Abstract
Advances in clinical genetic testing, including the introduction of exome sequencing, have uncovered the molecular etiology for many rare and previously unsolved genetic disorders, yet more than half of individuals with a suspected genetic disorder remain unsolved after complete clinical evaluation. A precise genetic diagnosis may guide clinical treatment plans, allow families to make informed care decisions, and permit individuals to participate in N-of-1 trials; thus, there is high interest in developing new tools and techniques to increase the solve rate. Long-read sequencing (LRS) is a promising technology for both increasing the solve rate and decreasing the amount of time required to make a precise genetic diagnosis. Here, we summarize current LRS technologies, give examples of how they have been used to evaluate complex genetic variation and identify missing variants, and discuss future clinical applications of LRS. As costs continue to decrease, LRS will find additional utility in the clinical space fundamentally changing how pathological variants are discovered and eventually acting as a single-data source that can be interrogated multiple times for clinical service.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13073-023-01194-3.
Keywords: Long-read sequencing, Genetic variation, Medical genetics, Structural variation, Mendelian disorders
Background
Despite the widespread use of exome sequencing (ES) in clinical practice, approximately half of individuals with a suspected Mendelian condition remain without a precise molecular diagnosis after a complete clinical evaluation. The application of short-read whole-genome sequencing (SR WGS), while offering much more uniform coverage across the genome, has only modestly increased the solve rate [1, 2]. There are likely multiple reasons for this, including incomplete gene–phenotype associations, incomplete ascertainment of individuals undergoing genetic testing, inadequate understanding of the regulatory landscape of genes, and technical limitations of sequencing. For example, short-read sequencing (SRS), despite its accuracy, does not reliably map sequence reads to repetitive regions of our genome, such as segmental duplications, tandem repeats, or low-complexity regions enriched for GC- or AT-rich DNA [3]. There are more than one thousand protein-coding genes associated with such regions, many of which are clinically relevant, where variation is simply not reliably assayed [4]. Moreover, numerous studies over the last few years have shown that most larger, more complex forms of human genetic variation—termed structural variations (SVs) for events >50 bp in size—are missed by SRS and ES because of their association with repetitive DNA. Technological advances and new methods, thus, are critical to more fully evaluate individuals who remain unsolved after comprehensive clinical evaluation.
Although not yet clinically available, long-read sequencing (LRS) represents a promising technology to evaluate individuals with unknown genetic etiology or those who have complex changes not fully resolved by prior evaluation. Most LRS commercial platforms now routinely deliver reads >10 kbp and up to several megabases [5]. Unlike SRS, which involves amplification of DNA, LRS typically analyzes native DNA; therefore, it may be regarded as 5-base sequencing, with the ability to determine the methylation status of CpG sites in addition to the standard four nucleotides identified by SRS. Currently, LRS platforms capable of 5-base sequencing are primarily produced by two companies: Pacific Biosciences (PacBio) and Oxford Nanopore Technologies (ONT). Because the two technologies differ fundamentally in the way they generate data, leading to differences in output and error rates, it is important to consider the nuances of both when selecting which platform to use (discussed below). At the time of this writing, a synthetic long-read product is being developed by Illumina, though is not yet widely available, so it will not be discussed in this review.
Because LRS technology is relatively new, there are few carefully controlled studies comparing LRS to SRS or ES [6]. Recent work has shown that LRS technologies typically identify ~25,000 SVs per human genome in contrast to SRS of the same samples, which depending on the SV discovery tools applied, only generates 3000–10,000 [6–10]. SV discovery using SRS lacks both sensitivity and specificity making it unreliable as a clinical test. Consequently, multiple groups have shown that LRS can be used to identify disease-causing variants missed by prior clinical testing in a modest number of cases [11–17]. This increased solve rate is derived given that LRS is able to access challenging regions of the genome refractory to analysis with SRS, which simplifies variant calling and resolution of complex SVs [4, 6, 18, 19]. More than 250 medically relevant genes are more accurately ascertained using LRS-based approaches when compared to SRS [20, 21]. In particular, LRS-based approaches can resolve complex SVs [14, 22–24], repeat expansions [25, 26], and differences in methylation [15] in medically relevant regions or cases that were not solved after standard clinical testing. Finally, LRS, specifically on the ONT platform, is unique in that the data is available for analysis in near real time, which has allowed for studies showing that a complete genome could be sequenced and analyzed in less than 8 h and WGS with targeted analysis for previously known variants could be completed within 3 h [27, 28]. Together, these studies suggest that systematic application of LRS to previously unsolved Mendelian cases might increase the overall rate of diagnosis.
Here, we provide an overview of LRS technology improvements, including the advantages and disadvantages of each technology, along with the advances that have increased coverage, throughput, and accuracy. Due to the rapid developments in LRS technology over the last few years, any review of this type is likely to be soon outdated. However, we ground this assessment on existing published data and flag potential projections. Using examples from the literature, we focus on cases of Mendelian variants that were identified with LRS and refractory to analysis with ES or SR WGS. We conclude with a discussion of how LRS may be used in the clinical setting in both the near and long term, including the use of LRS as a single data source to replace most clinical testing available today.
Long-read sequencing technologies
There are two commercially available technologies today, PacBio and ONT (Table 1), that routinely generate RNA or DNA reads greater than 10 kbp.
Table 1.
Comparison of PacBio and ONT sequencing technologies
| Sequencing technology | Platform | Supported flow cell | Data production | Read length (kbp) | Mean read accuracy (%) | Throughput per flow cell (Gbp) | Estimated Cost per Gbp (US$)a | Applications |
|---|---|---|---|---|---|---|---|---|
| Pacific Biosciences (PacBio) | Sequel II/IIec | SMRT Cell 8M | HiFi | 15-25 (up to 40) | >99 | 30-42 | 31-43 | WGS, Gene panels, cDNA sequencing, Methylation analysis, Metagenomics and microbiome analyses |
| Revioc | SMRT Cell 25M | >99 | Up to 90-126b | 8-11b | ||||
| Oxford Nanopore Technologies (ONT) | Flongle | R9.4.1 | Simplex, duplex | 1-100 (up to > 2000) | >95 | 1-2 | 118d | Amplicon or plasmid sequencing |
| MinION | R9.4.1, R10.4.1 | >95 | 15-25 | 29–51e | Amplicon sequencing, Sequencing of small genomes, Adaptive sampling, gene panel, cDNA and direct RNA sequencing, Metagenomic and microbiome analysis | |||
| GridION | R9.4.1, R10.4.1 | >95 | 15-25f | 29–51e | ||||
| PromethION | R9.4.1, R10.4.1 | >95 | 100-200 | 6–12g | WGS, cDNA and direct RNA sequencing, Adaptive sampling |
aPricing includes exclusively SMRTbell prep and sequencing reagents run on proprietary instruments.
bProjected estimate: Revio was launched in Q1 2023.
cThe Sequel IIe and Revio can process the raw sequencing data and generate HiFi reads on the instrument.
dAssumes output of 1.5 Gbp per flow cell using single library.
eAssumes output of 20 Gbp per flow cell using single library.
fGridION allows simultaneous sequencing on 5 flow cells.
gAssumes output of 150 Gbp per flow cell with three libraries.
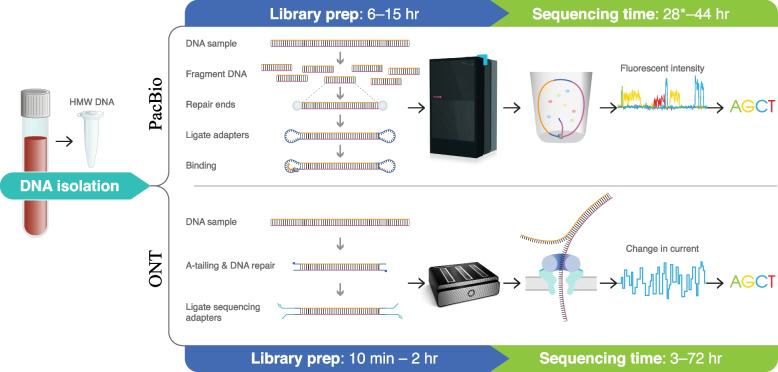
The technologies differ radically in how sequence data are generated (Fig. 1). PacBio sequencing depends upon a DNA polymerase tethered to the bottom of a well of picolitre volume known as a zero-mode waveguide (ZMW) (Fig. 1). Here, the DNA polymerase associates with a single molecule of native DNA incorporating fluorescently labelled deoxynucleoside triphosphates (dNTPs) as it polymerizes. The action of the polymerase liberates the fluorescently labelled phosphates allowing successive nucleotide incorporations to be directly assayed by a set of precisely positioned lasers and CCD cameras. The sequence data, as a result, has been referred to as single-molecule, real-time (SMRT) sequencing. PacBio offers two sequencing modes. The original, called continuous long-read (CLR) sequencing, was designed for maximizing the length of the sequence reads and typically involved the preparation of libraries greater than 30 kbp in length. In this case, the DNA polymerase typically passes through the DNA molecule only once, generating one single-pass read with a typically high error rate resulting in a read accuracy of ~85–92% [5].
Fig. 1.
Library preparation and sequencing workflow for both PacBio and ONT. PacBio workflow: DNA is first extracted from blood or cell lines and then sheared to the desired fragment size (typically at 15–25 kbp). After DNA end repair, fragments are ligated to adapters to form circular molecules called SMRTbells. Each SMRTbell is bound by a polymerase and loaded into a single-molecule, real-time (SMRT) cell. Once the sequencing library is loaded into the SMRT cells, each SMRTbell is immobilized at the bottom of one zero-mode waveguide (ZMW). Next, fluorescently labelled deoxynucleoside triphosphates (dNTPs) are added into the wells and sequencing begins. The polymerase starts incorporating dNTP to the new DNA strand. Each incorporated fluorescent dNTP remains briefly at the bottom of the well, where a light pulse from the bottom excites the fluorophore, which is captured by a camera; the fluorophore is then released after nucleotide incorporation. Erroneous stimulation of unincorporated dNTPs can rarely occur if they are particularly close to the bottom of the ZMWs. These occurrences contribute to the error rate of PacBio sequencing. Since modified bases slightly delay the action of the polymerase, CpG methylation can be identified. *Estimate for sequencing on Revio, which has not been extensively tested. ONT workflow: DNA extraction for ONT sequencing can depend on the desired read lengths and may be either a column-based or other extraction. Quality control steps could include an assay to evaluate contamination from the DNA extraction step and recovered fragment length. For DNA sequencing, libraries are typically prepared using either a rapid transposase-based kit, or a longer ligation-based prep that preserves fragment lengths. Libraries are loaded on the flow cell and run for the desired amount of time, with washes as needed based on flow cell performance. Sequencing data can be base called on the machine or transferred to a remote host for processing
The second sequencing mode, introduced in 2019 [29], uses high-fidelity (HiFi) reads (also referred to as circular consensus sequencing (CCS)) and, as the name suggests, is designed for accuracy instead of length. It works by targeting shorter fragments of DNA (10–30 kbp) and ligating a hairpin adapter (termed a SMRTbell) at both ends of the DNA fragment creating a circular molecule. As a result, the polymerase iterates through the reverse and forward strand of the molecule multiple times generating individual subreads (Fig. 1). These reads are combined, to generate a highly accurate consensus sequence that is estimated to be >99.9% accurate (QV >30). As a result of this CCS, HiFi sequencing is currently the most accurate LRS technology but is limited to comparatively shorter library sizes. The shorter the insert, the more accurate the consensus sequence that is generated because of an increase in the number of iterations. Because modified bases pass more slowly through the polymerases than non-modified bases, CpG methylation can be deduced from dwell time in the polymerase [30]. Given the advantage of highly accurate reads, PacBio is currently focused on HiFi production and CLR sequencing is considered outdated.
