Abstract
Transition metal complexes exhibiting thermally activated delayed fluorescence (TADF) remain underdeveloped for organic light-emitting diodes (OLEDs). Here, we describe a design of TADF Pd(II) complexes featuring metal-perturbed intraligand charge-transfer excited states. Two orange- and red-emitting complexes with efficiencies of 82 and 89% and lifetimes of 2.19 and 0.97 μs have been developed. Combined transient spectroscopic and theoretical studies on one complex reveal a metal-perturbed fast intersystem crossing process. OLEDs using the Pd(II) complexes show maximum external quantum efficiencies of 27.5 to 31.4% and small roll-offs down to 1% at 1000 cd m−2. Moreover, the Pd(II) complexes show exceptional operational stability with LT95 values over 220 hours at 1000 cd m−2, benefiting from the use of strong σ-donating ligands and the presence of multiple intramolecular noncovalent interactions beside their short emission lifetimes. This study demonstrates a promising approach for developing efficient and robust luminescent complexes without using the third-row transition metals.
The metal-perturbed excited state and intramolecular noncovalent interactions are combined to design luminescent Pd(II) complexes.
INTRODUCTION
The use of transition metal complexes for harvesting all the electrically generated singlet and triplet excitons underpins the performances of commercial organic light-emitting diodes (OLEDs) (1, 2). Owing to the facilely accessible metal-to-ligand charge transfer excited states, Ir(III) complexes can undergo fast intersystem crossing (ISC) from the lowest-lying singlet excited state (S1) to triplet manifold and subsequent radiative decay of the lowest-lying triplet excited state (T1) (3–8). It has been manifested that not only the spin-orbit coupling (SOC) constant of the metal atom but also its involvement into the lowest excited state is crucial for boosting phosphorescent emissions (9). In combination with the use of rigid multidentate ligands, the phosphorescent properties of Au(III) and Pt(II) complexes have also been greatly improved in recent years (10–12). Hitherto, however, only the third-row transition metal complexes have been capable of emanating efficient phosphorescence spanning the entire visible spectrum. It has been a formidable challenge to simultaneously attain decent phosphorescence efficiencies and short excited state lifetimes for the second-row transition metal complexes; the slow radiative decay rates bound to their triplet ligand-centered nature may prohibit the realization of stable devices (13). For example, Pd(II) complexes supported by tetradentate ligands have been reported to have moderate phosphorescence efficiencies but with extremely long emission lifetimes (14, 15). In the literature, strongly phosphorescent Pd(II) complexes for OLEDs have only been achieved by Li and coworkers for which a highly flat geometry has been judiciously designed to drive the intermolecular Pd-Pd and π-π associations (16, 17). Given the difficulty in precise manipulation of the molecule aggregation behavior, it is still paramount to develop mononuclear transition metal complexes that can exhibit efficient phosphorescence without relying on aggregation and allow for doped device fabrications.
As has been demonstrated on purely organic emitters, the thermally activated delayed fluorescence (TADF) provides a way for using the triplet excitons via reverse intersystem crossing (RISC) from the T1 to S1 state and subsequent radiation of S1 state (Fig. 1A) (18, 19). A small energy difference (ΔEST) between the S1 and T1 states is the crux for promoting the spin-forbidden and endothermic RISC (kRISC). In the meantime, this mechanism has been proven viable for luminescent transition metal complexes (20–22). Of particular note is that the participation of minor d orbital into the lowest-lying excited states is beneficial for boosting the RISC process through enhancing the SOC interactions, thereby enabling short-lived delayed fluorescence (9, 23–25). However, in comparison with the great number of phosphorescent metal complexes and purely organic TADF emitters, the quantity of TADF metal complexes has been much smaller. They are mainly limited to coinage metal complexes with only few reports on other metals (26–32). Hitherto, TADF emissions from d8 Pd(II) complexes have only been scarcely observed and the design principle remains elusive (15, 33–36). For the realization of efficient TADF from transition metal complexes, it is crucial to adjust both the ΔEST and SOC interaction between S1 and T1 states. This presents a difficulty in molecular design because both the electronic structure and coordination ability of the ligand(s) need to be carefully considered.
Fig. 1. Design concept and chemical structures.
(A) Illustration of the TADF mechanism and the factors affecting the RISC rate. (B) The proposed approach of MPICT excited state for the design of TADF emitters, which features a moderate SOC effect, small ΔEST value, and ease of color tuning. (C) Schematic illustration of the role of intramolecular noncovalent interactions between the perturbing metal motif and the lateral acceptor in improving the molecular stability. (D) Chemical structures of the Pd(II) complexes.
In this work, we demonstrate a design of TADF Pd(II) complexes by using donor-acceptor (D-A) type ligands in which only the donor moieties coordinate to the metal atom (Fig. 1B). The metal-ligand(s) (MLn) motif perturbs the dynamics of intraligand charge-transfer (ILCT) excited state through enhancing the SOC effect and reducing the ΔEST. Moreover, as illustrated in Fig. 1C, intramolecular noncovalent interactions have been integrated into the structural design, which can improve the stability of luminescent transition metal complexes (37, 38). Two orange and red TADF Pd(II) complexes (λmax = 598 to 637 nm in doped films) have been synthesized (Fig. 1D). Both complexes exhibit high photoluminescence quantum yields (PLQYs) of 82 and 89% and short emission lifetimes of 0.97 and 2.19 μs in doped films. OLEDs with maximum external quantum efficiencies (EQEs), little efficiency roll-offs, and exceptional operational lifetimes have been achieved for the new Pd(II) complexes.
