Abstract
Background
Our near-real-time safety monitoring of 16 adverse events (AEs) following COVID-19 mRNA vaccination identified potential elevation in risk for six AEs following primary series and monovalent booster dose administration. The crude association with AEs does not imply causality. Accordingly, we conducted robust evaluation of potential associations.
Methods
We conducted two self-controlled case series studies of COVID-19 mRNA vaccines (BNT162b2 and mRNA-1273) in U.S. Medicare beneficiaries aged ≥ 65 years. Adjusted incidence rate ratio (IRRs) and 95 % confidence intervals (CIs) were estimated following primary series doses for acute myocardial infarction (AMI), pulmonary embolism (PE), immune thrombocytopenia (ITP), disseminated intravascular coagulation (DIC); and following monovalent booster doses for AMI, PE, ITP, Bell’s Palsy (BP) and Myocarditis/Pericarditis (Myo/Peri).
Results
The primary series study included 3,360,981 individuals who received 6,388,542 primary series doses; the booster study included 6,156,100 individuals with one monovalent booster dose. The AMI IRR following BNT162b2 primary series and booster was 1.04 (95 % CI: 0.91 to 1.18) and 1.06 (95 % CI: 1.003 to 1.12), respectively; for mRNA-1273 primary series and booster, 1.01 (95 % CI: 0.82 to 1.26) and 1.05 (95 % CI: 0.998 to 1.11), respectively. The hospital inpatient PE IRR following BNT162b2 primary series and booster was 1.19 (95 % CI: 1.03 to 1.38) and 0.86 (95 % CI: 0.78 to 0.95), respectively; for mRNA-1273 primary series and booster, 1.15 (95 % CI: 0.94 to 1.41) and 0.87 (95 % CI: 0.79 to 0.96), respectively. The studies’ results do not support that exposure to COVID-19 mRNA vaccines elevate the risk of ITP, DIC, Myo/Peri, and BP.
Conclusion
We did not find an increased risk for AMI, ITP, DIC, BP, and Myo/Peri and there was not consistent evidence for PE after exposure to COVID-19 mRNA primary series or monovalent booster vaccines. These results support the favorable safety profile of COVID-19 mRNA vaccines administered in the U.S. elderly population.
Keywords: COVID-19 mRNA vaccines, COVID-19 vaccine safety, COVID-19 Pfizer-BioNTech vaccine, COVID-19 Moderna vaccine, Primary series, Monovalent booster
1. Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) heavily impacted both the United States (U.S.) and global populations. According to the Centers for Disease Control and Prevention (CDC), as of March 1, 2023, there have been a total of about 103 million COVID-19 cases, nearly 1.1 million related deaths, and 6 million related hospitalizations in the U.S [1], [2]. Of the observed COVID-19-related deaths, about 75 percent have occurred in individuals aged 65 and older [3]. In response, the U.S. Food and Drug Administration (FDA) has authorized (under emergency use authorization) or approved COVID-19 vaccines for prevention of COVID-19 including primary series (doses 1 and 2) and additional booster doses [4]. These include the Pfizer-BioNTech vaccine available for persons 6 months and older (BNT162b2), Moderna vaccine available for persons 6 months and older (mRNA-1273) and the Novavax vaccine (NVX-CoV2373) available for persons 12 years and older [5], [6], [7]. The monovalent vaccines available in the U.S. are based on the original strain of the SARS-CoV-2 virus [8]. Bivalent COVID-19 vaccine formulations of the BNT162b2 and mRNA-1273 have been authorized for use as booster doses by the U.S. FDA [8].
The FDA conducts active post-market surveillance to monitor the safety of COVID-19 vaccines (i) primary series and (ii) booster doses in all ages including those 65 years and older. Near real-time surveillance or rapid cycle analyses (RCA) is a sequential testing method to screen for an increased risk of adverse events (AEs) following vaccination as vaccination data accrue. Through this framework using the U.S. Centers for Medicare and Medicaid Services (CMS) Medicare database, the observed incidence rates of 16 pre-specified AEs were compared to historical AEs rates following administration of available COVID-19 mRNA vaccines during the study period.
This rapid screening method in persons 65 years and older identified statistically significant associations (signals) between the primary series BNT162b2 vaccine and acute myocardial infarction (AMI), pulmonary embolism (PE), disseminated intravascular coagulation (DIC), and immune thrombocytopenia (ITP) [9]. A signal detection study of COVID-19 monovalent booster dose vaccines (BNT162b2, mRNA-1273) detected signals for BNT162b2 COVID-19 vaccine and Bell’s Palsy (BP), as well as for mRNA-1273 COVID-19 vaccine and myocarditis/pericarditis (Myo/Peri). These six events may not be true safety concerns as the RCA cannot establish that the vaccines caused these AEs. While preliminary signal detection studies enable rapid safety screening, they can be subject to bias and confounding and must be further evaluated in signal evaluation studies that more rigorously adjust for various sources of confounding.
This manuscript summarizes results from two independent vaccine safety studies that used a self-controlled study design to account for time-invariant confounders. Specifically, we estimated the risk of AMI, PE, DIC and ITP following primary series vaccination with the COVID-19 mRNA vaccines, BNT162b2 and mRNA-1273, in a study referred to as the “primary series study” as well as the risk of AMI, PE, ITP, BP, and Myo/Peri following COVID-19 mRNA monovalent booster vaccination in a study referred to as the “booster study.”
2. Methods
2.1. Study designs, data sources and study periods
We define primary series as doses 1 and 2 of a COVID-19 mRNA vaccine, and the booster dose as a subsequent (or third) monovalent dose following a COVID-19 mRNA vaccine primary series. In these studies, COVID-19 bivalent mRNA vaccines were not evaluated. Third doses (or first monovalent booster doses) are hereafter referred to as “booster” doses for clarity. We compared the incidence of AEs within periods of hypothesized excess risk due to vaccine administration (risk interval) with their incidence during a control interval for COVID-19 primary series and booster vaccines, in two separate studies, among Medicare Fee-For-Service (FFS) individuals aged 65 years and older. Self-controlled case series (SCCS) or self-controlled risk interval (SCRI) analyses were conducted to compare exposed and unexposed periods within the same individual, and thus inherently adjusted for sources of time-invariant confounding [10]. The statistical analytical plan was pre-specified in the protocol and summarized in Table 1 . Graphic illustrations presenting the risk and control intervals for these studies are outlined in eFigures 1–3. Details of each study specifications are outlined in eTable 1 [11].
Table 1.
Summary of observation period and analyses for primary, secondary, exploratory and subgroup analyses and associated adjusted analyses, PRIMARY SERIES AND BOOSTER STUDIES.
| Specifications |
Primary Series Study |
Booster Study |
|||
|---|---|---|---|---|---|
| Primary Analysis | Secondary Analysis | Exploratory Analysis | Primary Analysis | Secondary Analysis | |
| Study Design | Self-controlled case series (SCCS) design with post-vaccination control intervals | SCCS design with pre- and post-vaccination control and post-vaccination risk intervals | Self-controlled (SCRI) design with pre-vaccination control interval and post-vaccination risk intervals | Consistent with primary series study primary analysis | Consistent with primary series study secondary analysis |
| Observation Period | Time of the first eligible COVID-19 vaccination dose through 90 days after the first eligible vaccination dose. | First day of the pre-vaccination control interval through 90 days after the first eligible vaccination dose. | Similar to the secondary analysis, observation period started the first day of the pre-vaccination control interval and extended to the end of the risk interval of the most recent vaccination dose. | Time of the first eligible booster dose COVID-19 vaccination through 90 days after this index vaccination. | Consistent with primary series secondary analysis. |
| Risk Interval | First Dose: Time of first-dose vaccination until the earlier of the end of the outcome-specific risk interval, or until the time of the second dose (if within the risk interval).Second Dose: Time of second dose vaccination until the end of the risk interval. | Consistent with definition used in the primary analysis. | Consistent with definition used in the primary analysis. | Time of first eligible booster dose vaccination through the earlier of the end of the outcome-specific risk interval | Consistent with definition used in the booster primary analysis. |
| Control Interval(s) | All remaining time during follow-up excluding risk interval time. | Pre-vaccination control interval covered the same length as the outcome-specific risk interval ending 15 days prior to the date of the first COVID-19 vaccination dose.Post-vaccination control interval consistent with definition from primary analysis. | Pre-vaccination control interval defined the same as secondary analysis.No post-vaccination control interval or defined observation period unlike primary and secondary analysis. | All remaining time during follow-up excluding risk interval time. | Pre-vaccination control interval covers same length as the outcome-specific risk interval ending 15 days prior to the date of the index booster COVID-19 vaccination.Post-vaccination control interval consistent with definition used in the primary/secondary analyses. |
| Adjusted Analyses | Primary-analysis adjustments performed to assess robustness of primary analysis risk estimates: | Consistent with primary analysis adjustments | Consistent with primary analysis adjustments | Primary-analysis adjustments: | Consistent with booster study primary analysis adjustments |
| (i) Seasonality adjustment using the pre-vaccination period | (i) Seasonality-adjusted analysis using the pre-vaccination period and the study period separately (PE-specific) | ||||
| (ii) PPV-adjusted analysis | (ii) Prior COVID-19 infection exclusion analysis | ||||
| (iii) Seasonality & PPV-adjusted analysis | (iii) Seasonality & Prior COVID-19 infection exclusion analysis | ||||
| (iv) Seasonality, PPV-adjusted & prior COVID-19 exclusion analysis | (iv) Concomitant vaccination exclusion analysis | ||||
| (v) Removal of primary series population restriction analysis | |||||
| (vi) PPV-adjusted analysis | |||||
| Sensitivity Analyses | The following sensitivity analyses were performed to test the robustness of specification choices: | None | None | None | None |
| (i) Different clean window lengths | |||||
| (ii) Alternative adverse event case definitions including restricting to Type I AMI only, restricting to inpatient cases (PE, ITP, DIC) or inpatient cases with the adverse event diagnosis as primary diagnosis (AMI, PE, ITP, DIC) | |||||
Abbreviations: AMI, acute myocardial infarction; ITP, immune thrombocytopenia; PE, pulmonary embolism; DIC, disseminated intravascular coagulation; Myo-/Peri, myocarditis/pericarditis; BP, Bell's Palsy.