Base calling is the first step needed to convert the raw sequencing data into a nucleotide sequence. In SMRT sequencing, as each nucleotide is incorporated by the polymerase, the fluorescent signal is recorded. The first base-calling step converts each fluorescent pulse into a base, generating a single long read (Fig. 1). This long read is then separated into subreads, each corresponding to a single polymerase pass through the DNA molecule. The alignment of subreads eventually generates a highly accurate consensus sequence. This correction method is allowed by the stochastic nature of PacBio errors, which decreases the possibility of having the same error in multiple subreads. Thus, discrepancy between subreads can be corrected with sufficient sequence coverage. Base calling is computationally intense; hence, the latest machines are capable of outputting CCS reads directly (Table 1). With the introduction of CCS, PacBio sequencing accuracy has become comparable to that of Illumina with the majority of residual errors confined to indels in homopolymers [29].
There are three different PacBio sequencing machines currently in use. The Sequel system (released in 2015) provides the lowest throughput, supporting SMRT cells with 1 million ZMW. It was originally designed for CLR sequencing and then adapted for HiFi. The Sequel II (released in 2019) and the Sequel IIe (2020) systems provide much higher throughput. Both support 8 million ZMWs (8M SMRT cells) and are optimized for HiFi sequencing. The Sequel IIe provides increased computational capacity compared to the previous model, which facilitates more rapid HiFi production and data processing. The Sequel II systems have become the current standard for SMRT sequencing in research laboratories. In Q1 2023, PacBio released a new machine called Revio, with capacity for 100 million ZMW (4 × 25 million reactions). The new design promises a 15-fold increase in throughput and a 4-fold reduction in cost with the potential of sequencing ~1300 human genomes per year. Since the Revio has not gone through extensive test and validation yet, we limit subsequent discussion to the Sequel II and IIe systems.
ONT sequencing, unlike most other sequencing technologies, does not depend on the action of DNA polymerase but rather an unwinding enzyme and pore protein that effectively threads single-stranded DNA or RNA molecules through a pore across a charged synthetic membrane (Fig. 1). As the molecule passes through the pore, changes in conductance are detected and are characteristic of particular nucleotide compositions. As a result, the sequence of the DNA or RNA molecule can be inferred. Library preparation is achieved through one of two methods. A rapid protocol exists and can be completed in approximately 10 min, with the drawback that random integration of adapter libraries shortens DNA fragments prior to sequencing. A second ligation-based protocol preserves the DNA fragment length and can be completed in approximately 1–2 h. In both cases, libraries are loaded onto a flow cell and can be run for as long as 72 h. Pores become unavailable over time; thus, the output of a sequencing run can be improved by washing and reloading of new libraries during the sequencing experiment. Methylation can also be determined based on differences in the current profile.
Similar to PacBio sequencing, raw sequencing output from the ONT machines has to be converted into nucleotide sequence through a base-calling process (Fig. 1). The current software used for ONT base calling is Guppy, which employs a recurrent neural network to determine sequence from raw signal. The speed and accuracy of base calling depends on which model is used, either “fast,” “high accuracy,” or “superior.” Because base calling is a computationally intensive process (most often performed on powerful graphical processing units (GPUs)), some users prefer a less accurate model that will complete quickly, such as the fast model (85–92% median read identity [31]). Alternatively, users who value accuracy over speed may choose the superior model (92–96% median read identity [31]). While several factors determine how much slower the superior model is than the fast model for a particular sample, our experience is that the superior model can be at least 10 times slower than the fast model on a high-end NVIDIA GPU. Methylation can be called concurrently by Guppy if a model trained to detect 5mC is used, resulting in slightly longer base-calling times and a slight improvement in base-calling accuracy. Changes to the signal file format and improvements to the base-caller architecture are anticipated that are likely to significantly decrease the amount of time and computational resources required for base calling.
One criticism of ONT sequencing in the past has been its lower accuracy when compared to SRS or PacBio HiFi. Improvements in chemistry, pore design, and base-calling models have increased per-read accuracy over time, with current single-nucleotide variant (SNV) recall at 30× coverage of 99.4%, and indel recall of 63–68% [32]. Indel recall increases only modestly as coverage increases, rising to 73–78%, for example, at 60× coverage [32]. There is not a well-described sequence bias in ONT sequencing as has been observed for HiFi PacBio data, which biases against regions enriched in GA/TC repeats [33]. However, a recent analysis showed that ONT is prone to base-calling errors for telomeric repeats and repeats that are represented by similar current profiles [34], while these errors are not present in equivalent PacBio sequences. Also, ONT does struggle to accurately resolve homopolymers longer than 5–7 nucleotides as the dwell time for a set of identical nucleotides in the pore is difficult to accurately determine [5]. Recently, ONT introduced a new pore, known as R10, which has a longer pore head, resulting in higher accuracy reads, with improvements in calling indels in homopolymers [35, 36].
There are several unique aspects of ONT sequencing. First, individual pores can be computationally controlled via software in real time—a sequencing mode known as adaptive sampling. This method works because signal from individual pores is sent to the controlling computer in real time allowing immediate base calling and alignment to a reference genome [37]. Therefore, during sequencing, it is possible to determine if the particular sequence maps to a region of interest. If not, the current at the pore can be reversed, the DNA molecule ejected, and a new molecule will begin sequencing. In this way, specific regions of the genome can be enriched or depleted during sequencing. Enrichment using adaptive sampling depends on several variables, such as fragment length, size of reference genome, and the ONT machine used. As an example, sequencing of a human genome with 10 kbp average fragment sizes results in 4–6× enrichment on a GridION over the region of interest [15]. Adaptive sampling recently became available on the PromethION [38] but has not been widely tested to determine its performance compared to the GridION. While the ONT platform, like PacBio, supports sequencing of complementary DNA (cDNA), another unique aspect is the ability to directly sequence native mRNA molecules using dedicated kits. This allows direct measurement of the length of a poly-A tail and, in principle, direct detection of mRNA modifications. Detecting RNA modifications using ONT sequencing is an emerging field of research, as more than 150 modifications are now known, but only a few can be reliability detected with current methods [39, 40]. Sequencing of other types of RNA molecules, such as tRNA, is an active area of research [41].
Multiple ONT sequencing platforms exist, with the PromethION being the largest device offered in either a 24- or 48-flow cell configuration (Table 1). Because a PromethION flow cell is capable of sequencing a human genome to 30–40-fold sequence coverage over a 72-h run with multiple washes, a single PromethION with 48 channels could sequence up to 98 human genomes per week. The GridION is a smaller physical device that is capable of sequencing five MinION flow cells simultaneously. Adaptive sampling is commonly performed on the MinION, and an adapter allows Flongle flow cells to be run here as well. The MinION, the smallest physical sequencer, is smaller than a typical stapler and can run both MinION and Flongle flow cells. A unique feature of ONT sequencing is portability in that the smaller devices, such as the Flongle or MinION, can be powered by a laptop, allowing them to be used in isolated areas or in resource-limited settings [42–44], and even in extremely remote locations, such as Antarctica [45] and the International Space Station [46].
Several polishing tools have been developed to improve the error rate of both PacBio CLR and ONT. They can be divided into hybrid tools, which combine SRS and LRS data, such as Hercules [47], proovread [48], LoRDEC [49], CoLoRMap [50], HG-CoLoR [51], and HALC [52]; and self-correction-based tools, such as FLAS [53] and LoRMA [54]. A recent comparison of several correction tools revealed that hybrid tools outperform nonhybrid methods, particularly when LRS coverage is low [55]. One downside of these polishing algorithms is the time they require for correcting LRS data, potentially requiring multiple days for a genome smaller than humans [55]. Therefore, more efficient methods will be required in the future to see these tools more routinely used. Alternatively, novel approaches, such as the newly released variation graphs-based tool called VeChat [56]; or DeepConsensus [57] from PacBio and Google, which use a deep-learning approach. DeepConsensus is available on the new Revio and is likely to improve error correction without the need for orthogonal data or additional compute resources.
Targeted long-read sequencing approaches
Because of the higher costs of LRS, there has been interest, especially early on, in developing and evaluating targeted long-read sequencing (T-LRS) methods. The simplest strategy is to use PCR to amplify a region or multiple regions of interest. This results in an over-enrichment of high-priority regions, with the disadvantage of loss of methylation information during the amplification process. In addition, it can be challenging to reliably amplify regions larger than 10 or 20 kbp requiring tedious optimization and primer redesign. As a proof of the efficacy of this approach, Loomis et al. showed that SMRT sequencing of long PCR products of FMR1 from patients with Fragile X syndrome allowed characterization of trinucleotide repeat expansions in patients with up to 750 repeats, an unachievable goal for short-read-based approaches [58].
Another T-LRS approach is hybridization capture. Typically, DNA is first sheared, and the DNA fragments are preselected according to the desired insert size (either <1 kbp or >1 kbp) [59]. The fragments containing regions of interest are then selected using a hybridization-based target enrichment kit. Once again, this step requires PCR amplification of the selected fragments to achieve sufficient DNA quantity for library preparation resulting in a loss of methylation signal and the amplification biases associated with PCR. Nevertheless, Wang and colleagues demonstrated the usefulness of this method by sequencing and characterizing a locus associated with reciprocal recurrent rearrangements associated with Potocki-Lupski syndrome (PTLS) and Smith-Magenis syndrome [59]. In three patients with PTLS, both known and novel breakpoints were characterized, which mapped within segmental duplications driving these rearrangements. Hybridization capture methods allow isolation of specific fragments of DNA, which could be theoretically sequenced on both PacBio and ONT instruments. However, ONT efforts are more focused on a computational method to sequence only specific regions of the human genome without prior sample treatment. This method will be discussed later.
To overcome limitations associated with PCR-based approaches, alternative strategies have been developed. CRISPR/Cas9-based target enrichment, for example, starts with a dephosphorylation step then uses an RNA-guided Cas9 digestion to expose new phosphorylation sites. The sequencing library then only is ligated to those molecules with free 5′ phosphorylation sites [60]. The CRISPR/Cas9-mediated approach was first validated by evaluating trinucleotide repeat expansions in individuals with Huntington’s disease (CAG repeats in HTT) and Fragile X [61]. Variations on this basic approach have been recently developed, including methods that perform digestion of dsDNA molecules not protected by Cas9 enzyme, and separate DNA molecules after cutting using pulsed-field gel electrophoresis (PFGE) [62, 63]. This approach has been successfully implemented on both the PacBio and ONT platforms. For example, Gabrieli and colleagues used Cas9-Assisted Targeting of Chromosome segments (CATCH) to target and sequence BRCA1 and its flanking regions on an ONT platform [62]. Instead, Walsh and colleagues designed guide RNA that targeted the BRCA1 and BRCA2 loci and utilized PacBio to sequence the fragments [63]. Both studies isolated the DNA fragments of interest with gel electrophoresis, but Gabrieli et al. used DNA amplification prior to sequencing (possibly due to a low number of isolated DNA).
Even though CRISPR/Cas9-mediated protocols have been successfully used in recent studies, difficulty in designing guide RNA that result in high yield have limited widespread adoption. Indeed, PacBio withdrew official support for such CRISPR/Cas9-mediated workflows in 2021. Currently, PacBio collaborates with Twist Biosciences, which offers hybridization capture-based panels: one targets 389 genes (~20 Mbp) difficult or impossible to fully characterize with SRS; a second covers 49 genes (2 Mbp) important for drug metabolism and therapeutic response; and it is also possible to design a custom panel. As previously discussed, these panels will not preserve methylation status, since DNA amplification is necessary.
Adaptive sampling in conjunction with ONT can be used to enrich or deplete specific regions of a genome during sequencing. This strategy has been successfully used for both human and nonhuman applications. It is strictly computational in nature requiring no additional experimental setup. It has been used to characterize multiple loci with repeats commonly associated with human disease, phasing of pathogenic variants, and characterizing complex rearrangements [15, 64, 65]. The decision to perform T-LRS over WGS is typically driven by cost, as smaller regions of the genome can be currently evaluated more inexpensively than the entire genome. It is also particularly useful in solving recessive cases of Mendelian disease when only one of the two pathogenic variants has been discovered and multiple cases can be multiplexed [15, 37, 38]. Moreover, at the end of last year, a T-LRS-based workflow was designed to target 59 loci associated with repeat expansion diseases and facilitate downstream data analysis [66]. As the cost of LRS continues to drop, it is likely that the use of T-LRS will wane and WGS will become the dominant technology for variant discovery. In our experience, we have moved away from T-LRS in favor of WGS approaches to assess other loci more comprehensively, including modifier loci, more uniformly. For all targeted sequencing approaches, it is important to remember that they depend on a priori knowledge of the disease-associated loci.
