Abstract
mRNA vaccines have won the race for early COVID-19 vaccine approval, yet improvements are necessary to retain this leading role in combating infectious diseases. A next generation of self-amplifying mRNAs, also known as replicons, form an ideal vaccine platform. Replicons induce potent humoral and cellular responses with few adverse effects upon a minimal, single-dose immunization. Delivery of replicons is achieved with virus-like replicon particles (VRPs), or in nonviral vehicles such as liposomes or lipid nanoparticles. Here, we discuss innovative advances, including multivalent, mucosal, and therapeutic replicon vaccines, and highlight novelties in replicon design. As soon as essential safety evaluations have been resolved, this promising vaccine concept can transform into a widely applied clinical platform technology taking center stage in pandemic preparedness.
Keywords: mRNA, replicon, self-amplifying RNA, lipid nanoparticles, vaccines, platform technology
The mRNA vaccine landscape – what is on the horizon?
Synthetic mRNA vaccines made their grand entrance during the coronavirus disease 2019 (COVID-19) pandemic after many years of fundamental and preclinical research. Given their rapid development, cell-free manufacturing (see Glossary) and high clinical efficacy, mRNA vaccines outcompeted conventional live-attenuated, inactivated, and protein-based subunit vaccines in the race for early vaccine approval [1]. However, the massive rollout of mRNA vaccines also revealed challenges in balancing the high administration dose with adverse effects, the requirement of prime–boost vaccinations, and the necessity of cold-chain storage. These challenges can be overcome by the next generation of mRNA vaccines based on self-amplifying mRNA, also known as replicon RNA [2., 3., 4.].
Whereas mRNA vaccines encode a protein of interest, replicons have been engineered as a molecular chassis encoding the gene of interest (GOI; transgene) and all essential elements allowing self-amplification of the replicon RNA. The rapid amplification of replicon RNA in target cells increases the expression of the protein of interest (e.g., a viral (glyco)protein) (Figure 1 ) and induces a protective immune response at a markedly lower initial RNA dose than conventional mRNA vaccines [2,3]. The self-amplifying replicon genes have been derived from a wide variety of positive-stranded RNA viruses. In this review, we focus on alpha- and flavivirus-based replicons as they are best studied for both human and veterinary applications [5,6]. Because the viral structural genes have been replaced by a transgene, the replicon RNA cannot spread in the environment, which is a key difference with chimeric or recombinant virus vaccines (Box 1 ) [7,8].
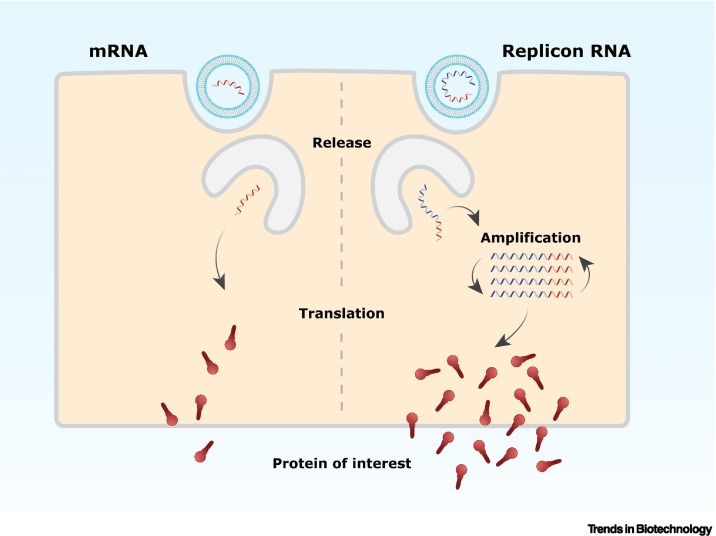
Figure 1.
Schematic representation of the protein of interest expression induced by a conventional mRNA and a replicon vaccine.
Once released in the cell, the mRNA is translated to produce the protein of interest. In contrast to mRNA, replicon RNA encodes alongside the protein of interest, self-amplifying genes (depicted in blue) that amplify the replicon RNA. This intracellular amplification will subsequently result in higher expression levels of the protein of interest.
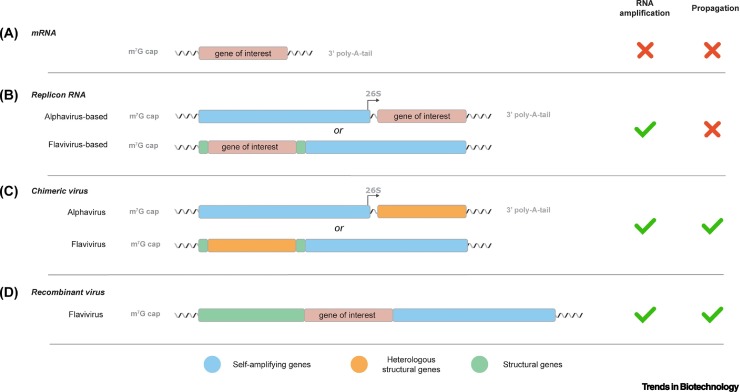
Box 1. Mode of action of (viral) nucleic acid vaccines.
mRNA
The structure of in vitro transcribed mRNA closely resembles cellular mRNA, and in general consists of a 5′-terminal 7-methylguanosine cap analog; a 5′ and 3′ untranslated region (UTR); the gene of interest (GOI); and a polyadenosine (poly-A) tail (Figure IA). To optimize the mRNA for vaccination purposes, modifications have been applied to increase the vector stability, translation efficacy, or immunogenicity. Since these nucleic acid vaccines encode solely the GOI, they are unable to replicate in, or spread to, neighboring cells.
Replicon RNA
Replicon RNA resembles in vitro transcribed mRNA, but additionally encodes viral replicase genes. These genes allow the rapid amplification of the mRNA and thereby increase the production of the GOI in comparison to non-amplifying mRNAs. The self-amplifying viral genes originated from viruses, for example, alphaviruses and flaviviruses (Figure IB). Alphavirus-based replicons contain a separated open-reading frame (ORF) upstream of the GOI that encodes all the replicase proteins. In contrast, in flavivirus-based replicons these replicase proteins are encoded in a single ORF downstream of the GOI. Since replicon RNA does not encode all alphavirus or flavivirus structural proteins, the RNA is propagation defective. As a result, replicon RNA vaccines are, similar to the non-amplifying mRNA vaccines, categorized as synthetic nucleic acid vaccines.
Chimeric virus
Similar to replicon RNA vaccines, chimeric virus vaccines are used to express protective antigens of a heterologous viral pathogen. The genomes of chimeric viruses encode their own replicase, but the structural genes are (partly) substituted for genes of a heterologous virus to induce protective immune responses against these structural proteins upon vaccination. Since both viral structural and replicase genes are maintained, viral propagation is observed in contrast to the non-propagative character of replicon vaccines. Therefore, chimeric virus vaccines are categorized as live-virus vaccines (Figure IC).
Recent chimeric alphavirus vaccine research focused on the development of a safe chimeric alphavirus vaccine platform based on the insect-restricted Eilat virus. The Eilat virus structural genes were replaced with the genes of highly pathogenic alphaviruses, such as VEEV, eastern equine encephalitis virus, and chikungunya virus [103].
For flaviviruses, the production of a chimeric vaccine was successfully demonstrated for the well-studied and attenuated YFV 17D strain. This strain was modified to express the structural genes of the heterologous West Nile virus, DENV, and Japanese encephalitis virus (JEV). The YFV 17D vaccine vector for DENV or JEV structural genes has been approved for human application [104., 105., 106., 107.].
