk of bias and GRADE tools.
Main results
Ten RCTs with 4052 participants and eyes with GA were included. Nine evaluated intravitreal (IVT) administrations against sham, and one investigated an intravenous agent against placebo. Seven studies excluded patients with prior MNV in the non‐study eye, whereas the three pegcetacoplan studies did not. The risk of bias in the included studies was low overall. We also synthesised results of two intravitreal agents (lampalizumab, pegcetacoplan) at monthly and every‐other‐month (EOM) dosing intervals.
Efficacy and safety of IVT lampalizumab versus sham for GA
For 1932 participants in three studies, lampalizumab did not meaningfully change BCVA given monthly (+1.03 letters, 95% confidence interval (CI) −0.19 to 2.25) or EOM (+0.22 letters, 95% CI −1.00 to 1.44) (high‐certainty evidence). For 1920 participants, lampalizumab did not meaningfully change GA lesion growth given monthly (+0.07 mm², 95% CI −0.09 to 0.23; moderate‐certainty due to imprecision) or EOM (+0.07 mm², 95% CI −0.05 to 0.19; high‐certainty). For 2000 participants, lampalizumab may have also increased MNV risk given monthly (RR 1.77, 95% CI 0.73 to 4.30) and EOM (RR 1.70, 95% CI 0.67 to 4.28), based on low‐certainty evidence. The incidence of endophthalmitis in patients treated with monthly and EOM lampalizumab was 4 per 1000 (0 to 87) and 3 per 1000 (0 to 62), respectively, based on moderate‐certainty evidence.
Efficacy and safety of IVT pegcetacoplan versus sham for GA
For 242 participants in one study, pegcetacoplan probably did not meaningfully change BCVA given monthly (+1.05 letters, 95% CI −2.71 to 4.81) or EOM (−1.42 letters, 95% CI −5.25 to 2.41), as supported by moderate‐certainty evidence. In contrast, for 1208 participants across three studies, pegcetacoplan meaningfully reduced GA lesion growth when given monthly (−0.38 mm², 95% CI −0.57 to −0.19) and EOM (−0.29 mm², 95% CI −0.44 to −0.13), with high certainty. These reductions correspond to 19.2% and 14.8% versus sham, respectively. A post hoc analysis showed possibly greater benefits in 446 participants with extrafoveal GA given monthly (−0.67 mm², 95% CI −0.98 to −0.36) and EOM (−0.60 mm², 95% CI −0.91 to −0.30), representing 26.1% and 23.3% reductions, respectively. However, we did not have data on subfoveal GA growth to undertake a formal subgroup analysis. In 1502 participants, there is low‐certainty evidence that pegcetacoplan may have increased MNV risk when given monthly (RR 4.47, 95% CI 0.41 to 48.98) or EOM (RR 2.29, 95% CI 0.46 to 11.35). The incidence of endophthalmitis in patients treated with monthly and EOM pegcetacoplan was 6 per 1000 (1 to 53) and 8 per 1000 (1 to 70) respectively, based on moderate‐certainty evidence.
Efficacy and safety of IVT avacincaptad pegol versus sham for GA
In a study of 260 participants with extrafoveal or juxtafoveal GA, monthly avacincaptad pegol probably did not result in a clinically meaningful change in BCVA at 2 mg (+1.39 letters, 95% CI −5.89 to 8.67) or 4 mg (−0.28 letters, 95% CI −8.74 to 8.18), based on moderate‐certainty evidence. Despite this, the drug was still found to have probably reduced GA lesion growth, with estimates of 30.5% reduction at 2 mg (−0.70 mm², 95% CI −1.99 to 0.59) and 25.6% reduction at 4 mg (−0.71 mm², 95% CI −1.92 to 0.51), based on moderate‐certainty evidence. Avacincaptad pegol may have also increased the risk of developing MNV (RR 3.13, 95% CI 0.93 to 10.55), although this evidence is of low certainty. There were no cases of endophthalmitis reported in this study.
Authors' conclusions
Despite confirmation of the negative findings of intravitreal lampalizumab across all endpoints, local complement inhibition with intravitreal pegcetacoplan meaningfully reduces GA lesion growth relative to sham at one year. Inhibition of complement C5 with intravitreal avacincaptad pegol is also an emerging therapy with probable benefits on anatomical endpoints in the extrafoveal or juxtafoveal GA population. However, there is currently no evidence that complement inhibition with any agent improves functional endpoints in advanced AMD; further results from the phase 3 studies of pegcetacoplan and avacincaptad pegol are eagerly awaited. Progression to MNV or exudative AMD is a possible emergent adverse event of complement inhibition, requiring careful consideration should these agents be used clinically. Intravitreal administration of complement inhibitors is probably associated with a small risk of endophthalmitis, which may be higher than that of other intravitreal therapies. Further research is likely to have an important impact on our confidence in the estimates of adverse effects and may change these. The optimal dosing regimens, treatment duration, and cost‐effectiveness of such therapies are yet to be established.
Keywords: Humans; Administration, Intravenous; Complement Inactivating Agents; Complement Inactivating Agents/adverse effects; Endophthalmitis; Geographic Atrophy; Geographic Atrophy/drug therapy; Macular Degeneration; Macular Degeneration/drug therapy
Plain language summary
Complement inhibitors for age‐related macular degeneration
Plain language summary title
What are the benefits and risks of medicines that block complement to treat age‐related macular degeneration (AMD)?
Key messages
• After one year of treatment, pegcetacoplan (a medicine that blocks complement) was shown to slow down the growth of patches of diseased retina in the eyes of people with a severe type of ‘dry’ AMD, but there is currently no evidence that it slows down sight loss or improves quality of life.
• Treating ‘dry’ AMD with pegcetacoplan and other medicines that block complement may result in more cases of ‘wet’ AMD, where abnormal blood vessels grow in the retina; these can leak blood or fluid and cause rapid vision loss, but can be treated if caught quickly.
• Future research in this area should focus on options and effects that are important to decision‐makers, such as:
‐ the benefits of blocking complement in different ways and for different periods of time;
‐ potential harms and costs;
‐ outcomes that are relevant to patients.
What is age‐related macular degeneration?
AMD is a leading cause of sight loss in adults, affecting almost 200 million people worldwide. There are two types of AMD: ‘dry’ and ‘wet’. AMD is at least partially due to genetic causes that are not fully understood or treatable. Most genetic changes linked to AMD have been found to affect complement, a major part of our immune system. When complement is too active, it can injure the retina (the light‐sensitive film lining the inside of our eyes) and cause AMD.
How is age‐related macular degeneration treated?
Currently, there is no cure for AMD, and while there are treatments available for the 'wet' type, no therapies are available for most of the 95% of patients with the 'dry' form. However, a new treatment option, pegcetacoplan, which blocks complement, has recently been approved in the US for treating severe 'dry' AMD.
What did we want to find out?
We wanted to find out if blocking complement with different medicines was better than sham or placebo (a fake treatment) at preventing or slowing down AMD.
What did we do?
We searched for studies that looked at complement blockers compared with sham or placebo in people with AMD across the world. We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 10 studies that involved 4052 people with geographic atrophy (a severe form of ‘dry’ AMD) that had lasted for at least a year. The biggest study was in 1881 people and the smallest study was in 30 people. The studies were conducted in countries around the world; most were done in the US or Europe. All studies were funded by pharmaceutical companies. Almost all studied medicines that blocked complement were given as injections into the eye (intravitreally).
The results of two medicines, lampalizumab and pegcetacoplan, were reported across three studies each. We combined the results of these studies to help give us an idea of the true effectiveness of these treatments when given monthly and every‐other‐month. We also looked at the results of all other studies, including a medicine called avacincaptad pegol.
Main results
We found that pegcetacoplan given every month or every‐other‐month reduces the growth of patches of diseased retina, but probably makes little to no difference to vision loss. We also found that avacincaptad pegol may reduce the growth of diseased retina, but also probably makes little to no difference in vision loss. We found that lampalizumab makes little to no difference to any of the outcomes of interest to patients and their clinicians.
Like other medicines given in the eye, we found that most medicines that block complement are probably associated with a small increase in the risk of serious eye infection or inflammation. We also found that blocking complement in the eye in most studies probably caused an increase in the risk of abnormal blood vessels growing in the retina; these can leak blood or fluid and cause rapid vision loss but can be treated if caught quickly.
What are the limitations of the evidence?
We are confident in our results for lampalizumab and pegcetacoplan to treat geographic atrophy. People in the studies were randomly placed into the different treatment groups. This means that differences between the groups are due to differences between the treatments rather than between the people. More information is needed to increase our confidence in the anatomical benefits of avacincaptad pegol, but early evidence is encouraging.
These findings relate only to treatment with intravitreal medicines for up to one year at most. Not all studies provided data about everything that patients and their clinicians may be interested in. Participants in the studies had severe ‘dry’ AMD, so our results may not be useful for people whose AMD is less severe or those who have the ‘wet’ form of AMD.
How up‐to‐date is this evidence?
This review updates our previous review. The evidence is up‐to‐date to 29 June 2022.
Summary of findings
Summary of findings 1. Summary of findings 1: Efficacy and safety of IVT lampalizumab 4‐weekly (monthly) versus sham for geographic atrophy (GA).
| Population: adults with GA due to AMD Setting: outpatient ophthalmology clinics around the world Intervention: lampalizumab 10 mg administered IVT every 4 weeks Comparison: sham treatment | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with comparatora | Risk with interventionb | |||||
| Change in BCVA from baseline at 1 year (ETDRS letters) | The mean BCVA change from baseline ranged across control groups from −5.68 letters (worst BCVA) to −3.93 letters (best BCVA) | The mean BCVA change from baseline in the intervention groups was on average 1.03 lettershigher (95% CI −0.19 to 2.25 letters) | — | 1182 (3) | ⨁⨁⨁⨁ High | |
| Change in untransformed GA lesion size from baseline at 1 year (mm²) | The mean GA lesion size change from baseline ranged across control groups from 1.90 mm² (best GA lesion size) to 2.06 mm² (worst GA lesion size) | The mean GA lesion size change from baseline in the intervention groups was on average 0.07 mm²higher (95% CI −0.09 to 0.23 mm²) | — | 1199 (3) | ⨁⨁⨁◯c Moderate | The mean square root‐transformed GA lesion size change from baseline in the intervention groups was on average 0.01 mm higher (95% −0.01 to 0.03) based on 1117 individuals from two studies. We have high certainty in this effect estimate. |
| Safety: Development of macular neovascularisation or exudative AMD at 1 year | 11 per 1000 | 19 per 1000 (8 to 47) |
RR 1.77 (0.73 to 4.30) |
1330 (3) | ⨁⨁◯◯d Low |
|
| Safety: Development of endophthalmitis at 1 year | 0 per 1000 | 4 per 1000 (0 to 77) |
RR 6.92 (0.36 to 133.73) |
1330 (3) | ⨁⨁⨁◯e Moderate |
|
| Loss of ≥ 15 letters BCVA at 1 year | 135 per 1000 | 116 per 1000 (85 to 159) |
RR 0.86 (0.63 to 1.18) |
1103 (2) | ⨁⨁⨁◯f Moderate | |
| Change in LLVA from baseline at 1 year (ETDRS letters) | The mean LLVA change from baseline ranged across control groups from −3.97 letters (worst LLVA) to −1.43 letters (best LLVA) | The mean LLVA change from baseline in the intervention groups was on average 0.20 letters higher (95% CI −1.07 to 1.46 letters) | — | 1068 (2) | ⨁⨁⨁⨁ High |
|
| Change in quality of life at 1 year (NEI VFQ‐25 composite score) | The mean NEI VFQ score change from baseline ranged across control groups from −3.14 (worst score) to −0.30 (best score) | The mean NEI VFQ score change from baseline in the intervention groups was on average 0.42higher (95% CI −1.12 to 1.95) | — | 983 (2) | ⨁⨁⨁⨁ High | |
| Abbreviations: AMD = age‐related macular degeneration; BCVA = best corrected visual acuity; CI = confidence interval; ETDRS = Early Treatment Diabetic Retinopathy Study; GA = geographic atrophy; GRADE = grading system for evidence and recommendations; IVT = intravitreal; LLVA = low luminance visual acuity; MD = mean difference; MNV = macular neovascularisation; NEI VFQ‐25 = National Eye Institute Visual Function Questionnaire 25; RR = risk ratio. | ||||||
| GRADE Certainty of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: Any estimate of effect is very uncertain. | ||||||
aAbsolute risks with comparator derived from the corresponding included studies. bFor the outcome endophthalmitis, we calculated the risk in the intervention group from the total number of events/total N reported in the intervention group in the included studies, rounded to the nearest integer. We calculated the confidence intervals by multiplying the 95% confidence limits of the relative effect with the corresponding risk (per 1000) and dividing by the RR. For the other dichotomous outcomes, we calculated the corresponding risk and confidence intervals by multiplying the RR and 95% confidence limits of the relative effect by the assumed risk.
Reason for downgrading certainty of evidence
cRated down (−1 level) for imprecision as the 95% CI overlaps no effect and fails to exclude important harm (i.e. a GA lesion size change of +0.22 mm2 from baseline relative to sham at 1 year). dRated down (−2 levels) for imprecision due to the very wide confidence intervals, as indicated by a ratio of the upper to lower boundary of the confidence interval greater than 3. eRated down (−1 level) for imprecision as the 95% CI overlaps no effect and fails to exclude important benefit or harm (i.e. an RR of under 0.75 or over 1.25). fRated down (−1 level) for imprecision as the 95% CI overlaps no effect and fails to exclude important benefit (i.e. an RR of under 0.75).
Summary of findings 2. Summary of findings 2: Efficacy and safety of IVT lampalizumab 6‐ to 8‐weekly (every other month) versus sham for geographic atrophy (GA).
| Population: adults with GA due to AMD Setting: outpatient ophthalmology clinics around the world Intervention: lampalizumab 10 mg administered IVT every 6 to 8 weeks Comparison: sham treatment | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with comparatora | Risk with interventionb | |||||
| Change in BCVA from baseline at 1 year (ETDRS letters) | The mean BCVA change from baseline ranged across control groups from −5.68 letters (worst BCVA) to −3.93 letters (best BCVA) | The mean BCVA change from baseline in the intervention groups was on average 0.22 lettershigher (95% CI −1.00 to 1.44 letters) | — | 1184 (3) | ⨁⨁⨁⨁ High | |
| Change in untransformed GA lesion size from baseline at 1 year (mm²) | The mean GA lesion size change from baseline ranged across control groups from 1.90 mm² (best GA lesion size) to 2.06 mm² (worst GA lesion size) | The mean GA lesion size change from baseline in the intervention groups was on average 0.07 mm²higher (95% CI −0.05 to 0.19 mm²) | — | 1207 (3) | ⨁⨁⨁⨁ High | The mean square root‐transformed GA lesion size change from baseline was on average 0.01 mm higher (95% −0.01 to 0.03) based on 1126 individuals from two studies. We have high certainty in this effect estimate. |
| Safety: Development of macular neovascularisation or exudative AMD at 1 year | 11 per 1000 | 19 per 1000 (7 to 47) |
RR 1.70 (0.67 to 4.28) |
1331 (3) | ⨁⨁◯◯c Low |
|
| Safety: Development of endophthalmitis at 1 year | 0 per 1000 | 3 per 1000 (0 to 62) |
RR 4.94 (0.24 to 102.78) |
1331 (3) | ⨁⨁⨁◯d Moderate |
|
| Loss of ≥ 15 letters BCVA at 1 year | 135 per 1000 | 132 per 1000 (97 to 180) |
RR 0.98 (0.72 to 1.33) |
1096 (2) | ⨁⨁⨁◯e Moderate | |
| Change in LLVA from baseline at 1 year (ETDRS letters) | The mean LLVA change from baseline ranged across control groups from −3.97 letters (worst LLVA) to −1.43 letters (best LLVA) | The mean LLVA change from baseline in the intervention groups was on average 0.27 letters lower (95% CI −1.33 to 0.79 letters) | — | 1065 (2) | ⨁⨁⨁⨁ High | |
| Change in quality of life at 1 year (NEI VFQ‐25 composite score) | The mean NEI VFQ score change from baseline ranged across control groups from −3.14 (worst score) to −0.30 (best score) | The mean LLVA change from baseline in the intervention groups was on average 0.28 lower (95% CI −2.82 to 2.26) | — | 1003 (2) | ⨁⨁⨁◯f Moderate | |
| Abbreviations: AMD = age‐related macular degeneration; BCVA = best corrected visual acuity; CI = confidence interval; ETDRS = Early Treatment Diabetic Retinopathy Study; GA = geographic atrophy; GRADE = grading system for evidence and recommendations; IVT = intravitreal; LLVA = low luminance visual acuity; MD = mean difference; MNV = macular neovascularisation; NEI VFQ‐25 = National Eye Institute Visual Function Questionnaire 25; RR = risk ratio. | ||||||
| GRADE Certainty of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: Any estimate of effect is very uncertain. | ||||||
aAbsolute risks with comparator derived from the corresponding included studies. bFor the outcome endophthalmitis, we calculated the risk in the intervention group from the total number of events/total N reported in the intervention group in the included studies, rounded to the nearest integer. We calculated the confidence intervals by multiplying the 95% confidence limits of the relative effect with the corresponding risk (per 1000) and dividing by the RR. For the other dichotomous outcomes, we calculated the corresponding risk and confidence intervals by multiplying the RR and 95% confidence limits of the relative effect by the assumed risk.
Reason for downgrading certainty of evidence
cRated down (−2 levels) for imprecision due to the very wide confidence intervals, as indicated by a ratio of the upper to lower boundary of the confidence interval greater than 3. dRated down (−1 level) for imprecision as the 95% CI overlaps no effect and fails to exclude important benefit or harm (i.e. an RR of under 0.75 or over 1.25). eRated down (−1 level) for imprecision as the 95% CI overlaps no effect and fails to exclude important benefit or harm (i.e. an RR of under 0.75 or over 1.25). fRated down (−1 level) for inconsistency due to evidence of substantial heterogeneity (I2 = 69%, Chi2 P = 0.07).
Summary of findings 3. Summary of findings 3: Efficacy and safety of IVT pegcetacoplan 4‐weekly (monthly) versus sham for geographic atrophy (GA).
| Population: adults with GA due to AMD Setting: outpatient ophthalmology clinics around the world Intervention: pegcetacoplan 15 mg administered IVT every 4 weeks Comparison: sham treatment | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with comparatora | Risk with interventionb | |||||
| Change in BCVA from baseline at 1 year (ETDRS letters) | The mean BCVA change from baseline ranged across the control group from −7.03 letters (worst BCVA) to −1.69 letters (best BCVA) | The mean BCVA change from baseline in the intervention group was on average 1.05 letterslower (95% CI −2.71 to 4.81 letters) | — | 164 (1) | ⨁⨁⨁◯c Moderate | |
| Change in untransformed GA lesion size from baseline at 1 year (mm²) | The mean GA lesion size change from baseline ranged across control groups from 1.88 mm² (best GA lesion size) to 2.11 mm² (worst GA lesion size) | The mean GA lesion size change from baseline in the intervention groups was on average 0.38 mm²lower (95% CI −0.57 to −0.19 mm²) | — | 967 (3) | ⨁⨁⨁⨁ High | The mean square root‐transformed GA lesion size change from baseline in the intervention group was on average 0.09 mm lower (95% −0.16 to −0.02) based on 242 individuals from one study. We have high certainty in this effect estimate. In a post hoc analysis involving only participants with extrafoveal GA, untransformed GA lesion size change from baseline in the intervention groups was on average 0.67 mm² lower (95% CI −0.98 to −0.36) based on 291 individuals from two studies. Due to the lack of prior specification for this subgroup analysis in the relevant studies, we cannot assess the certainty of the effect estimate. |
| Safety: Development of macular neovascularisation or exudative AMD at 1 year | 34 per 1000 | 152 per 1000 (14 to 1000) |
RR 4.47 (0.41 to 48.98) |
1003 (3) | ⨁⨁◯◯d Low | |
| Safety: Development of endophthalmitis at 1 year | 0 per 1000 | 6 per 1000 (1 to 53) |
RR 3.79 (0.42 to 34.05) |
1003 (3) | ⨁⨁⨁◯e Moderate |
|
| Loss of ≥ 15 letters BCVA at 1 year | No studies reported this outcome. | |||||
| Change in LLVA from baseline at 1 year (ETDRS letters) | The mean LLVA change from baseline ranged across the control group from −2.80 letters (worst LLVA) to 1.70 letters (best LLVA) | The mean LLVA change from baseline in the intervention group was on average 2.18 letters lower (95% CI −5.36 to 1.00 letters) | — | 164 (1) | ⨁⨁⨁◯f Moderate | |
| Change in quality of life at 1 year (NEI VFQ‐25 composite score) | No studies reported this outcome. | |||||
| Abbreviations: AMD = age‐related macular degeneration; BCVA = best corrected visual acuity; CI = confidence interval; ETDRS = Early Treatment Diabetic Retinopathy Study; GA = geographic atrophy; GRADE = grading system for evidence and recommendations; IVT = intravitreal; LLVA = low luminance visual acuity; MD = mean difference; MNV = macular neovascularisation; NEI VFQ‐25 = National Eye Institute Visual Function Questionnaire 25; RR = risk ratio. | ||||||
| GRADE Certainty of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: Any estimate of effect is very uncertain. | ||||||
aAbsolute risks with comparator derived from the corresponding included studies. bFor the outcome endophthalmitis, we calculated the risk in the intervention group from the total number of events/total N reported in the intervention group in the included studies, rounded to the nearest integer. We calculated the confidence intervals by multiplying the 95% confidence limits of the relative effect with the corresponding risk (per 1000) and dividing by the RR. For the other dichotomous outcomes, we calculated the corresponding risk and confidence intervals by multiplying the RR and 95% confidence limits of the relative effect by the assumed risk.
Reason for downgrading certainty of evidence
cRated down (−1 level) for imprecision as the 95% CI overlaps no effect and fails to exclude important benefit (i.e. a BCVA change of +5 letters from baseline relative to sham). dRated down (−2 levels) for imprecision due to the very wide confidence intervals, as indicated by a ratio of the upper to lower boundary of the confidence interval greater than 3. We did not further downgrade the certainty of evidence for inconsistency as the results of the studies were consistent in indicating an elevated risk of MNV. eRated down (−1 level) for imprecision as the 95% CI overlaps no effect and fails to exclude important benefit or harm (i.e. an RR of under 0.75 or over 1.25). fRated down (−1 level) for imprecision as the 95% CI overlaps no effect and fails to exclude important harm (i.e. an LLVA change of −5 letters from baseline relative to sham).
Summary of findings 4. Summary of findings 4: Efficacy and safety of IVT pegcetacoplan 8‐weekly (every other month) versus sham for geographic atrophy (GA).
| Population: adults with GA due to AMD Setting: outpatient ophthalmology clinics around the world Intervention: pegcetacoplan 15 mg administered IVT every 8 weeks Comparison: sham treatment | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with comparatora | Risk with interventionb | |||||
| Change in BCVA from baseline at 1 year (ETDRS letters) | The mean BCVA change from baseline ranged across the control group from −7.03 letters (worst BCVA) to −1.69 letters (best BCVA) | The mean BCVA change from baseline in the intervention group was on average 1.42 letterslower (95% CI −5.25 to 2.41 letters) | MD −1.42 (−5.25 to 2.41) |
158 (1) | ⨁⨁⨁◯c Moderate | |
| Change in untransformed GA lesion size from baseline at 1 year (mm²) | The mean GA lesion size change from baseline ranged across control groups from 1.88 mm² (best GA lesion size) to 2.11 mm² (worst GA lesion size) | The mean GA lesion size change from baseline in the intervention groups was on average 0.29 mm²lower (95% CI −0.44 to −0.13 mm²) | — | 963 (3) | ⨁⨁⨁⨁ High | The mean square root‐transformed GA lesion size change from baseline in the intervention group was on average 0.07 mm lower (95% −0.14 to 0.00) based on 158 individuals from one study. We have moderate certainty in this effect estimate due to imprecision that fails to exclude important benefit. In a post hoc analysis involving only participants with extrafoveal GA, untransformed GA lesion size change from baseline in the intervention groups was on average 0.60 mm² lower (95% CI −0.91 to −0.30) based on 288 individuals from two studies. Due to the lack of prior specification for this subgroup analysis in the relevant studies, we cannot assess the certainty of the effect estimate. |
| Safety: Development of macular neovascularisation or exudative AMD at 1 year | 34 per 1000 | 78 per 1000 (16 to 386) |
RR 2.29 (0.46 to 11.35) |
997 (3) | ⨁⨁◯◯d Low | |
| Safety: Development of endophthalmitis at 1 year | 0 per 1000 | 8 per 1000 (1 to 70) |
RR 4.77 (0.55 to 41.68) |
997 (3) | ⨁⨁⨁◯e Moderate |
|
| Loss of ≥ 15 letters BCVA at 1 year | No studies reported this outcome. | |||||
| Change in LLVA from baseline at 1 year (ETDRS letters) | The mean LLVA change from baseline ranged across the control group from −2.80 letters (worst LLVA) to 1.70 letters (best LLVA) | The mean LLVA change from baseline in the intervention group was on average 2.66 letters lower (95% CI −5.90 to 0.58 letters) | — | 158 (1) | ⨁⨁⨁◯f Moderate | |
| Change in quality of life at 1 year (NEI VFQ‐25 composite score) | No studies reported this outcome. | |||||
| Abbreviations: AMD = age‐related macular degeneration; BCVA = best corrected visual acuity; CI = confidence interval; ETDRS = Early Treatment Diabetic Retinopathy Study; GA = geographic atrophy; GRADE = grading system for evidence and recommendations; IVT = intravitreal; LLVA = low luminance visual acuity; MD = mean difference; MNV = macular neovascularisation; NEI VFQ‐25 = National Eye Institute Visual Function Questionnaire 25; RR = risk ratio. | ||||||
| GRADE Certainty of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: Any estimate of effect is very uncertain. | ||||||
aAbsolute risks with comparator derived from the corresponding included studies. bFor the outcome endophthalmitis, we calculated the risk in the intervention group from the total number of events/total N reported in the intervention group in the included studies, rounded to the nearest integer. We calculated the confidence intervals by multiplying the 95% confidence limits of the relative effect with the corresponding risk (per 1000) and dividing by the RR. For the other dichotomous outcomes, we calculated the corresponding risk and confidence intervals by multiplying the RR and 95% confidence limits of the relative effect by the assumed risk.
Reason for downgrading certainty of evidence
cRated down (−1 level) for imprecision as the 95% CI overlaps no effect and fails to exclude important harm (i.e. a BCVA change of −5 letters from baseline relative to sham). dRated down (−2 levels) for imprecision due to the very wide confidence intervals, as indicated by a ratio of the upper to lower boundary of the confidence interval greater than 3. We did not further downgrade the certainty of evidence for inconsistency as the results of the studies were consistent in indicating an elevated risk of MNV. eRated down (−1 level) for imprecision as the 95% CI overlaps no effect and fails to exclude important benefit or harm (i.e. an RR of under 0.75 or over 1.25). fRated down (−1 level) for imprecision as the 95% CI overlaps no effect and fails to exclude important harm (i.e. an LLVA change of −5 letters from baseline relative to sham).
Background
Age‐related macular degeneration (AMD) is the leading cause of irreversible sight loss in the elderly worldwide (Flaxman 2017). Although we know surprisingly little about its pathogenesis, mounting genetic and biological information has implicated overactivation of the complement system as a key driver of the disease. Complement inhibition is therefore a promising therapeutic strategy for this incurable and largely untreatable condition. We provide a critical evaluation of the evidence base of these agents on which patients, physicians and healthcare funders can base their treatment decisions.
Description of the condition
Biology
The retina is the innermost layer of the eye, responsible for converting light into neuronal impulses that are transmitted to the brain for visual processing. The retina consists of inner neurosensory layers, which include the light‐sensing rod and cone photoreceptor cells, and the retinal pigment epithelium (RPE) that supports and recycles these cells. The macula (5.5 mm in diameter), and its centre the fovea (0.35 mm in diameter), refer to an area of retina with the highest density of cone photoreceptors that are responsible for high acuity central vision. These tissues are supported by an underlying extracellular matrix that modulates local inflammatory responses (the Bruch’s membrane) (Booij 2010), and a nourishing vascular connective tissue (the choroid). With age, extracellular lipoprotein debris accumulate at the RPE–Bruch’s membrane interface in a process that is thought to be light‐dependent and fuelled by oxidative stress. These so‐called 'drusen' are visible on dilated eye examination as yellow dots and are the pathological hallmark of AMD.
Other than advancing age, the most consistent environmental risk factor for AMD is smoking (Chakravarthy 2010). AMD also has a significant heritable component, with people thought to be 5 to 10 times more likely to develop AMD if a parent or sibling is affected (Shahid 2012). We know now of over 52 common and rare genetic variants across 34 loci that explain 27% of AMD heritability (Fritsche 2016). Most of these genetic signals implicate the complement system. It is noteworthy that a single nucleotide polymorphism in the Complement Factor H gene, present in around a third of all Europeans (Karczewski 2020), increases the odds of AMD two‐ to three‐fold per allele (Edwards 2005; Hageman 2005; Haines 2005; Klein 2005; Despriet 2006). The number of complement gene variants that have been found to influence AMD risk is ever‐increasing (Gold 2006; Spencer 2007; Yates 2007; Raychaudhuri 2011; Seddon 2013; van de Ven 2013; Lorés‐Motta 2018).
Diagnosis
AMD is classified by severity into early, intermediate and advanced forms. Although there are varying definitions, patients with medium drusen (63 μm to 125 μm) and no pigmentary abnormalities are considered to have early AMD. Intermediate AMD is characterised by large drusen (> 125 μm) and/or pigmentary abnormalities (Ferris 2013). There are two advanced AMD subtypes: neovascular AMD (nAMD) and geographic atrophy (GA). These are often called 'dry' or 'wet' AMD, respectively. Early and intermediate AMD typically manifest during an individual's sixth decade of life, whereas GA and neovascular AMD tend to become more prevalent during the seventh and eighth decades.
nAMD results from the formation of abnormal vascular complexes within the retina, broadly termed macular or choroidal neovascularisation (MNV; CNV). These neovascular fibrous membranes are unstable and may leak serous fluid or rupture, leading to frank haemorrhage. nAMD may thus become exudative, leading to retinal toxicity and disciform subretinal scarring that destroys the architecture of local tissues. On the other hand, GA describes an extending atrophic zone characterised by well‐demarcated areas of photoreceptor, RPE, and choriocapillaris loss and thickened BM (Guillonneau 2017). This typically starts parafoveally and expands inwards, towards the fovea (Sarks 1988). RPE and photoreceptor cells may persist within these atrophic zones for some time but show altered morphology and activity (Litts 2015; Schaal 2015; Cao 2021).
nAMD can present with a variety of symptoms, such as difficulty seeing details, distorted straight lines, altered colour perception or contrast sensitivity, and even partial or complete loss of vision. If left untreated, the condition will rapidly progress to severe vision loss. In GA, these symptoms are insidious, and patients are often not aware until both eyes are affected. If the fovea is not affected, then central vision can be preserved. Central visual deterioration is typically more severe in patients with subfoveal GA (Colijn 2021), where the centre point of the fovea is affected. This type of GA is seen in approximately one‐third of patients at first diagnosis (Keenan 2018; Colijn 2021). However, subtle visual symptoms such as delayed dark adaptation may be present even in the early/intermediate phases of the disease, when central visual acuity is unaffected (Cocce 2018).
Drusen and atrophic areas can be seen clinically and on imaging techniques such as colour fundus photography (CFP), fundus autofluorescence (FAF) and optical coherence tomography (OCT). Where MNV is suspected, this may be confirmed using indocyanine green (ICG) angiography, fundus fluorescein angiography (FFA) or OCT‐angiography.
Prognosis
AMD is highly variable in its presentation and prognosis. Although its early and intermediate forms are not expected to affect central visual function, these may herald progression to advanced AMD. The advent of intravitreal vascular endothelial growth factor inhibitor (anti‐VEGF) therapy over the last decade has markedly improved visual outcomes in up to a third of patients with nAMD (Rofagha 2013). Nowadays, the most common causes of sight loss from AMD are macular atrophy and fibrosis (Chakravarthy 2018); until recently, there were no approved treatments for either.
GA is not benign: in patients with bilateral disease, the better seeing eye loses an average of six letters of best‐corrected visual acuity (BCVA) per year (Chakravarthy 2018). Over two‐thirds of people with bilateral GA become unable to drive within two years, and a fifth progress to blindness over six years (Chakravarthy 2018). The progression of GA is influenced by the size, focality, distance from the fovea and autofluorescence patterns of the atrophic areas at initial presentation (Chakravarthy 2018). Approximately 60% of patients with non‐central GA at initial detection will experience progression to subfoveal GA within a period of four years (Keenan 2018), with progression seen over a median of 2.5 years (Lindblad 2009). Bilaterality of disease is also a key risk factor for both GA and nAMD progression, and there is a high correlation of GA lesion enlargement rates between affected eyes (Chakravarthy 2018). As BCVA largely reflects central acuity of the fovea, its loss typically accompanies central GA (Keenan 2018). However, BCVA does not change linearly over time and does not capture the full extent of visual impairment in AMD (Sunness 1999; Balaskas 2022). Additionally, fluctuating fixation patterns may result in BCVA measurements that underestimate functional impairment in patients with subfoveal GA (Sunness 2005; Meleth 2011). Emerging tools such as microperimetry, low‐luminance visual acuity (LLVA), reading speed assessments, and patient‐reported outcomes may overcome these limitations and capture other functional manifestations that precede BCVA loss (Cocce 2018; Fleckenstein 2018; Balaskas 2022).
Impact on affected people or communities
AMD can have a profound impact on the health and wellbeing of affected individuals and caregivers, who are primarily family members (Gupta 2007; Soubrane 2007). Sight loss resulting from AMD may lead to physical, social, and financial isolation, as well as situational dependency (Hodge 2013). Sight loss does not only lead to functional impairment but also limits access to leisure activities, reducing quality of life (Brown 1999; Sharma 2000; Chia 2004; Vu 2005; Slakter 2005; Hassell 2006). Self‐reported anxiety and depression rates in patients with AMD are higher than the general population and reflect the severity of visual impairment (Augustin 2007; Jacob 2017). These experiences may be compounded by the burden of treatment for the exudative form of the condition, which requires the co‐ordination of frequent hospital visits for repeated eye injections. The direct and indirect costs of AMD on individuals and healthcare systems are substantial (Gupta 2007; Soubrane 2007; Coleman 2008; Brechner 2011; Schmier 2012). In the UK, the societal costs of detection, treatment and provision of social care for patients with AMD have previously been estimated at GBP £1.6 billion a year (Minassian 2009), but the indirect costs of AMD‐related visual impairment are likely to be much higher (Simkiss 2016). Sight‐restoring treatments for exudative AMD are considered cost‐effective through improvements in both morbidity and mortality (van Asten 2018; Mulligan 2019; Brown 2020). As the global prevalence of AMD is expected to increase due to our ageing populations, the personal and socioeconomic burden of AMD will likely accelerate.
Prevalence or incidence
AMD is a frequent disease, with an estimated 196 million individuals affected worldwide as of 2020, of which 11 million people are predicted to have the advanced form of the condition (Wong 2014). Recent studies of predominately white, European populations have estimated the prevalence of early or intermediate AMD at 25.3% and of advanced AMD at 2.4% in those 60 years and older (Li 2019). In cases of advanced AMD, neovascular forms are marginally (around 1.4 times) more common than GA (Li 2019), and typically result in greater visual impairment (Colijn 2017). There is no evidence for a gender difference in the prevalence of any stage of AMD after accounting for risk factors (Wong 2014). Although AMD is more prevalent in populations of European ancestry (12.3%) than in African (7.5%) or Asian (7.4%) populations, the number of cases in Europe are plateauing (Wong 2014; Colijn 2017; Creuzot‐Garcher 2022), and more projections anticipate growing numbers of affected individuals globally, especially in Asia (Wong 2014). By 2040, an estimated 288 million people will be living with the condition (Wong 2014).
