Abstract
PURPOSE:
To explore the utility of eye tracking glasses in patients with intermittent exotropia as a means for quantifying the occurrence of exotropia, defined as the percentage of time that the eyes are misaligned.
DESIGN:
Prospective observational study.
METHODS:
Eye tracking glasses were used to obtain 68 recordings in 44 ambulatory patients with a history of intermittent exotropia. Vergence angle was monitored for up to 12 hours to document the occurrence of exotropia.
RESULTS:
Intermittent exotropia was present in 31 of 44 patients. They had a mean exotropia of 19.3 ± 5.3° and a mean occurrence of 40% (range 3–99%). There was a moderate correlation between the magnitude of exotropia and its occurrence (r = 0.59). In 13 patients the occurrence of exotropia was <1%; they were deemed to have an exophoria only. In 35 of 44 cases, families reported an occurrence of intermittent exotropia greater than that measured by the eye tracking glasses.
CONCLUSIONS:
Eye tracking glasses may be a useful tool for quantifying the severity of intermittent exotropia and for defining more precisely its clinical features.
Acharacteristic feature of intermittent exotropia is that patients flip back and forth between two sensory states, often unaware of the transition. 1 Much of the time the eyes are fused, allowing normal binocular vision. Abruptly one eye diverges, triggering suppression and anomalous retinal correspondence. 2–7 In the exotropic state, patients explore their visual environment by fixating on targets of interest with either the right eye or the left eye. 8 Consequently, amblyopia is rare. 9 After a period of exotropia, normal eye alignment is restored often by looking at a near target to boost convergence tone. 10
When parents bring a child with intermittent exotropia to the clinic, the pediatric ophthalmologist faces a difficult management decision. There is pressure to correct the exotropia through eye muscle surgery. However, recurrence of the deviation is not uncommon.11–17 Without surgical intervention, episodes of exotropia may become more prolonged and frequent, resulting eventually in a constant deviation and potentially irreversible loss of stereovision. 18 The dilemma is that the natural history of intermittent exotropia is uncertain. 19 Patients may improve with time, remain stable, or get worse. 20–25 Even with better natural history data, providing a meaningful individual prognosis is problematic because each patient’s clinical course can be different.
The two most crucial parameters in measuring the severity of intermittent exotropia are its magnitude and rate of occurrence. While deviation size is relatively easy to measure, the percentage of time—the rate of occurrence—spent in a deviated state is more difficult to assess. Patients are often not aware when exotropia is present and clinicians are limited to brief periods of observation during office examinations.26 Investigators have developed various indices to assess control of intermittent exotropia, such as the revised Newcastle Control Score, 27 the Mayo Clinic Office-based Scale, 28 and the “Look And Cover, then Ten seconds of Observation Scale for Exotropia.”29 For patients without a manifest exotropia, they rely on breaking fusion with an occluder paddle to see how long it takes the subject to regain ocular alignment. It is not clear how well this measurement correlates, during normal binocular viewing, with the true rate of occurrence of exotropia. Moreover, these control scales are qualitative, using a point system to arrive at a numeric score rather than directly quantifying how much of the time ocular misalignment is present. Scores are sometimes inconsistent when testing is repeated during the course of a single day. 30
Eye tracking glasses permit investigators to record the fixations and eye movements of freely ambulating subjects. From the readout of binocular gaze direction, one can extract information about the ductions of each eye. Previously, we have used eye tracking glasses to profile the vergence behavior of normal subjects. 31 Here we use eye tracking glasses in patients with intermittent exotropia. The glasses were worn by subjects while they engaged in their normal daily activities, allowing us to gauge the severity of intermittent exotropia simply by measuring its occurrence, defined as the percentage of time that the eyes were deviated outward.
METHODS
PARTICIPANTS:
Eligible patients receiving care from a single pediatric ophthalmologist (J.C.H.) were invited to participate in this prospective, observational pilot study conducted to explore the utility of eye tracking glasses in the management of intermittent exotropia. Forty-four subjects were recruited between February 2020 and October 2022. The study adhered to the Declaration of Helsinki and was approved by the University of California, San Francisco Institutional Review Board. A parent or guardian provided informed consent for the participation of children under 7 years of age. Children between the ages of 7 and 17 years provided their assent, and in addition, a parent or guardian provided informed consent. Adults provided informed consent. In addition, separate written permission was obtained for any subject who agreed to allow publication or public presentation of a photograph of their face or videographic scene data. All data were deidentified, coded anonymously, encrypted, and stored on a computer in a secure location.
Subjects were referred for evaluation because they had been observed either by a family member or a clinician to have an exodeviation. They were eligible to participate in the study if clinical examination showed the presence of typical findings associated with intermittent exotropia. The inclusion criteria were: 1) 20/20 Snellen acuity in each eye; 2) no eye disease except strabismus; 3) an intermittent exotropia ≥10 prism diopters observed in the clinic or an exophoria ≥10 prism diopters with episodes of exotropia witnessed by parents; 4) ability to fuse at near, with stereopsis measuring at least 80 arc-sec by Randot circles; and 5) willingness and ability to wear the eye tracking glasses for at least an hour. Subjects with a spherical equivalent refractive error of ≤±2.00 D were tested without correction. If the spherical equivalent refractive error was ≥±2.00 D, subjects wore soft contact lenses or auxiliary spherical corrective lenses inserted into the eye tracking glasses. The corrective lenses were available from −5.00 sphere to + 3.00 sphere in steps of 0.50 D. Subjects with a refractive error outside this range who did not have contact lenses available were excluded from the study.