Recombinant virus
Similar to chimeric virus vaccines, recombinant virus vaccines are based on modified viral genomes to express an exogenous GOI (Figure ID). However, in contrast to chimeric virus vaccines, the recombinant viruses encode all the genes for viral replication and encapsidation/transmission. Despite the many applications of the alphavirus-based replicon platform, the cytopathic nature of the complete alphavirus genome make them less favorable as recombinant virus vaccines. In contrast, recombinant flavivirus vaccines have been considered feasible in their use as an immunotherapy or for the protection against bacterial or viral infectious diseases [108., 109., 110.]. Notwithstanding, other viruses such as vesicular stomatitis virus, adenovirus or measles virus have been described more frequently in their use as recombinant viral vectors and pose a suitable alternative [111., 112., 113.]. Similarly to chimeric viruses, recombinant-vectored viruses are categorized as live-virus vaccines.
Figure I.
Schematic overview of (viral) nucleic acid vectors.
(A) An mRNA molecule contains the coding sequence of the gene of interest (GOI) flanked by a 5′ m7G cap-analog and a 3′ poly-A-tail. Similar to the mRNA vector, (B) virus-based replicons encode the GOI but also self-amplification genes, allowing RNA amplification. Both mRNA and replicon vectors are propagation-deficient as they do not encode a complete structural gene cassette. This is in contrast to (C) chimeric and (D) recombinant virus vectors that encode a complete (heterologous) structural gene cassette. These genes facilitate viral propagation, not limiting transduction of the viral vector to a single cell.
Alt-text: Box 1
The replicon technology has several key advantages over traditional vaccines. First, as the transgene is synthetically derived and the replicon cannot spread, replicon manufacturing processes have low biocontainment restrictions and application is safe-by-design [9]. Second, the transgene can simply be inserted into the ‘plug-and-play’ replicon (e.g., the commercially available Simplicon plasmid of Merck/Sigma–Aldrich), allowing rapid application in case of emerging infectious disease outbreaks. Finally, due to the self-amplifying character of the replicon vaccine, both humoral and cellular immune responses are triggered, which promises induction of protective immunity with a single low-dose immunization [2,3,10]. Despite a thorough understanding of the biology and the benefits of replicon technology, commercial application of alphavirus- and flavivirus-based replicons has only just commenced.
Here, we provide an overview of current challenges in replicon formulation and delivery and the safety considerations that need to be overcome. We discuss future opportunities for mucosal and therapeutic vaccination as well as novel advances in replicon design that can be exploited to transform this promising vaccine concept into a widely applied clinical platform technology.
Developments in replicon vaccine formulation and delivery
The most straightforward replicon formulation is based on naked delivery of the nucleic acids to the target cell. Early studies showed that direct injection of a low dose (10 μg) of naked RNA was sufficient to establish potent cellular and humoral immune responses in mice [11]. However, throughout the years, studies continued to report the susceptibility of naked RNA to nucleases and thus their underperformance in clinical applications [12,13]. While the replicon RNA amplifies itself and mediates the production of the protein of interest reaching 15–20% of total cell protein, the high sensitivity to RNases and poor capacity for internalization into the host cell, compromise the full capabilities of the naked replicon RNA [8,14]. Hence, several more stable modalities have been developed and are still being optimized, such as VRPs, as well as nonviral delivery systems, such as liposomes and lipid nanoparticles.
VRPs
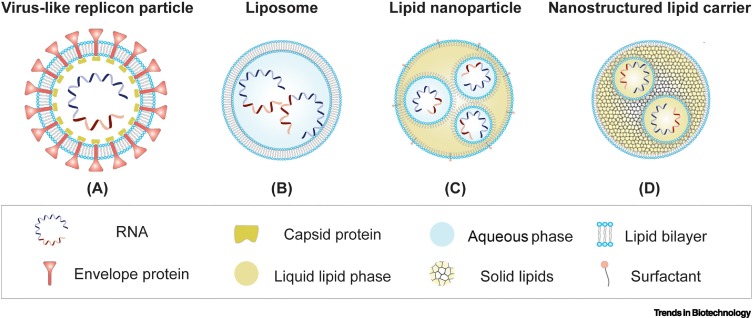
Replicon RNA is packaged into VRPs by the viral structural proteins expressed in trans from helper RNAs (Figure 2A). The natural tropism of these particles, which are structurally similar to a virus, enables the efficient delivery of the replicon to dendritic cells (direct priming) or target cells that indirectly prime antigen-presenting cells, resulting in the induction of a robust immune response [15,16]. Although the genetic cargo of the VRP consists of replicon RNA, which is limited to a single round of transduction, there is an anticipated risk of recombination between the replicon RNA and the trans-provided helper RNA resulting in the generation of replication-competent virus (RCV) during manufacturing [17]. RCV is an undesirable contamination in clinical products. To eliminate the risk for RCV formation, split-helper systems are used for VRP production with one helper RNA encoding the capsid protein and the other helper RNA the envelope glycoproteins. Both helpers lack subgenomic promoter sequences to reduce homologous overlap with the replicon. At least two independent, nonhomologous recombination events would be required to generate RCVs, but this has never been observed [18].
Figure 2.
Schematic overview of replicon delivery vehicles.
(A) In trans coexpression of replicon RNA and helper RNAs in a mammalian production cell line enables encapsulation of replicon RNA in virus-like replicon particles. Delivery vehicles can also be based on nonviral carriers that encapsulate the replicon RNA in (B) liposomes, (C) lipid nanoparticles, or (D) nanostructured lipid carriers in a cell-free manufacturing process. The delivery vehicles protect the replicon RNA and allow efficient delivery to target cells upon immunization.
Another potential concern for VRP delivery is antivector immunity induced after repetitive application of the highly immunogenic VRPs. Antivector immunity reduces the efficiency of vaccines as a consequence of antibody binding to the delivery vehicle. These expectations were based on interfering antivector responses in the adenovirus and vaccinia virus vector field [19,20]. Although vaccination with alphavirus or flavivirus VRPs can trigger vector-immune responses, these did not adversely influence the vaccine efficiency after repetitive vaccinations with the same VRP [10,21., 22., 23., 24.]. Another study showed that five repetitive vaccinations with the same VRP did not cause a decrease in serum antibody levels against a subsequent vaccination encoding a new protein of interest [22]. These results demonstrate that VRPs can repeatedly be applied against diverse antigens in a platform technology strategy without excessive interference of antivector immunity. However, to fully exclude antivector immunity, nonviral and lipid-based carriers for replicon RNA delivery can be considered.
Liposomes, lipid nanoparticles, and other nonviral carriers
Besides the VRP system, several alternative nucleic acid delivery methods based on chemical formulations have been developed and optimized over the years; namely, liposomes, liquid lipid nanoparticles (LNPs), and solid lipid nanoparticles (SNPs). These synthetic formulations improve vaccine stability, allow efficient replicon delivery, and rely on manufacturing processes without (mammalian) cell substrates. Most of these synthetic carriers are also extensively used in the pharmaceutical industry for the delivery of antibodies, peptides, or contrast substances [25].
