Abstract
The molecular mechanisms which govern the developmental specificity of human β-globin gene transcription have been studied in K562 cells, a human eyrthroleukemia line that expresses minimal β-globin. Protein-binding analysis reveals that the 5′ region contains three elements bound by trans-acting factors, beta-protein 1 (BP1) and beta-protein 2 (BP2). In vitro mutagenesis of each individual element in a beta-globin vector containing chloramphenicol acetyltransferase (pCAT) followed by transient transfection into K562 cells increased levels of CAT activity 5.5-fold higher than wild-type (wt) βCAT, consistent with their silencing role. Mutagenesis of all three elements, however, resulted in activity significantly lower than wt βCAT. BP1 and BP2 motifs have overlapping binding sites for high mobility group proteins (HMG1+2), DNA-bending factors, shown here to extrinsically bend the β-globin promoter. Theoretically, mutations in all beta-protein binding sites could affect the binding of HMG1+2 sufficiently to impede DNA–protein and/or protein–protein interactions needed to facilitate constitutive gene expression. Placing two turns of DNA between BP1 and BP2 motifs also increased expression 3-fold, indicative of spatial constraints required for optimal silencing. However, insertion of the HMG1+2 DNA-bending motif (also equivalent to two turns) facilitates β-silencing by re-establishment of BP1–BP2 proximity. Thus a combination of general DNA-bending and specific transcriptional factors appear to be involved in β-globin silencing in the embryonic/fetal erythroid stage.
INTRODUCTION
Investigations are underway to better elucidate the molecular mechanism(s) involved in tissue specificity and developmental regulation of the human β-globin gene. Interactions between DNA cis-acting elements and trans-acting regulatory factors are known to govern gene expression; however, the specific constellation of DNA sequences and soluble nuclear proteins involved in this regulation remain elusive.
The genes of the human beta-globin cluster are sequentially arranged reflecting their order of expression during development. The locus has been extensively mapped on chromosome 11 and encodes five functional genes: ɛ (embryonic stage), Gγ and Aγ (fetal stage), and δ and β (adult stage). Regions of high DNase I sensitivity, located far upstream of the adult β-globin gene extending upstream of the cluster itself, are collectively called the β-globin locus control region (βLCR), which has been shown to confer high-level, tissue-specific, copy-number-dependent, and integration-site independent gene expression (1). The βLCR is thought to interact at the individual promoter regions of downstream globin genes for developmentally-specific enhancement and regulation (2,3).
The specificity of βLCR hypersensitive sites (HS) interaction with individual β-globin promoters may be mediated by ubiquitous, tissue-specific or developmentally-specific trans-acting factors. An erythroid-specific activator of adult β-globin expression, the Erythroid Krüppel-like factor (EKLF), was identified and shown to direct high-level expression of the adult β-globin promoter in HEL, JK1 and OCIM1 erythroleukemic cell lines (4); however, its mere presence is not sufficient to activate the adult β-globin promoter in the primitive erythroid cell line K562, which expresses EKLF, yet does not express adult β-globin (5). Previously, we had shown that two factors, termed beta-protein 1 (BP1) and beta-protein 2 (BP2), appeared to function as repressors of adult β-globin gene transcription in K562 cells (6). BP1 protein was shown via competitive gel shift assay to bind to two separate sequence motifs: one between –553 and –527 bp upstream of the β-cap (DNA motif called BP1U) and the other between –302 and –294 bp (BP1 motif). The second factor, BP2, binds to the DNA sequence between –274 and –253 bp (BP2 motif). Overlapping and adjacent to BP1 and BP2 binding sites are the motifs for an architectural, chromosomal protein called high mobility group protein 1 and the closely related 2 (HMG1+2) (7). Structural features of HMG1+2 proteins have been well characterized (8–10) and include two hydrophilic domains each comprised of approximately 70 residues. Functionally, these factors activate the assembly of nucleoprotein complexes by interacting with DNA on the minor groove and sharply bending it (11). Such extrinsic, protein-induced bends in DNA appear to aggregate distant transcription factors bound at separate sequence motifs into close proximity. The synergism created between protein–protein complexes interacts with the basal initiation complex facilitating gene regulation (12–15).
In this study, we have focused on determining whether interactions between trans-acting factors BP1, BP2, and HMG1+2 and specific DNA elements in the 5′ flanking regulatory region of the adult β-globin gene contribute to the low level of β-globin expression in embryonic/fetal erythroid tissues. We have employed the K562 erythroleukemic cell line as a tissue culture model of embryonic/fetal erythroid development. Previous characterization of these cells revealed a very low level of adult β-globin gene expression, with high epsilon and gamma expression, as well as other primitive-stage erythroid characteristics such as i-antigen and lactate dehydrogenase isoenzyme expression (16,17). The lack of adult beta-globin expression is not due to any major β-globin gene deletions or rearrangements, since the β-globin gene cloned from these cells was demonstrated to be functional when used in heterologous expression systems such as COS or HeLa cells (18). Therefore, the differential expression of β-globin genes in K562 cells appears to be under the control of transcription factors; either (i) lack of functional activator(s) required for adult β-globin gene synthesis and/or (ii) abundance of in situ repressors that down-regulate adult expression.
