Abstract
Background and objectives:
The prevalence of pain syndromes that affect the territories innervated by the trigeminal nerve, such as headaches, is one of the highest and ranks second only to low back pain. A potential mechanism underlying this high prevalence may be a relatively weak endogenous pain modulation of trigeminal pain. Here, we sought to systematically compare endogenous pain modulation capabilities in the trigeminal region to those of extra-trigeminal regions in healthy subjects.
Methods:
Healthy, pain free subjects (n = 17) underwent a battery of quantitative sensory testing to assess endogenous pain inhibition and pain enhancement efficiencies within and outside the trigeminal innervated region. Measurements included conditioned pain modulation (CPM), temporal summation of pain (TSP) and spatial summation of pain (SSP).
Results:
Testing configurations that included trigeminal-innervated body regions displayed significantly weaker CPM when compared to extra-trigeminal innervated areas. SSP magnitude was smaller in the ophthalmic trigeminal innervation when compared to other body regions. TSP magnitude was not different between the different body regions tested.
Conclusions:
Our findings point to regional differences in endogenous pain inhibition and suggest that in otherwise healthy individuals, the trigeminal innervation is subjected to a weaker inhibitory pain control than other body regions. Such weaker endogenous pain control could play, at least in part, a role in mediating the high prevalence of trigeminal-related pain syndromes, including primary headaches and TMD pain.
Keywords: Conditioned pain modulation, temporal summation of pain, spatial summation of pain, healthy individuals
Introduction
The prevalence of pain syndromes that affect the territories innervated by the trigeminal nerve, such as headaches, is one of the highest and ranks second only to low back pain (1,2). A potential mechanism underlying this high prevalence may be a relatively weak endogenous pain modulation of trigeminal pain. Weaker or deficient pain modulation have been reported in numerous chronic pain syndromes that involve the trigeminal innervation, including trigeminal neuralgia (3), cluster headache (4), chronic posttraumatic headache (5), chronic tension-type headache (6,7), and persistent postendodontic pain (8). Yet it remains unclear whether in these cases a state of weak pain modulation preceded the pain and thus contributed to its pathophysiology, or rather was a consequence of these chronic pain conditions.
Clinical evidence supports both possibilities. For example, a weak ability to inhibit pain, as evaluated with the conditioned pain modulation (CPM) paradigm among pain-free people elected to undergo thoracotomy, predicted a higher risk for chronic post-thoracotomy pain (9). Thus, a pre-existing state of weak endogenous pain modulation may increase the propensity to develop persistent pain. The finding that migraine patients display deficient pain modulation, even during periods when they were pain free (10,11) further supports this possibility; in particular, the notion that an inherently weak pain modulation in the trigeminal region, as opposed to other body regions, may underlie the high prevalence of pain conditions related with this region.
Alternatively, persistent pain may itself promote changes in central nervous system processing that could lead to dysfunctional endogenous pain modulation. These include, among others, strengthening of processes that enhance central transmission of pain and/or impairment of descending circuits that inhibit pain transmission (12). This view is supported by the finding of an improvement in endogenous pain modulation following successful chronic pain management (13). Thus, pathologies in the trigeminal system or other cranial structures that lead to chronic or persistent pain may in turn weaken pain modulation of these regions.
A comparison of pain modulation indices between the trigeminal and extra-trigeminal body regions among pain-free, healthy subjects may help to determine potential anatomical differences in endogenous pain inhibitory pathways. Several studies have previously evaluated pain modulation capabilities involving the trigeminal region among healthy subjects (14–18). These studies employed mainly the CPM paradigm. It is assumed that the magnitude of pain inhibition during CPM reflects, at least in part, the efficacy of the diffuse noxious inhibitory control (DNIC) (19), although additional supraspinal mechanisms may also be involved in mediating CPM. In most of the above-mentioned studies, the conditioning stimulus was applied to areas outside the trigeminal region, a configuration that produced CPM effects regardless of whether the test stimulus was applied at, or outside, the trigeminal region. In one study (16), CPM was produced when both the conditioning and the test stimulus were applied at the trigeminal region, but its intensity was not compared to a configuration in which either stimulus was applied to an extra-trigeminal region.
A different experimental paradigm of pain modulation that evaluates pain facilitation rather than pain inhibition is temporal summation of pain (TSP). TSP, which is centrally mediated by frequency-dependent increments in spinal nociceptive neurons (20,21) was assessed in the trigeminal innervation (16), but was not compared, within the same subject, to that elicited in an extra-trigeminal region. In a recent study, repeated noxious stimulation over eight consecutive days induced stronger within-session sensitization when applied to the forehead than the forearm, suggesting increased susceptibility of the trigeminal region to hyper-excitability (22).
