Implications.
α-Lactalbumin, a milk protein, had evolved from c-type lysozyme, and it can associate with β4-galactosyltransferase 1 within the mammary epithelial cells. The acceptor specificity of this enzyme had changed by result of the association of two proteins to biosynthesize lactose.
Milk oligosaccharides are biosynthesized in addition to lactose, as lactose is the preferred acceptor for several glycosyltransferases present within the cells.
Milk oligosaccharides can be utilized as significant energy sources for the suckling neonates of monotremes and marsupials even though the small intestinal mucosa of these neonates lacks a lactase.
Lactose has become a predominant saccharide in the milk of most eutherians, resulting from a hypothetical increase in the expression level of α-lactalbumin. It has become to be utilized as a significant energy source for the suckling neonates, as a result of the acquisition of lactase in the microvilli of small intestinal cells.
Human milk contains the oligosaccharides at significant concentrations along with lactose among eutherians, which should be advantageous for symbiosis with beneficial colonic bifidobacteria.
Introduction
Among vertebrates, the only feature that distinguishes mammals is that they secrete milk. Living mammals consist of monotremes, marsupials, and eutherians. Monotremes only have oviparous mode reproduction, whereas marsupials have a short gestation period as they do not have the developed placenta and the females give birth to extremely immature newborns. Immediately after birth, the new-born kangaroo (a marsupial) enters the mother’s pouch to reach one of the nipples, and then, milk secretion starts from only this nipple among the four nipples. Eutherian females have a developed placenta to have a long gestation, and mothers deliver rather developed newborns. In eutherians, the nutrients are transferred from mother to fetus via placenta, while in marsupials the immature newborns receive them by suckling the mother’s milk after birth.
It is hypothesized that during the evolutionary step from the ancestor to mammals, the mammary gland had evolved from the ancestral gland to establish lactation step by step, while the milk-specific components, including caseins, α-lactalbumin, β-lactoglobulin, and lactose, would have been acquired at some stages during this evolutionary step.
It is speculated that the milk-specific proteins were acquired by molecular evolution from the other proteins, which existed in body fluids or body tissues in mammals and other vertebrates. It is interesting to perform investigations for determining the proteins from which milk-specific proteins evolved. Kawasaki (2011) of Pennsylvania State University has hypothesized that αs-, β-, and κ-caseins evolved from the enamel proteins in teeth, as the ancestral proteins, based on a comparison of these DNA sequences.
It has been found that α-lactalbumin, a milk protein, resembles lysozyme, which exists in body fluids, such as tear, saliva, and milk, in primary and tertiary structures (McKenzie and White, 1991). Lysozyme is an enzyme that cleaves the bond between N-acetylglucosamine and muramic acid of peptidoglycans of bacterial cell walls. It functions as an anti-infective agent against pathogenic bacteria. As lysozyme is an older protein that exists in avian egg whites and mammalian body fluids, the mammalian-specific new protein, α-lactalbumin, should have evolved from lysozyme. As described below, α-lactalbumin assembles with β4-galactosyltransferase 1 for the biosynthesis of lactose, a specific carbohydrate in milk (Rajput et al., 1996). There is no doubt that the acquisition of α-lactalbumin and caseins is a critical event for the establishment of lactation during the evolutionary step.
α-Lactalbumins
α-Lactalbumins are metalloproteins capable of binding different monovalent, divalent, and trivalent metal ions such as Na+, K+, Ca2+, Sc3+, Mn2+, Co2+, Cu2+, Zn2+, Sr2+, Y3+, Cd2+, Ba3+, Pb2+, La3+, Tb3+, and Lu3+ (Segawa and Sugai, 1983; Permyakov et al, 1985; Acharya et al, 1989; Ren et al, 1993; Aramini et al, 1996). Among them, Ca2+ is most commonly found in crystal structures of α-lactalbumins. There are two different Ca2+-binding sites, namely high affinity Ca2+-binding and low affinity Ca2+-binding sites. High affinity Ca2+-binding site is constituted of four or five aspartic acid residues with a conserved motif of Lys-x-x-Asp-Asp-Asp/Glu/Asn-x-x-Asp-Asp, consisting so-called “Ca2+-binding elbow”. Calcium ion is not necessarily required for the lactose synthase activity, but it significantly increase folding stability of α-lactalbumin. Ca2+-binding elbow is not found in mammalian lysozymes, but it is conserved among bird lysozymes. Hence, Nitta et al. (1988) assumed that gene duplication had occurred on Ca2+-binding-lysozyme-coding gene before splitting of avian and mammalian lineages and that the Ca2+-binding lysozyme is the ancestral protein of α-lactalbumin. It is also assumed that Ca2+-binding ability was lost during evolution among conventional (non-Ca2+ binding) lysozymes in mammalian species (Grobler et al., 1994). Whereas Zn2+ was found in α-lactalbumin to bind in a cleft located at the opposite side of Ca2+-binding elbow. Catalytic Glu-35 and Asp-53 are located in the corresponding cleft in c-type lysozyme (Ren et al., 1993). In fact, Zn2+ can bind to the cleft in c-type lysozyme, resulting in inhibition of the enzymatic, i.e., bacterial peptide glycan—hydrolyzing activity (Ostroy et al., 1978). Binding of Zn2+ induces a time-dependent conformational change in α-lactalbumin, and this change promotes lactose synthase activity (Kronman et al., 1981).
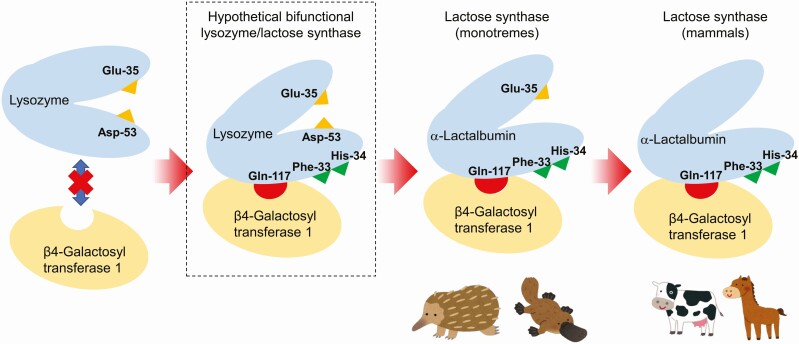
Urashima et al. (2012) proposed the hypothesis of the molecular evolution of c-type lysozyme to α-lactalbumin, as shown in Figure 1. The mutation occurred in lysozyme at positions that were not the catalytic sites, and then, at a time, the hypothetical bifunctional protein, which had two functions of lysozyme and α-lactalbumin, was acquired. As a result of the additional mutation Asp-53 was lost, while Glu-35 was retained. This protein should be equivalent to α-lactalbumins of living monotremes. Subsequently, Glu-35 was lost due to the additional mutation. The resulting proteins must be equivalent to α-lactalbumins of living marsupials and eutherians. It has been reported that the hypothetical bifunctional protein-like protein was separated from the milk of an echidna caught on Kangaroo Island, South Australia, in 1974 (Hopper and McKenzie, 1974), but this finding has never been reproduced since that time.