Description of the intervention
The complement system is a dynamic network of plasma and tissue proteins that exert broad immunological functions throughout the body. These include inactive circulating components that can be cleaved to expose enzymatically active domains (e.g. C2, C3, C4, Factor B), endogenous complement inhibitors (e.g. Factors I and H), and membrane‐bound complement receptors. Complement proteins are mainly produced by the liver but are also expressed at high concentrations in various eye tissues including the retina (Hallam 2020).
There are three pathways of complement activation: the classical, lectin, and alternative pathways. Each is characterised by separate recognition molecules and triggers (for example, by antibody‐antigen complexes or by carbohydrate molecules on pathogens and diseased cells). The alternative pathway is constitutively active through the spontaneous hydrolysis of C3 to C3(H2O). After initiation, proteins in their inactive form are cleaved into active fragments by the serine proteases of the respective pathways (i.e. C1s, MASPs, or Factor D).
All pathways converge at the level of C3. Cleaved C2, C4, Factor B, and C3(H2O) assemble with each other to form C3 convertases. These enzymatic complexes cleave C3 into active smaller (C3a) and larger (C3b) fragments. C3b molecules also join cell‐surface C3 convertases to form C5 convertases, which cleave C5 into and C5b. C3a and C5a are anaphylatoxins which recruit inflammatory cells and induce VEGF production from RPE cells (Nozaki 2006). However, the key effectors of the complement system are the opsonin C3b, which marks diseased cells for removal by immune cells, and C5b, which triggers the sequential assembly of C5b–C9 components (also known as membrane attack complex; MAC) on cell surfaces. MAC is a porous structure that exerts several highly pro‐inflammatory effects on host cells and, rarely, leads to cell lysis (Morgan 2016).
As the alternative pathway exhibits constant low‐level activation at stable state, it is uniquely placed to amplify the effects of the complement system irrespective of the primary trigger. Indeed, 80% to 90% of MAC formation involves alternative pathway activation (Harboe 2004). Alternative pathway activity is entirely dependent on the rates of the C3b breakdown and feedback cycles, which both require regulatory proteins to function. The enzyme that cleaves C3b into its inactive form is Factor I, which requires C3b to be complexed with Factor H or other cofactors to function (Tzoumas 2021).
Complement modulates several key inflammatory functions including phagocytosis, chemotaxis, and lysis of pathogens and diseased cells. It also contributes to tissue healing by modulating angiogenesis (Nozaki 2006; Kahr 2010), stem cell mobilisation (Lee 2010; Mastellos 2013), tissue remodelling (Yanamadala 2010), and clearing diseased cells (Keenan 2012). These functions extend to the eye, where complement is essential for the function and survival of retinal tissues (Hoh Kam 2013; Cerniauskas 2020). These functions are variably influenced by systemic and local inflammatory elements (Mohlin 2017). For example, C5a has been shown to either stimulate or prevent retinal/choroidal neovascularisation in animal models depending on the inflammatory insult (Nozaki 2006; Kahr 2010).
The complement system was first implicated in AMD through the observation of drusen‐like deposits in patients with complement‐mediated renal disease (Duvall‐Young 1989), and supported by the identification of complement components in the drusen of AMD patients in early histological analyses (Anderson 2010). AMD has since evolved as the most prominent example of immune system involvement in ageing and degeneration in the eye, fuelled by the identification of associations of genetic variants in complement proteins that modulate susceptibility to AMD (Copland 2018). These variants may negatively affect the ability of key regulatory molecules such as Factor I and Factor H to function (Hallam 2020), leading to impaired clearance of pro‐inflammatory immune cells (Calippe 2017), and an altered cellular response to oxidative stress (Weismann 2011; Shaw 2012; Cerniauskas 2020). Retinal tissue atrophy has also been shown to accelerate in the presence of risk‐associated AMD genotypes (Whitmore 2015; Tzoumas 2022).
There are several outstanding considerations for the development of complement therapeutics. Ideally, agents designed to inhibit the complement cascade would effectively prevent overactivation without compromising its normal functions. However, the effects of complete complement blockade in the eye are currently unknown. Preventing one step of the cascade could stop the formation of all downstream products, which may disrupt the fine balance of complement activation leading to adverse events. The route of administration is also critical as this will influence dosing, reversal regimens and perhaps even treatment efficacy and safety. Currently, oral, intravenous (IV), subcutaneous (SC), intravitreal (IVT), and subretinal administrations are being trialled.
How the intervention might work
The complement inhibitor development landscape is fast‐moving with several candidates progressing through clinical trials in AMD and other conditions (Mastellos 2019). Broadly speaking, these employ two strategies to suppress inflammation in the retina: blockage of complement activation or supplementation of endogenous regulatory activity (Zelek 2019).
The terminal pathway is considered a key therapeutic target as it is the common final pathway of the complement system. The first complement inhibitor to be studied in a randomised controlled trial (RCT) for AMD was eculizumab (Alexion Pharmaceuticals, a subsidiary of AstraZeneca), an anti‐C5 humanised monoclonal antibody (mAb) delivered intravenously (NCT00935883). LFG316/tesidolumab (initially developed by MorphoSys, later by Novartis), was a fully‐human anti‐C5 mAb delivered as IVT monotherapy (NCT01527500) and in combination with CLG561/NOV7 (MorphoSys/Novartis), an anti‐properdin humanised antibody fragment, for the treatment of GA (NCT02515942). Avacincaptad pegol (IVERIC bio), also known as Zimura, is an IVT‐delivered, anti‐C5, single‐strand, PEGylated nucleic acid aptamer that is being considered for GA as monotherapy (NCT02686658; NCT04435366), and in combination with an anti‐VEGF agent for nAMD (NCT03362190). Avacincaptad pegol is currently under US Food and Drug Administration (FDA) review for the treatment of GA, with an outcome expected in the third quarter of 2023.
Inhibition at the level of C3 represents an alternative strategy that may result in broader suppression. This may have the additional benefit of avoiding the upstream accumulation of C3 that may lead to rapid relapse on cessation of therapy (Hillmen 2021). The most promising candidate in this area is pegcetacoplan (Apellis Pharmaceuticals), also known as Syfovre, a PEGylated compstatin peptide analogue that prevents cleavage of C3 into its active components, as well as binding to and inactivating C3b. Pegcetacoplan has recently been approved by the FDA for treating GA based on its 24‐month phase 3 study data, but remains under review at the European Medicines Agency and the UK's Medicines and Healthcare products Regulatory Agency. Other anti‐C3 agents currently or previously developed for AMD include the compstatin analogues POT‐4 (Alcon) (NCT00473928) and AMY‐106 (Amyndas pharmaceuticals) (NCT03316521), the mAb NGM621 (NGM Biopharmaceuticals, NCT04014777), and the protease CB‐2782 (initially developed by Catalyst Biosciences, now by Vertex Pharmaceuticals). A concern of complement inhibition at this level is the possibility of increased infection risk, although clinical data have shown that systemic C3 and C5 inhibition result in similar safety profiles (Hillmen 2021).
It is also possible to inhibit complement even further upstream. The alternative pathway is a promising target given its outsized contribution to terminal pathway activation and high representation among genetic variants associated with AMD, so limiting its activation may yield therapeutic benefits. Lampalizumab (Roche) was an anti‐Factor D humanised mAb and the first IVT complement inhibitor to be investigated for the treatment of GA in late‐stage clinical trials (NCT02247479; NCT02247531). More recently, the small molecule danicopan/ALXN2040 (previously developed by Alexion Pharmaceuticals, now by AstraZeneca) is being developed as an oral anti‐Factor D therapy for GA (NCT05019521). IONIS‐FB‐LRx, a ligand‐conjugated antisense inhibitor of Factor B mRNA, is also in development for GA as a subcutaneously administered therapy (initially developed by Ionis Pharmaceuticals, later by Roche, NCT03815825).
Increasingly, the role of the classical and lectin pathways in AMD is being appreciated. The anti‐C1q antibody fragment ANX007 (Annexon) is undergoing a phase 2 trial for GA (NCT04656561). Other C1 inhibitor proteins (e.g. Berinert, Ruconest and Cinryze) and mAbs (e.g. sutimlimab, BIVV‐020 and PRO‐02) are in development for non‐ocular indications (Zelek 2019). Similarly, mAbs targeting the lectin pathway such as OMS721 and OMS906 (Omeros), anti‐mannan‐binding lectin serine protease (MASP)‐2 and MASP‐3 respectively, have been proposed for AMD.
An alternative to using synthetic agents to inhibit the complement system is to enhance the levels of naturally occurring complement inhibitors. This approach may be safer as it has been theorised that it would only result in complement inhibition in areas of inflammation. Investigational strategies to increase the local concentration of Factor I, the key alternative pathway regulator, include the subretinal viral‐based gene vector GT005 (developed by Gyroscope Therapeutics, a Novartis company; NCT03846193; NCT04437368; NCT04566445), as well as the recombinant IV CB‐4332 (initially developed by Catalyst Biosciences, now by Vertex Pharmaceuticals) and IVT GEM104 (initially developed by Gemini Therapeutics, now by Disc Medicine) agents. Factor H is the main co‐factor of Factor I and has additional functions in accelerating the decay of the C3 convertase of the alternative pathway. Recombinant Factor H supplementation with IVT GEM103 (Gemini Therapeutics/Disc Medicine) is also being attempted for AMD (NCT04566445; NCT04643886). JNJ‐1887, formerly referred to as AAVCAGsCD59 and HMR59 (initially developed by Hemera Biosciences, now by Janssen) is a gene therapy aimed at increasing levels of CD59, an endogenous MAC inhibitor, for GA that has recently completed phase 1 studies (NCT03144999).
Finally, there are emerging bi‐specific fusion mAbs that combine anti‐VEGF and complement inhibition for the treatment of nAMD such as the anti‐sCR1 IBI302 (Innovent Biologics) (NCT04820452), the anti‐C3 Ranifitin/APL‐2006 (Apellis Pharmaceuticals), and the anti‐C3b KNP‐301 (Kanaph Therapeutics).
Why it is important to do this review
The landscape of AMD treatment has changed significantly since our last review, with several complement inhibitors emerging as promising candidates for preventing or treating the condition. At the time of writing, one of these agents (pegcetacoplan) has already received regulatory approval in the US. In this updated review, we aim to evaluate the key functional, anatomical, and safety outcomes of RCTs for the treatment of advanced AMD to determine their efficacy. As the effectiveness of complement inhibitors in AMD becomes more established, we may narrow our focus to the most relevant drug classes, dosage regimens, clinical endpoints, and phenotypes for further analysis.
Objectives
The aim of this review was to assess the effects and safety of complement inhibitors in the prevention or treatment of advanced AMD.
Methods
Criteria for considering studies for this review
Types of studies
In this review, we included parallel‐group randomised trials comparing complement inhibitors to inactive (e.g. placebo or sham) or active control (e.g. different drug or drug combination). We did not consider cross‐over trials due to the degenerative nature of the condition and the potential for carry‐over effects of complement inhibition, and cluster‐randomised trials were also excluded as the intervention was applied to individuals. We excluded cohort or case‐control studies as these would not be suitable for evaluating the benefits of therapy. However, the inclusion of these studies may be re‐evaluated in future updates to assess any rare or long‐term adverse effects of complement inhibitors in AMD. No studies were excluded based on publication status or language of publication. Studies with a follow‐up period of 12 weeks or less were not included as it was deemed insufficient time to estimate treatment effects in a slowly progressive condition like AMD.
Types of participants
We included trials with participants who have advanced AMD that investigated the treatment of advanced AMD in the treatment and control arms, as we anticipated that most RCTs on the use of complement inhibitors in AMD would be in this category. We defined advanced AMD as GA or nAMD that could be extrafoveal, juxtafoveal, or subfoveal, as identified by clinical examination, ophthalmic imaging, or other validated criteria. We defined non‐advanced AMD as early age‐related maculopathy or drusen/pigmentary abnormalities without neovascularisation or central GA. We excluded studies where the treatment arm consisted of co‐intervention with an agent that is not a complement inhibitor, such as a VEGF inhibitor, as this could lead to confounding of results and would not allow us to reach a meaningful answer about the risks and benefits of complement inhibition.
Equity and special populations
We did not exclude trials based on the age, sex, ethnicity, genotype, educational status, or socioeconomic group of participants as the questions addressed by this review are of relevance to all patients with AMD.
Planning for mixed populations
We explored past ocular history as a source of variability across trials that included participants with a history of GA, nAMD, or both in either eye.
Types of interventions
We included studies that evaluated the efficacy of therapeutic agents aimed at treating or preventing advanced AMD by inhibiting the complement cascade. These agents were compared to active treatment, sham treatment, or no treatment. We did not discriminate based on the mode of administration, such as intravitreal, subretinal, suprachoroidal, or systemic. The target of the complement cascade and whether the investigational agent blocked complement activatory proteins or supplemented complement regulatory proteins was not considered as criteria for exclusion. All of these agents are referred to as complement inhibitors in this review. There were no restrictions on delivery, dose, duration, or concurrent interventions.
Types of outcome measures
Critical outcomes
Change in BCVA from baseline at one year
Change in untransformed GA lesion size from baseline at one year
-
Adverse events in the study eye at one year
Development of MNV or exudative AMD
Development of endophthalmitis
Important outcomes
Loss of ≥ 15 letters BCVA at one year
Change in low luminance visual acuity (LLVA) from baseline at one year
Change in square root‐transformed GA lesion size from baseline at one year
Change in quality of life at one year
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following databases for randomised controlled trials and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 29 June 2022.
Cochrane Central Register of Controlled Trials (CENTRAL 2022, Issue 6) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 29 June 2022) (Appendix 1).
MEDLINE Ovid (1946 to 29 June 2022) (Appendix 2).
Embase Ovid (1980 to 29 June 2022) (Appendix 3).
LILACS (Latin American and Caribbean Health Science Information database) (1982 to 29 June 2022) (Appendix 4).
Web of Science (1985 to 29 June 2022) (Appendix 5).
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 29 June 2022) (Appendix 6).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 29 June 2022) (Appendix 7).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 29 June 2022) (Appendix 8).
Searching other resources
We conducted a comprehensive search of the Science Citation Index and reached out to relevant companies to identify any ongoing or completed trials of complement inhibitors for the treatment or prevention of advanced AMD. In addition, we reviewed abstracts from major ophthalmology conferences and organisations, such as the Association for Research in Vision and Ophthalmology, the American Academy of Ophthalmology, the UK Royal College of Ophthalmologists' Annual Congress, The Macular Society, and the Retina Society, from 2014 onwards.
Data collection and analysis
Selection of studies
Two review authors (NT and GR) independently evaluated all titles and abstracts resulting from the searches using online review management software (Covidence). We obtained full copies of all the reports that potentially met the criteria for consideration in this review according to each review author's independent assessment. We discussed these reports and compiled a definitive list of selected studies.
Data extraction and management
Two review authors (NT and GR) independently collected information on study design and setting, participant characteristics (including disease severity and age), study eligibility criteria, details of the intervention(s) given, the outcomes assessed, the source of study funding, and any conflicts of interest stated by the investigators. We used a data collection form to ensure consistency in the process of data extraction and for comparing data extracted in duplicate. We resolved discrepancies by discussion between all four authors. When data were not available in the published report on the critical or important outcomes of interest to this review, we contacted the study authors and/or sponsors and asked for relevant data to overcome any selective reporting biases. When necessary, we extracted data from figures in the reports and contacted the authors to confirm or refute the accuracy of data so obtained. Where the same outcomes were presented across different reports, we compared these data and highlighted any inconsistencies. We also compared the magnitude and direction of effects reported by studies with how they are presented in our review, highlighting any differences.
Assessment of risk of bias in included studies
We used Chapter 7 (Boutron 2022) and Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions and the Cochrane Risk of Bias 1 (RoB 1) tool to guide the assessment of the methodological quality of each trial included in the review (Higgins 2011). Consequently, each of two review authors (NT and GR) independently considered the following for each trial:
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Masking (performance bias and detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other biases
These predefined domains cover all types of bias that are currently understood to affect the results of RCTs. We answered the signalling questions set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions to reach an overall risk of bias judgement, assigning one of three levels to each domain: 'low risk', 'unclear risk', or 'high risk' (Higgins 2011). We contacted the authors and/or sponsors of trials in which outcomes are categorised as unclear for additional information.
Bias may vary between different outcomes within the same study, for example for some outcomes, assessors, and participants may be more easily masked (e.g. grade of AMD on ophthalmic imaging) than for others (e.g. visual acuity). Therefore, we commented on bias at the level of outcomes rather than the study.
Having made these assessments independently, we discussed outcomes for each trial to agree on its overall bias level and whether to include the data. We presented all judgements and steps relating to bias in the Risk of bias in included studies and Included studies sections.
Measures of treatment effect
The critical outcomes of interest were: (A) change in BCVA from baseline to one year, (B) change in untransformed size of GA lesion from baseline to one year, and (C) pre‐specified adverse events in the study eye after one year. We calculated the mean differences (MDs) of BCVA (in ETDRS letters) and GA lesion size (in mm2) between baseline and follow‐up, and determined risk ratios (RRs) for the development of neovascular AMD or exudative AMD, and of endophthalmitis, in the study eye, as described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). We also summarised secondary outcomes as continuous data and calculated mean differences (MD). The only exception was the outcome of loss of ≥ 15 letters BCVA at one year, deemed as a clinically significant decline in vision, which was treated as a dichotomous outcome and for which we determined the RR. We performed statistical analyses using the Review Manager (RevMan) Web software (RevMan Web 2022).
Unit of analysis issues
Randomisation occurred at the level of the individual with the outcome assessed at the eye level in all included studies. In studies with more than one intervention group, we were careful to avoid including the same group of participants (e.g. those receiving sham injection) twice in the same meta‐analysis.
Dealing with missing data
We discussed the potential impact of missing data on the conclusions of the review in the Discussion section.
Assessment of heterogeneity
We assessed heterogeneity by calculating an I2 statistic and undertaking a Chi2 test as part of our meta‐analyses. As we anticipated low numbers of studies, we used a P‐value of 0.1 to address the null hypothesis of no significant heterogeneity. We also assessed methodological variability through careful review of the included studies.
Assessment of reporting biases
In order to minimise the impact of reporting biases in the studies, we conducted a comprehensive search for trials. To mitigate this potential selective outcome reporting, we explicitly defined our primary and secondary outcome measures ahead of time, as described previously. Additionally, we created a review outcome matrix, as described in the Outcome Reporting Bias In Trials (ORBIT) study (Kirkham 2018), which summarised the reporting status of each outcome in each trial (Table 5). In cases where we suspected that the outcome may have been recorded or analysed but not reported, we reached out to the authors and/or study sponsors to request the missing data.
1. Outcome matrix of included studies.
| Medicine (sponsor) | Target (route of administration) | Mechanism of action | Study name (stage) | Critical outcomes | Important outcomes | ||||||
| Change in BCVA from baseline at 1 year | Change in untransformed GA lesion size from baseline at 1 year | Safety: Development of macular neovascularisation or exudative AMD at 1 year | Safety: Development of endophthalmitis at 1 year | Loss of ≥ 15 letters BCVA at 1 year | Change in LLVA from baseline at 1 year | Change in square root‐transformed GA lesion size from baseline at 1 year | Change in quality of life at 1 year | ||||
| Lampalizumab (Roche) | Factor D (IVT) | Humanised mAb, inhibits Factor D and blocks AP C3 convertase | MAHALO (Phase 2) |
✓ | ✓ | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ |
| CHROMA (Phase 3) |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| SPECTRI (Phase 3) |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Pegcetacoplan (Apellis) | C3 (IVT) | Pegylated peptide, inhibits C3 cleavage | FILLY (Phase 2) |
✓ | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✕ |
| DERBY (Phase 3) |
✕ | ✓ | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | |||
| OAKS (Phase 3) |
✕ | ✓ | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | |||
| Avacincaptad pegol (IVERIC bio) | C5 (IVT) | Pegylated aptamer, inhibits C5 cleavage | GATHER1 (Phase 2/3) |
✓ | ✓ | ✓ | ✓ | ✕ | ✕ | ✓ | ✕ |
| Eculizumab (Alexion) | C5 (IVT) | Humanised mAb, inhibits C5 cleavage | COMPLETE (Phase 2) |
✓ | ✕ | ✓ | ✓ | ✓ | ✕ | ✓ | ✕ |
| LFG316/tesidolumab (Novartis) |
C5 (IVT) | Fully human mAb, inhibits C5 cleavage |
NCT01527500 (Phase 2) |
⒪ | ✓ | ✕ | ✓ | ✕ | ✕ | ✕ | ✕ |
| CLG561 ± LFG316 (Novartis) | Properdin (IVT) | Humanised mAb, inhibits properdin and reduces AP C3 convertase activity |
NCT02515942 (Phase 2) |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ |
Key
✓ Full reporting of results for treatment comparison of interest
⒪ Partial reporting of results for treatment comparison of interest
✕ No reporting of results for treatment comparison of interest
Abbreviations: AMD = age‐related macular degeneration; AP = alternative pathway of complement; BCVA = best‐corrected visual acuity; GA = geographic atrophy; IVT = intravitreal; IV = intravenous; LLVA = low luminance visual acuity; mAb = monoclonal antibody.
Data synthesis
We conducted meta‐analysis following the guidelines described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). In instances where meta‐analysis was not feasible or appropriate, we provided a structured summary. We determined weighted averages by calculating MDs for continuous outcomes and RRs for categorical outcomes. We obtained confidence intervals from the weighted averages, sample sizes, and other relevant measures of data spread, such as the standard error.
We used a random‐effects model for our meta‐analyses to account for any variability between studies. In instances where data were limited, such as in the case of having only two sources of data, we verified the robustness of our estimates by comparing these with a fixed‐effect model. We made sure to interpret the results of both models appropriately, following the guidelines provided by Riley 2011. We used the Mantel‐Haenszel method for dichotomous data and the inverse variance method for continuous data, in accordance with the default approach of RevMan Web.
In the presence of substantial heterogeneity, indicated by effect estimates in opposing directions or an I2 statistic greater than 50% and a significant Chi2 test (P < 0.1), we discussed the results narratively (Higgins 2003). We also provided a visual representation of the results through forest plots, which displayed the overlap between the confidence intervals of the studies.
Subgroup analysis and investigation of heterogeneity
We have considered the possibility of variation in the intervention effect for different populations and characteristics in the Discussion section of our report. Furthermore, to examine the potential modifiers of GA lesion size change, we performed a post hoc subgroup analysis for participants with extrafoveal GA only in the pegcetacoplan phase 3 studies. This analysis was deemed important based on our protocol, external data on the faster rates of GA progression in this group (Fleckenstein 2018), and the availability of data to support it. Unfortunately, disaggregated data for other subgroups in both these and other studies were not available for analysis.
Sensitivity analysis
We conducted sensitivity analyses for each outcome that was categorised as 'high risk' or 'unclear risk' in any trial, to assess the impact of including the data from these trials on the conclusions of the respective meta‐analysis. As the only data available were either from unpublished sources or industry‐funded studies, we did not perform sensitivity analyses based on publication type. We were mindful that the unpublished studies that we were able to locate may not accurately represent all the unpublished studies in this area.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings tables to present a clear and concise overview of the relative and absolute risks associated with the treatment or prevention of advanced AMD through complement inhibition. To ensure the accuracy and reliability of the information presented, two authors (NT and GR) independently evaluated the quality of the evidence for each outcome using the GRADE classification system (GRADEpro GDT). The choice of outcomes to be included in the tables was based on their clinical importance and not influenced by any anticipated or observed effects, or the likelihood that these outcomes had been examined in the reviewed studies.
Results
Description of studies
Please refer to the Included studies, Excluded studies, and Ongoing studies sections.
Results of the search
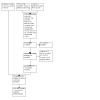
Our updated searches were last conducted on 29 June 2022, resulting in a total of 2521 records (Figure 1). After eliminating 1018 duplicate records, the Cochrane Information Specialist pre‐screened 1503 records and removed 660 records that were not relevant to the scope of the review. We then screened the remaining 843 records and further excluded 797 records based on the information in their title and abstract. Upon reviewing full‐text copies of 46 records, we included 32 reports from 10 studies in our analysis, as described in the Characteristics of included studies section. We excluded four reports from four studies for specific reasons, which are explained in the Characteristics of excluded studies section. We identified the following 10 ongoing studies (please refer to the Characteristics of ongoing studies section for further details).
1.
NCT03815825, a phase 2 study investigating the safety and efficacy of IONIS‐FB‐LRx in GA (Ionis Pharmaceuticals, Inc.)
NCT04435366, a phase 3 study investigating the safety and efficacy of avacincaptad pegol in GA (IVERIC bio, Inc.)
NCT04437368 and NCT04566445, phase 2 studies investigating the safety and efficacy of GT005 in GA (Gyroscope Therapeutics Limited, a Novartis company)
NCT04465955, a phase 2 study investigating the safety and efficacy of NGM621 in GA (NGM Biopharmaceuticals, Inc.)
NCT04643886, a phase 2 study investigating the safety and efficacy of GEM103 in GA (Gemini Therapeutics, Inc.)
NCT04656561, a phase 2 study investigating the safety and efficacy of ANX007 in GA (Annexon, Inc.)
NCT04820452, a phase 2 study investigating the safety and efficacy of IBI302 in nAMD (Innovent Biologics Co. Ltd.)
NCT05019521, a phase 2 study investigating the safety and efficacy of danicopan (ALXN2040) in GA (Alexion Pharmaceuticals)
NCT05230537, a phase 2 study investigating the safety and efficacy of iptacopan (LNP023) in early/intermediate AMD (Novartis Pharmaceuticals)
These studies, with reporting dates from 2023 to 2026, will be assessed in a future update of this review. We did not identify any ongoing studies of complement inhibitors for the treatment of non‐advanced AMD, or for the prevention of advanced AMD.
Included studies
All 10 included studies were of randomised, masked, parallel‐group design and evaluated complement inhibition at various levels (C3, C5, and Factor D) as compared to sham or placebo for the treatment of GA (Included studies). Sham injections were selected as comparator treatments as there are currently no approved treatments for the condition. There were no studies of the treatment of nAMD or non‐advanced AMD, or on the prevention of advanced AMD. Nine studies evaluated their agents as monotherapy, with the remaining study testing two complement inhibitors (CLG561 ± LFG316). Only the eculizumab phase 2 study investigated an intravenous (IV) agent, with the remaining studies using intravitreal (IVT) agents. All included studies were industry‐funded. All but one study (eculizumab phase 2) recruited from multiple study centres. Participants were majority female, white, and recruited from Europe and North America. Subgroup analyses based on complement genotype were only reported by five studies, including the lampalizumab phase 2 and 3, pegcetacoplan phase 2, and eculizumab phase 2 trials. Most studies did not report all our outcomes of interest (Table 5).
The eligibility criteria across the studies were similar, with most studies enrolling participants aged 50 years or older, with baseline BCVA of 24 letters or better, and GA lesion size ranging from 2.5 to 17.5 mm2. There were no restrictions on the type of GA due to age‐related macular degeneration AMD. Most participants across all studies had GA in both eyes at baseline, as summarised in Table 6. All studies allowed for multifocal GA, although several had additional GA lesion size requirements if this was the case (Included studies). However, there were some exceptions. The avacincaptad pegol study only recruited patients with GA located at least partially within 1500 μm of the foveal centre but not involving the centre point. This definition would encompass cases of extrafoveal or juxtafoveal GA, as defined clinically. Despite this eligibility criterion, a small proportion of the intention‐to‐treat population in this study was found to have subfoveal GA. Most studies had additional requirements for perilesional hyperautofluorescence to be present in the GA lesions (CLG561 ± LFG316 phase 2, pegcetacoplan phase 2 and 3, and lampalizumab phase 2 and 3). In practice, nearly all participants in the avacincaptad pegol phase 2/3 study also displayed perilesional hyperautofluorescence, as reflected in the Included studies section. The three studies on lampalizumab only enrolled participants with perilesional banded or diffuse patterns of hyperautofluorescence, which are considered high‐risk phenotypes, and with bilateral GA. Finally, the CLG561 ± LFG316 phase 2 study had no restrictions on BCVA and a higher minimum GA lesion size threshold, ranging from 8 to 16 mm2.
2. Baseline characteristics of patients in included studies.
| Avacincaptad pegol phase 2/3 (GATHER1)a | CLG561 ± LFG316 phase 2 | Eculizumab phase 2 (COMPLETE)b | Lampalizumab phase 2 (MAHALO) | Lampalizumab phase 3 (CHROMA and SPECTRI) | LFG316/tesidolumab phase 2 | Pegcetacoplan phase 2 (FILLY) | Pegcetacoplan phase 3 (DERBY and OAKS) | |
| ITT population, N | 286 | 114 | 30 | 129 | 1881 | 158 | 246 | 1208 |
| Mean age, years | 79 | 78 | 80 | 79 | 78 | 79 | 80 | 78 |
| Female, % | 70 | 59 | 52 | 44 | 60 | 61 | 63 | 61 |
| White ethnicity, % | 98 | 65 | NA | 98 | 97 | NA | 98 | 93 |
| Bilateral GA, % | 99 | NA | 60 | 100 | 100 | NA | 84 | NA |
| nAMD in fellow eye, % | 0 | 0 | 0 | 0 | 0 | 0 | 38 | NA |
| Mean GA lesion size at baseline, mm2 | 7.6 | NA | 6.4 | 8.7 | 8.1 | NA | 8.4 | 8.3 |
| Extrafoveal GA lesion, % | 95 | NA | NA | NA | 48 | NA | NA | 39 |
| Unifocal GA lesion, % | NA | NA | NA | NA | 22 | NA | NA | 30 |
| Mean BCVA at baseline, ETDRS letters | 69 | NA | 74 | 48 | 66 | 43 | 34 | 59 |
aThere were no available baseline characteristics for the 1 mg intervention group of the avacincaptad pegol phase 2/3 study.
bThe percentage of female participants was estimated from the enrolled population (n = 60), as this metric was unavailable for the analysed population (n = 30) of the eculizumab phase 2 study.
Abbreviations: BCVA = best‐corrected visual acuity; ETDRS = early treatment for diabetic retinopathy; GA = geographic atrophy; ITT = intention‐to‐treat; NA = not available.
Although all studies excluded participants with a prior history of nAMD or MNV in the study eye, only the three studies concerning pegcetacoplan did not exclude participants based on fellow eye status. The approach to handling participants with nAMD or MNV during the trial varied among the studies. In four studies (avacincaptad pegol phase 2/3, CLG561 ± LFG316 phase 2, eculizumab phase 2, and pegcetacoplan phase 2), participants were withdrawn from the study if nAMD or MNV developed in the study eye. On the other hand, the phase 2 and 3 lampalizumab and phase 3 pegcetacoplan studies allowed the participants to continue in the trial and offered the option of concurrent anti‐VEGF therapy. The study of eculizumab was the only one to specify that participants who developed nAMD or MNV in the fellow eye should be removed, although this did not occur.
Similar measurement instruments were used for all studies: Early Treatment Diabetic Retinopathy Study (ETDRS) charts at four metres were used for measuring BCVA, whereas fundus autofluorescence (FAF) was used for characterising GA lesion growth. A combination of other imaging modalities including CFP, OCT, FFA, or near‐infrared reflectance were also used to inform patient eligibility and assess efficacy and safety outcomes in all studies except the LFG316 monotherapy phase 2 trial. Adverse outcome reporting was informed by clinical examination and imaging in all participants, but there were differences in whether diagnoses of MNV and nAMD, or infectious and non‐infectious endophthalmitis, were grouped or reported separately. All four studies reporting LLVA outcomes used a regular ETDRS chart, but two (the lampalizumab phase 3 studies) did so under low luminance conditions, whereas two (pegcetacoplan phase 2 and CLG561 ± LFG316 phase 2 studies) used a neutral density filter.
In total, 4052 participants were enrolled. All studies undertook randomisation at the level of individuals, and identified and reported on one eye per person, the 'study eye', so there are no unit of analysis issues. The baseline characteristics of participants in each study are shown in Table 6. A full list of the included studies can be found in the Included studies section.
Excluded studies
We excluded four studies. Reasons for exclusion included early termination, study withdrawal, follow‐up duration of less than 12 weeks, and co‐intervention with a VEGF inhibitor in the treatment arm.
More information is presented in the Characteristics of excluded studies table.
Risk of bias in included studies
All included studies were of randomised, parallel‐group design with comparator groups. Thus, the overall risk of bias is low, increasing the confidence in the results of this review. Despite this, some RCTs are subject to higher degrees of bias than others. The risk of bias assessments detailed in the Included studies section, and summarised in Figure 2 and Figure 3, were compiled using the RoB 1 assessment tool (Higgins 2011), after thorough comparison of study reports, registrations, protocols, and statistical analysis plans.
2.