Liposomes consist of a charged lipid bilayer with an aqueous core that can capture hydrophilic molecules such as DNA and RNA [26] (Figure 2B). The surface of liposome complexes can easily be adapted with other moieties, such as polyethylene glycol (PEG)–lipid conjugates or small molecules (e.g., antibodies), to facilitate tissue-specific vaccine delivery. Although most phospholipids used in liposomes spontaneously self-assemble when exposed to water, the ability to scale up the production procedure, the efficient trapping of RNA, and the flexibility in liposomes size are confined [26]. Early-generation cationic liposomes used in the formulation of RNA might be challenged by the fact that RNA is sometimes exposed on the outside of the carrier compromising both the stability and toxicity of the vaccine [27]. In contrast to VRPs, the bare lipid exterior lacks immunogenic proteins, which prevents unfavorable antivector immunity [28].
A more flexible delivery platform for nucleic acids are LNPs, which are composed of a single layer of lipids combined with surfactants (Figure 2C). The core is not required to be aqueous, but can consist of liquid lipids (e.g., LNPs), solid lipids (e.g., SNPs), or a combination known as nanostructured-lipid carriers (NLCs). Similar to liposomes, the lipid membrane of LNPs allows for additional modification to either the surface as well as the drug cargo itself [27,29]. For example, in 1998 the implementation of ionizable lipids revolutionized the LNP characteristics and reduced innate immunogenicity towards the exterior lipid molecules used in early-generation lipid carriers. In contrast to liposomes, these newer-generation LNPs require a carefully controlled manufacturing process that first captures the RNA at low pH while an additional step neutralizes the lipid charge for effective in vivo delivery. As a result, a more precise cargo formulation, broader application due to the variability in core composition, and better control over LNP size are achieved. These LNPs are taken up by antigen-presenting cells via receptor-mediated endocytosis. Subsequently, the pH drop within the endosome results in protonation of the ionizable lipids and facilitates membrane fusion of the LNP and release of the RNA into the cytosol.
The delivery of the replicon RNA formulated in LNPs does however require precise and extensive testing as was observed in a study comparing the intradermal delivery of LNP-encapsulated replicon RNA and naked replicon RNA in vivo. Upon injection of a high-dose replicon RNA:LNP complex in mice, a short-lived peak in innate immune responses resulted in a lower replicon-mediated protein expression compared to an injection with a low-dose replicon RNA:LNP complex. This highlights that highly efficient delivery of the LNP-formulated RNA to the cells can sometimes induce a strong local innate immune response that counteracts the protein expression via mRNA translational blockage [30]. To better control the delivery of the replicon RNA, the use of SNPs or NLCs may be exploited. Both are developed to overcome the weaknesses of liposomes and LNPs, such as stability, large-scale production, and drug release. The hybrid composition of liquid and solid lipids in NLCs (Figure 2D) makes these superior to SNPs. The increased drug loading capacity, the prolonged drug release and ability to stockpile vaccines in preparation for a pandemic makes the NLCs a valuable delivery method [31., 32., 33.].
DNA-launched replicons
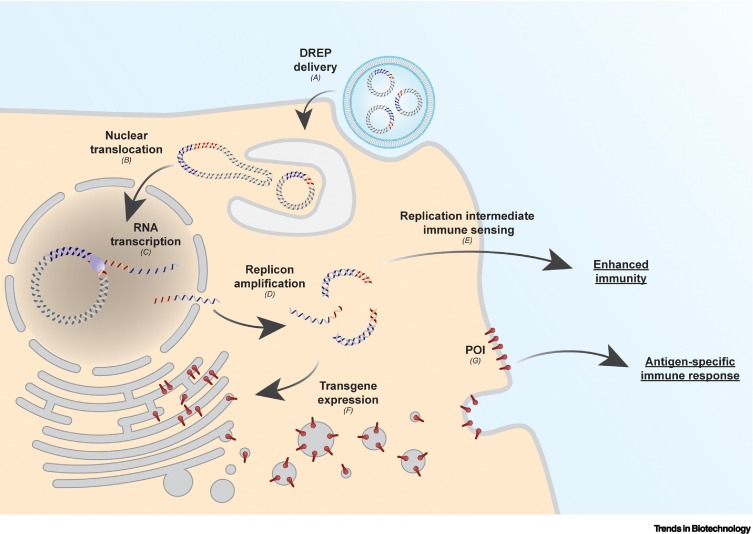
In the early race for nucleic acid vaccines, the primary focus was on DNA instead of RNA. In the case of replicon vaccines, a prerequisite of the in vitro transcription of replicon RNA is a DNA template. Since the DNA template can also be delivered directly to the cell itself, thereby surpassing the need of an in vitro RNA reaction, DNA-launched replicon (DREP) constructs were designed. A DREP is typically a circular double-stranded DNA molecule with a strong promoter (e.g., human cytomegalovirus immediate early promoter) enabling RNA polymerase II-driven transcription of replicon RNA in the nuclei of transfected cells [34., 35., 36.] (Figure 3 ). The replicon RNA is then transported to the cytoplasm as a capped mRNA and is translated to initiate self-amplification, similar to the direct delivery of in vitro transcribed replicon RNA [37]. A DREP shows less susceptibility to nucleases, is intrinsically more stable, and simplifies storage requirements in the logistic pipeline. However, the delivery of naked plasmid DNA to the nucleus is more challenging than the delivery of mRNA to the cytoplasm [38,39]. Nonetheless, combining advanced physical delivery techniques such as electroporation can aid the delivery of DNA to the nuclei and result in similar levels of immune response elicited by naked replicon RNA. Electroporation is mostly optimized for intradermal delivery of vaccines, which somewhat restricts their application [40]; however, DNA can efficiently be delivered by LNPs both in vitro and in vivo. It was observed that both protein of interest production and immunogenicity of LNP-delivered DNA was higher compared with naked DNA [41]. To improve nuclear DNA delivery, specific sequence elements can facilitate recognition by transcription factors and aid the translocation of the DNA via nuclear pores [42].
Figure 3.
Schematic overview of heterologous gene expression using a liposomal-delivered DNA-launched RNA replicon (DREP).
(A) Upon liposomal delivery to a cell, (B) the DREP migrates to the nucleus where (C) it serves as a template for the RNA polymerase II-mediated transcription of replicon RNA. (D) Subsequently, the replicon RNA is transported to the cytoplasm where the self-amplification, mediated by replicase proteins, occurs. (E) During amplification, cellular sensors recognize amplification intermediates (double-stranded RNA), enhancing host immunity. (F) At the same time, translation of the replicon RNA produces the protein of interest (POI). (G) This will induce an antigen-specific immune response.
A potential safety concern of DNA vaccines is the theoretical risk of DNA vector integration into the host genome or adverse (health) effects of prokaryotic elements. Fortunately, several studies have shown that the risk of plasmid integration is negligible under a variety of experimental conditions [43., 44., 45.]. Provided that incidents of integration described in literature are rare, actual integration might depend on factors such as the route of administration, the cell type, the expressed antigen, and the presence of prokaryotic elements [44,46,47]. In contrast to RNA vaccines, DNA vaccines typically require a prokaryotic host for plasmid propagation; therefore, functional elements such as a replication origin and an antibiotic resistance gene are encoded on the DNA vector. Research shows that the prokaryotic sequence elements could affect expression in the eukaryotic cells or cause DNA vector instability [48]. To tackle this, several strategies could be implemented in the DNA vector design, such as the use of ministring/minicircle DNA [49].