RESULTS
Design, synthesis, and structures
The metal-perturbation concept toward TADF is illustrated in Fig. 1B. The MLn has two main roles: (i) The M–N covalent bonding interaction leads to an admixture of d orbital to the highest occupied molecular orbital (HOMO) that is mainly localized on the carbazole moiety and induces enhanced SOC effect; and (ii) the steric effect of MLn forces the acceptor moiety to have a large torsion angle with respect to the donor plane, giving a small ΔEST. In addition, by this design, the emission colors can be facilely tuned by varying the noncoordinating electron acceptor unit. On the other hand, the spatial arrangement of the MLn and the lateral acceptor moiety allows for the engineering of noncovalent interactions between them, which can be exploited for strengthening the metal-ligand association (Fig. 1C). The structures of the Pd(II) complexes are shown in Fig. 1D. 3,6-Di-tert-butylcarbazole (Cz) was selected as the electron-rich segment of the D-A ligands. Dibenzo[a,c]phenazine (DBPZ) and triphenyltriazine (TRZ) were used as the acceptors. In the D-A ligands, namely, CzDBPZ and CzTRZ, the acceptor units are linked to the 1-position of Cz, leaving the N atom accessible for coordination with a metal ion. [(C^C^C)Pd]+ (C^CH^C = 1,3-bis(3'-butylimidazol-2'-ylidene)benzene) was introduced as the perturbing motifs. The substituted carbazoles were synthesized by following the methods reported elsewhere (39, 40). The complexes were synthesized by reacting the [(C^C^C)PdCl] precursor with the substituted carbazole in the presence of tBuONa (see details in the Supplementary Materials). After the reaction, the complexes were isolated by a dissolution-precipitation method (yields: 70 to 72%). The identity of the complexes was ascertained by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy, high-resolution mass spectrometry, and elemental analysis. It is interesting to note that the chemical shifts of the -CH2CH3 on the carbene ligands of Pd1 and Pd2 lie in the region below 0.5 parts per million in the 1H NMR spectra. This strong shielding effect is due to the presence of CH···π interactions between the butyl chains and the carbazolyl ligand, as evidenced by their x-ray structures (vide infra). Both complexes show moderate to good solubility in aprotic organic solvents such as toluene, chlorobenzene, dichloromethane (DCM), and tetrahydrofuran.
X-ray diffraction analyses were performed for both complexes. The crystallograhic data and key structural parameters are listed in tables S1 and S2. The lengths of Pd–N bonds are 2.105 and 2.084 Å for Pd1 and Pd2, respectively. As shown in Fig. 2 and figs. S1 to S3, both the pincer ligand and the DBPZ/TRZ are significantly twisted with respect to the carbazole plane in Pd1 and Pd2. For example, the [(C^C^C)Pd]+ and DBPZ moieties in Pd1 have torsion angles of 70.10° and 57.56°. In all, the twisted conformations for the complexes are beneficial for preferred HOMO and lowest unoccupied molecular orbital (LUMO) separation. As expected, there are strong intramolecular metal···π (hole) and π···π interactions between the MLn and DBPZ/TRZ in the crystal structures of Pd1 and Pd2, as revealed by the stacking with distances of 3.1 to 3.6 Å. Moreover, the alkyl chains of the N-heterocyclic carbene (NHC) ligand are found to clamp the carbazole plane with C–H···π interactions (Fig. 2A and fig. S4). As depicted in Fig. 2B and fig. S5, attractive van der Waals interactions are authenticated by the independent gradient model based on Hirshfeld partition (IGMH) analysis of Pd1 based on its optimized structure (41, 42). The secondary interactions are surmised to stabilize the metal-amide ligation in Pd1 and Pd2, accounting for their excellent thermal stability (vide infra) (37, 38). Therefore, the present work suggests a strategy for robust planar d8 metal complexes that does not require cumbersome syntheses.
Fig. 2. Structure and intramolecular noncovalent interactions of Pd1.
(A) Perspective views of the crystal structure of Pd1 showing the torsions of [(C^C^C)Pd]+ and DBPZ moieties with respect to the carbazole plane (left), the intramolecular π···π and Pd···π interactions (middle), and the C–H···π interactions between the butyl chains and the carbazole plane (right). (B) IGMH analysis of optimized structure of Pd1 showing the presence of multiple attractive noncovalent interactions.
Thermal and electrochemical properties
The thermal properties of both complexes were investigated by thermogravimetric analysis in nitrogen atmosphere (fig. S6). Impressively, Pd1 (Td = 438°C, the temperature corresponding to a weight loss of 5%) shows much higher stability that is unprecedented for a pincer-type Pd(II) complex and even superior to those supported by tetradentate ligands (43). The employment of strongly σ-donating NHC ligand and the presence of multiple intramolecular noncovalent interactions are conceived to impart the stability to Pd1 (vide infra). Electrochemical properties of the complexes were studied by cyclic voltammetry in DCM. As shown in fig. S7, both complexes show (quasi)reversible oxidation waves with half-potentials (E1/2,OX) of about 0.30 V versus Ag/AgCl. The oxidations are almost identical for the Pd(II) complexes and are assigned to be the carbazolyl ligand–centered processes, which are marginally affected by the substituent on the carbazolyl ligand. By referring to the redox couple of Fc+/Fc, the HOMO levels of Pd1 and Pd2 were determined to be −4.60 and −4.62 eV (table S3). By the addition of the optical bandgaps (determined from their absorption onsets), their LUMO levels were estimated to be −2.60 and −2.41 eV. The difference in LUMO levels of Pd1 and Pd2 is consistent with the acceptor variation on the carbazolyl ligand.
Photophysical properties
The absorption spectra of the two free ligands and the complexes in toluene solution (1 × 10−5 M) are shown in Fig. 3A, and the pertinent data are compiled in Table 1. The strong absorptions at ca. 280 and 300 nm for the ligands are assigned to π-π* transitions of the carbazole and the acceptor moieties (39, 40). The absorptions with maxima at ca. 360 to 420 nm are ascribed to intraligand charge-transfer transitions from the carbazole moiety to the lateral acceptor, which can be supported by the red shift with increasing electron-accepting strength from TRZ to DBPZ. The much higher molar absorptivity for CzDBPZ is due to the localized π-π* transitions of DBPZ. Upon coordination with a metal ion, the charge-transfer absorption bands shift to the lower-energy region by 130 to 145 nm (λabs: 550 nm for Pd1; 500 nm for Pd2). The sizeable bathochromic shifts mainly result from the uplifted HOMO levels caused by the Pd–N interaction. Therefore, the nature of the lowest excited states can be described as metal-perturbed intraligand charge-transfer (MPICT). The MPICT bands show negative solvatochromic properties (fig. S8), which can be interpreted by the opposite molecular dipole moments in the ground and excited states.
Fig. 3. Photophysical properties.