We utilized longitudinal claims and enrollment data from the Medicare database to obtain information on beneficiaries’ demographics, enrollment, vaccination history, medical history, AE occurrence, and nursing home residence status.
The study start date for the primary series and booster studies aligned with the earliest Emergency Use Authorization (EUA) date for the respective vaccines. Study end dates for both sets of vaccines were independently specified as the dates when the study achieved sufficient power to detect a minimum pre-specified risk, and to ensure at least 90 percent data completeness. For the primary series study, the start date was December 11, 2020 and the event-specific study end dates were April 16, 2021 for AMI, April 30, 2021 for PE and DIC, and May 7, 2021 for ITP. The booster study start date was August 12, 2021, and the event-specific study end dates were April 30, 2022 for AMI, PE and ITP, May 7, 2022 for Myo/Peri, and May 14, 2022 for BP.
2.2. Study populations, exposures, adverse events, and follow-up times
The primary series and booster studies both included elderly Medicare FFS beneficiaries who received at least one of the specified COVID-19 mRNA vaccine doses and experienced an incident AE occurrence during the follow-up. The booster study excluded individuals without an observed primary series vaccination in alignment with EUA authorized uses.
Exposure and AE definitions are found in eTables 1 and 2, respectively. The six AEs in both studies were identified from previously performed active monitoring surveillance analyses specific to the primary series and booster dose vaccines. The primary series study focused on the post-vaccination risk of AMI, DIC, and PE using a risk window of 1–28 days, and ITP using a risk window of 1–42 days post-vaccination. The booster study examined the risk of AMI and hospital inpatient PE using a 1–28 day post-vaccination risk window, BP and ITP using a 1–42 day post-vaccination risk window, and Myo/Peri using a 1–21 day post-vaccination risk window. While consistent event definitions were used for AMI and PE in the two studies, the booster study used a more restrictive hospital inpatient ITP event definition to improve the specificity of cases identified.
To ensure sufficient observation time, beneficiaries were required to accrue follow-up time during both the risk and control intervals, unless death occurred before the control window. Beneficiaries were followed until the earliest occurrence of observation period end, study period end, disenrollment, death, or a fourth vaccine dose. Continuous enrollment was required from the clean window prior to the occurrence of an AE through follow-up, and in the 365 days prior to the incident AE. A clean window is defined as an interval relative to an AE date used to define incident outcomes, where an outcome is considered incident only if no prior outcome occurs during that interval. To assess the effect of individual vaccine brands and prevent misclassification of vaccine doses, both studies excluded individuals with heterologous vaccination use, and vaccinations too close in proximity to previous vaccine doses.
2.3. Medical record review
To validate the claims-based AE definitions, medical record review (MRR) was conducted for cases identified from the primary series (AMI, PE (all care settings, hospital inpatient setting only), ITP (all care settings), DIC) and booster studies (BP, ITP (hospital inpatient setting only, primary diagnosis) Myo/Peri). For each case definition, medical records were obtained and adjudicated from a random sample of cases identified in both studies. Cases were then classified as true cases, non-cases, and indeterminate using standard clinical definitions when available [12], [13], [14], [15], [16], [17], [18]. When not available, case definitions for the AE were developed in consultation with specialist clinicians and consensus literature. For each AE definition, a positive predictive value (PPV) along with a corresponding 95 % confidence interval (CI) was estimated [19]. Table 3 presents classification decisions and PPV estimates by AE. These estimates were used to conduct a quantitative bias analysis (QBA) for each AE to assess the direction and magnitude of event misclassification [20].
Table 3.
Summary of medical record review case adjudication results and PPVs associated with adverse events.
| Outcome and Final Case Classifications | Risk and Control Cases Received* | Risk Cases† | Control Cases†† |
|---|---|---|---|
| AMI (Cases Requested: 125) | 92 | 50 | 42 |
| Confirmed case | 35 | 20 | 15 |
| Probable | 37 | 21 | 16 |
| Possible | 15 | 5 | 10 |
| Not a case | 3 | 2 | 1 |
| Unable to be determined | 2 | 2 | 0 |
| PPV (Confirmed + Probable)¶ | 80.00 % (95 % CI: 70.59, 86.96) | 85.42 % (95 % CI: 72.83, 92.75) | 73.81 % (95 % CI: 58.93, 84.70) |
| PE (Cases Requested: 179) | 101 | 59 | 42 |
| Confirmed case | 38 | 20 | 18 |
| Probable | 5 | 3 | 2 |
| Possible | 5 | 3 | 2 |
| Not a case | 46 | 29 | 17 |
| Unable to be determined | 7 | 4 | 3 |
| PPV (Confirmed + Probable)¶ | 45.74 % (95 % CI: 36.04, 55.78) | 41.82 % (95 % CI: 29.74, 54.97) | 51.28 % (95 % CI: 36.20, 66.13) |
| PE (IP) (Cases Requested: 42) | 42 | 23 | 19 |
| Confirmed case | 32 | 19 | 13 |
| Probable | 3 | 2 | 1 |
| Possible | 4 | 2 | 2 |
| Not a case | 3 | 0 | 3 |
| Unable to be determined | 0 | 0 | 0 |
| PPV (Confirmed + Probable)¶ | 83.33 % (95 % CI: 69.40, 91.68) | 91.30 % (95 % CI: 73.20, 97.58) | 73.68 % (95 % CI: 51.21, 88.19) |
| ITP (Cases Requested: 182) | 91 | 53 | 38 |
| Confirmed case | 2 | 1 | 1 |
| Probable | 1 | 1 | 0 |
| Possible | 6 | 2 | 4 |
| Not a case | 66 | 39 | 27 |
| Unable to be determined | 16 | 10 | 6 |
| PPV (Confirmed + Probable)¶ | 4.00 % (95 % CI: 1.37, 11.11) | 4.65 % (95 % CI: 1.28, 15.46) | 3.12 % (95 % CI: 0.55, 15.74) |
| DIC (Cases Requested: 128) | 90 | 48 | 42 |
| Confirmed case | 35 | 20 | 15 |
| Probable | 0 | 0 | 0 |
| Possible | 24 | 12 | 12 |
| Not a case | 23 | 11 | 12 |
| Unable to be determined | 8 | 5 | 3 |
| PPV (Confirmed) ¶ | 42.68 % (95 % CI: 32.54, 53.48) | 46.51 % (95 % CI: 32.51, 61.08) | 38.46 % (95 % CI: 24.89, 54.10) |
| BP (Cases Requested: 144) | 79 | 79 | N/A |
| Confirmed case | 3 | 3 | N/A |
| Probable | 7 | 7 | N/A |
| Possible | 10 | 10 | N/A |
| Not a case | 40 | 40 | N/A |
| Unable to be determined | 19 | 19 | N/A |
| PPV (Confirmed + Probable) ¶ | 12.66 % (95 % CI: 7.02, 21.76) | 12.66 % (95 % CI: 7.02, 21.76) | N/A |
Abbreviations: AMI, acute myocardial infarction; ITP, immune thrombocytopenia; PE, pulmonary embolism; DIC, disseminated intravascular coagulation; BP, Bell’s Palsy; PPV, positive predictive value; CI, Confidence Interval.
N/A Control Cases were not obtained for BP.
Cases that occurred during either the risk or the control interval.
Cases that occurred during the risk interval.
Cases that occurred during the control interval.
PPV Calculation excludes cases that we are unable to be determined/assigned a case classification based on MRR.
To facilitate timely analysis, PPV estimates from the primary series study MRR were utilized in the booster study QBA for AMI and PE. Additional MRR was initiated in the booster study for BP, ITP (hospital inpatient setting only, primary diagnosis), and Myo/Peri. The MRR for ITP (hospital inpatient setting, primary diagnosis) and Myo/Peri outcomes is ongoing, and the results are not available for this manuscript.
2.4. Statistical analyses
Descriptive statistics were calculated for categorical variables. The primary analysis used an SCCS study design with a post-vaccination control interval. In the primary analysis, follow-up included all time up to 90 days post-vaccination, with post-vaccination time in pre-specified risk windows considered exposed time and all remaining time considered control time. A conditional Poisson regression was used to estimate the incidence rate ratio (IRR) comparing rates in the risk and control intervals for each AE and the corresponding attributable risk (AR). The AR was calculated by the excess number of cases predicted from the regression model divided by the number of eligible vaccinations or person-time [21].
The secondary analysis included both pre-vaccination and post-vaccination control intervals and was performed to evaluate the robustness of risk estimates from the primary analysis to variations in the period used to estimate baseline risk. Additional control windows from 15 to 43 days pre-vaccination were included.
Given the high fatality rate of certain events, an adjustment to address bias from event-dependent observation time was conducted [22]. Adjustments to the primary and secondary analyses were performed to further investigate the impact of various potential sources of confounding: (i) a seasonality adjustment to account for potential time-varying confounding due to seasonal changes in incidence rates, (ii) an analysis using the PPV from MRR to conduct QBA to assess robustness of results to event misclassification, and (iii) an analysis excluding individuals with prior COVID-19 infection to account for the hypothesized association between the infection and AEs [23], [24], [25], [26], [27], [28], [29], [30].
Additionally, there were analyses unique to each evaluation. The primary series study included (i) an exploratory analysis using only the pre-vaccination control interval to assess robustness to temporal variations in baseline risk, and (ii) adjusted analyses varying the definition of events, including care settings as well as risk and control intervals. The booster study included (i) an analysis removing the requirement that a primary series be observed given the limited observability of primary series vaccinations in the Medicare population, and (ii) a PE-specific post-hoc analysis adjusting for seasonality using pandemic rather than pre-pandemic incidence rates.
All analyses were conducted using R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria), and SAS v. 9.4 (SAS Institute Inc., Cary, NC, United States).
This surveillance activity was conducted as part of the FDA public health surveillance mandate.
