Abstract
Messenger RNAs (mRNAs) that contain U-rich elements are targeted for rapid decay. Selective inhibition of this decay results in a rapid increase in steady state level. Thus, this is an important regulatory step in gene expression. Previously, we have found that these mRNAs are selectively stabilized by a specific mRNA binding protein called HuR. The mechanism of action of HuR is not well understood. It has been postulated that HuR stabilizes mRNA by the displacement or inhibition of factors that specifically cleave or deadenylate these mRNAs. In this paper, we report the identification and characterization of a novel endonuclease that cleaves within an HuR binding site in p27kip1 mRNA. The specificity of this endonuclease and its inhibition by HuR argue for it playing a role in the postranscriptional regulation of gene expression.
INTRODUCTION
The regulation of mRNA turnover in mammalian cells is a critical, yet poorly understood step in gene expression (1,2). Many short-lived mRNAs contain cis-acting elements that direct their rapid turnover. The first cis-acting sequence to be described was the U-rich or Shaw–Kamen element (3–5). In unstimulated cells, mRNAs that contain U-rich elements rapidly disappear. Upon appropriate stimulation, a specific mRNA–protein complex is formed, decay is blocked and a rapid rise in the steady state level of the mRNA ensues (6–9). Recently, significant insight has been gained into the identity of factors that stabilize the expression of U-rich element containing mRNAs. It has been found that the hypoxia induced stabilization of VEGF mRNA, the DNA damage induced stabilization of p21waf1 mRNA, the cell cycle regulation of cyclin mRNA and the stability of U-rich element reporters are mediated by a specific mRNA binding protein called HuR (10–16). HuR is a widely expressed member of the human family of Elav-like proteins, which were first discovered as tumor antigens (17–19). HuR contains three highly conserved RNA recognition motifs. The first and second domains are required for the specific recognition of the U-rich element, whereas the third domain binds to the poly(A) tail of mRNA (20–22). HuR is a nuclear protein; however, it can shuttle between the nucleus and the cytoplasm (12,13,23,24). Indeed, it is possible that the nucleo-cytoplasmic export of HuR may be intrinsically coupled to the stabilization of mRNA (14).
The precise mechanism of action of HuR, however, remains unclear. To further elucidate the mechanism of HuR, it is crucial to understand the decay pathway directed by the U-rich elements. There are two schools of thought (25–27). In the first, it is posited that a specific endonuclease cleaves the mRNA within the U-rich element. It is envisaged that the cleaved mRNA would then be susceptible to an exonuclease. In the second model, it is thought that an AU-rich element dependent deadenylase shortens the poly(A) tail and renders the main body mRNA susceptible to an exonuclease (25). Indeed, in vivo, the first discernable step in U-rich element directed decay is the shortening of the poly(A) tail. Thus, it is more likely that the decay process is initiated by a U-rich element dependent deadenylase. The development of an in vitro system that reconstituted U-rich element directed mRNA decay permitted the direct identification of decay factors (28). On purification, the catalytic decay activity was not retained; however, a specific U-element binding protein (AUF1) was identified (29). Although AUF1 does not appear to be either a specific endonuclease or a U-rich element dependent deadenylase, it is clearly involved in mRNA decay (30,31). Indeed, it is likely that AUF1 is an essential subunit of an mRNA decay complex.
We anticipate that HuR stabilizes mRNA by the inhibition or displacement of such decay complexes. In this paper, we report the identification and characterization of a specific endonuclease that cleaves mRNA within an HuR binding site. We show that this endonuclease is inhibited by HuR and suggest that it may be a trans-acting factor responsible for U-rich element directed mRNA decay.
MATERIALS AND METHODS
Materials
HeLa-S3 cells were obtained from National Cell Culture Center (Minneapolis). RNAs were synthesized by IDT Technologies (Bethesda, MA).