(A) Absorption spectra of the free D-A ligands (top) and the complexes (bottom) in toluene at room temperature. (B) Normalized emission spectra of Pd1 and Pd2 in degassed toluene (298 K, solid line; 77 K, dashed line). a.u., arbitrary units. (C) Normalized emission spectra of Pd1 and Pd2 in 5 wt % PMMA films (298 K, solid line; 77 K, dashed line). (D) Temperature-dependent transient PL characteristics of Pd1 in PMMA film. Inset: Arrhenius fit of the kTADF value versus temperature. (E) Boltzmann-type fitting of the emission lifetimes of Pd1 in PMMA film at various temperatures. (F) Fractions of TADF and phosphorescence as a function of temperature for Pd1.
Table 1. Photophysical data of the Pd(II) complexes.
| λabs (nm) (ε/103 M−1 cm−1)* | λem.RT (nm)† | λem.RT (nm)‡ | τRT (μs)§ | ΦRT (%)¶ | kr (s−1)# | knr (s−1)# | λem.77K (nm)‡ | τ77K (μs)§ | |
|---|---|---|---|---|---|---|---|---|---|
| Pd1 | 315 (29), 385 (20), 402 (24), 550 (3.0) | 665 | 637 | 0.97 (0.36 μs, 40%; 1.37 μs, 60%) | 89 | 9.2 × 105 | 1.1 × 105 | 634 | 70.6 (4.94 μs, 47%; 58.4 μs, 53%) |
| Pd2 | 307 (24), 397 (1.6), 500 (2.0) | 582 | 598 | 2.19 (0.70 μs, 51%; 4.46 μs, 49%) | 82 | 3.7 × 105 | 8.2 × 104 | 594 | 336 (9.60 μs, 29%; 470 μs, 71%) |
*Measured in diluted toluene at 298 K.
†Measured in degassed toluene at 298 K.
‡Measured in 5 wt % doped PMMA at 298 K and 77 K.
§Determined for the 5 wt % doped PMMA at 298 K in vacuum and calculated from the weighted averages of the two or three contributions.
¶Determined for the 5 wt % doped PMMA at 298 K in nitrogen atmosphere using an integrating sphere (±10%).
#Calculated for the 5 wt % doped PMMA at 298 K by kr = Φ/τ and knr = (1 − Φ)/τ.
Both complexes show broad and featureless emission bands with maxima (λem) at 665 and 582 nm (Fig. 3B and Table 1) in degassed toluene. The positive solvatochromic effect exemplified by Pd2 manifests the charge-transfer emitting state (fig. S9). To evaluate their potentials as OLED emitters, in-depth emission properties of the complexes in poly(methyl methacrylate) (PMMA) at 298 K and 77 K were studied. As depicted in Fig. 3C, the complexes in doped PMMA films exhibit structureless emissions at room temperature with λem of 637 and 598 nm and high PLQYs of 89 and 82%. The excited-state decay plots of the Pd(II) complexes in PMMA films at room temperature can be fitted using a biexponential function (Table 1). Akin to our previous studies on coinage metal complexes with a twisted conformation, the coexistence of various conformers is proposed (44). The average lifetimes of the complexes are 0.97 and 2.19 μs. Of particular note is the high radiative decay rate of 9.2 × 105 s−1 for Pd1. To the best of our knowledge, such large values have only been attained for the dinuclear and aggregate of Pd(II) complexes featuring triplet metal-metal–to–ligand charge transfer excited states (16, 17, 45).
To corroborate the emission mechanism of the present complexes in doped PMMA films, temperature-dependent (77 to 297 K) photoluminescence properties were probed for Pd1 (Fig. 3D). First, the emission with λem of 634 nm at 77 K is very close to its emission at room temperature, while the emission lifetime is increased by 73 times compared with that at room temperature, implying a distinct radiative decay rate (Table 1). This pronounced temperature dependence of radiative decay rate suggests the emissions to be TADF at room temperature and phosphorescence at 77 K. An Arrhenius analysis was used to fit the data in 197 to 297 K where TADF is the predominant emission (vide infra). The Arrhenius plot shows a linear dependence of ln(kTADF) as a function of 1/T with a slope of ΔEST/kB. The radiative rate constant (kTADF) at each temperature was estimated from the emission quantum yield and lifetime (table S4). The ΔEST value is determined to be 90 meV for Pd1 (Fig. 3D), rationalizing its favored TADF emission at room temperature. Moreover, the plot of the emission lifetimes as a function of temperature for Pd1 was also fitted using a Boltzmann-type equation (Fig. 3E), giving a ΔEST value of 73 meV. As depicted in Fig. 3F, the contribution of TADF emission increases as the temperature increases. At room temperature, TADF accounts for 98% of the total emission. Notably, it has been previously shown that CzTRZ has only prompt fluorescence due to its large ΔEST (ca. 0.5 eV) (39). Likewise, CzDPBZ is not TADF active (fig. S10 and table S5). Therefore, it is the metal coordination that activates the TADF from the ILCT state. It is worth noting that the relatively short emission lifetimes of phosphorescent Ir(III) and Pt(II) complexes are beneficial for their good device performances by reducing the device efficiency roll-off and improving the operational stability. However, it is a challenge to achieve fast radiative decays of T1 states for transition metal complexes without containing the third-row metal element. The present TADF Pd(II) complexes having short lifetimes of as short as 1 μs render them promising candidates as OLED emitters. Furthermore, given that the D-A–type ligands only coordinate with the metal ion in a monodentate manner, the present design of TADF Pd(II) complexes should be applicable to metal ions with various electronic configurations.
