Abstract
Background This study compared the ergonomics of surgeons during deep inferior epigastric perforator (DIEP) flap surgery using either baseline equipment (loupes, headlights, and an operating microscope) or an exoscope. Plastic surgeons may be at high risk of musculoskeletal problems. Recent studies indicate that adopting an exoscope may significantly improve surgeon postures and ergonomics.
Methods Postural exposures, using inertial measurement units at the neck, torso, and shoulders, were calculated in addition to the surgeons' subjective physical and cognitive workload. An ergonomic risk score on a scale of 1 (lowest) to 4 (highest) was calculated for each of the postures observed. Data from 23 bilateral DIEP flap surgeries (10 baseline and 13 exoscope) were collected.
Results The neck and torso risk scores decreased significantly during abdominal flap harvest and chest dissection, while right shoulder risk scores increased during the abdominal flap harvest for exoscope DIEP flap procedures compared with. Exoscope anastomoses demonstrated higher neck, right shoulder, and left shoulder risk scores. The results from the survey for the “surgeon at abdomen” showed that the usage of exoscopes was associated with decreased performance and increased mental demand, temporal demand, and effort. However, the results from the “surgeon at chest” showed that the usage of exoscopes was associated with lower physical demand and fatigue, potentially due to differences in surgeon preference.
Conclusion Our study revealed some objective evidence for the ergonomic benefits of exoscope; however, this is dependent on the tasks the surgeon is performing. Additionally, personal preferences may be an important factor to be considered in the ergonomic evaluation of the exoscope.
Keywords: exoscope, plastic surgery, ergonomics, postural exposure, inertial measurement unit
Musculoskeletal disorder (MSD) prevalence is high among surgeons, 1 2 resulting in lost productivity and decreased quality of surgical care. 3 In addition, the frequency of MSD within the surgical profession may also deter medical students from pursuing surgical specialties. 4 Studies have shown that surgeons' necks overall are the most at-risk body area intraoperatively. 5 6 Specifically, the use of surgical equipment such as loupes, 7 8 headlights, 9 and operating microscopes 10 may be associated with risk factors (e.g., higher neck postural exposure with the use of loupes, awkward neck postural exposure from microscope use, and cervical spine loading when using loupes and headlights).
Plastic surgeons and residents may be at high risk of musculoskeletal problems because of chronic work-related neck pain and discomfort. 11 12 13 14 This may be true especially during autologous breast reconstruction using deep inferior epigastric perforator (DIEP) flap surgery, involving harvesting the fat, skin, and blood vessels from the abdomen and reattaching it using microsurgery to the breast area after it is dissected to reconstruct the breast. Each DIEP flap surgery can be thought of in three main phases: (1) the abdominal flap harvest, (2) chest vessel dissection, and (3) anastomosis in which the harvested flap is reattached to the chest using microsurgery. The DIEP flap presents several ergonomic risks to surgeons, such as its long average duration, the use of loupes and headlights, and the use of a surgical microscope. Attempts to address the ergonomic issues associated with operating microscopes have included employing different imaging methods such as stereoscopic video displays during microsurgery. Video displays, when compared with traditional microscopes, have been shown to reduce neck angles but increased the shoulder flexion. 15
Adopting exoscopes in place of microscopes may significantly improve surgeon postures and ergonomics, in addition to offering high-resolution three-dimensional visualization and increased field-of-view adjustability. 16 17 Studies have shown additional ergonomic advantages with exoscope use. 18 19 20 21 Potential advantages with intraoperative exoscope use include decreased neck flexion and fatigue, which may hinder surgeon performance. 22 However, the utility of an exoscope as a potential ergonomic intervention to reduce the risk of MSDs among plastic surgeons (e.g., DIEP flap cases) is not yet well known.
The current study evaluated the ergonomics (e.g., surgeons' intraoperative postural exposure and workload) of DIEP flap surgery using a 3D exoscope compared with those using loupes, headlights, and microscopes for the various phases of the case.
Methods
Participants
Two surgeons who regularly perform DIEP flap autologous breast reconstruction surgeries at a quaternary care academic medical center (United States) participated during their regularly scheduled dual-surgeon DIEP flap cases. One surgeon's role was to work on the flap harvest for most of the surgeries (surgeon at abdomen), while the other surgeon's role was to work on the chest dissection and anastomosis for most of the surgeries (surgeon at chest). The surgeons were novices with exoscopes but had performed cadaveric practice sessions prior to the study. This study was approved by the medical center's institutional review board.