Preparation of HeLa cell extracts
HeLa cells were resuspended in hypotonic buffer [25 mM Tris (pH 7.5), 5 mM MgCl2, 10 mM NaCl, 2 mM DTT, 10 µg/ml leupeptin, 10 µg/ml aprotinin and 1 mM PMSF]. After incubation on ice for 10 min, cells were broken by Dounce homogenizer (10 strokes, ‘B’ pestle). After a further 10 min incubation on ice, the homogenate was centrifuged at 300 g for 10 min at 4°C. The supernatant ‘cytoplasmic fraction’ was removed and stored at –80°C. Further extracts were made by sequential extraction of the pellet with hypotonic buffer containing 40, 150, 450 and 1000 mM NaCl, respectively. In each case the homogenate was centrifuged at 15 000 g for 30 min. All fractions were stored at –80°C.
Preparation of labeled RNA
RNAs were labeled at the 5′ end using T4 polynucleotide kinase and [γ-32P]ATP. Reaction mixtures (20 µl) contained 50 mM Tris (pH 7.5), 5 mM MgCl2, 2.5 µM RNA, 50 µCi of [γ-32P]ATP (6000 Ci/mmol, Pharmacia, NJ) and T4 polynucleotide kinase (500 U/ml). Reactions were incubated at 37°C for 30 min. The labeled RNA was gel purified.
RNAs were labeled at the 3′ end using T4 RNA ligase and 5′-[32P]pCp. Reaction mixtures (20 µl) contained 50 mM Tris (pH 7.8), 10 mM MgCl2, 10 mM DTT, 1 mM ATP, 2.5 µM RNA, 50 µCi of 5′-[32P]pCp (6000 Ci/mmol) and T4 RNA ligase (1000 U/ml). Reactions were incubated at 37°C for 1.5 h. The labeled RNA was then gel purified.
Footprint analysis
Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.0), 500 µg/ml BSA, 500 µg/ml tRNA, 5 mM MgCl2, 50 µg/ml tRNA, 3 nM of labeled RNA and GST (negative control) or GST-HuR as indicated. Mixtures were pre-incubated at 37°C for 10 min and RNase T1 or lead acetate was then added to a final concentration of 0.5 U/ml or 10 mM, respectively. Reactions were further incubated for 10 min and then stopped by the addition of 40 µl of Stop Solution and one-tenth of the reaction was analyzed by 10% polyacrylamide/urea gel electrophoresis.
Endonuclease assay
Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.0), 100 mM NaCl, 5 mM MgCl2, 50 µg/ml tRNA, 3 nM RNA and protein extract as indicated. After 30 min of incubation at 37°C, 40 µl of Stop Solution (United States Biochemical, OH) containing formamide (final concentration 80%) was added to each reaction. After heating at 60°C for 5 min, one-tenth of each reaction mixture was analyzed by electrophoresis on a 10% polyacrylamide/50% urea gel. The gel was fixed in 1:8 acetic acid:water, dried and exposed to the XAR5 film at –80°C.
RESULTS
The identification of an endonuclease that cleaves adjacent to the HuR binding site
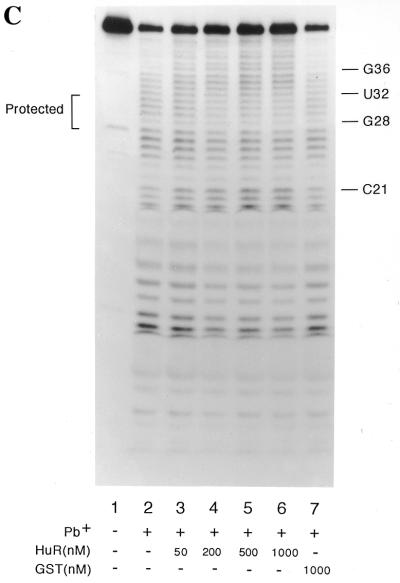
The 42 nt RNA that contains the HuR binding site used in these studies is derived from p27KIP1 mRNA (the entire sequence of the 42 nt oligomer is shown in Fig. 1A). p27kip1 encodes an inhibitor of cyclin dependent protein kinases and thus is an important negative regulator of cell growth (32,33). In previous studies, we identified an HuR binding site in the 5′-UTR of p27KIP1 mRNA (34). In the present study, we have further defined this HuR binding site using RNase T1 and lead footprint analysis. Figure 1B shows that the addition of HuR to the 42 nt RNA resulted in the hyposensitivity of residues G28, G23 and G18 and the hypersensitivity of residues G40, G38 and G36 to RNase T1 hydrolysis. The effects seen were proportional to the concentration of HuR and no effect was evident on addition of GST (Fig. 1B, lane 7). Protection against RNase T1 yields an exaggerated contact site since RNase T1 is a rather large molecule. To further define the minimal HuR/RNA contact site, we performed footprint analysis using lead hydrolysis. In this case, the addition of HuR resulted in the protection of residues G28, U29, U30, U31 and U32 (Fig. 1C, lanes 3–6). Again, hypersensitivity to lead hydrolysis was observed at residues U33 to G38. Thus, we concluded that the minimal HuR contact site encompasses residues G28 to U32.