AMBULATORY EYE TRACKING:
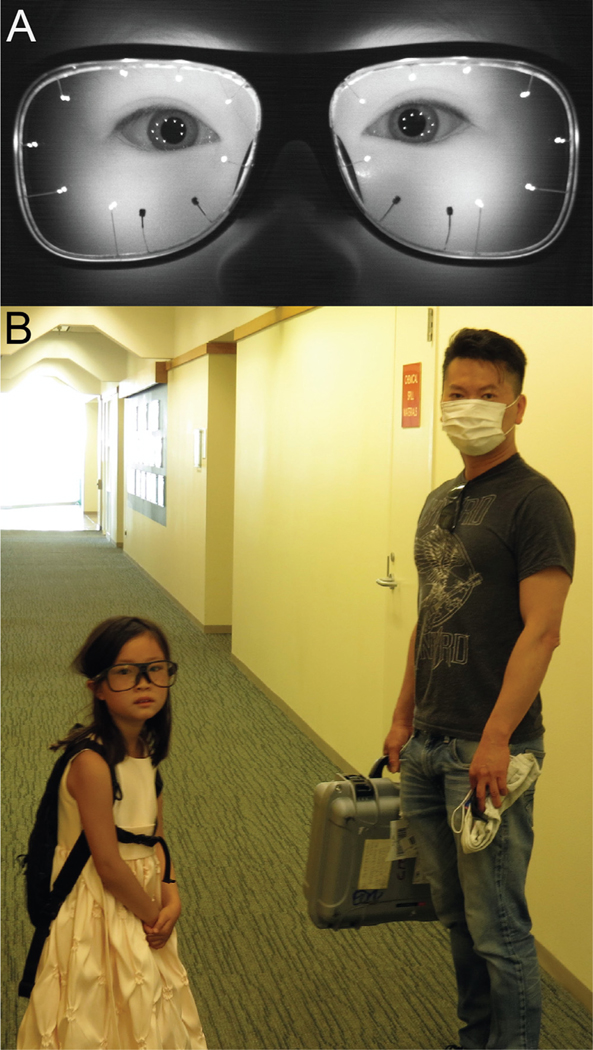
Ocular alignment was measured in ambulatory subjects using Tobii Pro Glasses 3 (www.tobiipro.com), a third-generation instrument worn like a pair of eyeglasses. Each plano lens contains 8 in-frared illuminators and 2 cameras embedded in the plastic (Figure 1, A). The instrument is calibrated by having the subject fixate a bull’s eye target at a distance between 50 and 100 cm for 2 sec. The glasses also contain a scene camera and a microphone. Each eye’s center of gaze (fixation point) in the scene is computed from the relative positions of the pupil center and the corneal reflections of the 8 illuminators. The center of gaze is superimposed on the live video stream provided by the scene camera and viewable in near real time on a tablet or computer screen via a wireless connection. Data are stored on a secure digital card after transfer via a cord from the eye tracking glasses to a small recording unit carried by the subject. The glasses are powered by a lithium ion battery inside the recording unit that has a lifetime of 100 minutes. To extend operating time up to 12 hours, an external rechargeable battery pack was connected to the recording unit. The recording unit and battery were placed inside a small knapsack, normally intended to contain a hydration bladder, to allow subjects unfettered mobility (Figure 1, B). A pair of slip-on infrared-blocking glasses were worn outdoors to prevent sunlight from washing out the reflections of the illuminators.
FIGURE 1.
Tobii Pro 3 eye tracking glasses. A. Eight infrared illuminators (bright objects) on metal stalks and 2 miniature cameras (dark squares, inferonasal) are embedded in the lenses. Gaze is calculated from the relative positions of the center of the pupil and the constellation of illuminator reflections on the cornea. A scene camera and microphone (not visible in this infrared photograph) are located on the bridge of the eyeglass frames. B. The same child leaving with the eye tracking glasses and the backpack containing the recording unit and battery.
If an eligible patient was identified during a clinic visit, the purpose of using eye tracking glasses to assess intermittent exotropia was explained. The family was asked to estimate the percentage of time that the patient manifested an exotropia, for later comparison with data obtained from the eye tracking glasses. If the patient chose to participate in the research, the glasses were placed on the subject and their use was demonstrated. Subjects could see how the glasses tracked their gaze position and understood that a history of eye movements in their visual and auditory environment would be recorded for later analysis. They were instructed to remove the eye tracking glasses before using a bathroom. The glasses resumed accurate tracking immediately once placed back on the face. Subjects left the clinic wearing the eye tracking glasses and were asked to wear them while carrying out their normal repertoire of activities. Depending on the goal of the recording and the patient’s level of co-operation, the glasses were worn for a variable time. Most patients returned home and wore the glasses until they went to bed. The equipment was shipped back the next day prepaid via UPS in a Nanuk 925 plastic protective case.