Figure 1.
Schematic picture of a hypothetical scenario of molecular evolution from lysozyme to α-lactalbumin in complex with β4-galactosyl transferase 1 (lactose synthase). Based on the observation that monotremes maintain Glu-35, a catalytic residue of lysozymes, it is hypothesized that acquisition of amino acid residues such as Gln-117 (hydrogen bonding to β4-galactosyl transferase 1), Phe-33 (forming the hydrophobic pocket), and His-34 (forming the hydrophobic pocket, hydrogen bounding to donor glucose) had occurred before entire loss of catalytic activity of lysozyme. From this point of view, presence of a hypothetical bifunctional lysozyme/lactose synthase can be predicted, but no clear evidence has yet found to date.
Which Is Predominant in Milk, Oligosaccharides or Lactose? What Is the Selective Advantage?
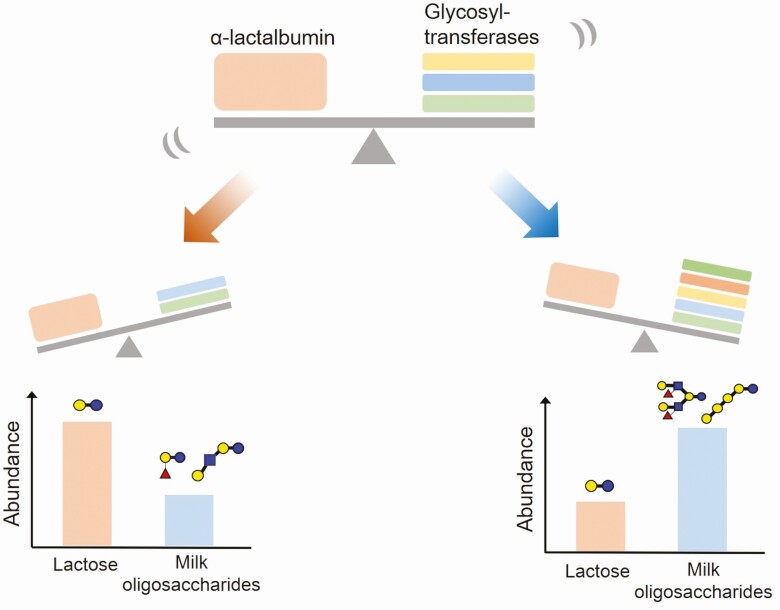
After the acquisition of α-lactalbumin, lactose biosynthesis started as a result of the assembly of this protein with β4-galactosyltransferase 1 within lactating mammary epithelial cells. As lactose could be the preferential acceptor substrate for several glycosyltransferases within these cells, the biosynthesis of milk oligosaccharides, which have a lactose unit at the reducing end, had also begun (Messer and Urashima, 2002; Urashima et al., 2022). Lactose can be co-present with oligosaccharides in the milk. The predominance of lactose or milk oligosaccharides in the carbohydrate fractions of milk should be controlled by the rate of lactose and milk oligosaccharide biosynthesis. If the reaction rate for the synthesis of lactose is greater than that of milk oligosaccharides, due to the increased expression of α-lactalbumin, lactose should be more abundant than oligosaccharides in milk. However, if the reaction rate for the biosynthesis of milk oligosaccharides is higher than that of lactose because of the higher activities of several glycosyltransferases, oligosaccharides should be predominant in milk. We assume that the key to this proposition is the balance between the expression rate of α-lactalbumin and several glycosyltransferases in the mammary epithelial cells (Figure 2).
Figure 2.
Which is predominant in milk, oligosaccharides or lactose? Lactose is biosynthesized as a result of the assembly of α-lactalbumin with β4-galactosyltransferase 1 within lactating mammary epithelial cells. As lactose could be the preferential acceptor substrate for several glycosyltransferases within these cells, the milk oligosaccharides, which have a lactose unit at the reducing end, are also biosynthesized. When the reaction rate for the synthesis of lactose is higher than that of milk oligosaccharides, due to the high expression of α-lactalbumin, lactose should be more abundant than oligosaccharides in milk, such as the eutherians. However, when the reaction rate for the biosynthesis of milk oligosaccharides is higher than that of lactose because of the higher activities of several glycosyltransferases, oligosaccharides should be predominant in milk, such as the monotremes and marsupials.
The ratio of milk oligosaccharides to lactose in milk varies among mammalian species (Messer and Urashima, 2002; Urashima et al., 2022). Milk oligosaccharides predominate over lactose in milk of monotremes (Messer and Kerry, 1973), marsupials (Messer and Green, 1979), and some species of eutherians, including bears (Urashima et al., 2020b), and raccoons (Urashima et al., 2020b), while lactose is a predominant saccharide in most eutherian milk (Jenness et al., 1964). In addition, even in the species in which lactose is predominant, the concentration of oligosaccharides in milk varies among species. For example, its concentration in human milk is 100 times higher than that in bovine milk (Urashima et al., 2013). The speculation that the concentrations of oligosaccharides and lactose in milk must be related to their physiological significance for neonates has attracted attention toward mammalian evolution and the living strategies of animals, such as the energy acquisition method by the suckling neonates and symbiosis with colonic beneficial bacteria.
Small Intestinal Digestion of Milk Oligosaccharides in Suckling Young of Monotremes and Marsupials
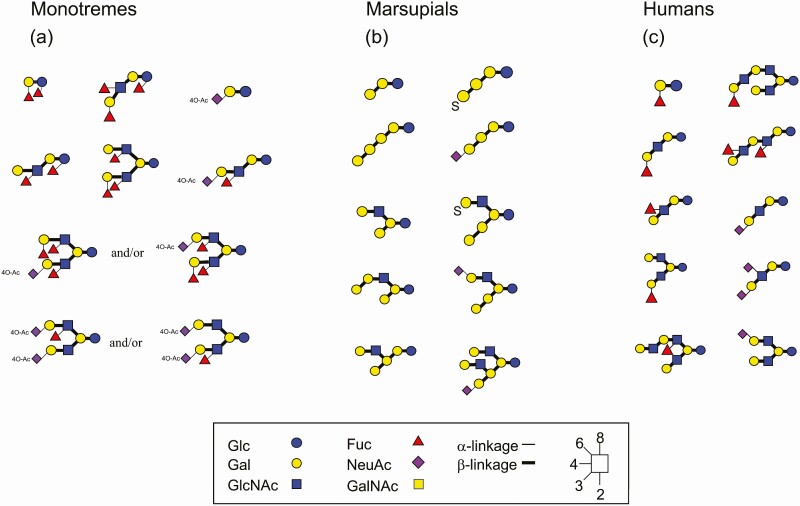
The milk oligosaccharides of monotremes of platypus and echidna as well as monotremes, including tammar wallaby, red kangaroo, common brushtail possum, koala, common wombat, eastern quoll, and tiger quoll, were characterized (Urashima et al., 2017). Some of them are shown in Figure 3a and b.
