Abstract
Objectives:
Exogenous estrogen is associated with growth of hepatocellular adenomas (HCAs), although the influence of progestin-only agents is unknown. We therefore evaluated the association of progestin-only agents on HCA progression compared to no hormone exposure and compared to estrogen exposure in female patients.
Study design:
In this single-center, retrospective cohort study of reproductive-aged female patients (ages 16–45) with diagnosed HCAs between 2003 and 2021, we evaluated radiographic HCA growth during discrete periods of well-defined exogenous hormone exposures.
Results:
A total of 34 patients were included. Nineteen (55.9%) had follow-up scans during periods without hormone exposure, 7 (20.6%) during estrogen exposure, and 8 (23.5%) during progestin-only exposure. Over a median follow-up of 11 months, percent change in sum of adenoma diameters from baseline to last available scan was −15.0% with progestin-only agents versus 29.4% with estrogen exposure (p = 0.04), and −7.4% with no hormonal exposure (p = 0.52 compared to progestin-only). Greater than 10% growth was observed in two individuals (25.0%) with progestin-only agent use (one patient on high-dose progestin for menorrhagia) versus five individuals (71.4%) with estrogen use (p = 0.13), and 7 (36.8%) with no exogenous hormone use (p = 0.68 vs progestin-only).
Conclusions:
During discrete periods of progestin-only use, HCA growth overall declined, similar to declining growth during periods without exogenous hormonal exposure. This differed from discrete periods of exogenous estrogen exposure, during which time HCAs demonstrated overall increased growth. Though larger studies are needed, these findings support recent guidance supporting progestin-only agents for female patients with HCAs seeking non-estrogen alternatives for contraception.
Implications:
In this small retrospective study, we observed overall decrease in HCA size during discrete periods of progestin-only contraception use, similar to that observed during periods without exogenous hormone exposure, supporting their use as a safe alternative to estrogen-containing contraceptives in this patient population.
Keywords: Contraceptive agents, Estrogens, Hepatocellular adenoma, Progestins, Women’s health
1. Introduction
Hepatocellular adenomas (HCAs) are benign liver lesions formed by proliferation of hepatocytes [1]. Less commonly they may result in malignant transformation and/or rupture. HCAs are diagnosed by computed tomography and/or magnetic resonance imaging and typically demonstrate early arterial phase enhancement with washout in later phases as well as intralesional fat or heterogeneity due to presence of blood products [2,3]. Exogenous estrogen exposure, particularly in the form of ethinylestradiol from use of oral combined hormonal contraceptive (CHC) pills, is an established risk factor for growth and rupture, with an annual incidence of approximately 3 to 4 for every 100,000 women using these agents [4–7]. Obesity is another risk factor for the development of HCAs, particularly as visceral fat is a source of estrone, with weight loss shown to result in HCA regression [8].
The influence of endogenous and exogenous estrogen on HCA development and growth are well-established clinically, with a higher prevalence in women using CHCs as compared to age-matched controls [9], and increase in HCA growth during pregnancy [10]. Cessation of CHCs also results in HCA regression [11]. The CDC Medical Eligibility Criteria for Contraceptive Use therefore recommends against the use of combined hormonal contraceptives in individuals with HCAs. As progesterone receptors have been identified in HCAs, the MEC guidance states that the risk of progestin-only contraception may outweigh the benefits [12, 13], although data on HCA growth following progestin-only exposure are lacking.
2. Methods
2.1. Study design/patient population
This is a single-center, retrospective cohort study of reproductive-aged females (ages 16–45) with imaging- and/or biopsy-confirmed HCA seen at our tertiary care center between 2003 to 2021. We identified patients with HCAs based on review of diagnosis terms and ICD coding in the UCSF radiology and pathology databases. Institutional Review Board approval (IRB 15-16868) was obtained. Informed consent was not deemed necessary given absence of patient identifiers or contact.
We collected patient age, sex assigned at birth, race, ethnicity, past medical history (specifically presence of diabetes mellitus, hyperlipidemia, obesity, and polycystic ovary syndrome), and history of any exogenous sex hormone use (type, duration, and dose) from electronic medical records. Through chart review we also obtained the type, date, and number of imaging studies, biopsies, and therapeutic interventions (defined as ablation, embolization, or surgical resection of HCAs) for each patient. We ascertained histologic characteristics from pathology reports.