Using in vitro mutagenesis of the DNA elements BP1U, BP1, BP2 and HMG1+2 followed by transient expression assays into hemin-induced K562 cells, we have examined the transcriptional regulatory activity of β-globin. Our studies confirm and extend the results of Berg and colleagues (6); the beta-protein elements are repressors of adult β-globin transcription. Additionally, the role of HMG1+2 as a DNA-bending factor for human adult β-globin gene was demonstrated. We propose a combinatorial model of silencing the expression of the human adult β-globin gene during the embryonic/fetal stages of development within the erythroid environment.
MATERIALS AND METHODS
DNA constructs
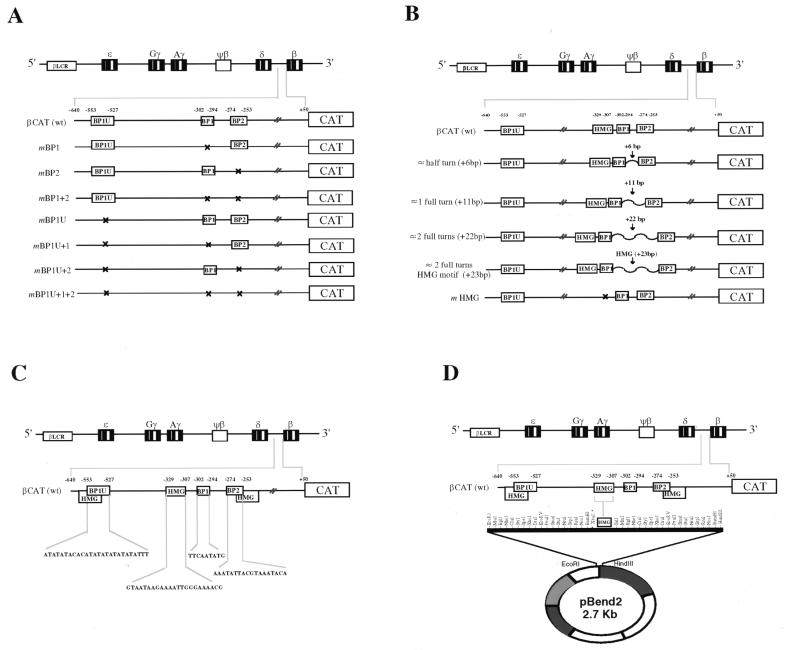
A series of point mutations within the 5′ upstream regulatory region of the adult beta-globin gene was constructed by a method originally described by Higuchi et al. (19). Mutations in the previously determined binding sites were introduced by two-step PCR site-specific mutagenesis. The BP1 upstream (BP1U) motif from position –553 to –527 was mutated to 5′-ACTAGGCCAGTCTGACTAGGCCTACCG-3′. The downstream BP1 (–302 to –294) and BP2 (–274 to –253) mutated sequences were 5′-TTGCTCGAC-3′ and 5′-TGCCGAGTCGGGCACGTT-3′, respectively. Lastly, mutations were also created in the HMG1+2 binding motif from position –329 to –307, 5′-TTCGGCAGTCATGAGACAACGTGC-3′. For each, bold-type print indicates the mutation, while the wild-type sequence is shown in Figure 1C for comparison. Primers corresponding to the binding sites to be mutated and encompassing 690 bp of the 5′ upstream regulatory region of β-globin (–640 to +50) were synthesized. Each mutated β-globin promoter was cloned upstream of the promoterless, enhancerless chloramphenicol acetyltransferase (CAT) gene (Fig. 1A). Subsequent insertion constructs were created to contain the addition of an approximate half turn (+6 bp), full turn (+11 bp) or two full turns (+22 bp) of random DNA (non-transcription factor-binding) sequences, respectively, between the downstream BP1 and BP2 sequence motifs contained in the pCAT vector. The addition of the 23 bp HMG1+2 sequence motif from β –329 to –307 (also corresponding to an approximate spacing of two full DNA turns) was also inserted to examine the effects of its re-introduction. In vitro mutagenesis of the HMG1+2 sequence motif was also performed and ligated into the pCAT vector (Fig. 1B). Sequences of the mutated and inserted cloned fragments were confirmed by standard sequencing methods.
Figure 1.
DNA constructs. DNA constructs are shown as schematic representations of the human β-globin gene cluster. (A) Wild-type (wt) and mutated βCAT constructs showing the mutations of the beta-protein sequence elements of the 5′ flanking regulatory region of the adult β-globin gene from –640 to +50 relative to the cap site (see text for details). (B) Insertional βCAT constructs of the same region as in (A), creating an approximate half, full or two full helical turns of DNA between the downstream BP1 and BP2 sequence motifs by the addition of 6, 11 or 22 bp of DNA corresponding to restriction endonuclease recognition sites. The insertion of the HMG1+2 motif also corresponds to roughly adding two full helical turns of DNA (details in text). (C) Wild-type BP1U, BP1, BP2 and HMG1+2 sequence motifs used as probes for EMSA. (D) Circular permutation assay construct. A blunt-ended 23 bp double-stranded oligonucleotide containing the HMG1+2 site (β-globin –329/–307) was cloned into the XbaI site of pBend2. Multiple digestions with different restriction enzymes produced fragments of the same length with the HMG1+2 motif located at different positions. Each was used as a probe for the circular permutation assay.