Quantity and quality of input DNA/RNA for long-read sequencing
LRS requires high molecular weight (HMW) DNA composed of long fragments and a higher input quantity compared to SRS. For optimal library preparation and sequencing, PacBio protocols ideally require 90% of fragments to be >10 kbp long and 50% to be >30 kbp long. 1 µg of HMW DNA is required for SMRT Cell 8M (Sequel II/IIe) and 2 µg for SMRT Cell 25M (Revio) (see PacBio website for complete protocols). ONT protocols require the amount of small fragments (<20 kbp) in the DNA sample to be the lowest possible, as shorter fragments would be preferentially sequenced. The minimum size threshold can be determined according to the purpose of the experiment, but to take full advantage of LRS, most DNA fragments should surpass at least 30-40 kbp (no theoretical upper limit for ONT read length). ONT protocols require 1.5-3 µg of input HMW DNA, and a low-input protocol, which requires a PCR step, is also available (see ONT website for complete protocols). This is optimal for certain conditions, but base modification signals will be lost during amplification and reads will be comparatively shorter. For both technologies, input DNA quality can be improved with a size selection aimed to remove shorter fragments, but this procedure requires a higher initial DNA amount because some will be lost during the process. HMW DNA for LRS should be extracted from fresh blood or cell pellets. Typically, 10 million cells or 500 µl of blood are sufficient to obtain 100-125 µg and 10-35 µg of DNA respectively using commercially available HMW DNA extraction kits.
ONT also has a protocol for ultra-long (UL) libraries. In this case, HMW DNA should be extracted with a dedicated phenol-chloroform-based protocol [67–69]. For UL libraries, the input DNA ranges from 20 to 40 µg. For both the technologies, older DNA extractions and samples that have been frozen and defrosted multiple times are less ideal for LRS due to DNA damage and fragmentation.
Both the ONT and PacBio Sequel II/IIe platforms are capable of transcriptome sequencing and can perform bulk and single-cell cDNA sequencing with different kits. Bulk sequencing using PacBio requires 300 ng of RNA with RNA integrity number (RIN) ≥7 while ONT requires 200 ng of total RNA for cDNA sequencing and 500 ng for direct RNA sequencing. Single-cell sequencing requires between 15 ng and 60-75 ng of cDNA, with the PCR cycles in the protocol adjusted according to the amount of starting material. cDNA sequencing is currently unavailable on Revio, but dedicated kits are expected in the near future (see Other Applications for more information).
Analysis of long-read sequencing data
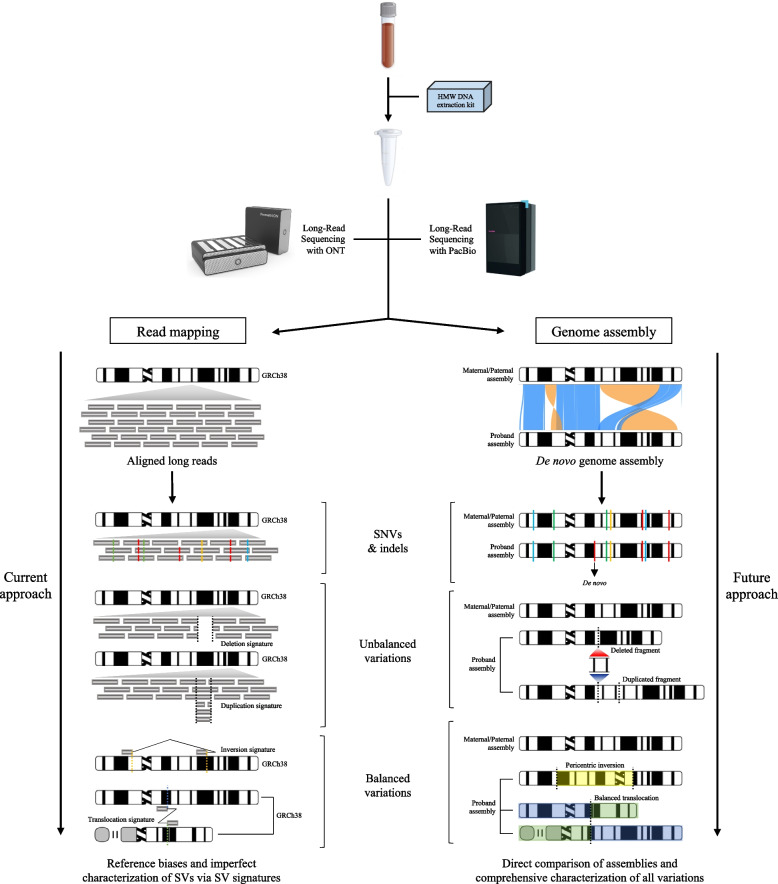
There are two basic approaches to identify variants using LRS. Like SRS, the most straightforward approach is read based—i.e., mapping the reads against a reference genome. Because read lengths are typically longer than most common repeat sequences (>10 kbp), the approach dramatically increases the sensitivity for SV detection. The first LRS-based studies reported >20,000 SVs per human sample [6, 7, 19], markedly higher than early data based on SRS (such as the 1000 Genomes Project), which reported only 2100–2500 SVs per genome [70] after rigorous filtering. Applying multiple SRS SV callers increases this number; for example, gnomAD-SV contains SV calls from SRS data of ~15,000 individuals and reported a median of 7,439 high-quality SVs per genome [8]. Read-based mapping approaches using LRS have improved with the release of specialized alignment tools optimized to handle longer and more error-prone data (BLASR [71], MHAP [72], NGMLR [73], and Minimap2 [74]) and software dedicated to variant discovery and phasing (WhatsHap [75], DeepVariant [76], Sniffles [73], PBSV [29], Phased-SV [6], and CuteSV [77]). While these tools continue to rapidly evolve, Minimap2 is particularly valuable for the alignment of large segments of DNA to define the breakpoints of large structural variants. DeepVariant shows excellent sensitivity for the discovery of SNVs while Sniffles and PBSV are considered current state of the art for the discovery of structural variants. LongPhase [78] can complement the analysis with variant phasing.
Unlike SRS, longer reads also enable reliable assembly-based discovery of variants. In principle, de novo genome assembly of long-read datasets has the potential to determine the complete or nearly complete telomere-to-telomere (T2T) DNA sequence of both haplotypes of a sample [4, 79, 80]. Recently, several genome assemblers have been developed for this purpose, such as HiCanu [33], Peregrine [81], wtdbg2 [82], Flye [83], Shasta [84], hifiasm [85, 86], and Verkko [87]—the latter is a hybrid assembly approach that combines the scaffolding potential of ONT with the high accuracy of HiFi. Genome assembly provides the most complete representation of a human genome and the potential to investigate the full spectrum of human genetic variation ranging from SNVs to fully sequence-resolved SVs, including copy number variants [88] (Fig. 2). Although close, complete T2T assembly of a diploid genome has yet to be achieved because of the challenges of traversing complex repetitive regions associated with acrocentric, centromeric, or segmentally duplicated DNA [79, 88]. The key to the assembly-based approach is correctly separating the long reads into the two constituent haplotypes underpinning each diploid genome. Over the last two years, two basic strategies have emerged depending on either the use of parental SR WGS data for trio-binning [89] or physical-based approaches where parental data are unavailable. The latter takes advantage of single-cell strand sequencing data (Strand-seq) [10] or high-throughput capture chromatin conformation (Hi-C) data [86] to identify SNV haplotypes obtained from SRS data from the same sample to then effectively phase LRS data and assembled contigs. Both methods effectively allow SNVs to be physically phased on a particular homologous chromosome. Strand-seq depends on replication and BRDU incorporation followed by degradation of the newly synthesized strand and single-cell sequencing technology to phase SNVs on the template strand for each chromosome; while Hi-C depends on crosslinking and proximity ligation to define SNVs and therefore build up locally phased haplotypes. This information is used to phase long-read sequences and assembled contigs to generate T2T chromosomes at the chromosomal level.
Fig. 2.
Read mapping versus de novo genome assembly for variant discovery. A traditional approach uses long-read mapping to a reference genome to identify SNVs, indels, and SV signatures, while de novo genome assembly reconstructs the two haplotypes of the sequenced individual and permits the direct comparison of assemblies (in clinical settings, ideally, parents versus proband). Genome assembly improves variant discovery, as all types of variations are fully sequence resolved and do not have to be inferred from SV signatures. Moreover, using a reference genome such as GRCh38 introduces biases due to the incompleteness of certain regions and misassembled complex loci. De novo genome assembly is the approach that we expect to substitute all the others and eventually be the gold standard method for variant discovery. (Visualization of assembly comparison adapted from SafFire [90].)
In 2021, the Human Genome Structural Variation Consortium (HGSVC) successfully assembled haplotype-resolved genomes of 32 human genome samples (64 haplotypes) sequenced with both CLR, HiFi PacBio, and Strand-seq as phasing technology. The authors developed a phased assembly variant (PAV) caller that enabled, for the first time, variant discovery (SNVs, indels, SVs) by direct comparison of two haplotypes of a single sample against the human reference genome. This study identified more than 100,000 SVs in the general human population providing the first comprehensive sequence-resolved map of human genome structural variation in linkage disequilibrium with flanking SNVs facilitating the discovery of new expression quantitative trait loci and disease associations [9]. Importantly, once linkage disequilibrium and breakpoints of common SVs were fully resolved, the analysis showed that new genotyping tools (e.g., PanGenie) [91] could be employed to go back to existing SRS datasets to make new associations. More than a year later [92], the Human Pangenome Reference Consortium (HPRC) assembled a more complete pangenome from 47 human genomes (94 haplotypes) using HiFi PacBio and parent–child Illumina WGS data. While not yet complete, the SV catalogs (as well as the underlying pangenomes) produced by the HGSVC and HPRC are providing a useful roadmap of “normal” human genetic variation to help focus on potentially pathogenic variants in human disease samples.
In addition to increased sensitivity for variant discovery, the sequencing of native DNA as opposed to amplified material (e.g., bridge amplification Illumina) has meant that methylation, and other modifications of the native DNA, may be determined (Fig. 3). Both PacBio and ONT have developed specialized tools: Primrose [93] uses a convolutional neural network to predict the 5-Methylcytosine (5mC) of CpG dinucleotides from polymerase kinetics during sequencing, while Nanopolish [94] uses a pre-trained hidden Markov model to distinguish 5mC from unmethylated cytosines based on subtle changes in the current. However, many other tools dedicated to 5mC detection and other base modifications were developed for ONT data, such as Tombo/Nanoraw [95], DeepSignal [96], DeepMod [97], and Megalodon [98], albeit now methylation detection is built into the current ONT base-caller, Guppy [99]. These methods circumvent some of the drawbacks of bisulfite sequencing (considered so far, the gold standard method for methylation analysis), such as protocol complexity, DNA degradation caused by bisulfite treatment, and read mapping limitations.
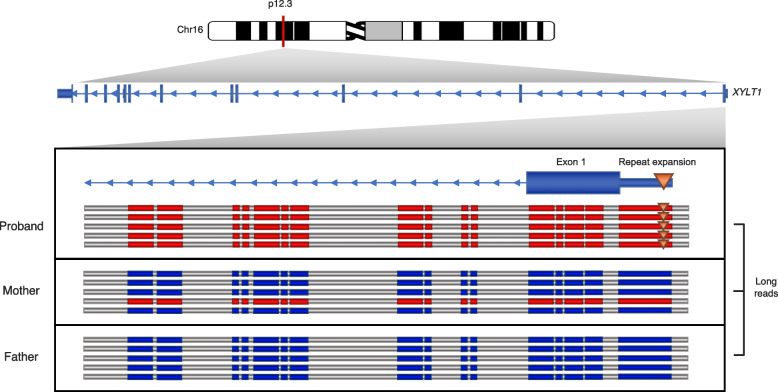
Fig. 3.