3. Results
3.1. Descriptive results
We evaluated the risk of AMI, PE, DIC, and ITP in a study of the primary series doses of COVID-19 mRNA vaccines (primary series study) and evaluated the risk of AMI, PE, ITP, BP, and Myo/Peri in a separate monovalent booster dose study (booster study). Table 2 summarizes the baseline characteristics of vaccinated individuals who developed an AE in the follow-up windows for both studies. Among 3,360,981 individuals who received 6,388,542 primary series doses and 6,156,100 individuals who received monovalent booster doses of either BNT162b2 or mRNA-1273, case counts were as follows: AMI (3,653 primary series, 16,042 booster), hospital inpatient PE (2,470 primary series, 5,085 booster), ITP (1,085 primary series, 88 booster), DIC (254 primary series), BP (3,268 booster), and Myo/Peri (1,295 booster). In both studies, BNT162b2 recipients compared to mRNA-1273 recipients were generally younger, less likely to reside in a rural area, less likely to be dual-eligible for Medicare and Medicaid, and more likely to reside in a nursing home. The primary series study and the booster study were conducted separately and in different time periods during the pandemic. The primary series study was conducted first and earlier in the pandemic era (2021) when a smaller proportion of the elderly population had received the primary series vaccines. The booster study was conducted later in the pandemic (2022) when a larger proportion of the elderly population had received a monovalent booster dose. Therefore, the data through date for each study is different, and the booster study has a larger population than the primary series study. The primary series study had a higher proportion of cases that were older, residing in a nursing home, and eligible for dual Medicare and Medicaid compared to the booster study (Table 2).
Table 2.
Descriptive summary of case characteristics for primary SCCS analysis, by vaccine brand and adverse event, PRIMARY SERIES and BOOSTER STUDIES.
| Patient Characteristics( % of Total) |
Primary Series COVID-19 Vaccinations |
Booster Dose COVID-19 Vaccinations |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
BNT162b2 |
mRNA-1273 |
BNT162b2 |
mRNA-1273 |
|||||||||||||||
| AMI | PE (IP) | ITP | DIC | AMI | PE (IP) | ITP | DIC | AMI | ITP | PE | Myo/Peri | BP | AMI | ITP | PE | Myo/Peri | BP | |
| Total | 2,783 | 1,684 | 668 | 175 | 870 | 786 | 417 | 79 | 8,101 | 49 | 2,622 | 683 | 1,674 | 7,941 | 39 | 2,463 | 612 | 1,594 |
| Age (years) | ||||||||||||||||||
| 65–74 | 18.29 | 21.14 | 30.24 | 31.43 | 23.10 | 25.70 | 30.22 | 35.44 | 29.68 | 57.14 | 34.97 | 42.75 | 45.64 | 32.44 | ** | 37.31 | 44.28 | 47.80 |
| 75–84 | 34.89 | 39.67 | 40.87 | 45.14 | 37.24 | 40.46 | 46.28 | 45.57 | 37.18 | ** | 39.97 | 39.24 | 37.28 | 40.46 | 43.59 | 41.17 | 39.87 | 38.08 |
| 85+ | 46.82 | 39.19 | 28.89 | 23.43 | 39.66 | 33.84 | 23.50 | 18.99 | 33.14 | ** | 25.06 | 18.01 | 17.08 | 27.10 | ** | 21.52 | 15.85 | 14.12 |
| Sex | ||||||||||||||||||
| Female | 57.17 | 59.68 | 46.41 | 54.86 | 54.14 | 56.74 | 47.72 | 55.70 | 49.91 | 55.10 | 57.70 | 52.42 | 58.30 | 46.42 | 46.15 | 55.66 | 52.78 | 55.46 |
| Male | 42.83 | 40.32 | 53.59 | 45.14 | 45.86 | 43.26 | 52.28 | 44.30 | 50.09 | 44.90 | 42.30 | 47.58 | 41.70 | 53.58 | 53.85 | 44.34 | 47.22 | 44.54 |
| Race/Ethnicity | ||||||||||||||||||
| Black | 7.55 | 9.38 | 4.19 | 13.71 | 10.11 | 6.11 | ** | ** | 5.80 | ** | 7.17 | 6.30 | 5.50 | 5.28 | ** | 6.29 | 5.39 | 4.58 |
| White | 87.32 | 87.59 | 88.62 | 75.43 | 82.07 | 90.97 | 90.65 | 74.68 | 87.75 | 83.67 | 87.99 | 87.26 | 84.77 | 87.81 | 89.74 | 89.08 | 87.58 | 86.07 |
| Other | 5.14 | 3.03 | 7.19 | 10.86 | 7.82 | 2.93 | ** | ** | 6.44 | ** | 4.84 | 6.44 | 9.74 | 6.91 | ** | 4.63 | 7.03 | 9.35 |
| Urban/Rural | ||||||||||||||||||
| Urban | 78.08 | 84.86 | 85.03 | 82.86 | 68.85 | 74.30 | 81.53 | 82.28 | 84.96 | ** | 86.61 | 86.97 | 86.86 | 73.76 | ** | 77.71 | 83.01 | 76.66 |
| Rural | 21.92 | 15.14 | 14.97 | 17.14 | 31.15 | 25.70 | 18.47 | 17.72 | 15.04 | ** | 13.39 | 13.03 | 13.14 | 26.24 | ** | 22.29 | 16.99 | 23.34 |
| HHS Region | ||||||||||||||||||
| Region 1 (Boston) | 8.95 | 8.55 | 8.83 | ** | ** | ** | ** | 0.00 | 8.37 | ** | 7.63 | 8.64 | 7.59 | 5.04 | ** | 5.16 | 7.84 | 5.14 |
| Region 2 (New York) | 12.18 | 10.63 | 9.88 | 13.71 | 2.99 | 5.60 | 9.59 | ** | 9.80 | ** | 8.28 | 10.83 | 9.98 | 9.68 | ** | 9.42 | 10.95 | 11.92 |
| Region 3 (Philadelphia) | 8.70 | 10.69 | 8.23 | 7.43 | 3.91 | 5.85 | 8.87 | ** | 10.37 | ** | 11.17 | 14.79 | 10.81 | 10.01 | ** | 11.45 | 14.05 | 11.23 |
| Region 4 (Atlanta) | 20.45 | 20.25 | 16.92 | 18.86 | 26.67 | 30.15 | 26.38 | 24.05 | 15.11 | ** | 15.18 | 13.03 | 14.64 | 20.75 | ** | 19.08 | 16.67 | 20.70 |
| Region 5 (Chicago) | 10.96 | 18.47 | 21.71 | 12.57 | 21.49 | 22.77 | 22.06 | 17.72 | 24.31 | ** | 26.62 | 21.96 | 24.25 | 20.85 | 33.33 | 24.16 | 19.93 | 17.82 |
| Region 6 (Dallas) | 18.40 | 12.23 | 11.98 | 20.57 | 26.90 | 13.87 | 10.79 | 22.78 | 10.07 | ** | 9.19 | 7.47 | 9.68 | 10.01 | ** | 7.51 | 7.19 | 10.48 |
| Region 7 (Kansas City) | 6.83 | 4.51 | 3.59 | ** | 4.83 | 5.47 | ** | ** | 5.80 | ** | 6.03 | 5.71 | 5.32 | 5.33 | ** | 5.72 | 3.59 | 5.71 |
| Region 8 (Denver) | 3.52 | 4.04 | 3.89 | ** | 3.33 | 2.80 | 4.08 | ** | 2.79 | ** | 3.47 | ** | ** | 2.14 | 0.00 | 2.64 | ** | 1.76 |
| Region 9 (San Francisco) | 6.61 | 7.54 | 11.23 | 16.00 | 6.55 | 9.16 | 12.47 | 17.72 | 9.65 | ** | 9.38 | 11.13 | 11.11 | 12.66 | ** | 11.53 | 15.03 | 13.05 |
| Region 10 (Seattle) | ** | 3.09 | ** | ** | 2.07 | 3.31 | 2.40 | ** | 3.57 | 0.00 | ** | 3.51 | 3.76 | 3.39 | 0.00 | 3.33 | 2.45 | 2.20 |
| Missing/Unknown | ** | 0.00 | ** | 0.00 | ** | ** | 0.00 | 0.00 | 0.16 | 0.00 | ** | ** | ** | 0.14 | 0.00 | 0.00 | ** | 0.00 |
| Dual-Eligibility Status | ||||||||||||||||||
| Dual-Eligible | 40.46 | 24.41 | 13.47 | 39.43 | 34.83 | 17.56 | 6.95 | 27.85 | 12.02 | ** | 9.50 | 8.05 | 8.42 | 9.82 | ** | 7.92 | 7.52 | 7.47 |
| Non-Dual-Eligible | 59.54 | 75.59 | 86.53 | 60.57 | 65.17 | 82.44 | 93.05 | 72.15 | 87.98 | ** | 90.50 | 91.95 | 91.58 | 90.18 | ** | 92.08 | 92.48 | 92.53 |
| Area Deprivation Index (ADI) Rank | ||||||||||||||||||