Review authors' judgements about each risk of bias domain across included studies.
3.

Review authors' judgements about each risk of bias domain for each included study.
Allocation
Selection bias, which refers to systematic differences in baseline characteristics between compared groups, can be mitigated using random sequence generation and allocation concealment in studies (Higgins 2011). Although all included studies were randomised using a web‐based system, four studies (avacincaptad pegol, eculizumab, lampalizumab phase 2, and LFG316 monotherapy) did not specify the procedure for selecting the study eye if both eyes were eligible, leading to unclear risk of bias in the randomisation process.
The randomisation strategies used in the studies varied, with all but one study (CLG561 ± LFG316) using restricted random assignments, such as minimisation (avacincaptad pegol) or stratified randomisation (all other studies), but this may compromise allocation concealment if investigators are aware of the size of the blocks and participant characteristics. We judged the risk of allocation bias to be unclear in the eculizumab and pegcetacoplan phase 2 studies, as they stratified randomisation by treatment group at single sites. The allocation concealment process of the LFG316 monotherapy trial was not described, making it impossible to determine the risk of allocation bias.
Blinding
After participants were enrolled, all included studies implemented masking procedures to ensure that both participants and personnel were kept unaware of the interventions received or administered. The use of appropriate comparators, in conjunction with masking, effectively prevented any performance bias from influencing the outcome measurements of any study. Furthermore, the clear dosing regimens and outcome reporting in all studies minimised the risk of misclassification bias and bias resulting from the use of adjunctive therapies.
Incomplete outcome data
Attrition bias can occur when there is an imbalance in missing outcome data between the study arms, regardless of the reason for missing data (Higgins 2011). In most of the studies included in this review, there was an unclear risk of attrition bias. For example, a considerably higher proportion of missing data was observed in the active treatment groups compared to the sham group in the avacincaptad pegol, CLG561 ± LFG316, and pegcetacoplan phase 2 trials. While the LFG316 monotherapy study had a balanced proportion of missing data, it was still substantial at around a third of the participants. The lampalizumab phase 2 study had balanced missing data across groups, but the primary analyses of BCVA and GA lesion size change used the last observation carried forward (LOCF) procedure, which could lead to an overestimation of treatment benefits. These studies failed to present any imputation strategies in their mixed‐effect analyses, raising questions about the validity of their results. The exception was the avacincaptad pegol study, which presented various mean imputation analyses for GA lesion size, but not for other outcomes. The eculizumab study had no missing data, but there were discrepancies in results reporting, leading to an unclear risk of bias assessment. On the other hand, the lampalizumab phase 3 and pegcetacoplan phase 3 studies had a low and balanced amount of attrition, making them a low risk in this domain.
Selective reporting
The assessment of seemingly objective outcomes such as change in BCVA from baseline, change in GA lesion size, and adverse event reporting can be subject to detection bias, as the lack of masking can influence the measurement of these outcomes (Higgins 2011). Only the LFG316 monotherapy study had unmasked outcome assessments for all domains, leading to a high risk of detection bias judgement. As the assessors of adverse events in the CLG561 ± LFG316 study were also not masked, we judged the study to have a high risk of bias in this domain. In the pegcetacoplan phase 2 study, assessors of adverse outcomes were also not masked, however the extensive reporting of the key adverse event of MNV/nAMD in study drug recipients provided reassurance and resulted in an unclear risk of bias judgement.
Other potential sources of bias
Regarding the risk of other biases, we evaluated the methods used in the studies to control for confounding factors. Most of the studies were deemed to have a low risk of confounding by indication, due to the use of multivariable analyses that considered key patient‐ and eye‐level prognostic indicators in their primary outcome assessments. However, the eculizumab study was found to have a high risk of bias in this domain, due to a significant imbalance in outcomes of interest at baseline and the use of univariable analyses. On the other hand, the LFG316 monotherapy study did not provide enough information on covariate adjustment, leading to an unclear risk of bias judgement in this area. We did not detect any other sources of bias in the studies. The randomised controlled trial design of the included studies, with concurrent enrolment and random assignment of treatments, eliminates the possibility of lead‐time and confounding by indication biases. While regression to the mean may occur, it is unlikely to be differential between the intervention and comparison groups.
Further details on our risk of bias assessments can be found in the Included studies section.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
For key analyses and grading of evidence certainty see:
Table 1: Efficacy and safety of intravitreal (IVT) lampalizumab four‐weekly versus sham for geographic atrophy (GA)
Table 2: Efficacy and safety of IVT lampalizumab six‐ to eight‐weekly versus sham for geographic atrophy (GA)
Table 3: Efficacy and safety of IVT pegcetacoplan four‐weekly versus sham for geographic atrophy (GA)
Table 4: Efficacy and safety of IVT pegcetacoplan eight‐weekly versus sham for geographic atrophy (GA)
Following independent review of study characteristics and risk of bias assessment across studies and outcomes, we decided to proceed with a meta‐analysis of the following studies:
-
Lampalizumab phase 2 and 3 studies (MAHALO, CHROMA, SPECTRI):
-
Pegcetacoplan phase 2 and 3 studies (FILLY, DERBY, OAKS):
1.1. Analysis.

Comparison 1: Efficacy and safety of IVT lampalizumab 4‐weekly versus sham for geographic atrophy (GA), Outcome 1: BCVA change at 1 year
1.2. Analysis.

Comparison 1: Efficacy and safety of IVT lampalizumab 4‐weekly versus sham for geographic atrophy (GA), Outcome 2: GA lesion size change at 1 year (mm2)
1.3. Analysis.

Comparison 1: Efficacy and safety of IVT lampalizumab 4‐weekly versus sham for geographic atrophy (GA), Outcome 3: Safety: Development of MNV or exudative AMD at 1 year
1.4. Analysis.

Comparison 1: Efficacy and safety of IVT lampalizumab 4‐weekly versus sham for geographic atrophy (GA), Outcome 4: Safety: Development of endophthalmitis at 1 year
2.1. Analysis.

Comparison 2: Efficacy and safety of IVT lampalizumab 6‐ to 8‐weekly versus sham for geographic atrophy (GA), Outcome 1: BCVA change at 1 year
2.2. Analysis.

Comparison 2: Efficacy and safety of IVT lampalizumab 6‐ to 8‐weekly versus sham for geographic atrophy (GA), Outcome 2: GA lesion size change at 1 year (mm2)
2.3. Analysis.

Comparison 2: Efficacy and safety of IVT lampalizumab 6‐ to 8‐weekly versus sham for geographic atrophy (GA), Outcome 3: Safety: Development of MNV or exudative AMD at 1 year
2.4. Analysis.

Comparison 2: Efficacy and safety of IVT lampalizumab 6‐ to 8‐weekly versus sham for geographic atrophy (GA), Outcome 4: Safety: Development of endophthalmitis at 1 year
3.1. Analysis.

Comparison 3: Efficacy and safety of IVT pegcetacoplan 4‐weekly versus sham for geographic atrophy (GA), Outcome 1: GA lesion size change at 1 year (mm2)
3.2. Analysis.

Comparison 3: Efficacy and safety of IVT pegcetacoplan 4‐weekly versus sham for geographic atrophy (GA), Outcome 2: Extrafoveal GA lesion size change at 1 year (mm2)
3.3. Analysis.

Comparison 3: Efficacy and safety of IVT pegcetacoplan 4‐weekly versus sham for geographic atrophy (GA), Outcome 3: Safety: Development of MNV or exudative AMD at 1 year
3.4. Analysis.

Comparison 3: Efficacy and safety of IVT pegcetacoplan 4‐weekly versus sham for geographic atrophy (GA), Outcome 4: Development of endophthalmitis at 1 year
4.1. Analysis.

Comparison 4: Efficacy and safety of IVT pegcetacoplan 8‐weekly versus sham for geographic atrophy (GA), Outcome 1: GA lesion size change at 1 year (mm2)
4.2. Analysis.

Comparison 4: Efficacy and safety of IVT pegcetacoplan 8‐weekly versus sham for geographic atrophy (GA), Outcome 2: Extrafoveal GA lesion size change at 1 year (mm2)
4.3. Analysis.

Comparison 4: Efficacy and safety of IVT pegcetacoplan 8‐weekly versus sham for geographic atrophy (GA), Outcome 3: Safety: Development of MNV or exudative AMD at 1 year
4.4. Analysis.