DATA ANALYSIS:
Data stored on the secure digital card from each recording session about ocular alignment were contained in a file named “gazedata.gz.” This file contained the horizontal , vertical , and depth spatial coordinates that encode the direction of gaze for each eye, sampled at 50 Hz. Using a custom script written in Python (https://osf.io/fesdp/?view_only=864465d515c649938f82d466731db2ef), the and components were transformed into the horizontal position for each eye measured in degrees by applying this function:
Positive values for horizontal eye position denote right gaze. The horizontal position data contained brief epochs where loss of tracking occurred in either eye, from blinks and other interruptions. To mitigate this problem, a filter was applied to interpolate over gaps lasting ≤25 data samples (0.5 sec). The median value of the surrounding 24 samples was applied to fill in each point in the gap. 32
Some position data were noisy because of inaccurate measurement by the tracking glasses. This error had many potential sources, including momentary loss of the corneal reflections from the infrared illuminators, extraneous ocular surface reflections, and inaccurate determination of the pupil center location. To replace outlier values in the position data, a filter was applied that compared each sample with the surrounding 24 samples. If the sample fell > 1 ° outside the median, it was substituted with the median value.
After application of these 2 filters, each subject’s vergence angle was calculated by subtracting the right eye position from the left eye position:
Negative values indicate ocular divergence. The history of each subject’s ocular alignment for the duration of their ambulatory recording was profiled by plotting a histogram, compiled using 0.2 ° bins, to show the amount of time that the subject’s eyes manifested any given vergence angle.
In a normal subject, the visual axes are either parallel at infinity (vergence angle = 0 °) or converged at near (positive vergence). In principle, negative vergence (exotropia) does not occur. However, calibration of the eye tracking glasses is not perfectly accurate. Testing in normal subjects has shown that an offset of up to 3 ° can be present when viewing a distant target. 31 This means that a readout of −3 ° can, in reality, correspond to orthotropic alignment at distance. To avoid the risk of confounding calibration error of the eye tracker with genuine exotropia, we set a threshold of ≤−5 ° for a peak in the histogram to qualify as exotropic.
Application of the data filters reduced the problem of gaps and noise, but incompletely. In normal subjects, steady fixation on a target produces a measurement that has an approximately Gaussian distribution of values, with a standard deviation ranging between 1 ° and 5 °. 31 The exact value depends on the vergence angle and on the quality of the recording in any given subject. In general, recording quality is better in adults. Tracker noise that remains even after implementing the two data filters increases the spread of measurements around peak vergence angle values. In addition, the relatively slow eye movement that occurs during frequent transitions between orthotropia and exotropia (and vice versa) generates intermediate values for the ocular deviation. 33 These factors contribute to a tendency for the flanks to merge between the peaks representing exotropia and orthotropia.
To quantify the occurrence of strabismus, the custom Python script fitted a Gaussian and an exponentially modified Gaussian function to the exotropia peak, if detected. It selected the better function based on a goodness-of-fit criterion (chi-square). 34 The fit domain was restricted to a minimum X value which corresponded to a Y value that fell to < 0.5% of the detected peak height. It was restricted to a maximum X value that was located at 0 °, or at the trough between the exotropia peak and the adjacent peak, depending upon which X value was less. Then, the area under the fitted exotropia peak was divided by the total duration of the filtered data. The quotient represented the occurrence of exotropia.