| 1–10 (low deprivation) | 9.85 | 11.70 | 15.87 | 9.71 | 7.47 | 9.54 | 20.38 | 13.92 | 12.16 | 24.49 | 12.74 | 16.84 | 15.89 | 11.30 | ** | 12.55 | 17.16 | 14.81 |
| 11–20 | 10.38 | 13.00 | 16.77 | 16.00 | 7.70 | 10.81 | 8.15 | ** | 13.66 | ** | 13.65 | 15.52 | 14.22 | 11.27 | ** | 12.42 | 14.22 | 12.42 |
| 21–30 | 12.15 | 15.20 | 14.22 | 10.86 | 9.43 | 10.05 | 13.67 | ** | 13.60 | ** | 14.65 | 18.45 | 15.05 | 11.67 | ** | 13.72 | 12.58 | 12.17 |
| 31–40 | 11.43 | 11.64 | 11.83 | 9.14 | 8.51 | 10.05 | 9.59 | ** | 12.49 | ** | 13.65 | 8.64 | 12.31 | 11.35 | ** | 11.29 | 9.48 | 10.73 |
| 41–50 | 10.74 | 10.39 | 9.13 | 13.14 | 11.84 | 12.47 | 11.03 | ** | 12.12 | ** | 12.59 | 9.37 | 13.44 | 11.13 | ** | 11.45 | 12.09 | 10.23 |
| 51–60 | 8.62 | 9.50 | 7.93 | 8.00 | 11.61 | 13.23 | 9.35 | ** | 9.55 | ** | 9.15 | 8.78 | 7.29 | 10.53 | ** | 10.68 | 10.13 | 10.98 |
| 61–70 | 9.99 | 8.79 | 8.23 | 9.14 | 11.61 | 10.43 | 7.91 | ** | 8.04 | ** | 6.67 | 5.86 | 6.63 | 9.92 | ** | 8.53 | 7.52 | 8.59 |
| 71–80 | 9.09 | 5.88 | 5.54 | ** | 10.57 | 7.89 | 7.19 | ** | 6.63 | ** | 6.56 | 4.39 | 5.68 | 8.73 | ** | 7.06 | 6.86 | 7.34 |
| 81–90 | 7.83 | 6.18 | 4.34 | ** | 9.89 | 7.51 | 6.00 | ** | 5.11 | 0.00 | 4.61 | 4.39 | 4.06 | 7.34 | 0.00 | 6.41 | 3.76 | 7.09 |
| 91–100 (high deprivation) | 5.93 | 4.28 | 3.74 | 9.14 | 8.16 | 5.47 | 3.36 | ** | 3.96 | ** | 3.24 | 3.66 | 3.46 | 4.36 | ** | 3.29 | 3.92 | 3.01 |
| Missing/Unknown | 3.99 | 3.44 | 2.40 | ** | 3.22 | 2.54 | 3.36 | ** | 2.67 | ** | 2.48 | 4.10 | 1.97 | 2.41 | ** | 2.60 | 2.29 | 2.63 |
| Nursing Home Status | ||||||||||||||||||
| Nursing Home | 54.04 | 36.46 | 14.97 | 47.43 | 44.37 | 21.25 | 5.52 | 35.44 | 7.39 | 0.00 | 7.13 | 6.59 | 3.76 | 4.39 | ** | 3.65 | 2.45 | 2.76 |
| Non-Nursing Home | 45.96 | 63.54 | 85.03 | 52.57 | 55.63 | 78.75 | 94.48 | 64.56 | 92.61 | 100.00 | 92.87 | 93.41 | 96.24 | 95.61 | ** | 96.35 | 97.55 | 97.24 |
| Medicare Status | ||||||||||||||||||
| Aged-in only | 81.39 | 85.57 | 89.07 | 79.43 | 80.69 | 87.66 | 89.69 | 77.22 | 87.2 | ** | 88.9 | 86.2 | 87.9 | 87.04 | ** | 88.55 | 87.91 | 87.26 |
| Disabled or ESRD | 18.61 | 14.43 | 10.93 | 20.57 | 19.31 | 12.34 | 10.31 | 22.78 | 12.78 | ** | 11.10 | 13.76 | 12.13 | 12.96 | ** | 11.45 | 12.09 | 12.74 |
| Medical Conditions (0–365 days prior to vaccination date) | ||||||||||||||||||
| Hospitalization | 49.59 | 44.00 | 27.54 | 57.71 | 41.26 | 34.86 | 19.66 | 44.30 | 30.53 | 38.78 | 30.09 | 31.48 | 18.58 | 27.49 | ** | 27.61 | 28.59 | 16.94 |
| Hypertension | 91.48 | 85.45 | 79.34 | 85.71 | 90.11 | 82.44 | 79.86 | 96.20 | 86.83 | 71.43 | 80.78 | 82.87 | 79.75 | 86.45 | 71.79 | 80.59 | 83.99 | 79.42 |
| Diabetes | 48.08 | 34.80 | 33.23 | 46.29 | 46.55 | 33.46 | 34.05 | 62.03 | 42.57 | 30.61 | 30.40 | 32.06 | 35.13 | 42.84 | ** | 31.26 | 33.82 | 36.95 |
| COPD | 30.94 | 26.84 | 20.51 | 30.29 | 28.62 | 25.45 | 18.47 | 21.52 | 23.07 | ** | 22.46 | 20.94 | 13.74 | 24.38 | ** | 22.86 | 20.26 | 13.93 |
| Asthma w/o COPD | 7.65 | 8.31 | 9.58 | 11.43 | 6.78 | 10.56 | 7.19 | ** | 8.76 | ** | 10.11 | 12.30 | 9.32 | 8.88 | ** | 10.23 | 10.78 | 8.66 |
| Charlson Comorbidity Index > 0 | 95.11 | 90.80 | 85.48 | 94.29 | 92.18 | 85.37 | 84.17 | 96.20 | 88.17 | 83.67 | 86.42 | 84.77 | 79.15 | 86.75 | 61.54 | 83.07 | 82.52 | 77.73 |
| Atrial Fibrillation | 36.58 | 24.29 | 31.14 | 42.29 | 33.68 | 20.23 | 27.82 | 43.04 | 29.26 | 32.65 | 20.67 | 34.11 | 15.11 | 27.88 | ** | 19.08 | 32.68 | 18.13 |
| Bronchiectasis | 1.44 | 2.79 | 2.69 | ** | ** | 2.80 | 3.60 | ** | 2.07 | ** | 3.28 | 2.34 | 2.03 | 1.70 | 0.00 | 2.68 | 2.45 | 1.63 |
| Coronary Revascularization | 1.01 | 1.31 | ** | ** | 1.49 | ** | ** | ** | 2.06 | 0.00 | 0.76 | 2.20 | 0.90 | 2.28 | 0.00 | 1.46 | 1.96 | 0.88 |
| Depression | 48.76 | 43.59 | 26.35 | 42.29 | 41.49 | 32.32 | 20.14 | 35.44 | 26.21 | 22.45 | 28.18 | 26.65 | 24.97 | 22.97 | ** | 25.90 | 21.73 | 24.97 |
| Gout | 9.95 | 8.37 | 10.18 | 15.43 | 10.46 | 8.40 | 9.35 | 16.46 | 10.05 | ** | 8.47 | 8.64 | 7.11 | 9.91 | ** | 8.16 | 10.13 | 7.53 |
| Interstitial Lung Disease | 5.50 | 4.99 | 4.49 | 8.57 | 4.83 | 5.85 | 5.52 | ** | 5.01 | ** | 6.22 | 4.98 | 3.29 | 4.73 | 0.00 | 5.77 | 4.25 | 2.45 |
| Impaired Mobility | 4.71 | 2.67 | ** | ** | 4.14 | 1.53 | 0.00 | ** | 1.26 | ** | 1.45 | ** | 0.72 | 0.96 | 0.00 | 1.10 | ** | 0.82 |
| Obesity | 22.10 | 23.87 | 23.35 | 27.43 | 25.06 | 24.05 | 22.30 | 35.44 | 23.31 | 24.49 | 29.52 | 23.87 | 25.75 | 25.15 | 28.21 | 30.09 | 26.47 | 25.72 |
| Pneumonia | 25.33 | 22.98 | 12.28 | 28.00 | 21.26 | 15.52 | 10.79 | 18.99 | 11.13 | ** | 11.37 | 12.01 | 6.57 | 9.84 | ** | 10.27 | 9.15 | 5.21 |
| Stroke | 4.60 | 3.44 | ** | ** | 3.10 | 2.29 | ** | 0.00 | 1.78 | 0.00 | 1.14 | 1.61 | 1.25 | 1.64 | 0.00 | 1.42 | ** | 0.75 |
| Neurological or Neurodevelopmental Conditions | 27.85 | 24.23 | 16.47 | 32.00 | 24.37 | 20.61 | 11.03 | 24.05 | 14.31 | ** | 15.22 | 11.86 | 14.64 | 12.29 | ** | 15.43 | 9.15 | 12.36 |
| Prior COVID-19 diagnosis (0–365 days prior to vaccination date) | ||||||||||||||||||
| Inpatient | 7.22 | 7.42 | 2.40 | 9.71 | 6.21 | 4.71 | 2.64 | ** | 2.21 | ** | 1.72 | 3.07 | 1.08 | 1.73 | ** | 1.38 | ** | 1.13 |
| Outpatient & Professional | 14.95 | 10.69 | 8.83 | 13.14 | 13.10 | 7.00 | 3.84 | ** | 5.90 | ** | 4.35 | 6.15 | 5.26 | 4.60 | ** | 3.90 | ** | 4.02 |
| None | 77.83 | 81.89 | 88.77 | 77.14 | 80.69 | 88.30 | 93.53 | 79.75 | 91.89 | 87.76 | 93.94 | 90.78 | 93.67 | 93.68 | 94.87 | 94.72 | 95.42 | 94.86 |
| Other vaccination (on the COVID-19 vaccination dose date) | ||||||||||||||||||
| Flu vaccination | ** | ** | 0.00 | 0.00 | ** | 0.00 | 0.00 | 0.00 | 12.97 | ** | 13.73 | 11.42 | 11.95 | 6.50 | ** | 7.19 | 7.03 | 7.28 |
| Pneumococcal vaccination | ** | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.15 | 0.00 | ** | ** | ** | 0.24 | ** | ** | ** | ** |
Abbreviations: AMI, acute myocardial infarction; ITP, immune thrombocytopenia; PE, pulmonary embolism; DIC, disseminated intravascular coagulation; Myo-/Peri, myocarditis/pericarditis; BP, Bell's Palsy; ADI, area deprivations index; ESRD, end-stage renal disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus 2019; IP, Hospital Inpatient.
*Patient characteristics are summarized as a percentage of the total population receiving the specific brand of vaccine and experiencing the outcome.