Apparatus
The DIEP flap surgery was performed as either a baseline or exoscope (ORBEYE, Olympus Corporation, Tokyo, Japan) surgical case. In baseline surgical cases, the surgeons used loupes with a headlight during abdominal flap harvest and chest dissection ( Fig. 1A ) and used a microscope (MM51, Mitaka USA, Denver, CO) for the microsurgical anastomosis ( Fig. 1B ). In the exoscope surgical case, the surgeons used the exoscope ( Fig. 1C ) (ORBEYE, Olympus Corporation, Tokyo, Japan) during the main phases of the surgery: abdominal flap harvest, chest dissection, and anastomosis. The exoscope features a camera mounted on a semirobotic arm that is controlled by the surgeon either by directly manipulating controls on the device or remotely by using a foot pedal. Images captured on the exoscope were displayed on a 4K-3D monitor, and the surgeons and their team wore 3D glasses to view the surgical field projection ( Fig. 1C ). The exoscope also had optical and digital zoom capabilities of up to 26× for visualization of small anatomical structures in detail, which was crucial for the microsurgical anastomosis. For this study, two exoscope units and four monitors were used simultaneously; one exoscope with two monitors (one each on the left and right side of the patient) was used at the chest for chest dissection and anastomosis and the second exoscope (ORBEYE, Olympus Corporation, Tokyo, Japan) also with two monitors was used at the abdomen for flap harvest. Each exoscope was used by an attending surgeon and a resident.
Fig. 1.
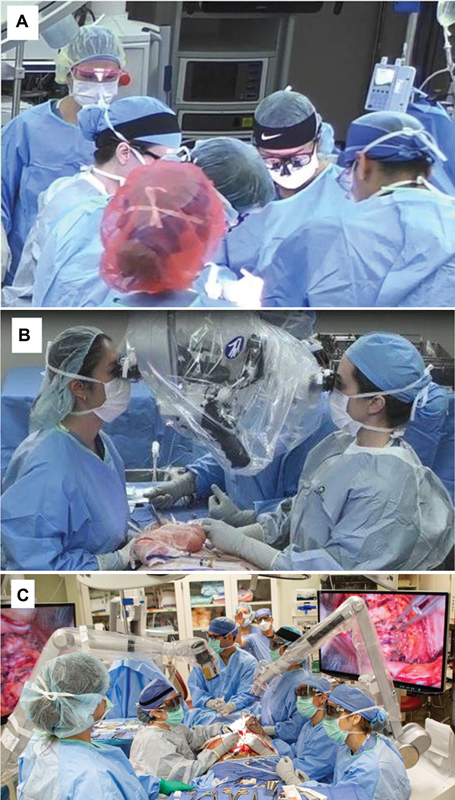
Baseline surgical procedure using ( A ) loupes and headlights and ( B ) microscope and ( C ) exoscope. (Reproduced with permission of Mayo Foundation for Medical Education and Research. All rights reserved.).
Data Collection Instrumentation
The data collection instrumentation consisted of inertial measurement units (IMUs; objective assessment) and an electronic survey (subjective assessment). IMU sensors (Opal, APDM, Inc., Portland, OR) were used to collect surgeons' postural exposure. Three IMUs were affixed to the surgeon's scrub top at their upper back and right/left shoulders to capture their intraoperative back and shoulders angles. The fourth IMU sensor was placed in an elastic headband worn by the surgeon with the sensor positioned at the back of their head to capture their intraoperative neck angle. 7 IMUs recorded accelerometer, gyroscope, magnetometer, and time (in UTC) data at a sampling rate of 128 Hz.
After each surgical procedure, the participants received an electronic survey that evaluated their intraoperative workload, including mental demand, physical demand, temporal demand, performance, effort, and frustration based on NASA-TLX, 23 distractions based on SURG-TLX, 24 degree of difficulty (difficulty) based on Global Operative Assessment of Laparoscopic Skills (GOALS), 25 and fatigue based on Borg CR-10 Scale 26 (all ranged from “0 = min” to “20 = max”). The surgeons were asked to complete the survey within 24 hours after the end of each surgical procedure.
Experimental Procedure
Prior to the start of each surgical case, study personnel were present in the operating room (OR) to place the IMUs on the participants and calibrate them following a method developed and explained in previous studies. 8 27 The start and end times of each phase of the surgery as well as the surgeon who performed the phase were recorded for both sides of the patient. The surgeons then completed the workload survey after each procedure.