Inclusion criteria were presence of two or more cross-sectional imaging reports prior to any procedural intervention (ablation, embolization, or surgical resection), with formal imaging review at our center. Only patients with clear documentation in the electronic medical record of presence or absence of hormonal exposure, type, and duration that during the time periods when two or more available imaging studies were performed for monitoring of HCA growth were included (Fig 1). Imaging characteristics were compared among patients during discrete periods of (1) no exogenous hormonal exposure, (2) exogenous estrogen exposure (estrogen plus progestin or estrogen only), and (3) progestin-only exposure. Individuals with discrete periods of hormonal exposure or absence of hormonal exposure at time of scans were analyzed, with some patients contributing data to multiple groups if they had multiple discrete periods within these three categories. Among the 27 patients meeting inclusion criteria, seven contributed data to multiple groups, for a total of 34 intervals of analyzed hormonal exposures. These seven patients included the following: one patient contributing to all three groups, two patients with estrogen exposure followed by no exposure, 1 patient with no exposure then progestin-only exposure, two separate discrete periods of progestin-only exposure, with interval no hormone exposure.
Fig. 1.
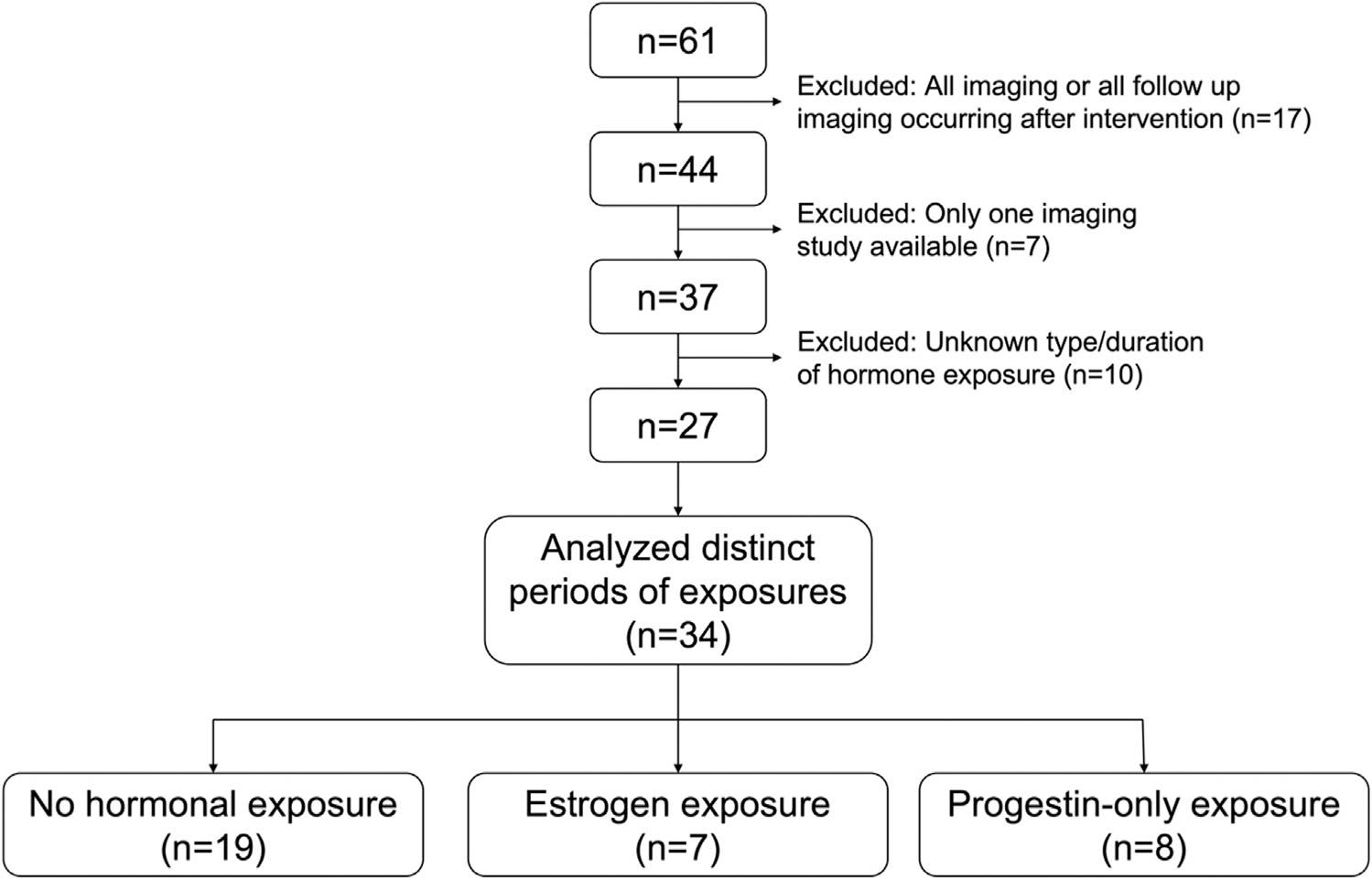
Patient selection and cohort population. Sixty-one female patients were identified with hepatocellular adenoma. Exclusions were procedural intervention prior to follow-up scan (n = 17), absence of two or more scans (n = 7), or unknown type or duration of hormonal exposure (n = 10). Of the 27 patients meeting study criteria, distinct periods of hormone exposure were analyzed (some individuals had multiple discrete periods of hormone exposure were counted in each respective group). The total number of periods of with discrete nonoverlapping periods of hormone exposure was 34 (19 intervals without hormone exposure, seven periods of estrogen exposure, and eight periods of progestin-only exposure).