Figure 3.
Some milk oligosaccharides of monotremes, marsupials and humans. CGF symbols are used to express individual monosaccharides and different colors, whereas different glycosidic linkages are shown by different bond angles in a clockwise format; i.e., 1-2 linkage (6:00 O’clock), 1-3 (7:30), 1-4 (9:00), and 1-6 (10:30). On the other hand, α and β anomers are represented by thin and thick lines, respectively.
As described above, milk oligosaccharides predominate over lactose in the milk of monotremes and marsupials during the early and middle lactation stages. Because the milk oligosaccharides predominate over lactose in the milk of monotremes, which have the ancestral characters not shared by other mammals, such as oviparous mode reproduction and cervical ribs and dwarf nephrons, it is hypothesized that the oligosaccharides would have been predominant saccharides in milk or milk-like secretions of primitive early mammals (Messer and Urashima, 2002; Urashima et al., 2022).
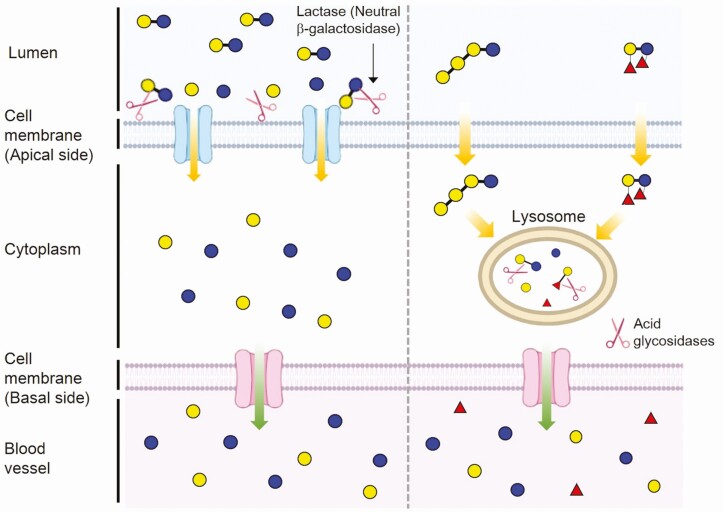
What is the physiological significance of milk oligosaccharides for the suckling young monotremes and marsupials? In eutherian neonates, lactose, a dominant saccharide in milk, is hydrolyzed by lactase in the microvilli of small intestinal epithelial cells to glucose and galactose; these monosaccharides are absorbed by the active transport system into the cell when they drink their mother’s milk. Glucose enters the circulation to be utilized as an energy source, whereas galactose is converted to glucose in the liver (Figure 4, left). This indicates that lactose is a significant energy source for young eutherians.
Figure 4.
The digestion and absorption of lactose by the suckling neonates of eutherians (left) and those of milk oligosaccharides by the neonates of monotremes and marsupials (right). (left) The predominant saccharide, lactose, is split into glucose and galactose by an intestinal lactase (neutral β-galactosidase) which is located in the membrane of the microvilli of the small intestinal brush border, and these monosaccharides are transported into the enterocytes via specific mechanisms. (right) The milk oligosaccharides enter the small intestinal cells via pinocytosis or endocytosis and are transferred to lysosomes in which they are hydrolyzed to monosaccharides by several glycosidases.
It has been found that the activity of small intestinal lactase is lacking in the suckling neonates of monotremes (Stewart et al., 1983) and Macropodidae marsupials (Walcott and Messer, 1980; Crisp et al., 1987; Messer et al., 1989), including wallabies and kangaroos. Based on the histochemical observations of the small intestine of the suckling echidna and tammar wallaby, it has been concluded that the milk oligosaccharides are transported by pinocytosis or endocytosis into the small intestinal cells and then enclosed in the supranuclear vacuoles to be moved to the lysosome. Oligosaccharides are then hydrolyzed to monosaccharides by lysosomal acidic glycosidases (Messer and Urashima, 2002; Urashima et al., 2022) (Figure 4, right). It is speculated that the oligosaccharides are utilized by the suckling young as an energy source; this mechanism must differ from that of lactose in milk by the eutherian young.
However, the activity of small intestinal lactase has been found in suckling brushtail possums during the late lactation stage (Crisp et al., 1989). It is no contradiction that lactose predominates over oligosaccharides in the mother’s milk of this species during late lactation (Crisp et al., 1989). This suggests that young brushtail possums mainly use milk oligosaccharides as the energy source in the early and middle suckling stages, while preferring lactose only in the late stage. The finding that small intestinal lactase activity exists even over a short period in the suckling brushtail possum indicates that this enzyme should have been acquired in the common ancestor of marsupials and eutherians after the divergence of monotremes. It is hypothesized that the young macropods and other marsupials in most suckling stages should prefer oligosaccharides to lactose in the mother’s milk as their energy source (Urashima et al., 2022).
What is the advantage for young marsupial in utilising oligosaccharides rather than lactose in milks? The osmotic pressure of the aqueous solution of the saccharides is expressed by the equation of πv = nRT (π: osmotic pressure in the solution, v: volume of the solution, n: molar concentration of the solute, R: proportional constant, T: absolute temperature). The osmotic pressure was proportional to the molar concentration of the solution. Because the molecular weights of monosaccharides and disaccharides are 180 and 342, respectively, the osmotic pressure of the aqueous solution of the monosaccharide is twice that of the disaccharide at 180 g/L. This shows that the osmotic pressure is less in the aqueous solution of the milk oligosaccharides than in that of lactose at the same w/w concentration. Even though tammar milk contains 14% of carbohydrates at the greatest concentration during the lactation period (Messer and Green, 1979), the osmotic pressure must not be so high because the milk predominantly contains oligosaccharides. The predominance of oligosaccharides over lactose is advantageous for marsupial and monotreme young because they function as a significant energy source in milk without stress due to high osmotic pressure (Urashima et al., 2022). It has been hypothesized that this is a selective advantage for survival.