Figure 1.
Sequence and footprint analysis of the HuR binding site. (A) The sequence of RNA oligomer used in these studies. The dots and upper arrows indicate residues that are hypo- and hyperhydrolysed respectively. The large arrow indicates the endonuclease cleavage site. (B) RNase T1 footprint analysis of the HuR binding site. Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 50 µg/ml tRNA, 3 nM labeled RNA (801 200 c.p.m./pmol) and purified GST or GST-HuR as indicated. Mixtures were incubated at 37°C for 10 min and RNase T1 was then added to a final concentration of 0.5 U/ml and the mixture further incubated for 10 min at 4°C. Reactions were then terminated and the reaction was analyzed by 10% polyacrylamide/urea gel electrophoresis. (C) Lead footprint analysis of the HuR binding site. Reactions were conducted as above except that lead acetate (10 mM) was substituted for RNase T1 after the pre-incubation.
Next, we sought to identify endonucleases that may cleave within the HuR binding site. Towards this end, we prepared a series of extracts from HeLa cells. One cannot anticipate the cellular location of a putative activity, so we fractionated HeLa cells into cytoplasmic and nuclear fractions and then the nuclear pellet was sequentially extracted with 40, 150, 450 mM and 1 M NaCl. This approach has the advantage that potential inhibitors may be adventitiously removed. The 42 nt RNA was incubated with each of these extracts and the products analyzed by gel electrophoresis. Figure 2 shows that incubation with the cytoplasmic extract resulted in significant cleavage to a 26 nt species. No such endonuclease activity was detectable in any of the other extracts. However, when assaying crude extracts, lack of an activity may be due to the presence of inhibitors. Thus, we investigated whether there were any specific inhibitors in the inactive extracts. To do this, we mixed the inactive fractions with the active cytoplasmic fraction and assayed for endonuclease activities. Figure 3 shows that there is indeed an inhibitory factor present in the 40 mM NaCl extract. This factor is quite potent in that 15 ng of the extract is sufficient for half-maximal inhibition of the endonuclease (Fig. 3B). Thus, we cannot conclude whether there is endonuclease activity in the 40 mM NaCl extracts. More importantly, however, these observations may explain why such specific endonucleases have been difficult to identify. In our case, we had fortuitously removed an inhibitor by fractionation. The inhibitory factor does not appear to be HuR, since the vast majority of HuR is present in the 450 mM salt extract. Thus, it may be a new mRNA stability factor. However, more definitive proof of this will require further characterization and purification.
Figure 2.

The identification of an endonuclease activity in extracts from HeLa cells. Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 50 µg/ml tRNA, 3 nM labeled RNA (801 200 c.p.m./pmol) and volume equivalents of the extracts as indicated. Lane 1 contained no extract, lanes 2–6 contained 300, 15, 30, 40, and 4 ng of cytoplasmic, 40, 150 and 450 mM salt and 1 M salt extracts, respectively. The reaction was incubated at 37°C for 30 min and then immediately analyzed by electrophoresis on a 10% polyacrylamide/50% urea gel. In lane T2, substrate RNA has been partially hydrolyzed with RNase T2 to serve as a marker.