2.2. Imaging analysis
HCA growth patterns were determined by cross sectional imaging using either contrast enhanced computed tomography or magnetic resonance imaging according to established radiologic criteria [14, 15]. Any imaging report noting equivocal findings were reviewed by a study investigator (R.P.L.) with expertise in HCA imaging. Imaging characteristics included size of largest HCA on baseline and follow-up imaging, presence of adenomatosis (10 or more lesions or “adenomatosis” noted in imaging report) [16], and HCA rupture. Baseline imaging was defined as first available cross-sectional imaging formally reviewed by radiologists at our center during discrete period of hormone exposure or absence of hormone exposure. Follow-up imaging refers to any imaging study after the baseline imaging during the same discrete period of exposure. Median time to follow up was calculated as time between the baseline imaging and the last follow up scan within the period of hormone exposure. To trend HCA growth, the sum of HCA diameters from first to last available follow-up scan was calculated, and median percent change compared by hormonal exposure.
2.3. Hepatocellular adenoma histology
We reviewed pathology reports from core biopsy, resection, and/or explant tissue. Gross and microscopic features were examined for HCA subtype and features of malignant transformation based on standardized histologic criteria [14, 15]. Absence of map-like pattern with glutamine synthase staining helped to exclude focal nodular hyperplasia. Positive C-reactive protein and/or serum amyloid A staining indicated inflammatory variant of HCA. Nuclear beta catenin staining, as opposed to membranous, and diffuse glutamine synthase staining indicated beta catenin-activated HCA. Loss of liver fatty acid binding protein staining in lesion tissue supported hepatocyte nuclear factor-1 alpha-inactivated HCA. Features of hepatocellular carcinoma were evaluated by reticulin, cluster of differentiation 34, and glypican-3 staining.
2.4. Statistical analysis
Descriptive statistics were reported by Wilcoxon test for continuous variables and Fisher’s exact test for dichotomous variables. Variables related to HCA growth or hormonal exposure were reported, including number of patients with missing data. Median and interquartile range were reported to handle non-normally distributed data. A significance level of 0.05 was considered for all analyses.
3. Results
3.1. Patient characteristics
There were 27 total patients with 34 discrete periods of hormonal exposure. Median patient age was 34 years; 15% were white, 20.6% were black, 8.8% Asian, and 17.6% Hispanic (Fig. 1, Table 1). Nineteen patients (55.9%) had discrete periods of no hormone exposure, seven (20.6%) had discrete periods of estrogen exposure, and eight (23.5%) had discrete periods of progestin-only exposure at time of available imaging. Estrogen-containing agents included combined oral contraceptives (n = 6) and the transdermal estradiol patch for menopausal symptoms after oophorectomy (n = 1). Progestin-only agents included hormonal intrauterine device (n = 3) for contraception, medroxyprogesterone injection (n = 1) for contraception, 5 milligram dose of oral medroxyprogesterone (n = 1) for amenorrhea, etonogestrel implant (n = 1) for contraception, and between 5 and 15 mg doses of norethindrone (n = 2) for treatment of menorrhagia.
Table 1.