Safety considerations that challenge vaccine registration
Widespread commercial application of replicon vaccines can have an impact on pathogen control strategies, but to reach full potential of the replicon technology, registration as a vaccine platform is required. The vaccine platform concept relies on licensing a single replicon vector, a standardized delivery method, and a well-defined process for inserting the transgene to generate different replicon vaccines which do not require extensive re-evaluation as part of the authorization procedure. Indeed, the United States Department of Agriculture (USDA) already registered alphavirus-based Venezuelan equine encephalitis virus (VEEV; vaccine strain TC83) replicon particles as veterinary platform technology (product code 9PP0.00), allowing rapid delivery of customized replicon vaccine for farmers [50]. EU regulations on veterinary platform technologies are currently in development to help increase the availability of vaccines [51]. Confidence in replicon platform registration in the veterinary field will help establishing similar procedures for the human vaccine field. Recently, the first human replicon vaccine was granted an Emergency Use Approval by Indian regulators (Central Drugs Standard Control Organization; CDSCO). This CDSCO-approved VEEV-TC83-based replicon vaccine expresses a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike gene and is encapsulated in LNPs (license number MF/BIO/22/000064 under PD/Vacc-06) [10,52., 53., 54.].
Global authorization of alphavirus- and flavivirus-based replicon vaccines is expected now that others have paved the way. The USDA-approved VEEV-TC83 replicon vaccine already showed successful utilization in the immunization of millions of animals against a swine coronavirus – porcine epidemic diarrhea virus [55]. Furthermore, a live-attenuated, chimeric flavivirus vector vaccine platform (ChimeriVax) is commercially available in Australia, Thailand, Mexico, the Philippines, and Brazil [56]. In this chimeric yellow fever virus (YFV) vectored vaccine, the YFV envelope gene is replaced by a heterologous flavivirus gene to induce protective immunity against Japanese encephalitis virus and dengue virus (DENV) in humans [57]. Whereas the Chimerivax live-attenuated vaccines still encode structural genes, replicon vaccines do not encode the structural alphavirus or flavivirus genes, cannot spread in the environment, and consequently are anticipated safer. Despite these recent successes, there are still challenges ahead for global authorization of alphavirus and flavivirus replicon vaccines.
At present, the main challenges involved in the global authorization are potential safety concerns regarding the replicative character of these vaccines. As for all self-amplifying vaccines, concerns have been raised over adverse events in vulnerable individuals. For example, replicon vaccines could persist in immunocompromised individuals as clearance may be less efficient. Another group of consideration is pregnant women, especially when using replicon vectors derived from viruses that cause congenital infections (e.g., VEEV and YFV) [58,59]. Nevertheless, it is expected that replicon RNA will not disseminate to the fetus, because the most common route of replicon vaccine administration, intramuscular injection, is associated with local distributions at the injection site. Intravenous injections can cause systemic distribution, but the placenta will hinder passage of the replicon [60,61]. Even if the replicon spreads to the fetus, it is not expected to affect the development of the fetus as was shown for an attenuated YFV17D vector vaccine [62,63]. However, additional preclinical and clinical studies are required to safeguard the implementation of replicon vaccines in vulnerable individuals.
Lastly, the ability of replicon vaccines to recombine with circulating viruses must be considered. This potential safety risk is based on reported genetic interactions between traditional live-attenuated vaccines and viruses in nature [64., 65., 66., 67., 68., 69., 70.]. Moreover, several recombination events have been described for alphaviruses of which the most intriguing has been the ancient recombination event between two distinct alphaviruses resulting in the pathogenic western equine encephalitis virus [71]. Although genetic interactions between flaviviruses have been described as well, these are less likely to occur [72,73]. Nevertheless, even if the incidence of recombination is considered low, the consequences of such an event need to be evaluated prior to the widespread distribution of replicon vaccines.
Revolutionizing replicon technology
A diverse spectrum of replicon vaccines has been developed, yet still new innovative advancements and applications arise. Here, we provide some examples of recent and current developments that can revolutionize the replicon vaccine field.
Multivalent replicon vaccines
Multivalent vaccines are useful tools to control and prevent the spread of cocirculating or seasonal pathogens as a greater number of protective antigens are presented to the immune system. In the case of multivalent replicon vaccines, a single replicon can encode multiple antigens, or multiple replicons each expressing a different antigen are mixed in one vaccine formulation. Both designs have shown protection against a mixture of seasonal influenza strains, and cocirculating Lassa and Ebola viruses [74,75]. Although multivalent replicon vaccines can be rapidly developed and formulated, optimizing the composition of the individual target antigens can be laborious and may require extensive testing. This is particularly important when crossreactivity against different virus isolates of a virus is observed and antibody-dependent enhancement leads to adverse effects in the vaccinated individual. For example, incomplete protection of a tetravalent live-attenuated DENV vaccine against all DENV subtypes resulted in an increased replication of non-neutralized DENV subtypes [76]. If antigen ratios are less important and a platform technology is in place, multivalent replicon vaccine formulations can be a great solution to effectively combat dynamic virus outbreaks, for example, SARS-CoV-2 with novel variants continuously emerging.
Replicons targeting the mucosa
It becomes increasingly clear that local immune responses in mucosal tissue are important in the protection against a wide range of pathogens [77]. Since the majority of viral pathogens enter the host via the mucosal surfaces such as the respiratory, gastrointestinal, or urogenital tract, mucosal vaccination deserves more attention. In the ongoing SARS-CoV-2 pandemic, vaccines are delivered via direct intramuscular injection and are mostly focused on systemic cellular and humoral immunity without conferring sufficient mucosal immunity [78,79]. As such, the full potential of reducing viral entry and shedding is not obtained.
In the past, the focus was on mucosal delivery of mostly live-attenuated vaccines as these showed an effective immune response for an array of pathogens including poliovirus [80], influenza A virus [81], and rotavirus [82]. However, the associated risk of reversion to virulence with live-attenuated vaccines, and the lack of well-defined mucosal adjuvants, held back the exploration of the mucosal administration route. The latter is mainly what self-adjuvating replicon vaccines may be able to tackle. In a recent study on intranasal vaccination with therapeutic VEEV-TC83 replicon particles, effective delivery of the replicon RNA and a long-lasting expression of the proteins of interest (anti-SARS-CoV-2 neutralizing antibodies) were able to prevent the onset of viral infection [83]. On top of that, recent advances in liposome formulation and LNP encapsulation for RNA vaccines, and the propensity of these molecules to be taken up by the mucosal tissue, creates new opportunities for replicon RNA application [84,85]. These add to the capabilities of mucosal vaccines to be more easily mass delivered via noninvasive methods, for instance via nasal sprays in humans, or via water and feed for livestock [86].
Therapeutic application of replicon vectors
In addition to the versatile application of alphavirus- and flavivirus-based replicon vectors as vaccine platforms, replicon vectors have also been utilized in cancer immunotherapy. These therapeutic replicon vaccines express tumor antigens to activate tumor-specific T cells and antibody responses. The antigen target can be a general tumor factor such as carcinoembryonic antigen, or a specific tumor-associated protein such as the human papillomavirus E6 and E7 antigens [87,88]. Expression of the antigens by replicon vectors demonstrated promising immune modulation and efficient tumor regression in recent clinical trials [87., 88., 89., 90., 91.]. These encouraging clinical results support additional studies to enhance immune responses and develop novel delivery strategies, for example, intratumoral injection of LNP-encapsulated replicon RNA [92].