Comparison 4: Efficacy and safety of IVT pegcetacoplan 8‐weekly versus sham for geographic atrophy (GA), Outcome 4: Development of endophthalmitis at 1 year
These analyses were selected due to the low risk of bias and low heterogeneity among the studies. For our meta‐analyses of outcomes, we employed a random‐effects model approach. In cases where data were limited to two or fewer sources, such as the effect of lampalizumab on the loss of ≥ 15 letters best‐corrected visual acuity (BCVA) at one year with only two phase 3 studies and the effect of pegcetacoplan on the development of macular neovascularisation (MNV) or exudative AMD with aggregated phase 3 study results, we conducted sensitivity analyses using a fixed‐effect models. Due to the limited number and methodological heterogeneity of the remaining studies, including the different approaches used to target the complement system using various modalities, we did not consider cross‐intervention meta‐analysis to be reasonable and so performed a descriptive analysis instead.
Overview of outcome reporting
Eight of 10 studies reported BCVA change at one year (lampalizumab phase 2 and 3, pegcetacoplan phase 2, avacincaptad pegol phase 2/3, CLG561 ± LFG316 phase 2, LFG316 phase 2, and eculizumab phase 2) and four studies also reported BCVA loss as a dichotomous outcome (lampalizumab phase 3, CLG561 ± LFG316 phase 2, and eculizumab phase 2). We considered a change of five letters BCVA as clinically significant in line with previous research suggesting that this is the minimum amount to be reasonably certain that the change is real (Beck 2007).
Nine of 10 studies (lampalizumab phase 2 and 3, pegcetacoplan phase 2 and 3, avacincaptad pegol phase 2/3, CLG561 ± LFG316 phase 2, and LFG316 phase 2) reported untransformed GA lesion growth over one year, and five reported square root‐transformed GA lesion growth over one year (lampalizumab phase 3, pegcetacoplan phase 2, avacincaptad pegol phase 2/3, and eculizumab phase 2). Currently, there is no scientific consensus on the threshold for clinically meaningful GA lesion size growth: epidemiological studies have estimated these at 1.09 mm2 untransformed (Colijn 2021) and 0.29 mm square root‐transformed (Keenan 2018) per year in untreated patients. We assumed a 20% change in these estimates per year as evidence of a meaningful effect, corresponding to 0.22 mm2 and 0.06 mm changes in respective measures of GA lesion growth.
All studies reported safety endpoints. The following reports do not distinguish between nAMD or MNV types, or between infectious and non‐infectious endophthalmitis, as there was diagnostic and reporting variability of these subgroups. The rate of GA progression to nAMD/MNV per year has been estimated at 7.4% (Chakravarthy 2018). Local immunosuppression with complement inhibitors could in theory increase the eye’s susceptibility to infection. The rate of endophthalmitis after IVT treatment has been reported at 2 per 1000 for corticosteroid, compared with 2 per 10,000 for anti‐VEGF (steroid injections versus anti‐VEGF, RR 6.9) (VanderBeek 2015).
Five of 10 studies reported low luminance visual acuity (LLVA) outcome data (lampalizumab phase 3, pegcetacoplan phase 2, avacincaptad pegol phase 2/3, and CLG561 ± LFG316 phase 2). As with BCVA, we considered a change of five letters in this outcome to be clinically meaningful. Only the two lampalizumab phase 3 studies have reported quality of life outcome data so far, with pegcetacoplan phase 3 studies expected to report in future. We considered a four‐point change in the overall National Eye Institute Visual Function Questionnaire 25 (NEI‐VFQ) as the minimum clinically meaningful within‐person change based on previous research (Submacular Surgery Trials Research Group 2007).
All outcomes reflect data at the study eye‐level; there were no unit of analysis issues. Mean differences are based on reported least‐square mean change using frequentist approaches for most studies, except for the eculizumab study that reported observed means only and the LFG316 monotherapy study that reported means from an unspecified Bayesian linear mixed model. There was insufficient outcome reporting to permit other subgroup analyses (e.g. by ethnicity, genomics, disease severity, or type of advanced AMD). As each of the lampalizumab and pegcetacoplan studies used one comparator group against which both intervention arms were compared, the number of participants presented in this section are not direct sums from the summary of findings tables (please refer to Table 1, Table 2, Table 3, and Table 4).
Efficacy and safety of IVT lampalizumab versus sham for GA
For lampalizumab, we considered week 48 data from phase 3 studies as approximating to one‐year outcome data. We conducted sensitivity analyses that excluded phase 2 data for BCVA and GA lesion size change due to the study's unclear risk of bias for these outcomes. The phase 2 study presented GA lesion change results using two statistical models, mixed model for repeated measures (MMRM) and ANOVA with last observation carried forward (LOCF) imputation. However, LOCF imputation has certain limitations that can impact the validity of the results obtained. When a person drops out of a study, MMRM assumes that the participant's missing data would have followed the trend of their treatment group whereas the LOCF method assumes that the participant's missing data would remain unchanged from the last recorded value. This assumption is not always accurate and may result in biased results, particularly in the case of progressive conditions such as AMD where the missing data point may have changed in the meantime. Furthermore, the lampalizumab phase 2 LOCF/ANOVA analyses dichotomise the covariate of baseline GA lesion size, which could result in significant loss of power and precision compared to the use of baseline GA lesion size as a continuous variable in the MMRM. Therefore, we opted to conduct meta‐analyses using MMRM data from the phase 2 study while also considering the impact of LOCF/ANOVA data. The phase 3 studies only provide MMRM data.
Change in BCVA from baseline
There were no meaningful changes in mean difference in BCVA over one year compared to sham for lampalizumab administered IVT every four weeks (mean difference (MD) +1.03 letters, 95% confidence interval (CI) ‐0.19 to 2.25) or every six to eight weeks (MD +0.22 letters, 95% CI ‐1.00 to 1.44) for 1932 participants with GA (please refer to Analysis 1.1 and Analysis 2.1). Substantial heterogeneity is unlikely (I² = 0%, P = 0.80; I² = 0%, P = 0.50). On sensitivity analyses excluding phase 2 data, these estimates were largely unchanged (MD +0.93, 95% CI −0.33 to 2.19; MD +0.03, 95% CI −1.24 to 1.29).
In the phase 3 studies, there were probably no meaningful changes in the proportion of participants who lost 15 letters or more of BCVA over one year compared to sham for lampalizumab administered IVT every four weeks (RR 0.86, 95% CI 0.63 to 1.18) or every six to eight weeks (RR 0.98, 95% CI 0.72 to 1.33) for 1103 and 1096 participants with GA, respectively. We have moderate certainty in these estimates due to imprecision. Substantial heterogeneity is unlikely (I² = 0%, P = 0.64; I² = 0%, P = 0.74), and our estimates did not change using fixed‐effect analyses. Although this outcome is not reported for the phase 2 study, further data would be very unlikely to change our confidence in the estimate of effect.
Change in GA lesion size from baseline
Using phase 2 and 3 MMRM data, there were no meaningful changes in mean difference in untransformed GA lesion size over one year compared to sham for lampalizumab administered IVT every four weeks (MD +0.07 mm², 95% CI ‐0.09 to 0.23; moderate certainty due to imprecision) or every six to eight weeks (MD +0.07 mm², 95% CI −0.05 to 0.19; high certainty) for 1920 participants with GA (please refer to Analysis 1.2, Analysis 2.2, Table 1, and Table 2). Heterogeneity is unlikely for both the four‐weekly (P = 0.25; I² = 28%) and six‐ to eight‐weekly (P = 0.95; I² = 0%) intervention arms using MMRM data.
Using phase 2 LOCF and phase 3 MMRM data, there were no meaningful changes in mean difference in untransformed GA lesion size over one year compared to sham for lampalizumab administered IVT every four weeks (MD +0.02 mm², 95% CI −0.19 to 0.23; moderate certainty due to imprecision) or every six to eight weeks (MD +0.07 mm², 95% CI −0.05 to 0.18; high certainty) for 1920 participants with GA. Moderate heterogeneity is likely for the four‐weekly (I² = 58%, P = 0.09) but not the six‐ to eight‐weekly (I² = 0%, P = 0.93) arm estimate. This suggests that the LOCF imputation method of the phase 2 study overestimated the therapeutic benefit of four‐weekly lampalizumab in slowing down untransformed GA lesion size growth. On sensitivity analyses excluding phase 2 data, these estimates were largely unchanged (MD +0.08 mm², 95% CI −0.10 to 0.25; MD +0.07, 95% CI −0.05 to 0.19).
The mean difference in square root‐transformed GA lesion size over one year compared to sham for lampalizumab administered IVT every four weeks (MD +0.01 mm, 95% ‐0.01 to 0.03) and every six weeks (MD +0.01 mm, 95% ‐0.01 to 0.03) showed no meaningful changes for 1797 participants with GA in the phase 3 studies. Our confidence in this result is high. Although the phase 2 study did not report this outcome, additional data are unlikely to alter our estimation of the effect.
Adverse events
Of 2000 participants analysed, those receiving lampalizumab may have had a meaningful increase in the risk of nAMD/MNV compared to sham over a year, both at four‐weekly (RR 1.77, 95% CI 0.73 to 4.30) and at six‐ to eight‐weekly (RR 1.70, 95% CI 0.67 to 4.28) intervals (Analysis 1.3 and Analysis 2.3). There was probably also an increase in the risk of endophthalmitis over a year compared to sham for lampalizumab administered either at four‐weekly (RR 6.92, 95% CI 0.36 to 133.73) or six‐ to eight‐weekly (RR 4.94, 95% CI 0.24 to 102.78) intervals (Analysis 1.4 and Analysis 2.4). There was no substantial heterogeneity (I² = 0%, P = 0.75) in the MNV estimate for four‐weekly administration. It was not possible to calculate heterogeneity for the remaining comparisons. Our certainty in these effects is downgraded due to imprecision (Table 1 and Table 2).
Change in LLVA from baseline
There were no meaningful changes in mean difference in LLVA over one year compared to sham for lampalizumab administered IVT every four weeks (MD +0.20 letters, 95% CI ‐1.07 to 1.46) or every six weeks (MD ‐0.27 letters, 95% CI ‐1.33 to 0.79) for 1594 participants with GA in the phase 3 studies. Substantial heterogeneity is unlikely (I2 = 0%, P = 0.29; I2 = 38%, P = 0.20). We have high certainty in these effect estimates (Table 1 and Table 2).
Change in quality of life from baseline
There were no meaningful changes in mean difference in NEI VFQ‐25 composite score, which ranges from 0 to 100, over one year compared to sham for lampalizumab administered IVT every four weeks (MD +0.42, 95% CI ‐1.12 to 1.95; high certainty) or every six weeks (MD ‐0.28, 95% CI ‐2.82 to 2.26; moderate certainty due to inconsistency) for 1496 participants with GA in the phase 3 studies (Table 1 and Table 2). Substantial heterogeneity is likely in the six‐weekly administration groups (I2 = 69%, P = 0.07), but not the four‐weekly group (I2 = 15%, P = 0.28). This heterogeneity is due to different directions of effect seen with six‐weekly administration of lampalizumab across the phase 3 studies for reasons unknown. Additional metrics of quality of life, such as separate NEI VFQ‐25 near and distance scores and the Functional Reading Independence (FRI) index score, were not meaningfully changed by intervention (Heier 2020).
Efficacy and safety of IVT pegcetacoplan versus sham for GA
Each study on pegcetacoplan had its own distinct outcome reporting, with the exception of MNV or exudative AMD development, which was reported together for the DERBY and OAKS studies. We carried out a sensitivity analysis excluding phase 2 study data due to ‘unclear risk’ of bias in GA lesion size and adverse event domains. We also undertook a post hoc analysis of GA lesion size change in participants with extrafoveal GA only, a characteristic associated with faster disease progression (Fleckenstein 2018).
Change in BCVA from baseline
In the phase 2 study, there were probably no meaningful changes in mean difference in BCVA over one year compared to sham for pegcetacoplan administered IVT every four weeks (MD +1.05 letters, 95% CI −2.71 to 4.81) or every eight weeks (MD −1.42 letters, 95% CI −5.25 to 2.41) for 242 participants with GA. We have moderate certainty in these estimates due to imprecision. In future, phase 3 BCVA outcome data are likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Change in GA lesion size from baseline
IVT pegcetacoplan caused a meaningful decrease in the rate of untransformed GA lesion growth over one year compared to sham when administered every four weeks (MD −0.38 mm², 95% CI −0.57 to −0.19) or every eight weeks (MD −0.29 mm², 95% CI −0.44 to −0.13) for 1208 participants with GA (please refer to Analysis 3.1 and Analysis 4.1). These represent 19.2% and 14.8% reductions versus sham, respectively. We have high certainty in these effect estimates, and further research is unlikely to change our confidence in these results (Table 3 and Table 4). Substantial heterogeneity is unlikely (I2 = 25%, P = 0.26 for the four‐weekly arm; I2 = 0%, P = 0.57 for the eight‐weekly arm). Sensitivity analyses excluding phase 2 data did not change the estimates substantially (MD −0.34, 95% CI −0.52 to −0.15 for the four‐weekly arm; MD −0.27, 95% CI −0.43 to −0.11 for the eight‐weekly arm).
In a post hoc analysis of participants with extrafoveal GA only, pegcetacoplan may have had a greater effect on untransformed GA lesion size change over one year for both four‐weekly (MD −0.67 mm², 95% CI −0.98 to −0.36) and eight‐weekly (MD −0.60 mm², 95% CI −0.91 to −0.30) administrations for 446 participants with GA across both phase 3 studies (Analysis 3.2 and Analysis 4.2). This represents a 26.2% and 23.3% reduction versus sham, respectively. Substantial heterogeneity is unlikely (I2 = 59%, P = 0.26 for the four‐weekly arm; I2 = 0%, P = 0.57 for the eight‐weekly arm). The results of this subgroup analysis are uncertain, as it was not planned beforehand in the relevant studies (Table 3 and Table 4). Additionally, the criteria used to define extrafoveal GA in this study have not been reported in any published materials. Lastly, we cannot confidently conclude if pegcetacoplan's effect varies in this subgroup as we lack disaggregated data on participants with non‐extrafoveal GA, making it difficult to formally test for subgroup differences.
In the phase 2 study, IVT pegcetacoplan also achieved a meaningful difference in square root‐transformed GA lesion size over one year compared to sham when administered every four weeks (MD −0.09 mm, 95% −0.16 to −0.02) and probably also every eight weeks (MD −0.07 mm, 95% CI −0.14 to 0.00) for 242 participants with GA. We have high certainty in the four‐weekly effect estimate, but moderate certainty in the six‐ to eight‐weekly effect estimate due to overlap with no effect. A meaningful reduction was also reported in square root‐transformed extrafoveal GA lesion size in the four‐weekly (−0.14 mm, 95% CI −0.25 to −0.03; high certainty) and probably also the eight‐weekly group (−0.07 mm, 95% CI −0.22 to 0.08; moderate certainty due to imprecision) for 77 people with GA in the phase 2 study. These outcomes have not yet been reported for the phase 3 studies.
Adverse events
Of 1502 participants analysed, those receiving pegcetacoplan may have had an increase in the risk of MNV or exudative AMD over a year compared to sham, both at four‐weekly (RR 4.47, 95% CI 0.41 to 48.98) and at eight‐weekly (RR 2.29, 95% CI 0.46 to 11.35) intervals. However, the evidence for this association is limited by imprecision due to very wide confidence intervals, leading to a low level of certainty (Analysis 3.3, Analysis 4.3, Table 3, and Table 4). Although there is evidence of heterogeneity in the four‐weekly (I2 = 82%, P = 0.02) and eight‐weekly (I2 = 59%, P = 0.12) groups between studies, the effect estimates are in the same direction and confidence intervals overlap. Therefore, these results are consistent in indicating an elevated risk of MNV. Our estimates were significantly impacted by the exclusion of phase 2 data in sensitivity analyses (RR 1.68, 95% CI 0.92 to 3.07 for the four‐weekly arm and RR 1.30, 95% CI 0.69 to 2.46 for the eight‐weekly arm). Fixed‐effect analyses including phase 2 and 3 data also showed variations (RR 2.60, 95% 1.50 to 4.50 for the four‐weekly arm and RR 1.64, 95% CI 0.91 to 2.96 for the eight‐weekly arm). These discrepancies due to the increased occurrence of MNV or exudative AMD in phase 2 study participants, which could reflect variations in the number of patients with a history of nAMD in the fellow eye or the presence of the double‐layer sign, a possible indicator of sub‐RPE fluid, on OCT imaging. It is likely that further research will alter these intervention estimates.
Furthermore, we found a probable increase in the risk of endophthalmitis over a year when comparing pegcetacoplan administered at four‐weekly (RR 3.79, 95% CI 0.42 to 34.05) or eight‐weekly (RR 4.77, 95% CI 0.55 to 41.68) intervals to sham (Analysis 3.4 and Analysis 4.4). We have moderate certainty in these effects due to imprecision (Table 3 and Table 4). Substantial heterogeneity is unlikely (I2 = 0% for both groups). Sensitivity analyses excluding phase 2 data did not change our estimates substantially (RR 2.97, 95% CI 0.12 to 72.54 for the four‐weekly arm; RR 6.97, 95% CI 0.36 to 134.06 for the eight‐weekly arm). Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Change in LLVA from baseline
There were probably no meaningful changes in mean difference in LLVA over one year compared to sham for pegcetacoplan administered IVT every four weeks (MD −2.18 letters, 95% CI −5.36 to 1.00) or every eight weeks (MD −2.66 letters, 95% CI −5.90 to 0.58) for 242 participants with GA in the phase 2 study. We have moderate certainty in these effect estimates due to imprecision (Table 3 and Table 4). Phase 3 data are not yet available, but further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Efficacy and safety of IVT avacincaptad pegol versus sham for GA
Due to the overlap in the comparator groups of the phase 2/3 avacincaptad pegol intervention arms and the unavailability of further data from the sponsor or investigators, we were unable to conduct a meta‐analysis of the 2 mg and 4 mg doses in this study. However, we present the results of each dosing regimen individually.
Change in BCVA from baseline
There were probably no meaningful changes in mean difference in BCVA over one year compared to sham for avacincaptad pegol administered IVT at 2 mg (MD +1.39 letters, 95% CI −5.89 to 8.67) or at 4 mg (MD −0.28 letters, 95% CI −8.74 to 8.18) every four weeks for 260 participants with GA in the Avacincaptad pegol Phase 2/3 (GATHER1). We have moderate certainty in these estimates as the associated confidence intervals overlap no effect and fail to exclude important benefit or important harm. In future, BCVA outcomes from the phase 3 trial (GATHER2; NCT04435366) are likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Change in GA lesion size from baseline
Avacincaptad pegol probably resulted in a meaningful decrease in the rate of untransformed GA lesion growth over one year when administered IVT at 2 mg (MD −0.70 mm², 95% CI −1.99 to 0.59) or at 4 mg (MD −0.71 mm², 95% CI −1.92 to 0.51) every four weeks compared to sham for 260 participants with predominantly extrafoveal or juxtafoveal GA in the phase 2/3 study. These represent 30.5% and 25.6% reductions versus sham, respectively. We have moderate certainty in these estimates given the overlap of confidence intervals with no effect. Further research is likely to have an important impact on our confidence in the estimate of effect.
Similarly, the agent may have resulted in a meaningful decrease in the rate of square root‐transformed GA lesion growth over one year at 2 mg (MD −0.11 mm, 95% CI −0.32 to 0.10) and 4 mg (MD −0.12 mm, 95% CI −0.33 to 0.08) compared to sham for 260 participants with GA. We have a low level of certainty in this estimate due to the overlapping confidence intervals that we calculated from the standard errors reported in the study, which differ from those presented in the original publication (for 2 mg, 95% CI 0.030 to 0.190; for 4 mg, 95% CI 0.038 to 0.209). The reason for the discrepancy in precision between the two analyses is unclear as the statistical methods used in the published reports are not adequately described and our attempts to seek clarification from the corresponding study author or sponsor were unsuccessful. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Adverse events
IVT avacincaptad pegol at 1 mg, 2 mg, or 4 mg may have increased the risk of MNV or exudative AMD substantially compared to sham over a year (RR 3.13, 95% CI 0.93 to 10.55) in the 286 participants analysed in the phase 2/3 study. We have low certainty in this effect as the confidence intervals overlap no effect and fail to exclude meaningful harm, and there is a serious risk of bias in this outcome. Only pooled sham rates were reported, so it was not possible to derive RRs at different intervention doses. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. There were no cases of endophthalmitis reported in this study.
There were probably no meaningful changes in mean difference in LLVA over one year compared to sham for avacincaptad pegol administered IVT at 2 mg (MD +0.38 letters, 95% CI −8.91 to 9.67) or 4 mg (MD −1.44 letters, 95% CI −11.00 to 8.12 letters) every four weeks for 260 participants with GA in the phase 2/3 study. We have moderate certainty in this estimate as the confidence intervals overlap no effect and fail to exclude meaningful benefit or harm. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Efficacy and safety of IVT CLG561 ± LFG316 versus sham for GA
Change in BCVA from baseline
There were probably no meaningful changes in mean difference in BCVA over one year compared to sham for CLG561 administered IVT as 10 mg monotherapy (MD −0.20 letters, 95% CI −13.18 to 12.78) or as 5 mg in combination with LFG316 5 mg (MD −0.38 letters, 95% CI −13.03 to 12.27) every four weeks for 96 participants with GA. We have moderate certainty in these estimates as the associated confidence intervals overlap no effect and fail to exclude important benefit or important harm.
There were probably no meaningful changes in the proportion of participants who lost 15 letters or more of BCVA over one year compared to sham for CLG561 as monotherapy (RR 1.17, 95% CI 0.17 to 7.79). Administration of CLG561 in combination with LFG316 probably increased the risk of losing 15 letters or more of BCVA over one year (RR 2.00, 95% CI 0.39 to 10.22) for 100 participants with GA. We have moderate certainty in these estimates as the associated confidence intervals overlap no effect.
Change in GA lesion size from baseline
CLG561 administered IVT as 10 mg monotherapy every four weeks probably resulted in a meaningful reduction in the rate of untransformed GA lesion growth over one year (MD −0.29 mm², 95% CI −1.19 to 0.61), but probably made little to no difference when given as 5 mg in combination with LFG316 5 mg every four weeks (MD +0.06 mm², 95% −0.81 to 0.93) compared to sham for 96 participants with GA. We have moderate certainty in these estimates as the confidence intervals overlap no effect.
Adverse events
Of 114 participants analysed, those receiving CLG561 ± LFG316 IVT every four weeks probably had a meaningful increase in the risk of endophthalmitis compared to sham over a year (RR 1.58, 95% CI 0.07 to 37.88). We have moderate certainty in this effect as the confidence intervals overlap no effect and fail to exclude meaningful benefit or harm. There were no new cases of nAMD/MNV reported in this study.
Change in LLVA from baseline
There were probably no meaningful changes in mean difference in LLVA over one year compared to sham for CLG561 administered IVT at 2 mg as 10 mg monotherapy (MD +2.20 letters, 95% CI −9.53 to 13.93) or as 5 mg in combination with LFG316 5 mg (MD −0.36 letters, 95% CI −11.92 to 11.20) every four weeks for 96 participants with GA in the study. We have moderate certainty in this estimate due to imprecision.
Efficacy and safety of IVT LFG316 monotherapy versus sham for GA
Change in BCVA from baseline
It is unclear if LFG316 monotherapy resulted in a meaningful change in mean difference in BCVA over one year when administered IVT at 5 mg every four weeks (MD +0.87 letters, 95% −4.53 to 6.27) compared to sham for 112 participants with GA; we have very low certainty in this estimate as the associated confidence intervals fail to exclude important benefit or important harm, there was a serious risk of bias, and information was partially reported – we had to extrapolate mean differences and standard deviations (SDs) from the reported BCVA estimates of each group at baseline and day 337. Any estimate of effect is very uncertain.
Change in GA lesion size from baseline
For 77 participants with GA, LFG316 administered IVT at 5 mg every four weeks may have resulted in a meaningful increase in the rate of untransformed GA lesion growth over one year compared to sham, either using a Bayesian statistical approach (MD +0.37 mm², 95% CI −0.14 to 0.88), or by MMRM sensitivity analysis (MD +0.15 mm², 95% −0.25 to 0.55). There was a discrepancy between the clinical study report on the Novartis clinical trials report website and the NCT registry entry regarding the reporting timeframe (337 versus 505 days), but the magnitude of change indicates that they are most likely day 337 mean differences. We have low certainty in these estimates as the associated confidence intervals overlap no effect, and there was a serious risk of bias.
Adverse events
Adverse event results for one year are unavailable, therefore we evaluated data at 18 months. Of 150 participants analysed, LFG316 IVT administered at 5 mg or 10 mg every four weeks may have reduced the risk of MNV or exudative AMD compared to sham at 18 months (RR 0.53, 95% CI 0.03 to 8.23). The intervention may have increased the risk of endophthalmitis substantially compared to sham at 18 months (RR 2.60, 95% CI 0.13 to 53.17). We have low certainty in these estimates as the confidence intervals overlap no effect and fail to exclude meaningful benefit or harm, and there is a serious risk of bias.
Efficacy and safety of IV eculizumab versus placebo for GA
Change in BCVA from baseline
Eculizumab may have made little to no difference in mean difference in BCVA over one year when administered IV at 600 mg to 1200 mg every one to two weeks compared to placebo (MD −2.20 letters, 95% −7.56 to 3.16) for 30 participants with GA. Those receiving eculizumab may have had a meaningful reduction in the risk of ≥ 15 letter BCVA loss compared to placebo (RR 0.50, 95% CI 0.03 to 7.19). We have low certainty in these estimates because of the serious risk of bias, and the associated confidence intervals overlap no effect and fail to exclude important benefit or harm.
Change in GA lesion size from baseline
Untransformed GA lesion size was not reported over one year for this study. It is unclear if eculizumab had an effect on untransformed GA lesion growth at six months (MD +0.01 mm², 95% −0.10 to 0.12) compared to placebo for 30 participants with GA. We have very low certainty in this estimate as the study is associated with a serious risk of bias, it addressed a restricted version of the review outcome, and the associated confidence intervals overlap no effect.
Eculizumab may have made little to no difference to the outcome of square root‐transformed GA lesion size at one year, either at the low dose (MD −0.02 mm, 95% −0.17 to 0.13) or high dose (MD +0.03 mm, 95% −0.19 to 0.25) when compared to placebo for 30 patients with GA. We have a low certainty in this estimate as the study is associated with a serious risk of bias, and the associated confidence intervals overlap no effect.
Adverse events
Of 30 participants analysed, the main report states that there were no patients who developed MNV, exudative AMD, or endophthalmitis over one year. However, a secondary publication states that one participant in the placebo group did in fact develop exudative AMD (RR 0.17, 95% 0.01 to 3.94), thus eculizumab may have reduced the risk of nAMD/MNV. We have low certainty in this estimate for the aforementioned reasons of bias and imprecision.
Discussion
Summary of main results
The discovery of complement gene variants that significantly increase the risk of AMD has fuelled investigation into strategies to suppress local inflammation for the treatment of this debilitating disease, culminating in the recent approval of the first‐ever treatment for by a major regulatory agency. In this systematic review and meta‐analysis, we evaluate the latest evidence for complement inhibitors in the treatment or prevention of AMD. In 10 parallel‐group RCTs with inactive comparator arms, 4052 patients with GA were treated with complement blocking medicines and were followed up for at least one year. All but one of these agents were administered by the IVT route.
Factor D inhibition with lampalizumab
Despite promising initial results, our meta‐analysis of phase 2 and 3 data confirms with moderate‐to‐high certainty that lampalizumab, given as a 10 mg IVT injection monthly or every six to eight weeks, has little to no effect on anatomical (Analysis 1.2 and Analysis 2.2), functional (Analysis 1.1 and Analysis 2.1), or quality of life endpoints for participants with GA (Table 1 and Table 2). By undertaking a sensitivity analysis we show that the LOCF imputation method used in the phase 2 study may have biased the effect estimates of untransformed GA lesion size change from baseline relative to the MMRM analysis (Analysis 1.2 and Analysis 2.2), a widely accepted statistical technique for longitudinal RCTs that is employed in the phase 3 studies. We also demonstrate that lampalizumab may increase the risk of MNV or exudative AMD, even though there were no participants with a history of nAMD in either eye in the studies (Analysis 1.3 and Analysis 2.3). Additionally, we demonstrate that the risk of endophthalmitis is probably increased in recipients of lampalizumab relative to sham (4 per 1000 with monthly and 3 per 1000 with every‐other‐month administrations); this is an imprecise estimate that fails to exclude meaningful harm higher than that associated with IVT corticosteroid (2 per 1000) or anti‐VEGF (2 per 10,000) administrations (Analysis 1.4 and Analysis 2.4) (VanderBeek 2015).
Factor D is the enzyme that propagates the C3 feedback cycle, activating the alternative pathway and enhancing the amplification of the classical and lectin pathways of complement. Therefore, it remains unclear why blocking Factor D with lampalizumab was not an effective therapeutic strategy for this population. As lampalizumab binds to the self‐inhibitory exosite of Factor D (Katschke 2012), rather than its catalytic centre, it is possible that complete inhibition was not achieved – even small amounts of Factor D are sufficient to facilitate complement activity (Wu 2018). Alternatively, it has been suggested that C3 activation by Factor D can be bypassed by other plasma proteases (Irmscher 2018). Finally, it has been proposed that Factor D has a high turnover rate (initially by Undar 2002) requiring high levels of an inhibitor for target saturation, although we could not find experimental validation of this claim and we assume that this limitation would apply to the targeting of all complement molecules. Nevertheless, lampalizumab may still have achieved sufficient Factor D inhibition to disrupt the ocular immune response to injury and infection, which could have been the reason for the higher rates of MNV and endophthalmitis seen. Trials of lampalizumab for AMD have been discontinued, but the ongoing phase 2 trial of oral danicopan for the treatment of GA may further inform the efficacy and safety of Factor D inhibition in the eye.
C3 inhibition with pegcetacoplan