Several additional precautions were taken to avoid misinterpreting small negative vergence peaks, generated by tracker noise or calibration error, as true exotropia. First, as mentioned previously, peak location was required to be ≤−5 ° In addition, a potential exotropia peak had to reach a height ≥ 2% of the height of the maximum peak present in the recording data. Finally, an exotropia peak was required to have a height of 2% above the surrounding baseline (“prominence”). 35 There were 13 subjects whose data lacked an exotropia peak fulfilling these requirements. Their exotropia occurrence was stipulated to be < 1%. Enforcing these threshold criteria could potentially result in failure to detect a real exotropia that occurred rarely (< 1%) or that had a small amplitude (between 0 and −4.9 °). Consequently, a clinician should manually review the vergence histogram, along with the automated calculation of exotropia occurrence, to ensure that data are correctly interpreted.
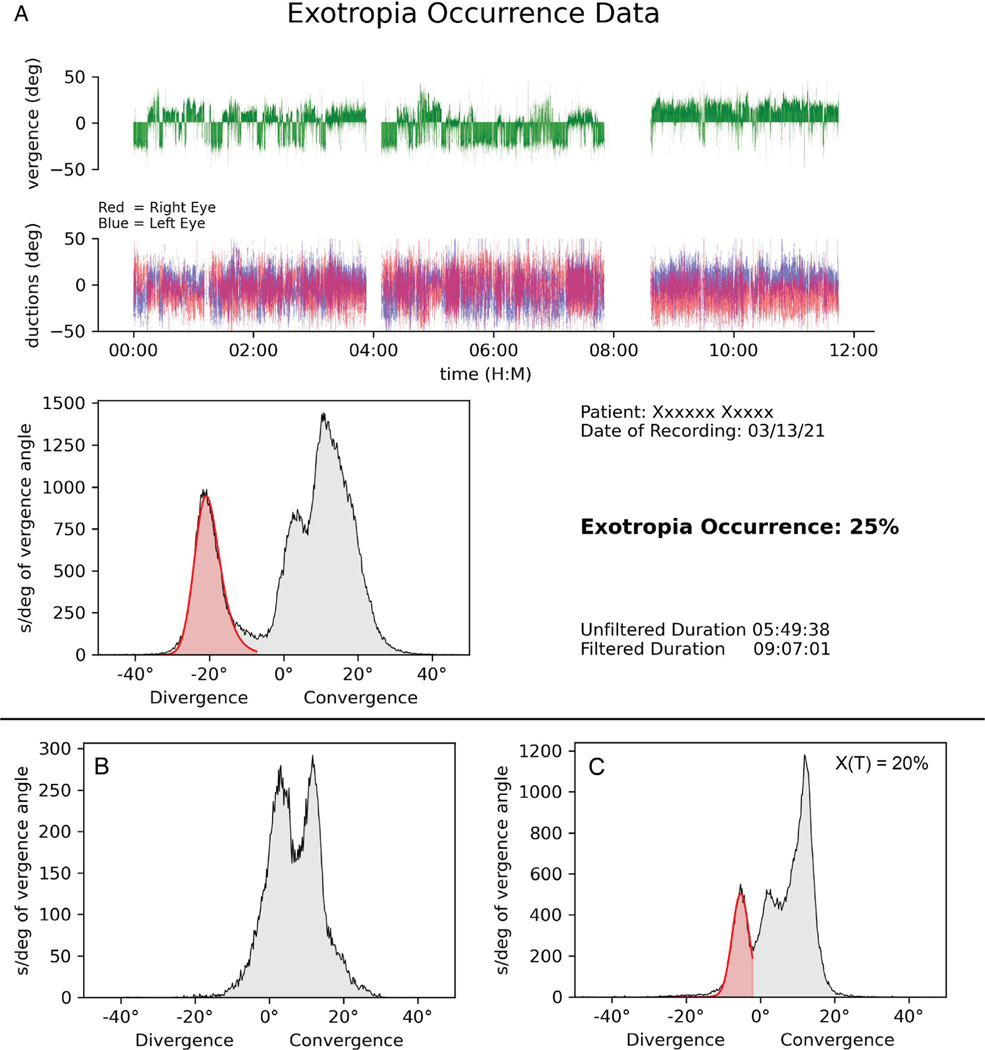
The data ( Figure 2, A) showing the vergence angle history and the percentage of time that exotropia was present were shared with patients and their families.
FIGURE 2.
Pre- and postoperative recordings of exotropia occurrence. A. Example of data printout from the custom Python script, recorded from the child in Figure 1 . Green trace shows vergence angle, with epochs of either convergence or exotropia, depending on the activity in which the child was engaged. She experienced ~140 epochs of exotropia in 547 minutes, an average of 1 episode nearly every 4 minutes. Two gaps in the data represent intervals when she temporarily removed the tracking glasses. Traces below show the ductions of each eye, with positive values representing right gaze. The histogram was compiled from the vergence data (green trace). It shows 3 peaks, corresponding to near orthotropia (11 °), distance orthotropia (4 °), and intermittent exotropia (−21 °). Red shading is the automated fit to the exotropia peak, revealing a deviation occurrence of 25%. B. Vergence angle histogram 2 months after eye muscle surgery shows no exotropia peak. C. Recording 14 months postsurgery shows recurrence of intermittent exotropia, at reduced amplitude.
RESULTS
Sixty-eight recordings were made in 44 patients, 19 females and 25 males, ranging in age from 3 to 79 years, with a median age of 9 years. Thirteen children were ≤7 years of age. Some patients were recorded more than once, either to monitor their intermittent exotropia through serial follow-up visits or to document the outcome of eye muscle surgery.
All patients were referred with a diagnosis of intermittent exotropia, but exotropia was detected by the eye tracking glasses in only 31 of 44 patients. Measurement with the prism cover test in these 31 patients showed a mean exotropia of 19.3 ± 5.3°, with a range from 12 ° to 31 °. The remaining 13 patients had an exophoria, with exotropia manifest < 1% of the time.
For the initial recording in the 44 patients, the eye tracking glasses were worn an average of 5 hours, 35 minutes, yielding an average of 4 hours, 53 minutes of filtered data. The maximum time any patient wore the glasses was 12 hours, 5 minutes, which yielded 10 hours, 43 minutes of filtered data. Among those with exotropia, the mean occurrence of an ocular deviation was 40 ± 32%, with a range from 3% to 99%. In each patient, the eye tracker recorded values of exotropia that had an approximately Gaussian distribution. The mean standard deviation of these Gaussian peaks was 4.2 ± 1.9 ° (n = 31).
Figure 2, A shows the data printout from a 6-year-old girl with a history of intermittent exotropia since 1 year of age. The prism/cover test revealed a 35 to 40 prism diopters right exotropia that the parents estimated was present 20% of the time. Ambulatory eye tracking over nearly 12 hours yielded 9 hours 7 minutes of usable data after filtering. There were 3 distinct peaks in her vergence profile. The scene camera revealed that the large peak at 11 ° corresponded to near activities, such as using a smartphone or working on a computer. There was a smaller peak at 4 ° generated by distance activities, such as playing or walking. The cardinal finding was a third peak at −21 ° representing exotropia. It was present 25% of the time.
Review of the scene videotape (Supplemental Videos 1 and 2) showed that exotropia occurred predominately while the patient was engaged in activities that involved fixation at distance. The peak centered at 4 °, which represented or-thotropic alignment during fixation at distance, had an area amounting to 19% of the total recording. Therefore, when the child looked at distant targets, more than half the time (25% vs 19%) an exotropia was present.