Pathogenic GGC repeat expansion in the 5′ untranslated region (UTR) of XYLT1. This variation was characterized in a patient known to have Baratela-Scott syndrome caused by expansion of a maternal premutation allele and paternally inherited deletion [15]. The expansion leads to hypermethylation (red) of the UTR and surrounding area. The father did not carry the expansion; however, some level of methylation was detected in the mother, who was heterozygous for a premutation allele
Simultaneous methylation and genetic variant characterization are particularly relevant to the study of human disease. Pathogenic repeat expansions, for example, are frequently associated with hypermethylation of the promoters and their genes leading to the loss of expression [25, 100, 101]. Moreover, individuals with pathogenic repeat expansions but showing leaking transcription/translation and possibly less extensive hypermethylation are often less severely affected [102–104]. Recently, Miller et al. 2021 confirmed that a known case of Baratela-Scott syndrome caused by a repeat expansion and associated hypermethylation could be evaluated by T-LRS and methylation analysis with Nanopolish. Notably, the authors showed that hypermethylation was detected for the premutation allele carried by the mother, a level of detail not achievable with prior methods (Fig. 3). With respect to cancer, methylation characterization is key. Different methylation profiles are frequently associated with different cell types and the pathogenic properties of various tumors often associate with methylation of tumor suppressor genes [105–107]. In such cases, it is critical that relevant tissues be ascertained for methylation and somatic changes.
Other applications
Outside of strict genetic variant discovery, LRS has provided new biological insights and opportunities related to health more broadly. ONT has the potential to sequence both cDNA [108] and native RNA molecules [109]. While both provide insight into the structure of longer isoforms and full-length transcripts, the latter does not involve conversion to cDNA or subsequent amplification so there is the potential to directly assess RNA modifications. This method, called direct RNA sequencing, has been used to study RNA from bacteria and viruses [110–113] as well as that of humans [114, 115]. However, as recently discussed by Jain et al., some limitations still prevent widespread use of this technology [116]. For instance, direct RNA sequencing requires a high amount of starting material, long RNA transcripts are underrepresented, and base-calling accuracy is below that of DNA sequencing. Thus, direct RNA sequencing may not be sufficient to accurately identify all open reading frames and splice sites [116].
In contrast, PacBio is limited to cDNA sequencing, and its full-length isoform sequencing protocol, termed Iso-Seq, has successfully been used to characterize splicing events, detect fusion genes, and identify tissue- and allele-specific isoforms both at the bulk [117–120] and single-cell/single-nuclei level [121–124]. One limitation of PacBio Iso-Seq is its limited output, which substantially increases the cost of this assay. For this reason, a new protocol called single-cell MAS-ISO-seq was recently developed by PacBio in collaboration with 10X Genomics and the Broad Institute [125]. This method concatenates single-cell cDNA molecules generated by 10X Genomics technology into single fragments that can be used for LRS, increasing throughput by >15-fold. Given the decrease in cost and additional information regarding isoforms that this new method allows, in February 2023, it was announced that MAS-ISO-seq will be soon adapted for bulk sequencing.
In addition to RNA sequencing, LRS has also complemented SRS studies of the microbiome and provided several critical advantages. Chen et al. showed that the use of the two technologies improved microbe genome assembly and SV detection (particularly for insertions and inversions) [126]. The study also demonstrated how microbial SVs could be used as a fingerprint and track the flora of the gut microbiome given the high structural diversity between individuals. Further applications, among others, include the study of virus-human integration [127] and virus surveillance [128, 129], including SARS-CoV-2.
Human pathogenic variant discoveries
The number of reports using either PacBio or ONT to successfully identify pathogenic variants missed by clinical testing has dramatically increased over the last few years [11–17, 23–26, 130, 131]. While large-scale and systematic studies evaluating the power of LRS to identify variants in unsolved cases are lacking, efforts to date have suggested that one of the biggest gains will be the resolution of disease-associated SVs. An early study showed that 85% of common SVs had not been reported in previous large SRS studies [18]. Specifically, 92% of insertions and 69% of deletions were novel. Since deletions and insertions are variants of large effect and a known cause of genetic disease [132], this report suggested that the application of LRS would be beneficial to the study of unsolved Mendelian cases, particularly those with negative ES and SR WGS. Several groups have subsequently applied LRS to detect SVs missed or not fully clarified with ES or SR WGS (Table 2).
Table 2.
Example disease-associated variations resolved by LRS; additional examples in Additional file 1: Table S1
| Variant class | Associated disease - locus of interest | Long-read sequencing technology | Previous approaches | Details | Citation |
|---|---|---|---|---|---|
| Trinucleotide repeat expansion | Neuronal intranuclear inclusion disease, oculopharyngeal myopathy with leukoencephalopathy, oculopharyngodistal myopathy | HiFi WGS | SR WGS | Characterization of trinucleotide repeat expansion in a candidate locus | [25] |
| Single-nucleotide variant | Angelman syndrome | T-LRS ONT | SR WGS | Identification of the parent of origin of a pathogenic de novo variant | [133] |
| Structural variations | Duchenne muscular dystrophy | ONT WGS | T-SRS, T-LRS | Identification and characterization of a pathogenic complex SV | [13] |
| Hereditary cancer | ONT WGS | SR WGS | Reinterpretation and characterization of SVs in cancer patients | [134] | |
| Retinitis pigmentosa | ONT WGS | T-SRS | Identification of two likely pathogenic SVs | [17] | |
| Complex β-globin genes with SV clusters | T-LRS HiFi | MLPA | Haplotype characterization of complex SV-rich loci; breakpoint characterization of deletions, inversions, and duplications | [22] |
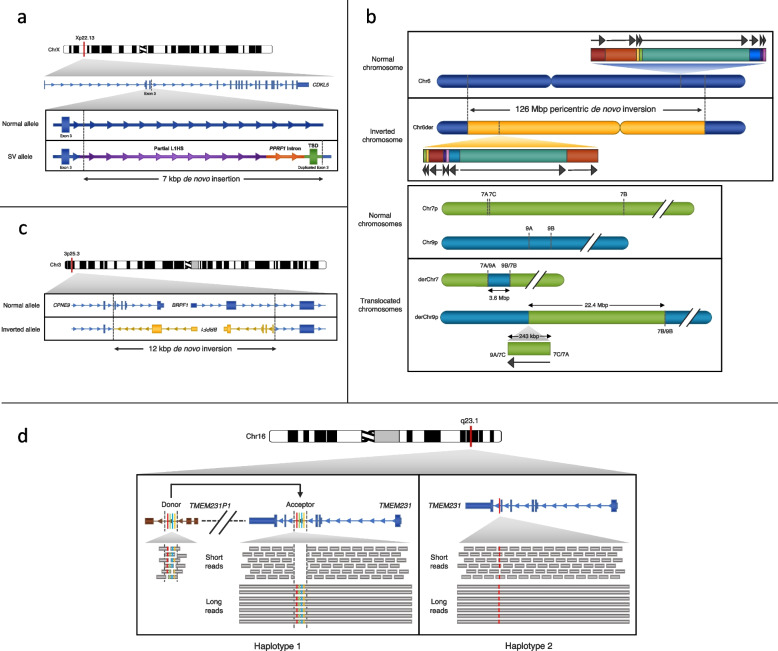
Hiatt and colleagues, for example, evaluated six probands with neurodevelopmental disorders and their unaffected parents using PacBio sequencing [14]. Analysis of the LRS data revealed a de novo ~7 kbp insertion (Fig. 4a) in CDKL5 in one proband (n. 6) and a complex de novo SV in a second patient (n. 4) (Fig. 4b). Analysis of the LRS data allowed the authors to fully characterize the insertion as a ~4.3 kbp 5′ truncated, retrotransposed L1 repeat (including a poly[A] tail) with ~2.6 kbp of sequence identical to an intron of the nearby gene PPEF1, and a 119 bp target-site duplication that included a copy of exon 3 from CDKL5. The presence of the duplicated exon 3 was predicted to cause a frameshift in the transcript and inclusion was confirmed by RT-PCR. CDKL5 was previously associated with early infantile epileptic encephalopathy 2 (OMIM #300672), a condition that overlapped with the proband’s phenotype. In proband 4, a large complex de novo SV affecting chromosomes 6, 7, and 9 was identified. Examination of the proband’s haplotype-resolved genome assembly revealed a 126-Mbp pericentric-inverted fragment with eight additional breakpoints and eight rearranged fragments inside, some of which were inverted (Fig. 4b). Six genes were predicted to be disrupted by the presence of the 10 breakpoints on chromosome 6, but the region was not previously associated with neurodevelopmental disorder phenotypes. In the same sample, two translocations between chromosomes 7 and 9 were identified with part of the translocated region of chromosome 7 also inverted. The expected effect of this complex rearrangement is the disruption of DGKB (chromosome 7) and MLLT3 (chromosome 9). A decreased transcription of MLLT3 was observed from qPCR data, while DGKB could not be tested due to its low level of expression in blood. Balanced translocations involving chromosomes 4 and 9 and disrupting MLTT3 were previously reported in patients with phenotypes partially overlapping that of the proband [135, 136].
Fig. 4.
Examples of SNVs and SVs characterized by LRS that were difficult to fully resolve using SRS. a De novo insertion containing an additional copy of CDKL5 exon 3 identified by Hiatt et al. in an individual with intellectual disability [14]. b Complex chromosomal rearrangement including a 126-Mbp pericentric chromosome 6 inversion that contained a 9.3-Mbp region composed of eight segments rearranged in position and orientation identified by Hiatt et al. in an individual with intellectual disability [14]. The same individual also carried two insertional translocations between chromosomes 7 and 9. c De novo inversion predicted to disrupt both CPNE9 and BRPF1 identified by Mizuguchi et al. in two monozygotic twins with Dravet syndrome [16]. d A gene conversion identified by Watson et al. in a fetus with Meckel-Gruber syndrome that did not map well by SRS [12]. LRS identified a likely pathogenic SNV in the intron 5 splice donor site (G, red; haplotype 2) of TMEM231 in trans with a cluster of four missense SNVs (G, red; A, green; C, blue; and T, yellow; haplotype 1)
These examples highlight the complex nature of some of the genetic variants underlying disease that are difficult to detect or fully resolve using SRS. While these events represent promising candidates, they are not easily classified under existing American College of Medical Genetics (ACMG) recommendations [137]. As sequence-resolved complex rearrangements become more commonplace, additional updates to ACMG guidelines will be required that take into account events involving multiple breakpoints that likely alter transcriptional and regulatory control. It is reassuring that retrospective analysis using SRS data shows, in fact, that orthogonal sequence platforms confirm most of the new breakpoints being identified by LRS. However, discordance among SRS calling tools, incorrect or incomplete classification of size and class of variants, and the stringent requirement to minimize false positives have made discovery using SRS data extremely challenging. Such increased resolution for complex pathogenic events, including sequence resolution of cytogenetic/karyotypic rearrangements [15], is why long-read WGS (LR WGS) represents a potential alternative to SR WGS as a single clinical test.
Another recent compelling study by Mizuguchi and colleagues evaluated two monozygotic twins suspected to have Dravet syndrome with negative ES [16]. HiFi WGS revealed a de novo 12-kbp inversion that involved the first two exons of BRPF1 and CPNE9 in both probands confirmed by PCR (Fig. 4c). Disruptions of BRPF1 are associated with a specific form of intellectual disability consistent with the proband’s phenotype (OMIM: 617333). Use of LRS data allowed the authors to determine that the inversion breakpoints mapped to a (TA)n simple repeat element and to a mammalian-wide interspersed repeat (MIR) element, which is part of the short interspersed nuclear element (SINE) family. Due to the absence of indels at the junctions or sequence homology, nonhomologous end joining was proposed as the likely mechanism. The variant was deemed to be pathogenic because of the association of variants in BRPF1 with intellectual disability. It is worth noting that the SV caller used in this study (PBSV) miscalled this 12-kbp inversion (called twice as a deletion and as an insertion of different size) and further examination of reads alignment was required to characterize the variant. Complete sequence assembly of the phased haplotype in conjunction with Strand-seq would have likely fully resolved the pathogenic variant of interest [10].