Figure 3.
A factor in the 40 mM NaCl extract inhibits the endonuclease activity. (A) Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 50 µg/ml tRNA, 3 nM labeled RNA (801 200 c.p.m./pmol) and 300 ng of cytoplasmic extract (lanes 2–6). Reactions were incubated at 37°C for 30 min in the presence of 40 mM (30 ng, lane 3), 150 mM (60 ng, lane 4), 450 mM (80 ng, lane 5) or 1000 mM (8 ng, lane 6) extracts. (B) Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 50 µg/ml tRNA, 3 nM labeled RNA (801 200 c.p.m./pmol) and 300 ng of cytoplasmic extract (lanes 2–8). Reaction mixtures were incubated at 37°C for 30 min with the indicated amounts of 40 mM NaCl extract. In lane T2, substrate RNA has been partially hydrolyzed with RNase T2 to serve as a marker.
Properties of the endonuclease activity
The endonuclease activity displayed a clear time course with significant cleavage after only 4 min of incubation (Fig. 4A). The amount of the 26 nt cleavage product was also proportional to the amount of extract added (Fig. 4B). Pre-treatment of the extract with microccocal nuclease (35) did not affect the endonuclease activity but it was quantitatively inactivated by treatment with heat, SDS or SDS and proteinase K (Fig. 5A). Thus, it is unlikely that the activity requires an essential RNA component. The reaction proceeded in the absence of Mg2+, but was markedly stimulated by 5 mM Mg2+ (Fig. 5B). The Mg2+ independent activity was unaffected by the addition of 10 mM EDTA (data not shown). The reaction was unaffected by the addition of ATP (data not shown). The activity was not significantly stimulated by the addition of salt (NaCl), but was inhibited by salt concentrations in excess of 100 mM (Fig. 5C). These properties indicated to us that the endonuclease activity is proteinacious in nature.
Figure 4.
The endonucleolytic activity is proportional to the time of incubation and the concentration of the extract. (A) Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 50 µg/ml tRNA, 3 nM of labeled RNA (801 200 c.p.m./pmol) and 300 ng of cytoplasmic extract (lanes 2–8). Reactions were incubated at 37°C for the time indicated. (B) Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 50 µg/ml tRNA, 3 nM labeled RNA (801 200 c.p.m./pmol) and the indicated amount of the cytoplasmic extract. Reactions were incubated at 37°C for 30 min. In lane T2, substrate RNA has been partially hydrolyzed with RNase T2 to serve as a marker.
Figure 5.



Properties of the endonuclease activity. (A) Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 50 µg/ml RNA, 3 nM labeled RNA (801 200 c.p.m./pmol) and no extract (lane 1), 300 ng of cytoplasmic extract (lane 2) or 300 ng of cytoplasmic extract pre-treated with: (lane 3), 40 U/ml of micrococcal nuclease at 37°C for 30 min followed by the addition of EGTA to inactivate the micrococcal nuclease (34); (lane 4), 100 µg/ml of proteinase K at 37°C for 30 min in the presence of 0.1% of SDS; (lane 5), 0.1% SDS at 37°C for 30 min; (lane 6) incubation at 100°C for 2 min; (lane 7) incubation at 37°C for 30 min. That the micrococcal nuclease was active under these conditions is demonstrated by the quantitative hydrolysis of the RNA substrate (lane M). In lane T2, substrate RNA has been partially hydrolyzed with RNase T2 to serve as a marker. (B) Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.5), 100 mM NaCl, 50 µg/ml tRNA, 3 nM of labeled RNA (801 200 c.p.m./pmol), 300 ng of cytoplasmic extract (lanes 2–7) and the indicated concentration of MgCl2. (C) Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.5), 5 mM MgCl2, 50 µg/ml tRNA, 3 nM labeled RNA (801 200 c.p.m./pmol), 300 ng of cytoplasmic extract (lanes 2–10) and the indicated concentration of NaCl. In lane T2, substrate RNA has been partially hydrolyzed with RNase T2 to serve as a marker.