The parents requested surgical treatment of the intemittent exotropia. A bilateral 7-mm medial rectus muscle resection was performed. On postoperative day 4 there were 12 to 15 prism diopters of esotropia. By postoperative month 2, cover testing showed 4 prism diopters of esophoria. Immediately following this examination, the child wore the tracking glasses for 80 minutes while visiting the hospital cafeteria. The data showed 2 peaks, corresponding to activities accompanied by vergence at near and far ( Figure 2, B). The exotropia peak recorded preoperatively was absent.
An examination 14 months postsurgery showed an exophoria of 20 prism diopters. The parents denied observing any evidence of exotropia. However, ambulatory eye tracking conducted over 5 hours revealed recurrence of an exotropia peak, accounting for 20% of the total recording (Figure 2, C). This result was unexpected, because no episodes of exotropia were witnessed during the 20-minute clinic visit conducted just before the recording session. The parents probably missed the exotropia because its amplitude was reduced. They declined further eye muscle surgery. A follow-up eye tracking session was scheduled for 6 months to monitor the child’s intermittent exotropia.
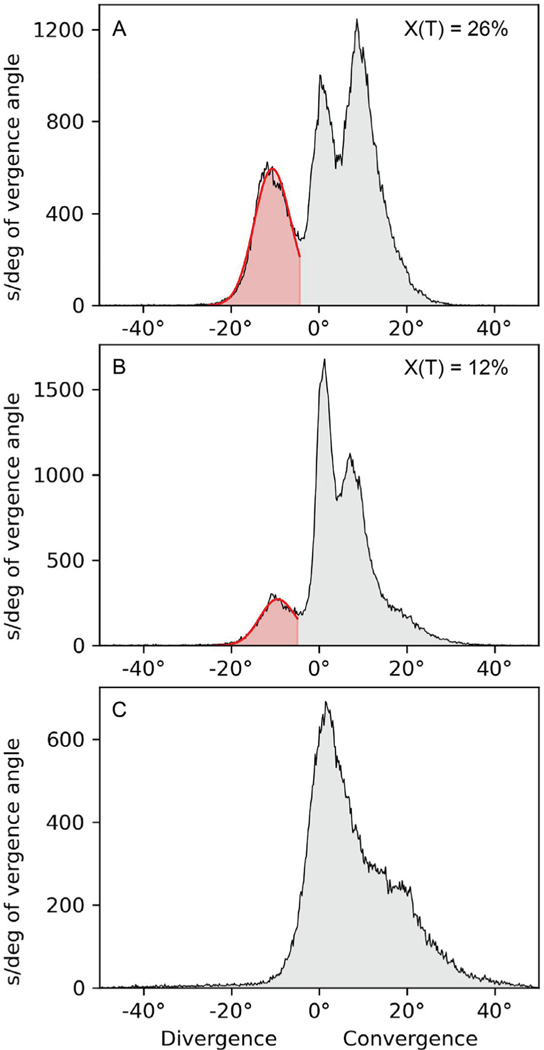
Figure 3, A shows the vergence angle histogram from a 4-year-old boy with 30 prism diopters of intermittent exotropia present since 6 months of age. The child’s mother estimated that the condition manifested 50% of the time. Data collected over 10 hours showed an exotropia occurrence of 26%. The child’s cycloplegic refraction was plano in both eyes. To test the response to induced accommodation, a recording was made over 7 hours with −2.50 D lenses placed in the eye tracking glasses.36 They reduced the exotropia occurrrence to 12% ( Figure 3, B). However, the child’s compliance with minus lenses eyeglasses was inconsistent, leading his mother to request eye muscle surgery. A bilateral 6-mm medial rectus muscle resection was performed. A week after surgery there were 12 prism diopters of esotropia. This overcorrection was treated with glasses con-taining 5 prism diopters base out in each eye. Five months after surgery, clinical examination showed 4 prism diopters of esophoria. Ambulatory eye tracking over 3.5 hours confirmed that no exotropia was present ( Figure 3, C).
FIGURE 3.
Minus lenses versus eye muscle surgery. A. Recording in a 4-year-old boy showing 3 peaks, with an exotropia peak at −11 ° that had an occurrence of 26%. B. Recording after placement of −2.50 diopter lenses in the tracking glasses. They reduced the exotropia peak to −10 ° with an occurrence of 12%. C. Recording 5 months postsurgery showing absence of exotropia peak.
Figure 4 shows more examples of data from patients referred because of intermittent exotropia. The eye tracking glasses detected an exotropia in some subjects (Figure 4, A through D), but not in others (Figure 4, E through H). In those with exotropia, surgery was declined for a variety of reasons, including low rate of deviation occurrence, aversion to surgery, or concern about recurrence.
FIGURE 4.
Typical eye tracking glasses recordings. A through D. Four subjects with varying occurrence of exotropia. E through H. Four subjects without exotropia detectable by the eye tracking glasses, but exophoria on alternate cover test (E = 11 °, F = 12.5 °, G = 9 °, and H = 15 °).
Figure 5 shows examples of recordings conducted before and after bilateral medial rectus muscle resection for intermittent exotropia. In each child, clinical examination after surgery showed elimination of strabismus. This finding was confirmed by postoperative recording with the eye tracking glasses.
FIGURE 5.
Eye tracking before and after extraocular muscle surgery. Top row shows preoperative recordings in 4 children; bottom row shows recordings done (A) 5 months, (B) 4 months, (C) 3 months, and (D) 12 months postsurgery.