**Display of specific cell count discloses small cell size < 11; suppressed to protect patient confidentiality.
For all six AEs included in either the primary series or booster study, seasonality patterns varied, indicating the need for a seasonality adjustment comparing incidence rates during corresponding calendar months (data not shown). For the booster study, we observed the largest variation in seasonality patterns for PE, with elevated incidence rates of PE comparing the pre-pandemic (2018–2019) and pandemic (2020–2022) periods. Given that the main seasonality adjustment was made using pre-pandemic incidence rates, a PE-specific seasonality adjustment using the pandemic period rates was performed to assess the impact on risk estimation (eFigure 4).
Descriptive analyses of cases in the primary series study found that AMI and DIC exhibited a high case fatality rate of 34 % and 67 %, respectively, indicating the need to adjust for curtailed observation time (eTable 3). AMI, hospital inpatient PE, and DIC had a high proportion of cases with prior medically attended COVID-19 infection, ranging from 28 % to 36 % (eTable 4). Case fatality rates were not estimated for the booster study.
3.2. Medical record review
Table 3 summarizes results of MRR to verify outcomes for the primary series and the booster studies. MRR was conducted for a sample of cases with AMI, PE, ITP, DIC, and BP events. AMI (PPV: 80.00 % (95 % CI: 70.59–86.96 %)) and hospital inpatient PE (PPV: 83.33 % (95 % CI: 69.40–91.68 %)) claims-based definitions had the highest PPVs, indicating relatively accurate identification of cases. Comparatively, the all-care-setting PE (PPV: 45.74 % (95 % CI: 36.04–55.78 %)) and DIC (PPV: 42.68 % (95 % CI: 32.54–53.48 %)) event definitions had lower PPV estimates in identifying true disease cases. The all-care-setting BP (PPV: 12.66 % (95 % CI: 7.02–21.76 %)) event definition used in the booster study and all-care-setting ITP (PPV: 4.00 % (95 % CI: 1.37–11.11 %)) event definition used in the primary series study had the lowest PPVs of all AEs, pointing to high misclassification of these events.
3.3. Inferential results
Results from the primary analyses are described below for the primary series and booster studies. Secondary and exploratory analyses for the primary series study (eTable 7 and eTable 8, respectively) as well as secondary analyses for the booster study are included in the supplemental material (eTable 11).
3.3.1. Acute myocardial infarction (AMI)
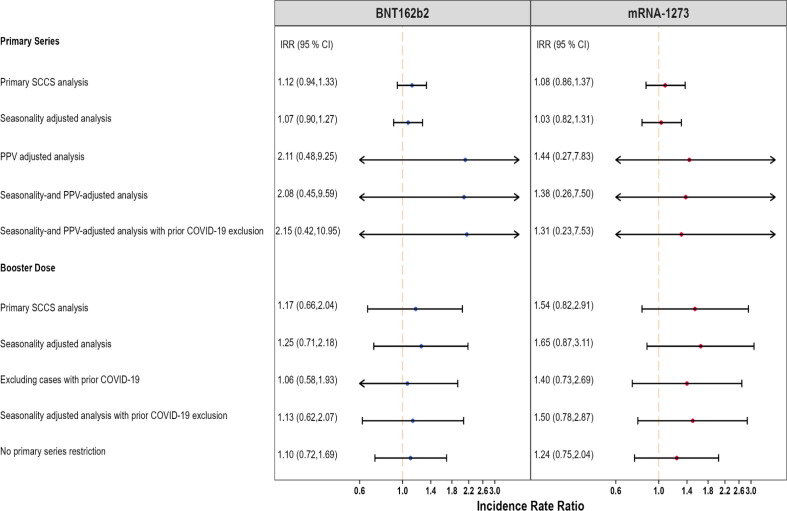
AMI was evaluated in both primary series and booster studies. We did not find consistent results for AMI risk following administration of the BNT162b2 or mRNA-1273 vaccines in both primary series and booster studies (Fig. 1 ).
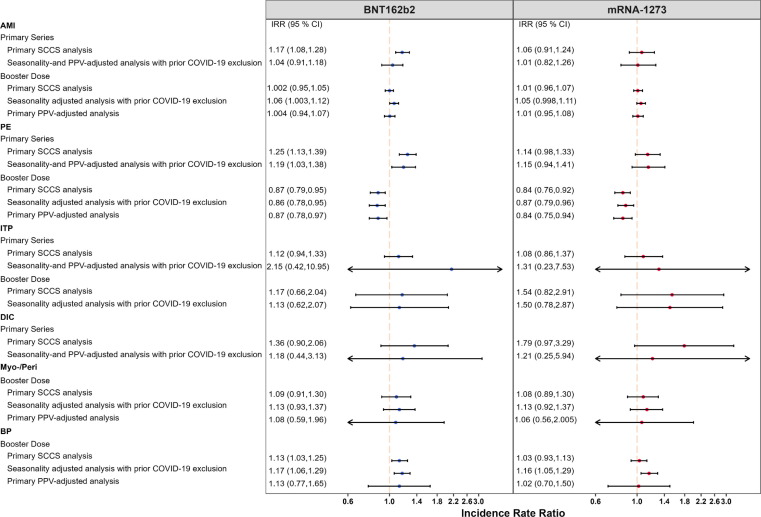
Fig. 1.
Summary of SCCS analysis with post-vaccine control interval assessing the Incidence Rate Ratio (IRR) of Acute Myocardial Infarction (AMI), Pulmonary Embolism (PE), Immune Thrombocytopenia (ITP), Disseminated Intravascular Coagulation (DIC), Myocarditis/Pericarditis (Myo-/Peri), and Bell’s Palsy (BP) adjusted for seasonality, event-dependent observation time, interval-specific PPV, and excluding cases with prior COVID-19 diagnosis, PRIMARY SERIES AND BOOSTER STUDIES *Fig. 1 displays incident rate ratios and corresponding 95 % confidence intervals from eTables 5, 6, 9, and 10.
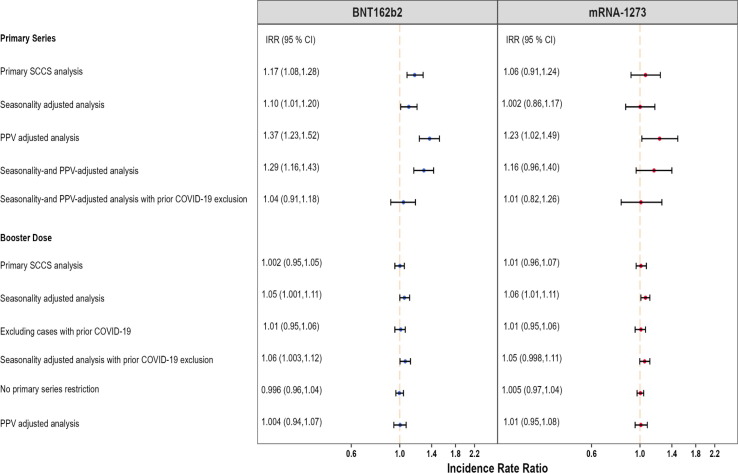
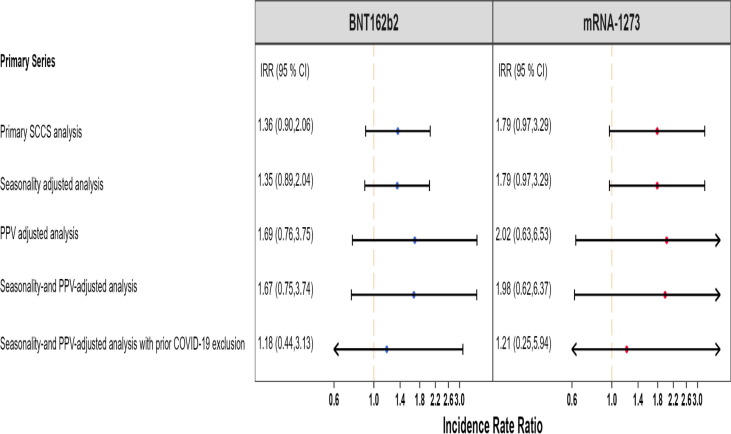
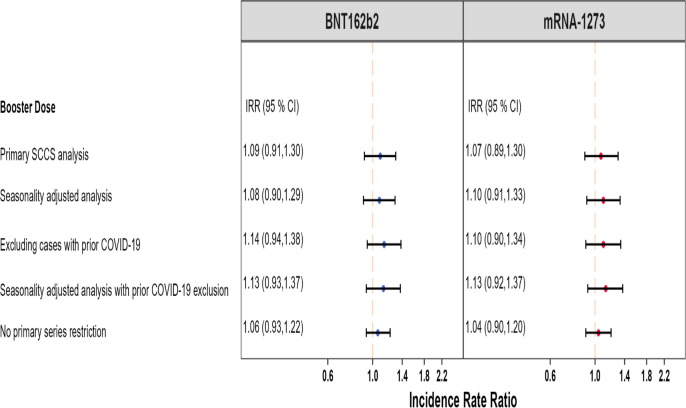
We detected a small but statistically significant elevated risk of AMI following the BNT162b2 primary series (IRR: 1.17, 95 % CI: 1.08 to 1.28) (Fig. 1). However, this effect was no longer statistically significant when accounting for seasonality, adjusting for outcome misclassification using MRR-derived PPVs, and excluding individuals with prior COVID-19 infection (IRR: 1.04, 95 % CI: 0.91 to 1.18). We did not observe evidence of elevated risk following the BNT162b2 booster dose (IRR: 1.00, 95 % CI: 0.95 to 1.05) in the primary analysis. After adjusting for seasonality and exclusion of cases with prior COVID-19 infection, a marginally statistically significant elevation in risk was observed following the BNT162b2 booster dose (IRR: 1.06, 95 % CI: 1.003 to 1.12). In the adjusted analysis, the AR per 100,000 doses following BNT162b2 primary series vaccination (AR: 3.33, 95 % CI: −8.80 to 14.02, eTable 6) was similar to the AR after booster dose (AR: 3.91, 95 % CI: 0.19 to 7.63, eTable 10).