Physiological Significance of Human Milk Oligosaccharides
It is hypothesized that lactose has become a dominant saccharide in eutherian milk, probably due to the increase in the expression of α-lactalbumin in the lactating mammary glands. As described above, suckling eutherian young use lactose, but not milk oligosaccharides, as the energy source in the mother’s milk using intestinal lactase in the digestive tract. Even though milk oligosaccharides are not digested and absorbed in the small intestine in most eutherian young, the milk of some eutherian species, including humans, still contains milk oligosaccharides as well as the predominant lactose. Why have the oligosaccharides remained in the eutherian milk, even though these have no direct nutritional values for the young? What are the advantages of eutherian milk containing oligosaccharides? We discuss this by highlighting a human case.
Human mature milk and colostrum contain 1.2-1.3% and 2.2-2.4% of human milk oligosaccharides (HMOs), respectively, that constitute the third largest solid component after lactose and lipid. Accordingly, while lactose accounts for 80 % of the free carbohydrates in human milk, HMOs comprise up to 20% of them (Urashima et al., 2018, 2020a). Thus, HMOs should not be considered a minor component of human milk. To date, approximately 250 molecular species of HMOs have been separated, and more than 200 chemical structures have been characterized (Urashima et al., 2018, 2020a). Some of the representing HMOs are shown in Figure 3c.
A large portion of HMOs reach the colon without being absorbed in the small intestine (Engfer et al., 2000). HMOs thus do not serve as the energy source for suckling infants, but instead they exert a prebiotic effect in the intestine and promote the growth of specific beneficial bacteria, especially bifidobacteria. Indeed, the colonic microbiota of breastfed infants is generally rich in HMO-utilizing bifidobacteria (Sakanaka et al., 2019), which is directly linked with the increased amounts of short-chain fatty acids and aromatic lactic acids in the intestines, thereby consequently benefiting human health (Henrick et al., 2021; Laursen et al., 2021). Thus, the ability of bifidobacteria to utilize HMOs should provide a selective advantage for the bacteria to establish a symbiotic relationship with humans during infancy.
The metabolic pathways of bifidobacteria to utilize HMOs have been elucidated mainly in the last 20 years. The enzymes characterized so far to be responsible for HMO utilization are 1,2-α-l-fucosidase, 1,3/4-α-l-fucosidase, 2,3/6-α-sialidase, lacto-N-biosidase, 1,4-β-galactosidase, 1,3-β-galactosidase, and β-N-acetylglucosaminidase, galacto-N-biose/lacto-N-biose I phosphorylase, fucosyllactose transporters, LNnT transporter, and transporters for HMO degradation products (Kitaoka et al., 2012; Sakanaka et al., 2020). The homology search against the public database showed that the homologues of the HMO utilization genes are almost exclusively present in the genomes of infant-type Bifidobacterium species (Bifidobacterium bifidum, Bifidobacterium longum subspecies infantis, Bifidobacterium breve, B. longum subspecies longum, Bifidobacterium catenulatum subspecies kashiwanohense, and Bifidobacterium pseudocatenulatum). However, the prevalence of these homologues considerably varies not only at the species level but also at the strain level, indicating that bifidobacteria have acquired the varied HMO utilization pathways during co-evolution with humans (Sakanaka et al., 2020). In general, B. bifidum and B. longum subspecies infantis are equipped with the gene sets required for utilization of almost all HMO molecules. By contrast, B. breve, B. longum subspecies longum, B. catenulatum subspecies kashiwanohense, and B. pseudocatenulatum carry a limited set of genes required for HMO utilization.
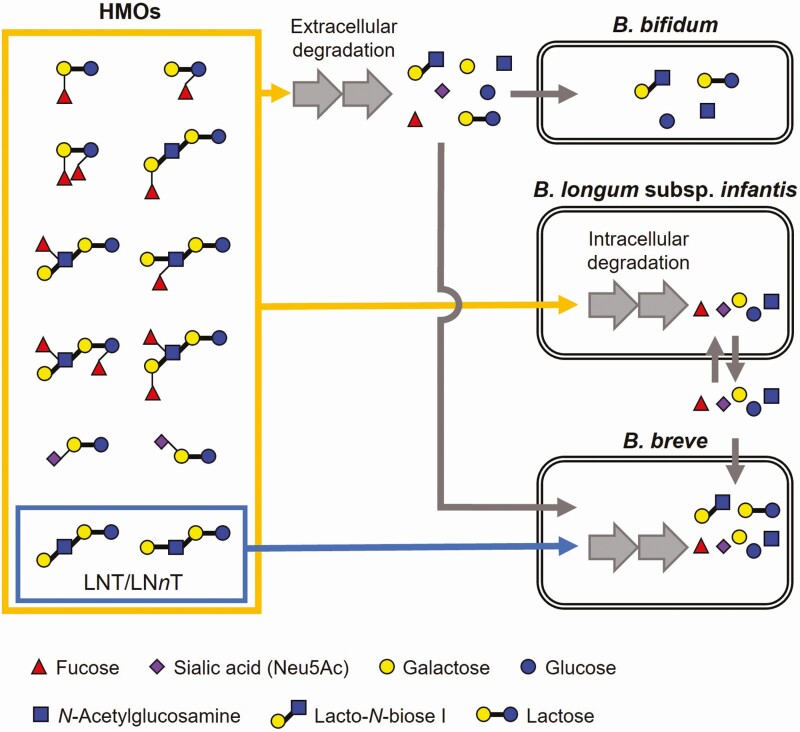
Infant-type bifidobacteria have evolved two distinct strategies to digest HMOs, i.e., extracellular or intracellular glycosidase-dependent types (Figure 5). Bifidobacterium bifidum has a repertoire of cell wall-anchored glycosidases, which enables the bacterium to liberate mono- and disaccharides Neu5Ac, fucose, lactose, lacto-N-biose I [Galβ1-3GlcNAc, LNB], Gal, GlcNAc, and Glc from HMOs outside the cells (Sakanaka et al., 2020). Interestingly, B. bifidum does not utilize all of the degraded sugars and leaves Neu5Ac, fucose, and Gal unconsumed due to the lack of specific transporters for these monosaccharides. Accordingly, B. bifidum shares these HMO degradation products with other Bifidobacterium species (Tannock et al., 2013; Gotoh et al., 2018; Ojima et al., 2022a).
Figure 5.