Data Processing
Joint angles were calculated from the IMU data using a custom program in MATLAB (MathWorks, Natick, MA). 27 Joint angles were defined as deviation of a body segment from the vertical axis. The average joint angle across each surgical phase was calculated for each body segment. Additionally, the joint angles for each body part during the surgery was divided to four risk areas 7 using a modified Rapid Upper Limb Assessment (RULA) 28 where larger joint angle deviations from neutral were allocated to higher risk scores. 8 Postural risk scores on a scale of 1 (lowest) to 4 (highest) were then calculated for each body segment and each phase of the surgery based on a methodology described by Norasi et al. 8
Statistical Analysis
Statistical analysis was performed in SPSS (IBM, Armonk, NY). Paired t -tests were used to compare the joint angles of the participants between the DIEPs performed on the left and right sides of the patient. Risk scores associated with each body segment were compared between the baseline and the exoscope using a t -test after checking data normality. To determine any changes in the frequency of participants adjusting the exoscope using the camera controls, the percentage of each phase that the right arm elevation angle was greater than 45 degrees was calculated and any trends over time was identified using a least-squares linear regression. The normality of the skin-to-skin duration of surgical procedures was also checked and t -test was used to evaluate the difference in this dependent variable between baseline and exoscope surgical procedures. Furthermore, operating time, flap harvest, chest dissection, and anastomosis time were compared between baseline and exoscope. The Kruskal–Wallis test was used for chest dissection completion time because of violation of the normality assumption.
Additionally, the Kruskal–Wallis test was employed to investigate the effects of surgical procedure (baseline and exoscope) on the nine discrete subscales of the survey for each surgeon individually. All tests had a significance level of 0.05.
Results
Objective Evaluation Using IMUs (Postural Risk Scores)
A total of 23 bilateral DIEP flap surgeries (10 baseline and 13 exoscope) were included in this study.
A paired t -test comparing joint angles observed during the left and right DIEPs showed that there were no significant differences in postures for any of the phases (abdominal flap harvest, chest dissection, anastomosis); therefore, we did not distinguish laterality in any further analyses.
Joint angles for each body segment, neck, torso, and right and left upper arms, during each phase of the DIEP were compared between the baseline and the exoscope cases ( Table 1 ).
Table 1. The comparison of neck, torso, and right and left upper arm average deviation angles between baseline and exoscope cases for three phases of the surgery (abdominal flap harvest, chest dissection, and anastomosis).
| Baseline, mean (SD) [degrees] | Exoscope, mean (SD) [degrees] | p -Value | |
|---|---|---|---|
| Abdominal flap harvest | |||
| Neck | 30.3 (6.8) | 14.4 (2.4) | <0.001 |
| Torso | 17.9 (6.7) | 12.5 (3.9) | 0.008 |
| Right upper arm | 15.0 (4.9) | 22.0 (11.3) | 0.009 |
| Left upper arm | 16.7 (3.8) | 21.3 (15.5) | NSS |
| Chest dissection | |||
| Neck | 28.0 (8.9) | 15.5 (4.2) | <0.001 |
| Torso | 19.8 (6.7) | 14.3 (5.0) | 0.006 |
| Right upper arm | 21.8 (8.5) | 25.8 (10.7) | NSS |
| Left upper arm | 20.8 (5.4) | 25.4 (9.7) | NSS |
| Anastomosis | |||
| Neck | 12.5 (5.1) | 17.5 (5.3) | 0.009 |
| Torso | 13.0 (4.3) | 13.4 (3.5) | NSS |
| Right upper arm | 23.1 (9.9) | 31.9 (9.5) | 0.013 |
| Left upper arm | 19.0 (5.9) | 29.9 (5.7) | <0.001 |
Abbreviations: NSS, not statistically significant; SD, standard deviation.
There were significant decreases in the surgeons' neck angle and torso angle for exoscope cases compared with the baseline during the abdominal flap harvest and the chest dissection phases; however, there were modest but statistically significant increases in the neck angles for exoscope cases compared with baseline during the anastomosis (all p -values < 0.05). Additionally, the exoscope cases were associated with increased upper arm elevation angles during the abdominal flap harvest (only right upper arm) and the anastomosis (both upper arms) (all p -values < 0.05). Although the right arm elevation angles during abdominal flap harvest were higher in the exoscope, the percentage of time spent in the most extreme right arm elevation angles (>45 degrees) gradually decreased as more cases were performed. Although not statistically significant, there was a negative slope in the right arm angles as more cases were performed (slope = −1.4 degrees/case, p = 0.054). Furthermore, the right arm angles during the anastomosis using the exoscope significantly decreased as more cases were performed. A linear regression showed a statistically significant negative slope with the more cases being performed (slope= −4.1 degrees/case, p < 0.001), suggesting that the surgeons reached up less often to position or adjust the exoscope camera over time.
Postural risk scores for each body segment during each section of the DIEP were compared between the baseline and the exoscope DIEP cases as well. The percentage of time that the participants spent in each risk score by body segment and phase of the DIEP is illustrated in Fig. 2 . Average risk scores by body segment and phase are illustrated in Fig. 3 and Fig. 4 .