Here, we sought to systematically compare endogenous pain modulation capabilities in the trigeminal region to those of extra-trigeminal regions in healthy subjects to test whether pain inhibition is inherently weaker in the former. Pain inhibition and pain enhancement paradigms were tested a) within the trigeminal region only, b) in a configuration that includes the trigeminal and extra-trigeminal regions, and c) outside the trigeminal region.
Methods
Subjects
Participants were 17 healthy subjects (eight males, nine females, mean age 25.5 ± 1.59 years). Exclusion criteria for both groups were: Acute or chronic pain; present or previous pathology in the testing sites; bruises or any other skin lesions on the hands; diseases causing potential neural damage (e.g. diabetes); systemic and mental illnesses, and communication disabilities. Informed consent was obtained from all subjects after they received a full explanation of the goals and protocols of the study. The experiment was approved by the institutional review board of the Tel-Aviv University.
Equipment
Thermal stimulator:
Heat stimuli were delivered using two Peltier-based computerized thermal stimulators (TSA II, Medoc Ltd., Ramat Ishay, Israel), with 3 × 3 cm contact probes. According to the principles of the Peltier element, a passage of current through the Peltier element produces temperature changes at rates determined by an active feedback system. As soon as the target temperature is attained, probe temperature actively reverts to a preset adaptation temperature by passage of an inverse current. The adaptation (baseline) temperature was set to 35°C.
Visual analog scale (VAS):
Perceived pain was rated using a VAS. The VAS consisted of a 15 cm plastic ruler with an inner slider. Moving the slider exposes a horizontal red bar (the visual side) to the subject, while the side facing the experimenter displays an analogue scale with values between 0 and 10. The end points were set as “no pain sensation” and “the most intense pain sensation imaginable”.
Testing procedures
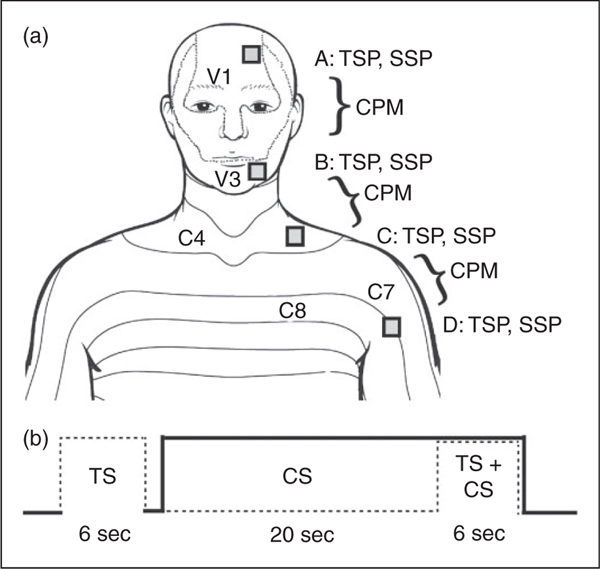
Each subject was invited to a single testing session that lasted approximately 2 hours. Testing took place in a quiet room with temperature maintained at 22 ± 2°C. The subjects sat in a comfortable armchair. All subjects were trained in the measurements prior to the actual testing. Figure 1 describes the experimental protocol. Testing always started with the evaluation of the stimulus-response functions from which the temperatures eliciting 5–6 on the VAS were extracted (see Figure 2). This procedure was done on two trigeminal body regions: the forehead (area A), lower cheek (B) as well as two extra-trigeminal regions: The neck, just above the clavicle (C) and the dorsal surface of the upper arm (D). In each of these regions, the stimulation intensities were adjusted for each subject individually to achieve the desired VAS to account for possible differences in the sensitivity of these regions to noxious stimuli. After a 5 minute break, testing of conditioned pain modulation (CPM), temporal summation of pain (TSP) and spatial summation of pain (SSP) paradigms (see below) commenced in a random order with 5–7 minutes’ break between each test. CPM was tested in the following configurations (in random order): a) A–B; trigeminal-trigeminal (test stimulus in the forehead and conditioning stimulus in the cheek), b) B–C; trigeminal-spinal (test stimulus in the cheek and conditioning stimulus in the lower neck), and c) C–D; spinal-spinal (test stimulus in the lower neck and conditioning stimulus in the arm). A 10cm separation was maintained between the test and conditioning stimulus at each of these configurations in order to neutralize as much as possible any confounding factors related to the distance between the regions (23). TSP and SSP were measured in the same four locations, A–D (Figure 1). The four testing sites were selected due to several considerations: First, it was important to compare CPM between combinations of sites that are within the trigeminal region, include the trigeminal region and outside the trigeminal region. A combination that includes both the intra-trigeminal and extra-trigeminal necessitated a site that is adjacent, that is, the neck area. Second, since it was important to maintain the same separation distance between pairs of regions for the CPM test, the upper arm was chosen to pair with the neck, as the regions outside trigeminal innervation.