Characterization of the reaction product
There was a possibility that the activity that we had detected was not an endonuclease but the product of ‘stalled’ exonuclease activity. One could envisage that a 3–5 exonuclease may progressively remove nucleotides from the 3′ end but then stall at some secondary structure yielding a fragment that would resemble a cleavage product. A bona fide endonuclease should therefore produce two fragments from one cleavage. In the experiments above the RNA is labeled at the 5′ end and thus the 3′ fragment cannot be seen. To further characterize the putative endonuclease activity, we labeled the RNA at the 3′ end using [32P]pCp and RNA ligase. If indeed our activity was an endonuclease the 3′ fragment of the cleavage should now be seen. Moreover it should be 17 nt since the uncleaved substrate is 43 nt (the substrate is 1 nt longer due to the labeling with [32P]pCp) and the cleavage occurs at nucleotide 26. Incubation of the 3′-labeled substrate with the extract resulted in the formation of a 17 nt product (Fig. 6). Thus, we conclude that the activity is indeed an endonuclease. Next, we sought to determine the nature of the termini left by the endonuclease. To do this, we used the 3′ end-labeled substrate and tested whether the 5′ cleavage site was sensitive to phosphomonoesterase. Such sensitivity would indicate that the endonuclease had left 5′ phosphate and 3′-OH termini. Prior to the reaction, we removed the 3′ phosphate from the 3′-labeled RNA so that the sensitivity of this phosphate would not confound our analysis (the RNA is still labeled since it is the phosphate in the phospodiester bond that it is labeled). The 17 nt reaction product was gel purified and treated with calf intestine phosphatase. There was no difference in the migration of the reaction product incubated with and without phosphatase (Fig. 6B). This was not because that the phosphatase was inactive, since qualitative removal of the phosphate from the substrate RNA was achieved under the same conditions (Fig. 6B, lanes 1 and 2). Thus, we conclude that the endonuclease cleaves the RNA to yield 3′ phosphate and 5′-OH termini. There is a possibility that the 5′ terminus is a phosphate but was removed from the reaction product by a phosphatase activity in the extract. However, we think it is unlikely since we see only the reaction product, there is no sign of a precursor species. In view of the fact that a phosphate is present at the 3′ terminus, we can now make a more accurate determination of the cleavage site. The most accurate size markers will be produced by partial digestion of the RNA with enzymes that leave a 3′ phosphate. In Figure 6C, the migration of the reaction product from 5′ end-labeled RNA is compared with ‘ladders’ made by partial digestion of the RNA with RNase T1 and RNase T2. From this, we conclude that the endonuclease cleaves after U26 (Fig. 1).
Figure 6.
Analysis of the endonuclease reaction product. (A) Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 50 µg/ml tRNA, 3 nM 3′ end-labeled RNA and extract (300 ng) as indicated. In lane T2, substrate RNA has been partially hydrolyzed with RNase T2 to serve as a marker. (B) The 3′ end-labeled RNA (….GpUpGp*Cp ) was first treated with phosphomonoesterase to remove the 3′ phosphate yielding GpUpGp*C. This was then incubated with cytoplasmic extract as described above. The reaction product was isolated and incubated at 37°C for 1 h in the absence (lane 3) or presence (lane 4) of calf intestine phosphatase. As a positive control, the 3′ end-labeled substrate RNA (…..GpUpGp*Cp) was incubated in the absence (lane 1) or presence (lane 2) of calf intestine phosphatase under the same conditions. (C) The 5′ labeled RNA was incubated with extract as described above and the reaction product (lane 3) was compared with a partial RNase T1 (lane 1) and RNase T2 (lane 2) digest.
Specificity of the endonuclease activity
The endonuclease that we have identified cleaves at U26. This is quite remarkable since the sequence that surrounds the cleavage site (GUUUUG) is also reiterated at nucleotides 13–18, yet this site is not cleaved. To further investigate the specificity of the endonuclease, we synthesized a mutant RNA in which residues 23–28 were changed from GUUUUG to GAAAAC (Fig. 1). No cleavage at A26 of the mutant RNA was observed. However, at high extract concentration, cleavage at the upstream GUUUUG (U16) was observed (Fig. 7). Thus, from these observations we concluded that the core recognition signal might be GUUUUG in the appropriate context. We did not investigate the sequence specificity of the endonuclease in more detail. More definite analysis of the sequence specificity will require the purification of the endonuclease.