The future of replicons and replicons of the future
The replicon vector technology is constantly advancing. One recent development is the establishment of a bipartite replicon vector system [93]. Whereas a monopartite replicon encodes the self-amplifying genes and the transgene on one RNA strand, the bipartite replicon expresses the protein of interest from a trans-amplifying RNA. This carries a safety advantage for proteins of interest that may otherwise package the replicon vector in a monopartite system, for example, glycoprotein of rabies virus [94]. Furthermore, this modular system allows pre-production of the replicon vector and fast, on demand production of the variable RNA for the protein of interest.
New strategies are explored to improve the efficiency of the replicon vector by reducing the replicon-induced interferon activation, which inhibits translation. Elements such as the vaccinia virus E3L protein (Simplicon expression plasmid of Merck/Sigma–Aldrich) and Middle East respiratory syndrome coronavirus ORF4a protein have been inserted in the VEEV-TC83-based replicon to evade innate immune recognition [95]. It was demonstrated that these elements can abate the nonlinear dose dependency and enhance immunogenicity. However, putative recombination events involving such a replicon may potentially generate viruses that are more pathogenic than the wild-type virus the replicon was derived from, and thus strict safety testing is required before deliberate release of such replicons in the environment is allowed.
We also foresee developments in a few other areas. First, there is a drive for miniaturization of the replicon to reduce the length of the mRNA molecule. The replicons used most widely are based on the alphavirus replicases, which consist of several interacting multifunctional proteins encoded by a relatively long 8–9-kb gene cassette. A shorter replicon RNA molecule will ease manufacturing, increase RNA delivery efficiency, and reduce administration dose and possibly production cost. Arguably, not all functions within the alphavirus replicase are required for RNA self-amplification [96]. Through rational design in combination with directed evolution experiments, smaller replicases may be generated that outperform the canonical alphavirus replicase. To take this further, in theory an RNA-dependent RNA polymerase (RdRp) in combination with the essential 5′ and 3′ noncoding regions at the replicon RNA termini is sufficient for self-amplification. Viral genomes with RdRps sized ~2 kb are present in nature [97] and these may inspire future replicon design.
Second, de novo designed replicons may in the future not be subject to current genetically modified organism (GMO) legislation. With the implementation of artificial intelligence in the design of proteins, we expect it will be possible to engineer small RdRps (based on sequence information of all viral RdRp in public databases) using deep learning algorithms [98]. Various de novo assembled proteins have been realized, demonstrating advantages in protein size and stability compared to natural proteins [99]. In a similar way, novel synthetic RdRps not derived from, or associated with, any particular virus family may be engineered. If successful, a replicon encoding such RdRp plus a transgene (e.g., coronavirus spike gene) will have a similar legal status as the currently licensed mRNA vaccines. In other words, these sophisticated ‘RNA machines’ will not be subject to GMO legislation and/or registration procedures that may now restrict, or at least delay, market authorization of alphavirus- and flavivirus-based replicons.
Lastly, circular RNAs (circRNAs) are a novel recent direction in the mRNA vaccine field [100., 101., 102.]. As these circRNAs lack free ends susceptible to exonuclease degradation, they have an increased stability compared to capped, polyadenylated, linear mRNA vectors. Furthermore, circRNA in eukaryotic cell lines led to increased protein expression levels and improved protein production half-life compared to the linear mRNA counterpart [102]. Although the delivery of circRNA vectors in mice was promising, optimization was shown to be essential for specific tissues [100]. To improve RNA stability, the development of replicon circRNA vaccines can be explored.
Concluding remarks
From all recent advances in the delivery, efficacy, and safety of nucleic acid vaccines, a clear role emerges for self-amplifying mRNA vaccines in the battle against current and future infectious disease outbreaks, and in the rapidly growing field of cancer immunotherapy. The most attractive feature is that replicons encode their own replication machinery to boost their copy numbers directly after administration in target cells. The self-amplifying nature dramatically lowers the required initial mRNA dose and consequently reduces adverse effects in patients. However, the current application of replicon technology barely touches the surface of what the system is capable of (see Outstanding questions). Our view is that it is possible to revise or simplify regulatory procedures to license replicon vaccine platforms without extensive safety re-evaluations every time the GOI changes. The paradigm shift in the vaccine field from protein to mRNA will definitely help to mature replicon technology in general and promote the further improvement of these sophisticated RNA machines.
Outstanding questions.
How can the most efficient replicon delivery vehicle per application be predicted?
What type of data is needed to guarantee the environmental safety of replicon vaccines?
In which way can regulatory procedures be simplified or revised to obtain authorization for replicons as a human vaccine platform technology?
Can optimized self-amplifying mRNAs eventually outcompete nonamplifying mRNAs?
Will artificial intelligence tools be able to revolutionize the de novo design of miniaturized replicons?
Alt-text: Outstanding questions
Declaration of interests
No interests are declared.
Glossary
- Antibody-dependent enhancement
the increased severity of disease as a result of pre-existing, non-neutralizing antibodies against the pathogen.
- Carcinoembryonic antigen
a protein found in a wide range of cells that is often associated with certain tumors and fetal development when detected at elevated levels.
- Cell-free manufacturing
the production of biological compounds (e.g., RNA, DNA, or proteins) without the interference of a living cell.
- Circular RNA (circRNA)
novel type of mRNA vectors that are synthetically derived by ligation of in vitro transcribed linear RNA fragments.
- Helper RNA
in vitro transcribed RNA that encodes and supplements structural proteins to the replicon mRNA, allowing encapsulation of the mRNA in VRPs.
- Intramuscular injection
administration of a biological compound directly into specifically selected muscles.
- Intravenous injection
administration of a biological compound directly into the vein.
- Ministring DNA
a small linear, covalently closed plasmid DNA without any undesirable bacterial sequences.
- Multivalent vaccine
a vaccine that aims to induce protective immunity against various pathogens or serotypes.
- Nanostructured-lipid carrier (NLC)
LNP that consists of solid lipids, liquid lipids, surfactants, water, and the active pharmaceutical ingredient.
- Plug-and-play principle (replicon)
straightforward and effortless exchange of genetic elements of an existing platform technology (often from a fully synthetic origin).
- Replication-competent virus (RCV)
a propagation-competent virus that originates from a propagation-defective viral vector (replicon) which acquired structural genes for propagation via an RNA recombination event.
- Replicon RNA
an mRNA molecule that encodes the genetic elements necessary for the self-amplification and transient protein of interest expression.
- RNA-dependent RNA polymerase (RdRp)
a versatile enzyme that catalyzes genomic RNA replication and transcription.
- Safe-by-design principle (replicon)
a genetic design that is self-limiting and cannot escape the host cell.
- Solid lipid nanoparticles (SNPs)
a lipid nanoparticle that consists of solid lipids with a crystalline matrix containing the active ingredient.
- Transgene
a gene that is artificially introduced into the genome of the target (micro)organism.
- Vaccine platform
technology that allows the development of multiple vaccines based on the same building blocks with the aim to speed the registration process and the availability of future vaccines
- Virus-like replicon particle (VRP)
single-cycle, propagation-defective particle, that mimics the wild-type virus’ outer shell to deliver the encapsulated replicon mRNA into cells.
References
- 1.World Health Organization Status of COVID-19 vaccines within WHO EUL/PQ evaluation process. 2021. https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_01March2021.pdf Published online January 20, 2021.