Tissue culture and transfections
K562 cells were maintained in 90% RPMI 1640 medium supplemented with 10% fetal bovine serum plus glutamine, penicillin and streptomycin (Biofluids, Rockville, MD) and maintained in a CO2 incubator. Transient transfections were carried out using 20 µg of each DNA construct. To normalize for the efficiency of transfection, a beta-galactosidase (β-gal) reporter gene driven by an SV40 promoter, pSV-β-galactosidase (Promega Corp., Madison, WI) was co-transfected as an internal control into the cells at one-tenth the concentration of the βCAT constructs. Both constructions were introduced into 3.2 × 107 K562 cells via electroporation (Bio-Rad, Hercules, CA) at a capacitance of 960 µF at 220 V. Following delivery of a single pulse, cell suspensions were chilled on ice for 15 min, then resuspended in RPMI media (as described above) with the addition of 30 mM hemin to induce hemoglobin synthesis (20). Cells were incubated at 37°C for 48 h before harvesting. Following harvest, cell lysates were extracted using a previously described method (6).
CAT assays
The protein concentrations of the cell lysates were measured by the Bio-Rad dye-binding assay (Bio-Rad, Hercules, CA). Fifty micrograms was assayed for β-gal activity to determine transfection efficiency. Additionally, 50 mg of supernatant was used for the CAT assay with the addition of 7 µl of 4 mM acetyl coenzyme A plus 4 µl of 14C chloramphenicol (50 µCi/ml; Dupont, NEN, Boston, MA). This mixture was allowed to incubate at 37°C for 15 h and was analyzed by thin layer chromatography (TLC) as previously described (6). After autoradiography, results were quantified by phosphoimaging (Fujifilm BAS-1500, Fuji Medical System, Stanford, CT). Each sample was normalized and expressed relative to pCAT control vector containing SV40 promoter and enhancer sequences (pCAT-3 Control Vector) (Promega Corp., Madison, WI).
Electrophoretic mobility shift assay (EMSA)
Characterization of BP1, BP2 and HMG1+2 protein interactions with the β-globin gene was performed using EMSA. A modified procedure of Latchman was followed (21). K562 nuclear extract was prepared as described by Dignam et al. (22) and 3 µg of extract was incubated with 5× binding buffer (10 mM HEPES pH 7.9, 4 mM MgCl2, 0.5 mM EDTA, 40 mM KCl, 6% glycerol), 2 mg BSA and 2 µg poly(dI–dC) for 30 min. Approximately 10 fmol of end-labeled DNA corresponding to either BP1U, BP1, BP2 or HMG1+2 sequence motifs were added to the mixture and allowed to incubate for an additional 30 min in a final volume of 20 µl (see Fig. 1C for probe sequences). DNA–protein complexes were resolved by 4% non-denaturing polyacrylamide gel electrophoresis in 0.5× TBE buffer. The gel was run for ~2 h at 150 V in 0.5× TBE buffer. Autoradiography was performed using Kodak BIOMAX MR film and exposed overnight at –70°C. For gel shift competition assays, 100–1000× molar excess of either specific competitor (to the β-globin BP1U, BP1, BP2 or HMG1+2 sequence motifs) or non-specific unlabeled competitor was added to the binding reaction.
Circular permutation assay
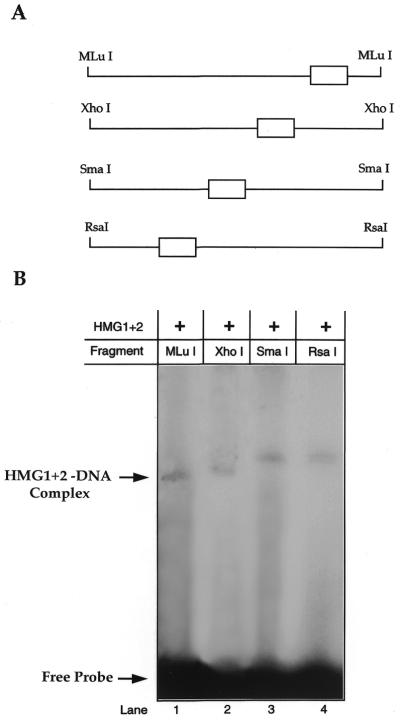
This assay, described by Wu et al. (23), can identify whether protein-induced DNA-bending occurs. The HMG1+2 motif at position –329 to –307 of the β-globin gene was cloned into the pBR322-derived pBend2 vector (24) (Fig. 1D). Multiple digestions with different restriction endonucleases produced various fragments, each totaling 163 bp in length with the HMG1+2 motif (shown as an open box) located at different positions along the fragments (Fig. 4A). Each fragment was 5′ end labeled using T4-polynucleotide kinase and [γ-32P]dATP (10 µCi/ml; Dupont, NEN, Boston, MA). Each labeled probe (20 000 c.p.m.) was incubated with purified HMG1+2 protein (3 µg) (Wako, Richmond, VA) followed by EMSA reaction as described above. The bend angle (α) was estimated from the equation µM/µE = cos(α/2), where µM/µE is the ratio of the most slowly migrating DNA–protein complex to the fastest migrating complex (25).
Figure 4.
Circular permutation assay. Alteration of DNA conformation by HMG1+2 factor determined by circular permutation assay. (A) Multiple digestions of cloned HMG1+2 motif in the pBend2 (see Fig. 1D) produced probes of equal length bounded by the indicated restriction enzyme sites. The open box indicates the location of the HMG1+2 motif along each probe. (B) Resolution of various probes on 4% polyacrylamide gel after incubation with purified HMG1+2 factor. The mobilities of the fastest (lane 1) and slowest (lane 3) DNA–protein complexes were used to estimate a DNA bending angle (α) of 54° as described in Materials and Methods.