Figure 7.
Mutation of the GUUUUG motif to GAAAAC abrogates cleavage by the endonuclease activity. Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 50 µg/ml tRNA, 3 nM of 5′ end-labeled wild-type (lanes 1–4) or mutant (lanes 5–8) RNA and cytoplasmic extract as indicated. In lane T1, substrate RNA has been partially hydrolyzed with RNase T1 to serve as a marker.
The endonuclease is inhibited by HuR
So far we have identified and characterized a specific endonuclease that cleaves mRNA within an HuR binding site. Next, we sought to determine if the action of the endonuclease would be blocked by HuR. Thus, we added purified recombinant HuR to the cytoplasmic extracts and monitored its effect on the endonuclease activity. This experiment is possible since there is very little endogenous HuR in the cytoplasmic fraction. Figure 8 shows that the efficiency of cleavage was significantly blocked on addition of 200 nM HuR. On the other hand, no effect was observed on addition of 1000 nM GST.
Figure 8.
HuR inhibits the endonuclease activity. Reaction mixtures (10 µl) contained 50 mM Tris (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 50 µg/ml tRNA, 3 nM labeled RNA (801 200 c.p.m./pmol). Reactions were pre-incubated in the absence or presence of the indicated concentrations of GST-HuR or GST at 37°C for 10 min. After 10 min cytoplasmic extract (300 ng) was added to the indicated reactions. In lane T2, substrate RNA has been partially hydrolyzed with RNase T2 to serve as a marker.
DISCUSSION
HuR increases the half-life of mRNAs that contain U-rich decay elements (11–14). Our current model for the action of HuR is that it displaces or inhibits trans-acting factors that catalyze the U-rich element directed mRNA decay (11). To date there has been little information on trans-acting decay factors that may be displaced by HuR. In this report, we have identified and characterized a novel RNA endonuclease that cleaves within an HuR binding site in p27KIP1 mRNA. The specificity of this endonuclease and the observation that its cleavage efficiency is significantly blocked by HuR, argue that it plays a role in mRNA decay. However, definite proof of this must await the purification of the activity and cloning of the gene(s) that encode it.
In the interim, however, it is useful to consider the current biochemical model of U-rich element directed mRNA decay in the light of the current observation. Two models seek to explain the U-rich element directed turnover of mRNA. The first model postulates that a specific endonuclease cleaves the U-rich element and the cleaved mRNA becomes susceptible to exonucleases. The major obstacle for this model has been the difficulty in identifying the putative endonuclease. Thus, the most significant contribution of the present study is the identification of a specific endonuclease. Moreover, our observation that there are potent inhibitors of the endonuclease suggests why such endonucleases may have been elusive. In addition, this observation may facilitate the identification of other specific endonucleases involved in the U-rich element directed mRNA decay.
The second model proposes that there is a U-rich element directed deadenylase. Following the removal of the poly(A) tail, the mRNA is rendered susceptible to exonuclease action. The strength of this model stems from the observation that the removal of the poly(A) tail is the first event in the decay of mRNA. In addition, the existence of a U-rich element dependent deadenylase has been demonstrated in Xenopus oocytes (36) and in HeLa cell extracts (28,37–39). However, little is known as to the mechanism of exonuclease action. Indeed, it is thought that the deadenylase does not completely deadenylate the poly(A) tail, thus the activation of the exonuclease remains obscure.
Endonucleases that specifically cleave mRNAs have been previously reported. An endonuclease that specifically cleaves Xlhbox2B mRNA has been identified in Xenopus and Drosophila extracts (40). The endonuclease that has been implicated in the estrogen-regulated decay of Xenopus albumin mRNA has been identified and cloned (41,42). The mRNA elements recognized by these endonucleases do not appear to correspond to HuR binding sites. Thus, it is likely that they participate in different processing pathways.