Transient spectroscopy
A notable merit of TADF metal complexes is the accelerated ISC and RISC process and thus short-lived TADF. To decipher the role of metal perturbation in promoting the ISC rates of the present complexes, femtosecond and nanosecond transient absorption (fs/ns-TA) spectra of Pd1 as well as their kinetics (λex = 400 nm) were measured in toluene. As depicted in Fig. 4A, broad excited-state absorption (ESA) bands at 440 to 500 nm and 550 to 650 nm grow rapidly within 0.80 ps, which corresponds to the generation of a charge-transfer state based on the characteristic absorptions of the radical anion of DBPZ (46). Then, accompanied by the drop of the ESA peak at 640 nm until 475 ps, the ESA peaks at 493 and 585 nm are further increased slightly (Fig. 4B). This evolution is attributed to the ISC process, which can be supported by the subsequent much slower decay (Fig. 4C). The kinetics at 585 nm are extracted from fs-TA spectra and fitted best by a triexponential function, giving three time constants of 0.40, 49.4, and 8010 ps, respectively (Fig. 4D). The first two time constants are associated with the population into the MPICT state (S1) and the ISC process, respectively. The long lifetime and triplet nature of the ESA peak at 440 nm are proved by the ns-TA spectra and oxygen quenching experiment (fig. S11). Fitting kinetics in the ns-TA spectra gives a time constant of 69 ns for the triplet state decay, which should contain mainly RISC (T1 → S1) and ISC (T1 → S0) processes. On the basis of the results of fs/ns-TA experiments, the kISC value for Pd1 is estimated to be 2.0 × 1010 s−1, revealing the role of metal perturbation in boosting the spin-flip process. Notably, the ultrafast S1 structural relaxation or solvent relaxation in the time scale of sub-to-several picoseconds was not observed in our measurements (47). Solvent reorganization is conceived to be not pronounced because of the weak solvation capability of toluene.
Fig. 4. Transient spectroscopy.
(A to C) Fs-TA evolution spectra and (D) kinetics at 595 nm of Pd1 in toluene (concentration = 0.1 mM, λex = 400 nm).
Theoretical studies
To understand the influence of metal coordination on the emissive properties of the ILCT states, density functional theory/time-dependent density functional theory (DFT/TDDFT) calculations on CzDBPZ and Pd1 were performed. In its optimized S0 geometry, the torsion between the DBPZ and Cz moieties in CzDBPZ is characterized by a dihedral angle of 43.67° (Fig. 5). DFT calculations show that the LUMO is localized on the acceptor moiety (DBPZ). The HOMO of CzDBPZ is mainly located on the donor moiety (Cz) but with a minor spread over DBPZ. TDDFT calculations show that both the S1 and T1 states exhibit electron transition from HOMO to LUMO, which are of a charge-transfer character with an admixture of π-π* of DBPZ. Therefore, CzDBPZ has a large ΔEST value of 0.66 eV, accounting for its TADF inactivity. For Pd1, the torsion angle of DBPZ with respect to the carbazole plane increases to 63.68° (Fig. 5). Although the LUMO of Pd1 is dominated by DBPZ as in CzDBPZ, there is a notable difference in its HOMO compared with the free ligand. In addition to the principal contribution from the carbazole unit, the HOMO consists of 4d orbital of the Pd atom. The coordination interaction results in a significant increase in the HOMO level from −5.75 eV for free ligand to −4.68 V for Pd1. This change, together with the larger D-A torsion, is conceived to lead to a vanished HOMO distribution on the DBPZ moiety. According to the TDDFT calculations based on its optimized S0 geometry, the S1 and T1 states of Pd1 correspond to an electronic transition from HOMO to LUMO, exhibiting a predominant charge-transfer character from the carbazole unit and metal atom to the DBPZ moiety. Hence, this results in a small ΔEST of 0.04 eV. Besides, the SOC interaction between the S1 and T1 states is markedly increased from 0.21 cm−1 for CzDBPZ to 3.07 cm−1 for Pd1. Therefore, the changes in both SOC and ΔEST boost the RISC and efficient TADF for the present complexes.
Fig. 5. Theoretical calculations.
The optimized ground-state geometries, frontier molecular orbitals, and the excited-state energy levels of CzDBPZ and Pd1.
Electroluminescence
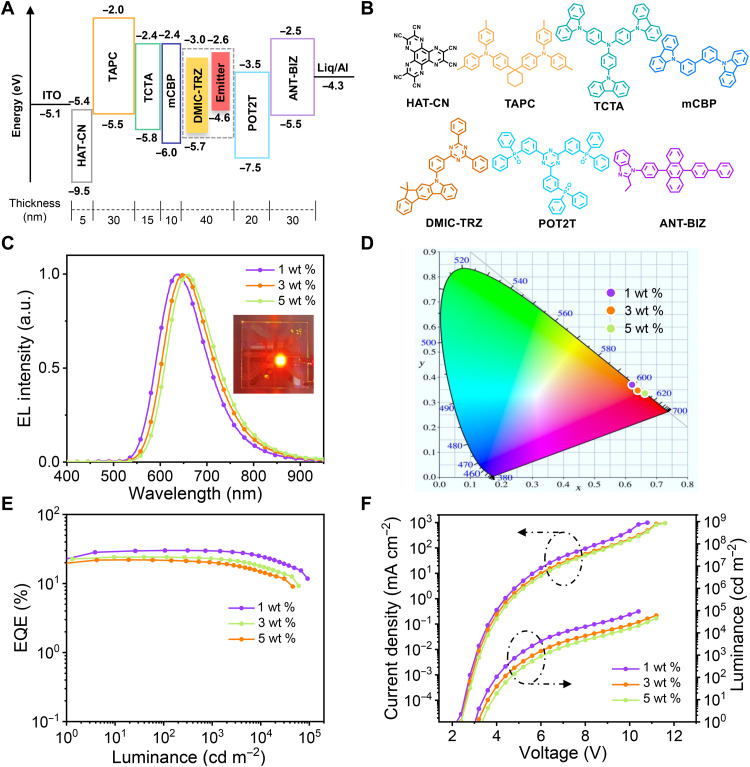
The good thermal stability, high TADF efficiencies, and short emission lifetimes of Pd1 and Pd2 prompted us to study their electroluminescence performances in vacuum-deposited OLEDs. As shown in Fig. 6A, the devices with a configuration of ITO (indium tin oxide)/HAT-CN (1,4,5,8,9,11-hexaazatriphenylenehexacarbonitrile) (5 nm)/TAPC (1,1-bis[(di-4-tolylamino)phenyl]cyclohexane) (30 nm)/TCTA [tris(4-carbazolyl-9-ylphenyl)amine] (15 nm)/mCBP (3,30-di(9H-carbazol-9-yl)-1,10-biphenyl) (10 nm)/DMIC-TRZ {5-(3-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole}: Pd(II) complex (40 nm)/POT2T [(1,3,5-triazine-2,4,6-triyl)tris(benzene-3,1-diyl)(tris(diphenylphosphine oxide)] (20 nm)/ANT-BIZ {1-(4-(10-([1,10-biphenyl]-4-yl)anthracen-9-yl)phenyl)-2-ethyl-1H-benzo[d]-imidazole} (30 nm)/Liq (8-hydroxyquinolinolato-lithium) (2 nm)/Al (aluminum) (100 nm) were first fabricated. The emitting layer consists of DMIC-TRZ doped with Pd1 or Pd2 at different concentrations. HAT-CN serves as the hole-injection layer. TAPC and ANT-BIZ are used as the hole- and electron-transport layers, respectively. TCTA, mCBP, and POT2T act