Fig. 2.
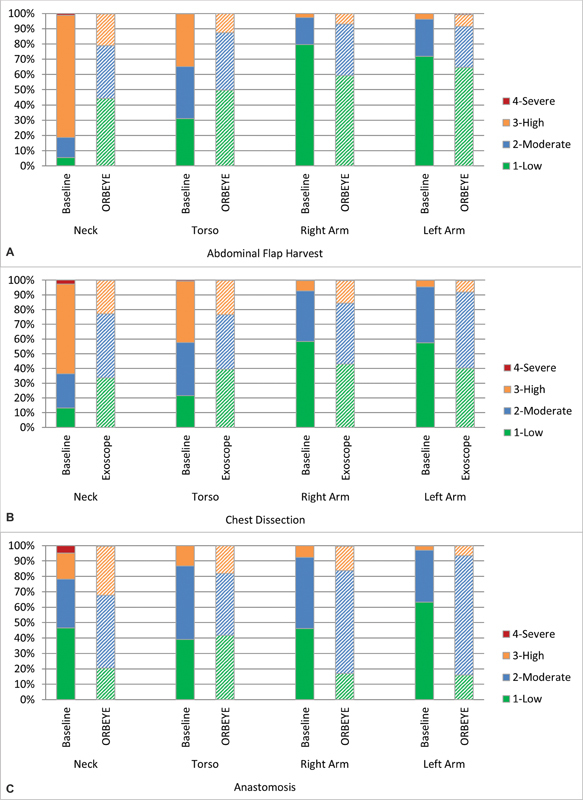
Percentage of time spent in each risk category for each body segment compared between the baseline and exoscope cases during the ( A ) abdominal flap harvest, ( B ) chest dissection, and ( C ) anastomosis.
Fig. 3.
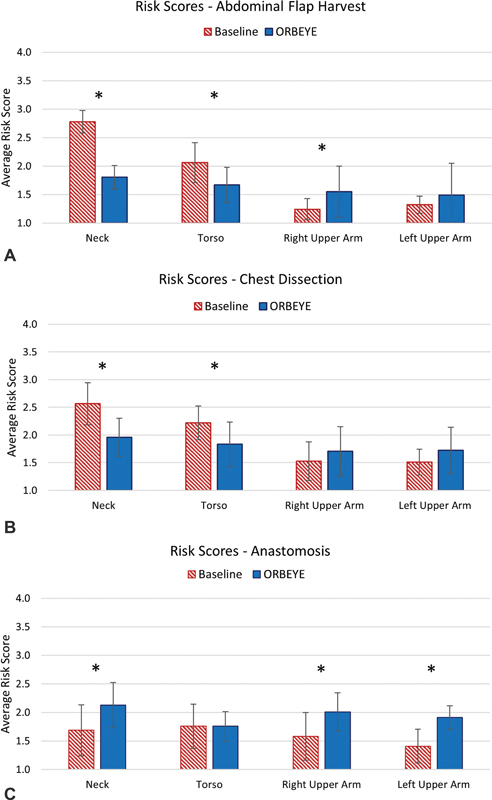
Mean (SD) risk scores for the baseline and exoscope during the ( A ) abdominal flap harvest, ( B ) chest dissection, and ( C ) anastomosis.
Fig. 4.
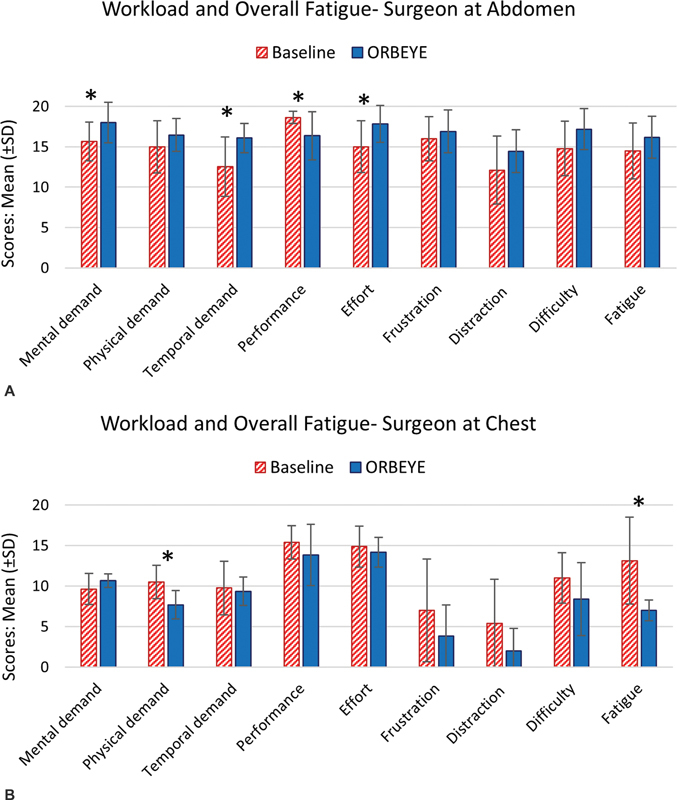
Mean (±SD) of the nine subscales of the survey for the ( A ) surgeon at abdomen and ( B ) surgeon at chest. Asterisks represent statistical differences ( p < 0.05).