Figure 6 examines the relationship between exotropia magnitude and occurrence. Magnitude was measured at distance with the prism cover test and occurrence with the eye tracking glasses. A larger deviation was correlated moderately with a greater percentage of time that it was manifest (r = 0.59). This result confirms a previous study, which measured exotropia control by using the Mayo Clinic Office-based Scale (0 = phoria to 5 = constant exotropia). 37 The authors reported a weak association at distance (r = 0.27) and a slightly stronger association at near (r = 0.37).
FIGURE 6.
Magnitude of exotropia is correlated with the percentage of time that it is present (r = 0.59, P <.001). Green circles, representing data from 13 patients with exophoria only (ie, exotropia < 1%), were not included in calculation of the Pearson correlation coefficient.
Figure 7 compares the measurement by the eye tracking glasses of the occurrence of intermittent exotropia with the assessment provided by family members based on their observations. In 35 of 44 patients, family members overestimated the occurrence of intermittent exotropia. In the 13 patients who had only an exophoria, parents reported observing an exotropia a mean of 22% of the time.
FIGURE 7.
Comparison of exotropia occurrence measured by the eye tracking glasses with the rate estimated by family members. Green circles indicate 13 patients with exophoria only. In 35 of 44 cases, family members thought that an exotropic deviation occurred more often than measured by the eye tracking glasses.
DISCUSSION
The invention of eye tracking glasses has made it possible to quantify the percentage of time that an intermittent exotropia is present. This information may be useful for planning the care of patients. When an exotropia is highly prevalent, one does not need an instrument to show it. But when an intermittent exotropia is infrequent, our data reveal that patients and families often overestimate its severity. In 13 of 44 patients referred for treatment of intermittent exotropia, the tracking glasses measured an occurrence of < 1% ( Figure 7 ). Consequently, surgery was deferred and the patients were scheduled instead for a follow-up ambulatory eye tracking session to monitor their deviation.
Previously, assessment of the occurrence of intermittent exotropia has depended on an indirect method: the use of “control” scores. 38 The validity of such metrics hinges on whether the ability to regain alignment after deliberate fusion interruption is an accurate measure of the frequency of spontaneous exotropia. This assumption may not be true in some patients. Moreover, the Mayo Clinic Office-based Scale and the Newcastle Control Score rely in part on estimates of the frequency of exotropia, made either in the office or at home by parents. Such estimates are sometimes inaccurate, at least when provided by parents ( Figure 7 ). 39
In this pilot study, patients wore the eye tracking glasses for a variable length of time, depending on the subject and the situation. Wear time was sometimes only an hour, when the recording was conducted at the clinic right after an examination. The occurrence of exotropia depends to some extent on the activity in which the patient is engaged. Exotropia is less likely, for example, during near work. The longer the glasses are worn, the more likely they will capture a full picture of a subject’s ocular motor behavior, and therefore accurately reflect the true occurrence of exotropia. For this reason, we encouraged patients to wear the glasses for the rest of the day, when possible, after setting up a recording session.
In a patient who has a manifest ocular deviation, the prism cover test measures the mean value of the exotropia. 40 Under binocular, free-viewing conditions, exotropia magnitude fluctuates constantly. In a study of 25 patients with intermittent exotropia, we recorded a mean horizontal standard deviation of 2.43 °. 41 This value was low, because it was obtained under ideal conditions: patients fixated a stationary target with their head in a chin/forehead rest. Eye position was recorded with video trackers having a precision better than 1 °. In contrast, in the present study, head and gaze were unconstrained and subjects were free to alternate fixation. Changes in gaze angle, target distance, or eye of fixation increase the variability of exotropia. 42–47 Further-more, transitions between exotropia and orthotropia gave rise to intermediate values for the ocular deviation, which were captured by the eye tracker. All these factors contributed to the high mean standard deviation of exotropia (4.2 °) that we measured in our patients.
Instrument error was another major factor, because it introduced noise into the measurement of vergence angle during ambulatory monitoring. This error depends on recording quality, which varies from one patient to another. Even in normal subjects assiduously fixating a stationary target, measurement of vergence angle by the eye tracking glasses typically has a standard deviation of 2 °to 3 °. 31
The variability of data obtained with the eye tracking glasses—because of a combination of real biologic fluctuation in ocular alignment and spurious instrument noise—produced wide peaks in the histograms of vergence angle. In addition, calibration error could introduce an offset of up to 3 ° between measured and true vergence angle. As a result, even in patients without exotropia, the left flank of the peak representing orthotropia during distance fixation often strayed into negative territory (Figure 4, E, F, and H). If one were simply to adopt a cutoff of 0 °, an exotropia would be identified erroneously in many subjects. To overcome this problem, we required a peak in the histogram to have a value ≤−5 ° to qualify as an exotropia. This requisite did not result in any instances of false exclusion, because intermittent exotropia has a relatively large magnitude. In a study of 37 patients with intermittent exotropia, only one subject had a deviation smaller than 5 °. 47 In such a case, admittedly, it might be difficult to differentiate cleanly between exotropia and orthotropia.
The potential for an offset of up to 3 ° in the calibration of the eye tracking glasses means that one should not rely upon them to measure exotropia magnitude. The data they provide regarding the occurrence of exotropia are useful for deciding if surgery should be performed, but the surgical dose should still be based on the prism cover test.