RESULTS
Functional studies of adult β-globin gene upstream elements
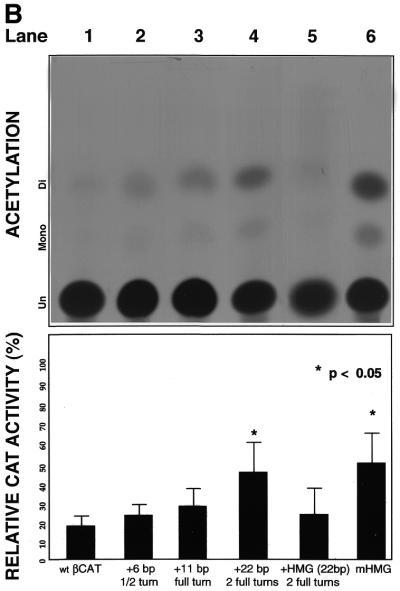
Analysis of mutational βCAT constructs. Three previously identified DNA elements (BP1U motif at position –553/–527, BP1 at position –302/–294, and BP2 at –274/–253) were studied in order to define their roles in β-globin gene regulation. In vitro mutagenesis of each element cloned 5′ of the pCAT reporter vector was performed followed by transient transfection into K562 cells. Each experiment was repeated at least five times to ensure reproducibility. The reference (wt) βCAT construct resulted in minimal gene activity (13% CAT activity) as expected in this embryonic/fetal environment. In contrast, marked increases of CAT activity were observed with individual mutated βCAT constructs, ranging from 49 to 68% CAT activity (P<0.001). Therefore, these distal promoter elements are confirmed negative regulators of β-globin expression (Fig. 2A). Mutagenesis of any one or a combination of two of the beta-protein binding motifs conferred CAT induction. However, to our surprise, simultaneous mutation of all three silencer motifs caused the greatest decrease in gene activity; activity even lower than that of the reference (wt) βCAT (5% versus 13%, P<0.05). The β-globin motifs for BP1U and BP2 both have overlapping binding sites with the chromosomal HMG1+2, non-sequence-specific DNA-binding factors. A third HMG1+2 motif is located 4 bp 5′ of the BP1 motif (Fig. 1C).
Figure 2.
CAT assays. Results for mutation constructions transiently transfected into hemin-induced K562 cells. TLC was performed following incubation of cell lysates from transfected cells with the CAT assay mixture. Bar graphs show quantitation results obtained from phosphoimaging: percentages indicate amount of CAT gene activity relative to 100% control. Error bars indicate the standard error of the mean of at least five independent experiments. (A) βCAT constructions containing sequence alteration mutations of the beta-protein motifs (see Fig. 1A). (B) βCAT mutation constructions containing insertions between downstream BP1 and BP2 (see Fig. 1B).
Insertion of βCAT constructs. Inserting an approximate half (+6 bp), full (+11 bp) or two full (+22 bp) turns of DNA between downstream BP1 and BP2 was constructed and placed 5′ of the pCAT gene for subsequent functional analysis. The addition of an approximate half or full turn of DNA (sequence corresponding to restriction endonuclease recognition sites for FnuHI and/or EcoRI) revealed no marked effect on β-silencing compared to reference βCAT (Fig. 2B, lanes 1–3). However, inserting a non-transcriptionally active 22 bp fragment roughly equivalent to the addition of two full turns of DNA increased significantly β-expression by 3-fold compared to wt βCAT (Fig. 2B, lane 4), while the insertion of a 23 bp HMG1+2-specific fragment from β–329/–307 site effectively restored silencing (Fig. 2B, lane 5). On the other hand, the mutagenesis of the non-overlapping HMG motif (β–329/–307) abrogates β-silencing (Fig. 2B, lane 6).
Structural study of protein–DNA interactions of the adult β-globin gene
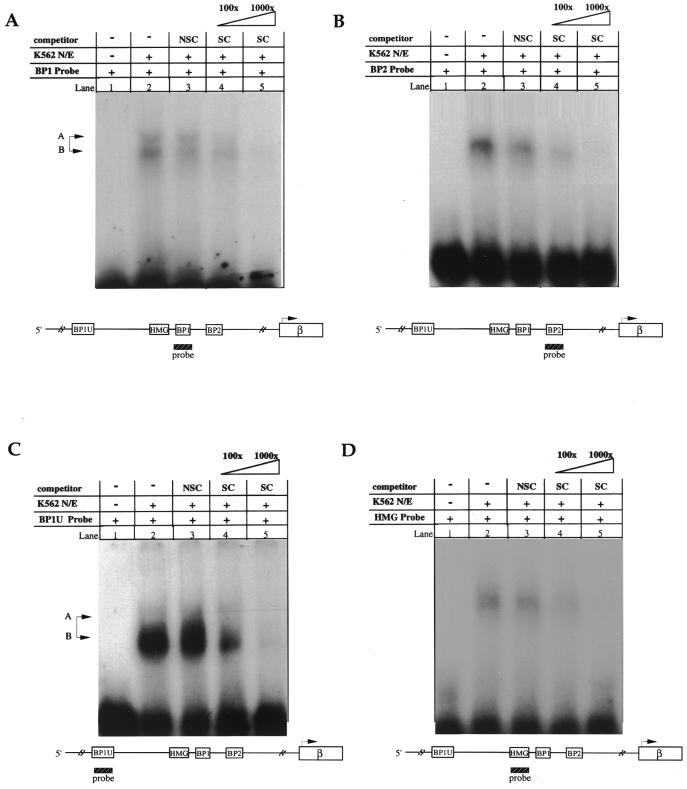
EMSA. A determination of DNA–protein binding specificity was performed using EMSA adding either molar excess of unlabeled DNA corresponding to the same sequence as the probe (the specific competitor) or non-specific competitor (e.g. AP2). BP1 binding (Fig. 3A, lane 2) decreased by a factor of 1.8 (Fig. 3A, lane 4) and 7.5 (Fig. 3A, lane 5) in the presence of 100× and 1000× excess unlabelled specific competitor, respectively. Non-specific competitor at either 100× excess (Fig. 3A, lane 3) or 1000× excess (data not shown) did not compete for BP1 binding. BP2 (Fig. 3B), BP1U (Fig. 3C) and HMG1+2 (Fig. 3D) all demonstrated quantitatively similar results; significant DNA–protein complex formation could be competed by the addition of specific competitor [fold decreases: BP2, 4.5 and 15.4; BP1U, 1.4 and 10.5; HMG1+2, 1.7 and 3.4 (all at 100× or 1000× excess, respectively)], without a significant decrease in binding when excess non-specific competitor was used. In order to further define specificity, competitive EMSA were carried out using the mutated sequences for each beta-protein motif and HMG1+2 motif that were created for the functional studies in this report (as described in Materials and Methods). Thus, BP1 was competed with cold mBP1, BP2 was competed with cold mBP2 and HMG1+2 was competed with cold mHMG1+2. In these experiments, the addition of 100× or 1000× excessive mutant sequence decreased the binding <0.1 fold in all instances as judged by computer-assisted imaging analysis (data not shown).