The results of the statistical analyses for the postural risk scores ( Table 2 ) were consistent with the results of average angles ( Table 1 ). Additionally, the percentage of time spent in a high-risk neck angle (postural risk score 3 or 4) decreased from 82% to 21% during the abdominal flap harvest and from 63% to 22% during the chest dissection but increased from 21.7% to 32.2% during the anastomosis. Torso angle also showed a similar decrease in high-risk postures (postural risk score of 3 or 4) when using the exoscope, from 35% to 13% during the abdominal flap harvest and from 42% to 23% during the chest dissection phase ( Fig. 2 ).
Table 2. The comparison of neck, torso, and right and left upper arm risk scores between baseline and exoscope DIEP cases for three phases of the surgery (abdominal flap harvest, chest dissection, and anastomosis) with a minimum of 1 and a maximum of 4.
| Baseline, mean (SD) | Exoscope, mean (SD) | p -Value | |
|---|---|---|---|
| Abdominal flap harvest | |||
| Neck | 2.78 (0.20) | 1.80 (0.20) | <0.001 |
| Torso | 2.06 (0.35) | 1.67 (0.31) | 0.001 |
| Right upper arm | 1.24 (0.19) | 1.55 (0.45) | 0.004 |
| Left upper arm | 1.32 (0.15) | 1.49 (0.55) | NSS |
| Chest dissection | |||
| Neck | 2.56 (0.38) | 1.95 (0.34) | <0.001 |
| Torso | 2.22 (0.30) | 1.83 (0.40) | 0.002 |
| Right upper arm | 1.52 (0.34) | 1.70 (0.44) | NSS |
| Left upper arm | 1.51 (0.23) | 1.72 (0.42) | NSS |
| Anastomosis | |||
| Neck | 1.69 (0.44) | 2.12 (0.40) | 0.005 |
| Torso | 1.77 (0.38) | 1.75 (0.25) | NSS |
| Right upper arm | 1.57 (0.41) | 2.01 (0.33) | 0.002 |
| Left upper arm | 1.39 (0.30) | 1.91 (0.21) | <0.001 |
Abbreviations: NSS, not statistically significant; SD, standard deviation.
Also, comparing the skin-to-skin duration of baseline and exoscope surgical procedures using a t -test showed that the skin-to-skin duration of exoscope surgical procedures was significantly longer than baseline surgical procedures ( p = 0.0273). The mean (standard deviation (SD)) of skin-to-skin surgical duration was 552.1 (70.3) minutes for the baseline cases, compared with 635.5 (92.5) minutes for the exoscope cases. However, the results of the statistical analyses did not show a significant difference between baseline and exoscope for the three surgical phases. The mean (SD) duration for abdominal flap harvest were 143.25 (42.34) and 140.08 (53.70) minutes for baseline and exoscope, respectively. The values for chest dissection were 90.80 (37.39) and 70.95 (43.08) minutes for baseline and exoscope, respectively. Finally, the mean (SD) anastomosis duration were 78.41 (17.43) and 79.44 (18.40) minutes for baseline and exoscope, respectively. The longer duration of the exoscope cases may have been due to the additional time needed for the exoscope equipment setup during surgery. It should also be noted that since two phases of the surgery are often being performed simultaneously, a shorter or longer duration of any individual surgical phase may not be reflected in the skin-to-skin duration.
Subjective Evaluation (Surveys)
Fig. 4 illustrates the mean (±SD) of the nine questions in the survey by surgeon. The Kruskal–Wallis test revealed that for the surgeon harvesting the abdominal flap, the mental demand ( p = 0.0346), temporal demand ( p = 0.0321), and effort ( p = 0.0414) were all higher during exoscope cases compared with baseline cases. The surgeon's self-perceived performance for exoscope cases was lower than for baseline cases ( p = 0.0499). Furthermore, for the surgeon performing the chest dissection and anastomosis, the physical demand ( p = 0.0255) and fatigue ( p = 0.0188) were lower during exoscope cases compared with baseline cases. There was no statistically significant difference in other workload subscales between exoscope and baseline surgical procedures for the two surgeons.