Serial ambulatory recording of ocular alignment in a larger cohort of patients could refine our knowledge of the natural history of intermittent exotropia. It could also provide further data regarding the utility of minus lenses, alternate patching, vision therapy, and the best operative strategy. Ambulatory recording also potentially allows one to define clinical features of intermittent exotropia that cannot be easily quantified during an office examination. For example, how many times does a subject switch between orthotropia and exotropia during the course of a day? Which eye is favored, under natural viewing, for fixation on visual targets? What is the precise relationship between the occurrence of exotropia and the distance from the subject of the target being fixated? What behaviors and activities are associated with loss of fusion?
In 1961, Holter introduced continuous electrocardiographic monitoring in active subjects over long periods of time. 48 It enhanced the diagnosis and treatment of arrhythmias that were elusive during office visits. As eye tracking glasses become easier to use and lower in cost, ambulatory recording may eventually become a routine outpatient test, permitting measurement of the exact occurrence of this disorder in each patient.
Supplementary Material
Funding/Support:
This work was supported by National Eye Institute grants EY029703 (to J.C.H.) and EY02162 (Vision Core Grant) and by an unrestricted grant from Research to Prevent Blindness. Financial Disclosures: J.C.H. receives honoraria from lecturing and serving on grant review panels, and from royalties from publications. The other authors have no conflict of interests. They have no investment in the company that manufactures the eye tracking glasses, nor have they received any payment from it. All authors contributed to carrying out the experiments, interpreting the data, and preparation of the manuscript. All authors attest that they meet the current ICMJE criteria for authorship.
Footnotes
Supplemental Material available at AJO.com.
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest.
REFERENCES
- 1.Hatt SR, Mohney BG, Leske DA, Holmes JM. Variability of control in intermittent exotropia. Ophthalmology. 2008;115:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joosse MV, Simonsz HJ, van Minderhout EM, Mulder PG, de Jong PT. Quantitative visual fields under binocular viewing conditions in primary and consecutive divergent strabismus. Graefes Arch Clin Exp Ophthalmol. 1999;237:535–545. [DOI] [PubMed] [Google Scholar]
- 3.Jampolsky A.Physiology of intermittent exotropia. Am Orthopt J. 1963;13:5–13. [PubMed] [Google Scholar]
- 4.Cooper J, Record CD. Suppression and retinal correspondence in intermittent exotropia. Br J Ophthalmol. 1986;70:673–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Economides JR, Adams DL, Horton JC. Perception via the deviated eye in strabismus. J Neurosci. 2012;32:10286–10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper J, Feldman J. Panoramic viewing, visual acuity of the deviating eye, and anomalous retinal correspondence in the intermittent exotrope of the divergence excess type. Am J Optom Physiol Opt. 1979;56:422–429. [DOI] [PubMed] [Google Scholar]
- 7.Herzau V.How useful is anomalous correspondence? Eye (London). 1996;10(pt 2):266–269. [DOI] [PubMed] [Google Scholar]
- 8.Economides JR, Adams DL, Horton JC. Eye choice for acquisition of targets in alternating strabismus. J Neurosci. 2014;34:14578–14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohney BG, Huffaker RK. Common forms of childhood exotropia. Ophthalmology. 2003;110:2093–2096. [DOI] [PubMed] [Google Scholar]
- 10.Ahn SJ, Yang HK, Hwang JM. Binocular visual acuity in intermittent exotropia: role of accommodative convergence. Am J Ophthalmol. 2012;154:981–986 e3. [DOI] [PubMed] [Google Scholar]
- 11.Buck D, Powell CJ, Sloper JJ, Taylor R, Tiffin P, Clarke MP. Surgical intervention in childhood intermittent exotropia: current practice and clinical outcomes from an observational cohort study. Br J Ophthalmol. 2012;96:1291–1295. [DOI] [PubMed] [Google Scholar]
- 12.Heo H, Lambert SR. Effect of age on reoperation rate in children undergoing exotropia surgery. Acta Ophthalmol. 2021;99:e1206–e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekdawi NS, Nusz KJ, Diehl NN, Mohney BG. Postoperative outcomes in children with intermittent exotropia from a population-based cohort. J AAPOS. 2009;13:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker JD. Twenty-year follow-up of surgery for intermittent exotropia. J AAPOS. 2008;12:227–232. [DOI] [PubMed] [Google Scholar]
- 15.Pineles SL, Ela-Dalman N, Zvansky AG, Yu F, Rosen-baum AL. Long-term results of the surgical management of intermittent exotropia. J AAPOS. 2010;14:298–304. [DOI] [PubMed] [Google Scholar]
- 16.Repka MX, Lum F, Burugapalli B. Strabismus, strabismus surgery, and reoperation rate in the United States: analysis from the IRIS Registry. Ophthalmology. 2018;125:1646–1653. [DOI] [PubMed] [Google Scholar]
- 17.Repka MX, Chandler DL, Holmes JM, et al. The relationship of age and other baseline factors to outcome of initial surgery for intermittent exotropia. Am J Ophthalmol. 2020;212:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abroms AD, Mohney BG, Rush DP, Parks MM, Tong PY. Timely surgery in intermittent and constant exotropia for superior sensory outcome. Am J Ophthalmol. 2001;131:111–116. [DOI] [PubMed] [Google Scholar]