- 2.Vogel A.B., et al. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol. Ther. 2018;26:446–455. doi: 10.1016/j.ymthe.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Alwis R., et al. A single dose of self-transcribing and replicating RNA-based SARS-CoV-2 vaccine produces protective adaptive immunity in mice. Mol. Ther. 2021;29:1970–1983. doi: 10.1016/j.ymthe.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voigt E.A., et al. A self-amplifying RNA vaccine against COVID-19 with long-term room-temperature stability. NPJ Vaccines. 2022;7:136. doi: 10.1038/s41541-022-00549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundstrom K. Self-amplifying RNA viruses as RNA vaccines. Int. J. Mol. Sci. 2020;21:1–29. doi: 10.3390/ijms21145130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hikke M.C., Pijlman G.P. Veterinary replicon vaccines. Annu. Rev. Anim. Biosci. 2017;5:89–109. doi: 10.1146/annurev-animal-031716-032328. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki R., et al. Production of single-round infectious chimeric flaviviruses with DNA-based Japanese encephalitis virus replicon. J. Gen. Virol. 2014;95:60–65. doi: 10.1099/vir.0.058008-0. [DOI] [PubMed] [Google Scholar]
- 8.Kamrud K.I., et al. Development and characterization of promoterless helper RNAs for the production of alphavirus replicon particle. J. Gen. Virol. 2010;91:1723–1727. doi: 10.1099/vir.0.020081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meechan P.J., Potts J. 6th edition. Centers for Disease Control and Prevention; 2020. Biosafety in Microbiological and Biomedical Laboratories.https://stacks.cdc.gov/view/cdc/97733 Published online June 2020. [Google Scholar]
- 10.Erasmus J.H., et al. An alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci. Transl. 2020;eabc9396:1–12. doi: 10.1126/scitranslmed.abc9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou X., et al. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine. 1994;12:1510–1514. doi: 10.1016/0264-410x(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 12.Diken M., et al. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 2011;18:702–708. doi: 10.1038/gt.2011.17. [DOI] [PubMed] [Google Scholar]
- 13.Démoulins T., et al. Self-amplifying pestivirus replicon RNA encoding influenza virus nucleoprotein and hemagglutinin promote humoral and cellular immune responses in pigs. Front. Immunol. 2021;11:1–18. doi: 10.3389/fimmu.2020.622385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geall A.J., et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. U. S. A. 2012;109:14604–14609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rayner J.O., et al. Alphavirus vectors and vaccination. Rev. Med. Virol. 2002;12:279–296. doi: 10.1002/rmv.360. [DOI] [PubMed] [Google Scholar]
- 16.Huckriede A., et al. Induction of cytotoxic T lymphocyte activity by immunization with recombinant Semliki Forest virus: indications for cross-priming. Vaccine. 2003;22:1104–1113. doi: 10.1016/j.vaccine.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Weiss B.G., Schlesinger S. Recombination between Sindbis virus RNAs. 1991;65:4017–4025. doi: 10.1128/jvi.65.8.4017-4025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pushko P., et al. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 19.Sharpe S., et al. Induction of simian immunodeficiency virus (SIV)-specific CTL in rhesus macaques by vaccination with modified vaccinia virus Ankara expressing SIV transgenes: Influence of pre-existing anti-vector immunity. J. Gen. Virol. 2001;82:2215–2223. doi: 10.1099/0022-1317-82-9-2215. [DOI] [PubMed] [Google Scholar]
- 20.Barouch D.H., et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 21.Aberle J.H., et al. Humoral and cellular immune response to RNA immunization with flavivirus replicons derived from tick-borne encephalitis virus. J. Virol. 2005;79:15107–15113. doi: 10.1128/JVI.79.24.15107-15113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uematsu Y., et al. Lack of interference with immunogenicity of a chimeric alphavirus replicon particle-based influenza vaccine by preexisting antivector immunity. Clin. Vaccine Immunol. 2012;19:991–998. doi: 10.1128/CVI.00031-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walczak M., et al. Heterologous prime-boost immunizations with a virosomal and an alphavirus replicon vaccine. Mol. Pharm. 2011;8:65–77. doi: 10.1021/mp1002043. [DOI] [PubMed] [Google Scholar]
- 24.White L.J., et al. An immunogenic and protective alphavirus replicon particle-based dengue vaccine overcomes maternal antibody interference in weanling mice. J. Virol. 2007;81:10329–10339. doi: 10.1128/JVI.00512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musielak E., et al. Synthesis and potential applications of lipid nanoparticles in medicine. Materials. 2022;15:682. doi: 10.3390/ma15020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenchov R., et al. Lipid nanoparticles - from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15:16982–17015. doi: 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- 27.Xue H., et al. Lipid-based nanocarriers for RNA delivery. Curr. Pharm. Des. 2015;21:3140–3147. doi: 10.2174/1381612821666150531164540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W.C., Huang L. Non-viral vector as vaccine carrier. Adv. Genet. 2005;54:315–337. doi: 10.1016/S0065-2660(05)54013-6. [DOI] [PubMed] [Google Scholar]
- 29.Buschmann M.D., et al. Nanomaterial delivery systems for mRNA vaccines. Vaccines (Basel) 2021;9:1–30. doi: 10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huysmans H., et al. Expression kinetics and innate immune response after electroporation and LNP-mediated delivery of a self-amplifying mRNA in the skin. Mol. Ther. Nucleic Acids. 2019;17:867–878. doi: 10.1016/j.omtn.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghasemiyeh P., Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res. Pharm. Sci. 2018;13:288–303. doi: 10.4103/1735-5362.235156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra V., et al. Solid lipid nanoparticles: emerging colloidal nano drug delivery systems. Pharmaceutics. 2018;10:1–21. doi: 10.3390/pharmaceutics10040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerhardt A., et al. A flexible, thermostable nanostructured lipid carrier platform for RNA vaccine delivery. Mol. Ther. Methods Clin. Dev. 2022;25:205–214. doi: 10.1016/j.omtm.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varnavski A.N., et al. Stable high-level expression of heterologous genes in vitro and in vivo by noncytopathic DNA-based Kunjin virus replicon vectors. J. Virol. 2000;74:4394–4403. doi: 10.1128/jvi.74.9.4394-4403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berglund P., et al. Enhancing immune responses using suicidal DNA vaccines. Nat. Biotechnol. 1998;16:562–565. doi: 10.1038/nbt0698-562. [DOI] [PubMed] [Google Scholar]
- 36.Agapov E. v, et al. Noncytopathic Sindbis virus RNA vectors for heterologous gene expression. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12989–12994. doi: 10.1073/pnas.95.22.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallen K.J., et al. A novel, disruptive vaccination technology: self-adjuvanted RNActive ® vaccines. Hum. Vaccine Immunother. 2013;9:2263–2276. doi: 10.4161/hv.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johansson D.X., et al. Intradermal electroporation of naked replicon rna elicits strong immune responses. PLoS One. 2012;7:1–7. doi: 10.1371/journal.pone.0029732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gómez-Aguado I., et al. Nucleic acid delivery by solid lipid nanoparticles containing switchable lipids: plasmid DNA vs. messenger RNA. Molecules. 2020;25:5995. doi: 10.3390/molecules25245995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ljungberg K., Liljeström P. Self-replicating alphavirus RNA vaccines. Expert Rev. Vaccines. 2014;14:177–194. doi: 10.1586/14760584.2015.965690. [DOI] [PubMed] [Google Scholar]
- 41.Mucker E.M., et al. Lipid nanoparticle formulation increases efficiency of DNA-vectored vaccines/immunoprophylaxis in animals including transchromosomic bovines. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-65059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dean D.A., et al. Sequence requirements for plasmid nuclear import. Exp. Cell Res. 1999;253:713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nichols W., et al. Potential DNA vaccine integration into host cell genome. Ann. N. Y. Acad. Sci. 1995;772:30–39. doi: 10.1111/j.1749-6632.1995.tb44729.x. [DOI] [PubMed] [Google Scholar]
- 44.Ledwith B.J., et al. Plasmid DNA vaccines: investigation of integration into host cellular DNA following intramuscular injection in mice. Intervirology. 2000;43:258–272. doi: 10.1159/000053993. [DOI] [PubMed] [Google Scholar]
- 45.Manam S., et al. Plasmid DNA vaccines: tissue distribution and effects of DNA sequence, adjuvants and delivery method on integration into host DNA. Intervirology. 2000;43:273. doi: 10.1159/000053994. [DOI] [PubMed] [Google Scholar]
- 46.Doerfler W. Adenoviral vector DNA- and SARS-CoV-2 mRNA-based Covid-19 vaccines: possible integration into the human genome - are adenoviral genes expressed in vector-based vaccines? Virus Res. 2021;302 doi: 10.1016/j.virusres.2021.198466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Würtele H., et al. Illegitimate DNA integration in mammalian cells. Gene Ther. 2003;10:1791–1799. doi: 10.1038/sj.gt.3302074. [DOI] [PubMed] [Google Scholar]
- 48.Williams J.A., et al. Plasmid DNA vaccine vector design: impact on efficacy, safety and upstream production. Biotechnol. Adv. 2009;27:353–370. doi: 10.1016/j.biotechadv.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nafissi N., et al. DNA ministrings: highly safe and effective gene delivery vectors. Mol. Ther. Nucleic Acids. 2014;3 doi: 10.1038/mtna.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.United States Department of Agriculture Veterinary Biological Products. 2023. https://www.aphis.usda.gov/animal_health/vet_biologics/publications/currentprodcodebook.pdf Published online February 2, 2023.