Figure 3.
EMSA. (A) BP1, (B) BP2, (C) BP1U and (D) HMG1+2 DNA–protein complexes using K562 nuclear extracts. On each autoradiograph, lane 1 indicates free probe (no K562 nuclear extract or competitor added), lane 2 the specific β-globin target sequence binding to the soluble nuclear factor without competitor and lanes 3–5 contain the addition of molar excess of either non-specific competitor (NSC, the binding site for transcription factor AP2 at 100× excess) or specific competitor (SC, unlabeled probe at the indicated molar excess). Each factor binds with specificity to its target β-globin sequence. The BP1 protein (A and C) binds as a doublet, as indicated by the production of two bands (A and B) on the gel.
Circular permutation analysis. Since HMG1+2 factors are capable of bending DNA (11), we tested the ability of the HMG1+2 motif from β–329/–307 to cause protein-induced DNA-bending for the β-promoter. Synthetic oligonucleotides were created, annealed and cloned into the pBEND2 vector (Fig. 1D). Cleavage with MluI, XhoI, SmaI and RsaI enzymes produced 163 bp long fragments and each was end-labeled and incubated with purified HMG1+2 factor for analysis by EMSA (Fig. 4A). SmaI probe has the HMG1+2 motif relatively in the center of the 163 bp long fragment and thus has the highest retardation band when bound by HMG1+2 purified factor compared to all other probes which have regressing bands (Fig. 4B). Collectively, this representation denotes extrinsic DNA-bending. Intrinsic, self-induced DNA-bending is not seen with this motif, for the free probe bands of all four lanes remain at a constant position. A bend angle of 54° was calculated based on these experiments which were performed in triplicate (as indicated in Materials and Methods).
DISCUSSION
We have studied human β-globin gene regulation using the human K562 eyrthroleukemia cell line as our tissue culture model. These cells, originally isolated from an adult CML patient in erythroleukemic blast crisis (26), nevertheless express low levels of adult beta-globin (16,17), yet maintain a high embryonic/fetal environment. This lack of expression appears to be under the control of transcription factors, either an abundance of gene repressors which interact with DNA sequences in the β-globin promoter to down-regulate adult expression, and/or the lack of formation of an effective activator complex.
Ubiquitous and erythroid-specific transcription factors bind to the adult β-globin gene. Within the distal promoter, beyond the first 200 nt (relative to the β-cap site) are DNA sequences which had been previously identified (6,27) and confirmed here to silence β-globin gene expression. These motifs are bound by BP1 and BP2 factors, and have overlapping or adjacent elements for HMG1+2.
The HMG1+2 factors are evolutionarily conserved chromosomal proteins (105–106 molecules per cell nucleus) each with two homologous HMG-box domains, A and B, an acidic C-terminal tail, C (28). Within HMG1 protein, the HMG-box A has been shown to interact with other proteins (15), while gel retardation and DNA supercoiling assays have indicated that the HMG-box B region is essential for the preferential binding to DNA (29,30). A single HMG domain may cover 20 bp of DNA, binding in a non-sequence-specific manner, yet recognizing and having an affinity for local DNA structure (i.e. four-way junctions) (31). Furthermore, computational modeling studies suggest the side chain of Phe-102 in Box B is inserted into the minor groove of DNA to cause protein-induced DNA-bending (29,32). In this study, we demonstrate by circular permutation analysis, the HMG1+2 factors lead to extrinsic DNA-bending specifically within the –329/–307 β-globin gene sequence motif (Fig. 4B).