Figure 1.
Experimental configuration. (a) In each of the four testing sites, adjusting stimulation temperature was followed by testing of CPM, temporal summation of pain (TSP), and spatial summation of pain (SSP). (b) CPM testing protocol timeline.
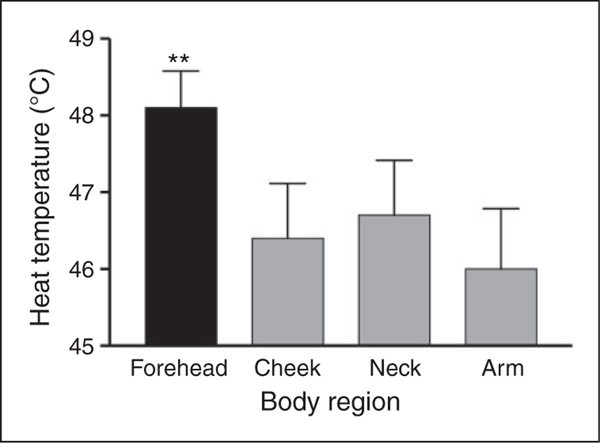
Figure 2.
The heat temperature needed to elicit VAS scores of 5–6 was higher in the forehead than that required in all other three testing regions (**p < 0.01), indicating that this region was the least sensitive to heat-pain. Bars denote group mean temperature ± SEM.
Measurements
Suprathreshold perceived pain intensity:
Perceived pain intensity was measured with the method of magnitude estimation, using a thermal stimulator and a VAS. Subjects received a series of thermal stimuli and were asked to rate their pain following each stimulus on the VAS by moving its inner slider. The stimulus intensities presented in an ascending manner used for magnitude estimation rose (rate of rise 2°C/sec) from a baseline temperature of 35°C to a destination temperature ranging between 37°C to the intensity eliciting 8 on the VAS, at which it remained for 1sec and then returned to baseline. An inter-stimulus interval of at least 30 seconds was maintained to avoid any changes in skin sensitivity and to allow for adequate VAS scoring. A stimulus-response function was obtained, and the temperature eliciting a VAS value of 5–6 was extracted for subsequent testing (24). This stimulus intensity was administered again to verify that a VAS rating of 5–6 was induced, or to further adjust the intensity (slightly increase or decrease the temperature) until such a rating was obtained. This procedure was repeated for each of the four testing regions (A, B, C, and D; Figure 1).
Conditioned pain modulation (CPM):
CPM was measured by applying a noxious stimulus to one body region (the “test stimulus”, TS), and evaluating its perceived intensity alone and in the presence of another noxious stimulus applied to another body region (“conditioning stimulus”, CS). To minimize any attentional biases towards either the TS or the CS, the two stimuli were similar in magnitude, modality, and areal extent; the TS and CS were delivered via the probes of the thermal stimulators at an intensity equivalent to 5–6 on VAS (individually adjusted). The initial TS lasted for 6 seconds and the CS for 26 seconds, so that the second application of the TS (for 6 seconds) occurred after 20 seconds of conditioning (Figure 1(b)). The magnitude of CPM was calculated by subtracting the VAS rating of the test stimulus in the presence of the conditioning stimulus, from the VAS rating of the test stimulus when presented alone (24). CPM was measured three times, in each of the stimulation configurations; A–B, B–C and C–D (see Figure 1(a)). For unifying purposes, and for comparisons with most studies using CPM, the CS was always the distal of the two stimulated body regions.
Spatial summation of pain (SSP):
SSP was measured by applying a noxious stimulus at an intensity equivalent to VAS 5–6 (individually adjusted) to a particular body region, for a duration of 6 seconds. The stimulus was applied twice, once using a stimulation area of 3 × 3 cm (9 cm2) and once using a stimulation area of 1.5 × 1.5 cm (2.25 cm2) with 45 seconds between them. Subjects were instructed to rate the perceived pain evoked by each of the two applications separately. The magnitude of SSP was calculated by subtracting the VAS rating of the small surface from that of the large one (24). SSP was measured within the same four body regions used to evaluate CPM.
Temporal summation of pain (TSP):
TSP was measured by applying a noxious stimulus at an intensity equivalent to VAS 5–6 (individually adjusted) to a particular body region for a duration of 20 seconds. During this time, the subjects were instructed to rate the perceived pain intensity on the VAS at 1, 10 and 15 seconds of application. The magnitude of TSP was calculated by subtracting the last VAS rating from the first one (24). As with the SSP measurements, TSP was also measured within the same regions used to evaluate CPM.