Through our meta‐analysis of phase 2 and 3 study data we confirm that pegcetacoplan, given as a 15 mg IVT injection monthly or every‐other‐month, results in a meaningful decrease in untransformed GA lesion size relative to sham over one year in the overall treated population (growth rate reductions of 19.2% for monthly and 14.8% for every‐other‐month administrations; Table 3, Table 4, Analysis 3.1, and Analysis 4.1). Post hoc phase 3 analyses also show that these effects may be at least doubled in a smaller number of participants with extrafoveal GA at both frequencies of administration (growth rate reductions of 26.2% for monthly and 23.3% for every‐other‐month administrations; Analysis 3.2 and Analysis 4.2), although the smaller phase 2 study suggests a more modest increase on square root‐transformed GA lesion size growth (Steinle 2021). These results align with the faster progression of extrafoveal GA observed in a previous epidemiological study (Schmitz‐Valckenberg 2016), and may indicate rapid growth of GA lesions in this subgroup rather than a specific effect of pegcetacoplan. However, we cannot confirm the method used to distinguish extrafoveal GA in the study and as it was not pre‐specified, we cannot comment on the certainty of the effect estimate. The lack of data also prevents us from determining differences in pegcetacoplan's efficacy for those without extrafoveal GA. Additionally, pegcetacoplan probably does not achieve a meaningful change in the functional endpoints of BCVA and LLVA change from baseline at one year in the phase 2 study. The lower bounds of these 95% confidence intervals suggest that meaningful benefit (i.e. fewer than five ETDRS letters of BCVA/LLVA change) in these parameters at one year may still be reported by the phase 3 studies. Nevertheless, it is likely that any functional benefits of inhibiting C3 in advanced AMD will not be immediately evident. To account for this, the phase 3 studies are planned to report outcomes such as BCVA, LLVA, reading speed, and quality of life at month 24, which should allow adequate time for any benefits to become apparent.
We also show with low certainty that this medication may increase the risk of MNV or exudative AMD, higher than the rate reported with lampalizumab. This large effect size is primarily driven by phase 2 data (Analysis 3.3 and Analysis 4.3). This heterogeneity may be explained by the higher proportion of exudative AMD history in the fellow eye (39% versus 0% for other studies), or a high proportion of subclinical MNV in the study eye; a post hoc analysis identified that separation between the RPE and Bruch’s membrane (identifiable on OCT imaging as the 'double‐layer' sign), which may indicate treatment‐naïve MNV, was a highly significant biomarker for exudative AMD induction following pegcetacoplan therapy (present in 73% of cases; Wykoff 2021). However, we cannot exclude the possibility that the properties and biological actions of the agent itself could have triggered this adverse event: pegcetacoplan is an analogue of the peptide compstatin that blocks C3 and C3b to prevent complement amplification, downstream C5 activation, and generation of inflammatory effectors (Mastellos 2019). Although its PEGylation prolongs tissue half‐life and reduces immunogenicity (Gupta 2019), this modification has been shown to trigger CNV in mice (Lyzogubov 2011). Inhibition of C3 has also been linked to CNV induction by increasing the proportion of alternatively activated macrophages involved in generating new blood vessels preclinically (Cao 2011; Ruan 2015). Finally, we show that pegcetacoplan therapy probably results in an increase in the risk of endophthalmitis (6 per 1000 with monthly and 8 per 1000 with every‐other‐month administrations; Analysis 3.4 and Analysis 4.4). Despite their imprecision, these effect estimates suggest a risk beyond that associated with the IVT route of administration alone (VanderBeek 2015). The long‐term risks of these adverse events require further clarification. Further research, possibly from phase 4 trials, is necessary to increase our certainty of these risks and will likely change these effect estimates.
C5 inhibition with avacincaptad pegol
We undertook a descriptive analysis of the functional and anatomical phase 2/3 study endpoints and confirm that avacincaptad pegol, given as one or two 2 mg IVT injections, probably reduces untransformed GA lesion growth to a meaningful extent relative to sham over one year in participants with predominantly extrafoveal or juxtafoveal GA (untransformed growth rate reductions of 30.5% for 2 mg and 25.6% for 4 mg administrations). We have moderate certainty in these effects due to imprecision. The medicine may also meaningfully decrease square root‐transformed GA lesion growth over the same period, but we have a low certainty in this estimate due to imprecision and inconsistencies of reporting.
It is noteworthy that 95% of the avacincaptad pegol study participants were diagnosed with extrafoveal or juxtafoveal GA. Given the lack of clarity regarding the criteria used to identify extrafoveal GA in the pegcetacoplan studies, we suggest exercising caution when comparing the rate of reduction in extrafoveal GA growth between studies. Nevertheless, the reduction in untransformed extrafoveal GA lesion growth achieved with pegcetacoplan in a similar population may be comparable. If these estimates are accurate, this may indicate that the anatomical benefits of complement inhibitors are primarily achieved through inhibition of membrane attack complex (MAC), whether by blocking C3 or C5 (Kim 2021). Nevertheless, C5 inhibition is not expected to benefit from the broader immunosuppression that can be achieved by blocking C3, such as the reduced production of opsonins and anaphylatoxins that drive immune cell recruitment to the retina.
Although there were no cases of endophthalmitis reported, this study was underpowered to detect a difference in this outcome. Notably, avacincaptad pegol may cause an increase in the induction of MNV or exudative AMD (Effects of interventions), despite the exclusion of participants with a history of exudative AMD in either eye from the study. There is currently insufficient published data to conclude that participants who developed this adverse effect had subclinical MNV features that contributed to their increased risk. It is unclear whether the PEGylated component of avacincaptad pegol could again have contributed to this risk (Lyzogubov 2011), or whether MNV is a class effect of complement inhibition. Such a mechanism could plausibly act through inhibition of MAC by C3 or C5 inhibition; in mice models of laser‐induced CNV, MAC has been shown to be essential for choroidal angiogenesis (Bora 2005). Further data from the ongoing phase 3 study of avacincaptad pegol (GATHER2) are needed to improve our confidence in the effect estimates of the above risks and benefits of this agent.
Other therapeutic strategies
CLG561 (a properdin inhibitor) administered IVT as monotherapy or in combination with LFG316 (a C5 inhibitor) probably had no effect on GA lesion growth and had neutral‐to‐harmful effects on BCVA change depending on how the outcome was measured, i.e. as a continuous or binary outcome. The sample size was likely insufficient to conclude on the effect of pooled CLG561 ± LFG316 intervention on MNV induction risk, although therapy with these agents may have increased the risk of endophthalmitis. The medicine did not proceed with clinical development for any indication.
We judged that the LFG316/tesidolumab monotherapy study has a serious risk of bias across several domains (Figure 2 and Figure 3), which lowered our certainty of its effects. It was therefore not possible to comment on its effect on BCVA and MNV induction, although therapy with this agent may have resulted in an increased risk of endophthalmitis relative to sham and may have accelerated GA lesion growth. The latter effect could be due to population differences, as this is not consistent with the findings of CLG561/LFG316 combination therapy. The study was terminated early due to poor efficacy, and trials of the agent in exudative and non‐exudative AMD have been discontinued.
Eculizumab may have made little to no difference to GA lesion growth or BCVA outcomes compared to sham. Although the intravenous (IV) route is theoretically safer in terms of MNV and endophthalmitis outcomes, there were insufficient participants to reliably detect these outcomes. It is unlikely that eculizumab was dosed long enough to observe any benefits (less than six months). However, we cannot dismiss the possibility that its ineffectiveness may have been due to systemic administration, which may have hindered its diffusion beyond the Bruch's membrane or blood‐retina barriers. Eculizumab is no longer in development for AMD.
Future perspectives
Despite significant progress in the development and study of complement inhibitors for the treatment of AMD over the last decade, there remain several unanswered questions regarding the potential clinical use of these agents:
First, it is still unclear which therapeutic approach is the most effective to limit GA advancement, with several targets, drug designs, and modes of administration under scrutiny. Divergence of biological and methodological approaches to complement inhibition across studies meant that it was not appropriate to undertake a network meta‐analysis comparing different agents in this review update. Although a particular complement inhibition approach may in future emerge as superior overall, decision‐makers should remain mindful of the potential influence of patient characteristics on treatment outcomes; different targets or combinations thereof may be favourable in different AMD subpopulations. Subgroup analyses stratified by AMD phenotype and/or genotype may be highly informative in this regard, but unfortunately were not possible in this update given the lack of data. Although a few inconsequential genetic markers have been reported in a handful of complement inhibitor trials so far, AMD is a genetically complex disease, so it is unlikely that a single common genetic variant will have a substantial influence on disease risk or treatment response (Fritsche 2016). Furthermore, the genetic variants that predispose to AMD development do not necessarily correlate with disease progression and so, by extension, may not influence treatment response. A measure of polygenic risk due to complement overactivation, informed by a functional understanding of protein consequences and validated on deeply phenotyped cohorts, will be necessary to reliably stratify patients by genotype in future trials.
Next, it is still unclear when and at what dosage complement inhibitors should be initiated, and when treatment should be stopped. These are essential considerations to maximise patient benefit while avoiding harm and wasted resources. It is known that complement is involved early in the pathogenesis of AMD (Weismann 2011), and that microstructural changes caused by genetic deficiencies in endogenous complement regulators may precede overt disease by decades (Tzoumas 2022). Treating earlier in the disease course may avert irreversible retinal cell death and atrophy. Additionally, total complement system inhibition may not be required, reducing the risk of adverse events. However, consensus thresholds for starting and initiating therapy will likely be necessary before this approach can be pursued given the high prevalence of early and intermediate AMD. The identification and validation of quantifiable biomarkers of disease progression can help in this regard. For example, post hoc analyses of the pegcetacoplan phase 2 study have shown that the agent probably reduces the progression of incomplete RPE and outer retinal atrophy (Nittala 2022). Future updates to this review may consider this and other subclinical biomarkers as important endpoints.
It is also important to consider whether we are matching treatment with pathology. We do not yet know whether GA growth reductions of more than 30% are achievable with complement inhibition, or whether targeting additional mechanisms will be necessary to reduce growth rates further. Variation in complement genes does not explain all advanced AMD heritability (Fritsche 2016), and contributions may be different for early/intermediate disease subtypes (Winkler 2020). Also, the effect of most of these risk variants on AMD progression has not yet been confirmed (Heesterbeek 2020). Ongoing trials of bi‐specific molecules (e.g. inhibiting complement and vascular endothelial growth factor (VEGF)) will inform this consideration.
Additionally, the acceptability of the above treatment‐emergent adverse effects is yet to be determined. Although exudative AMD induced by complement inhibition was readily treated with anti‐VEGF therapies in the above trials, it is unclear whether this will be reversible. Patients and clinicians will need to consider whether periodic and perhaps concurrent anti‐VEGF treatment to mitigate against this adverse event is tolerable and effective. While these studies were not designed to detect infrequent adverse events, the likely higher incidence of endophthalmitis among recipients of lampalizumab and pegcetacoplan compared to those receiving IVT administration of anti‐VEGF or corticosteroids in other studies (VanderBeek 2015) warrants caution. We have not examined other adverse event outcomes, but no additional safety signals have been reported that would not be expected from an IVT therapy. Nevertheless, it is likely that longer‐term studies following market authorisation will be required to clarify the risk to patients.
Furthermore, we have yet to clarify what patient compliance will be. It is regrettable that quality of life outcomes have only been reported for the two lampalizumab phase 3 studies. Nonetheless, variation in the adherence of recipients to these medicines is already evident, with marked or imbalanced attrition in the treatment arms of pegcetacoplan phase 2, avacincaptad pegol, CLG561 ± LFG316, and LFG316 monotherapy studies. For the remaining studies, it should not be assumed that high levels of adherence will be maintained outside of a clinical trial setting. Adherence to treatment with complement inhibitors will likely depend on individual differences in how recipients of care perceive the balance of functional, anatomical, and safety outcomes. Patients will likely show a clear preference for functional over anatomical endpoints; although novel measures such as LLVA, reading speed, and microperimetry may be useful surrogates for function, BCVA remains the gold standard by which the efficacy of treatment is judged, and remains widely accepted in clinics and by regulatory authorities. Demonstrating a meaningful improvement in this endpoint will be critical to the adoption of these therapies by patients and clinicians. Gene therapy for complement inhibition provides a potential solution for reducing the treatment demands on patients, but its safety, efficacy, and longevity have yet to be fully established.
Finally, the cost‐effectiveness of these agents is yet to be established. Both direct and indirect economic expenses associated with care will remain a significant factor in the adoption of these medicines. These will have different implications for patients, clinicians, and regulatory agencies across countries and regions that employ different funding mechanisms. The above considerations of treatment initiation/cessation, management of adverse events, and use of concomitant medications must also be taken into account in these cost calculations, and weighed up against the economic burden of continued vision loss (Wittenborn 2013).
Overall completeness and applicability of evidence
Data on our critical outcomes were largely complete, but most studies did not address our important outcomes. Due to biological differences between different stages and subtypes of AMD, the results of our study are not applicable to other forms of AMD beyond GA, or to other causes of retinal degeneration.
Quality of the evidence
We conducted this review according to the processes described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Although we considered most study data to be of high quality, there are a few potential limitations that should be considered when extrapolating the results of these studies to clinical practice:
1. Missing data. The presence of missing data in medical research often leads to partially observed datasets, which can pose a significant threat to the validity of any study. The mechanism by which data become missing can vary. As it is not possible to determine if the missing data are missing completely at random (MCAR), missing at random (MAR), or missing not at random (MNAR), it is important to explore how inferences may vary under these differing assumptions.
Commonly used methods such as listwise deletion (used exclusively for the eculizumab phase 2, lampalizumab phase 3, LFG316 phase 2, and pegcetacoplan phase 2 studies), where participants containing at least one missing observation are removed, result in a loss of statistical power as a large portion of the data is discarded. This method makes no use of the missing data or its relationship to other observed variables, potentially leading to biased and imprecise measurements. As discussed above, the LOCF method, sometimes used in longitudinal studies (such as the lampalizumab phase 2 study), should be avoided as it can distort means and measures of precision.
The mean imputation method used in the avacincaptad pegol phase 2/3 study, where missing values are replaced by the general mean of the observed series, does not add any new information and alters the underlying distribution to be more peaked at the mean. This does not improve bias or precision for any missingness mechanism. Regression mean imputation, used in the CLG561 ± LFG316 phase 2 study, accounts for the observed measurements recorded on other variables, potentially improving bias for MCAR and MAR missingness mechanisms. However, the problem of reduced standard error remains as the variation of the regression line estimation is not accounted for, leading to imprecise estimates.
The multiple random imputation method used only by the lampalizumab phase 2 study for sensitivity analyses improves precision for MCAR and MAR missingness mechanisms, but is not robust for MNAR. Future studies may wish to consider exploring multiple imputation and MNAR methods for sensitivity analyses (Kenward 2007; Spratt 2010; Harrell 2015; Galimard 2018; Hughes 2019). Full details on the sensitivity analyses of the pegcetacoplan phase 3 studies are eagerly awaited.
2. Data transformation. We included both untransformed and square root‐transformed GA lesion size as outcomes in this review update given ongoing debate about their respective utility. The square root transformation was initially proposed over a decade ago as a way of eliminating the effect of baseline values on statistical tests of GA lesion size progression, following the observation that growth rates were no longer significantly associated with baseline lesion size in Pearson and Spearman correlation tests using this method (Feuer 2013). This is a valid consideration for parametric methods with a normality assumption (e.g. t‐tests, ANOVA, and ANCOVA), but may not work for data with asymmetric or skewed distributions (Harrell 2015). None of the included studies explicitly present or discuss the distribution of their continuous outcomes or covariates, thus it is difficult to establish whether such a transformation would be appropriate. However, linearity is not realistic to assume; a pooled analysis of two major longitudinal studies showed considerable variation in GA progression rates amongst participants (Colijn 2021). Transformations can overcome these problems, but a better approach may be to use flexible statistical methods that are not sensitive to the distribution of variables (e.g. nonparametric methods that only assume smoothness, such as spline functions) (Harrell 2015). Future studies may wish to examine the need for such transformations or alternative statistical methods with fewer assumptions as sensitivity analyses.
3. Measurement error. All studies relied primarily on FAF or other imaging modalities for the detection, monitoring, and measurement of GA area. As these are not automated trial endpoints, there is a risk of imprecision. It is not clear what the devices’ margins of error are, and no study has explicitly commented on or attempted to quantify observer variability. Additionally, there is a lack of clarity as to whether the assessments of GA using OCT and FAF imaging are consistent with each other. This consideration extends to the assessment of adverse events; the two pegcetacoplan phase 3 studies report that six out of 52 investigator‐determined cases of study eye new MNV/exudative AMD diagnosed by investigators had not been confirmed by the reading centre. We have nevertheless included these events in the above analyses.
Potential biases in the review process
We acknowledge the risk of bias inherent in only evaluating outcomes over a year, particularly in a degenerative condition such as AMD that may take years to manifest. This choice has been informed by the primary endpoints and, where available, power calculations undertaken by the authors of the included studies which indicate that a 12‐month treatment period is sufficient to measure clinically significant effects given an appropriate sample size. Evidence of therapeutic efficacy at one year also remains a clinically relevant measure required by regulators. Nevertheless, we recognise that adverse events occurring before one year may be of interest; these may be explored in a future update that focuses on the adverse effects of complement inhibitors beyond the critical outcomes of macular neovascularisation and endophthalmitis.
Additionally, we recognise the limitations of BCVA as a functional endpoint measure for trials of retinal diseases, but this decision is in line with current regulatory preferences in the absence of other validated functional endpoints or surrogates (Csaky 2017). Nevertheless, we have supplemented our assessment of BCVA with emerging endpoints such as LLVA and National Eye Institute Visual Function Questionnaire 25 (NEI VFQ‐25) composite score, even though these are unreported by most studies.
Finally, we acknowledge the risk of publication bias as a threat to the validity of any systematic review. To reduce the risk of this, we contacted the authors and/or sponsors of eight studies (avacincaptad pegol, eculizumab, lampalizumab, and pegcetacoplan) to enquire about any unpublished data mentioned in online publications and presentations and to gain clarification on the study protocols and statistical analysis plans. Unfortunately, we only received clarification from the sponsor of the pegcetacoplan studies.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this systematic review and meta‐analysis of complement inhibition for AMD is the first and only study of its kind published since the initial reporting of RCTs in this field.
Authors' conclusions
Implications for practice.
In people with geographic atrophy (GA), complement inhibition with pegcetacoplan favourably reduces lesion size growth over one year. This is based on data from three parallel‐group, masked randomised controlled trials (RCTs) with comparator arms, representing high‐quality evidence. There is also moderate‐certainty evidence to suggest that avacincaptad pegol probably reduces lesion size growth over one year in patients with extrafoveal or juxtafoveal GA. Due to imprecision, the effect estimate may change with further research; phase 3 study results are required. There is currently little to no evidence that complement inhibition with any medicine results in a meaningful benefit in any of our chosen functional and quality of life outcomes at one year. Ongoing research on pegcetacoplan and avacincaptad pegol is likely to have an important impact on these assessments. Complement inhibition was associated with important safety outcomes: treatment with pegcetacoplan may have increased the incidence of macular neovascularisation (MNV) or exudative age‐related macular degeneration (AMD) (low‐certainty evidence), and probably increased the risk of endophthalmitis (moderate‐certainty evidence). Treatment with avacincaptad pegol may have also increased the incidence of MNV or exudative AMD (low‐certainty evidence), whereas its effect on endophthalmitis risk is yet to be established. It is unclear whether these adverse effects are related to the treatment modality, target, population characteristics, or a feature of complement inhibition in general. Larger studies are required to clarify the safety of these agents. Studies in people with early or intermediate AMD are needed because the evidence is from studies in people with GA, a form of advanced AMD.
Implications for research.
This review highlights the need for large RCTs to evaluate the effectiveness of complement inhibitors in improving functional, anatomical, and safety outcomes in people diagnosed with GA. Future trials need to be rigorous in design and delivery, with subsequent reporting to include high‐quality descriptions of all aspects of methodology to enable appraisal and interpretation of results. Consideration should also be given to the comparative benefits of C3 and C5 inhibition in similar populations, the potential harms and costs of treatment, and the adoption of critical outcomes to include patient‐based measures.
What's new
| Date | Event | Description |
|---|---|---|
| 14 June 2023 | New citation required and conclusions have changed | First evidence‐based recommendations on the potential efficacy and safety of complement inhibitors for the treatment of advanced AMD. |
| 14 June 2023 | New search has been performed | This review has been extensivley updated following new literature searches in June 2022. |
History
Protocol first published: Issue 9, 2011 Review first published: Issue 1, 2014
Acknowledgements
The authors wish to acknowledge the contributions of Professor Usha Chakravarthy and Dr Gareth McKay to the initial review of this topic, on which our methods are based. The authors are also grateful to Ms Anupa Shah, Dr Jennifer Evans, and Ms Iris Gordon of Cochrane Eyes and Vision for their invaluable help throughout the editorial process, as well as to Ms Sue Marcus and Ms Jenny Bellorini for their help at the copy‐editing stage.
We also thank the peer reviewers for the 2023 update:
Dr Christiana Dinah, London North West University Healthcare NHS Trust; Imperial College London
Mr Leslie Choi, Evidence Synthesis Development Editor; Cochrane Central Executive Team
Dr Jennifer Evans and Professor Gianni Virgili, Co‐ordinating Editors for Cochrane Eyes and Vision, signed‐off the review for publication.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Macular Degeneration] explode all trees #2 MeSH descriptor: [Retinal Degeneration] this term only #3 MeSH descriptor: [Retinal Neovascularization] this term only #4 MeSH descriptor: [Choroidal Neovascularization] this term only #5 MeSH descriptor: [Macula Lutea] explode all trees #6 maculopath* #7 (macul* or retina* or choroid*) near/3 degener* #8 (macul* or retina* or choroid*) near/3 neovasc* #9 macula* near/2 lutea #10 AMD or ARMD or CNV #11 geographic near/2 atroph* #12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 #13 MeSH descriptor: [Complement System Proteins] explode all trees #14 (cascad* or inhibit* or pathway*) near/3 complement #15 C3 or C5 #16 lampalizumab or solaris #17 compstatin #18 POT 4 or POT4 #19 Avacincaptad pegol or ARC‐1905 or Zimura #20 Tesidolumab or LFG316 #21 CLG561 or APL‐2 or Fovista or Pegpleranib or Pegcetacoplan or HMR 59 or HMR59 or Iptacopan #22 #13 or #14 or #15 #16 or #17 or #18 or #19 or #20 or #21 #23 #12 and #22
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp macular degeneration/ 14. retinal degeneration/ 15. retinal neovascularization/ 16. choroidal neovascularization/ 17. exp macula lutea/ 18. maculopath$.tw. 19. ((macul$ or retina$ or choroid$) adj3 degener$).tw. 20. ((macul$ or retina$ or choroid$) adj3 neovasc$).tw. 21. (macula$ adj2 lutea).tw. 22. (AMD or ARMD or CNV).tw. 23. (geographic adj2 atroph$).tw. 24. or/13‐23 25. exp complement system proteins/ 26. (complement adj3 (cascad$ or inhibit$ or pathway$)).tw. 27. (C3 or C5).tw. 28. (eculizumab or soliris).tw. 29. lampalizumab.tw. 30. compstatin.tw. 31. (POT 4 or POT4).tw. 32. (Avacincaptad pegol or ARC‐1905 or Zimura).tw. 33. (Tesidolumab or LFG316).tw. 34. (CLG561 or APL‐2 or Fovista or Pegpleranib or Pegcetacoplan or HMR 59 or HMR59 or Iptacopan).tw. 35. or/25‐34 36. 24 and 35 37. 12 and 36
Appendix 3. EMBASE Ovid search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp retina degeneration/ 34. retina neovascularization/ 35. subretinal neovascularization/ 36. maculopath$.tw. 37. ((macul$ or retina$ or choroid$) adj3 degener$).tw. 38. ((macul$ or retina$ or choroid$) adj3 neovasc$).tw. 39. exp retina macula lutea/ 40. (macula$ adj2 lutea$).tw. 41. (AMD or ARMD or CNV).ti,ab. 42. (geographic adj2 atroph$).tw. 43. or/33‐42 44. exp complement/ 45. (complement adj3 (cascad$ or inhibit$ or pathway$)).tw. 46. (C3 or C5).tw. 47. (eculizumab or soliris).tw. 48. lampalizumab.tw. 49. compstatin.tw. 50. (POT 4 or POT4).tw. 51. (Avacincaptad pegol or ARC‐1905 or Zimura).tw. 52. (Tesidolumab or LFG316).tw. 53. (CLG561 or APL‐2 or Fovista or Pegpleranib or Pegcetacoplan or HMR 59 or HMR59 or Iptacopan).tw. 54. or/44‐53 55. 43 and 54 56. 32 and 55
Appendix 4. LILACS search strategy
Macular Degeneration OR AMD OR nAMD OR ARMD and Complement OR C3 OR C5 OR Eculizumab OR Soliris OR Lampalizumab OR Avacincaptad pegol OR ARC‐1905 OR Zimura OR Tesidolumab OR LFG316 Compstatin OR POT 4 OR POT4 OR CLG561 OR APL‐2 OR Iptacopan
Appendix 5. Web of Science search strategy
#5 #3 AND #4 #4 TS= (Complement OR C3 OR C5 OR Eculizumab OR Soliris OR Lampalizumab OR Avacincaptad pegol OR ARC‐1905 OR Zimura OR Tesidolumab OR LFG316 Compstatin OR POT 4 OR POT4 OR CLG561 OR APL‐2 OR Iptacopan) #3 #1 OR #2 #2 TS= (AMD OR nAMD OR ARMD) #1 TS= (macula* degenerat*)
Appendix 6. ISRCTN search strategy
(Macular Degeneration OR AMD OR nAMD OR ARMD) AND (Complement OR C3 OR C5 OR Eculizumab OR Soliris OR Lampalizumab OR Avacincaptad pegol OR ARC‐1905 OR Zimura OR Tesidolumab OR LFG316 Compstatin OR POT 4 OR POT4 OR CLG561 OR APL‐2 OR Iptacopan)
Appendix 7. ClinicalTrials.gov search strategy
(Macular Degeneration OR AMD OR nAMD OR ARMD) AND (Complement OR C3 OR C5 OR Eculizumab OR Soliris OR Lampalizumab OR Avacincaptad pegol OR ARC‐1905 OR Zimura OR Tesidolumab OR LFG316 Compstatin OR POT 4 OR POT4 OR CLG561 OR APL‐2 OR Iptacopan)
Appendix 8. WHO ICTRP search strategy
Condition = Macular Degeneration OR AMD OR nAMD OR ARMD AND Intervention = Complement OR C3 OR C5 OR Eculizumab OR Soliris OR Lampalizumab OR Avacincaptad pegol OR ARC‐1905 OR Zimura OR Tesidolumab OR LFG316 Compstatin OR POT 4 OR POT4 OR CLG561 OR APL‐2 OR Iptacopan
Data and analyses
Comparison 1. Efficacy and safety of IVT lampalizumab 4‐weekly versus sham for geographic atrophy (GA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 BCVA change at 1 year | 3 | 1182 | Mean Difference (IV, Random, 95% CI) | 1.03 [‐0.19, 2.25] |
| 1.2 GA lesion size change at 1 year (mm2) | 3 | 1199 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.09, 0.23] |
| 1.3 Safety: Development of MNV or exudative AMD at 1 year | 2 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.73, 4.30] |
| 1.4 Safety: Development of endophthalmitis at 1 year | 2 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 6.92 [0.36, 133.73] |
Comparison 2. Efficacy and safety of IVT lampalizumab 6‐ to 8‐weekly versus sham for geographic atrophy (GA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 BCVA change at 1 year | 3 | 1184 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐1.00, 1.44] |
| 2.2 GA lesion size change at 1 year (mm2) | 3 | 1207 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.05, 0.19] |
| 2.3 Safety: Development of MNV or exudative AMD at 1 year | 2 | 1331 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.67, 4.28] |
| 2.4 Safety: Development of endophthalmitis at 1 year | 2 | 1331 | Risk Ratio (M‐H, Random, 95% CI) | 4.94 [0.24, 102.78] |
Comparison 3. Efficacy and safety of IVT pegcetacoplan 4‐weekly versus sham for geographic atrophy (GA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 GA lesion size change at 1 year (mm2) | 3 | 967 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.57, ‐0.19] |
| 3.2 Extrafoveal GA lesion size change at 1 year (mm2) | 2 | 291 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐0.98, ‐0.36] |
| 3.3 Safety: Development of MNV or exudative AMD at 1 year | 2 | 1003 | Risk Ratio (M‐H, Random, 95% CI) | 4.47 [0.41, 48.98] |
| 3.4 Development of endophthalmitis at 1 year | 3 | 1003 | Risk Ratio (M‐H, Random, 95% CI) | 3.79 [0.42, 34.05] |
Comparison 4. Efficacy and safety of IVT pegcetacoplan 8‐weekly versus sham for geographic atrophy (GA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 GA lesion size change at 1 year (mm2) | 3 | 963 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.44, ‐0.13] |
| 4.2 Extrafoveal GA lesion size change at 1 year (mm2) | 2 | 288 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.91, ‐0.30] |
| 4.3 Safety: Development of MNV or exudative AMD at 1 year | 2 | 997 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [0.46, 11.35] |