The use of LRS on the ONT platform has become more prevalent in the clinical research community because of lower startup costs, lower materials costs, faster turnaround times, and greater flexibility (Table 1). There is a wide range of devices that vary in throughput (Table 1) ideal a variety of clinical (and field) applications where either whole-genome or targeted sequencing may be performed without the expense or footprint of large machines. The ONT platform is particularly nimble with respect to turnaround time [27, 28]. For example, Watson and colleagues used a targeted approach to evaluate an individual with suspected Meckel-Gruber syndrome (MKS), an autosomal recessive ciliopathy [12]. The condition is typically perinatally lethal and presents with cranial abnormalities, polydactyly, and other congenital malformations. The DNA of a fetus suspected to be affected by MKS initially underwent an SRS assay targeting 223 genes associated with pediatric neurological disorders, including 29 genes associated with MKS and Joubert syndrome. Two likely pathogenic heterozygous variants (1 SNV and 1 deletion) were identified in TMEM231 with SRS. The long-range PCR product was subsequently sequenced using ONT and confirmed only the SNV. Moreover, T-LRS revealed a cluster of four SNVs absent in the SRS data. The same cluster was previously reported in the literature as the result of a gene conversion between TMEM231 and a downstream pseudogene [138]. Sanger sequencing confirmed the gene conversion in the fetus (Fig. 4d). SRS data had incorrectly mapped the SNV cluster to the downstream pseudogene instead of TMEM231. This incorrect assignment essentially eliminated the SNVs from consideration and resulted in reduced coverage within the converted region of TMEM231, mimicking a heterozygous deletion. LRS analysis also revealed that the two variants identified were in trans and inherited from the healthy carrier parents, providing a good example of the additional information that LRS can provide in the clinical setting and how it may be used to overcome SRS mapping limitations.
Recent work has also shown high concordance between SVs identified by clinical testing and LRS. For example, Miller and colleagues evaluated 30 individuals with known SVs, using adaptive sampling on the ONT platform [15]. The authors reported 100% concordance with known SVs identified by clinical testing and LRS. In all eight individuals with known complex structural rearrangements, T-LRS identified the known aberrations and identified additional as well. They also showed that systematic evaluation of missing variant cases, or those with a single pathogenic variant in a gene associated with a recessive condition or no pathogenic variants found in suspected X-linked or dominant disorders, using LRS is high yield.
Summary and concluding remarks
In this review, we focused on the utility and advantages of LRS with respect to clinical research and human health. In short, both PacBio and ONT offer a more complete view of human variation and identify disease-causing variants missed by evaluation with both clinical and research SRS pipelines. These technologies led to the first complete human genome sequence [4], threefold improved SV discovery [6, 7, 9], more complete RNA sequencing [108, 109, 125], and modified base characterization of the human genome [30, 94]. PacBio offers greater sequencing accuracy, comparable to that of SRS, while ONT provides longer reads (up to >2 Mbp), rapid turnaround, and direct RNA sequencing.
Advances in SRS technology have been driven by massive increases in parallelization per machine to decrease cost and increase throughput per human genome. LRS technology, in contrast, has been focused on increasing read length and sequence accuracy. The recent launch of Revio by PacBio, which promises a highly accurate sequence of a human genome for $1000 in materials per human sample, represents an important shift in strategy and will allow more researchers to access high-quality LR WGS data. We also anticipate similar improvements with ONT, including duplex sequencing and advances in chemistry and pore structure to improve sequencing accuracy and increase output. Duplex sequencing allows for the sequencing of both strands of DNA, resulting in an increase in accuracy, but potentially sacrificing output and thus coverage [139].
At present, the two LRS technologies appear to be complementary and useful for different purposes. It is, however, tempting to speculate when LR WGS might emerge as a single test for clinical samples. Despite being more expensive than SR WGS, LR WGS advantages are clear: improved variant discovery (particularly for SVs), physical phasing of genomes, simultaneous discovery of methylation differences and genetic variants without additional experiments, and the ability to reanalyze a single dataset based on clinical suspicion. It is, in principle, the most comprehensive test currently available as it has the potential to fully sequence resolve both maternal and paternal chromosomes of a patient. If de novo assemblies of patient genomes and their parents become routine, it fundamentally changes how variants are discovered. Instead of read-based discovery, parent-to-offspring comparison of fully resolved chromosomes can be made to discover genetic and epigenetic changes of both small and large effect (Fig. 2). As the disadvantages, including, cost, throughput, and computational overhead are resolved, LRS will become a more attractive option to human genetics researchers and clinicians alike.
Recent efforts have tried to build automated and standardized pipelines for the screening and analysis of SVs [66, 140]. Mitsuhashi et al. focused on the analysis of large-scale chromosomal rearrangements, while Miyatake et al. on the analysis of repeat expansions. Both strategies call variations from read alignment to a reference, use a small control dataset (27 and 33 individuals) to remove common benign variations, and provide a list of prioritized candidate SVs for further manual investigation. Some type of variants (e.g., small deletions in Mitsuhashi et al.) are removed by the filtering step or by design (Miyatake et al. workflow is a targeted approach to only study repeat expansion loci associated to disease) to focus on the variant type of interest. These two workflows are very useful to simplify downstream analysis but also highlight the need for dedicated pipelines per SV type and larger control datasets. In fact, a current limitation of LRS is data interpretation, particularly for SVs of unknown significance and ultra-rare variants. At the moment, large databases of LRS samples from population controls comparable to SRS samples do not exist; SRS databases such as gnomAD-SV [8] cannot be used to assess frequency of a particular variant and the tolerance to mutation of many of the genes being accessed for the first time are unknown (e.g., there are no pLI [probability of being loss-of-function intolerant] [141] or LOEUF [loss-of-function observed/expected upper bound fraction] [142] scores for duplicate genes). Also, it is extremely important to have all populations represented in control datasets to perform a thorough screening of common variations.
Several efforts are underway to begin to characterize the normal pattern of variation (Table 3) in the general population using LRS. We expect that the increasing use of LRS in clinical studies and matched population controls will deepen our knowledge and interpretation of SVs and the decrease in sequencing cost will lead to progressively larger studies, as already seen for some circumscribed populations [143]. Together, these efforts will lead to improved outcomes for individuals with suspected Mendelian conditions who today remain unsolved after comprehensive evaluation, uncover novel biological processes that may be amenable to directed therapies, and allow development of improved clinical tests to reduce the time required to make precise genetic diagnoses.
Table 3.
Consortia using long-read sequencing
| Project / consortium | Description | Estimated samples involved for LRS | Sequencing technology employed |
|---|---|---|---|
| HGSVC | The Human Genome Structural Variation Consortium aims to create a high-quality map of human structural variation analyzing the genomes of individuals from different human populations and develop new discovery/analysis methods. | 69+ | PacBio, ONT |
| HPRC | The Human Pangenome Reference Consortium aims to develop a novel genome reference able to include all human genome variations and represent the full diversity of the human populations. | 350+ | PacBio, ONT |
| GREGoR | The GREGoR Consortium aims to substantially increase the number of Mendelian disorders with a known genetic cause focusing on the study of clinical cases. | 500+ | PacBio, ONT |
| ONT 1000 Genomes Project | The ONT 1000 Genomes Project aims to create a comprehensive genomic dataset of a large, diverse group of persons and provide an extensive catalog of structural variations. | 500+ | ONT |
| All of Us | The All of Us project aims to create a collection of genomic data from a large number of individuals in the United States. | 12,000+ | PacBio, ONT |
Supplementary Information
Additional file 1: Table S1. Additional examples of disease-associated variations resolved by LRS.
Acknowledgements
The authors thank G.A. Logsdon for discussion and assistance with the figures, K.M. Munson for technical assistance and real-world benchmarks, A.L. Miller for figure preparation, and T. Brown for assistance in editing this manuscript.
Abbreviations
- ACMG
American College of Medical Genetics
- CATCH
Cas9-Assisted Targeting of Chromosome segments
- CCS
Circular consensus sequencing
- cDNA
Complementary DNA
- CLR
Continuous long-read
- dNTPs
Deoxynucleoside triphosphates
- ES
Exome sequencing
- GPU
Graphical processing units
- HGSVC
Human Genome Structural Variation Consortium
- Hi-C
High-throughput capture chromatin conformation
- HiFi
High-fidelity
- HMW
High molecular weight
- HPRC
Human Pangenome Reference Consortium
- Iso-Seq
Isoform sequencing
- LITS
Large-insert targeted sequencing
- LOEUF
Loss-of-function observed/expected upper bound fraction
- LR WGS
Long-read whole-genome sequencing
- LRS
Long-read sequencing
- MKS
Meckel-Gruber syndrome
- MLPA
Multiplex ligation-dependent probe amplification
- MIR
Mammalian-wide interspersed repeat
- ONT
Oxford Nanopore Technologies
- PacBio
Pacific Biosciences
- PAV
Phased assembly variant
- PFGE
Pulsed field gel electrophoresis
- pLI
Probability of being loss-of-function intolerant
- PTLS
Potocki-Lupski syndrome
- RIN
RNA integrity number
- SINE
Short interspersed nuclear element
- SMRT
Single-molecule, real-time
- SNV
Single-nucleotide variant
- SR WGS
Short-read whole-genome sequencing
- SRS
Short-read sequencing
- Strand-seq
Single-cell strand sequencing
- SV
Structural variation
- T2T
Telomere-to-telomere
- T-LRS
Targeted long-read sequencing
- T-SRS
Targeted short-read sequencing
- ZMW
Zero-mode waveguide
- 5mC
5-Methylcytosine
Authors’ contributions
Writing, main draft, figures, and revisions: F.K.M., D.E.M., E.E.E. All authors read and approved the final manuscript.
Funding
D.E.M. is supported by NIH grant DP5OD033357. This work was supported, in part, by US National Institutes of Health (NIH) grants (R01HG002385 and R01MH101221) and the Simons Foundation (SFARI #810018EE) to E.E.E. E.E.E. is an investigator of the Howard Hughes Medical Institute (HHMI).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
E.E.E. is a scientific advisory board (SAB) member of Variant Bio, Inc. DEM is on a SAB at Oxford Nanopore Technologies (ONT), is engaged in a research agreement with ONT, and ONT has paid for him to travel to speak on their behalf. The remaining author declares that he does not have any competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Francesco Kumara Mastrorosa and Danny E. Miller contributed equally.