Data analysis
Data were analyzed using SPSS statistic software version 21. Variables were described as means ± SD. Analysis of variance (ANOVA) with corrected two-tailed post hoc t-tests were used to evaluate the effect of body region/stimulation configuration on the magnitude (delta values) of CPM, TSP, and SSP. In addition, pair-wise comparisons between the VAS rating of the TS alone and the TS in the presence of the CS were conducted within each configuration to establish the presence of a significant CPM. Similarly, pair-wise comparisons between the pain rating of the large and the small stimulation areas were used to establish the presence of a significant SSP. Differences in the number of subjects that could not exhibit any positive CPM between the configurations were evaluated with the chi-square test. Repeated measures ANOVA was used to evaluate differences between body regions in the time trend of TSP. Correlations between pairs of variables were calculated using Pearson’s correlation coefficient. p <0.05 was considered significant.
Results
Effect of body region on temperature eliciting VAS 5–6
Figure 2 presents the temperatures needed to elicit VAS scores 5–6 in the four body regions tested. These pain-eliciting temperatures were significantly affected by body region (F(3, 48) = 4.7, p <0.01). Post-hoc analyses revealed that the temperature needed to elicit VAS 5–6 in the forehead was higher than that required in all other three testing regions (p <0.01, for each) indicating that this region was the least sensitive to heat-pain.
Conditioned pain modulation (CPM)
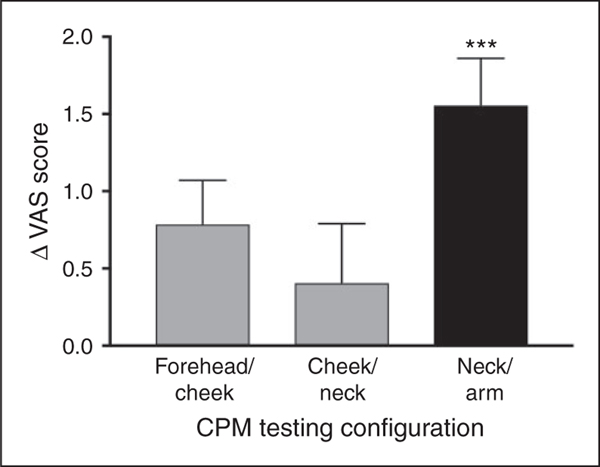
Figure 3 presents the magnitude of CPM (ΔVAS) in the three testing configurations. When the pain rating of the TS alone was compared to that of the TS in the presence of the CS, a significant difference existed only in the extra-trigeminal, arm-neck configuration (5.42 ± 0.66 vs. 3.85 ± 1.74; p < 0.0001), suggesting the presence of a significant CPM. However, the configurations that involved the trigeminal region did not show any significant CPM effect; neither the neck-cheek configuration (5.44 ± 1.10 vs. 5.03 ± 1.70; p = 0.33) nor the cheek-forehead configuration (5.41 ± 0.82 vs. 4.72 ± 1.74; p = 0.12). Thus, these regions did not sustain CPM. In the same vein, we observed differences in the propensity to exhibit CPM between the trigeminal and extra-trigeminal configurations. The relative number of subjects that did not exhibit positive CPM (ΔVAS > 0), namely that were non-responsive, was 2/17 (11.8%) in the extra-trigeminal arm-neck configuration, which was lower than that observed in the neck-cheek and cheek-forehead configurations (8/17 (47.1%) for both; p < 0.05 for both comparisons). One-way ANOVA of CPM magnitudes revealed a borderline effect of configuration (F(2,49) = 2.7, p = 0.075). Corrected post-hoc analyses revealed that CPM magnitude in the extra-trigeminal arm-neck configuration (1.53 ± 1.2 VAS units) was larger than in the configuration that included the trigeminal region (neck-cheek; 0.41 ± 1.6 VAS units, p < 0.05 and cheek-forehead configuration; 0.52 ± 1.3 VAS units, p = 0.07) with the latter two configurations displaying similar CPM magnitudes (p = 0.47).
Figure 3.
Only CPM in the extra-trigeminal configuration (hand-neck) existed, producing a significant pain reduction of the test stimulus by the conditioning stimulus (***p < 0.0001). Bars denote group mean of CPM magnitude (delta between test stimulus alone and test stimulus in the presence of the conditioning stimulus) ± SEM.