There was no statistically significant increased risk of AMI following the mRNA-1273 primary series in both the primary analysis (IRR:1.06, 95 % CI: 0.91 to 1.24) and the adjusted analysis accounting for seasonality, PPV adjustment, and excluding cases with prior COVID-19 infection (IRR:1.01, 95 % CI: 0.82 to 1.26) (Fig. 1). The estimates from the booster study were consistent with no statistically significant increase in risk observed in the primary analysis (IRR: 1.01, 95 % CI: 0.96 to 1.07) or the adjusted analysis (IRR: 1.05, 95 % CI: 0.998 to 1.11).
Fig. 2 presents a more detailed summary of all the AMI analyses and results in both studies.
Fig. 2.
Summary of SCCS analysis with post-vaccine control interval assessing the Incidence Rate Ratio (IRR) of Acute Myocardial Infarction (AMI) adjusted for seasonality, event-dependent observation time, interval-specific PPV, and excluding cases with prior COVID-19 diagnosis, PRIMARY SERIES AND BOOSTER STUDIES.
3.3.2. Pulmonary embolism (PE)
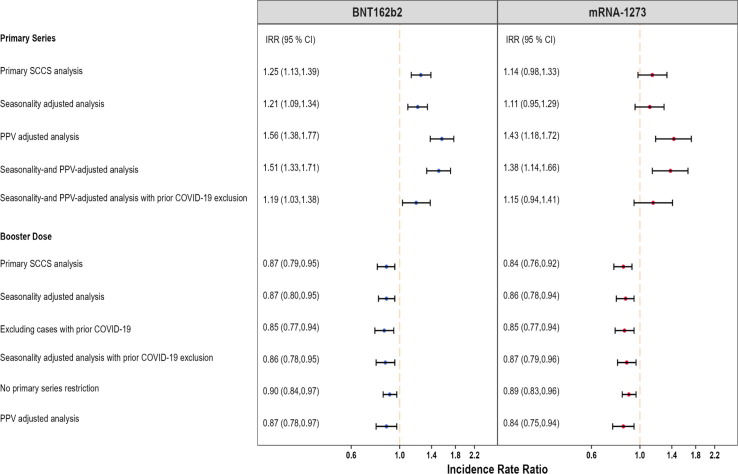
Hospital inpatient PE was evaluated in both primary series and booster studies. We detected a small but statistically significant elevated risk of hospital inpatient PE following BNT162b2 vaccination (IRR: 1.25, 95 % CI: 1.13 to 1.39) in the primary series study, which remained significant in analyses adjusting for seasonality and PPV, and excluding individuals with prior COVID-19 infection (Fig. 1). However, the booster study showed a statistically significant reduction in hospital inpatient PE risk associated with BNT162b2 (IRR: 0.87, 95 % CI: 0.79 to 0.95), which remained consistent after adjusting for seasonality and PPV, and excluding individuals with prior COVID-19 infection (IRR: 0.86, 95 % CI: 0.78 to 0.95) and in most additional analyses (Fig. 3 ). In the adjusted analysis, AR per 100,000 doses after primary series (AR: 3.50, 95 % CI: 0.55 to 6.05, eTable 6) was higher than that following the booster dose (AR: −3.34, 95 % CI: −5.40 to −1.29, eTable 10).
Fig. 3.
Summary of SCCS analysis with post-vaccine control interval assessing the Incidence Rate Ratio (IRR) of Pulmonary Embolism (PE) adjusted for seasonality, event-dependent observation time, interval-specific PPV, and excluding cases with prior COVID-19 diagnosis, PRIMARY SERIES AND BOOSTER STUDIES.
In the primary series study, we did not detect a statistically significant elevated risk of hospital inpatient PE following mRNA-1273 in the primary analysis (IRR: 1.14, 95 % CI: 0.98 to 1.33) or in the analysis adjusting for seasonality and PPV, and prior COVID-19 infection (IRR: 1.15, 95 % CI: 0.94 to 1.41) (Fig. 1). The booster study showed a statistically significant decrease in hospital inpatient PE risk in the primary analysis (IRR: 0.84, 95 % CI: 0.76 to 0.92) and analyses adjusting for seasonality and PPV, and excluding individuals with prior COVID-19 infection (IRR: 0.87, 95 % CI: 0.79 to 0.96) and in all other adjustments (Fig. 3). In the adjusted analysis, AR per 100,000 doses after primary series (AR: 2.58, 95 % CI: −1.24 to 5.69, eTable 6) was higher than that following the booster dose (AR: −2.71, 95 % CI: −4.61 to −0.81, eTable 10).
Fig. 3 presents a more detailed summary of all the hospital inpatient PE analyses and results in both studies.
3.3.3. Bell’s Palsy (BP)
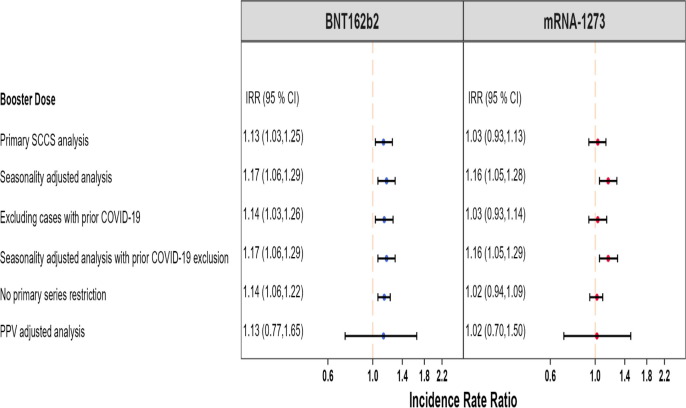
BP was evaluated in the booster study only. A small but statistically significant elevation in BP risk was detected following a booster dose of BNT162b2 vaccination (IRR: 1.13, 95 % CI: 1.03 to 1.25), and it remained consistent across additional analyses such as adjustment for seasonality and prior COVID-19 infection exclusion (IRR: 1.17, 95 % CI: 1.06 to 1.29) (Fig. 5 ). There was no statistically significant elevation in BP risk after a booster dose of mRNA-1273 vaccination (IRR: 1.03, 95 % CI: 0.93 to 1.13), and it remained consistent across some additional analyses although a statistically significant result was detected for the primary analysis adjusted for seasonality and exclusion for prior COVID-19 infection (IRR: 1.16, 95 % CI: 1.05 to 1.29) (Fig. 5). However, after adjusting for outcome misclassification using MRR-derived PPV, there was no longer a statistically significant elevation in BP risk following a booster dose of BNT162b2 vaccination (IRR: 1.13, 95 % CI: 0.77 to 1.65) and mRNA-1273 vaccination (IRR: 1.02, 95 % CI: 0.70 to 1.50) (Fig. 5) (eTable 12). In the adjusted analysis, AR per 100,000 doses post-BNT162b2 was (AR: 3.73, 95 % CI: 1.38 to 6.08) larger than that post-mRNA-1273 (AR: 3.16, 95 % CI: 1.02 to 5.03) (eTable 10).
Fig. 5.
Summary of SCCS analysis with post-vaccine control interval assessing the Incidence Rate Ratio (IRR) of Bell’s Palsy (BP) adjusted for seasonality, event-dependent observation time, interval-specific PPV, and excluding cases with prior COVID-19 diagnosis, BOOSTER STUDY.
Fig. 5 presents a more detailed summary of all the BP analyses and results in the booster study.
3.3.4. Other adverse events (ITP, DIC, and Myo/Peri)
ITP was evaluated in both primary series and booster studies. We did not find a statistically significant increase in ITP risk in any of the analyses in either primary series or booster study after exposure to mRNA vaccines. Following primary series of BNT162b2 and mRNA-1273, the seasonality and PPV-adjusted analyses with prior COVID-19 exclusion resulted in IRR: 2.15 (95 % CI: 0.42 to 10.95), and IRR: 1.31 (95 % CI: 0.23 to 7.53), respectively. Following booster dose of BNT162b2 and mRNA-1273, the seasonality analyses with prior COVID-19 exclusion resulted in IRR: 1.13 (95 % CI: 0.62 to 2.07) and IRR: 1.50 (95 % CI: 0.78 to 2.87), respectively (Fig. 7).
Fig. 7.
Summary of SCCS analysis with post-vaccine control interval assessing the Incidence Rate Ratio (IRR) of Immune Thrombocytopenia (ITP) adjusted for seasonality, event-dependent observation time, interval-specific PPV, and excluding cases with prior COVID-19 diagnosis, PRIMARY SERIES AND BOOSTER STUDIES.
DIC was evaluated only in the primary series study, and we did not detect a statistically significant increase in DIC risk in the analyses for either vaccine. Following primary series of BNT162b2 and mRNA-1273, the seasonality and PPV-adjusted analyses with prior COVID-19 exclusion resulted in IRR: 1.18 (95 % CI: 0.44 to 3.13) and IRR: 1.21 (95 % CI: 0.25 to 5.94), respectively (Fig. 6 ).
Fig. 6.
Summary of SCCS analysis with post-vaccine control interval assessing the Incidence Rate Ratio (IRR) of Disseminated Intravascular Coagulation (DIC) adjusted for seasonality, event-dependent observation time, interval-specific PPV, and excluding cases with prior COVID-19 diagnosis, PRIMARY SERIES STUDY.
Myo/Peri was evaluated only in the booster study, and similarly we did not observe a statistically significant increase in Myo/Peri risk in the analyses for either vaccine. Following booster dose of BNT162b2 and mRNA-1273, the seasonality analyses with prior COVID-19 exclusion resulted in IRR: 1.13 (95 % CI: 0.93 to 1.37) and IRR: 1.13 (95 % CI: 0.92 to 1.37), respectively (Fig. 4).
Fig. 4.
Summary of SCCS analysis with post-vaccine control interval assessing the Incidence Rate Ratio (IRR) of Myocarditis/Pericarditis (Myo-/Peri) adjusted for seasonality, event-dependent observation time, interval-specific PPV, and excluding cases with prior COVID-19 diagnosis, BOOSTER STUDY.
Fig. 4, Fig. 6, Fig. 7 present a more detailed summary of all the analyses and results for ITP, DIC, and Myo/Peri, respectively, in both studies.