as exciton-blocking layers. The corresponding energy-level diagram and chemical structures of the materials are also shown in Fig. 6. The key device data of Pd1 are summarized in Table 2. Complex Pd1 exhibits saturated red electroluminescence with maxima (λEL) at 640 to 662 nm (Fig. 6C) and CIEx [the Commission Internationale de L'Eclairage (CIE)-x color coordinate] >0.63 (Fig. 6D). The device doped with 1 wt % of Pd1 shows the highest EQE, current efficiency, and power efficiency of 30.1%, 29.6 cd A−1, and 27.5 lm W−1, respectively (Fig. 6E and Table 2). Notably, this EQE is only lower than those of yellow-orange devices based on Pd(II) aggregates in the context of luminescent Pd(II) complexes (16, 17). In particular, the EQE of Pd1 remains as high as 29.8% at 1000 cd m−2, corresponding to a very small efficiency roll-off of 1.0%. This is mainly ascribed to its short emission lifetime. By the same token, the maximum luminance reaches 92,000 cd m−2 (Fig. 6F). The red shifts in λEL and decreased efficiencies with increasing doping concentration are likely due to the intermolecular interactions. As illustrated in fig. S12 and table S6, the devices based on Pd2 show orange emissions (λEL = 576 to 583 nm) with maximum EQEs of up to 28.8% and the efficiency roll-offs at 1000 cd m−2 are also small. The efficiency roll-offs for both Pd1 and Pd2 are markedly improved compared with the state-of-the-art phosphorescent mononuclear Pd(II) complexes (14, 15).
Fig. 6. Structure and performances of OLEDs doped with Pd1.
(A) Device structure with the energy-level diagram. (B) Chemical structures of organic materials used for the devices. (C) Electroluminescence spectra at 1000 cd m−2. (D) Electroluminescence color coordinates in the CIE 1931 chromaticity diagram. Values were taken at 1000 cd m−2. (E) EQE-luminance characteristics. (F) Current density-voltage-luminance characteristics.
Table 2. EL performances of the TADF-OLEDs using Pd1 as the emitter.
| Conc. | λmax (nm) | L (cd m−2)* | CE (cd A−1)† | PE (lm W−1)‡ | EQE (%)§ | CIE (x, y)]¶ | |||
|---|---|---|---|---|---|---|---|---|---|
| Maximum | 1000 cd m−2 | Maximum | 1000 cd m−2 | Maximum | 1000 cd m−2 | ||||
| 1 wt % | 640 | 92,000 | 29.6 | 28.9 | 27.5 | 18.1 | 30.1 | 29.8 | 0.63, 0.37 |
| 3 wt % | 645 | 60,500 | 16.7 | 15.8 | 14.7 | 8.9 | 24.2 | 23.0 | 0.65, 0.35 |
| 5 wt % | 662 | 45,600 | 11.9 | 11.0 | 10.0 | 5.6 | 22.0 | 19.9 | 0.66, 0.34 |
*Maximum luminance.
†Current efficiency.
‡Power efficiency.
§External quantum efficiency.
¶CIE coordinates at 1000 cd m−2.
The operational stability of Pd1 and Pd2 has been tested at an initial luminance (L0) of 1000 cd m−2 (constant current density: 5 and 2 mA cm−2 for Pd1 and Pd2, respectively). Devices with the configuration ITO/HAT-CN (5 nm)/TBBD (N4,N4,N4',N4'-tetra(4-biphenylyl)-biphenyl-4,4'-diamine) (30 nm)/o-SFAF (N-([1,1'-biphenyl]-2-yl)-N-(9,9-dimethyl-9H-fluoren-2-yl)-9,9'-spirobi[fluoren]-2-amine) (15 nm)/DMIC-TRZ: Pd(II) complex (45 nm)/ANT-BIZ (40 nm)/Liq (2 nm)/Al (100 nm) were vacuum-deposited and encapsulated in a glove box. The chemical structures of the used organic materials in the OLEDs are displayed in fig. S13. As summarized in figs. S14 and S15 and tables S7 and S8, these devices also show high efficiencies with maximum EQEs up to 31.4%. The LT95 (the time required for the luminance to drop to the 95% of the initial intensity) values for Pd1 and Pd2 are 220 and 436 hours, respectively (Fig. 7). The corresponding LT90 and LT85 values for Pd1 are 1010 and 2370 h (fig. S16). Together, the high efficiencies and the performances are superior to previous Pd(II) complexes supported by tetradentate ligands (14, 15, 33). The operational stability of Pd1 and Pd2 is on a par with the recent achievements on TADF Au(III) complexes, which show LT90 longer than 1000 hours at 1000 cd m−2 (32). Moreover, as shown in table S9, the overall device performances of Pd1 are comparable to or better than those of the representative red-emitting Ir(III) and Pt(II) complexes in the literature, among which Pd1 is the rare emitter showing both a high maximum EQE (>30%) and a long operational lifetime. As aforementioned, the short TADF lifetimes and the strong Pd–C bonds together with the intramolecular noncovalent interactions are conceived to play crucial roles in preventing the emitter degradation.
Fig. 7. Operational stability of devices doped with Pd1 or Pd2.
Relative luminance versus operating time at an initial luminance of 1000 cd m−2.
DISCUSSION
We have presented a metal-perturbation strategy for the design of TADF d8 Pd(II) complexes by ligating the donor moiety of D-A–type ligands to the metal center in a monodentate manner. The composition of minor 4d orbital in the frontier molecular orbitals significantly enhances the SOC between the coupling singlet and triplet excited states. The coordination with a metal-perturbing motif also induces a larger torsion between the donor and acceptor segments. This structural feature results in effective separation of the HOMO and LUMO and thus small singlet-triplet energy splitting (ΔEST) down to less than 100 meV, in contrast to the much larger ΔEST of ca. 500 meV for the free ligands. Both these effects are beneficial for enticing efficient and short-lived delayed fluorescence. On the other hand, by varying the noncoordinating acceptor moiety, the emission colors can be tuned without changing the coordination sphere.