HMG-mediated deformation of DNA facilitates the assembly of functional nucleoprotein complexes (33). These factors modulate the binding affinity of various transcription factors to their cognate sites (34–37) for gene regulation. Therefore, either activation or silencing of various genes has been reported. For instance, a protein containing the HMG-box domains is involved in the activation of the human ɛ-globin gene (38,39), while the Drosophila HMG1-like protein, DSP1, appears to repress transcription (13–15) by binding directly to the TATA binding protein (TBP) and forming a stable ternary complex with TBP and the DNA, displacing TFIIA from binding. These and other data suggest a more general role of HMG in gene regulation through its inhibition or facilitation of RNA polymerase II complex formation and/or binding.
We found full repression of adult β-globin gene expression required intact BP1U, BP1 and BP2 sequence motifs (Fig. 2A). However, simultaneous mutagenesis of all three silencing motifs paradoxically resulted in even lower transcriptional activity than normally seen in this system (Fig. 2A). We posit that simultaneous mutagensis of all three beta-protein motifs also abolished the overlapping recognition site for HMG1+2 binding, DNA-bending factors whose architectural role in globin regulation would be altered to the extent that even the low level of β-expression would not be permitted.
A limitation of most studies of human β-globin activation has been the lack of a continuous cell that expresses meaningful levels of β-globin mRNA. We have previously reported that a wt β-globin promoter (–640/+50 regulatory region) is sufficient to confer high levels of reporter gene activity when transiently expressed in a mouse erythroleukemia (MEL) cell line (40), since these cells express very high levels of adult mouse β-globin. However, studies of the role of individual (or joint) β-globin silencer motifs placed into MEL cells might be difficult to discern given the near maximal β-globin gene expression.
Placing 6 (an approximate half turn of DNA) or 11 bp (a full turn of DNA) between the BP sites does not alter β-silencing. However, once an approximate two full turns (+22 bp) of DNA were inserted between these motifs, a statistically significant increase in gene expression was observed (Fig. 2B, lanes 1–4). These results suggest that a spatial constraint exists between the (downstream) BP1 and BP2 elements for optimal silencing and supports the existence of possible protein–protein interactions. Interestingly, however, the insertion of the β–329/–307 HMG1+2 motif, between the BP1 and BP2 elements, which also corresponds to an approximate spacing of two full DNA turns, significantly decreased gene activity (Fig. 2B, lane 5). Given the role of HMG1+2 as a DNA-bending factor, the addition of HMG1+2 motif between the beta-protein sites restored the requisite spacing of the BP1 and BP2 silencing motifs by permitting protein-induced DNA bending, and thus the protein–protein interactions to cooperatively down-regulate transcription. The importance of the HMG1+2 DNA-bending motif to these protein–protein complexes was further confirmed by in vitro mutagenesis of this motif (β–329 to –307) (Fig. 2B, lane 6), as silencing was abolished, although BP1 and BP2 were still able to bind to their respective motifs. This result shows the importance of DNA-bending in beta-globin regulation. Indeed, Chase et al. (41) have demonstrated that a HMG-like factor termed HIG(I)Y binds to the β–550 region and causes protein-induced extrinsic bending of the region.
Nuclear extracts derived from K562 cells readily form protein–DNA interactions in vitro to the beta-protein motifs (6), indicating both the abundance and ability of these transcription factors to bind to naked DNA, which has been confirmed and extended in the current work. The gel shift of BP1 binding sites (Fig. 3A and C) located at positions –553 to –527 and –302 to –294, produce double bands on the gel, behavior that may be indicative of the protein binding as a dimer given the dyad symmetrical nature of the BP1U DNA sequence motif (6) (see Fig. 1C). Additionally, the binding of BP2 and the HMG1+2 factors from K562 cells were also observed. For each protein–DNA interaction, sequence-specific binding was determined using molar excess of specific and non-specific competitors, including the mutated target sequence competitors originally used in the mutagenic functional assays. Each interaction was shown to exhibit sequence-specific DNA-binding (Fig. 3). Within the chromatin context in vivo protein–DNA formation cannot be detected in the β-promoter in the K562 cellular environment. Using a DMS in vivo DNA-footprinting technique, Ikuta and Kan (42) and Kim and Rodgers (43) showed that while there are numerous γ-globin proximal promoter specific footprints, there are no detectable protein–DNA interactions within the β-globin proximal promoter region in K562 cells. Hence, erythroid-specific and basal-level positive transcription factors, while present, do not appear to bind to the adult β-globin gene promoter in K562 cells.
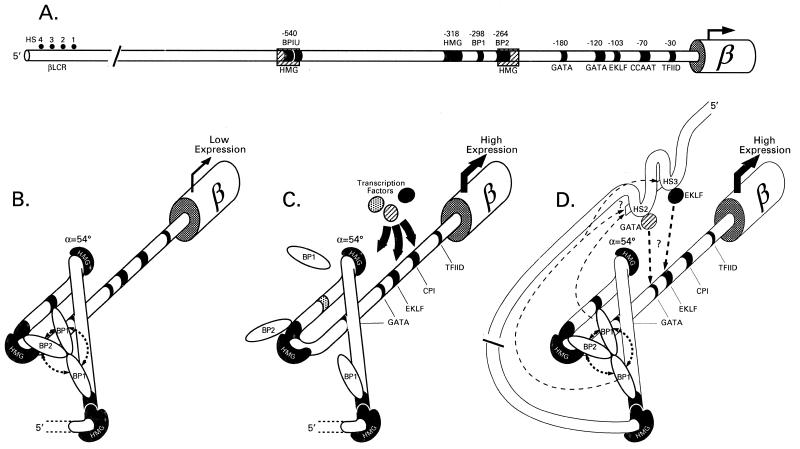
Based on these results, we present a model for β-globin gene regulation in which β-specific DNA motifs are bound by various transcription factors (Fig. 5A). HMG-mediated DNA-bending allows for the assembly of a functional nucleoprotein complex; bringing into close proximity the long-range β-globin silencing factors BP1 and BP2 to form a stable silencing complex, effectively impairing the binding and subsequent function of positive regulators (i.e. EKLF, GATA, TFIID), to silence the gene (Fig. 5B). However, when a DNA silencing motif is mutated, the corresponding transcription factor is thus unable to bind to its target sequence. This destabilizes the DNA–protein and protein–protein interactions of the whole silencing complex eliminating the silencing stability and steric hindrance, allowing access of the positive regulators to the promoter to induce gene expression (Fig. 5C).