Spatial summation of pain
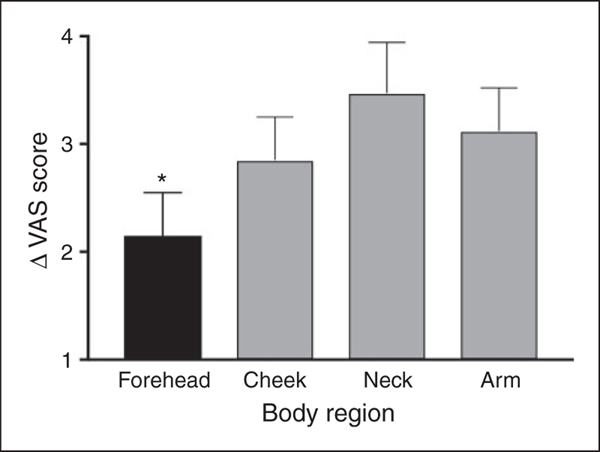
Figure 4 illustrates the magnitude of SSP (ΔVAS for large-small area) in the four tested regions. When the pain rating of the large stimulation area was compared to that of the small area, significant differences existed in all the body regions; the forehead (5.41 ± 0.8 vs. 3.25 ± 2.06; p < 0.0001), cheek (5.44 ± 1.0 vs. 2.58 ± 1.80; p < 0.0001), neck (5.41 ± 0.60 vs. 1.94 ± 2.11; p < 0.0001) and hand (6.01 ± 0.60 vs. 2.12 ± 1.52; p < 0.0001). This suggests the presence of effective SST in both the trigeminal and extra-trigeminal regions. One way ANOVA of the SSP magnitude revealed a significant global effect of body region (F(3,48)=4.03, p < 0.01). Post-hoc analyses revealed that the magnitude of SSP was smaller in the forehead compared to the cheek, neck, and arm (p < 0.05, for each comparison). All other regions were not significantly different from each other, except for a trend towards the cheek having diminished SSP compared to the neck SSP (p = 0.066).
Figure 4.
The magnitude of SSP was smaller in the forehead compared to the cheek, neck, and arm (*p < 0.05 for each comparison). All other regions were not significantly different from each other except for a trend towards the cheek having less SSP compared to the neck (p = 0.066). Bars denote group mean of SSP magnitude (delta between VAS rating of the small and large stimulation areas) ± SEM.
Temporal summation of pain
Repeated measures ANOVA revealed a significant global effect of time on the pain ratings during tonic stimulation (F(2,94)=4.9, p < 0.01) with no effect of body region (F(3,94)=0.25, p = 0.78). The interaction between time and body region was also not significant (F(3,94)=1.31, p = 0.27). This suggests that the change in pain ratings with time occurred in a similar manner among all the tested body regions. One way ANOVA on the magnitude of TSP (delta VAS first-last) also did not yield significant differences between the regions (F(3,94)=1.31, p = 0.27, not shown).
Discussion
The purpose of this study was to test for possible differences in endogenous pain modulation capacities between body regions inside and outside the innervated territory of the trigeminal system. The results showed that CPM did not exist in configurations that included body regions innervated by the trigeminal system, the forehead and the cheek. These regions also had the lowest sensitivity to pain, with the forehead in particular requiring a higher temperature to induce the same level of pain and presenting the least SSP compared to other regions. These results suggest that although the trigeminal region is not uniform in its sensitivity to noxious stimuli, it is subject to less efficient endogenous pain inhibition than other body regions.
The lack of trigeminal-related CPM effect was also manifested as a higher rate of failed (non-responsive) CPM trials inside the trigeminal innervation (47%) compared to failed trials outside the trigeminal innervation (~12%), which further supports the notion that endogenous pain inhibition in the trigeminal region may be inherently weaker compared to those serving extra-trigeminal regions. Our results are supported by a recent study in which a longitudinal heat pain paradigm, conducted over eight consecutive days, induced a stronger increase in pain ratings when the stimuli were administered to the forehead compared to the forearm, suggesting a greater tendency for sensitization in the trigeminal region (22). Our CPM findings are further supported by the study of Aymanns et al. (25), which demonstrated inhibition of the trigeminal blink reflex by homotopic low frequency stimulation at the forehead but not after a similar heterotopic stimulation in infraorbital and mental nerve areas, indicating lack of suppressive effects of heterotopic stimulation within one side of the face.
A few studies compared CPM within versus outside the trigeminal regions in healthy individuals; however, the conditioning stimulus was always applied outside the trigeminal region. In these studies, CPM was similar whether the test stimulus was applied at, or outside, the trigeminal region (14–18). These studies thus partly contradict our data showing the lack of CPM when the conditioning stimulus was applied to the neck (i.e. outside the trigeminal region) while the test stimulus was applied to the trigeminal-innervated cheek. The use of different stimulation modalities for the conditioning (14,15,17,18) or the test stimulus (14,15,17) than those used in our study, and for much longer durations, may explain the different results. For example, Oono et al. (16) employed a prolonged conditioning stimulus of 30 minutes’ duration to induce a trigeminal-related CPM effect, which may reinforce the probability of CPM and its magnitude. Thus CPM in the trigeminal regions may indeed be weak, but protocols that enhanced CPM may, in some instances, evoke such a response in this region. It is noteworthy that in the study of Youssef et al. (18), the conditioning stimulus elicited CPM but only to a mechanical test stimulus applied to the cheek region, and not to a similarly-applied thermal test stimulus, thus supporting our claim. Taken together, while previous studies suggest that trigeminal CPM could be elicited, it likely requires a much more prolonged conditioning, and to only a subset of test stimulation modalities (mechanical but not thermal), all of which support the notion that the trigeminal regions is subjected to a less efficient pain modulation that relies on DNIC neural circuitry.