Cohort demographic characteristics and prevalence of medical comorbidities by hormonal exposure in female patients with hepatocellular adenomas from 2003 to 2021 (n = 34)
| Characteristic | All patients (n = 34) | No hormone exposure (n = 19) | Estrogen exposure (n = 7) | Progestin-only exposure (n = 8) | p-valuea |
|---|---|---|---|---|---|
|
| |||||
| Age, median years (IQR) | 33.5 (26–36.8) | 35 (32.5–40) | 25 (23–26.5) | 32.5 (23–34.5) | < 0.01 |
| Race, n (%) | |||||
| White | 17 (50%) | 11 (58%) | 3 (43%) | 3 (38%) | 0.65 |
| Black | 7 (21%) | 4 (21%) | 2 (29%) | 1 (13%) | 0.86 |
| Asian | 3 (9%) | 2 (11%) | 0 (0%) | 1 (13%) | 1.00 |
| Other | 7 (21%) | 2 (11%) | 2 (29%) | 3 (38%) | 0.20 |
| Hispanic ethnicity, n (%) | 6(l8%) | 2 (11%) | 2 (29%) | 2 (25%) | 0.50 |
| Diabetes Mellitus, n (%) | 8 (24%) | 2 (11%) | 3 (43%) | 3 (38%) | 0.10 |
| Dyslipidemia, n (%) | 9 (27%) | 5 (26%) | 1 (14%) | 3 (38%) | 0.77 |
| PCOS, n (%) | 9 (27%) | 3 (16%) | 2 (29%) | 4 (50%) | 0.22 |
| BMI, median kg/m2 (IQR) | 28.7 (25.9 – 37.4) b | 28.0 (23.4 – 33.7) b | 31.7 (27.5 – 42.3) | 30.4 (26.6 – 37.0) | 0.27 |
| BMI > 30 kg/m 2, n (%) | 14 (41%) b | 6 (32%) b | 4 (57%) | 4 (50%) | 0.46 |
Statistical analysis comparing “No hormone exposure,” “Estrogen exposure,” and “Progestin-only exposure.”
One patient in no hormone exposure group did not have BMI available.Abbreviations: IQR, Interquartile Range; PCOS, polycystic ovary syndrome; BMI,body mass index. p-values for n (%) based on Fisher’s exact test; p-values for median (IQR) based on Wilcoxon tests.
Median age was older (35 years) in patients without hormone exposure, as compared to those with progestin-only (33 years) and estrogen exposures (25 years, p < 0.01) (Table 1). There was no statistically significant difference in prevalence of diabetes mellitus, dyslipidemia, and polycystic ovary syndrome (PCOS) by hormone exposure.
3.2. Hepatocellular adenoma characteristics
There was no statistically significant difference in median largest HCA and sum of HCA diameters at baseline imaging during periods of progestin-only exposure compared to no hormone exposure or estrogen exposure (Table 2). Median follow-up, defined as time between baseline scan and last follow-up scan, was 11, 9, and 15 months in the no hormone, estrogen, and progestin-only groups, with median number of available cross-sectional imaging of 4, 3, and 3.5, respectively. Median percent change in sum diameters between baseline and last follow-up scan was −7.4% during no hormone exposure, 29.4% with estrogen exposure, and − 15.0% with progestin-only exposure. There was a statistically significant lower percent change in sum of adenoma diameters with progestin-only compared with estrogen exposure (p = 0.04), but no statistically significant difference between periods of progestin-only and no hormone exposures (p = 0.52). Greater than 10% growth was seen in two patients (25.0%) in the progestin-only group, compared with seven (36.8%) patients during no hormone exposure (p = 0.68) and five (71.4%) in estrogen exposed group (p = 0.13 vs progestin-only exposure group). Of the two patients with HCA growth during progestin-only exposure, one included high dose progestin use to treat heavy menstrual bleeding (up to 15 mg of norethindrone). The second patient was using a hormonal intrauterine device, though also had a diagnosis of morbid obesity with a BMI of 57 kg/m2. Within person trends in HCA growth are demonstrated graphically in Figure 2 A–C. No patients in this study developed malignant transformation.
Table 2.
Hepatocellular Adenoma Histology, Size, and Growth by Hormonal Exposure in Females with HCAs from 2003–2021 (n = 34)
| Characteristic | No hormone exposure (n = 19) | Estrogen exposure (n = 7) | Progestin-only exposure (n = 8) | Progestin-only vs no hormone exposure p-value | Progestin-only vs estrogen exposure p-value |
|---|---|---|---|---|---|
|
| |||||
| Type of HCA, n (%) | |||||
| Beta catenin-activated | 1 (5%) | 0 (0%) | 0 (0%) | 1.00 | |
| Inflammatory | 5 (26%) | 3 (43%) | 1 (13%) | 0.63 | 0.28 |
| HNF-1 alpha inactivated | 3 (l6%) | 0 (0%) | 2 (25%) | 0.62 | 0.47 |
| Unclassified | 3 (l6%) | 1 (14%) | 2 (25%) | 0.62 | 1.00 |
| Unspecified | 2 (l1%) | 0 (0%) | 0 (0%) | 1.00 | - |
| Adenomatosis (≥ 10 HCAs), n (%) | 1 (5%) | 0 (0%) | 2 (25%) | 0.20 | 0.47 |
| Largest baseline adenoma, median cm (IQR) | 4.0 (2.2 – 4.9) | 3.3 (1.7 – 3.8) | 3.3 (2.8 – 3.7) | 0.49 | 0.98 |
| Number of imaging reports, median number (IQR) | 4 (2 – 6.5) | 3 (2 – 3.5) | 3.5 (3 – 7.25) | 0.70 | 0.28 |
| Duration of follow-up, median years (IQR) | 0.9 (0.5 – 4.0) | 0.8 (0.4 – 2.8) | 1.3 (0.9 – 2.4) | 0.70 | 0.28 |
| Sum of HCAs at baseline, median cm (IQR) | 4.0 (2.5 – 7.4) | 3.3 (1.7 – 5.9) | 4.4 (3.3 – 6.8) | 0.78 | 0.63 |
| Sum of HCAs at last scan, median cm (IQR) | 4.5 (2.3 – 7.0) | 7.0 (3.9 – 7.2) | 5.2 (2.5 – 6.2) | 0.95 | 0.56 |
| Change in sum of HCA diameters, median % change (IQR) | −7.4 (-17.6 – 27.9) | 29.4 (17.4 – 140.4) | −15.0 (-24.4 – 5.9) | 0.52 | 0.04 |
| > 10% increase in sum of HCA diameters, n (%) | 7 (37%) | 5 (71%) | 2 (25%) | 0.68 | 0.13 |
HCA, hepatocellular adenoma; HNF, hepatocyte nuclear factor. IQR, interquartile range. p-values for n (%) based on Fisher’s exact test; p-values for median (IQR) based on exact Wilcoxon tests.