- 19.Hatt SR, Gnanaraj L. Interventions for intermittent exotropia. Cochrane Database Syst Rev. 2013;9:CD003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romanchuk KG, Dotchin SA, Zurevinsky J. The natural history of surgically untreated intermittent exotropia-looking into the distant future. J AAPOS. 2006;10:225–231. [DOI] [PubMed] [Google Scholar]
- 21.Rutstein RP DA Corliss. The clinical course of intermittent exotropia. Optom Vis Sci. 2003;80:644–649. [DOI] [PubMed] [Google Scholar]
- 22.Nusz KJ, Mohney BG, Diehl NN. The course of intermittent exotropia in a population-based cohort. Ophthalmology. 2006;113:1154–1158. [DOI] [PubMed] [Google Scholar]
- 23.Cotter SA, Mohney BG, Chandler DL, et al. Three-year observation of children 12 to 35 months old with untreated intermittent exotropia. Ophthalmic Physiol Opt. 2020;40:202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohney BG, Cotter SAPediatric Eye Disease Investigator Group Writing Committee. Three-year observation of children 3 to 10 years of age with untreated intermittent exotropia. Ophthalmology. 2019;126:1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buck D, Powell CJ, Rahi J, et al. The improving outcomes in intermittent exotropia study: outcomes at 2 years after diagnosis in an observational cohort. BMC Ophthalmol. 2012;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatt SR, Leske DA, Holmes JM. Awareness of exodeviation in children with intermittent exotropia. Strabismus. 2009;17:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buck D, Clarke MP, Haggerty H, et al. Grading the severity of intermittent distance exotropia: the revised Newcastle Control Score. Br J Ophthalmol. 2008;92:577. [DOI] [PubMed] [Google Scholar]
- 28.Mohney BG, Holmes JM. An office-based scale for assessing control in intermittent exotropia. Strabismus. 2006;14:147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Kim DH, Ahn H, Lim HT. Proposing a new scoring system in intermittent exotropia: towards a better assessment of control. Can J Ophthalmol. 2017;52:235–239. [DOI] [PubMed] [Google Scholar]
- 30.Prile SM, Kim J, Moon Y, Lim HT. Assessing variability of control within a single day in intermittent exotropia. J Pediatr Ophthalmol Strabismus. 2020;57:378–383. [DOI] [PubMed] [Google Scholar]
- 31.Dilbeck MD, Gentry TN, Economides JR, Horton JC. Quotidian profile of vergence angle in ambulatory subjects monitored with wearable eye tracking glasses. Translational Vis. Sci. & Tech. 2023;12(2):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niehorster DC, Hessels RS, Benjamins JS. GlassesViewer: Open-source software for viewing and analyzing data from the Tobii Pro Glasses 2 eye tracker. Behav Res Methods. 2020;52:1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Economides JR, Adams DL, Horton JC. Capturing the moment of fusion loss in intermittent exotropia. Ophthalmology. 2017;124:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golubev A.Exponentially modified peak functions in biomedical sciences and related disciplines. Comput Math Methods Med. 2017;2017:7925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helman A.The Finest Peaks - Pominence and Other Mountain Measures. Trafford Publishing; 2005. [Google Scholar]
- 36.Chen AM, Erzurum SA, Chandler DL, et al. Overminus lens therapy for children 3 to 10 years of age with intermittent exotropia: a randomized clinical trial. JAMA Ophthalmol. 2021;139:464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Superstein R, Dean TW, Holmes JM, et al. Relationship among clinical factors in childhood intermittent exotropia. J AAPOS. 2017;21:268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thuma TBT, Zhang QE, Sharpe J, Gunton KB. Understanding the use of the Newcastle, PEDIG, and LACTOSE Control scores among pediatric ophthalmologists for intermittent exotropia. J Pediatr Ophthalmol Strabismus. 2022. doi: 10.3928/01913913-20220425-02. [DOI] [PubMed] [Google Scholar]
- 39.Oatts JT. Intermittent exotropia. American Academy of Ophthalmology ONE Network. Posted online April 9, 2020. https://www.aao.org/disease-review/intermittent-exotropia-2.
- 40.Hatt SR, Leske DA, Liebermann L, Mohney BG, Holmes JM. Variability of angle of deviation measurements in children with intermittent exotropia. J AAPOS. 2012;16:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Economides JR, Adams DL, Horton JC. Variability of ocular deviation in strabismus. JAMA Ophthalmol. 2016;134: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore S.The prognostic value of lateral gaze measurements in intermittent exotropia. Am Orthopt J. 1969;19:69–71. [PubMed] [Google Scholar]
- 43.Carlson MR, Jampolsky A. Lateral incomitancy in intermittent exotropia: cause and surgical therapy. Arch Ophthalmol. 1979;97:1922–1925. [DOI] [PubMed] [Google Scholar]
- 44.Repka MX, Arnoldi KA. Lateral incomitance in exotropia: fact or artifact? J Pediatr Ophthalmol Strabismus. 1991;28:125–128 discussion 129–130. [DOI] [PubMed] [Google Scholar]
- 45.Graeber CP, Hunter DG. Changes in lateral comitance after asymmetric horizontal strabismus surgery. JAMA Ophthalmol. 2015;133:1241–1246. [DOI] [PubMed] [Google Scholar]
- 46.Kushner BJ, Morton GV. Distance/near differences in intermittent exotropia. Arch Ophthalmol. 1998;116:478–486. [DOI] [PubMed] [Google Scholar]
- 47.Adams DL, Economides JR, Horton JC. Incomitance and eye dominance in intermittent exotropia. Invest Ophthalmol Vis Sci. 2017;58:4049–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holter NJ. New method for heart studies. Science. 1961;134:1214–1220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.