References
- 1.Kingsmore SF, Cakici JA, Clark MM, Gaughran M, Feddock M, Batalov S, et al. A randomized, controlled trial of the analytic and diagnostic performance of singleton and trio, rapid genome and exome sequencing in ill infants. Am J Hum Genet. 2019;105(4):719–33. doi: 10.1016/j.ajhg.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costain G, Walker S, Marano M, Veenma D, Snell M, Curtis M, et al. Genome sequencing as a diagnostic test in children with unexplained medical complexity. JAMA Network Open. 2020;3(9):e2018109. doi: 10.1001/jamanetworkopen.2020.18109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vollger MR, Guitart X, Dishuck PC, Mercuri L, Harvey WT, Gershman A, et al. Segmental duplications and their variation in a complete human genome. Science. 2022;376(6588):eabj6965. doi: 10.1126/science.abj6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nurk S, Koren S, Rhie A, Rautiainen M, Bzikadze AV, Mikheenko A, et al. The complete sequence of a human genome. Science. 2022;376(6588):44–53. doi: 10.1126/science.abj6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logsdon GA, Vollger MR, Eichler EE. Long-read human genome sequencing and its applications. Nat Rev Genet. 2020;21(10):597–614. doi: 10.1038/s41576-020-0236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaisson MJP, Sanders AD, Zhao X, Malhotra A, Porubsky D, Rausch T, et al. Multi-platform discovery of haplotype-resolved structural variation in human genomes. Nat Commun. 2019;10(1):1784. doi: 10.1038/s41467-018-08148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Audano PA, Sulovari A, Graves-Lindsay TA, Cantsilieris S, Sorensen M, Welch AE, et al. Characterizing the major structural variant alleles of the human genome. Cell. 2019;176(3):663–675.e19. doi: 10.1016/j.cell.2018.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins RL, Brand H, Karczewski KJ, Zhao X, Alföldi J, Francioli LC, et al. A structural variation reference for medical and population genetics. Nature. 2020;581(7809):444–51. doi: 10.1038/s41586-020-2287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebert P, Audano PA, Zhu Q, Rodriguez-Martin B, Porubsky D, Bonder MJ, et al. Haplotype-resolved diverse human genomes and integrated analysis of structural variation. Science. 2021;372(6537):eabf7117. doi: 10.1126/science.abf7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porubsky D, Ebert P, Audano PA, Vollger MR, Harvey WT, Marijon P, et al. Fully phased human genome assembly without parental data using single-cell strand sequencing and long reads. Nat Biotechnol. 2021;39(3):302–8. doi: 10.1038/s41587-020-0719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaché C, Puechberty J, Faugère V, Darmaisin F, Liquori A, Baux D, et al. A 4.6 Mb inversion leading to PCDH15-LINC00844 and BICC1-PCDH15 fusion transcripts as a new pathogenic mechanism implicated in usher syndrome type 1. Front Genet. 2020;11:623. doi: 10.3389/fgene.2020.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson CM, Dean P, Camm N, Bates J, Carr IM, Gardiner CA, et al. Long-read nanopore sequencing resolves a TMEM231 gene conversion event causing Meckel-Gruber syndrome. Human Mutation. 2020;41(2):525–31. doi: 10.1002/humu.23940. [DOI] [PubMed] [Google Scholar]
- 13.Xie Z, Sun C, Zhang S, Liu Y, Yu M, Zheng Y, et al. Long-read whole-genome sequencing for the genetic diagnosis of dystrophinopathies. Ann Clin Transl Neurol. 2020;7(10):2041–6. doi: 10.1002/acn3.51201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiatt SM, Lawlor JMJ, Handley LH, Ramaker RC, Rogers BB, Partridge EC, et al. Long-read genome sequencing for the molecular diagnosis of neurodevelopmental disorders. Human Genet Genomics Adv. 2021;2(2):100023. doi: 10.1016/j.xhgg.2021.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller DE, Sulovari A, Wang T, Loucks H, Hoekzema K, Munson KM, et al. Targeted long-read sequencing identifies missing disease-causing variation. Am J Hum Genet. 2021;108(8):1436–49. doi: 10.1016/j.ajhg.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizuguchi T, Okamoto N, Yanagihara K, Miyatake S, Uchiyama Y, Tsuchida N, et al. Pathogenic 12-kb copy-neutral inversion in syndromic intellectual disability identified by high-fidelity long-read sequencing. Genomics. 2021;113(1 Pt 2):1044–53. doi: 10.1016/j.ygeno.2020.10.038. [DOI] [PubMed] [Google Scholar]
- 17.Sano Y, Koyanagi Y, Wong JH, Murakami Y, Fujiwara K, Endo M, et al. Likely pathogenic structural variants in genetically unsolved patients with retinitis pigmentosa revealed by long-read sequencing. J Med Genet. 2022;59(11):1133–1138. doi: 10.1136/jmedgenet-2022-108428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaisson MJP, Huddleston J, Dennis MY, Sudmant PH, Malig M, Hormozdiari F, et al. Resolving the complexity of the human genome using single-molecule sequencing. Nature. 2015;517(7536):608–11. doi: 10.1038/nature13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huddleston J, Chaisson MJP, Steinberg KM, Warren W, Hoekzema K, Gordon D, et al. Discovery and genotyping of structural variation from long-read haploid genome sequence data. Genome Res. 2017;27(5):677–85. doi: 10.1101/gr.214007.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandelker D, Schmidt RJ, Ankala A, McDonald Gibson K, Bowser M, Sharma H, et al. Navigating highly homologous genes in a molecular diagnostic setting: a resource for clinical next-generation sequencing. Genet Med. 2016;18(12):1282–9. doi: 10.1038/gim.2016.58. [DOI] [PubMed] [Google Scholar]
- 21.Wagner J, Olson ND, Harris L, McDaniel J, Cheng H, Fungtammasan A, et al. Curated variation benchmarks for challenging medically relevant autosomal genes. Nat Biotechnol. 2022;40(5):672–80. doi: 10.1038/s41587-021-01158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rangan A, Hein MS, Jenkinson WG, Koganti T, Aleff RA, Hilker CA, et al. Improved characterization of complex β-globin gene cluster structural variants using long-read sequencing. J Mol Diagn. 2021;23(12):1732–40. doi: 10.1016/j.jmoldx.2021.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Bruels CC, Littel HR, Daugherty AL, Stafki S, Estrella EA, McGaughy ES, et al. Diagnostic capabilities of nanopore long-read sequencing in muscular dystrophy. Ann Clin Transl Neurol. 2022;9(8):1302–9. doi: 10.1002/acn3.51612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boerkoel PK, Dixon K, Fitzsimons C, Shen Y, Huynh S, Schlade-Bartusiak K, et al. Long-read genome sequencing resolves a complex 13q structural variant associated with syndromic anophthalmia. Am J Med Genet Part A. 2022;188(5):1589–94. doi: 10.1002/ajmg.a.62676. [DOI] [PubMed] [Google Scholar]
- 25.Ishiura H, Shibata S, Yoshimura J, Suzuki Y, Qu W, Doi K, et al. Noncoding CGG repeat expansions in neuronal intranuclear inclusion disease, oculopharyngodistal myopathy and an overlapping disease. Nat Genet. 2019;51(8):1222–32. doi: 10.1038/s41588-019-0458-z. [DOI] [PubMed] [Google Scholar]
- 26.Mangin A, de Pontual L, Tsai YC, Monteil L, Nizon M, Boisseau P, et al. Robust detection of somatic mosaicism and repeat interruptions by long-read targeted sequencing in myotonic dystrophy type 1. Int J Mol Sci. 2021;22(5):2616. doi: 10.3390/ijms22052616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galey M, Reed P, Wenger T, Beckman E, Chang IJ, Paschal CR, et al. 3-hour genome sequencing and targeted analysis to rapidly assess genetic risk. Genet Genomic Med; 2022 [cited 2022 Nov 15]. Available from: 10.1101/2022.09.09.22279746.
- 28.Gorzynski JE, Goenka SD, Shafin K, Jensen TD, Fisk DG, Grove ME, et al. Ultrarapid Nanopore genome sequencing in a critical care setting. N Engl J Med. 2022;386(7):700–2. doi: 10.1056/NEJMc2112090. [DOI] [PubMed] [Google Scholar]
- 29.Wenger AM, Peluso P, Rowell WJ, Chang PC, Hall RJ, Concepcion GT, et al. Accurate circular consensus long-read sequencing improves variant detection and assembly of a human genome. Nat Biotechnol. 2019;37(10):1155–62. doi: 10.1038/s41587-019-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flusberg BA, Webster DR, Lee JH, Travers KJ, Olivares EC, Clark TA, et al. Direct detection of DNA methylation during single-molecule, real-time sequencing. Nat Methods. 2010;7(6):461–5. doi: 10.1038/nmeth.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perešíni P, Boža V, Brejová B, Vinař T. Nanopore base calling on the edge. Bioinformatics. 2021;37(24):4661–7. doi: 10.1093/bioinformatics/btab528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shafin K, Pesout T, Chang PC, Nattestad M, Kolesnikov A, Goel S, et al. Haplotype-aware variant calling with PEPPER-Margin-DeepVariant enables high accuracy in nanopore long-reads. Nat Methods. 2021;18(11):1322–32. doi: 10.1038/s41592-021-01299-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nurk S, Walenz BP, Rhie A, Vollger MR, Logsdon GA, Grothe R, et al. HiCanu: accurate assembly of segmental duplications, satellites, and allelic variants from high-fidelity long reads. Genome Res. 2020;gr.263566.120. [DOI] [PMC free article] [PubMed]
- 34.Tan KT, Slevin MK, Meyerson M, Li H. Identifying and correcting repeat-calling errors in nanopore sequencing of telomeres. Genome Biology. 2022;23(1):180. doi: 10.1186/s13059-022-02751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sereika M, Kirkegaard RH, Karst SM, Michaelsen TY, Sørensen EA, Wollenberg RD, et al. Oxford Nanopore R10.4 long-read sequencing enables the generation of near-finished bacterial genomes from pure cultures and metagenomes without short-read or reference polishing. Nat Methods. 2022;19(7):823–6. doi: 10.1038/s41592-022-01539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolmogorov M, Billingsley KJ, Mastoras M, Meredith M, Monlong J, Lorig-Roach R, et al. Scalable Nanopore sequencing of human genomes provides a comprehensive view of haplotype-resolved variation and methylation. bioRxiv; 2023 [cited 2023 Apr 6]. p. 2023.01.12.523790. Available from: 10.1101/2023.01.12.523790v2. [DOI] [PMC free article] [PubMed]
- 37.Payne A, Holmes N, Clarke T, Munro R, Debebe BJ, Loose M. Readfish enables targeted nanopore sequencing of gigabase-sized genomes. Nat Biotechnol. 2021;39(4):442–50. doi: 10.1038/s41587-020-00746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Payne A, Munro R, Holmes N, Moore C, Carlile M, Loose M. Barcode aware adaptive sampling for GridION and PromethION Oxford Nanopore sequencers. bioRxiv; 2022 [cited 2022 Nov 29]. p. 2021.12.01.470722. Available from: 10.1101/2021.12.01.470722v2.