Figure 5.
Combinatorial model of adult β-globin gene repression. (A) Wild-type β-globin gene showing known transcription factor DNA-binding sites. (B) Model shows interaction between these DNA binding sites with HMG1+2, BP1 and BP2 factors. Collectively, they provide DNA–protein and protein–protein interactions needed to form a stable silencing complex. (C) Modification of the model in which disruption of the stable DNA–protein, protein–protein silencing complex (seen here by in vitro mutagenesis of functional assays by the DNA mutation of BP1 binding) activates the β-globin gene in K562 environment. (D) Silencing disruption in erythroid cells via βLCR interaction with the β-promoter, leading to gene activation.
How does this model relate to β-globin gene activation in definitive adult erythropoiesis? The current view holds that a holocomplex of the individual elements in the βLCR (HS 4321) is formed [for a review see Enver et al. (44)] which interacts in embryonic life with elements in the proximal ɛ-globin promoter, and sequentially and effectively move to the downstream fetal (γ-) and then to the adult (β-)globin gene during ontogeny. Within the βLCR, hypersensitive site 2 (HS2) and hypersensitive site 3 (HS3) both have BP1-like and BP1U-like sequence motifs of ~90% sequence homology which are within 100–200 bp of GATA-1 and EKLF binding motifs (unpublished data). In definitive adult erythropoiesis, our model would favor the recruitment of the BP1 protein to these βLCR sites from the adult β-globin gene promoter causing a disturbance of the stable BP–HMG–βDNA silencing complex. This in turn might facilitate the recruitment of EKLF (and presumably other activators) from neighboring sites within the HS2 and/or HS3 to the proximal β-globin promoter sites leading to gene activation now relieved of the steric hindrance from the silencing complex (Fig. 5D). A number of observations in erythroid cells support this model: (i) the presence of in vivo DNA fingerprints corresponding to the putative BP1U site in HS3 in the interspecies human–mouse cell hybrid line HU11 that expresses β-globin, and their absence in K562 cells (45); (ii) the presence of in vivo DNA footprints corresponding to EKLF, GATA-1 and NF-E2 in HS2 of K562 cells, while the absence of these footprints in the proximal promoter of β-globin gene in these cells (42); and (iii) the demonstration of the potential recruitment of EKLF from HS2 and HS3 sites to the promoter of β-globin concomitant with β-globin expression (46,47).
This model, if confirmed, may have implications relevant to the understanding of the control of developmentally regulated multigene loci. A deeper appreciation of the developmental control of globin gene regulation in particular may point to novel genetic approaches to reverse γ- to β-globin switch.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank Drs Ida Owens and Samuel Adeniyi-Jones for their thoughtful review and comments. This work was supported in part by the National Institutes of Health Grant DK53533 (P.E.B).
REFERENCES
- 1.Grosveld F., Greaves,D. and Kollis,G. (1987) Cell, 51, 975–985. [DOI] [PubMed] [Google Scholar]
- 2.Tuan D., Solomon,W., London,I. and Lee,D. (1989) Proc. Natl Acad. Sci. USA, 86, 2554–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orkin S. (1995) Eur. J. Biochem., 231, 271–281. [DOI] [PubMed] [Google Scholar]
- 4.Bieker J. (1996) DNA Cell Biol., 15, 347–352. [DOI] [PubMed] [Google Scholar]
- 5.Bieker J., Ouyang,L. and Chen,X. (1998) Ann. N.Y. Acad. Sci., 30, 64–69. [DOI] [PubMed] [Google Scholar]
- 6.Berg P., Williams,D., Qian,R., Cohen,R., Mittelman,M. and Schechter,A. (1989) Nucleic Acids Res., 17, 8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian R., Chen,Y., Song,Q. and Hu,Y. (1993) Sci. China B, 36, 81–88. [PubMed] [Google Scholar]
- 8.Jantzen H., Admon,A., Bell,S. and Tjian,R. (1990) Nature, 344, 830–836. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi M., Falciola,L., Ferrari,S. and Lilley,D. (1992) EMBO J., 11, 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Read C., Cary,P., Crane-Robinson,C., Driscoll,P. and Norman,D. (1993) Nucleic Acids Res., 21, 3427–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paull T., Haykinson,M. and Johnson,R. (1993) Genes Dev., 7, 1521–1534. [DOI] [PubMed] [Google Scholar]
- 12.Grosschedl R. (1995) Curr. Opin. Cell Biol., 7, 362–370. [DOI] [PubMed] [Google Scholar]
- 13.Lehming N., Le Saux,A., Schuller,J. and Ptashne,M. (1998) Proc. Natl Acad. Sci. USA, 95, 7322–7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirov N., Lieberman,P. and Rushlow,C. (1996) EMBO J., 15, 7079–7087. [PMC free article] [PubMed] [Google Scholar]
- 15.Sutrias-Grau M., Bianchi,M. and Bernues,J. (1999) J. Biol. Chem., 274, 1628–1634. [DOI] [PubMed] [Google Scholar]
- 16.Rutherford T., Clegg,J., Higgs,D., Jones,R., Thompson,J. and Weatherall,D. (1981) Proc. Natl Acad. Sci. USA, 78, 348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benz E., Murnane,M., Mazur,E., Jenko,T., Synder,E., Forget,B. and Hoffman,R. (1980) Proc. Natl Acad. Sci. USA, 77, 3509–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fordis M., Anagnou,N., Dean,A., Nienhuis,A. and Schechter,A. (1984) Proc. Natl Acad. Sci. USA, 81, 4485–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higuchi R., Krummel,B. and Saiki,R. (1988) Nucleic Acids Res., 16, 7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean A., Ley,T., Humphries,R., Fordis,M. and Schechter,A. (1983) Proc. Natl Acad. Sci. USA, 80, 5515–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latchman D. (1993) In Hames,B and Rickwood,D. (eds), Transcription Factors, A Practical Approach. Oxford University Press, New York.