HMO utilization strategies adopted by B. bifidum, B. longum subspecies infantis, and B. breve, representative infant-type species. B. bifidum extracellularly degrades HMOs into mono- and disaccharides to assimilate them by itself and also to cross-feed the degradants to other bifidobacteria, especially B. breve. B. longum subspecies infantis import almost all HMOs as intact forms and degrades them inside the cells. During the process, a part of the liberated monosaccharides is expelled from the cell, which is then preferentially utilized by B. breve to outcompete B. longum subspecies infantis. The ability of B. breve to rapidly consume mono- and disaccharides in the environment could partly explain the dominance and prevalence of B. breve in the intestine of breastfed infants living the industrialized lifestyle (Ojima et al., 2022a; Olm et al., 2022).
Bifidobacterium longum subspecies infantis harbors a set of ATP-binding cassette transporters and intracellular glycosidases that confers to this subspecies the ability to utilize almost all HMO molecules (Sela et al., 2008; Sakanaka et al., 2020). Thus, the subspecies internalizes the HMO molecules as intact forms (Figure 5). This apparently selfish characteristic enables B. longum subspecies infantis to dominate the infant colonic microbiota in some cases (Olm et al., 2022). This subspecies is however hardly detected in infants living industrialized lifestyles, and in that case, B. breve, whose HMO utilization ability is essentially limited to LNT and LNnT only, is alternatively found to dominate the infant colonic microbiota regardless of the presence of B. bifidum. A recent study showed that B. breve outcompetes B. longum subspecies infantis when B. breve arrives earlier than or at the same time as B. longum subspecies infantis in an HMO-rich environment (Ojima et al., 2022a). B. breve can benefit from the so-called priority effect by utilizing the monosaccharides, especially fucose, that are transiently released from B. longum subspecies infantis cells during the HMO assimilation process (Figure 5).
B. longum subspecies longum, B. catenulatum subspecies kashiwanohense, and B. pseudocatenulatum adopt intracellular HMO degradation strategy, similar to B. longum subspecies infantis (Sakanaka et al., 2020). One exception is lacto-N-biosidase-positive strains of B. longum subspecies longum, which degrades LNT extracellularly into LNB and lactose like B. bifidum (Yamada et al., 2017). Bifidobacterium catenulatum subspecies kashiwanohense and B. pseudocatenulatum show overlapping but distinct preferences for fucosyl HMOs over other HMOs, for which specific variants of fucosyllactose transporter homologues are responsible (Sakanaka et al., 2019; Ojima et al., 2022b). The functions (specificities) of HMO transporters are thus distantly diversified even in the same homologue. Although we have no clear answer to the question about how diversified specificities of the transporter influence the microbiota formation and composition, it may be an evolutionary consequence within Bifidobacterium species/strains to share the niches in the infant colonic ecosystems.
In addition, it has been recently found that HMOs have the other biological functions of inhibiting infections by pathogenic viruses and bacteria, strengthening colonic barrier function, coordinating immune modulation, and stimulating brain activity (Urashima et al., 2020a, 2021b). Determination of the biofunction of human milk oligosaccharides will contribute to the development of the functional foods, including infant formulas.
Few Cases Where Oligosaccharides Predominate Over Lactose in the Milk of Eutherian Species
As described above, oligosaccharides predominate over lactose in the milk of monotremes and marsupials, while lactose predominates over milk oligosaccharides in most eutherians. However, some eutherian milks have been found to contain oligosaccharides as the predominant carbohydrates (Messer and Urashima, 2002; Urashima et al., 2020b). Among the species of Caniformia including bears (Urashima et al., 2020b), seals (Urashima et al., 2020b), raccoons (Urashima et al., 2020b), minks (Urashima et al., 2020b), and skunks (Urashima et al., 2020b), oligosaccharides predominate over lactose in their milk; in contrast, it has been shown that lactose is more dominant than the oligosaccharides in house dog milk (Bubb et al., 1999). The high ratio of milk oligosaccharides to lactose is conspicuous in Ursidae and Procyonidae (Urashima et al., 2020b).
Why is the high ratio of milk oligosaccharides to lactose in milk specific to Caniformia species among eutherians? The suckling neonates of some species prefer fats to carbohydrates in the milk as energy for their growth, while those of other species prefer carbohydrates. The preference for fats or carbohydrates varies depending on species. It has been shown that there is a tendency for high-fat milk to contain low concentrations of saccharides. The high dependence on milk fats as an energy source should be related to the adaptive strategy for the living environment of the animals. The lactation period is very short in seals, and the rate of increase in the body weight of the young is high. This is because the young must store subcutaneous fat quickly, to maintain their body temperature in marine environments. High-fat milk is preferable for rapid storage of subcutaneous fat in young sucklings (Oftedal et al., 1996).
Bears deliver newborns when they are hibernating with fasting. Although mothers do not have access to food, they must maintain a constant blood sugar concentration for survival. Due to this physiological restriction, it is advantageous for the female to give a high concentration of fats in milk to the young rather than the carbohydrates, as they have a rich store in subcutaneous fat (Ramsay and Dunbrack 1986; Oftedal et al., 1993).
The seal and bear young do not depend on milk carbohydrates for energy. It is assumed that a decrease in the concentrations of milk carbohydrates is achieved by the downregulation of α-lactalbumin in lactating mammary glands. It is hypothesized that as the activities of the glycosyltransferases remain in the mammary glands, the milk produced contains a high ratio of oligosaccharides to lactose.
Thus, the reason for the high ratio of oligosaccharides to lactose in milk is explained in the case of bears and seals, but it may not be applicable for raccoons and other Caniformia species. Why is this notable feature specific to the Caniformia species of Carnivora among eutherians? This aspect must be explored in future studies. Only the milk of domestic dogs contains more lactose than oligosaccharides among the Caniformia. This might be related to their breeding by humans (Urashima et al., 2021a).
Conclusion
α-Lactalbumin, which evolved from c-type lysozyme, assembled with β4-galactosyltransferase 1, and then, the substrate specificity of the transferase changes from GlcNAc to Glc to cause the biosynthesis of lactose. As the lactose unit is the preferential substrate for some glycosyltransferases in mammary cells, the biosynthesis of milk oligosaccharides, which have a reducing lactose unit, takes place. Because oligosaccharides predominate over lactose in the milk of living monotremes, which have the primitive features of the ancestor mammals, it is hypothesized that oligosaccharides had predominated over lactose in the milk-like secretions of the common ancestors.
The predominant milk oligosaccharides are utilized as an energy source in suckling monotremes and marsupials. Oligosaccharides are absorbed by pinocytosis or endocytosis in the small intestinal cells and then transported into the lysosome to be digested by the actions of acid glycosidases to monosaccharides. This metabolic system of oligosaccharides differs from that of lactose, which uses lactase in the microvilli of epithelial cells in eutherian neonates. The predominance of oligosaccharides over lactose is advantageous for marsupial and monotreme young because they function as a significant energy source in milk without stress due to high osmotic pressure.