Several experimental confounding factors may have contributed to the regional differences observed in CPM. First, CPM can be influenced by the distance between the test and conditioning stimuli (23); however, these distances were identical among all the configurations. Second, the longitudinal location of the test versus the conditioning may also affect CPM intensity. However, the conditioning stimulus was always located distally to the test stimulus in all the CPM configurations. Third, although the intensity of the test and conditioning stimuli may affect CPM, these were similarly adjusted to achieve a VAS rating of 5–6 so that the painfulness of these stimuli was similar across the regions. Indeed, a higher temperature was needed to elicit VAS rating of 5–6 in the forehead than other regions. Nevertheless, a failed CPM was also observed in the cheek-neck configuration, in which the temperatures of the test stimuli was the same as those used in the extra-trigeminal configuration. Fourth, it may be argued that the lack of CPM in the forehead-cheek configuration was due to the conditioning stimulus not being extra-segmental to the test stimulus. However, this possibility is also unlikely considering that extra-segmental arrangement in the neck-cheek configuration also failed to evoke CPM. Taken together, factors related to the experimental paradigm were unlikely to underlie the inefficient CPM detected in the trigeminal innervation territory.
The deficient trigeminal CPM we observed may be due to neural as well as behavioral mechanisms. Considering that CPM partly reflects the capacity of descending pain inhibition mediated via the brainstem, such as DNIC, the descending control of information arriving from the trigeminal regions may be weaker than that of the rest of the body (26). Additionally, the representation of the trigeminal region within brainstem structures involved in pain modulation may be limited compared to that of the soma (26). Alternatively, but not mutually exclusive, is the possibility that the spatial organization of nociceptive information in the brainstem is different than that in the spinal cord (27). Further work, however, is required to determine whether the poor trigeminal CPM we identified using cutaneous thermal stimuli also applies to the modulation of pain arising from deeply-innervated tissues such as the meninges and muscles (for headache pain) or joints (for TMD pain).
With respect to the behavioral mechanism, previous studies suggest that stimuli applied to the face area may be more threatening. For example, when the hand was placed inside the peripersonal space of the face, the blink reflex was increased in magnitude compared to when the hand was placed outside (28). Although we did not use the defensive blink reflex as an index, subjects may have perceived the stimuli to the forehead and cheek as more threatening than the stimuli applied to the neck or upper arm. This may have counteracted the inhibitory capacity of the conditioning stimuli. In the same vein, attention may affect the magnitude of CPM (e.g. (23,29) and stimulation of the face may increase the attention of the subject to the test stimuli, thus weakening CPM.
Growing evidence supports the concept that chronic pain is related to dysfunction in descending pain inhibition. Diminished pain inhibition, as evaluated using the CPM paradigm, was indeed found among chronic pain patients of various etiologies, including those associated with trigeminal innervations. Thus, patients with trigeminal neuralgia (3), temporomandibular disorder (30,31), headache (4–7) and persistent postendodontic pain (8) failed to exhibit significant CPM. Recent imaging studies done on patients with migraine and trigeminal neuralgia reveal altered neural connectivity (32,33) and structural cortical and subcortical changes (34,35) in neuroanatomical regions that were implicated in reduced pain (36,37). The results of the present study conducted in healthy, pain-free subjects raise the possibility that pain inhibition capability is inherently weak in the trigeminal region compared to other body regions. Thus, the trigeminal nociceptive system, due to its weak inhibitory control, may be more susceptible than the spinal nociceptive system to change under pathophysiological conditions: It is possible that once a pathological process takes place in structures related to the trigeminal system, the already weak inhibition fails to restrain the emergence and chronification of the pain. The inherent susceptibility of the trigeminal region may also be the reason why medication overuse often results in headache that is also associated with dysfunctional inhibitory control (38).