In the meantime, it has been shown that intramolecular noncovalent interactions can be exploited for improving the molecular stability. Because of these electronic and geometric features, the newly synthesized Pd(II) complexes have demonstrated appealing device performances with high efficiencies and very little efficiency roll-offs. The exceptional operational stability of the Pd(II) complexes implies their potential for practical use. The present strategy is conceived to broaden the choice of metals and ligands for the design of robust TADF metal complexes.
MATERIALS AND METHODS
Synthesis and characterization
CzDBPZ and CzTRZ were synthesized according to literature procedures (39, 40). Pd1 and Pd2 were synthesized and characterized according to the methods described in the Supplementary Text.
Temperature-dependent lifetime fitting analysis
The emission lifetimes at various temperatures were analyzed using literature methods (21, 48). The Boltzmann-type equation is shown in Eq. 1, where k is the radiative decay rate constant of S1 or T1 state, kB is Boltzmann’s constant, and T is temperature
| (1) |
The kTADF values can also be fitted to a two-level model using Eq. 2 (48). The Arrhenius plot shows a linear dependence of ln kTADF as a function of 1/T. The intercept is collectively determined by the S1 → T1 ISC rate constant (kISC) and the radiative rate of the singlet excited state (kfl). The radiative rate constant (kTADF) can be estimated from the emission quantum yield and lifetime
| (2) |
Using the fluorescence and phosphorescence rates and ∆𝐸ST values (Boltzmann analysis), the contribution of TADF and direct phosphorescence to the observed emission can be estimated using Eq. 3 (49).
| (3) |
Computational methods
All geometry optimizations and electronic property calculations were performed with the Gaussian 09 program package (50). The ground-state (S0) structures were optimized by the DFT method with the PBE0 (51) functional and mixed basis sets in which the SDD (52) pseudopotential set was used for metal atom (Pd) and the DEF2-SVP (53) basis set was used for the C, H, N, and Pd atoms (51). The excited-state properties were obtained by the TDDFT method at the same level with the S0 calculations. The SOCs were calculated using the PySOC code (54). No solvent was applied to all calculations, and the results were analyzed further with GaussView.
Device fabrication and characterization
The ITO-coated glass substrates were consecutively ultrasonicated with acetone, alcohol, and deionized water in turn, then desiccated in high-purity nitrogen gas flow, followed by 20 min ultraviolet (UV) light-ozone treatment in a UV-ozone surface processor (PL16 series, Sen Lights Corporation). The Liq as electron injection layer and Al as cathode layer were deposited at rates of 0.1 and 3 Å/s, respectively, in vacuum at a pressure of 5 × 10−5 Pa. The emitting area of the device is about 0.09 cm2. The current density–voltage–luminance (J-V-L), L-EQE curves, and electroluminescence spectra were measured using a Keithley 2400 source meter and an absolute EQE measurement system (C9920-12, Hamamatsu Photonics, Japan). For operational stability studies, the devices were encapsulated with glass lids by UV curing adhesive in a nitrogen-filled glove box and then taken out from the glove box for lifetime tests. The luminance of the working devices in the normal direction was measured using an OLED lifetime testing system (FS-MP64, Suzhou FSTAR Scientific Instrument Co. Ltd., China) under a constant current density.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (nos. 22271196 and 52130308), the Guangdong Basic and Applied Basic Research Foundation (nos. 2021A1515010175 and 2021A1515110392), the China Postdoctoral Science Foundation (2021M702254), and the Science, Technology and Innovation Commission of Shenzhen Municipality (nos. JCYJ20180507182244027 and ZDSYS20210623091813040). K.L. acknowledges support from the Department of Science and Technology of Guangdong Province (2019QN01C617).
Author contributions: K.L. conceived and directed the project. J.-G.Y. and X.F. synthesized all materials. J.-G.Y. performed photophysical measurements and prepared the draft of the paper. N.L. fabricated and tested the OLEDs. J.L. and M.-D.L. performed the femtosecond transient-absorption (fs-TA) studies. C.J. and J.Z. assisted some steady-state spectroscopy measurements. X.-F.S. and G.C. performed the theoretical calculations. K.L. and C.Y. analyzed all the data and wrote the paper. All authors discussed the results and commented on the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S19
Tables S1 to S9
REFERENCES AND NOTES
- 1.Baldo M. A., O'Brien D. F., You Y., Shoustikov A., Sibley S., Thompson M. E., Forrest S. R., Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395, 151–154 (1998). [Google Scholar]
- 2.Ma Y., Zhang H., Shen J., Che C.-M., Electroluminescence from triplet metal—Ligand charge-transfer excited state of transition metal complexes. Synth. Met. 94, 245–248 (1998). [Google Scholar]
- 3.Chi Y., Chou P.-T., Transition-metal phosphors with cyclometalating ligands: Fundamentals and applications. Chem. Soc. Rev. 39, 638–655 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Fan C., Yang C., Yellow/orange emissive heavy-metal complexes as phosphors in monochromatic and white organic light-emitting devices. Chem. Soc. Rev. 43, 6439–6469 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Evans R. C., Douglas P., Winscom C. J., Coordination complexes exhibiting room-temperature phosphorescence: Evaluation of their suitability as triplet emitters in organic light emitting diodes. Coord. Chem. Rev. 250, 2093–2126 (2006). [Google Scholar]
- 6.Li K., Tong G. S. M., Wan Q., Cheng G., Tong W.-Y., Ang W.-H., Kwong W.-L., Che C.-M., Highly phosphorescent platinum(ii) emitters: Photophysics, materials and biological applications. Chem. Sci. 7, 1653–1673 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yam V. W.-W., Law A. S.-Y., Luminescent d8 metal complexes of platinum(II) and gold(III): From photophysics to photofunctional materials and probes. Coord. Chem. Rev. 414, 213298 (2020). [Google Scholar]