4. Discussion
Of six AEs evaluated in two independent population-based studies including the U.S. elderly population, no statistically significant increase in risk was identified for ITP, DIC, and Myo/Peri following COVID-19 mRNA vaccination for primary series and monovalent booster. These findings were robust to multiple analytic methods and adjustments.
The potential increased risk of AMI following the BNT162b2 primary series vaccine was reduced to a null effect after analytic adjustments. Following the booster BNT162b2 vaccine, the null effect from the primary analysis became a marginally significantly elevated risk after analytic adjustments, and the increased risk was very small. In both the primary and adjusted analyses, there was no evidence of a statistically significantly increased AMI risk after exposure to the primary series and booster doses of mRNA-1273 vaccine.
There was not consistent evidence of an increased risk of hospital inpatient PE following COVID-19 mRNA vaccination between the primary series and booster studies. Following the primary series of BNT162b2 vaccination we observed a small but statistically significant increase in hospital inpatient PE risk that was robust to analytical adjustments; however, the risk following mRNA-1273 primary series was not statistically significantly elevated. In contrast, exposure to both mRNA vaccines booster doses showed a statistically significant protective effect against hospital inpatient PE risk that was largely robust to analytical adjustments. The increased risk of PE following BNT162b2 primary series and its decreased risk following booster doses of both mRNA vaccines were both small. The protective effect following a booster dose when there was a small but elevated PE risk following the primary series would be unexpected if there were a true increased PE risk following COVID-19 mRNA vaccination. This adds to the level of uncertainty about an increased PE risk.
The primary series study did not evaluate BP risk since it did not signal in the signal detection study. The booster study showed a small increased risk of BP after exposure to BNT162b2 and mRNA-1273 booster dose vaccines, which was statistically significant for the former but not the latter. For both vaccines, the small elevated risk was statistically significant after some adjustments such as seasonality and exclusion of cases with prior COVID-19 infection but not after other adjustments such as PPV-adjustment. The PPV for BP was quite low at 12.6 %, which increases the uncertainty about the observed small magnitude of increased risk because of a high degree of outcome misclassification for BP in the booster study.
One potential explanation for some of the statistically significant results associated with AMI, PE, and BP after exposure to the COVID-19 mRNA vaccines in these two studies could be attributed to the fact that the studies implemented multiple analyses and designs and they have a large sample size which could increase the probability of detecting statistically significant but not necessarily clinically significant results.
The results from both primary series and booster studies contribute to the safety profile of COVID-19 mRNA vaccines [26], [31], [32], [33], [34], [35], [36], [37] and they are largely consistent with the results of other studies. Using an SCCS study design, Jabagi, Botton, and Bertrand (2022) did not observe an increased risk of AMI, PE, and stroke within 14 days after BNT162b2 vaccine doses among French vaccine recipients aged 75 years or older [33]. Whiteley et al. (2022) found lower risk of arterial and venous thrombotic events after BNT162b2 COVID-19 vaccination in those aged 70 years and older [37]. Two UK and Scotland studies using the SCCS study design also found no evidence for elevated risk of thrombocytopenia, venous thromboembolism, myocardial infarction, or hemorrhagic events following BNT162b2 vaccine administration in the general population [26], [35]. A Danish study on frontline workers found no association of thrombosis and thrombotic events with the BNT162b2 vaccine [32]. Welsh et al. (2021) assessed thrombocytopenia cases (including ITP) reported to the Vaccine Adverse Event Reporting System (VAERS) and found that the risk of ITP following mRNA vaccines did not exceed expected historical rates [36]. Berild et al. (2022) found increased rates of several thromboembolic and thrombocytopenia outcomes following mRNA vaccines, however this result was not robust to sensitivity analyses and was smaller compared to post vaccination risk following ChAdOx1 vaccines [38]. Similarly, a number of case reports and studies have indicated a small but elevated risk of Myo/Peri in young males 16–24 years following COVID-19 mRNA vaccination; however, no strong evidence has indicated an elevated risk among individuals aged 65 years and older [39], [40].
The evidence of PE risk following COVID-19 mRNA vaccination in our studies is mixed, and this observation is similarly reflected in the current literature. Despite case reports documenting the occurrence of PE cases following COVID-19 vaccination with a proposed etiology of inflammatory response in susceptible patients [41], [42], multiple studies suggest a lack of evidence for a strong association between elevated risk of venous thromboembolism events as well as PE, specifically following the BNT162b2 vaccine [26], [32], [33], [35], [37], [43]. This elevated risk has been more commonly associated with the ChAdOx1 nCoV-19 vaccine [37]. While Burn et al. (2022) detected a slightly elevated PE risk following both vaccines, the risk was substantially higher following COVID-19 infection [43]. Several studies have similarly shown an increased PE risk following COVID-19 infection [27], [31]. Our investigation into the seasonality of PE during the pandemic suggests a strong correlation between spikes in COVID-19 infection and PE occurrence. When excluding PE cases with evidence of prior medically attended COVID-19 infection, the increased PE risk following mRNA vaccination attenuated but remained elevated. This however conflicts with the protective PE effect observed following booster doses for both mRNA vaccines which suggests that the exclusion of cases with evidence of prior COVID-19 infection might not have completely accounted for this effect. Although the predominant circulating COVID-19 variants may impact PE risk following infection, Law et al. (2022) compared the incidence of PE in COVID-19 positive patients between various variant waves of COVID-19 (i.e., original, Delta, and Omicron periods) and concluded that the risk of PE after COVID-19 infection with the respective variants was not statistically different [44]. The risk of AEs following COVID-19 infection with various circulating strains of SARS-CoV-2 requires further investigation and does not fall within the scope of the two independent SCCS studies. Furthermore, the booster study was conducted later in the pandemic (2022) during which a rise in authorization and use of at-home Over the Counter (OTC) COVID-19 diagnostic tests was observed [45], [46]. Hence, increased use of these alternative COVID-19 detection methods may have led to a lower proportion of prior COVID-19 infections being detected during the booster study period. Taken together, these observational studies do not provide conclusive evidence for increased PE risk following COVID-19 mRNA vaccination.
The claims-based definition used in the booster study for BP had a low PPV, pointing to a high level of outcome misclassification. Hence, the statistically non-significant results from the PPV-adjusted analyses for BP are consistent with the lack of evidence in the literature about elevated BP risk following COVID-19 mRNA vaccines booster doses. Wan et al. (2022) did not find a statistically significant increase in BP risk associated with BNT162b2 vaccination in a study conducted in China [47]. While several case reports have also cited BP cases following COVID-19 mRNA vaccines, Renoud et al. (2021) suggest that the reporting rate of BP after COVID-19 mRNA vaccination is comparable to other viral vaccines from a disproportionality analysis conducted in the World Health Organization (WHO) pharmacovigilance database [48]. Shemer (2021) did not identify a statistically significant association between BP risk and COVID-19 mRNA vaccination [49]. Tamaki (2021) suggests that the risk of BP is seven times more likely after SARS-CoV-2 infection than after COVID-19 vaccination [50]. The risk of AMI, PE and BP secondary to SARS-CoV-2 infections were reported in several studies to be substantially higher than post-vaccination risk estimates [50], [51]. Challenges in confirming prior COVID-19 infection in vaccinees, especially near the time of vaccination, may complicate our ability to obtain accurate estimate of some of these outcomes.
These two observational studies have several strengths. The SCCS study design inherently adjusts for potential time-invariant confounders which may draw from between-individual comparisons. The large size of the CMS Medicare population provides more power for the study to evaluate rare AEs with more precision. The Medicare database is a large, population-based database containing information on beneficiaries’ demographics and longitudinal information on health care services utilization across care settings, thus more comprehensively capturing people’s baseline health conditions and across time. Further, since a large proportion of the U.S. elderly population is enrolled in Medicare and beneficiary attrition is minimal once eligible, our findings are highly generalizable to the U.S. population aged 65 years and older. While implementation of multiple study designs and analytic methods in the observational studies examines the robustness of the risk estimates, it can also increase the likelihood of detecting statistically significant results due to chance alone.
Potential vaccine exposure misclassification cannot be ruled out in the studies given the current evidence with respect to under-reporting of COVID-19 vaccine administration in medical claims data sources [52]. However, the impact of exposure misclassification on the risk estimates may not be large since the study population in both studies was comprised of only vaccinated individuals. Outcome misclassification cannot be ruled out either especially in regards to the use of ‘rule-out diagnosis’ in administrative claims. Also, MRR results were not available at the time of the two studies to confirm the outcome status for all AEs in these studies; and some outcomes for which MRR was conducted had low PPV. In particular, ITP in all care settings had a very low PPV at 4 %, indicating low reliability of the unadjusted analysis, which is additionally reflected in the uncertainty in the PPV-adjusted analysis. Misspecification of risk and control intervals could also bias the estimates in either direction. Finally, since residual and unmeasured confounding in observational studies cannot be fully ruled out, the results carry a certain level of uncertainty.
In these two studies of the U.S. elderly we did not find an increased risk for AMI, ITP, DIC, BP, and Myo/Peri; and the results did not show consistent evidence of an elevated risk for PE after exposure to COVID-19 mRNA vaccines. These results support the safety profile of COVID-19 mRNA vaccines administered to the U.S. elderly and are consistent with the conclusion that the benefits of COVID-19 vaccination outweigh the risks of disease.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Funding was provided by the US Food and Drug Administration.
We would like to thank Purva Shah, Amei Hao, Yeerae Kim, and Mahasweta Mitra for providing statistical programming and writing support. We would like to thank Kristin Sepúlveda for program management.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.06.014.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- 1.Centers for Disease Control and Prevention. Trends in Number of COVID-19 Cases and Deaths in the US Reported to CDC, by State/Territory; 2022; https://covid.cdc.gov/covid-data-tracker/#trends_weeklydeaths_select_00. Accessed 1/9/2023.