Fig. 2.
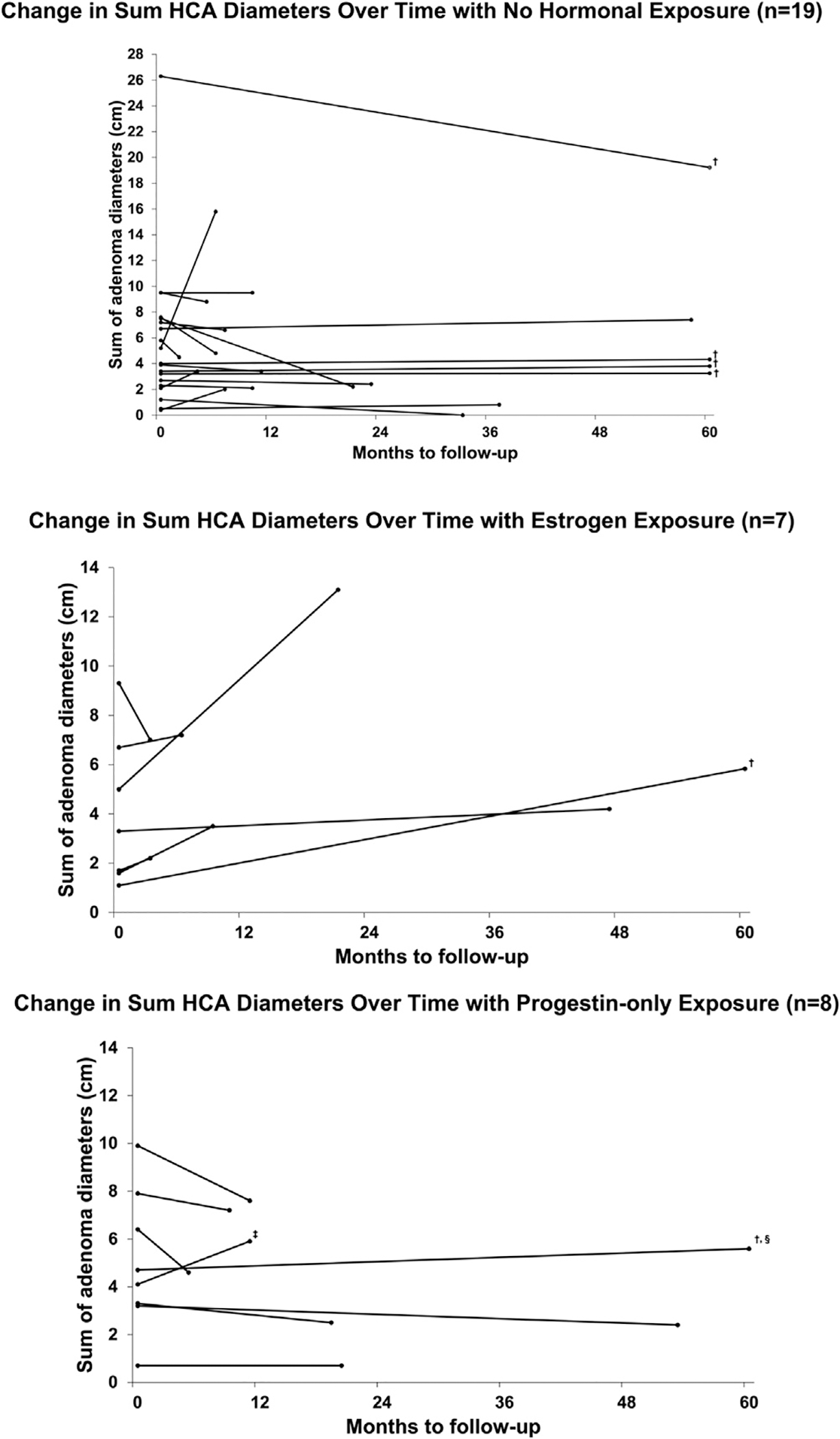
Change in sum of adenoma diameters from baseline to follow-up by hormonal exposure. Lines represent individual patients. Baseline imaging was designated as first scan available, and final imaging designated as last scan during period of exposure. (A) No hormone exposure (n = 19). (B) Estrogen exposure (n = 7). (C) Progestin-only exposure (n = 8). † Truncated at 5 years from first scan. ‡ Up to 15 milligrams oral norethindrone for menorrhagia. § hormonal intrauterine device.