Interestingly, the weak CPM we found in the trigeminal region was neither associated with enhanced TSP or SSP in this region, nor with increased sensitivity to noxious stimuli. These finding imply that reduced inhibitory control does not necessitates the presence of increased pain excitability and sensitivity, at least under normal conditions. Previous studies assessing these traits did not consistently find correlations between them among healthy subjects (24). It thus seems that the inhibitory and excitatory components of the pain modulatory system operate in parallel to each other, or that yet unknown factors mediate between these components. Although the inherently weak CPM in the trigeminal regions may not necessitates enhanced excitability in normal conditions (as herein), it may contribute to enhanced excitability in pathological conditions, as frequently found in chronic pain conditions.
The results of the study should be considered in light of several limitations. First, as CPM was measured with the conditioning stimulus applied distal to the test stimulus, further studies are needed to verify the weaker trigeminal inhibitory control by testing CPM also in configurations where the conditioning stimulus is applied proximal to the test stimulus. Second, testing an additional experimental paradigm that evaluates descending inhibition is warranted to substantiate the pain modulation profile of the trigeminal region. Third, assessing the amount of threat subjects perceive during stimulation of the face as opposed to other body regions may help to identify behavioral confounds when testing the trigeminal region. In summary, the findings point to a regional difference in endogenous pain inhibition and suggest that the trigeminal innervation is subjected to a weaker inhibitory pain control than other body regions. Weaker pain control could play a role in mediating the high prevalence of trigeminal-related pain syndromes, including primary headaches and TMD pain.
Key findings.
Systematic comparisons of endogenous pain modulation capabilities using quantitative sensory testing revealed differences between trigeminal and extra-trigeminal regions in healthy individuals.
Conditioned pain modulation (CPM) in trigeminal innervated areas is weaker when compared to CPM in extra-trigeminal body regions.
The magnitude spatial summation of pain (SSP) in the ophthalmic trigeminal innervation is smaller compared to that observed in the mandibular and extra-trigeminal innervation territories.
Weaker trigeminal CPM is not associated with enhanced temporal summation of pain (TSP).
Weaker control of trigeminal pain could play, at least in part, a role in mediating the high prevalence of trigeminal-related pain syndromes, including primary headaches and temporomandibular (TMD) pain.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported in part by grants from the NIH/NINDS (NS086830, NS078263 to DL).
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.US-CDC. Health, United States, 2016; Charterbook on Trends in the Health of Americans, https://www.cdc.gov/nchs/data/hus/hus16.pdf#listfigures. [Google Scholar]
- 2.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonard G, Goffaux P, Mathieu D, et al. Evidence of descending inhibition deficits in atypical but not classical trigeminal neuralgia. Pain 2009; 147: 217–223. [DOI] [PubMed] [Google Scholar]
- 4.Perrotta A, Serrao M, Ambrosini A, et al. Facilitated temporal processing of pain and defective supraspinal control of pain in cluster headache. Pain 2013; 154: 1325–1332. [DOI] [PubMed] [Google Scholar]
- 5.Defrin R, Riabinin M, Feingold Y, et al. Deficient pain modulatory systems in patients with mild traumatic brain and chronic post-traumatic headache: Implications for its mechanism. J Neurotrauma 2015; 32: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pielsticker A, Haag G, Zaudig M, et al. Impairment of pain inhibition in chronic tension-type headache. Pain 2005; 118: 215–223. [DOI] [PubMed] [Google Scholar]
- 7.Sandrini G, Rossi P, Milanov I, et al. Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia 2006; 26: 782–789. [DOI] [PubMed] [Google Scholar]
- 8.Nasri-Heir C, Khan J, Benoliel R, et al. Altered pain modulation in patients with persistent postendodontic pain. Pain 2015; 156: 2032–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain 2008; 138: 22–28. [DOI] [PubMed] [Google Scholar]
- 10.Nahman-Averbuch H, Granovsky Y, Coghill RC, et al. Waning of “conditioned pain modulation”: A novel expression of subtle pronociception in migraine. Headache 2013; 53: 1104–1115. [DOI] [PubMed] [Google Scholar]
- 11.Weissman-Fogel I, Sprecher E, Granovsky Y, et al. Repeated noxious stimulation of the skin enhances cutaneous pain perception of migraine patients in-between attacks: Clinical evidence for continuous sub-threshold increase in membrane excitability of central trigeminovascular neurons. Pain 2003; 104: 693–700. [DOI] [PubMed] [Google Scholar]