- 2.Centers for Disease Control and Prevention. New admissions of patients with confirmed COVID-19; 2023; https://covid.cdc.gov/covid-data-tracker/#new-hospital-admissions. Accessed 1/9/2023, 2023.
- 3.Centers for Disease Control and Prevention. Demographic trends of COVID-19 cases and deaths in the US reported to CDC; 2022; https://covid.cdc.gov/covid-data-tracker/#demographics. Accessed 1/9/2023.
- 4.Centers for Disease Control and Prevention. Overview of COVID-19 Vaccines; 2022; https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/overview-COVID-19-vaccines.html. Accessed November 30, 2022.
- 5.U.S. Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccines; 2022; https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccines. Accessed 1/9/2023.
- 6.U.S. Food and Drug Administration. Moderna COVID-19 Vaccines; 2022; https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccines. Accessed 1/9/2023.
- 7.U.S Food and Drug Administration. Novavax COVID-19 Vaccine, Adjuvanted; 2022; https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/novavax-covid-19-vaccine-adjuvanted. Accessed 1/9/2023.
- 8.U.S Food and Drug Administration. Coronavirus (COVID-19) update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 vaccines for use as a booster dose; 2022; https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use. Accessed 1/9/2023.
- 9.U.S. Food and Drug Administration. Initial results of near real-time safety monitoring of COVID-19 vaccines in persons aged 65 years and older; 2022; https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/initial-results-near-real-time-safety-monitoring-covid-19-vaccines-persons-aged-65-years-and-older. Accessed 11/30/2022, 2022.
- 10.Petersen I., Douglas I., Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354 doi: 10.1136/bmj.i4515. [DOI] [PubMed] [Google Scholar]
- 11.Wong Hui-Lee ZKC, Ellen Tworkoski, Mao Hu, Deborah Thompson, Patricia Lloyd, Rositsa Dimova, et al. Assessment of acute myocardial infarction, pulmonary embolism, disseminated intravascular coagulation and immune thrombocytopenia following COVID-19 vaccination; July 30, 2021; https://bestinitiative.org/wp-content/uploads/2021/09/C-19-Vaccine-Safety-AMI-PE-DIC-ITP-Protocol-2021.pdf, 2022.
- 12.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) Glob Heart. 2018;13(4):305–338. doi: 10.1016/j.gheart.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Fuller C.C., Nambudiri V.E., Spencer-Smith C., et al. Medical chart validation of inpatient diagnosis codes for transfusion-related acute lung injury 2013–2015. Transfusion. 2021;61(3):754–766. doi: 10.1111/trf.16251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wise R.P., Bonhoeffer J., Beeler J., et al. Thrombocytopenia: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5717–5724. doi: 10.1016/j.vaccine.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 15.Taylor F.B., Jr., Toh C.H., Hoots W.K., Wada H., Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 16.Toh C.H., Hoots W.K. The scoring system of the scientific and standardisation committee on disseminated intravascular coagulation of the international society on thrombosis and haemostasis: a 5-year overview. J Thrombosis Haemostasis: JTH. 2007;5(3):604–606. doi: 10.1111/j.1538-7836.2007.02313.x. [DOI] [PubMed] [Google Scholar]
- 17.Rath B., Gidudu J.F., Anyoti H., et al. Facial nerve palsy including Bell's palsy: Case definitions and guidelines for collection, analysis, and presentation of immunisation safety data. Vaccine. 2017;35(15):1972–1983. doi: 10.1016/j.vaccine.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Sexson Tejtel S.K., Munoz F.M., Al-Ammouri I., et al. Myocarditis and pericarditis: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499–1511. doi: 10.1016/j.vaccine.2021.11.074. [DOI] [PubMed] [Google Scholar]
- 19.Wilson E.B. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22(158):209–212. [Google Scholar]
- 20.Rubin D.B., Schenker N. Multiple imputation for interval estimation from simple random samples with ignorable nonresponse. J Am Stat Assoc. 1986;81(394):366–374. [Google Scholar]
- 21.Cox C., Li X. Model-based estimation of the attributable risk: a loglinear approach. Comput Stat Data Anal. 2012;56(12):4180–4189. doi: 10.1016/j.csda.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrington C.P., Whitaker H.J., Hocine M.N. Case series analysis for censored, perturbed, or curtailed post-event exposures. Biostatistics (Oxford, England) 2009;10(1):3–16. doi: 10.1093/biostatistics/kxn013. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharjee S., Banerjee M. Immune thrombocytopenia secondary to COVID-19: a systematic review. SN Comprehensive Clin Med. 2020;2(11):2048–2058. doi: 10.1007/s42399-020-00521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clerkin K.J., Fried J.A., Raikhelkar J., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 25.Deutch MR, Holmberg MJ, Gissel T, Hollingdal M. Pulmonary embolism after discharge for COVID-19: A report of two cases. JRSM Cardiovasc Dis 2021; 10: 20480040211034998. [DOI] [PMC free article] [PubMed]
- 26.Hippisley-Cox J., Patone M., Mei X.W., et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374 doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho F.K., Man K.K.C., Toshner M., et al. Thromboembolic risk in hospitalized and nonhospitalized COVID-19 patients: a self-controlled case series analysis of a nationwide cohort. Mayo Clin Proc. 2021;96(10):2587–2597. doi: 10.1016/j.mayocp.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsoularis I., Fonseca-Rodríguez O., Farrington P., Lindmark K., Fors Connolly A.-M. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398(10300):599–607. doi: 10.1016/S0140-6736(21)00896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modin D., Claggett B., Sindet-Pedersen C., et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142(21):2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 31.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. 2021; 385(12): 1078–1090. [DOI] [PMC free article] [PubMed]
- 32.Hviid A., Hansen J.V., Thiesson E.M., Wohlfahrt J. Association of AZD1222 and BNT162b2 COVID-19 vaccination with thromboembolic and thrombocytopenic events in frontline personnel: a retrospective cohort study. Ann Int Med. 2022;175(4):541–546. doi: 10.7326/M21-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jabagi M.J., Botton J., Bertrand M., et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA. 2022;327(1):80–82. doi: 10.1001/jama.2021.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein N.P., Lewis N., Goddard K., et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326(14):1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson C.R., Shi T., Vasileiou E., et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welsh K.J., Baumblatt J., Chege W., Goud R., Nair N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS) Vaccine. 2021;39(25):3329–3332. doi: 10.1016/j.vaccine.2021.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whiteley W.N., Ip S., Cooper J.A., et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLoS Med. 2022;19(2) doi: 10.1371/journal.pmed.1003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dag Berild J., Bergstad Larsen V., Myrup Thiesson E., et al. Analysis of thromboembolic and thrombocytopenic events after the AZD1222, BNT162b2, and MRNA-1273 COVID-19 vaccines in 3 Nordic countries. JAMA Netw Open. 2022;5(6) doi: 10.1001/jamanetworkopen.2022.17375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oster M.E., Shay D.K., Su J.R., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US From December 2020 to August 2021. JAMA. 2022;327(4):331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Clinical considerations: myocarditis and pericarditis after receipt of mRNA COVID-19 vaccines among adolescents and young adults; 2022; https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html. Accessed 1/9/2023.
- 41.Curcio R, Gandolfo V, Alcidi R, et al. Vaccine-induced massive pulmonary embolism and thrombocytopenia following a single dose of Janssen Ad26.COV2.S vaccination. Int J Infect Dis: IJID: Off Publ Int Soc Infect Dis 2022; 116: 154–156. [DOI] [PMC free article] [PubMed]
- 42.Malik B., Kalantary A., Rikabi K., Kunadi A. Pulmonary embolism, transient ischaemic attack and thrombocytopenia after the Johnson & Johnson COVID-19 vaccine. BMJ Case Reports CP. 2021;14(7) doi: 10.1136/bcr-2021-243975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burn E, Li X, Delmestri A, et al. Thrombosis and thrombocytopenia after vaccination against and infection with SARS-CoV-2: a population-based cohort analysis. medRxiv. 2021: 2021.2007.2029.21261348.
- 44.Law N., Chan J., Kelly C., Auffermann W.F., Dunn D.P. Incidence of pulmonary embolism in COVID-19 infection in the ED: ancestral, Delta, Omicron variants and vaccines. Emerg Radiol. 2022;29(4):625–629. doi: 10.1007/s10140-022-02039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. Food and Drug Administration. At-Home OTC COVID-19 Diagnostic Tests. General Information About At-Home OTC COVID-19 Diagnostic Tests. Available at: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/home-otc-covid-19-diagnostic-tests.
- 46.U.S. Food and Drug Administration. In Vitro Diagnostic EUAs - Antigen Diagnostic Tests for SARS-CoV-2. Individual EUAs for Antigen Diagnostic Tests for SARS-CoV-2. Available at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2#IndividualEUAs.
- 47.Wan E.Y.F., Chui C.S.L., Lai F.T.T., et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1):64–72. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renoud L., Khouri C., Revol B., et al. Association of facial paralysis with mRNA COVID-19 vaccines: a disproportionality analysis using the world health organization pharmacovigilance database. JAMA Intern Med. 2021;181(9):1243–1245. doi: 10.1001/jamainternmed.2021.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shemer A., Pras E., Einan-Lifshitz A., Dubinsky-Pertzov B., Hecht I. Association of COVID-19 vaccination and facial nerve palsy: A case-control study. JAMA Otolaryngol-Head Neck Surg. 2021;147(8):739–743. doi: 10.1001/jamaoto.2021.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamaki A., Cabrera C.I., Li S., et al. Incidence of bell palsy in patients with COVID-19. JAMA Otolaryngol-Head Neck Surg. 2021;147(8):767–768. doi: 10.1001/jamaoto.2021.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lassen M.H., Modin D., Skaarup K.G., et al. Risk of acute myocardial infarction, stroke and thromboembolism following COVID-19 vaccination compared to testing positive for COVID-19 infection: a nationwide cohort study of 4.6 mio individuals. Eur Heart J. 2022;43 [Google Scholar]
- 52.Centers for Medicare & Medicaid Services. Assessing the completeness of medicare claims data for measuring COVID-19 vaccine administration; 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.