4. Discussion
Endogenous and exogenous estrogen exposure, including use of combined hormonal contraception, are established risk factors for HCA development and growth, although data on progestin-only exposure on HCA growth are limited [4,5,8]. In this study we report HCA growth patterns in female patients with well-defined radiographic features of HCAs and detailed corresponding periods of exogenous hormone duration and dosing. We found that HCA growth was highest and overall increased during periods of estrogen exposure, while HCA growth overall declined during periods of progestin-only exposure with similar growth patterns to those observed during periods without exogenous hormone use. Fewer patients had adenoma growth over 10% during periods of progestin-only use as compared to periods with estrogen exposure or no exogenous hormone use.
Estrogen receptors are present within HCAs and both exogenous and endogenous estrogen are well established risk factors for the development and progression of these lesions. Hormonal contraceptives containing ethinylestradiol increase HCA growth, with a higher prevalence of HCAs in women using CHCs as compared to age-matched controls. HCA regression also occurs upon cessation of ethinyl estradiol use [9, 11]. Endogenous hormonal influences are also evident, with HCA growth observed during pregnancy, and regression of lesions following delivery [10, 17]. These prior in adenoma growth during periods of exogenous estrogen exposure observations are consistent with our study noting overall increase.
Progesterone receptors are also evident within HCAs, raising the possibility that progestin exposure could also promote HCA growth [12]. A case report of a patient using at least 10 mg of daily norethindrone acetate (NET-A) for treatment of endometriosis showed increase in HCA growth following exposure [18]. Interestingly, in a clinical trial published in 2007, NET-A administered in doses ranging from 10 to 40 mg resulted in conversion to a small amount of ethinyl estradiol (E2) [19]. Such findings underlie the current CDC MEC guidelines which designate progestin-only agents as a category 3 for patients with HCAs, meaning that risks may outweigh the benefits [13].
However, these higher doses of NET-A, such as that used in the case report of endometriosis, are higher than contemporary progestin-only contraceptive preparations. Thus, the Reproductive Health in Liver Disease Guidance on behalf of the American Association for the Study of Liver Diseases supports the use of progestin-only contraception as an alternative to estrogen-containing agents for female patients with HCAs [20]. The key finding of the current study was that HCA growth was no greater during periods of progestin-only exposure as compared to periods without exogenous hormone use and of the two patients with any HCA growth, one was exposed to a high-dose progestin preparation, using up to 15 mg norethindrone for noncontraceptive purposes. Our findings align with a recent publication aimed at creating an HCA risk score in relation to exogenous estrogen exposure and obesity. While this study was not designed to evaluate progestin-only exposures, 17 patients did use progestin-only contraception following their diagnosis of HCAs, including two with subcutaneous implants, seven with intrauterine devices and 8 with progestin-only pills. Of these only 1 of 17 had HCA growth [8].
Although the current study was not powered to adjust for potential confounders, we did note that obesity was numerically lower in the unexposed group at 32% as compared to the estrogen (57%) and progestin (50%) exposed groups. As reported in prior studies, including recently published data, obesity does promote HCA growth with weight loss resulting in HCA regression. The effect of obesity on HCA appears to be due to increased aromatase activity, with consequent increase in circulating estrone levels. Importantly, growth patterns in the current study were not different between the progestin-only and unexposed groups, despite the higher percentage of obesity among our patients exposed to progestins. Obesity may have contributed to the one patient who had HCA growth with the hormonal IUD, who met criteria for morbid obesity with a BMI of 56.8 kg/m2. Larger studies evaluating hormonal exposures would benefit from adjustment for obesity measures.