- 12.Chapman CR and Vierck CJ. The transition of acute postoperative pain to chronic pain: An integrative overview of research on mechanisms. J Pain 2017; 18: 359.e1–359.e38. [DOI] [PubMed] [Google Scholar]
- 13.Kosek E and Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain 2000; 88: 69–78. [DOI] [PubMed] [Google Scholar]
- 14.Ellrich J and Treede RD. Characterization of blink reflex interneurons by activation of diffuse noxious inhibitory controls in man. Brain Res 1998; 803: 161–168. [DOI] [PubMed] [Google Scholar]
- 15.Rehberg B, Baars JH, Kotsch J, et al. Comparison of trigeminal and spinal modulation of pain and nociception. Int J Neurosci 2012; 122: 298–304. [DOI] [PubMed] [Google Scholar]
- 16.Oono Y, Baad-Hansen L, Wang K, et al. Effect of conditioned pain modulation on trigeminal somatosensory function evaluated by quantitative sensory testing. Pain 2013; 154: 2684–2690. [DOI] [PubMed] [Google Scholar]
- 17.Kothari SF, Baad-Hansen L, Oono Y, et al. Somatosensory assessment and conditioned pain modulation in temporomandibular disorders pain patients. Pain 2015; 156: 2545–2555. [DOI] [PubMed] [Google Scholar]
- 18.Youssef AM, Macefield VG and Henderson LA. Pain inhibits pain; human brainstem mechanisms. Neuroimage 2016; 124: 54–62. [DOI] [PubMed] [Google Scholar]
- 19.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010; 23: 611–615. [DOI] [PubMed] [Google Scholar]
- 20.Herrero JF, Laird JM and Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: Much ado about something? Prog Neurobiol 2000; 61: 169–203. [DOI] [PubMed] [Google Scholar]
- 21.Coste J, Voisin DL, Luccarini P, et al. A role for wind-upin trigeminal sensory processing: Intensity coding of nociceptive stimuli in the rat. Cephalalgia 2008; 28: 631–639. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt K, Schunke O, Forkmann K, et al. Enhanced short-term sensitization of facial compared with limb heat pain. J Pain 2015; 16: 781–790. [DOI] [PubMed] [Google Scholar]
- 23.Defrin R, Tsedek I, Lugasi I, et al. The interactions between spatial summation and DNIC: Effect of the distance between two painful stimuli and attentional factors on pain perception. Pain 2010; 151: 489–495. [DOI] [PubMed] [Google Scholar]
- 24.Gruener H, Zeilig G, Laufer Y, et al. Differential pain modulation properties in central neuropathic pain after spinal cord injury. Pain 2016; 157: 1415–1424. [DOI] [PubMed] [Google Scholar]
- 25.Aymanns M, Yekta SS and Ellrich J. Homotopic long-term depression of trigeminal pain and blink reflex within one side of the human face. Clin Neurophysiol 2009; 120: 2093–2099. [DOI] [PubMed] [Google Scholar]
- 26.Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Brain Res Rev 2002; 40: 29–44. [DOI] [PubMed] [Google Scholar]
- 27.Dubner R and Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci 1983; 6: 381–418. [DOI] [PubMed] [Google Scholar]
- 28.Sambo CF, Liang M, Cruccu G, et al. Defensive peripersonal space: The blink reflex evoked by hand stimulation is increased when the hand is near the face. J Neurophysiol 2012; 107: 880–889. [DOI] [PubMed] [Google Scholar]
- 29.Moont R, Pud D, Sprecher E, et al. ‘Pain inhibits pain’ mechanisms: Is pain modulation simply due to distraction? Pain 2010; 150: 113–120. [DOI] [PubMed] [Google Scholar]
- 30.Oono Y, Wang K, Baad-Hansen L, et al. Conditioned pain modulation in temporomandibular disorders (TMD) pain patients. Exp Brain Res 2014; 232: 3111–3119. [DOI] [PubMed] [Google Scholar]
- 31.King CD, Wong F, Currie T, et al. Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain 2009; 143: 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mainero C, Boshyan J and Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 2011; 70: 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Liu M, Lan L, et al. Altered periaqueductal grayresting state functional connectivity in migraine and the modulation effect of treatment. Sci Rep 2016; 6: 20298. doi: 10.1038/srep20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeSouza DD, Davis KD and Hodaie M. Reversal of insular and microstructural nerve abnormalities following effective surgical treatment for trigeminal neuralgia. Pain 2015; 156: 1112–1123. [DOI] [PubMed] [Google Scholar]
- 35.Desouza DD, Moayedi M, Chen DQ, et al. Sensorimotor and pain modulation brain abnormalities in trigeminal neuralgia: A paroxysmal, sensory-triggered neuropathic pain. PLoS One 2013; 8: e66340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schweinhardt P and Bushnell MC. Pain imaging in health and disease – how far have we come? J Clin Invest 2010; 120: 3788–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis KD and Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol 2013; 8: 518–534. [DOI] [PubMed] [Google Scholar]
- 38.Michels L, Christidi F, Steiger VR, et al. Pain modulationis affected differently in medication-overuse headache and chronic myofascial pain – a multimodal MRI study. Cephalalgia 2017; 37: 764–779. [DOI] [PubMed] [Google Scholar]