Discussion
The exoscope was effective in improving postures and reducing the ergonomic risk of the neck and torso compared with baseline (i.e., loupes, headlights) during the abdominal flap harvest and the chest dissection phases. Exoscope use was associated with significantly less neck flexion and torso flexion angles during abdominal flap harvest and chest dissection in comparison to the use of loupes and headlights. During the abdominal flap harvest phase, the neck and torso risk scores in the exoscope cases were significantly reduced compared with the baseline. Using a threshold average RULA value of 2.5 or higher to determine high-risk postures, 29 the neck angles during the baseline abdominal flap harvest and chest dissection can be categorized as posing a high ergonomic risk to participants. Use of the exoscope improved postures and reduced the ergonomic risks during these procedures such that the high-risk neck angles observed during the baseline abdominal flap harvest and chest dissection phases were no longer observed during the exoscope cases. The torso risk score associated with the baseline abdominal flap harvest and chest dissection cases using loupes and headlights were not considered to be high-risk using the RULA; however, they were further reduced in the exoscope cases.
While the exoscope was associated with an increase in joint angles of the right upper arm during the abdominal flap harvest, as well as the neck and left and right upper arms during the anastomosis, these increases did not present a high degree of ergonomic risk to surgeons. Elevated arm angles observed during abdominal flap harvest could be due to the overhead exoscope visual field, lighting, and zooming adjustments made by the surgeon using their right hand. It may also reflect the ability of the exoscope to allow the surgeon to sit during surgery, something that the traditional equipment of loupes, headlights, and microscope did not afford. On the other hand, the ability to sit while either dissecting the abdominal flap and chest vessels or performing the anastomosis may be beneficial to reducing lower body fatigue and discomfort associated with performing surgery.
Inclusion of a survey in the study allowed exploration of surgeons' perceived cognitive and physical workload. The results for the “surgeon at abdomen,” who mostly performed the abdominal flap harvest task, showed that her mental demand, temporal demand, and effort were all higher during the exoscope cases, and her performance was lower during exoscope cases compared with the baseline. The results for the “surgeon at chest,” who mostly performed the chest dissection and anastomosis tasks, revealed that her physical demand and fatigue were significantly lower during exoscope cases compared with baseline. These findings indicate that there may be other workload drivers in addition to the difference in postural exposure (e.g., neck angle, torso angle) between exoscope and baseline cases that should be considered. Such factors may include (1) the effects of learning curve for both the surgeon 30 31 and surgical team, (2) smaller focal area during the use of exoscope compared with loupes, (3) longer skin-to-skin duration of exoscope cases (83 minutes longer on average) compared with baseline (although the phase-by-phase comparison did not show any significant difference in duration between baseline and exoscope), (4) managing multiple monitors due to setup and integration of two exoscope units within the OR, and (5) the need to adjust the exoscope cameras during the surgery. These potential underlying causes may have negatively affected workload during exoscope surgical procedures.
While this study focused on the ergonomics of the two primary surgeons, experimenters and OR staff noted additional findings based on researcher's observation. Setting up two ORBEYE exoscope units presented a challenge due to the amount of equipment involved. Additionally, surgical staff and residents expressed space concerns due to the two exoscope units and four screens present, even though the 4K-3D screens provide the residents and medical students with a high-quality view of the surgical field without being obstructed by the surgical team in real time.
There are limitations to this study that should be considered when generalizing these findings. Data were collected from two primary surgeons at one hospital during a single, complex surgery (DIEP flap). Similar studies with larger samples of surgeons with additional surgical expertise will help verify these findings. While this study focused solely on the primary attending surgeons, it should be acknowledged that the exoscope may have also affected the postural exposure and workload of other surgical team members, such as the residents and surgical assistant. These factors should be noted in comparing the findings for the exoscope and baseline surgical procedures. It should be noted that due to the long durations of procedures, our evaluation using IMU sensors was confined to joint angles as the deviation of the body segment from the vertical axis running inferosuperior to the body. However, the IMU sensors allowed us to capture surgeons' postural exposure in the restricted and challenging environment of the OR during long DIEP flap surgeries. Lastly, there could be learning taking place while using the exoscope. While this was not the focus of this study, future work should focus on exploring the learning curve associated with using the exoscope.
Conclusion
The findings of this study showed that the intraoperative exoscope significantly reduces the postural exposure and the corresponding ergonomic risk at the neck and torso during the abdominal flap harvest and chest dissection when compared with baseline DIEP flap cases where loupes and headlights were used. Conversely, the right arm during the abdominal flap and the neck, right arm, and left arm during the anastomosis were associated with higher joint angles when using the exoscope. Subjective measures showed that the “surgeon at abdomen” experienced greater mental demand, temporal demand, and effort as well as lower performance when using the exoscope. The “surgeon at chest” experienced lower physical demand and lower fatigue when using the exoscope. The exoscope improved postures at the neck and torso during the abdominal flap harvest and chest dissection phases using the objective postural data from the IMUs; however, the subjective data showed mixed results and may reflect the participants' personal preferences and characteristics.