Another potentially relevant confounder is polycystic ovary syndrome (PCOS), a common endocrinopathy affecting 10 % to 15% of reproductive-aged female patients which is marked by several clinical features that could promote HCA growth. PCOS patients have higher endogenous levels of both circulating androgens and estrogens, higher prevalence of obesity, as well as common use of combined oral contraception to regulate menses. The prevalence of PCOS in our study was quite high at 26%, suggesting that this condition may confer increased risk for HCA, although the relationship of PCOS with HCAs remains limited to case reports [21, 22]. Dedicated cohort studies evaluating the risk of HCA development and growth in PCOS are warranted, including adjustment for combined hormonal contraceptive use and BMI.
We do acknowledge the small number of female patients with progestin-only exposure in our study, though we were attentive to the need for inclusion of only those with detailed characterization of hormonal doses and durations which is often poorly recorded in medication lists. Larger studies including a breadth of progestin-only contraceptives are needed, including formulations with relatively higher doses than others, such as the medroxyprogesterone acetate injection. A strength of our study was the comprehensive radiographic review which allowed us to provide detailed characterization of adenoma growth patterns during these discrete periods of hormonal exposures. The reasons for patients continuing exogenous estrogen exposure is not known although referring providers may await subspecialist recommendations prior to changing potentially culprit medications. There were fewer serial images during exogenous estrogen exposure (up to 3.5 vs 6–7 for the unexposed and progestin-only groups), likely due to cessation of these exogenous agents following confirmation of HCA growth. As noted above, we were not powered to adjust for potential important confounders, such as BMI and PCOS, though PCOS prevalence was highest among progestin-only users, and obesity was similar between progestin and estrogen exposed groups, which we expect would have biased our results towards more increased growth in the progestin exposed groups. Patients in this study were also seen in a tertiary care setting which may limit generalizability to those followed in the community setting. However, we would not anticipate the biology of the HCAs to differ in that regard in response to different hormonal preparations, and obesity as a relevant potential confounder was seen in 41% of patients, similar to CDC estimates of obesity prevalence among young adults in the U.S. of ~ 36% [23]. However, larger studies are clearly needed to address definitive hormonal effects on HCAs, including ability to adjust for all relevant confounders.
In summary, we found no increased risk of HCA growth in female patients taking progestin-only agents for contraceptive purposes, with median HCA size decreasing in the progestin-only and no hormone exposure groups, and increasing in the estrogen exposure group. These results support the use of such agents as an alternative to estrogen-containing contraceptive options. Larger studies are needed to evaluate the impact of different sex hormones (both contraceptive and non-contraceptive formulations) on HCA growth, as well as the potential association of higher dose progestin-only agents on the risk of HCA growth and related complications.
Funding:
M.S. is supported by National Institute of Diabetes and Digestive and Kidney Diseases [K23 DK111944 & R03 DK131238] and by core facilities within the UCSF Liver Center [P30 DK026743].
Footnotes
Conflicts of interest: RPL has received consulting fees from Neptune Medical, Burlingame CA, outside the presented work. MS is the site principal investigator for clinical trials funded by Zydus pharmaceuticals and GSK.
References
- [1].Nault JC, Bioulac-Sage P, Zucman-Rossi J. Hepatocellular benign tumors-from molecular classification to personalized clinical care. Gastroenterology 2013;144:888–902. doi: 10.1053/j.gastro.2013.02.032. [DOI] [PubMed] [Google Scholar]
- [2].Patacsil SJ, Noor M, Leyva A. A review of benign hepatic tumors and their imaging characteristics. Cureus 2020;12:e6813. doi:7759/cureus.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blachar A. Hepatic adenomas: Imaging and pathologic findings. Radiogr a Rev Publ Radiol Soc North Am Inc 2001;21:874–7. doi: 10.1148/radiographics.21.4.g01jl04877. [DOI] [PubMed] [Google Scholar]