- 39.Boccaletto P, Machnicka MA, Purta E, Piatkowski P, Baginski B, Wirecki TK, et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46(1):303–7. doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leger A, Amaral PP, Pandolfini L, Capitanchik C, Capraro F, Miano V, et al. RNA modifications detection by comparative Nanopore direct RNA sequencing. Nat Commun. 2021;12(1):7198. doi: 10.1038/s41467-021-27393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas NK, Poodari VC, Jain M, Olsen HE, Akeson M, Abu-Shumays RL. Direct nanopore sequencing of individual full length tRNA strands. ACS Nano. 2021;15(10):16642–53. doi: 10.1021/acsnano.1c06488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoenen T, Groseth A, Rosenke K, Fischer RJ, Hoenen A, Judson SD, et al. Nanopore sequencing as a rapidly deployable Ebola outbreak tool. Emerg Infect Dis. 2016;22(2):331–4. doi: 10.3201/eid2202.151796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaffer L. Portable DNA sequencer helps farmers stymie devastating viruses. Proc Natl Acad Sci. 2019;116(9):3351–3. doi: 10.1073/pnas.1901806116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vandenbogaert M, Kwasiborski A, Gonofio E, Descorps-Declère S, Selekon B, NkiliMeyong AA, et al. Nanopore sequencing of a monkeypox virus strain isolated from a pustular lesion in the Central African Republic. Sci Rep. 2022;12(1):10768. doi: 10.1038/s41598-022-15073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson SS, Zaikova E, Goerlitz DS, Bai Y, Tighe SW. Real-Time DNA Sequencing in the Antarctic Dry Valleys Using the Oxford Nanopore Sequencer. J Biomol Tech. 2017;28(1):2–7. doi: 10.7171/jbt.17-2801-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro-Wallace SL, Chiu CY, John KK, Stahl SE, Rubins KH, McIntyre ABR, et al. Nanopore DNA sequencing and genome assembly on the International Space Station. Sci Rep. 2017;7(1):18022. doi: 10.1038/s41598-017-18364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Firtina C, Bar-Joseph Z, Alkan C, Cicek AE. Hercules: a profile HMM-based hybrid error correction algorithm for long reads. Nucleic Acids Research. 2018;46(21):e125. doi: 10.1093/nar/gky724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hackl T, Hedrich R, Schultz J, Förster F. proovread : large-scale high-accuracy PacBio correction through iterative short read consensus. Bioinformatics. 2014;30(21):3004–11. doi: 10.1093/bioinformatics/btu392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salmela L, Rivals E. LoRDEC: accurate and efficient long read error correction. Bioinformatics. 2014;30(24):3506–14. doi: 10.1093/bioinformatics/btu538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haghshenas E, Hach F, Sahinalp SC, Chauve C. CoLoRMap: Correcting Long Reads by Mapping short reads. Bioinformatics. 2016;32(17):i545–51. doi: 10.1093/bioinformatics/btw463. [DOI] [PubMed] [Google Scholar]
- 51.Morisse P, Lecroq T, Lefebvre A. Hybrid correction of highly noisy long reads using a variable-order de Bruijn graph. Bioinformatics. 2018;34(24):4213–22. doi: 10.1093/bioinformatics/bty521. [DOI] [PubMed] [Google Scholar]
- 52.Bao E, Lan L. HALC: High throughput algorithm for long read error correction. BMC Bioinformatics. 2017;18(1):204. doi: 10.1186/s12859-017-1610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao E, Xie F, Song C, Song D. FLAS: fast and high-throughput algorithm for PacBio long-read self-correction. Bioinformatics. 2019;35(20):3953–60. doi: 10.1093/bioinformatics/btz206. [DOI] [PubMed] [Google Scholar]
- 54.Salmela L, Walve R, Rivals E, Ukkonen E. Accurate self-correction of errors in long reads using de Bruijn graphs. Bioinformatics. 2017;33(6):799–806. doi: 10.1093/bioinformatics/btw321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H, Jain C, Aluru S. A comprehensive evaluation of long read error correction methods. BMC Genomics. 2020;21(6):889. doi: 10.1186/s12864-020-07227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo X, Kang X, Schönhuth A. VeChat: correcting errors in long reads using variation graphs. Nat Commun. 2022;13(1):6657. doi: 10.1038/s41467-022-34381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baid G, Cook DE, Shafin K, Yun T, Llinares-López F, Berthet Q, et al. DeepConsensus improves the accuracy of sequences with a gap-aware sequence transformer. Nat Biotechnol. 2022;1:1–7. doi: 10.1038/s41587-022-01435-7. [DOI] [PubMed] [Google Scholar]
- 58.Loomis EW, Eid JS, Peluso P, Yin J, Hickey L, Rank D, et al. Sequencing the unsequenceable: Expanded CGG-repeat alleles of the fragile X gene. Genome Res. 2013;23(1):121–8. doi: 10.1101/gr.141705.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang M, Beck CR, English AC, Meng Q, Buhay C, Han Y, et al. PacBio-LITS: a large-insert targeted sequencing method for characterization of human disease-associated chromosomal structural variations. BMC Genomics. 2015;16(1):214. doi: 10.1186/s12864-015-1370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilpatrick T, Lee I, Graham JE, Raimondeau E, Bowen R, Heron A, et al. Targeted nanopore sequencing with Cas9-guided adapter ligation. Nat Biotechnol. 2020;38(4):433–8. doi: 10.1038/s41587-020-0407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Höijer I, Tsai YC, Clark TA, Kotturi P, Dahl N, Stattin EL, et al. Detailed analysis of HTT repeat elements in human blood using targeted amplification-free long-read sequencing. Human Mutation. 2018;39(9):1262–72. doi: 10.1002/humu.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gabrieli T, Sharim H, Fridman D, Arbib N, Michaeli Y, Ebenstein Y. Selective nanopore sequencing of human BRCA1 by Cas9-assisted targeting of chromosome segments (CATCH) Nucleic Acids Research. 2018;46(14):e87. doi: 10.1093/nar/gky411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walsh T, Casadei S, Munson KM, Eng M, Mandell JB, Gulsuner S, et al. CRISPR–Cas9/long-read sequencing approach to identify cryptic mutations in BRCA1 and other tumour suppressor genes. J Med Genet. 2021;58(12):850–2. doi: 10.1136/jmedgenet-2020-107320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller DE, Hanna P, Galey M, Reyes M, Linglart A, Eichler EE, et al. Targeted long-read sequencing identifies a retrotransposon insertion as a cause of altered GNAS exon A/B methylation in a family with autosomal dominant pseudohypoparathyroidism type 1b (PHP1B) J Bone Miner Res. 2022;37(9):1711–9. doi: 10.1002/jbmr.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller DE, Lee L, Galey M, Kandhaya-Pillai R, Tischkowitz M, Amalnath D, et al. Targeted long-read sequencing identifies missing pathogenic variants in unsolved Werner syndrome cases. Jl Med Genet. 2022;59(11):1087–94. doi: 10.1136/jmedgenet-2022-108485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyatake S, Koshimizu E, Fujita A, Doi H, Okubo M, Wada T, et al. Rapid and comprehensive diagnostic method for repeat expansion diseases using nanopore sequencing. Genom Med. 2022;7(1):1–15. doi: 10.1038/s41525-022-00331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sambrook J, Russell DW. Purification of nucleic acids by extraction with Phenol:Chloroform. Cold Spring Harb Protoc. 2006;2006(1):pdb.prot4455. doi: 10.1101/pdb.prot4455. [DOI] [PubMed] [Google Scholar]
- 68.Quick J. Ultra-long read sequencing protocol for RAD004. 2018 [cited 2023 Mar 29]; Available from: https://www.protocols.io/view/ultra-long-read-sequencing-protocol-for-rad004-mrxc57n.
- 69.Logsdon G. HMW gDNA purification and ONT ultra-long-read data generation. 2022 [cited 2023 Mar 29]; Available from: https://protocols.io/view/hmw-gdna-purification-and-ont-ultra-long-read-data-b55tq86n.
- 70.Auton A, Abecasis GR, Altshuler DM, Durbin RM, Abecasis GR, Bentley DR, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaisson MJ, Tesler G. Mapping single molecule sequencing reads using basic local alignment with successive refinement (BLASR): application and theory. BMC Bioinformatics. 2012;13(1):238. doi: 10.1186/1471-2105-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berlin K, Koren S, Chin CS, Drake JP, Landolin JM, Phillippy AM. Assembling large genomes with single-molecule sequencing and locality-sensitive hashing. Nat Biotechnol. 2015;33(6):623–30. doi: 10.1038/nbt.3238. [DOI] [PubMed] [Google Scholar]
- 73.Sedlazeck FJ, Rescheneder P, Smolka M, Fang H, Nattestad M, von Haeseler A, et al. Accurate detection of complex structural variations using single-molecule sequencing. Nat Methods. 2018;15(6):461–8. doi: 10.1038/s41592-018-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34(18):3094–100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin M, Patterson M, Garg S, Fischer SO, Pisanti N, Klau GW, et al. WhatsHap: fast and accurate read-based phasing. bioRxiv; 2016 [cited 2022 Oct 23]. p. 085050. Available from: 10.1101/085050v2.
- 76.Poplin R, Chang PC, Alexander D, Schwartz S, Colthurst T, Ku A, et al. A universal SNP and small-indel variant caller using deep neural networks. Nat Biotechnol. 2018;36(10):983–7. doi: 10.1038/nbt.4235. [DOI] [PubMed] [Google Scholar]
- 77.Jiang T, Liu Y, Jiang Y, Li J, Gao Y, Cui Z, et al. Long-read-based human genomic structural variation detection with cuteSV. Genome Biology. 2020;21(1):189. doi: 10.1186/s13059-020-02107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin JH, Chen LC, Yu SC, Huang YT. LongPhase: an ultra-fast chromosome-scale phasing algorithm for small and large variants. Bioinformatics. 2022;38(7):1816–22. doi: 10.1093/bioinformatics/btac058. [DOI] [PubMed] [Google Scholar]
- 79.Jarvis ED, Formenti G, Rhie A, Guarracino A, Yang C, Wood J, et al. Semi-automated assembly of high-quality diploid human reference genomes. Nature. 2022;19:1–13. doi: 10.1038/s41586-022-05325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vollger MR, Logsdon GA, Audano PA, Sulovari A, Porubsky D, Peluso P, et al. Improved assembly and variant detection of a haploid human genome using single-molecule, high-fidelity long reads. Ann Hum Genet. 2020;84(2):125–40. doi: 10.1111/ahg.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chin CS, Khalak A. Human genome assembly in 100 minutes. bioRxiv; 2019 [cited 2022 Oct 22]. p. 705616. Available from: 10.1101/705616v1.
- 82.Ruan J, Li H. Fast and accurate long-read assembly with wtdbg2. Nat Methods. 2020;17(2):155–8. doi: 10.1038/s41592-019-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019;37(5):540–6. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 84.Shafin K, Pesout T, Lorig-Roach R, Haukness M, Olsen HE, Bosworth C, et al. Nanopore sequencing and the Shasta toolkit enable efficient de novo assembly of eleven human genomes. Nat Biotechnol. 2020;38(9):1044–53. doi: 10.1038/s41587-020-0503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng H, Concepcion GT, Feng X, Zhang H, Li H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods. 2021;18(2):170–5. doi: 10.1038/s41592-020-01056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng H, Jarvis ED, Fedrigo O, Koepfli KP, Urban L, Gemmell NJ, et al. Haplotype-resolved assembly of diploid genomes without parental data. Nat Biotechnol. 2022;40(9):1332–5. doi: 10.1038/s41587-022-01261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rautiainen M, Nurk S, Walenz BP, Logsdon GA, Porubsky D, Rhie A, et al. Verkko: telomere-to-telomere assembly of diploid chromosomes. bioRxiv; 2022 [cited 2022 Oct 22]. p. 2022.06.24.497523. Available from: 10.1101/2022.06.24.497523v1. [DOI] [PMC free article] [PubMed]
- 88.Chaisson MJP, Wilson RK, Eichler EE. Genetic variation and the de novo assembly of human genomes. Nat Rev Genet. 2015;16(11):627–40. doi: 10.1038/nrg3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koren S, Rhie A, Walenz BP, Dilthey AT, Bickhart DM, Kingan SB, et al. De novo assembly of haplotype-resolved genomes with trio binning. Nat Biotechnol. 2018;36(12):1174–82. doi: 10.1038/nbt.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vollger MR. SafFire. 2022 [cited 2022 Dec 8]. Available from: https://github.com/mrvollger/SafFire.
- 91.Ebler J, Ebert P, Clarke WE, Rausch T, Audano PA, Houwaart T, et al. Pangenome-based genome inference allows efficient and accurate genotyping across a wide spectrum of variant classes. Nat Genet. 2022;54(4):518–25. doi: 10.1038/s41588-022-01043-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liao WW, Asri M, Ebler J, Doerr D, Haukness M, Hickey G, et al. A draft human pangenome reference. Genomics; 2022 [cited 2022 Nov 15]. Available from: 10.1101/2022.07.09.499321.
- 93.Primrose. PacBio; 2022 [cited 2022 Dec 8]. Available from: https://github.com/PacificBiosciences/primrose.
- 94.Simpson JT, Workman RE, Zuzarte PC, David M, Dursi LJ, Timp W. Detecting DNA cytosine methylation using nanopore sequencing. Nat Methods. 2017;14(4):407–10. doi: 10.1038/nmeth.4184. [DOI] [PubMed] [Google Scholar]
- 95.Stoiber M, Quick J, Egan R, Lee JE, Celniker S, Neely RK, et al. De novo identification of DNA modifications enabled by genome-guided nanopore signal processing. bioRxiv; 2017 [cited 2022 Nov 18]. p. 094672. Available from: https://www.biorxiv.org/content/10.1101/094672v2.
- 96.Ni P, Huang N, Zhang Z, Wang DP, Liang F, Miao Y, et al. DeepSignal: detecting DNA methylation state from Nanopore sequencing reads using deep-learning. Bioinformatics. 2019;35(22):4586–95. doi: 10.1093/bioinformatics/btz276. [DOI] [PubMed] [Google Scholar]
- 97.Liu Q, Fang L, Yu G, Wang D, Xiao CL, Wang K. Detection of DNA base modifications by deep recurrent neural network on Oxford Nanopore sequencing data. Nat Commun. 2019;10(1):2449. doi: 10.1038/s41467-019-10168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.nanoporetech/megalodon. Oxford Nanopore Technologies; 2022 [cited 2022 Dec 8]. Available from: https://github.com/nanoporetech/megalodon.