Acknowledgments
The authors would like to acknowledge the support and participation of the surgeons involved in this study. We would also like to thank the OR staff and Olympus for their technical support. This study was funded in part by Olympus and by Mayo Clinic's Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Funding Statement
Funding This study was funded by a research grant from Olympus, which included a loan of equipment. Olympus had no involvement in the study execution or interpretation of results.
Conflict of Interest None declared.
Disclosures
Olympus had no involvement in the study execution or interpretation of results.
References
- 1.Damodaran O, Lee J, Lee G. Microscope in modern spinal surgery: advantages, ergonomics and limitations. ANZ J Surg. 2013;83(04):211–214. doi: 10.1111/ans.12044. [DOI] [PubMed] [Google Scholar]
- 2.Epstein S, Sparer E H, Tran B N. Prevalence of work-related musculoskeletal disorders among surgeons and interventionalists: a systematic review and meta-analysis. JAMA Surg. 2018;153(02):e174947. doi: 10.1001/jamasurg.2017.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis W T, Fletcher S A, Guillamondegui O D. Musculoskeletal occupational injury among surgeons: effects for patients, providers, and institutions. J Surg Res. 2014;189(02):207–2.12E8. doi: 10.1016/j.jss.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Sergesketter A R, Lubkin D T, Shammas R L. The impact of ergonomics on recruitment to surgical fields: a multi-institutional survey study. J Surg Res. 2019;236:238–246. doi: 10.1016/j.jss.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer A J, Hallbeck M S, Morrow M M. Measuring ergonomic risk in operating surgeons by using wearable technology. JAMA Surg. 2020;155(05):444–446. doi: 10.1001/jamasurg.2019.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Money S R, Morrow M M. Impact of procedure type, case duration, and adjunctive equipment on surgeon intraoperative musculoskeletal discomfort. J Am Coll Surg. 2020;230(04):554–560. doi: 10.1016/j.jamcollsurg.2019.12.035. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Wang T, Weidner T K, Madura J A, II, Morrow M M, Hallbeck M S. Intraoperative musculoskeletal discomfort and risk for surgeons during open and laparoscopic surgery. Surg Endosc. 2021;35(11):6335–6343. doi: 10.1007/s00464-020-08085-3. [DOI] [PubMed] [Google Scholar]
- 8.Norasi H, Tetteh E, Money S R. Intraoperative posture and workload assessment in vascular surgery. Appl Ergon. 2021;92:103344. doi: 10.1016/j.apergo.2020.103344. [DOI] [PubMed] [Google Scholar]
- 9.Nimbarte A D, Sivak-Callcott J A, Zreiqat M, Chapman M. Neck postures and cervical spine loading among microsurgeons operating with loupes and headlamp. IEE Trans Occup. 2013;1(04):215–223. [Google Scholar]
- 10.Lakhiani C, Fisher S M, Janhofer D E, Song D H. Ergonomics in microsurgery. J Surg Oncol. 2018;118(05):840–844. doi: 10.1002/jso.25197. [DOI] [PubMed] [Google Scholar]
- 11.Sivak-Callcott J A, Diaz S R, Ducatman A M, Rosen C L, Nimbarte A D, Sedgeman J A. A survey study of occupational pain and injury in ophthalmic plastic surgeons. Ophthal Plast Reconstr Surg. 2011;27(01):28–32. doi: 10.1097/IOP.0b013e3181e99cc8. [DOI] [PubMed] [Google Scholar]
- 12.Khansa I, Khansa L, Westvik T S, Ahmad J, Lista F, Janis J E. Work-related musculoskeletal injuries in plastic surgeons in the United States, Canada, and Norway. Plast Reconstr Surg. 2018;141(01):165e–175e. doi: 10.1097/PRS.0000000000003961. [DOI] [PubMed] [Google Scholar]
- 13.Howarth A L, Hallbeck S, Mahabir R C, Lemaine V, Evans G RD, Noland S S. Work-related musculoskeletal discomfort and injury in microsurgeons. J Reconstr Microsurg. 2019;35(05):322–328. doi: 10.1055/s-0038-1675177. [DOI] [PubMed] [Google Scholar]