- 8.Rausch A. F., Homeier H. H. H., Yersin H., Organometallic Pt(II) and Ir(III) triplet emitters for OLED applications and the role of spin-orbital coupling: A study based on high-resolution optical spectroscopy. Top Organomet. Chem. 29, 193–235 (2010). [Google Scholar]
- 9.Li K., Chen Y., Wang J., Yang C., Diverse emission properties of transition metal complexes beyond exclusive single phosphorescence and their wide applications. Coord. Chem. Rev. 433, 213755 (2021). [Google Scholar]
- 10.W.-P. To, Tong G. S.-M., Lu W., Ma C., Liu J., Chow A. L.-F., Che C.-M., Luminescent organogold(III) complexes with long-lived triplet excited states for light-induced oxidative C-H bond functionalization and hydrogen production. Angew. Chem. Int. Ed. 51, 2654–2657 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Kui S. C. F., Chow P. K., Cheng G., Kwok C.-C., Kwong C. L., Low K.-H., Che C.-M., Robust phosphorescent platinum(II) complexes with tetradentate O^N^C^N ligands: High efficiency OLEDs with excellent efficiency stability. Chem. Commun. 49, 1497–1499 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Wong Y.-W., Wong H.-L., Wong Y.-C., Chan M.-Y., Yam V. W.-W., Versatile synthesis of luminescent tetradentate cyclometalated alkynylgold(III) complexes and their application in solution-processable organic light-emitting devices. Angew. Chem. Int. Ed. 56, 302–305 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Sudheendran Swayamprabha S., Dubey D. K., Shahnawaz R. A., Kumar Yadav M. R., Nagar A., Sharma F.-C., Tung J.-H. J., Approaches for long lifetime organic light emitting diodes. Adv. Sci. 8, 2002254 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow P. K., Ma C.; W.-P. To, Tong G. S. M., Lai S.-L., Kui S. C. F., Kwok W.-M., Che C.-M., Strongly phosphorescent palladium(II) complexes of tetradentate ligands with mixed oxygen, carbon, and nitrogen donor atoms: Photophysics, photochemistry, and applications. Angew. Chem. Int. Ed. 52, 11775–11779 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Zhu Z.-Q., Fleetham T., Turner E., Li J., Harvesting all electrogenerated excitons through metal assisted delayed fluorescent materials. Adv. Mater. 27, 2533–2537 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Cao L., Klimes K., Ji Y., Fleetham T., Li J., Efficient and stable organic light-emitting devices employing phosphorescent molecular aggregates. Nat. Photon. 15, 230–237 (2021). [Google Scholar]
- 17.Cao L., Zhu Z.-Q., Klimes K., Li J., Efficient and stable molecular-aggregate-based organic light-emitting diodes with judicious ligand design. Adv. Mater. 33, 2101423 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Uoyama H., Goushi K., Shizu K., Nomura H., Adachi C., Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Dias F. B., Bourdakos K. N., Jankus V., Moss K. C., Kamtekar K. T., Bhalla V., Santos J., Bryce M. R., Monkman A. P., Triplet harvesting with 100% efficiency by way of thermally activated delayed fluorescence in charge transfer OLED emitters. Adv. Mater. 25, 3707–3714 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Czerwieniec R., Yu J., Yersin H., Blue-light emission of Cu(I) complexes and singlet harvesting. Inorg. Chem. 50, 8293–8301 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Yersin H., Rausch A. F., Czerwieniec R., Hofbeck T., Fischer T., The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 255, 2622–2652 (2011). [Google Scholar]
- 22.Deaton J. C., Switalski S. C., Kondakov D. Y., Young R. H., Pawlik T. D., Giesen D. J., Harkins S. B., Miller A. J. M., Mickenberg S. F., Peters J. C., E-type delayed fluorescence of a phosphine-supported Cu2(μ-NAr2)2 diamond core: Harvesting singlet and triplet excitons in OLEDs. J. Am. Chem. Soc. 132, 9499–9508 (2010). [DOI] [PubMed] [Google Scholar]
- 23.W.-P. To, Cheng G., Tong G. S. M., Zhou D., Che C.-M., Recent advances in metal-TADF emitters and their application in organic light-emitting diodes. Front. Chem. 8, 653 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G., Zhu Z.-Q., Chen Q., Li J., Metal complex based delayed fluorescence materials. Org. Electron. 69, 135–152 (2019). [Google Scholar]
- 25.Mahoro G. U., Fernandez-Cestau J., Renaud J.-L., Coto P. B., Costa R. D., Gaillard S., Recent advances in solid-state lighting devices using transition metal complexes exhibiting thermally activated delayed fluorescent emission mechanism. Adv. Optical Mater. 8, 2000260 (2020). [Google Scholar]
- 26.Di D., Romanov A. S., Yang L., Richter J. M., Rivett J. P. H., Jones S., Thomas T. H., Abdi Jalebi M., Friend R. H., Linnolahti M., Bochmann M., Credgington D., High-performance light-emitting diodes based on carbene-metal-amides. Science 356, 159–163 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Hamze R., Peltier J. L., Sylvinson D., Jung M., Cardenas J., Haiges R., Soleilhavoup M., Jazzar R., Djurovich P. I., Bertrand G., Thompson M. E., Eliminating nonradiative decay in Cu(I) emitters: >99% quantum efficiency and microsecond lifetime. Science 363, 601–606 (2019). [DOI] [PubMed] [Google Scholar]
- 28.To W.-P., Zhou D., Tong G. S. M., Cheng G., Yang C., Che C.-M., Highly luminescent pincer gold(III) aryl emitters: Thermally activated delayed fluorescence and solution-processed OLEDs. Angew. Chem. Int. Ed. 56, 14036–14041 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Chan K.-T., Lam T.-L., Yu D., Du L., Phillips D. L., Kwong C.-L., Tong G. S.-M., Cheng G., Che C.-M., Strongly luminescent tungsten emitters with emission quantum yields of up to 84 %: TADF and high-efficiency molecular tungsten OLEDs. Angew. Chem. Int. Ed. 58, 14896–14900 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Wong C.-Y., Tang M.-C., Li L.-K., Leung M.-Y., Tang W.-K., Lai S.-L., Cheung W.-L., Ng M., Chan M.-Y., Yam V. W.-W., Carbazolylgold(III) complexes with thermally activated delayed fluorescence switched on by ligand manipulation as high efficiency organic light-emitting devices with small efficiency roll-offs. Chem. Sci. 13, 10129–10140 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pander P., Daniels R., Zaytsev A. V., Horn A., Sil A., Penfold T. J., Williams J. A. G., Kozhevnikov V. N., Dias F. B., Exceptionally fast radiative decay of a dinuclear platinum complex through thermally activated delayed fluorescence. Chem. Sci. 12, 6172–6180 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou D., Tong G. S. M., Cheng G., Tang Y.-K., Liu W., Ma D., Du L., Chen J.-R., Che C.-M., Stable tetradentate gold(III)‐TADF emitters with close to unity quantum yield and radiative decay rate constant of up to 2 × 106s−1: High‐efficiency green OLEDs with operational lifetime (LT90) longer than 1800 h at 1000 cd m−2. Adv. Mater. 34, 2206598 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Zhu Z.-Q., Park C.-D., Klimes K., Li J., Highly efficient blue OLEDs based on metal-assisted delayed fluorescence Pd(II) complexes. Adv. Opt. Mater. 7, 1801518 (2019). [Google Scholar]