- 99.Nanopore sequencing data analysis. Oxford Nanopore Technologies. [cited 2022 Dec 8]. Available from: http://nanoporetech.com/nanopore-sequencing-data-analysis.
- 100.LaCroix AJ, Stabley D, Sahraoui R, Adam MP, Mehaffey M, Kernan K, et al. GGC Repeat expansion and exon 1 methylation of XYLT1 is a common pathogenic variant in Baratela-Scott Syndrome. Am J Hum Genet. 2019;104(1):35–44. doi: 10.1016/j.ajhg.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nobile V, Pucci C, Chiurazzi P, Neri G, Tabolacci E. DNA Methylation, Mechanisms of FMR1 inactivation and therapeutic perspectives for Fragile X Syndrome. Biomolecules. 2021;11(2):296. doi: 10.3390/biom11020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim K, Hessl D, Randol JL, Espinal GM, Schneider A, Protic D, et al. Association between IQ and FMR1 protein (FMRP) across the spectrum of CGG repeat expansions. PLOS ONE. 2019;14(12):e0226811. doi: 10.1371/journal.pone.0226811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Budimirovic DB, Schlageter A, Filipovic-Sadic S, Protic DD, Bram E, Mahone EM, et al. A genotype-phenotype study of high-resolution FMR1 nucleic acid and protein analyses in Fragile X patients with neurobehavioral assessments. Brain Sciences. 2020;10(10):694. doi: 10.3390/brainsci10100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boggs AE, Schmitt LM, McLane RD, Adayev T, LaFauci G, Horn PS, et al. Optimization, validation and initial clinical implications of a Luminex-based immunoassay for the quantification of Fragile X Protein from dried blood spots. Sci Rep. 2022;12(1):5617. doi: 10.1038/s41598-022-09633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 106.McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168(4):613–28. doi: 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 107.Casado-Pelaez M, Bueno-Costa A, Esteller M. Single cell cancer epigenetics. Trends in Cancer. 2022;8(10):820–38. doi: 10.1016/j.trecan.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 108.Oikonomopoulos S, Wang YC, Djambazian H, Badescu D, Ragoussis J. Benchmarking of the Oxford Nanopore MinION sequencing for quantitative and qualitative assessment of cDNA populations. Sci Rep. 2016;24(6):31602. doi: 10.1038/srep31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garalde DR, Snell EA, Jachimowicz D, Sipos B, Lloyd JH, Bruce M, et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat Methods. 2018;15(3):201–6. doi: 10.1038/nmeth.4577. [DOI] [PubMed] [Google Scholar]
- 110.Depledge DP, Srinivas KP, Sadaoka T, Bready D, Mori Y, Placantonakis DG, et al. Direct RNA sequencing on nanopore arrays redefines the transcriptional complexity of a viral pathogen. Nat Commun. 2019;10(1):754. doi: 10.1038/s41467-019-08734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith AM, Jain M, Mulroney L, Garalde DR, Akeson M. Reading canonical and modified nucleobases in 16S ribosomal RNA using nanopore native RNA sequencing. PLOS ONE. 2019;14(5):e0216709. doi: 10.1371/journal.pone.0216709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pust MM, Davenport CF, Wiehlmann L, Tümmler B. Direct RNA Nanopore sequencing of Pseudomonas aeruginosa Clone C Transcriptomes. J Bacteriol. 2022;204(1):e00418–21. doi: 10.1128/JB.00418-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vacca D, Fiannaca A, Tramuto F, Cancila V, La Paglia L, Mazzucco W, et al. Direct RNA Nanopore sequencing of SARS-CoV-2 extracted from critical material from swabs. Life (Basel). 2022;12(1):69. doi: 10.3390/life12010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Workman RE, Tang AD, Tang PS, Jain M, Tyson JR, Razaghi R, et al. Nanopore native RNA sequencing of a human poly(A) transcriptome. Nat Methods. 2019;16(12):1297–305. doi: 10.1038/s41592-019-0617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mulroney L, Wulf MG, Schildkraut I, Tzertzinis G, Buswell J, Jain M, et al. Identification of high-confidence human poly(A) RNA isoform scaffolds using nanopore sequencing. RNA. 2022;28(2):162–76. doi: 10.1261/rna.078703.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jain M, Abu-Shumays R, Olsen HE, Akeson M. Advances in nanopore direct RNA sequencing. Nat Methods. 2022;19(10):1160–4. doi: 10.1038/s41592-022-01633-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dainis A, Tseng E, Clark TA, Hon T, Wheeler M, Ashley E. argeted long-read RNA sequencing demonstrates transcriptional diversity driven by splice-site variation in MYBPC3. Circulation: Genom Precis Med. 2019;12(5):e002464. doi: 10.1161/CIRCGEN.119.002464. [DOI] [PubMed] [Google Scholar]
- 118.Huang KK, Huang J, Wu JKL, Lee M, Tay ST, Kumar V, et al. Long-read transcriptome sequencing reveals abundant promoter diversity in distinct molecular subtypes of gastric cancer. Genome Biology. 2021;22(1):44. doi: 10.1186/s13059-021-02261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miller AR, Wijeratne S, McGrath SD, Schieffer KM, Miller KE, Lee K, et al. Pacific biosciences fusion and long isoform pipeline for cancer transcriptome–based resolution of isoform complexity. J Mol Diagn. 2022;24(12):1292–1306. doi: 10.1016/j.jmoldx.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 120.Veiga DFT, Nesta A, Zhao Y, Mays AD, Huynh R, Rossi R, et al. A comprehensive long-read isoform analysis platform and sequencing resource for breast cancer. Sci Adv. 2022;8(3):eabg6711. doi: 10.1126/sciadv.abg6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mincarelli L, Uzun V, Rushworth SA, Haerty W, Macaulay IC. Combined single-cell gene and isoform expression analysis in haematopoietic stem and progenitor cells. bioRxiv; 2020 [cited 2022 Nov 23]. p. 2020.04.06.027474. Available from: 10.1101/2020.04.06.027474v1. [DOI] [PMC free article] [PubMed]
- 122.Joglekar A, Prjibelski A, Mahfouz A, Collier P, Lin S, Schlusche AK, et al. A spatially resolved brain region- and cell type-specific isoform atlas of the postnatal mouse brain. Nat Commun. 2021;12(1):463. doi: 10.1038/s41467-020-20343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koboldt DC, Miller KE, Miller AR, Bush JM, McGrath S, Leraas K, et al. PTEN somatic mutations contribute to spectrum of cerebral overgrowth. Brain. 2021;144(10):2971–8. doi: 10.1093/brain/awab173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Palmer CR, Liu CS, Romanow WJ, Lee MH, Chun J. Altered cell and RNA isoform diversity in aging Down syndrome brains. Proc Natl Acad Sci. 2021;118(47):e2114326118. doi: 10.1073/pnas.2114326118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Al’Khafaji AM, Smith JT, Garimella KV, Babadi M, Sade-Feldman M, Gatzen M, et al. High-throughput RNA isoform sequencing using programmable cDNA concatenation. bioRxiv; 2021 [cited 2022 Nov 17]. p. 2021.10.01.462818. Available from: 10.1101/2021.10.01.462818v1.
- 126.Chen L, Zhao N, Cao J, Liu X, Xu J, Ma Y, et al. Short- and long-read metagenomics expand individualized structural variations in gut microbiomes. Nat Commun. 2022;13(1):3175. doi: 10.1038/s41467-022-30857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou L, Qiu Q, Zhou Q, Li J, Yu M, Li K, et al. Long-read sequencing unveils high-resolution HPV integration and its oncogenic progression in cervical cancer. Nat Commun. 2022;13(1):2563. doi: 10.1038/s41467-022-30190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Quick J, Loman NJ, Duraffour S, Simpson JT, Severi E, Cowley L, et al. Real-time, portable genome sequencing for Ebola surveillance. Nature. 2016;530(7589):228–32. doi: 10.1038/nature16996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Park SY, Faraci G, Ward PM, Emerson JF, Lee HY. High-precision and cost-efficient sequencing for real-time COVID-19 surveillance. Sci Rep. 2021;11(1):13669. doi: 10.1038/s41598-021-93145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Au CH, Ho DN, Ip BBK, Wan TSK, Ng MHL, Chiu EKW, et al. Rapid detection of chromosomal translocation and precise breakpoint characterization in acute myeloid leukemia by nanopore long-read sequencing. Cancer Genetics. 2019;1(239):22–5. doi: 10.1016/j.cancergen.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 131.Watson CM, Crinnion LA, Lindsay H, Mitchell R, Camm N, Robinson R, et al. Assessing the utility of long-read nanopore sequencing for rapid and efficient characterization of mobile element insertions. Lab Invest. 2021;101(4):442–9. doi: 10.1038/s41374-020-00489-y. [DOI] [PubMed] [Google Scholar]
- 132.Gilissen C, Hehir-Kwa JY, Thung DT, Van De Vorst M, Van Bon BWM, Willemsen MH, et al. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511(7509):344–7. doi: 10.1038/nature13394. [DOI] [PubMed] [Google Scholar]
- 133.Watson CM, Jackson L, Crinnion LA, Bonthron DT, Sheridan E. Long-read sequencing to resolve the parent of origin of a de novo pathogenic UBE3A variant. J Med Genet. 2022;59(11):1082–1086. doi: 10.1136/jmedgenet-2021-108314. [DOI] [PubMed] [Google Scholar]
- 134.Thibodeau ML, O’Neill K, Dixon K, Reisle C, Mungall KL, Krzywinski M, et al. Improved structural variant interpretation for hereditary cancer susceptibility using long-read sequencing. Genet Med. 2020;22(11):1892–7. doi: 10.1038/s41436-020-0880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pramparo T, Grosso S, Messa J, Zatterale A, Bonaglia MC, Chessa L, et al. Loss-of-function mutation of the AF9/MLLT3 gene in a girl with neuromotor development delay, cerebellar ataxia, and epilepsy. Hum Genet. 2005;118(1):76–81. doi: 10.1007/s00439-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 136.Striano P, Elia M, Castiglia L, Galesi O, Pelligra S, Striano S. A t(4;9)(q34;p22) Translocation associated with partial epilepsy, mental retardation, and dysmorphism. Epilepsia. 2005;46(8):1322–4. doi: 10.1111/j.1528-1167.2005.64304.x. [DOI] [PubMed] [Google Scholar]
- 137.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. 2015; Available from: https://www.acmg.net/docs/standards_guidelines_for_the_interpretation_of_sequence_variants.pdf. [DOI] [PMC free article] [PubMed]
- 138.Maglic D, Stephen J, Malicdan MCV, Guo J, Fischer R, Konzman D, et al. TMEM231 gene conversion associated with Joubert and Meckel-Gruber syndromes in the same family. Human Mutation. 2016;37(11):1144–8. doi: 10.1002/humu.23054. [DOI] [PubMed] [Google Scholar]
- 139.Silvestre-Ryan J, Holmes I. Pair consensus decoding improves accuracy of neural network basecallers for nanopore sequencing. Genome Biol. 2021;22(1):38. doi: 10.1186/s13059-020-02255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mitsuhashi S, Ohori S, Katoh K, Frith MC, Matsumoto N. A pipeline for complete characterization of complex germline rearrangements from long DNA reads. Genome Medicine. 2020;12(1):67. doi: 10.1186/s13073-020-00762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–91. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–43. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Beyter D, Ingimundardottir H, Oddsson A, Eggertsson HP, Bjornsson E, Jonsson H, et al. Long-read sequencing of 3,622 Icelanders provides insight into the role of structural variants in human diseases and other traits. Nat Genet. 2021;53(6):779–86. doi: 10.1038/s41588-021-00865-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Additional examples of disease-associated variations resolved by LRS.
Data Availability Statement
Not applicable.