| 4.4 Development of endophthalmitis at 1 year | 3 | 997 | Risk Ratio (M‐H, Random, 95% CI) | 4.77 [0.55, 41.68] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Avacincaptad pegol Phase 2/3 (GATHER1).
| Study characteristics | ||
| Methods | Study design: parallel‐group Randomisation: web‐based, minimised by site, size of baseline GA, and pattern of FAF at the junctional zone. Undertaken in 2 parts with different randomisation ratios for 1 mg vs 2 mg, and 2 mg vs 4 mg drug administrations. Masking: participants, care providers, investigators, outcomes assessors Intention‐to‐treat: yes; modified – all patients who received at least 1 injection of the assigned treatment or sham Statistical methods: marginal means estimated from mixed model for repeated measures, covariates not specified but include an adjustment for randomisation group Missing data: missing outcome values were likely handled by listwise deletion for primary analyses. Sensitivity analyses using mean imputation were explored and data are presented. |
|
| Participants | Total number analysed: 286 Setting: multicentre Diagnostic tool: FAF, OCT, digital colour fundus photograph, near infrared reflectance, and fluorescein angiography Age: ≥ 50 years (mean 79 years) Key ocular eligibility criteria (study eye):
Key ocular eligibility criteria (both eyes):
Although perilesional hyperautofluorescence was not a criterion for eligibility, it was documented at baseline in 99% of participants in each study arm, although the pattern of hyperautofluorescence has not been reported |
|
| Interventions | Agent: avacincaptad pegol 1 mg, 2 mg, or 4 mg (administered as 1 mg/100 μL or 2 mg/100 μL injections) Route of delivery: IVT Frequency of delivery: every 4 weeks Duration of treatment: up to 18 months Controls: sham injection(s) |
|
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
|
| Notes | Ocular history of exudative AMD in the fellow eye is exclusionary. Patients who developed exudative AMD in the study eye were withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Web‐based randomisation. However, if both eyes were eligible for the study it is unclear which was selected as the study eye. |
| Allocation concealment (selection bias) | Low risk | Minimisation is a controversial randomisation practice, particularly when it is used without any random component as appears to be the case here, as it depends on data that can be determined. Nevertheless, it is unlikely to have introduced significant bias in this study given that randomisation was web‐based, the trial involved multiple centres, and evaluating physicians, reading centres, and sponsors were all masked. |
| Masking of outcome assessment (detection bias) BCVA | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) GA lesion size | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) Adverse event reporting | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) BCVA | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) GA lesion size | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) Adverse event reporting | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available but the published reports include all expected outcomes, including those that were pre‐specified. However, safety data are only reported for a subset of the ITT population (260 as opposed to 286 participants), excluding participants who received 1 mg of the agent – it is unclear whether this analysis was pre‐specified. |
| Incomplete outcome data (attrition bias) BCVA | Unclear risk | The proportion of missing outcome data was higher in the intervention groups (2 mg Q4W 19%, 4 mg Q4W 30%) compared to pooled sham (13%), with over half of withdrawals in the 4 mg Q4W group being due to patient request. This may be related to outcomes and may have compromised the missingness assumption of the mixed‐effect model. There were no imputation strategies considered for this outcome. |
| Incomplete outcome data (attrition bias) GA lesion size | Low risk | The proportion of missing outcome data was higher in the intervention groups (2 mg Q4W 19%, 4 mg Q4W 30%) compared to pooled sham (13%), with over half of withdrawals in the 4 mg Q4W group being due to patient request. This may be related to outcomes and may have compromised the missingness assumption of the mixed‐effect model. Nevertheless, various sensitivity analyses with imputation (including using the mean value of the sham arm) showed that change in GA size was robust across both 2 mg Q4W and 4 mg Q4W intervention groups. |
| Incomplete outcome data (attrition bias) Adverse event reporting | Unclear risk | The proportion of missing outcome data was higher in the intervention groups (2 mg Q4W 19%, 4 mg Q4W 30%) compared to pooled sham (13%), with over half of withdrawals in the 4 mg Q4W group being due to patient request. This may be related to outcomes. There were no imputation strategies considered for this outcome. |
| Other bias | Low risk | We did not identify other potential threats to validity. |
CLG561 ± LFG316 Phase 2.
| Study characteristics | ||
| Methods | Study design: parallel‐group Randomisation: web‐based, with no constraints Masking: participants, outcomes assessors Intention‐to‐treat: yes; modified – all patients who received at least one injection of the assigned treatment or sham and have baseline and at least one post‐baseline value of GA lesion area in the study eye Statistical methods:
Missing data: missing values of GA lesion size change were handled by regression mean imputation for primary analyses |
|
| Participants | Total number analysed: 114 Setting: multicentre Diagnostic tool: FAF, colour fundus photograph, and fluorescein angiography Age: ≥ 50 years (mean 78 years) Key ocular eligibility criteria (study eye):
Key ocular eligibility criteria (both eyes):
|
|
| Interventions | Agent: CLG561 10 mg/100 µL, or CLG561 5 mg/50 µL + LFG316 5 mg/50 µL Route of delivery: IVT Frequency of delivery: every 4 weeks Duration of treatment: up to 11 months Controls: sham injection |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
|
| Notes | Ocular history of exudative AMD in the fellow eye is exclusionary. Patients who developed exudative AMD in the study eye were withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation. As per the study protocol, both eyes must have GA but only one eye must meet the other inclusion/exclusion criteria to be eligible for study participation. |
| Allocation concealment (selection bias) | Low risk | There were no constraints on randomisation so the investigator/participant could not know or influence the intervention group before an eligible participant entered the study. |
| Masking of outcome assessment (detection bias) BCVA | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) GA lesion size | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) Adverse event reporting | High risk | No masking of outcome assessment, and the outcome measurement is likely to be influenced by lack of masking. |
| Masking of participants and personnel (performance bias) BCVA | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) GA lesion size | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) Adverse event reporting | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Selective reporting (reporting bias) | Low risk | The study protocol of the extension study is available, and all the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Incomplete outcome data (attrition bias) BCVA | Unclear risk | The proportion of missing outcome data overall was higher in the CLG561 group (19%) compared to CLG561 + LFG316 (8%) and sham (10%). Most of these were due to adverse events. This may have compromised the missingness assumptions of the analysis. There were no imputation strategies considered. |
| Incomplete outcome data (attrition bias) GA lesion size | Unclear risk | The proportion of missing outcome data overall was higher in the CLG561 group (19%) compared to the CLG561 + LFG316 (8%) and sham (10%) groups. Most of these withdrawals were due to adverse events. This may have compromised the missingness assumptions of the analysis. Imputation based on linear regression of day 253+ values is considered, but this would be subject to the same limitations, and its parameters/results are not clearly reported. |
| Incomplete outcome data (attrition bias) Adverse event reporting | Unclear risk | The proportion of missing outcome data overall was higher in the CLG561 group (19%) compared to CLG561 + LFG316 (8%) and sham (10%). Most of these were due to adverse events. This may have compromised the missingness assumptions of the analysis. There were no imputation strategies considered. |
| Other bias | Low risk | We did not identify other potential threats to validity. |
Eculizumab Phase 2 (COMPLETE).
| Study characteristics | ||
| Methods | Study design: parallel‐group Randomisation: blocked and stratified by treatment group. Masking: participants, care providers, investigators, outcomes assessors Intention‐to‐treat: yes Statistical methods: two‐sample t test between treated and untreated groups, and ANOVA between low‐dose, high‐dose, and placebo groups Missing data: missing outcome values were handled by listwise deletion for primary analyses. No missing data considerations were specified. |
|
| Participants | Total number analysed: 30 participants – 30 eyes analysed for primary analyses, and additional 18 fellow eyes analysed as sensitivity analysis Setting: single centre Diagnostic tool: FAF, OCT, digital colour fundus photograph, near infrared reflectance, and fluorescein angiography Age: ≥ 50 years (mean 80 years) Key ocular eligibility criteria (study eye):
Key ocular eligibility criteria (both eyes):
|
|
| Interventions | Agent/frequency:
Route of delivery: IV Duration of treatment: 24 weeks Controls: saline infusion |
|
| Outcomes | Primary outcome measures a:
Secondary outcome measure:
a The primary and secondary endpoints for this clinical trial were assessed at 6 months, however patients were monitored for a full year and data from this follow‐up period are available for analysis. |
|
| Notes | All participants received a meningococcal vaccine at least 15 days before the initiation of treatment. Ocular history of exudative AMD in the fellow eye is exclusionary. Patients who developed exudative AMD in the study or fellow eye were withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process, including blocked variables, to permit judgement. Unclear how study eye was chosen if both eyes met inclusion criteria. |
| Allocation concealment (selection bias) | Unclear risk | Blocked/stratified randomisation based only on treatment group and within one site introduces a risk that the unmasked clinical co‐ordinator could know or influence the intervention group before the eligible participant entered the study. |
| Masking of outcome assessment (detection bias) BCVA | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) GA lesion size | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) Adverse event reporting | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) BCVA | Low risk | Quote: “All subjects and study personnel other than the clinical coordinator were masked to treatment assignment.” Garcia Filho et al. 2014. Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) GA lesion size | Low risk | Quote: “All subjects and study personnel other than the clinical coordinator were masked to treatment assignment.” Garcia Filho et al. 2014. Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) Adverse event reporting | Low risk | Quote: “All subjects and study personnel other than the clinical coordinator were masked to treatment assignment.” Garcia Filho et al. 2014. Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but the published reports include all expected outcomes, including those that were pre‐specified. |
| Incomplete outcome data (attrition bias) BCVA | Low risk | No missing outcome data. |
| Incomplete outcome data (attrition bias) GA lesion size | Low risk | No missing outcome data. |
| Incomplete outcome data (attrition bias) Adverse event reporting | Unclear risk | There is a discrepancy in the quoted rates of new nAMD/MNV as an adverse event in the placebo group between the two study reports. |
| Other bias | High risk | Participants in the intervention arm had 28% larger GA lesion size and 10% worse BCVA at baseline compared to the placebo group. Baseline imbalance in these factors that are strongly related to outcome measures can cause bias in the intervention effect estimate as analyses were not adjusted for these and other relevant covariates. |
Lampalizumab Phase 2 (MAHALO).
| Study characteristics | ||
| Methods | Study design: parallel‐group Randomisation: web‐based, blocked and stratified by baseline GA lesion size (dichotomised at 10 mm2) Masking: participants, outcomes assessors Intention‐to‐treat: yes Statistical methods:
Missing data: missing values of BCVA and GA lesion size change were handled by last observation carried forward (LOCF) for primary analyses. Missing values of GA lesion size change were additionally explored through multiple random imputation using Markov Chain Monte Carlo (MCMC) sampling in sensitivity analyses. |
|
| Participants | Total number analysed: 129 Setting: multicentre Diagnostic tool: FAF, near‐infrared imaging, and digital colour fundus photograph Age: 60 to 89 years (mean 79 years) Key ocular eligibility criteria (study eye):
Key ocular eligibility criteria (both eyes):
|
|
| Interventions | Agent: lampalizumab 10 mg/100 μL Route of delivery: IVT Frequency of delivery: every 4 or 8 weeks Duration of treatment: up to 18 months Controls: sham injection |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
|
| Notes | Ocular history of exudative AMD in the fellow eye is exclusionary. Patients who developed exudative AMD during the study were not withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Web‐based randomisation. However, if both eyes met the inclusion criteria, it is unclear which eye was designated as the study eye. |
| Allocation concealment (selection bias) | Low risk | Blocked randomisation based only on GA lesion size but across multiple sites makes it unlikely that the investigator/participant would know or influence the intervention group before an eligible participant entered the study. |
| Masking of outcome assessment (detection bias) BCVA | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) GA lesion size | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) Adverse event reporting | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) BCVA | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) GA lesion size | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) Adverse event reporting | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Selective reporting (reporting bias) | Low risk | The protocol for the extension study is available, and all the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Incomplete outcome data (attrition bias) BCVA | Unclear risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. As AMD outcomes tend to deteriorate with time, the use of last observation carried forward (LOCF) procedures for imputation is inappropriate as this could have biased the effect estimate in favour of the drug. However, it is unclear whether the time interval is sufficient for this to affect BCVA. |
| Incomplete outcome data (attrition bias) GA lesion size | Unclear risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. Various sensitivity analyses and imputation methods to assess the impact of missing data showed that these did not significantly influence the study outcomes. Although derived using inappropriate last observation carried forward (LOCF) procedures, the GA lesion size outcomes are supported by linear mixed‐effect models. |
| Incomplete outcome data (attrition bias) Adverse event reporting | Low risk | Missing outcome data were balanced in numbers across intervention and sham groups, with similar reasons for missing data across groups. All adverse events were reported as participants were withdrawn from the study. |
| Other bias | Low risk | We did not identify other potential threats to validity. |
Lampalizumab Phase 3 (CHROMA).
| Study characteristics | ||
| Methods | Study design: parallel‐group Randomisation: web‐based, blocked and stratified by CFI biomarker status, baseline BCVA (20/50 or better vs worse than 20/50), sex, and microperimetry eligibility Masking: participants, care providers, investigators, outcomes assessors Intention‐to‐treat: yes; except for microperimetry measurements Statistical methods: marginal means estimated from mixed model for repeated measures adjusted for treatment group, time, treatment‐by‐time interaction, baseline GA area, baseline GA lesion location, baseline GA lesion contiguity, baseline BCVA category, sex, CFI biomarker status (overall population only), and study Missing data: missing outcome values were handled by listwise deletion for primary analyses ‐ specifically, participants without baseline measures or at least one post‐baseline measure were excluded. Imputation analyses were not considered. |
|
| Participants | Total number analysed: 1881 Setting: multicentre Diagnostic tool: FAF, OCT, digital colour fundus photograph, near infrared reflectance, and fluorescein angiography Age: ≥ 50 years (mean 78 years) Key ocular eligibility criteria (study eye):
Key ocular eligibility criteria (both eyes):
|
|
| Interventions | Agent: lampalizumab 10 mg/100 μL Route of delivery: IVT Frequency of delivery: every 4 or 6 weeks Duration of treatment: up to 44 weeks Controls: sham injection |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
|
| Notes | Ocular history of exudative AMD in the fellow eye is exclusionary. Patients who developed exudative AMD during the study were not withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation. If both eyes met the inclusion criteria, the eye with the worst visual acuity as determined at screening by the investigator and patient was designated as the study eye, followed by the eye with the larger GA lesion. |
| Allocation concealment (selection bias) | Low risk | Blocked and stratified based on several criteria and across multiple sites makes it unlikely that the investigator/participant would know or influence the intervention group before an eligible participant entered the study. |
| Masking of outcome assessment (detection bias) BCVA | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) GA lesion size | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) Adverse event reporting | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) BCVA | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) GA lesion size | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) Adverse event reporting | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Selective reporting (reporting bias) | Low risk | The study protocols are available, and all the pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. Although plans to evaluate the potential impact of missing data as specified in the study protocols were not reported, this is unlikely to have introduced significant bias given the overall negative results of the trials. |
| Incomplete outcome data (attrition bias) BCVA | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Incomplete outcome data (attrition bias) GA lesion size | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Incomplete outcome data (attrition bias) Adverse event reporting | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Other bias | Low risk | We did not identify other potential threats to validity. |
Lampalizumab Phase 3 (SPECTRI).
| Study characteristics | ||
| Methods | See Lampalizumab Phase 3 (CHROMA) | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Lampalizumab Phase 3 (CHROMA) |
| Allocation concealment (selection bias) | Low risk | See Lampalizumab Phase 3 (CHROMA) |
| Masking of outcome assessment (detection bias) BCVA | Low risk | See Lampalizumab Phase 3 (CHROMA) |
| Masking of outcome assessment (detection bias) GA lesion size | Low risk | See Lampalizumab Phase 3 (CHROMA) |
| Masking of outcome assessment (detection bias) Adverse event reporting | Low risk | See Lampalizumab Phase 3 (CHROMA) |
| Masking of participants and personnel (performance bias) BCVA | Low risk | See Lampalizumab Phase 3 (CHROMA) |
| Masking of participants and personnel (performance bias) GA lesion size | Low risk | See Lampalizumab Phase 3 (CHROMA) |
| Masking of participants and personnel (performance bias) Adverse event reporting | Low risk | See Lampalizumab Phase 3 (CHROMA) |
| Selective reporting (reporting bias) | Low risk | See Lampalizumab Phase 3 (CHROMA) |
| Incomplete outcome data (attrition bias) BCVA | Low risk | See Lampalizumab Phase 3 (CHROMA) |
| Incomplete outcome data (attrition bias) GA lesion size | Low risk | See Lampalizumab Phase 3 (CHROMA) |
| Incomplete outcome data (attrition bias) Adverse event reporting | Low risk | See Lampalizumab Phase 3 (CHROMA) |
| Other bias | Low risk | We did not identify other potential threats to validity. |
LFG316 Phase 2.
| Study characteristics | ||
| Methods | Study design: parallel‐group Randomisation: method not specified Masking: participants only Intention‐to‐treat: yes Statistical methods: for GA lesion size growth, a Bayesian linear mixed‐effect model for repeated measures with unspecified priors or covariates was used Missing data: missing outcome values were likely handled by listwise deletion for primary analyses. No missing data considerations were specified. |
|
| Participants | Total number analysed: 158 Setting: multicentre Diagnostic tool: FAF Age: ≥ 55 (mean 79 years) Key ocular eligibility criteria (study eye):
Key ocular eligibility criteria (both eyes):
No select concurrent ocular and systemic conditions |
|
| Interventions | Agent: LFG316 5 mg/50 µL or 10 mg/50 µL Route of delivery: IVT Frequency of delivery: every 4 weeks Duration of treatment: up to 2 years Controls: sham injection |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
|
| Notes | The study was terminated early following an interim analysis for efficacy of the LFG316 5 mg/50 µL formulation, and not due to any safety issues or concerns. Ocular history of exudative AMD in the fellow eye was exclusionary, but it was unclear if patients who developed exudative AMD during the study were withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although not explicit, randomisation of a multicentre study of this size is very likely to have been web‐based. However, it is unclear how study eye selection proceeded if both eyes were eligible. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process to permit judgement. |
| Masking of outcome assessment (detection bias) BCVA | High risk | No masking of outcome assessment, and the outcome measurement is likely to be influenced by lack of masking. As there is no published protocol or manuscript, it is unclear whether/how this risk of bias was minimised. |
| Masking of outcome assessment (detection bias) GA lesion size | High risk | No masking of outcome assessment, and the outcome measurement is likely to be influenced by lack of masking. As there is no published protocol or manuscript, it is unclear whether/how this risk of bias was minimised. |
| Masking of outcome assessment (detection bias) Adverse event reporting | High risk | No masking of outcome assessment, and the outcome measurement is likely to be influenced by lack of masking. As there is no published protocol or manuscript, it is unclear whether/how this risk of bias was minimised. |
| Masking of participants and personnel (performance bias) BCVA | Low risk | Incomplete masking (participants only), but the review authors judge that the outcome is not likely to be influenced by lack of masking. |
| Masking of participants and personnel (performance bias) GA lesion size | Low risk | Incomplete masking (participants only), but the review authors judge that the outcome is not likely to be influenced by lack of masking. |
| Masking of participants and personnel (performance bias) Adverse event reporting | Low risk | Incomplete masking (participants only), but the review authors judge that the outcome is not likely to be influenced by lack of masking. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. A key outcome (mean change in BCVA from baseline) that would be expected to have been reported for such a study is reported incompletely (as mean BCVA at different time points), so that it cannot be entered in a meta‐analysis. |
| Incomplete outcome data (attrition bias) BCVA | Unclear risk | A similar but significant proportion of participants in both treatment and sham arms did not complete the study (30% and 25%, respectively), and a similar number did so due to adverse events/death (8% and 5%, respectively). There were no sensitivity analyses to assess the impact of missing data. |
| Incomplete outcome data (attrition bias) GA lesion size | Unclear risk | A similar but significant proportion of participants in both treatment and sham arms did not complete the study (30% and 25%, respectively), and a similar number did so due to adverse events/death (8% and 5%, respectively). There were no sensitivity analyses to assess the impact of missing data. |
| Incomplete outcome data (attrition bias) Adverse event reporting | Unclear risk | A similar but significant proportion of participants in both treatment and sham arms did not complete the study (30% and 25%, respectively), and a similar number did so due to adverse events/death (8% and 5%, respectively). There were no sensitivity analyses to assess the impact of missing data. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists; the covariates adjusted for in GA lesion growth analyses are not specified. |
Pegcetacoplan Phase 2 (FILLY).
| Study characteristics | ||
| Methods | Study design: parallel‐group Randomisation: web‐based, blocked by treatment allocation within each site Masking: participants, outcomes assessors Intention‐to‐treat: yes; modified – all patients who received at least one injection of the assigned treatment or sham and have baseline and at least one post‐baseline value of GA lesion area in the study eye Statistical methods: marginal means estimated from mixed model for repeated measures adjusted for treatment group, visit, baseline value of the endpoint, and the interaction terms of treatment*visit and visit*baseline. Missing data: missing outcome values were likely handled by listwise deletion for primary analyses. Sensitivity analyses on per protocol population, data within a specified time period of last injection, and month 12 completers were explored and data are presented. Imputation analyses were not considered. |
|
| Participants | Total number analysed: 246 Setting: multicentre Diagnostic tool: FAF, OCT, digital colour fundus photograph, and fluorescein angiography Age: ≥ 50 years (mean 80 years) Key ocular eligibility criteria (study eye):
Key ocular eligibility criteria (both eyes):
|
|
| Interventions | Agent: pegcetacoplan 15 mg/100 μL Route of delivery: IVT Frequency of delivery: every 4 or 8 weeks Duration of treatment: up to 12 months Controls: sham injection |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
|
| Notes | Ocular history of exudative AMD in the fellow eye is NOT exclusionary. Patients who developed exudative AMD in the study eye were withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation. As per the study protocol, if both eyes met the inclusion criteria, the eye with the worst visual acuity at screening was designated as the study eye. If both eyes had the same visual acuity, the right eye was selected as the study eye. |
| Allocation concealment (selection bias) | Unclear risk | Blocked randomisation based on unspecified criteria and within each site introduces a risk that the investigator/participant could know or influence the intervention group before the eligible participant entered the study. |
| Masking of outcome assessment (detection bias) BCVA | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) GA lesion size | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) Adverse event reporting | Unclear risk | No masking of adverse outcome assessment, and it is unclear whether outcome measurement could have been influenced by lack of masking given the findings; the reported association of MNV/nAMD with the treatment is discussed extensively across two reports. |
| Masking of participants and personnel (performance bias) BCVA | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) GA lesion size | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) Adverse event reporting | Low risk | Incomplete masking (assessing physicians were not masked), but the review authors judge that the outcome is not likely to be influenced by lack of masking. |
| Selective reporting (reporting bias) | Low risk | The study protocols are available, and all the pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Incomplete outcome data (attrition bias) BCVA | Unclear risk | The proportion of missing outcome data overall was higher in the intervention groups (Q4W 40%, Q8W 24%) compared to sham (15%), with a large proportion due to adverse events (20% of Q4W group vs 5% sham group). This may have compromised the missingness assumption of the mixed‐effect model. Imputation strategies are considered in the protocol but not presented. |
| Incomplete outcome data (attrition bias) GA lesion size | Unclear risk | The proportion of missing outcome data overall was higher in the intervention groups (Q4W 40%, Q8W 24%) compared to sham (15%), with a large proportion due to adverse events (20% of Q4W group vs 5% sham group). This may have compromised the missingness assumption of the mixed‐effect model. Imputation strategies are considered in the protocol but not presented. |
| Incomplete outcome data (attrition bias) Adverse event reporting | Low risk | Although the proportion of missing outcome data due to adverse events was higher in the intervention groups as compared to placebo, all safety data were collected and analysed for all randomised patients who received at least one injection. |
| Other bias | Low risk | We did not identify other potential threats to validity. |
Pegcetacoplan Phase 3 (DERBY).
| Study characteristics | ||
| Methods | Study design: parallel‐group Randomisation: web‐based, stratified by GA lesion area at screening (< 7.5 mm2 vs ≥ 7.5 mm2) and presence of CNV in the fellow eye Masking: participants, investigators, outcomes assessors Intention‐to‐treat: yes; modified – all patients who received at least 1 injection of the assigned treatment or sham and have baseline and at least 1 post‐baseline value of GA lesion area in the study eye. Sensitivity analyses will also use the per‐protocol sets. Statistical methods: marginal means estimated from mixed model for repeated measures adjusted for treatment, presence of CNV in the fellow eye, baseline GA lesion area, time, and the interaction term of treatment*time Missing data: missing outcome values were likely handled by listwise deletion for primary analyses. Sensitivity analyses using multiple imputation will be explored, but data are not yet presented. |
|
| Participants | Total number analysed: 1208 Setting: multicentre Diagnostic tool: FAF, OCT, digital colour fundus photograph, near infrared reflectance, and fluorescein angiography Age: ≥ 60 years (mean 78 years) Key ocular eligibility criteria (study eye):
Key ocular eligibility criteria (both eyes):
|
|
| Interventions | Agent: pegcetacoplan 15 mg/100 μL Route of delivery: IVT Frequency of delivery: every 4 or 8 weeks Duration of treatment: up to 24 months Controls: sham injection |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
|
| Notes | Ocular history of exudative AMD in the fellow eye is NOT exclusionary. Patients who developed exudative AMD in the study eye were not withdrawn, and concurrent anti‐VEGF therapy was allowed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation. If both eyes met the inclusion criteria, the eye with the worst visual acuity at the screening visit was designated as the study eye. If both eyes had the same visual acuity, the right eye was selected as the study eye. |
| Allocation concealment (selection bias) | Low risk | Stratified randomisation based on two criteria but across multiple sites makes it unlikely that the investigator/participant would know or influence the intervention group before the eligible participant entered the study. |
| Masking of outcome assessment (detection bias) BCVA | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) GA lesion size | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of outcome assessment (detection bias) Adverse event reporting | Low risk | Masking of outcome assessment ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) BCVA | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) GA lesion size | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Masking of participants and personnel (performance bias) Adverse event reporting | Low risk | Masking of participants and key study personnel ensured, and unlikely that the masking could have been broken. |