In eutherians, lactose has become a dominant saccharide in milk, probably because of an increase in the expression levels of α-lactalbumin in lactating mammary glands. Lactose is utilized as an energy source for neonates by digestion with lactase in the small intestine. It is speculated that lactase was acquired in the common ancestor of marsupials and eutherians, but only eutherians and some marsupial species at the late lactation stage use this enzyme for the digestion of lactose in milk. In eutherians, milk oligosaccharides are not absorbed and utilized as energy sources by the young, but they remain in the milk. Their concentrations in milk vary depending on the eutherian species.
In humans, milk oligosaccharides are utilized by bifidobacteria to colonize the infant colon. By the acquisition of genes encoding transporters and glycosidases, which are related to the metabolism of human milk oligosaccharides, infant-type bifidobacteria could colonize the colon.
A trial to explore the evolution of lactose and milk oligosaccharides will continue along with the evolution of the mammals.
Acknowledgement
We thank Dr. Michael Messer of the University of Sydney for the collaboration study for 30 years.
About the Authors
Tadasu Urashima was born in 1957 in Hiroshima, Japan and graduated from the School of Agriculture, Tokyo University of Agriculture and Technology in 1980. He received his PhD from Tohoku University, Graduate School of Agriculture in 1986. He started his professional career in 1986 as an Assistant Professor at Obihiro University of Agriculture and Veterinary Medicine and promoted to Associate Professor in 1994. He was a Professor at Obihiro University since 2003 to 2022, and then has been a honorary Professor. His main research interests are in comparative aspects of milk oligosaccharides in a variety of mammalian species.

Risa Horiuchi is an Assistant Professor in the Division of Food Science, Department of Life and Food Sciences at Obihiro University of Agriculture and Veterinary Medicine (Japan). She has a PhD in life science from the Toyo University. Her research interests focus on the structure–function relationship analysis of oligosaccharides and glycans in milk.

Mikiyasu Sakanaka is a microbiologist working as a Program-Specific Associate Professor at Kyoto University (Japan). His research focuses on the interplay, symbiosis, and co-evolution between gut microbes and the host, mediated by dietary compounds. He received PhD from Graduate School of Agriculture, Hokkaido University (Japan) in 2015.

Takane Katayama graduated from Kyoto University in 1994 and received his PhD in the field of applied molecular microbiology under the supervision of Professor Hidehiko Kumagai at Kyoto University. The title of his doctoral thesis is “Studies on expression of tyrosine phenol-lyase gene in Erwinia herbicola”. After the postdoctoral training at the same lab, he was appointed an assistant professor at Graduate School of Biostudies, Kyoto University in 2002, when he started research on sugar metabolism of the genus Bifidobacterium in the laboratory of Professor Kenji Yamamoto. He isolated novel glycosidase genes whose products act on human-derived glycans such as human milk oligosaccharides and mucin O-glycans. The findings prompted him to consider a host glycan-mediated symbiotic relationship between gut microbes and humans. He revealed that infant gut-associated Bifidobacterium species specifically possess a gene set dedicated for assimilation of human milk oligosaccharides, the third most abundant non-digestible component contained in human milk. His research has contributed to understanding the mechanism of how bifidobacteria-rich microbiota is formed in the gut of breastfed infants.

Kenji Fukuda received a PhD degree from Hokkaido University in 2002 under the supervision of Professor Atsuo Kimura whose research expertise was in the field of molecular enzymology. He worked as a Postdoc in Professor Birte Svensson’s laboratory at Carlsberg laboratory and Technical University of Denmark until the end of 2004. He started his career at Obihiro University of Agriculture and Veterinary Medicine in Japan from 2005, and then appointed to the current position in 2021. His research interests are in exploration, characterization, and application of bioactive substances such as oligosaccharides, polysaccharides, and peptides found in raw and fermented dairy products, under the concept of “One Health”.

Contributor Information
Tadasu Urashima, Department of Food and Life Science, Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Hokkaido 080-8555, Japan.
Risa Horiuchi, Department of Food and Life Science, Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Hokkaido 080-8555, Japan.
Mikiyasu Sakanaka, Graduate School of Biostudies, Kyoto University, Kyoto 606-8502, Japan.
Takane Katayama, Graduate School of Biostudies, Kyoto University, Kyoto 606-8502, Japan.
Kenji Fukuda, Department of Food and Life Science, Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Hokkaido 080-8555, Japan; Research Center for Global Agromedicine, Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Hokkaido 080-8555, Japan.
Conflict of interest statement. Employment of MS at Kyoto University is supported in part by Morinaga Milk Industry Co., Ltd. The authors declare no other conflict of interest.