- 14.Kokosis G, Dellon L A, Lidsky M E, Hollenbeck S T, Lee B T, Coon D. Prevalence of musculoskeletal symptoms and ergonomics among plastic surgery residents: results of a national survey and analysis of contributing factors. Ann Plast Surg. 2020;85(03):310–315. doi: 10.1097/SAP.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 15.Yu D, Green C, Kasten S J, Sackllah M E, Armstrong T J.Effect of alternative video displays on postures, perceived effort, and performance during microsurgery skill tasks Appl Ergon 201653(Pt A):281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad F I, Mericli A F, DeFazio M V. Application of the ORBEYE three-dimensional exoscope for microsurgical procedures. Microsurgery. 2020;40(04):468–472. doi: 10.1002/micr.30547. [DOI] [PubMed] [Google Scholar]
- 17.Garneau J C, Laitman B M, Cosetti M K, Hadjipanayis C, Wanna G. The use of the exoscope in lateral skull base surgery: advantages and limitations. Otol Neurotol. 2019;40(02):236–240. doi: 10.1097/MAO.0000000000002095. [DOI] [PubMed] [Google Scholar]
- 18.Murai Y, Sato S, Yui K. Preliminary clinical microneurosurgical experience with the 4K3-dimensional microvideoscope (ORBEYE) system for microneurological surgery: observation study. Oper Neurosurg (Hagerstown) 2019;16(06):707–716. doi: 10.1093/ons/opy277. [DOI] [PubMed] [Google Scholar]
- 19.Amoo M, Henry J, Javadpour M. Beyond magnification and illumination: preliminary clinical experience with the 4K 3D ORBEYE™ exoscope and a literature review. Acta Neurochir (Wien) 2021;163(08):2107–2115. doi: 10.1007/s00701-021-04838-8. [DOI] [PubMed] [Google Scholar]
- 20.Ariffin M HM, Ibrahim K, Baharudin A, Tamil A M. Early experience, setup, learning curve, benefits, and complications associated with exoscope and three-dimensional 4K hybrid digital visualizations in minimally invasive spine surgery. Asian Spine J. 2020;14(01):59–65. doi: 10.31616/asj.2019.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu T, Toyota S, Nakagawa K. Retrosigmoid approach in the supine position using ORBEYE: a consecutive series of 14 cases. Neurol Med Chir (Tokyo) 2021;61(01):55–61. doi: 10.2176/nmc.tn.2020-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwan K, Schneider J R, Du V. Lessons learned using a high-definition 3-dimensional exoscope for spinal surgery. Oper Neurosurg (Hagerstown) 2019;16(05):619–625. doi: 10.1093/ons/opy196. [DOI] [PubMed] [Google Scholar]
- 23.Hart S G, Staveland L E. Oxford; North-Holland: 1988. Development of NASA-TLX (Task Load Index): results of empirical and theoretical research; pp. 139–183. [Google Scholar]
- 24.Wilson M R, Poolton J M, Malhotra N, Ngo K, Bright E, Masters R S. Development and validation of a surgical workload measure: the surgery task load index (SURG-TLX) World J Surg. 2011;35(09):1961–1969. doi: 10.1007/s00268-011-1141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vassiliou M C, Feldman L S, Andrew C G. A global assessment tool for evaluation of intraoperative laparoscopic skills. Am J Surg. 2005;190(01):107–113. doi: 10.1016/j.amjsurg.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Borg G AV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(05):377–381. [PubMed] [Google Scholar]
- 27.Morrow M MB, Lowndes B, Fortune E, Kaufman K R, Hallbeck M S. Validation of inertial measurement units for upper body kinematics. J Appl Biomech. 2017;33(03):227–232. doi: 10.1123/jab.2016-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAtamney L, Nigel Corlett E. RULA: a survey method for the investigation of work-related upper limb disorders. Appl Ergon. 1993;24(02):91–99. doi: 10.1016/0003-6870(93)90080-s. [DOI] [PubMed] [Google Scholar]
- 29.Wang T, Law K E, Harless C, Nguyen M-D, Hallbeck MS. Surgeon postures during deep inferior epigastric perforator flap breast reconstruction procedures: a pilot study. Proc Hum Factors Ergon Soc Annu Meet. 2020;64(01):632–633. [Google Scholar]
- 30.Liu N, Greenberg J A. Robotics vs laparoscopy-are they truly rivals? JAMA Surg. 2020;155(05):388. doi: 10.1001/jamasurg.2020.0052. [DOI] [PubMed] [Google Scholar]
- 31.Moschovas M C, Bhat S, Sandri M. Comparing the approach to radical prostatectomy using the multiport da Vinci Xi and da Vinci SP robots: a propensity score analysis of perioperative outcomes. Eur Urol. 2021;79(03):393–404. doi: 10.1016/j.eururo.2020.11.042. [DOI] [PubMed] [Google Scholar]