- 34.Zach P. W., Freunberger S. A., Klimant I., Borisov S. M., Electron-deficient near-infrared Pt(II) and Pd(II) benzoporphyrins with dual phosphorescence and unusually efficient thermally activated delayed fluorescence: First demonstration of simultaneous oxygen and temperature sensing with a single emitter. ACS Appl. Mater. Interfaces 9, 38008–38023 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Li G., Chen Q., Zheng J., Wang Q., Zhan F., Lou W., Yang Y. -F., She Y., Metal-assisted delayed fluorescent Pd(II) complexes and phosphorescent Pt(II) complex based on [1,2,4]triazolo[4,3-a]pyridine-containing ligands: Synthesis, characterization, electrochemistry, photophysical studies, and application. Inorg. Chem. 58, 14349–14360 (2019). [DOI] [PubMed] [Google Scholar]
- 36.She Y., Xu K., Fang X., Yang Y.-F., Lou W., Hu Y., Zhang Q., Li G., Tetradentate platinum(II) and palladium(II) complexes containing fused 6/6/6 or 6/6/5 metallocycles with azacarbazolylcarbazole-based ligands. Inorg. Chem. 60, 12972–12983 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Feng X., Yang J.-G., Miao J., Zhong C., Yin X., Li N., Wu C., Zhang Q., Chen Y., Li K., Yang C., Au···H–C interactions support a thermally activated delayed fluorescence (TADF) gold(I) complex for OLEDs with little efficiency roll-off and good stability. Angew. Chem. Int. Ed. 61, e202209451 (2022). [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Miao J., Xiong J., Li K., Yang C., Rigid bridge-confined double-decker platinum(II) complexes towards high-performance red and near-infrared electroluminescence. Angew. Chem. Int. Ed. 61, e202113718 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Wu C., Liu W., Li K., Cheng G., Xiong J., Teng T., Che C.-M., Yang C., Face-to-face orientation of quasiplanar donor and acceptor enables highly efficient intramolecular exciplex fluorescence. Angew. Chem. Int. Ed. 60, 3994–3998 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Jiang C., Miao J., Zhang D., Wen Z., Yang C., Li K., Acceptor-donor-acceptor π-stacking boosts intramolecular through-space charge transfer towards efficient red TADF and high-performance OLEDs. Research 2022, 9892802 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu T., Chen Q., Independent gradient model based on hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 43, 539–555 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Lu T., Chen F., Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Li G., Zheng J., Zhao X., Fleetham T., Yang Y.-F., Wang Q., Zhan F., Zhang W., Fang K., Zhang Q., She Y., Tuning the excited state of tetradentate Pd(II) complexes for highly efficient deep-blue phosphorescent materials. Inorg. Chem. 59, 13502–13516 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Yang J.-G., Song X.-F., Cheng G., Wu S., Feng X., Cui G., To W.-P., Chang X., Chen Y., Che C.-M., Yang C., Li K., Conformational engineering of two-coordinate gold(I) complexes: Regulation of excited-state dynamics for efficient delayed fluorescence. ACS Appl. Mater. Interfaces 14, 13539–13549 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Lin J., Zou C., Zhang X., Gao Q., Suo S., Zhuo Q., Chang X., Xie M., Lu W., Highly phosphorescent organopalladium(ii) complexes with metal-metal-to-ligand charge-transfer excited states in fluid solutions. Dalton Trans. 48, 10417–10421 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Dey D., Bose A., Chakraborty M., Basu S., Magnetic field effect on photoinduced electron transfer between dibenzo[a,c]phenazine and different amines in acetonitrile−water mixture. J. Phys. Chem. A 111, 878–884 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Gernert M., Balles-Wolf L., Kerner F., Müller U., Schmiedel A., Holzapfel M., Marian C. M., Pflaum J., Lambert C., Steffen A., Cyclic (amino)(aryl)carbenes enter the field of chromophore ligands: Expanded π system leads to unusually deep red emitting CuI compounds. J. Am. Chem. Soc. 142, 8897–8909 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Hamze R., Shi S., Kapper S. C., Muthiah Ravinson D. S., Estergreen L., Jung M. C., Tadle A. C., Haiges R., Djurovich P. I., Peltier J. L., Jazzar R., Bertrand G., Bradforth S. E., Thompson M. E., “Quick-silver” from a systematic study of highly luminescent, two-coordinate, d10 coinage metal complexes. J. Am. Chem. Soc. 141, 8616–8626 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Czerwieniec R., Leitl M. J., Homeier H. H. H., Yersin H., Cu(I) complexes-thermally activated delayed fluorescence. Photophysical approach and material design. Coord. Chem. Rev. 325, 2–28 (2016). [Google Scholar]
- 50.M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö . Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian 09, Revision D.01 (Gaussian Inc., 2009).
- 51.Adamo C., Barone V., Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999). [Google Scholar]
- 52.Andrae D., Häußermann U., Dolg M., Stoll H., Preuß H., Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theoret. Chim. Acta. 77, 123–141 (1990). [Google Scholar]
- 53.Weigend F., Ahlrichs R., Balanced basis sets of split valence, triple ζ valence and quadruple ζ valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Gao X., Bai S., Fazzi D., Niehaus T., Barbatti M., Thiel W., Evaluation of spin-orbit couplings with linear-response time-dependent density functional methods. J. Chem. Theory Comput. 13, 515–524 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S19
Tables S1 to S9