References
- Acharya, K.R., Stuart D.I., Walker N.P., Lewis M., and Phillips D.C.. . 1989. Refined structure of baboon α-lactalbumin at 1.7 Å resolution. Comparison with C-type lysozyme. J. Mol. Biol. 208:99–127. doi: 10.1016/0022-2836(89)90091-0. [DOI] [PubMed] [Google Scholar]
- Aramini, J.M., Hiraoki T., Grace M.R., Swaddle T.W., Chiancone E., and Vogel H.J.. . 1996. NMR and stopped-flow studies of metal ion binding to α-lactalbumins. Biochim. Biophys. Acta 1293:72–82. doi: 10.1016/0167-4838(95)00223-5. [DOI] [PubMed] [Google Scholar]
- Bubb, W.A., Urashima T., Kohso K., Nakamura T., Arai I., and Saito T.. . 1999. Occurrence of an unusual lactose sulfate in dog milk. Carbohydr. Res. 318:123–128. doi: 10.1016/s0008-6215(99)00102-0. [DOI] [PubMed] [Google Scholar]
- Crisp, E.A., Cowan P.E., and Messer M.. . 1989. Intestinal lactase (β-galactosidase) and other disaccharidase activities of suckling and adult common brushtail possums, Trichosurus vulpecula (Marsupialia: Phalangeridae). Reprod. Fertil. Dev. 1(4):309–314. doi: 10.1071/rd9890309. [DOI] [PubMed] [Google Scholar]
- Crisp, E.A., Czolij, R.R., Messer, M. 1987. Absence of beta-galactosidase (lactase) activity from intestinal brush borders of suckling macropods: implications for mechanism of lactose absorption. Comp. Biochem. Physiol. B88: 923–927. doi: 10.1016/0305-0491(87)90265-3 [DOI] [PubMed] [Google Scholar]
- Engfer, M.B., Stahl B., Finke B., Sawatzki G., and Daniel H.. . 2000. Human milk oligosaccharides are resident to enzymatic hydrolysis in the upper gastrointestinal tract. Am. J. Clin. Nutr. 71:1589–1596. doi: 10.1093/ajcn/71.6.1589. [DOI] [PubMed] [Google Scholar]
- Gotoh, A., Katoh T., Sakanaka M., Ling Y., Yamada C., Asakuma S., Urashima T., Tomabechi Y., Katayama-Ikegami A., Kurihara S., . et al. 2018. Sharing of human milk oligosaccharides degradants within bifidobacterial communities in faecal cultures supplemented with Bifidobacterium bifidum. Sci. Rep. 8:13958–13971. doi: 10.1038/s41598-018-32080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobler, J.A., Rao K.R., Pervaiz S., and Brew K.. . 1994. Sequences of two highly divergent canine type c lysozymes: implications for the evolutionary origins of the lysozyme/α-lactalbumin superfamily. Arch. Biochem. Biophys. 313:360–366. doi: 10.1006/abbi.1994.1399. [DOI] [PubMed] [Google Scholar]
- Henrick, B.M., Rodriquez, L., Lakshmikanth, T.et al. 2021. Bifidobacteria – mediated immune system imprinting early in life. Cell 184(15): 3884–3898.e11. doi: 10.1016/j.cell.2021.05.030. [DOI] [PubMed] [Google Scholar]
- Hopper, K.E., and McKenzie H.A.. . 1974. Comparative studies of α-lactalbumin and lysozyme: echidna lysozyme. Mol. Cell. Biochem. 3:93–108. doi: 10.1007/BF01659181. [DOI] [PubMed] [Google Scholar]
- Jenness, R.E., Regehr E.A., and Sloan R.E.. . 1964. Comparative biochemical studies of milks -Ⅱ. Dialyzable carbohydrates. Comp Biochem Physiol 13:339–352. doi: 10.1016/0010-406x(64)90028-3. [DOI] [PubMed] [Google Scholar]
- Kawasaki, K., Lafont A., and Sire J.. . 2011. The evolution of milk casein genes from tooth genes before the origin of mammals. Mol. Biol. Evol. 28:2053–2061. doi: 10.1093/molbev/msr020. [DOI] [PubMed] [Google Scholar]
- Kitaoka, M. 2012. Bifidobacterial enzymes involved in the metabolism of human milk oligosaccharides. Adv. Nutr. 3:422S–429S. doi: 10.3945/an.111.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronman, M.J., Sinha S.K., and Brew K.. . 1981. Characteristics of the binding of Ca2+ and other divalent metal ions to bovine α-lactalbumin. J. Biol. Chem. 256:8582–8587. doi: 10.1016/S0021-9258(19)68884-8 [DOI] [PubMed] [Google Scholar]
- Laursen, M.F., Sakanaka M., von Burg N., Mörbe U., Andersen D., Moll J.M., Pekmez C.T., Rivollier A., Michaelsen K.F., Mølgaard C., . et al. 2021. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat. Microbiol. 6(11):1367–1382. doi: 10.1038/s41564-021-00970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie, H.A., and White F.H. Jr.. 1991. Lysozyme and α-lactalbumin: structure, function, and interrelationships. Adv. Protein Chem. 41:174–315. doi: 10.1016/s0065-3233(08)60198-9 [DOI] [PubMed] [Google Scholar]
- Messer, M., Crisp, E.A., Czolij, R. 1989. Lactose digestion in suckling macropods. In: Grigg, G., Jarman, P., Hume, I. editors. Kangaroos, Wallabies and Rat Kangaroos. Australia:Surry Beatty & Sons Pty Ltd, NSW, Vol 1, p. 217–221. [Google Scholar]
- Messer, M., and Green B.. 1979. Milk carbohydrates of marsupials Ⅱ. Quantative and qualitative changes in milk carbohydrates during lactation in the tammar wallaby (Macropus eugenii). Aust. J. Biol. Sci. 32:519–531. doi: 10.1071/bi9790519. [DOI] [PubMed] [Google Scholar]
- Messer, M., and Kerry K.R.. . 1973. Milk carbohydrate of the echidna and the platypus. Science 180:201–203. doi: 10.1126/science.180.4082.201. [DOI] [PubMed] [Google Scholar]
- Messer, M., and Urashima T.. . 2002. Evolution of milk oligosaccharides and lactose. Trends Glycosci. Glycotech. 14(77):153–176. doi: 10.4052/tigg.14.153. [DOI] [Google Scholar]
- Nitta, K., Tsuge H., Shimazaki K., and Sugai S.. . 1988. Calcium-binding lysozymes. Biol. Chem. Hoppe-Seyler 369:671–675. doi: 10.1515/bchm3.1988.369.2.671. [DOI] [PubMed] [Google Scholar]
- Oftedal, O.T., Alt G., Widdowson E.M., and Jukubasz M.R.. . 1993. Nutrition and growth of suckling black bears (Ursus americanus) during their mother’s winter fast. Br. J. Nutr. 70:59–79. doi: 10.1079/bjn19930105. [DOI] [PubMed] [Google Scholar]
- Oftedal, O.T., Bowen W.D., and Bonness D.J.. . 1996. Lactation performance and nutrient deposition in pups of the harp seal, Phoca groenlandica, on ice floes off southeast Labrador. Physiol. Zool. 69:635–657. .https://www.jstor.org/stable/30164220 [Google Scholar]