- 51.European Medicines Agency Guideline on Data Requirements for Vaccine Platform Technology Master Files. 2021. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-data-requirements-vaccine-platform-technology-master-files-vptmf_en.pdf Published online January 28, 2022.
- 52.Hawman D.W., et al. SARS-CoV2 variant-specific replicating RNA vaccines protect from disease following challenge with heterologous variants of concern. eLife. 2022;e75537:1–21. doi: 10.7554/eLife.75537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawman D.W., et al. Replicating RNA platform enables rapid response to the SARS-CoV-2 Omicron variant and elicits enhanced protection in naïve hamsters compared to ancestral vaccine. eBioMedicine. 2022;83:1–14. doi: 10.1016/j.ebiom.2022.104196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Central Drugs Standard Control Organization Approved COVID-19 Vaccines as on 04.10.2022. 2022. https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadPublic_NoticesFiles/Approved%20COVID-19%20vaccines%20as%20on%2004.10.2022.pdf
- 55.Veen R. Vander, et al. Rapid development of an efficacious swine vaccine for novel H1N1. PLoS Curr. Influenza. 2009;1:1–7. doi: 10.1371/currents.RRN1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Condit R.C., et al. Unique safety issues associated with virus vectored vaccines: potential for and theoretical consequences of recombination with wild type virus strains. Vaccines (Basel) 2016;34:6610–6616. doi: 10.1016/j.vaccine.2016.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monath T.P., et al. Clinical proof of principle for ChimeriVaxTM: recombinant live, attenuated vaccines against flavivirus infections. Vaccine. 2002;20:1004–1018. doi: 10.1016/s0264-410x(01)00457-1. [DOI] [PubMed] [Google Scholar]
- 58.Charlier C., et al. Arboviruses and pregnancy: maternal, fetal, and neonatal effects. Lancet Child Adolesc. Health. 2017;1:134–146. doi: 10.1016/S2352-4642(17)30021-4. [DOI] [PubMed] [Google Scholar]
- 59.Platt D.J., et al. Zika virus-related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aao7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colmenero P., et al. Recombinant Semliki Forest virus vaccine vectors: the route of injection determines the localization of vector. Gene Ther. 2001;8:1307–1314. doi: 10.1038/sj.gt.3301501. [DOI] [PubMed] [Google Scholar]
- 61.Morris-Downes M.M., et al. Semliki Forest virus-based vaccines: persistence, distribution and pathological analysis in two animal systems. Vaccine. 2001;19:1978–1988. doi: 10.1016/s0264-410x(00)00428-x. [DOI] [PubMed] [Google Scholar]
- 62.Kum D.B., et al. A yellow fever–Zika chimeric virus vaccine candidate protects against Zika infection and congenital malformations in mice. NPJ Vaccines. 2018;3:56. doi: 10.1038/s41541-018-0092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.da Silva F.C., et al. Yellow fever vaccination in a mouse model is associated with uninterrupted pregnancies and viable neonates except when administered at implantation period. Front. Microbiol. 2020;11:1–9. doi: 10.3389/fmicb.2020.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee S.W., et al. Attenuated vaccines can recombine to form virulent field viruses. Science. 2012;337:188. doi: 10.1126/science.1217134. [DOI] [PubMed] [Google Scholar]
- 65.Wenhui L., et al. Complete genome sequence of a novel variant porcine reproductive and respiratory syndrome virus (PRRSV) strain: evidence for recombination between vaccine and wild-type PRRSV strains. J. Virol. 2012;86:9543. doi: 10.1128/JVI.01341-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Camus-Bouclainville C., et al. Genome sequence of SG33 strain and recombination between wild-type and vaccine myxoma viruses. Emerg. Infect. Dis. 2011;17:633–638. doi: 10.3201/eid1704.101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Becher P., et al. RNA recombination between persisting pestivirus and a vaccine strain: generation of cytopathogenic virus and induction of lethal disease. J. Virol. 2001;75:6256–6264. doi: 10.1128/JVI.75.14.6256-6264.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kew O.M., et al. Policy and practice circulating vaccine-derived polioviruses: current state of knowledge. Bull. World Health Organ. 2004;82:16–23. [PMC free article] [PubMed] [Google Scholar]
- 69.Chong Y.L., et al. The effect of vaccination on the evolution and population dynamics of avian paramyxovirus-1. PLoS Pathog. 2010;6:1–11. doi: 10.1371/journal.ppat.1000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moussatché N., et al. When good vaccines go wild: feral orthopoxvirus in developing countries and beyond. J. Infect. Dev. Ctries. 2008;2:156–173. doi: 10.3855/jidc.258. [DOI] [PubMed] [Google Scholar]
- 71.Hahn C.S., et al. Western equine encephalitis virus is a recombinant virus. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5997–6001. doi: 10.1073/pnas.85.16.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Twiddy S.S., Holmes E.C. The extent of homologous recombination in members of the genus Flavivirus. J. Gen. Virol. 2003;84:429–440. doi: 10.1099/vir.0.18660-0. [DOI] [PubMed] [Google Scholar]
- 73.McGee C.E., et al. Stability of yellow fever virus under recombinatory pressure as compared with chikungunya virus. PLoS One. 2011;6:1–13. doi: 10.1371/journal.pone.0023247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Magini D., et al. Self-amplifying mRNA vaccines expressing multiple conserved influenza antigens confer protection against homologous and heterosubtypic viral challenge. PLoS One. 2016;11:1–25. doi: 10.1371/journal.pone.0161193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pushko P., et al. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J. Virol. 2001;75:11677–11685. doi: 10.1128/JVI.75.23.11677-11685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shukla R., et al. Antibody-dependent enhancement: a challenge for developing a safe dengue vaccine. Front. Cell. Infect. Microbiol. 2020;10:1–12. doi: 10.3389/fcimb.2020.572681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morens D.M., et al. Rethinking next-generation vaccines for coronaviruses, influenzaviruses, and other respiratory viruses. Cell Host Microbe. 2023;31:146–157. doi: 10.1016/j.chom.2022.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bleier B.S., et al. COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol. Head Neck Surg. 2021;164:305–307. doi: 10.1177/0194599820982633. [DOI] [PubMed] [Google Scholar]
- 79.Singanayagam A., et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022;22:183–195. doi: 10.1016/S1473-3099(21)00648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dauer C.C. Poliomyelitis in the United States, 1954. Public Health Rep. 1955;70:1125–1127. [PMC free article] [PubMed] [Google Scholar]