| Selective reporting (reporting bias) | Low risk | The study protocol was made available on request and the study’s pre‐specified primary and safety outcomes of interest in the review have been reported in the pre‐specified way. The study is ongoing and secondary endpoints such as BCVA, LLVA, and quality of life measures are not due to be reported until Q4 2022. |
| Incomplete outcome data (attrition bias) GA lesion size | Low risk | Missing outcome data balanced in numbers across pooled intervention (11%) and sham (12%) arms. Although reasons for missing data across groups has not yet been published, the proportions of treatment‐emergent adverse events are similar across pooled intervention (77%) and sham (72%) arms. A full description of imputation methods is anticipated. |
| Incomplete outcome data (attrition bias) Adverse event reporting | Low risk | Missing outcome data balanced in numbers across pooled intervention (11%) and sham (12%) arms. Although reasons for missing data across groups has not yet been published, the proportions of treatment‐emergent adverse events are similar across pooled intervention (77%) and sham (72%) arms. A full description of imputation methods is anticipated. |
| Other bias | Low risk | We did not identify other potential threats to validity. |
Pegcetacoplan Phase 3 (OAKS).
| Study characteristics | ||
| Methods | See Pegcetacoplan Phase 3 (DERBY) | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Pegcetacoplan Phase 3 (DERBY) |
| Allocation concealment (selection bias) | Low risk | See Pegcetacoplan Phase 3 (DERBY) |
| Masking of outcome assessment (detection bias) BCVA | Low risk | See Pegcetacoplan Phase 3 (DERBY) |
| Masking of outcome assessment (detection bias) GA lesion size | Low risk | See Pegcetacoplan Phase 3 (DERBY) |
| Masking of outcome assessment (detection bias) Adverse event reporting | Low risk | See Pegcetacoplan Phase 3 (DERBY) |
| Masking of participants and personnel (performance bias) BCVA | Low risk | See Pegcetacoplan Phase 3 (DERBY) |
| Masking of participants and personnel (performance bias) GA lesion size | Low risk | See Pegcetacoplan Phase 3 (DERBY) |
| Masking of participants and personnel (performance bias) Adverse event reporting | Low risk | See Pegcetacoplan Phase 3 (DERBY) |
| Selective reporting (reporting bias) | Low risk | See Pegcetacoplan Phase 3 (DERBY) |
| Incomplete outcome data (attrition bias) GA lesion size | Low risk | See Pegcetacoplan Phase 3 (DERBY) |
| Incomplete outcome data (attrition bias) Adverse event reporting | Low risk | See Pegcetacoplan Phase 3 (DERBY) |
| Other bias | Low risk | We did not identify other potential threats to validity. |
AE: adverse event AMD: age‐related macular degeneration BCVA: best‐corrected visual acuity BD: twice a day administration CFB: complement factor B CFH: complement factor H CFI: complement factor I CNV: choroidal neovascularisation ELISA: enzyme‐linked immunosorbent assay ETDRS: early treatment for diabetic retinopathy FAF: fundus autofluorescence FRI: Functional Reading Independence GA: geographic atrophy IOP: intraocular pressure IV: intravenous IVT: intravitreal LLD: low luminance deficit (calculated as BCVA score minus LLVA score) LLVA: low luminance visual acuity mAb: monoclonal antibody MAC: membrane attack complex MNRead: Minnesota low‐vision reading test MNV: macular neovascularisation N/A: not applicable nAMD: neovascular age‐related macular degeneration NEI VFQ‐25: National Eye Institute Visual Function Questionnaire 25 OCT: optical coherence tomography Q4W: every four weeks Q8W: every eight weeks RPE: retinal pigment epithelium VEGF: vascular endothelial growth factor VFQ‐25: Visual Functioning Questionnaire‐25
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| NCT01157065 | Follow‐up duration of 12 weeks or less |
| NCT01624636 | Study was terminated after 4 patients enrolled due to uncertainty regarding the risk/benefit ratio of treating patients with AMD using systemic anti‐C5 therapy, following reports of meningococcal infections in patients receiving intravenous eculizumab |
| NCT03362190 | Treatment arm consisted of co‐intervention with a complement inhibitor and an anti‐VEGF agent |
| NCT03446144 | Withdrawn before participant enrolment (business objective change, no safety or efficacy concerns) |
Characteristics of ongoing studies [ordered by study ID]
NCT03815825.
| Study name | GOLDEN STUDY: A study to assess safety and efficacy of multiple doses of IONIS‐FB‐LRx in participants with geographic atrophy secondary to age‐related macular degeneration (AMD) |
| Methods | Phase 2, controlled, randomised, double‐blind, parallel‐group study conducted at multiple centres |
| Participants | Adults 50 years and older with well‐demarcated GA due to AMD in at least one eye and BCVA of 35 letters or better on the ETDRS chart |
| Interventions | IONIS‐FB‐LRx at multiple ascending doses administered subcutaneously every 4 weeks, compared to placebo matching solution administered subcutaneously every 4 weeks |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | January 2019 |
| Contact information | Ionis Pharmaceuticals Inc., CA, USA |
| Status | Recruiting |
| Required reporting date | October 2023 |
| Notes | — |
NCT04435366.
| Study name | A phase 3 safety and efficacy study of intravitreal administration of Zimura complement C5 inhibitor) |
| Methods | Phase 3, controlled, randomised, double‐blind, parallel group study conducted at multiple centres |
| Participants | Adults 50 years and older with GA due to AMD in at least one eye. Participants with prior history of exudative AMD or CNV are excluded. |
| Interventions | Zimura 2 mg administered every 4 weeks for 12 months, followed by Zimura 2 mg administered every 8 weeks for a further 12 months, as compared to sham injection every 4 weeks for 24 months |
| Outcomes | Primary outcome measure:
Secondary outcome measures: N/A |
| Starting date | June 2020 |
| Contact information | IVERIC bio, Inc. |
| Status | Active, not recruiting |
| Required reporting date | July 2023 |
| Notes | Evidence of CNV in either eye is exclusionary. However, if a participant develops CNV in the study eye during the trial, they remain in the study and are allowed to receive concurrent anti‐VEGF therapy. |
NCT04437368.
| Study name | EXPLORE: a phase II study to evaluate the safety and efficacy of two doses of GT005 (EXPLORE) |
| Methods | Phase 2, controlled, randomised, open‐label, outcomes‐assessor masked study at multiple centres |
| Participants | Adults 55 years and older with bilateral AMD and GA in the study eye and BCVA of 24 letters or better on the ETDRS chart. All participants must have a CFI rare variant genotype. Up to 25% of the enrolled study population are permitted to have CNV in the fellow eye. |
| Interventions | GT005 administered as a single subretinal injection at low dose, or GT005 administered as a single subretinal injection at high dose, compared to an untreated control group |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | June 2020 |
| Contact information | Gyroscope Therapeutics Limited, Stevenage, UK |
| Status | Recruiting |
| Required reporting date | February 2024 |
| Notes | — |
NCT04465955.
| Study name | A study of NGM621 in participants with geographic atrophy (CATALINA) |
| Methods | Phase 2, controlled, randomised, double‐blind, parallel‐group study at multiple centres. |
| Participants | Adults 55 years and older with GA due to AMD in at least one eye and BCVA of 34 letters or better on the ETDRS chart. Participants with prior history of exudative AMD or CNV are excluded. |
| Interventions | NGM621 administered as a single intravitreal injection every 4 weeks or every 8 weeks, compared to sham injection at an equivalent frequency of administration |
| Outcomes | Primary outcome measures:
Secondary outcome measures: N/A |
| Starting date | July 2020 |
| Contact information | NGM Biopharmaceuticals Inc., CA, USA |
| Status | Active, not recruiting |
| Required reporting date | December 2023 |
| Notes | — |
NCT04566445.
| Study name | HORIZON: a phase II study to evaluate the safety and efficacy of two doses of GT005 |
| Methods | Phase 2, controlled, randomised, open‐label, outcomes‐assessor masked study at multiple centres |
| Participants | Adults 55 years and older with bilateral AMD and GA in the study eye and BCVA of 24 letters or better on the ETDRS chart. Participants must either have GA that is non‐foveal or a CFI rare variant genotype. Up to 25% of the enrolled study population are permitted to have CNV in the fellow eye. |
| Interventions | GT005 administered as a single subretinal injection at medium dose, or GT005 administered as a single subretinal injection at high dose, compared to an untreated control group |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | September 2020 |
| Contact information | Gyroscope Therapeutics Limited, Stevenage, UK |
| Status | Recruiting |
| Required reporting date | October 2025 |
| Notes |
NCT04643886.
| Study name | A multiple dose study of repeat intravitreal injections of GEM103 in dry age‐related macular degeneration |
| Methods | Phase 2a, open‐label, multiple dose study at multiple centres |
| Participants | Adults 50 years and older with GA due to AMD and BCVA of 24 to 83 letters on the ETDRS chart. All participants must have one of the two pre‐specified genetic profiles. Participants with prior history of exudative AMD or CNV are excluded. |
| Interventions | GEM103 administered as repeat intravitreal injections in participants with Genetic Profile A as compared to participants with Genetic Profile B |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | November 2020 |
| Contact information | Gemini Therapeutics Inc., MA, USA |
| Status | Terminated (no further benefit) |
| Required reporting date | February 2023 |
| Notes | — |
NCT04656561.
| Study name | A study investigating the efficacy and safety of intravitreal injections of ANX007 in patients with geographic atrophy (ARCHER) |
| Methods | Phase 2, controlled, randomised, double‐blind, parallel group study conducted at multiple centres |
| Participants | Adults 50 years and older with well‐demarcated GA due to AMD in at least one eye and BCVA of 24 to 83 letters on the ETDRS chart |
| Interventions | ANX007 administered every 4 weeks or every 8 weeks, compared to sham injection every 4 weeks or every 8 weeks |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | December 2020 |
| Contact information | Annexon, Inc. |
| Status | Active, not recruiting |
| Required reporting date | June 2024 |
| Notes | — |
NCT04820452.
| Study name | A study of IBI302 in patients with nAMD |
| Methods | Phase 2, randomised, double‐blind, parallel group study at multiple centres, with active comparator |
| Participants | Adults 50 years or older with active subfoveal or parafoveal CNV secondary to neovascular AMD in at least one eye and BCVA of 24 to 73 letters on the ETDRS chart. Participants with prior anti‐VEGF therapy within 3 months are excluded. |
| Interventions | IBI302 administered intravitreally at 2 mg or 4 mg doses monthly for 3 months (loading phase) then every other month, compared to aflibercept 2 mg injection |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | March 2021 |
| Contact information | Innovent Biologics (Suzhou) Co. Ltd. |
| Status | Active, not recruiting |
| Required reporting date | Unknown |
| Notes | — |
NCT05019521.
| Study name | A study of danicopan in participants with geographic atrophy secondary to age‐related macular degeneration |
| Methods | Phase 2, controlled, randomised, double‐blind, parallel group study at multiple centres |
| Participants | Adults 60 years and older with GA due to AMD in at least one eye. Participants with prior history of intravitreal anti‐vascular endothelial growth factor therapy and a known/suspected complement deficiency are excluded |
| Interventions | Danicopan administered as an oral tablet at 100 mg twice a day, 200 mg twice a day, or 400 mg four times a day, as compared to placebo oral tablet at an equivalent frequency of administration |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | August 2021 |
| Contact information | Alexion Pharmaceuticals, MA, USA |
| Status | Recruiting |
| Required reporting date | February 2025 |
| Notes | — |
NCT05230537.
| Study name | A masked, placebo‐controlled study to assess iptacopan in age‐related macular degeneration |
| Methods | Phase 2, controlled, randomised, double‐blind, parallel group study conducted at multiple centres |
| Participants | Adults 50 years and older with high‐risk early or intermediate AMD in at least one eye |
| Interventions | Iptacopan (LNP023) oral capsules administered twice daily compared to placebo oral capsules |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | February 2022 |
| Contact information | Novartis Pharmaceuticals, Basel, Switzerland |
| Status | Recruiting |
| Required reporting date | August 2026 |
| Notes | — |
AE: adverse event AMD: age‐related macular degeneration BCVA: best‐corrected visual acuity BD: twice a day administration CFB: complement factor B CFH: complement factor H CFI: complement factor I CNV: choroidal neovascularisation ELISA: enzyme‐linked immunosorbent assay ETDRS: early treatment for diabetic retinopathy FAF: fundus autofluorescence FRI: functional reading independence GA: geographic atrophy IVT: intravitreal LLD: low luminance deficit (calculated as BCVA score minus LLVA score) LLVA: low luminance visual acuity MNRead: Minnesota low‐vision reading test OCT: optical coherence tomography VFQ‐25: Visual Functioning Questionnaire‐25
Differences between protocol and review
2022 Update (A) − We made the following amendments to the protocol originally set out in Williams 2014, confirmed with Cochrane Eyes and Vision, before any trials were included in the review and before the searches for the update were completed:
-
Types of studies:
We excluded cohort or case‐control studies as these would not be suitable for the evaluation of the beneficial effects of therapy, although their inclusion may be revised in future updates as rare or long‐term adverse effects of complement inhibitors in age‐related macular degeneration (AMD) are identified. We did not exclude studies based on publication status or language.
We excluded studies with a short follow‐up of 12 weeks or less as we considered this timeframe insufficient to demonstrate clinical benefit in any subtype of AMD.
We excluded studies where the treatment arm consisted of co‐intervention with a complement inhibitor and an anti‐vascular endothelial growth factor (VEGF) agent as this could lead to confounding of results.
Types of outcome measures: We revised our primary and secondary outcomes to critical and important outcomes following updated guidance from Cochrane Eyes and Vision. The updated outcomes include clinically relevant anatomical, functional, patient‐reported (quality of life), and safety endpoints that reflect the current priorities of the medical and scientific communities in this field. These outcomes represent a subset of the pre‐defined outcomes of the original review protocol. We removed the outcomes that we did not carry forward to this update (i.e. best‐corrected visual acuity (BCVA) maintenance, BCVA ≥ 30 letters loss, BCVA worse than +1.0 logMAR, contrast sensitivity, reading speed, and retinal morphology) as they are not currently clinically accepted biomarkers for monitoring disease and/or do not offer any additional information relative to the other outcomes. We would not describe these omitted outcomes as critical or important to decision‐makers. Of note, we selected one year as the threshold for outcome assessment given that this represents a clinically significant and pragmatic timeline within which to assess the benefits of treatment. In the absence of this timeframe, we comment on the same outcomes at other time points. As it is difficult to pre‐define adverse effects, we limited our assessment of safety to the critical outcomes of new macular neovascularisation (MNV)/exudative AMD and endophthalmitis given their visually significant nature and growing concern regarding their association with complement inhibition amongst the medical and scientific community.
Data synthesis: We revised the methods for data synthesis following updated guidance from Cochrane Eyes and Vision.
Summary of findings: We revised our review to include summary of findings (SoF) tables for a given comparison of interventions providing key information concerning the magnitudes of relative and absolute effects of the interventions examined, the amount of available evidence and the certainty of available evidence. As SoF tables are limited to a maximum of seven outcomes we chose to highlight untransformed, but not square root‐transformed, geographic atrophy (GA) lesion growth as we considered this to be the most useful and valid outcome for people making decisions about health care. Nevertheless, we have included additional data on square root‐transformed GA lesion growth in the comments of the respective SoF table row.
2022 Update (B) − We made the following amendments to the protocol, confirmed with Cochrane Eyes and Vision, after trials were included but before data analysis:
-
Planning for mixed populations:
-
We planned several provisions for mixed populations that were not necessary for this review update as our included studies only presented data on one advanced AMD subtype. These included:
to analyse separately the data from studies that included both advanced and non‐advanced AMD;
to exclude studies involving both advanced and non‐advanced AMD patients unless they reported results separately for the advanced AMD patients;
to analyse separately the data from studies of advanced AMD that included both GA and neovascular AMD (nAMD) when possible, as the underlying disease processes are different, and we would expect variation in the primary endpoints for efficacy and safety;
to exclude studies of advanced AMD involving both GA and nAMD patients unless they reported results separately for the GA patients.
We planned to analyse the influence of genetic variation on treatment response. However, we did not consider this to be informative in this update as there were scarce data available on participants with different genetic markers.
-
Types of participants: We planned to include studies of participants with non‐advanced AMD that looked at the prevention of advanced AMD in the treatment and control arms, but did not identify any such studies. We planned to exclude studies of participants with causes of GA and neovascularisation other than AMD, but did not identify any such studies.
-
Measurements of treatment effect:
We planned to calculate the standardised mean difference if the scale used to measure secondary outcomes varied between studies for any continuous outcome, but did not encounter this issue in any of our included studies.
We planned to seek the advice of a statistician on how to deal with issues of data multiplicity or skew, but did not suspect these issues in any of our included studies.
-
Dealing with missing data:
-
We planned to conduct the following sensitivity analyses to examine any systematic bias caused by exclusion of participants after randomisation:
the worst‐case scenario, i.e. assuming either those participants lost to follow‐up lost 15 or more letters of visual acuity or that they all developed advanced AMD;
the best‐case scenario, i.e. either that none of those lost to follow‐up lost 15 or more letters of visual acuity at one‐year follow‐up or that none developed advanced AMD.
However, these analyses were either not possible or not considered necessary in our review given that no studies investigated the prevention of advanced AMD, and the outcome of ≥ 15 letters BCVA loss at one year was only reported for two studies of a discontinued agent that failed to show therapeutic benefit with high certainty for continuous BCVA outcome assessment (lampalizumab phase 3).
-
Unit of analysis issues: The following unit of analysis provisions were planned but not required in this review update: If both eyes are treated, we planned to seek advice as to whether to analyse data from one eye (e.g. the eye with better visual acuity, the one with worse visual acuity, or an average), analyse data from both eyes using adjusted analyses, or select one eye to analyse at random and repeat the analysis on the fellow eye to ensure that answers are consistent. Trials may compare outcomes between each participant's treated eye and their fellow eye. If such paired study designs are encountered, we planned to seek statistical advice. In this situation we would have considered, for example, combining paired and unpaired trial results using the generic inverse variance method. These provisions were not necessary for any of the included studies in our review update.
Assessment of reporting biases: We planned to present a funnel plot for each outcome of five or more study results included in the meta‐analysis, which in the event was not necessary as a maximum of three studies were included in each meta‐analysis. This would have involved plotting effect size on the horizontal axis and the standard error of each trial on the vertical axis. We planned to judge funnel plot asymmetry by visual inspection, deciding whether this was due to publication bias or the tendency of smaller studies to produce different effect sizes for various reasons as outlined in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). We planned to present a full description of funnel plot interpretation with the cautionary note that such interpretation will be subjective and probably speculative. For any funnel plot that either NT or GR thought was asymmetrical and for which 10 or more trials were included, we planned to seek statistical advice as to whether and how to formally test for funnel plot asymmetry. Ultimately, this was not necessary.
-
Subgroup analysis:
We considered different classes of complement inhibitors as separate comparisons, not subgroups ‐ e.g. by drug class as suggested in the original protocol. It could be misleading to pool data on complement inhibitors acting at different levels of the system (e.g. C3, C5, Factor D), with different delivery routes (e.g. intravitreal, intravenous), schedules (e.g. monthly, every‐other‐month), mechanisms (e.g. monoclonal antibodies, small molecule inhibitors), and in populations with different baseline characteristics (e.g. extent of foveal involvement, disease severity, fellow eye status). Given the small number of studies and the heterogeneous nature of their interventions and/or populations as above, we did not consider that pooling of data on different complement inhibitors would be appropriate. Precisely defined subgroup analyses of this type could be considered in future updates as more data become available.
We planned to perform subgroup analyses of subsets of participants (e.g. by ethnicity) or disease characteristics (e.g. by MNV type) within studies, but in the event this was not possible as sufficient details to extract these data were not published in the included reports beyond the separately reported extrafoveal GA lesion size changes for participants in the pegcetacoplan phase 3 studies.
No changes to the protocol were made on the basis of the findings of the studies or the synthesis.
Contributions of authors
Draft the protocol: NT, MW, DS
Study selection: NT, GR, DS
Extract data from studies: NT, GR
Enter data into RevMan: NT
Carry out the analysis: NT
Interpret the analysis: NT, DS
Disagreement resolution: DS
Draft the final review: NT, GR, MW, DS
Sources of support
Internal sources
No sources of support provided
External sources
-
Nikolaos Tzoumas was supported by an NIHR Academic Clinical Fellowship (ACF‐2021‐01‐008), UK
Non‐financial support
-
Public Health Agency, UK
The HSC Research and Development (R&D) Division of the Public Health Agency funds the Cochrane Eyes and Vision editorial base at Queen's University Belfast.
Declarations of interest
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors. Nikolaos Tzoumas: no disclosures. George Riding: no disclosures. Michael A Williams: support to attend meetings from Novartis and Bayer Pharmaceuticals, ad hoc speaker for AbbVie, Novartis and Bayer Pharmaceuticals, education grant received from Bayer Pharmaceuticals. David HW Steel: Alcon (C, F), Bayer Pharmaceuticals (F), Boehringer Ingelheim (F), BVI Medical (C), Dutch Ophthalmic Research Centre [DORC] (C, F), Gyroscope Therapeutics (C), Roche (C). The financial relationships of the authors did not influence the collection, analysis or interpretation of data, the writing of the report, or the decision to submit the article for publication.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Avacincaptad pegol Phase 2/3 (GATHER1) {published data only (unpublished sought but not used)}
- Csaky KG, Westby K, Rezaei K. Avacincaptad pegol, a novel C5 inhibitor, significantly reduces the mean rate of geographic atrophy growth in a pivotal clinical trial. Investigative Ophthalmology and Visual Science 2020;61(7):ARVO E-abstract 1943.
- Jaffe GJ, Westby K, Csaky KG, Monés J, Pearlman JA, Patel SS, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology 2021;128(4):576-86. [DOI] [PubMed] [Google Scholar]
- Sheth V. Avacincaptad pegol, a novel C5 inhibitor, demonstrates a continued reduction in the mean rate of geographic atrophy growth: 18-month results from the GATHER1 clinical trial. Investigative Ophthalmology and Visual Science 2021;62(8):ARVO E-abstract 333.
CLG561 ± LFG316 Phase 2 {unpublished data only}
- NCT02515942. CLG561 proof-of-concept study as a monotherapy and in combination with LFG316 in subjects with geographic atrophy (GA). clinicaltrials.gov/ct2/show/NCT02515942 (first received 19 December 2018).
Eculizumab Phase 2 (COMPLETE) {published data only}
- Garcia Filho CA, Yehoshua Z, Gregori G, Nunes RP, Penha FM, Moshfeghi AA, et al. Change in drusen volume as a novel clinical trial endpoint for the study of complement inhibition in age-related macular degeneration. Ophthalmic Surgery, Lasers and Imaging Retina 2014;45(1):18-31. [DOI] [PubMed] [Google Scholar]
- Stetson PF, Yehoshua Z, Garcia Filho CA, Portella Nunes R, Gregori G, Rosenfeld PJ. OCT minimum intensity as a predictor of geographic atrophy enlargement. Investigative Ophthalmology and Visual Science 2014;55(2):792-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehoshua Z, Amorim Garcia Filho CA, Nunes RP, Gregori G, Penha FM, Moshfeghi AA, et al. Comparison of geographic atrophy growth rates using different imaging modalities in the COMPLETE study. Ophthalmic Surgery, Lasers & Imaging Retina 2015;46(4):413-22. [DOI] [PubMed] [Google Scholar]
- Yehoshua Z, Amorim Garcia Filho CA, Nunes RP, Gregori G, Penha FM, Moshfeghi AA, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology 2014;121(3):693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lampalizumab Phase 2 (MAHALO) {published data only (unpublished sought but not used)}
- Hariri A, Nittala MG, Sadda SR. Outer retinal tubulation as a predictor of the enlargement amount of geographic atrophy in age-related macular degeneration. Ophthalmology 2015;122(2):407-13. [DOI] [PubMed] [Google Scholar]
- Kimel M, Leidy NK, Tschosik E, Dolan C, Souied EH, Varma R, et al. Functional Reading Independence (FRI) Index: a new patient-reported outcome measure for patients with geographic atrophy. Investigative Ophthalmology and Visual Science 2016;57(14):6298-304. [DOI] [PubMed] [Google Scholar]
- Sivaprasad S, Tschosik E, Kapre A, Varma R, Bressler NM, Kimel M, et al. Reliability and construct validity of the NEI VFQ-25 in a subset of patients with geographic atrophy from the phase 2 Mahalo Study. American Journal of Ophthalmology 2018;190:1-8. [DOI] [PubMed] [Google Scholar]
- Yaspan BL, Williams DF, Holz FG, Regillo CD, Li Z, Dressen A, et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Science Translation Medicine 2017;9:eaaf1443. [DOI] [PubMed] [Google Scholar]
Lampalizumab Phase 3 (CHROMA) {published data only (unpublished sought but not used)}
- Heier JS, Pieramici D, Chakravarthy U, Patel SS, Gupta S, Lotery A, et al. Visual function decline resulting from geographic atrophy: results from the Chroma and Spectri phase 3 trials. Ophthalmology Retina 2020;4(7):673-88. [DOI] [PubMed] [Google Scholar]
- Holz FG, Sadda SR, Busbee B, Chew EY, Mitchell P, Tufail A, et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri phase 3 randomized clinical trials. JAMA Ophthalmology 2018;136(6):666-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02745119. Long-term safety of lampalizumab intravitreal (ITV) injections in participants with geographic atrophy (GA) secondary to age-related macular degeneration (OMASPECT). clinicaltrials.gov/ct2/show/NCT02745119 (first received 20 April 2016).
- Pieramici D, Holz F, Heier JS, Sadda SR, Busbee BG, Chew EY, et al. Lampalizumab for geographic atrophy (GA) in age-related macular degeneration (AMD): pooled results of the Chroma and Spectri phase 3 randomized clinical trials (RCTs). Investigative Ophthalmology and Visual Science 2018;59(9):ARVO E-abstract 4948.
Lampalizumab Phase 3 (SPECTRI) {published data only}
- Heier JS, Pieramici D, Chakravarthy U, Patel SS, Gupta S, Lotery A, et al. Visual function decline resulting from geographic atrophy: results from the Chroma and Spectri phase 3 trials. Ophthalmology Retina 2020;4(7):673-88. [DOI] [PubMed] [Google Scholar]
- Holz FG, Sadda SR, Busbee B, Chew EY, Mitchell P, Tufail A, et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri phase 3 randomized clinical trials. JAMA Ophthalmology 2018;136(6):666-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02745119. Long-term safety of lampalizumab intravitreal (ITV) injections in participants with geographic atrophy (GA) secondary to age-related macular degeneration (OMASPECT) . clinicaltrials.gov/ct2/show/NCT02745119 (first received 20 April 2016).
- Pieramici D, Holz F, Heier JS, Sadda SR, Busbee BG, Chew EY, et al. Lampalizumab for geographic atrophy (GA) in age-related macular degeneration (AMD): pooled results of the Chroma and Spectri phase 3 randomized clinical trials (RCTs). Investigative Ophthalmology and Visual Science 2018;59(9):ARVO E-abstract 4948.
LFG316 Phase 2 {unpublished data only}
- NCT01527500. Intravitreal LFG316 in patients with age-related macular degeneration (AMD). clinicaltrials.gov/ct2/show/record/NCT01527500 (first received 27 July 2018).
Pegcetacoplan Phase 2 (FILLY) {published and unpublished data}10.1016/j.ophtha.2019.07.011
- Bogunovic H, Lachinov D, Mai J, Reiter GS, Riedl S, Vogl WD, et al. Predictive identification of the fastest progressing geographic atrophy lesions based on deep learning in the phase 2 FILLY clinical trial of pegcetacoplan. Investigative Ophthalmology and Visual Science 2021;62(8):ARVO E-abstract 129.
- Chakravarthy U, Ip M, Nittala M, Metlapally R, Ribiero R, Sadda S. Impact of pegcetacoplan on progression of nascent atrophy in age-related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2021;62(8):ARVO E-abstract 1213.
- Liao DS, Grossi FV, El Mehdi D, Gerber MR, Brown DM, Heier JS, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology 2020;127(2):186-95. [DOI] [PubMed] [Google Scholar]
- Liao DS, Metapally R, Joshi P. Pegcetacoplan treatment for geographic atrophy due to age-related macular degeneration: a plain language summary of the FILLY study. Immunotherapy 2022;14(13):995-1006. [DOI] [PubMed] [Google Scholar]
- Nittala MG, Metlapally R, Ip M, Chakravarthy U, Holz FG, Staurenghi G, et al. Association of pegcetacoplan with progression of incomplete retinal pigment epithelium and outer retinal atrophy in age-related macular degeneration: a post hoc analysis of the FILLY randomized clinical trial. JAMA Ophthalmology 2022;140(3):243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl S, Vogl WD, Mai J, Reiter GS, Lachinov D, Grechenig C, et al. The effect of pegcetacoplan treatment on photoreceptor maintenance in geographic atrophy monitored by artificial intelligence-based OCT analysis. Ophthalmology Retina 2022;6(11):1009-18. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U, Mai J, Reiter GS, Riedl S, Lachinov D, Vogl WD, et al. AI-based quantification of photoreceptor maintenance in the treatment of geographic atrophy secondary to AMD in the FILLY trial. Investigative Ophthalmology and Visual Science 2021;62(8):ARVO E-abstract 236.
- Steinle N, Hamdani M. Evaluation of baseline factors on progression in a large phase-2 clinical trial for geographic atrophy (FILLY Study). Investigative Ophthalmology and Visual Science 2019;60(9):ARVO E-abstract 973.