- Ojima, M.N., Asao Y., Nakajima A., Katoh T., Kitaoka M., Gotoh A., Hirose J., Urashima T., Fukiya S., Yokota A., . et al. 2022b. Diversification of a fucosyllactose transporter within the genus Bifidobacterium. Appl. Environ. Microbiol. 88(2):e0143721. doi: 10.1128/AEM.01437-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojima, M.N., Jiang, L., Arzamasov, A.A., et al. 2022a. Priority effect shape the structure of infant-type Bifidobacterium communities on human milk oligosaccharides. ISME J. 16:2265–2279. doi: 10.1038/s41396-022-01270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olm, M.R., Dahan D., Carter M.M., Merrill B.D., Yu F.B., Jain S., Meng X., Tripathi S., Wastyk H., Neff N., . et al. 2022. Robust variation in infant gut microbiome assembly across a spectrum of lifestyles. Science 376(6598):1220–1223. doi: 10.1126/science.abj2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroy, F., Gams R.A., Glickson J.D., and Lenkinski R.E.. . 1978. Inhibition of lysozyme by polyvalent metal ions. Biochim. Biophys. Acta 527:56–62. doi: 10.1016/0005-2744(78)90255-3. [DOI] [PubMed] [Google Scholar]
- Permyakov, E.A., Morozova L.A., and Burstein E.A.. . 1985. Cation binding effects on the pH, thermal and urea denaturation transitions in α-lactalbumin. Biophys. Chem. 21:21–31. doi: 10.1016/0301-4622(85)85003-1. [DOI] [PubMed] [Google Scholar]
- Rajput, B., Shaper N.L., and Shaper J.H.. . 1996. Transcriptional regulation of murine β1,4-galactosyltransferase in somatic cells. Analysis of a gene that serves both a housekeeping and a mammary gland-specific function. J. Biochem. 271:5131–5142. doi: 10.1074/jbc.271.9.5131. [DOI] [PubMed] [Google Scholar]
- Ramsay, M.A., and Dunbrack R.L.. . 1986. Physiological constraints on life history phenomena: The example of small bear cubs at birth. Am. Nat. 127:735–743. doi: 10.1086/284522. [DOI] [Google Scholar]
- Ren, J., Stuart D.I., and Acharya K.R.. . 1993. α-lactalbumin possesses a distinct zinc binding site. J. Biol. Chem. 268:19292–19298. doi: 10.1016/S0021-9258(19)36512-3. [DOI] [PubMed] [Google Scholar]
- Sakanaka, M., Gotoh A., Yoshida K., . et al. 2020. Varied pathways of infant gut-associated Bifidobacterium to assimilate human milk oligosaccharides: Prevalence of the gene set and its correlation with bifidobacteria-rich microbiota formation. Nutrients. 12:71–91. doi: 10.3390/nu12010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka, M., Hansen M.E., Gotoh A., Katoh T., Yoshida K., Odamaki T., Yachi H., Sugiyama Y., Kurihara S., Hirose J., . et al. 2019. Evolutionary adaptation in fucosyllactose uptake systems supports bifidobacteria-infant symbiosis. Sci. Adv. 5:eaaw7696. doi: 10.1126/sciadv.aaw7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa, T., and Sugai S.. . 1983. Interactions of divalent metal ions with bovine, human, and goat α-lactalbumins. J. Biochem. 93:1321–1328. doi: 10.1093/oxfordjournals.jbchem.a134266. [DOI] [PubMed] [Google Scholar]
- Sela, D.A., Chapman J., Adeuya A., . et al. 2008. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA 195:18964–18969. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, I.M., Messer M., Walcott P.J., Gadiel P.A., and Griffiths M.. . 1983. Intestinal glycosidase activities in one adult and two suckling echidnas: absence of a neutral lactase (β-D-galactosidase). Aust. J. Biol. Sci. 36:139–146. doi: 10.1071/bi9830139. [DOI] [PubMed] [Google Scholar]
- Tannock, G.W., Lawley B., Munro K., Gowri Pathmanathan S., Zhou S.J., Makrides M., Gibson R.A., Sullivan T., Prosser C.G., Lowry D., . et al. 2013. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl. Environ. Microbiol. 79(9):3040–3048. doi: 10.1128/AEM.03910-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urashima, T. 2021b. Science of human milk oligosaccharides. Glycoforum. 24(5):A14. doi: 10.322285/glycoforum.24A14. [DOI] [Google Scholar]
- Urashima, T., Fukuda K., and Messer M.. . 2012. Evolution of milk oligosaccharides and lactose: a hypothesis. Animal. 6(3):369–374. doi: 10.1017/S1751731111001248. [DOI] [PubMed] [Google Scholar]
- Urashima, T., Hirabayashi J., Sato S., and Kobata A.. . 2018. Human milk oligosaccharides as essential tools for basic and application studies on galectins. Trends Glycosci. Glycotechnol. 30(172):SE51–SE65. doi: 10.4052/tigg.1734.1SE. [DOI] [Google Scholar]
- Urashima, T., Katayama, T., Fukuda, K., Hirabayashi, J. 2020a. Human milk oligosaccharides and innate immunity. In: Barchi, J. Jr. editor. Comprehensive Glycoscience. 2nd ed. Elsevier. doi: 10.1016/B978-0-12-819475-1.00009-2. [DOI] [Google Scholar]
- Urashima, T., Katayama T., Sakanaka M., Fukuda K., and Messer M.. . 2022. Evolution of milk oligosaccharides: origin and selectivity of the ratio of milk oligosaccharides to Lactose among mammls. Biochim. Biophys. Acta – General Subjects. 1866:130012.doi: 10.1016/j.bbagen.2021.130012. [DOI] [PubMed] [Google Scholar]
- Urashima, T., Messer, M. 2017. Evolution of milk oligosaccharides and their function in monotremes and marsupials. In: Pontarotti, P. editor. Evolutionary Bsiology: self/nonself evolution, species and complex traits evolution, methods and concepts. Springer: Switzerland; p. 237–256.doi: 10.1007/978-3-319-61569-1_12. [DOI] [Google Scholar]
- Urashima, T., Mineguchi, Y., Fukuda, K.et al. 2020b. Evolution of milk oligosaccharides of carnivora and artiodactyla: Significance of the ratio of oligosaccharides to lactose in milk. In: Pontarotti, P. editor. Evolutionary Biology – a transdisciplinary approach. Springer: Switzerland; p. 359–377.doi: 10.1007/978-3-030-57246-4_15. [DOI] [Google Scholar]
- Urashima, T., Sato S., Nio-Kobayashi J., and Hirabayashi J.. . 2021a. Do milk oligosaccharides get altered under breeding condition? An underlying mechanism for structural conversion and diversification. Glycoforum. 24(4):A11. doi: 10.32285/glycoforum.24A11. [DOI] [Google Scholar]
- Urashima, T., Taufik E., Fukuda K., and Asakuma S.. . 2013. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci. Biotechnol. Biochem. 77:455–466. doi: 10.1271/bbb.120810. [DOI] [PubMed] [Google Scholar]
- Walcott, P.J., and Messer M.. . 1980. Intestinal lactase (β-galactosidase) and other glycosidase activities in suckling and adult tammar wallaby (Macropus eugenii). Aust. J. Biol. Sci. 33:521–530. doi: 10.1071/bi9800521. [DOI] [PubMed] [Google Scholar]
- Yamada, C., Gotoh A., Sakanaka M., M.Hattie,Stubbs K.A., Katayama-Ikegami A., Hirose J., Kurihara S., Arakawa T., Kitaoka M., . et al. 2017. Molecular insight into evolution of symbiosis between breast-fed infants and a member of the human gut microbiome Bifidobacterium longum. Cell Chem. Biol. 24(4): 515–524.e5.e5. doi: 10.1016/j.chembiol.2017.03.012. [DOI] [PubMed] [Google Scholar]