- 22.Dignam J., Lobovitz,R. and Roeder,R. (1983) Nucleic Acids Res., 11, 1475–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H. and Crothers,D. (1984) Nature, 308, 509–513. [DOI] [PubMed] [Google Scholar]
- 24.Kim J., Zwieb,C., Wu,C. and Adhya,S. (1989) Gene, 85, 15–23. [DOI] [PubMed] [Google Scholar]
- 25.Thompson J. and Landy,A. (1988) Nucleic Acids Res., 16, 9687–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lozzio C. and Lozzio,B. (1975) Blood, 45, 321–334. [PubMed] [Google Scholar]
- 27.Ebb D., Tang,D., Drew,L., Chin,K., Berg,P. and Rodgers,G. (1998) Blood Cells Mol. Dis., 24, 356–369. [DOI] [PubMed] [Google Scholar]
- 28.Ramstein J., Locker,D., Bianchi,M. and Leng,M. (1999) Eur. J. Biochem., 260, 692–700. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka K., Saito,K., Tanabe,T., Yamamoto,A., Ando,Y., Nakamura,Y., Shirakawa,H. and Yoshida,M. (1999) Biochemistry, 38, 589–595. [DOI] [PubMed] [Google Scholar]
- 30.Stros M. (1998) J. Biol. Chem., 273, 10355–10361. [PubMed] [Google Scholar]
- 31.Pöhler J., Norman,D., Bramham,J., Bianchi,M. and Lilley,D. (1998) EMBO J., 17, 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito K., Kikuchi,T. and Yoshida,M. (1999) Protein Eng., 12, 235–242. [DOI] [PubMed] [Google Scholar]
- 33.Wolffe A. (1999) Nature Genet., 22, 215–217. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X. and Chada,K. (1998) Keio J. Med., 47, 73–77. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C., Krieg,S. and Shapiro,D. (1999) Mol. Endocrinol., 13, 632–643. [DOI] [PubMed] [Google Scholar]
- 36.Romine L., Wood,J., Lamia,L., Prendergast,P., Edwards,D. and Nardulli,A. (1998) Mol. Endocrinol., 12, 664–674. [DOI] [PubMed] [Google Scholar]
- 37.Boonyaratanakornkit V., Melvin,V., Prendergast,P., Altmann,M., Ronfani,L., Bianchi,M., Taraseviciene,L., Nordeen,S., Allegretto,E. and Edwards,D. (1998) Mol. Cell Biol., 18, 4471–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dyer M., Naidoo,R., Hayes,R., Larson,C., Verdine,G. and Baron,M. (1996) Mol. Cell Biol., 16, 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dyer M., Hayes,P. and Baron,M. (1998) Mol. Cell Biol., 18, 2617–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang D., Hardison,R. and Rodgers,G. (1997) Blood, 90, 421–427. [PubMed] [Google Scholar]
- 41.Chase M., Hankins,W., Williams,D., Bi,Z., Strovel,J., Obriecht,C. and Berg,P. (1999) Am. J. Hematol., 60, 27–35. [DOI] [PubMed] [Google Scholar]
- 42.Ikuta T. and Kan,Y.W. (1991) Proc. Natl Acad. Sci. USA, 88, 10188–10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim I.H. and Rodgers,G.P. (1997) Anal. Biochem., 254, 1–8. [DOI] [PubMed] [Google Scholar]
- 44.Enver T., Raich,N., Ebens,A.J., Papayannopoulou,T., Costantini,F. and Stamatoyannopoulos,G. (1990) Nature, 344, 309–313. [DOI] [PubMed] [Google Scholar]
- 45.Strauss E. and Orkin,S. (1992) Proc. Natl Acad. Sci. USA, 89, 5809–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J., Lee,C. and Chung,J. (1999) Proc. Natl Acad. Sci. USA, 96, 10051–10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J., Lee,C. and Chung,J. (1998) Proc. Natl Acad. Sci. USA, 95, 969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]