- Steinle NC, Pearce I, Monés J, Metlapally R, Saroj N, Hamdani M, et al. Impact of baseline characteristics on geographic atrophy progression in the FILLY trial evaluating the complement C3 inhibitor pegcetacoplan. American Journal of Ophthalmology 2021;227:116-24. [DOI] [PubMed] [Google Scholar]
- Vogl WD, Bogunovic H, Mai J, Reiter GS, Riedl S, Lachinov D, et al. Topographic effects on AI-quantified regional progression in the FILLY trial of pegcetacoplan (APL-2) for treatment of geographic atrophy secondary to AMD. Investigative Ophthalmology and Visual Science 2021;62(8):ARVO E-abstract 128.
- Vogl WD, Riedl S, Mai J, Reiter GS, Lachinov D, Bogunović H, et al. Predicting topographic disease progression and treatment response of pegcetacoplan in geographic atrophy quantified by deep learning. Ophthalmology Retina 2023;7(1):4-13. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Grossi F. APL-2, a complement C3 inhibitor, slows the growth of geographic atrophy secondary to AMD: 18-month results of a phase 2 trial (FILLY). Investigative Ophthalmology and Visual Science 2018;59(9):ARVO E-abstract 72.
- Wykoff CC, Rosenfeld PJ, Waheed NK, Singh RP, Ronca N, Slakter JS, et al. Characterizing new-onset exudation in the randomized phase 2 FILLY trial of complement inhibitor pegcetacoplan for geographic atrophy. Ophthalmology 2021;128(9):1325-36. [DOI] [PubMed] [Google Scholar]
Pegcetacoplan Phase 3 (DERBY) {published and unpublished data}
- Goldberg R, Heier JS, Wykoff CC, Staurenghi G, Singh RP, Steinle N, et al. Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the phase 3 OAKS and DERBY studies. Investigative Ophthalmology and Visual Science 2022;63(7):ARVO E-abstract 1500.
- Wykoff CC. Treatment of geographic atrophy secondary to age-related macular degeneration with pegcetacoplan: updates on the randomized phase 3 DERBY and OAKS trials. American Academy of Ophthalmology Annual Meeting, New Orleans, LA, United States 2021.
Pegcetacoplan Phase 3 (OAKS) {published and unpublished data}
- Goldberg R, Heier JS, Wykoff CC, Staurenghi G, Singh RP, Steinle N, et al. Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the phase 3 OAKS and DERBY studies. Investigative Ophthalmology and Visual Science 2022;63(7):ARVO E-abstract 1500.
- Wykoff CC. Treatment of geographic atrophy secondary to age-related macular degeneration with pegcetacoplan: updates on the randomized phase 3 DERBY and OAKS trials. American Academy of Ophthalmology Annual Meeting, New Orleans, LA, United States 2021 2021.
References to studies excluded from this review
NCT01157065 {published data only}
- NCT01157065. Evaluation of AL-78898A in exudative age-related macular degeneration (RACE). clinicaltrials.gov/ct2/show/NCT01157065 (first received 5 July 2010).
NCT01624636 {published data only}
- NCT01624636. Safety and tolerability of intravenous LFG316 in wet age-related macular degeneration (AMD). clinicaltrials.gov/ct2/show/NCT01624636 (first received 21 June 2012).
NCT03362190 {published data only}
- NCT03362190. ZIMURA in combination with LUCENTIS in patients with neovascular age related macular degeneration (NVAMD). clinicaltrials.gov/ct2/show/NCT03362190 (first received 5 December 2017).
NCT03446144 {published data only}
- NCT03446144. Safety and efficacy of IONIS-FB-Lrx in up to 120 patients 55 and older with geographic atrophy (GA) secondary to age-related macular degeneration (AMD). clinicaltrials.gov/ct2/show/NCT03446144 (first received 26 February 2018).
References to ongoing studies
NCT03815825 {published data only}
- NCT03815825. GOLDEN STUDY: a study to assess safety and efficacy of multiple doses of IONIS-FB-LRx in participants with geographic atrophy secondary to age-related macular degeneration (AMD). clinicaltrials.gov/ct2/show/NCT03815825 (first received 24 January 2019).
NCT04435366 {published data only}
- NCT04435366. A phase 3 safety and efficacy study of intravitreal administration of zimura (complement C5 inhibitor). clinicaltrials.gov/ct2/show/NCT04435366 (first received 17 June 2020).
NCT04437368 {published data only}
- NCT04437368. EXPLORE: a phase II study to evaluate the safety and efficacy of two doses of GT005 (EXPLORE). clinicaltrials.gov/ct2/show/NCT04437368 (first received 18 June 2020).
NCT04465955 {published data only}
- NCT04465955. A study of NGM621 in participants with geographic atrophy (CATALINA). clinicaltrials.gov/ct2/show/NCT04465955 (first received 10 July 2020).
NCT04566445 {published data only}
- NCT04566445. HORIZON: a phase II study to evaluate the safety and efficacy of two doses of GT005. clinicaltrials.gov/ct2/show/NCT04566445 (first received 28 September 2020).
NCT04643886 {published data only}
- NCT04643886. A multiple dose study of repeat intravitreal injections of GEM103 in dry age-related macular degeneration. clinicaltrials.gov/ct2/show/NCT04643886 (first received 25 November 2020).
NCT04656561 {published data only}
- NCT04656561. A study investigating the efficacy and safety of intravitreal injections of ANX007 in patients with geographic atrophy (ARCHER). clinicaltrials.gov/ct2/show/NCT04656561 (first received 7 December 2020).
NCT04820452 {published data only}
- NCT04820452. A study of IBI302 in patients with nAMD. clinicaltrials.gov/ct2/show/NCT04820452 (first received 29 March 2021).
NCT05019521 {published data only}
- NCT05019521. A study of danicopan in participants with geographic atrophy secondary to age-related macular degeneration. clinicaltrials.gov/ct2/show/NCT05019521 (first received 25 August 2021).
NCT05230537 {published data only}
- NCT05230537. A masked, placebo-controlled study to assess iptacopan in age-related macular degeneration. clinicaltrials.gov/ct2/show/NCT05230537 (first received 9 February 2022).
Additional references
Anderson 2010
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Progress in Retinal and Eye Research 2010;29(2):95-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Augustin 2007
- Augustin A, Sahel JA, Bandello F, Dardennes R, Maurel F, Negrini C, et al. Anxiety and depression prevalence rates in age-related macular degeneration. Investigative Ophthalmology and Visual Science 2007;48(4):1498-503. [DOI] [PubMed] [Google Scholar]
Balaskas 2022
- Balaskas K, Glinton S, Keenan TDL, Faes L, Liefers B, Zhang G, et al. Prediction of visual function from automatically quantified optical coherence tomography biomarkers in patients with geographic atrophy using machine learning. Scientific Reports 2022;12(1):15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 2007
- Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology 2007;114(10):1804-9. [DOI] [PubMed] [Google Scholar]
Booij 2010
- Booij JC, Baas DC, Beisekeeva J, Gorgels TG, Bergen AA. The dynamic nature of Bruch's membrane. Progress in Retinal and Eye Research 2010;29(1):1-18. [DOI] [PubMed] [Google Scholar]
Bora 2005
- Bora PS, Sohn JH, Cruz JM, Jha P, Nishihori H, Wang Y, et al. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. Journal of Immunology 2005;174(1):491-7. [DOI] [PubMed] [Google Scholar]
Boutron 2022
- Boutron I, Page MJ, Higgins JPT, Altman DG, Lundh A, Hróbjartsson A. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Brechner 2011
- Brechner RJ, Rosenfeld PJ, Babish JD, Caplan S. Pharmacotherapy for neovascular age-related macular degeneration: an analysis of the 100% 2008 medicare fee-for-service part B claims file. American Journal of Ophthalmology 2011;151:887-95. [DOI] [PubMed] [Google Scholar]
Brown 1999
- Brown GC. Vision and quality-of-life. Transactions of the American Ophthalmological Society 1999;97:473-511. [PMC free article] [PubMed] [Google Scholar]
Brown 2020
- Brown GC, Brown MM, Rapuano S, Boyer D. Cost-utility analysis of VEGF inhibitors for treating neovascular age-related macular degeneration. American Journal of Ophthalmology 2020;218:225-41. [DOI] [PubMed] [Google Scholar]
Calippe 2017
- Calippe B, Augustin S, Beguier F, Charles-Messance H, Poupel L, Conart JB, et al. Complement factor H inhibits CD47-mediated resolution of inflammation. Immunity 2017;46(2):261-72. [DOI] [PubMed] [Google Scholar]
Cao 2011
- Cao X, Shen D, Patel MM, Tuo J, Johnson TM, Olsen TW, et al. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathology International 2011;61(9):528-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2021
- Cao D, Leong B, Messinger JD, Kar D, Ach T, Yannuzzi LA, et al. Hyperreflective foci, optical coherence tomography progression indicators in age-related macular degeneration, include transdifferentiated retinal pigment epithelium. Investigative Ophthalmology and Visual Science 2021;62(10):ARVO E-abstract 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cerniauskas 2020
- Cerniauskas E, Kurzawa-Akanbi M, Xie L, Hallam D, Moya-Molina M, White K, et al. Complement modulation reverses pathology in Y402H-retinal pigment epithelium cell model of age-related macular degeneration by restoring lysosomal function. Stem Cells Transl Med 2020;9(11):1585-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chakravarthy 2010
- Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmology 2010;10(6):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chakravarthy 2018
- Chakravarthy U, Bailey CC, Johnston RL, McKibbin M, Khan RS, Mahmood S, et al. Characterizing disease burden and progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology 2018;125(6):842-9. [DOI] [PubMed] [Google Scholar]
Chia 2004
- Chia EM, Wang JJ, Rochtchina E, Smith W, Cumming RR, Mitchell P. Impact of bilateral visual impairment on health-related quality of life: the Blue Mountains Eye Study. Investigative Ophthalmology and Visual Science 2004;45(1):71-6. [DOI] [PubMed] [Google Scholar]
Cocce 2018
- Cocce KJ, Stinnett SS, Luhmann UFO, Vajzovic L, Horne A, Schuman SG, et al. Visual function metrics in early and intermediate dry age-related macular degeneration for use as clinical trial endpoints. American Journal of Ophthalmology 2018;189:127-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coleman 2008
- Coleman AL, Yu F. Eye-related medicare costs for patients with age-related macular degeneration from 1995 to 1999. Ophthalmology 2008;115(1):18-25. [DOI] [PubMed] [Google Scholar]
Colijn 2017
- Colijn JM, Buitendijk GHS, Prokofyeva E, Alves D, Cachulo ML, Khawaja AP, et al, European Eye Epidemiology (E3) consortium. Prevalence of age-related macular degeneration in Europe: The past and the future. Ophthalmology 2017;124(12):1753-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colijn 2021
- Colijn JM, Liefers B, Joachim N, Verzijden T, Meester-Smoor MA, Biarnés M, et al. Enlargement of geographic atrophy from first diagnosis to end of life. JAMA Ophthalmology 2021;139(7):743-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Copland 2018
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 1 October 2022. Available at www.covidence.org.
Creuzot‐Garcher 2022
- Creuzot-Garcher CP, Srour M, Baudin F, Daien V, Dot C, Nghiem-Buffet S, et al. Incidence and prevalence of neovascular age-related macular degeneration in France between 2008 and 2018: The LANDSCAPE study. Ophthalmology 2022;2(1):100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Csaky 2017
- Csaky K, Ferris FL 3rd, Chew EY, Nair P, Cheetham JK, Duncan JL. Report from the NEI/FDA endpoints workshop on age-related macular degeneration and inherited retinal diseases. Investigative Ophthalmology and Visual Science 2017;58(9):3456-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022 . Available from www.training.cochrane.org/handbook.
Despriet 2006
- Despriet DD, Klaver CC, Witteman JC, Bergen AA, Kardys I, Maat MP, et al. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA 2006;296(3):301-9. [DOI] [PubMed] [Google Scholar]
Duvall‐Young 1989
- Duvall-Young J, MacDonald MK, McKechnie NM. Fundus changes in (type II) mesangiocapillary glomerulonephritis simulating drusen: a histopathological report. British Journal of Ophthalmology 1989;73(4):297-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2005
- Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science 2005;308(5720):421-4. [DOI] [PubMed] [Google Scholar]
Ferris 2013
- Ferris FL 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, et al. Clinical classification of age-related macular degeneration. Ophthalmology 2013;120(4):844-51. [DOI] [PubMed] [Google Scholar]
Feuer 2013
- Feuer WJ, Yehoshua Z, Gregori G, Penha FM, Chew EY, Ferris FL, et al. Square root transformation of geographic atrophy area measurements to eliminate dependence of growth rates on baseline lesion measurements: a reanalysis of age-related eye disease study report no. 26. JAMA Ophthalmology 2013;131(1):110-1. [DOI] [PubMed] [Google Scholar]
Flaxman 2017
- Flaxman SR, Bourne RR, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Global Health 2017;5(12):1221-34. [DOI] [PubMed] [Google Scholar]
Fleckenstein 2018
- Fleckenstein M, Mitchell P, Freund KB, Sadda S, Holz FG, Brittain C, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology 2018;125(3):369-90. [DOI] [PubMed] [Google Scholar]
Fritsche 2016
- Fritsche LG, Igl W, Bailey JN, Grassmann F, Sengupta S, Bragg-Gresham JL, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nature Genetics 2016;48(2):134-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galimard 2018
- Galimard JE, Chevret S, Curis E, Resche-Rigon M. Heckman imputation models for binary or continuous MNAR outcomes and MAR predictors. BMC Medical Research Methodology 2018;18(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gold 2006
- Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, et al, AMD Genetics Clinical Study Group. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nature Genetics 2006;38(4):458-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 24 September 2022. Available at gradepro.org.
Guillonneau 2017
- Guillonneau X, Eandi CM, Paques M, Sahel JA, Sapieha P, Sennlaub F. On phagocytes and macular degeneration. Progress in Retinal and Eye Research 2017;61:98-128. [DOI] [PubMed] [Google Scholar]
Gupta 2007
- Gupta OP, Brown GC, Brown MM. Age-related macular degeneration: the costs to society and the patient. Current Opinion in Ophthalmology 2007;18(3):201-5. [DOI] [PubMed] [Google Scholar]
Gupta 2019
- Gupta V, Bhavanasi S, Quadir M, Singh K, Ghosh G, Vasamreddy K, et al. Protein PEGylation for cancer therapy: bench to bedside. Journal of Cell Communication and Signaling 2019;13(3):319-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hageman 2005
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America 2005;102(20):7227-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haines 2005
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 2005;308(5720):419-21. [DOI] [PubMed] [Google Scholar]
Hallam 2020
- Hallam TM, Marchbank KJ, Harris CL, Osmond C, Shuttleworth VG, Griffiths H, et al. Rare genetic variants in complement factor I lead to low FI plasma levels resulting in increased risk of age-related macular degeneration. Investigative Ophthalmology and Visual Science 2020;61(6):ARVO E-abstract 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harboe 2004
- Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clinical and Experimental Immunology 2004;138(3):439-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrell 2015
- Harrell FE. Multivariable Modeling Strategies. In: Regression Modeling Strategies. Springer Series in Statistics. Springer Cham, 2015. [DOI: 10.1007/978-3-319-19425-7_4] [DOI] [Google Scholar]
Hassell 2006
- Hassell JB, Lamoureux EL, Keeffe JE. Impact of age related macular degeneration on quality of life. British Journal of Ophthalmology 2006;90(5):593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heesterbeek 2020
- Heesterbeek TJ, Lorés-Motta L, Hoyng CB, Lechanteur YTE, den Hollander AI. Risk factors for progression of age-related macular degeneration. Ophthalmic and Physiological Optics 2020;40(2):140-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heier 2020
- Heier JS, Pieramici D, Chakravarthy U, Patel SS, Gupta S, Lotery A, et al. Visual function decline resulting from geographic atrophy: results from the Chroma and Spectri phase 3 trials. Ophthalmology Retina 2020;4(7):673-88. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2019
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hillmen 2021
- Hillmen P, Szer J, Weitz I, Röth A, Höchsmann B, Panse J, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. New England Journal of Medicine 2021;384:1028-37. [DOI] [PubMed] [Google Scholar]
Hodge 2013
- Hodge S, Eccles F. Loneliness, Social Isolation and Sight Loss: A literature review conducted for Thomas Pocklington Trust. Lancaster University. eprints.lancs.ac.uk/id/eprint/68597/1/loneliness_social_isolation_and_sight_loss_final_report_dec_13.pdf accessed 7 October 2022.
Hoh Kam 2013
- Hoh Kam J, Lenassi E, Malik TH, Pickering MC, Jeffery G. Complement component C3 plays a critical role in protecting the aging retina in a murine model of age-related macular degeneration. American Journal of Pathology 2013;183(2):480-92. [DOI] [PubMed] [Google Scholar]
Hughes 2019
- Hughes RA, Heron J, Sterne JAC, Tilling K. Accounting for missing data in statistical analyses: multiple imputation is not always the answer. International Journal of Epidemiology 2019;48(4):1294-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irmscher 2018
- Irmscher S, Döring N, Halder LD, Jo EAH, Kopka I, Dunker C, et al. Kallikrein cleaves C3 and activates complement. Journal of Innate Immunity 2018;10(2):94-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacob 2017
- Jacob L, Spiess A, Kostev K. Prevalence of depression, anxiety, adjustment disorders, and somatoform disorders in patients with age-related macular degeneration in Germany. German Medical Science 2017;15:Doc04. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahr 2010
- Kahr WH. Complement halts angiogenesis gone wild. Blood 2010;116(22):4393-4. [DOI] [PubMed] [Google Scholar]
Karczewski 2020
- Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020;581(7809):434-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katschke 2012
- Katschke KJ Jr, Wu P, Ganesan R, Kelley RF, Mathieu MA, Hass PE, et al. Inhibiting alternative pathway complement activation by targeting the factor D exosite. Journal of Biological Chemistry 2012;287(16):12886-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keenan 2012
- Keenan TD, Clark SJ, Unwin RD, Ridge LA, Day AJ, Bishop PN. Mapping the differential distribution of proteoglycan core proteins in the adult human retina, choroid, and sclera. Investigative Ophthalmology and Visual Science 2012;53(12):7528-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keenan 2018
- Keenan TD, Agrón E, Domalpally A, Clemons TE, Asten F, Wong WT, et al. Progression of geographic atrophy in age-related macular degeneration: AREDS2 report number 16. Ophthalmology 2018;125(12):1913-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kenward 2007
- Kenward MG, Carpenter J. Multiple imputation: current perspectives. Statistical Methods in Medical Research 2007;16(3):199-218. [DOI] [PubMed] [Google Scholar]
Kim 2021
- Kim BJ, Mastellos DC, Li Y, Dunaief JL, Lambris JD. Targeting complement components C3 and C5 for the retina: key concepts and lingering questions. Progress in Retinal and Eye Research 2021;83:100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2018
- Kirkham JJ, Altman DG, Chan AW, Gamble C, Dwan KM, Williamson PR. Outcome reporting bias in trials: a methodological approach for assessment and adjustment in systematic reviews. BMJ 2018;362:k3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 2005
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005;308(5720):385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2010
- Lee HM, Wysoczynski M, Liu R, Shin DM, Kucia M, Botto M, et al. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia 2010;24(3):573-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2019
- Li JQ, Welchowski T, Schmid M, Mauschitz MM, Holz FG, Finger RP. Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis. British Journal of Ophthalmology 2020;104(8):1077-84. [DOI] [PubMed] [Google Scholar]
Lindblad 2009
- Lindblad AS, Lloyd PC, Clemons TE, Gensler GR, Ferris FL 3rd, Klein ML, et al. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Archives of Ophthalmology 2009;127(9):1168-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Litts 2015
- Litts KM, Messinger JD, Dellatorre K, Yannuzzi LA, Freund KB, Curcio CA. Clinicopathological correlation of outer retinal tubulation in age-related macular degeneration. JAMA Ophthalmology 2015;133(5):609-12. [DOI] [PubMed] [Google Scholar]
Lorés‐Motta 2018
- Lorés-Motta L, Paun CC, Corominas J, Pauper M, Geerlings MJ, Altay L, et al. Genome-wide association study reveals variants in CFH and CFHR4 associated with systemic complement activation: Implications in age-related macular degeneration. Ophthalmology 2018;125(7):1064-74. [DOI] [PubMed] [Google Scholar]
Lyzogubov 2011
- Lyzogubov VV, Tytarenko RG, Liu J, Bora NS, Bora PS. Polyethylene glycol (PEG)-induced mouse model of choroidal neovascularization. Journal of Biological Chemistry 2011;286(18):16229-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mastellos 2013
- Mastellos DC, Deangelis RA, Lambris JD. Complement-triggered pathways orchestrate regenerative responses throughout phylogenesis. Seminars in Immunology 2013;25(1):29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mastellos 2019
- Mastellos DC, Ricklin D, Lambris JD. Clinical promise of next-generation complement therapeutics. Nature Reviews Drug Discovery 2019;18(9):707-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meleth 2011
- Meleth AD, Mettu P, Agrón E, Chew EY, Sadda SR, Ferris FL, et al. Changes in retinal sensitivity in geographic atrophy progression as measured by microperimetry. Investigative Ophthalmology and Visual Science 2011;52(2):1119-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Minassian 2009
- Minassian D, Reidy A. Future Sight Loss UK 2: An epidemiological and economic model for sight loss in the decade 2010-2020. www.rnib.org.uk/professionals/health-social-care-education-professionals/knowledge-and-research-hub/knowledge-and-research/future-sight-loss-uk-2/ (accessed 31 August 2022).
Mohlin 2017
- Mohlin C, Sandholm K, Ekdahl KN, Nilsson B. The link between morphology and complement in ocular disease. Molecular Immunology 2017;89:84-99. [DOI] [PubMed] [Google Scholar]
Morgan 2016
- Morgan BP. The membrane attack complex as an inflammatory trigger. Immunobiology 2016;221(6):747-51. [DOI] [PubMed] [Google Scholar]
Mulligan 2019
- Mulligan K, Seabury SA, Dugel PU, Blim JF, Goldman DP, Humayun MS. Economic value of anti-vascular endothelial growth factor treatment for patients with wet age-related macular degeneration in the United States. JAMA Ophthalmology 2020;138(1):40-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nittala 2022
- Nittala MG, Metlapally R, Ip M, Chakravarthy U, Holz FG, Staurenghi G, et al. Association of pegcetacoplan with progression of incomplete retinal pigment epithelium and outer retinal atrophy in age-related macular degeneration: a post hoc analysis of the FILLY randomized clinical trial. JAMA Ophthalmology 2022;140(3):243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nozaki 2006
- Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proceedings of the National Academy of Sciences of the United States of America 2006;103(7):2328-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raychaudhuri 2011
- Raychaudhuri S, Iartchouk O, Chin K, Tan PL, Tai AK, Ripke S, et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nature Genetics 2011;43(12):1232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:594. [DOI] [PubMed] [Google Scholar]
Rofagha 2013
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013;120(11):2292-9. [DOI] [PubMed] [Google Scholar]
Ruan 2015
- Ruan CC, Ge Q, Li Y, Li XD, Chen DR, Ji KD, et al. Complement-mediated macrophage polarization in perivascular adipose tissue contributes to vascular injury in deoxycorticosterone acetate-salt mice. Arteriosclerosis, Thrombosis, and Vascular Biology 2015;35(3):598-606. [DOI] [PubMed] [Google Scholar]
Sarks 1988
- Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye 1988;2(Pt 5):552-77. [DOI] [PubMed] [Google Scholar]
Schaal 2015
- Schaal KB, Freund KB, Litts KM, Zhang Y, Messinger JD, Curcio CA. Outer retinal tubulation in advanced age-related macular degeneration: optical coherence tomographic findings correspond to histology. Retina 2015;35(7):1339-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmier 2012
- Schmier JK, Covert DW, Lau EC. Patterns and costs associated with progression of age-related macular degeneration. American Journal of Ophthalmology 2012;154:675-81. [DOI] [PubMed] [Google Scholar]
Schmitz‐Valckenberg 2016
- Schmitz-Valckenberg S, Nadal J, Fimmers R, Lindner M, Holz FG, Schmid M, et al. Modeling visual acuity in geographic atrophy secondary to age-related macular degeneration. Ophthalmologica 2016;235(4):215-24. [DOI] [PubMed] [Google Scholar]
Seddon 2013
- Seddon JM, Yu Y, Miller EC, Reynolds R, Tan PL, Gowrisankar S, et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nature Genetics 2013;45(11):1366-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shahid 2012
- Shahid H, Khan JC, Cipriani V, Sepp T, Matharu BK, Bunce C, et al. Age-related macular degeneration: the importance of family history as a risk factor. British Journal of Ophthalmology 2012;96(3):427-31. [DOI] [PubMed] [Google Scholar]
Sharma 2000
- Sharma S, Brown GC, Brown MM, Shah GK, Snow K, Brown H, et al. Converting visual acuity to utilities. Canadian Journal of Ophthalmology 2000;35(5):267-72. [DOI] [PubMed] [Google Scholar]
Shaw 2012
- Shaw PX, Zhang L, Zhang M, Du H, Zhao L, Lee C, et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proceedings of the National Academy of Sciences of the United States of America 2012;109(34):13757-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simkiss 2016
- Simkiss P, Dennison D, Edwards E, Edwards R, Flynn K, Lee H, et al. The State of the Nation Eye Health 2016. RNIB. www.rnib.org.uk/stateofthenation (accessed 31 August 2022).
Slakter 2005
- Slakter JS, Stur M. Quality of life in patients with age-related macular degeneration: impact of the condition and benefits of treatment. Survey of Ophthalmology 2005;50(3):263-73. [DOI] [PubMed] [Google Scholar]
Soubrane 2007
- Soubrane G, Cruess A, Lotery A, Pauleikhoff D, Monès J, Xu X, et al. Burden and health care resource utilization in neovascular age-related macular degeneration: findings of a multicountry study. Archives of Ophthalmology 2007;125(9):1249-54. [DOI] [PubMed] [Google Scholar]
Spencer 2007
- Spencer KL, Hauser MA, Olson LM, Schmidt S, Scott WK, Gallins P, et al. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Human Molecular Genetics 2007;16(16):1986-92. [DOI] [PubMed] [Google Scholar]
Spratt 2010
- Spratt M, Carpenter J, Sterne JA, Carlin JB, Heron J, Henderson J, et al. Strategies for multiple imputation in longitudinal studies. American Journal of Epidemiology 2010;172(4):478-87. [DOI] [PubMed] [Google Scholar]
Steinle 2021
- Steinle NC, Pearce I, Monés J, Metlapally R, Saroj N, Hamdani M, et al. Impact of baseline characteristics on geographic atrophy progression in the FILLY trial evaluating the complement C3 inhibitor pegcetacoplan. American Journal of Ophthalmology 2021;227:116-24. [DOI] [PubMed] [Google Scholar]
Submacular Surgery Trials Research Group 2007
- Submacular Surgery Trials Research Group. Evaluation of minimum clinically meaningful changes in scores on the National Eye Institute Visual Function Questionnaire (NEI-VFQ) SST Report Number 19. Ophthalmic Epidemiology 2007;14(4):205-15. [DOI] [PubMed] [Google Scholar]
Sunness 1999
- Sunness JS, Gonzalez-Baron J, Applegate CA, Bressler NM, Tian Y, Hawkins B, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology 1999;106(9):1768-79. [DOI] [PubMed] [Google Scholar]
Sunness 2005
- Sunness JS, Applegate CA. Long-term follow-up of fixation patterns in eyes with central scotomas from geographic atrophy that is associated with age-related macular degeneration. American Journal of Ophthalmology 2005;140:1085-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tzoumas 2021
- Tzoumas N, Hallam D, Harris CL, Lako M, Kavanagh D, Steel DH. Revisiting the role of factor H in age-related macular degeneration: Insights from complement-mediated renal disease and rare genetic variants. Survey of Ophthalmology 2021;66(2):378-401. [DOI] [PubMed] [Google Scholar]
Tzoumas 2022
- Tzoumas N, Kavanagh D, Cordell HJ, Lotery AJ, Patel PJ, Steel DH. Rare complement factor I variants associated with reduced macular thickness and age-related macular degeneration in the UK Biobank. Human Molecular Genetics 2022;31(16):2678-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Undar 2002
- Undar A, Eichstaedt HC, Clubb FJ Jr, Fung M, Lu M, Bigley JE, et al. Novel anti-factor D monoclonal antibody inhibits complement and leukocyte activation in a baboon model of cardiopulmonary bypass. Annals of Cardiothoracic Surgery 2002;74(2):355-62. [DOI] [PubMed] [Google Scholar]
van Asten 2018
- Asten F, Michels CT, Hoyng CB, Wilt GJ, Klevering BJ, Rovers MM, et al. The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration — a cost-effectiveness analysis from a societal perspective. PLOS One 2018;13:e0197670. [DOI] [PMC free article] [PubMed] [Google Scholar]
van de Ven 2013
- de Ven JP, Nilsson SC, Tan PL, Buitendijk GH, Ristau T, Mohlin FC, et al. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nature Genetics 2013;45(7):813-7. [DOI] [PubMed] [Google Scholar]
VanderBeek 2015
- VanderBeek BL, Bonaffini SG, Ma L. The association between intravitreal steroids and post-injection endophthalmitis rates. Ophthalmology 2015;122(11):2311-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vu 2005
- Vu HT, Keeffe JE, McCarty CA, Taylor HR. Impact of unilateral and bilateral vision loss on quality of life. British Journal of Ophthalmology 2005;89(3):360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weismann 2011
- Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, Charbel IP, et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 2011;478(7367):76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitmore 2015
- Whitmore SS, Sohn EH, Chirco KR, Drack AV, Stone EM, Tucker BA, et al. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Progress in Retinal and Eye Research 2015;45:1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winkler 2020
- Winkler TW, Grassmann F, Brandl C, Kiel C, Günther F, Strunz T, et al. Genome-wide association meta-analysis for early age-related macular degeneration highlights novel loci and insights for advanced disease. BMC Medical Genomics 2020;13:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wittenborn 2013
- Wittenborn JS, Zhang X, Feagan CW, Crouse WL, Shrestha S, Kemper AR, et al. The economic burden of vision loss and eye disorders among the United States population younger than 40 years. Ophthalmology 2013;120(9):1728-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2014
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health 2014;2(2):e106-16. [DOI] [PubMed] [Google Scholar]
Wu 2018
- Wu X, Hutson I, Akk AM, Mascharak S, Pham CT, Hourcade DE, et al. Contribution of adipose-derived factor D/adipsin to complement alternative pathway activation: Lessons from lipodystrophy. Journal of Immunology 2018;200(8):2786-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wykoff 2021
- Wykoff CC, Rosenfeld PJ, Waheed NK, Singh RP, Ronca N, Slakter JS, et al. Characterizing new-onset exudation in the randomized phase 2 FILLY trial of complement inhibitor pegcetacoplan for geographic atrophy. Ophthalmology 2021;128(9):1325-36. [DOI] [PubMed] [Google Scholar]
Yanamadala 2010
- Yanamadala V, Friedlander RM. Complement in neuroprotection and neurodegeneration. Trends in Molecular Medicine 2010;16(2):69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yates 2007
- Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, et al. Complement C3 variant and the risk of age-related macular degeneration. New England Journal of Medicine 2007;357(6):553-61. [DOI] [PubMed] [Google Scholar]
Zelek 2019
- Zelek WM, Xie L, Morgan BP, Harris CL. Compendium of current complement therapeutics. Molecular Immunology 2019;114:341-52. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Williams 2011
- Williams MA, McKay GJ, Chakravarthy U. Complement inhibitors for age-related macular degeneration. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD009300. [DOI: 10.1002/14651858.CD009300] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2014
- Williams MA, McKay GJ, Chakravarthy U. Complement inhibitors for age-related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD009300. [DOI: 10.1002/14651858.CD009300.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