- [4].Deneve JL, Pawlik TM, Cunningham S, Clary B, Reddy S, Scoggins CR, et al. Liver cell adenoma: A multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol 2009;16:640–8. doi: 10.1245/s10434-008-0275-6. [DOI] [PubMed] [Google Scholar]
- [5].Bieze M, Phoa SSKS, Verheij J, van Lienden KP, van Gulik TM. Risk factors for bleeding in hepatocellular adenoma. Br J Surg 2014;101:847–55. doi: 10.1002/bjs.9493. [DOI] [PubMed] [Google Scholar]
- [6].Rooks JB, Ory HW, Strauss LT, Greenspan JR, Tyler CW, Ishak KG, et al. Epidemiology of hepatocellular adenoma: The role of oral contraceptive use. JAMA J Am Med Assoc 1979;242:644–8. doi: 10.1001/jama.1979.03300070040020. [DOI] [PubMed] [Google Scholar]
- [7].Bioulac-Sage P, Balabaud C, Zucman-Rossi J. Focal nodular hyperplasia, hepatocellular adenomas: Past, present, future. Gastroenterol Clin Biol 2010;34:355–8. doi: 10.1016/j.gcb.2010.03.014. [DOI] [PubMed] [Google Scholar]
- [8].Demory A, Péron J-M, Calderaro J, Selves J, Mokrane F-Z, Amaddeo G, et al. Body weight changes and duration of estrogen exposure modulate the evolution of hepatocellular adenomas after contraception discontinuation. Hepatology 2022. n/a. doi: 10.1002/hep.32734. [DOI] [PubMed] [Google Scholar]
- [9].Edmondson HA, Henderson B, Benton B. Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med 1976;294:470–2. doi: 10.1056/nejm197602262940904. [DOI] [PubMed] [Google Scholar]
- [10].Gaspersz MP, Klompenhouwer AJ, Broker MEE, Thomeer MGJ, van Aalten SM, Steegers E, et al. Growth of hepatocellular adenoma during pregnancy: A prospective study. J Hepatol 2020;72:119–24. doi: 10.1016/j.jhep.2019.09.011. [DOI] [PubMed] [Google Scholar]
- [11].Sinclair M, Schelleman A, Sandhu D, Angus PW. Regression of hepatocellular adenomas and systemic inflammatory syndrome after cessation of estrogen therapy. Hepatology 2017;66:989–91. doi: 10.1002/hep.29151. [DOI] [PubMed] [Google Scholar]
- [12].Torbenson M, Lee J-H, Choti M, Gage W, Abraham SC, Montgomery E, et al. Hepatic adenomas: analysis of sex steroid receptor status and the Wnt signaling pathway. Mod Pathol an Off J United States Can Acad Pathol Inc 2002;15:189–96. doi: 10.1038/modpathol.3880514. [DOI] [PubMed] [Google Scholar]
- [13].Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. U.S. medical eligibility criteria for contraceptive use, 2016. In: MMWR Recomm Rep, 65; 2016. p. 1–103. doi: 10.15585/mmwr.rr6503a1. [DOI] [PubMed] [Google Scholar]
- [14].Belghiti J, Cauchy F, Paradis V, Vilgrain V. Diagnosis and management of solid benign liver lesions. Nat Rev Gastroenterol Hepatol 2014;11:737–49. doi: 10.1038/nrgastro.2014.151. [DOI] [PubMed] [Google Scholar]
- [15].Marrero JA, Ahn J, Rajender Reddy K. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol 2014;109:1328–47 quiz 1348. doi: 10.1038/ajg.2014.213. [DOI] [PubMed] [Google Scholar]
- [16].Flejou J-F, Barge J, Menu Y, Degott C, Bismuth H, Potet F, et al. Liver adenomatosis: An entity distinct from liver adenoma? Gastroenterology 1985;89:1132–8. doi: 10.1016/0016-5085(85)90220-3. [DOI] [PubMed] [Google Scholar]
- [17].Haring MPD, Spijkerboer CS, Cuperus FJC, Duiker EW, de Jong KP, de Haas RJ, et al. Behavior and complications of hepatocellular adenoma during pregnancy and puerperium: a retrospective study and systematic review. HPB 2021;23:1152–63.doi: 10.1016/j.hpb.2021.04.019. [DOI] [PubMed] [Google Scholar]
- [18].Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol 2017;30:422–4. doi: 10.1016/j.jpag.2016.12.002. [DOI] [PubMed] [Google Scholar]
- [19].Chu MC, Zhang X, Gentzschein E, Stanczyk FZ, Lobo RA. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab 2007;92:2205–7. doi: 10.1210/jc.2007-0044. [DOI] [PubMed] [Google Scholar]
- [20].Sarkar M, Brady CW, Fleckenstein J, Forde KA, Khungar V, Molleston JP, et al. Reproductive health and liver disease: practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2020. doi: 10.1002/hep.31559. [DOI] [PubMed] [Google Scholar]
- [21].Toso C, Rubbia-Brandt L, Negro F, Morel P, Mentha G. Hepatocellular adenoma and polycystic ovary syndrome. Liver Int 2003;23:35–7. doi: 10.1034/j.1600-0676.2003.01776.x. [DOI] [PubMed] [Google Scholar]
- [22].Triantafyllopoulou M, Whitington PF, Melin-Aldana H, Benya EC, Brickman W. Hepatic adenoma in an adolescent with elevated androgen levels. J Pediatr Gastroenterol Nutr 2007;44:640–2. doi: 10.1097/MPG.0b013e31802e9a4a. [DOI] [PubMed] [Google Scholar]
- [23].Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief 2017:1–8. [PubMed] [Google Scholar]