The identification of this endonuclease has implications for the regulation of p27KIP1 gene expression. It is thought that the p27KIP1 gene is regulated primarily at the level of protein turnover (43). Our observation suggests that p27KIP1 may also be regulated via changes in mRNA stability. Indeed, recent data has suggested that the half-life of p27KIP1 mRNA is regulated by dexamethasone (44). However, there is also evidence that p27kip1 may be regulated at the translational level (45). It is important to note that the HuR binding site described here is in the 5′-UTR of p27kip1 mRNA. Thus, it is possible that the endonuclease activity may also have a direct effect on mRNA translation. In any event, the activity discussed here will provide a useful reagent to further investigate the post-transcriptional regulation of p27KIP1 expression.
Acknowledgments
ACKNOWLEDGEMENTS
We thank A. Koff and S.S. Millard for their very valuable comments on the manuscript. This work was supported by NIH-R01-HL 58510 and NCI core grant no. P30-CA08748.
REFERENCES
- 1.Sachs A.B. (1993) Cell, 74, 413–421. [DOI] [PubMed] [Google Scholar]
- 2.Ross J. (1995) Microbiol. Rev., 59, 423–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw G. and Kamen,R. (1986) Cell, 46, 659–667. [DOI] [PubMed] [Google Scholar]
- 4.Treisman R. (1985) Cell, 42, 889–902. [DOI] [PubMed] [Google Scholar]
- 5.Shyu A.-B., Greenberg,M.E. and Belasco,J.G. (1989) Genes Dev., 3, 60–72. [DOI] [PubMed] [Google Scholar]
- 6.Malter J.S. (1989) Science, 246, 664–666. [DOI] [PubMed] [Google Scholar]
- 7.Vakalopoulou E., Schaack,J. and Shenk,T. (1991) Mol. Cell. Biol., 11, 3355–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiavi S.C., Belasco,J.G. and Greenberg,M.E. (1992) Biochim. Biophys. Acta, 1114, 95–106. [DOI] [PubMed] [Google Scholar]
- 9.Lindsten T. (1991) Mol. Cell. Biol., 11, 3288–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myer V.E., Fan,X.C. and Steitz,J.A. (1997) EMBO J., 16, 2130–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy N.S., Chung,S., Furneaux,H. and Levy,A.P. (1998) J. Biol. Chem., 273, 6417–6423. [DOI] [PubMed] [Google Scholar]
- 12.Fan X.C. and Steitz,J.A. (1998) EMBO J., 17, 3448–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng S.S., Chen,C.Y., Xu,N. and Shyu,A.B. (1998) EMBO J., 17, 3461–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W., Furneaux,H., Cheng,H., Caldwell,M.C., Hutter,D., Liu,Y., Holbrook,N. and Gorospe,M. (2000) Mol. Cell. Biol., 20, 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma W.J., Cheng,S., Campbell,C., Wright,A. and Furneaux,H. (1996) J. Biol. Chem., 271, 8144–8151. [DOI] [PubMed] [Google Scholar]
- 16.Wang W., Caldwell,M.C., Lin,S., Furneaux,H. and Gorospe,M. (2000) EMBO J., 19, 2340–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo A., Dalmau,J., Manley,G., Rosenfeld,M., Wong,E., Henson,J., Posner,J.B. and Furneaux,H.M. (1991) Cell, 67, 325–333. [DOI] [PubMed] [Google Scholar]
- 18.Levine T.D., Gao,F., King,P.H., Andrews,L.C. and Keene,J.D. (1993) Mol. Cell. Biol., 13, 3494–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Good P.J. (1995) Proc. Natl Acad. Sci. USA, 92, 4557–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma W.J., Chung,S. and Furneaux,H. (1997) Nucleic Acids Res., 25, 3564–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung S., Jiang,L., Cheng,S. and Furneaux,H. (1996) J. Biol. Chem., 271, 11518–11524. [DOI] [PubMed] [Google Scholar]