- 81.Belshe R.B., et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine in children. N. Engl. J. Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 82.Bernasconi V., et al. Mucosal vaccine development based on liposome technology. J. Immunol. Res. 2016;2016 doi: 10.1155/2016/5482087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J.Q., et al. Intranasal delivery of replicating mRNA encoding neutralizing antibody against SARS-CoV-2 infection in mice. Signal Transduct. Target. Ther. 2021;6:1–8. doi: 10.1038/s41392-021-00783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Phua K.K.L., et al. Intranasal mRNA nanoparticle vaccination induces prophylactic and therapeutic anti-tumor immunity. Sci. Rep. 2014;4:4–10. doi: 10.1038/srep05128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li M., et al. Engineering intranasal mRNA vaccines to enhance lymph node trafficking and immune responses. Acta Biomater. 2017;64:237–248. doi: 10.1016/j.actbio.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 86.Walsh H.L., et al. Veterinary and Aquatic Diseases. Adv. Exp. Med. Biol. Adv. Microbiol. Infect. Dis. Public Health. 2020;3:811–830. [Google Scholar]
- 87.van de Wall S., et al. Potent therapeutic efficacy of an alphavirus replicon DNA vaccine expressing human papilloma virus E6 and E7 antigens. Oncoimmunology. 2018 doi: 10.1080/2162402X.2018.1487913. Published online July 26, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morse M.A., et al. An alphavirus vector overcomes the presence of neutralizing antibodies and elevated numbers of Tregs to induce immune responses in humans with advanced cancer. J. Clin. Investig. 2010;120:3234–3241. doi: 10.1172/JCI42672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Komdeur F.L., et al. First-in-Human phase I clinical trial of an SFV-based RNA replicon cancer vaccine against HPV-induced cancers. Mol. Ther. 2021;29:611–625. doi: 10.1016/j.ymthe.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crosby E.J., et al. Long-term survival of patients with stage III colon cancer treated with VRP-CEA(6D), an alphavirus vector that increases the CD8+ effector memory T cell to Treg ratio. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2020-001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crosby E.J., et al. Vaccine-induced memory CD8þ T cells provide clinical benefit in HER2 expressing breast cancer: a mouse to human translational study. Clin. Cancer Res. 2019;25:2725–2736. doi: 10.1158/1078-0432.CCR-18-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y., et al. Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat. Cancer. 2020;1:882–893. doi: 10.1038/s43018-020-0095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beissert T., et al. A trans-amplifying RNA vaccine strategy for induction of potent protective immunity. Mol. Ther. 2020;28:119–128. doi: 10.1016/j.ymthe.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y.N., et al. A novel rabies vaccine based on infectious propagating particles derived from hybrid VEEV-Rabies replicon. eBioMedicine. 2020;56 doi: 10.1016/j.ebiom.2020.102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blakney A.K., et al. Innate inhibiting proteins enhance expression and immunogenicity of self-amplifying RNA. Mol. Ther. 2021;29:1174–1185. doi: 10.1016/j.ymthe.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan Y.B., et al. Targeting the alphavirus virus replication process for antiviral development. Antivir. Res. 2023;210:1–8. doi: 10.1016/j.antiviral.2022.105494. [DOI] [PubMed] [Google Scholar]
- 97.Wolf Y.I., et al. Origins and evolution of the global RNA virome. mBio. 2018;9:1–31. doi: 10.1128/mBio.02329-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dauparas J., et al. Robust deep learning-based protein sequence design using ProteinMPNN. Science. 2022;378:49–56. doi: 10.1126/science.add2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Korendovych I.v., DeGrado W.F. De novo protein design, a retrospective. Q. Rev. Biophys. 2020;53:1–33. doi: 10.1017/S0033583519000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meganck R.M., et al. Tissue-dependent expression and translation of circular RNAs with recombinant AAV vectors in vivo. Mol. Ther. Nucleic Acids. 2018;13:89–98. doi: 10.1016/j.omtn.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bai Y., et al. Research progress on circular RNA vaccines. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1091797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wesselhoeft R.A., et al. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 2018;9:2629. doi: 10.1038/s41467-018-05096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Erasmus J.H., et al. A chikungunya fever vaccine utilizing an insect-specific virus platform. Nat. Med. 2017;23:192–199. doi: 10.1038/nm.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Monath T.P., et al. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J. Infect. Dis. 2003;188:1213–1230. doi: 10.1086/378356. [DOI] [PubMed] [Google Scholar]
- 105.Arroyo J., et al. ChimeriVax-West Nile virus live-attenuated vaccine: preclinical evaluation of safety, immunogenicity, and efficacy. J. Virol. 2004;78:12497–12507. doi: 10.1128/JVI.78.22.12497-12507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guirakhoo F., et al. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVaxTM-DEN2) vaccine: phase I clinical trial for safety and immunogenicity: effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all. Hum. Vaccines. 2006;2:60–67. doi: 10.4161/hv.2.2.2555. [DOI] [PubMed] [Google Scholar]
- 107.Biedenbender R., et al. Phase II, randomized, double-blind, placebo-controlled, multicenter study to investigate the immunogenicity and safety of a West Nile virus vaccine in healthy adults. J. Infect. Dis. 2011;203:75–84. doi: 10.1093/infdis/jiq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McAllister A., et al. Recombinant yellow fever viruses are effective therapeutic vaccine for treatment of murine experimental solid tumors and pulmonary metastases. J. Virol. 2000;74:9197–9205. doi: 10.1128/jvi.74.19.9197-9205.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nogueira R.T., et al. Recombinant yellow fever virus elicit CD8+ T cell responses and protective immunity against Trypanosoma cruzi. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sanchez-Felipe L., et al. A single-dose live-attenuated YF17D-vectored SARS-CoV-2 vaccine candidate. Nature. 2021;590:320–325. doi: 10.1038/s41586-020-3035-9. [DOI] [PubMed] [Google Scholar]
- 111.Cross R.W., et al. A recombinant VSV-vectored vaccine rapidly protects nonhuman primates against heterologous lethal Lassa fever. Cell Rep. 2022;40 doi: 10.1016/j.celrep.2022.111094. [DOI] [PubMed] [Google Scholar]
- 112.Fischer L., et al. ChAdOx1-vectored Lassa fever vaccine elicits a robust cellular and humoral immune response and protects guinea pigs against lethal Lassa virus challenge. Npj Vaccines. 2021;6 doi: 10.1038/s41541-021-00291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mateo M., et al. A single-shot Lassa vaccine induces long-term immunity and protects cynomolgus monkeys against heterologous strains. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abf6348. [DOI] [PubMed] [Google Scholar]