- 22.Abe R., Sakashita,E., Yamamoto,K. and Sakamoto,H. (1996) Nucleic Acids Res., 24, 4895–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atasoy U., Watson,J., Patel,D. and Keene,J.D. (1998) J. Cell Sci., 111, 3145–3156. [DOI] [PubMed] [Google Scholar]
- 24.Fan X.C. and Steitz,J.A. (1998) Proc. Natl Acad. Sci. USA, 95, 15293–15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C.Y.A. and Shyu,A.B. (1995) Trends Biochem. Sci., 20, 465–470. [DOI] [PubMed] [Google Scholar]
- 26.Shyu A.-B., Belasco,J.G. and Greenberg,M.E. (1991) Genes Dev., 5, 221–231. [DOI] [PubMed] [Google Scholar]
- 27.Peltz S.W., Brewer,G., Bernstein,P., Hart,P.A. and Ross,J. (1991) Crit. Rev. Eukaryot. Gene Exp., 1, 99–126. [PubMed] [Google Scholar]
- 28.Brewer G. (1991) Mol. Cell. Biol., 11, 2460–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W., Wagner,B.J., Ehrenman,K., Schaefer,A.W., DeMaria,C.T., Crater,D., DeHaven,K., Long,L. and Brewer,G. (1993) Mol. Cell. Biol., 13, 7652–7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeMaria C.T. and Brewer,G. (1996) J. Biol. Chem., 271, 12179–12184. [DOI] [PubMed] [Google Scholar]
- 31.Loflin P., Chen,C.Y. and Shyu,A.B. (1999) Genes Dev., 13, 1884–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koff A. and Polyak,K. (1995) Prog. Cell Cycle Res., 1, 141–147. [DOI] [PubMed] [Google Scholar]
- 33.Polyak K., Kato,J.Y., Solomon,M.J., Sherr,C.J., Massague,J., Roberts,J.M. and Koff,A. (1994) Genes Dev., 8, 9–22. [DOI] [PubMed] [Google Scholar]
- 34.Millard S.S., Vidal,A., Markus,M. and Koff,A. (2000) Mol. Cell. Biol., in press. [Google Scholar]
- 35.Furneaux H.M., Perkins,K.K., Freyer,G.A., Arenas,J. and Hurwitz,J. (1985) Proc. Natl Acad. Sci. USA, 82, 4351–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voeltz G.K. and Steitz,J.A. (1998)Mol. Cell. Biol., 18, 7537–7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford L.P., Watson,J., Keene,J.D. and Wilusz,J. (1999) Genes Dev., 13, 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brewer G. (1998) J. Biol. Chem., 273, 34770–34774. [DOI] [PubMed] [Google Scholar]
- 39.Brewer G. and Ross,J. (1988) Mol. Cell. Biol., 8, 1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown B.D., Zipkin,I.D. and Harland,R.M. (1993) Genes Dev., 7, 1620–1631. [DOI] [PubMed] [Google Scholar]
- 41.Dompenciel R.E., Garnepudi,V.R. and Schoenberg,D.R. (1995) J. Biol. Chem., 270, 6108–6118. [DOI] [PubMed] [Google Scholar]
- 42.Chernokalskaya E., Dompenciel,R. and Schoenberg,D.R. (1997) Nucleic Acids Res., 25, 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagano M., Tam,S.W., Theodoras,A.M., Beer-Romero,P., Del Sal,G., Chau,V., Yew,P.R., Draetta,G.F. and Rolfe,M. (1995) Science, 269, 682–685. [DOI] [PubMed] [Google Scholar]
- 44.Baghdassarian N., Peiretti,A., Devaux,E., Bryon,P.A. and Ffrench,M. (1999) Cell Growth Differ., 10, 405–412. [PubMed] [Google Scholar]
- 45.Millard S.S., Yan,J.S., Nguyen,H., Pagano,M., Kiyokawa,H. and Koff,A. (1997) J. Biol. Chem., 272, 7093–7098. [DOI] [PubMed] [Google Scholar]