Abstract
From 2 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) household transmission studies (enrolling April 2020 to January 2022) with rapid enrollment and specimen collection for 14 days, 61% (43/70) of primary cases had culturable virus detected ≥6 days post-onset. Risk of secondary infection among household contacts tended to be greater when primary cases had culturable virus detected after onset. Regardless of duration of culturable virus, most secondary infections (70%, 28/40) had serial intervals <6 days, suggesting early transmission. These data examine viral culture as a proxy for infectiousness, reaffirm the need for rapid control measures after infection, and highlight the potential for prolonged infectiousness (≥6 days) in many individuals.
Keywords: SARS-CoV-2, household transmission, secondary infection risk, viral culture
Using data from 2 SARS-CoV-2 household transmission studies with daily specimen collection, we examine viral culture as a proxy for infectiousness, reaffirm the need for rapid control measures after infection, and highlight the potential for prolonged infectiousness in many individuals.
(See the Editorial Commentary by Schiffer on pages 1339–42.)
As the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic evolves, there is continued need to understand the duration of infectiousness and how it impacts transmission. Several studies have reported that individuals with symptomatic uncomplicated SARS-CoV-2 virus infection, across multiple virus variants, shed cultureable virus 4–11 days after illness onset [1–3]. However, it remains unclear whether viral culture is an adequate marker of infectiousness, if there are other clinical or individual markers for infectiousness, and how the period of infectiousness contributes to the risk of secondary transmission [4, 5].
We combined 2 studies conducting daily prospective follow-up of persons acutely infected with SARS-CoV-2 and their household contacts. We described the frequency and duration of culturable virus detection in primary cases and assessed whether the duration of culturable virus detection was associated with household transmission and the timing of secondary infection.
METHODS
From April 2020 to January 2022, 2 case-ascertained household transmission studies were conducted in Nashville, Tennessee and San Francisco, California. Ethical approvals were provided by Vanderbilt University and the University of California-San Francisco (UCSF); the Centers for Disease Control and Prevention (CDC) reviewed these activities and relied on these approvals. Activities were conducted consistent with applicable federal law and CDC policy (see 45 C.F.R. part 46; 21 C.F.R. part 56).
We recruited individuals acutely ill with laboratory-confirmed SARS-CoV-2 infection and enrolled them and their household contacts within 7 days of illness onset (defined as first day of symptom onset or date of positive polymerase chain reaction [PCR] test for asymptomatic cases) [6, 7]. Primary cases and their household contacts were instructed to self-collect nasal swabs daily for at least 14 days after illness onset or enrollment. Specimens were tested for SARS-CoV-2 using reverse transcription-quantitative PCR (RT-qPCR). Whole-genome sequencing was conducted on positive specimens from San Francisco at UCSF following the ARTIC Network amplicon-based sequencing protocol adapted for SARS-CoV-2 [8]. Sequences were generated using a MinION instrument (Oxford Nanopore Technologies) and assembled using the nCoV-2019 novel coronavirus bioinformatics protocol [8, 9]. Viral lineages were assigned using Phylogenetic Assignment of Named Global Outbreak Lineages [10]. Seven households enrolled from San Francisco were missing viral lineage data, and lineage was assumed based on the dominant circulating variant at enrollment. We assumed that SARS-CoV-2 viruses detected in the Nashville households were not variants of concern or interest (non-VOC/VOI) given that all Nashville households were enrolled before August 2020 and the first case with a documented VOC was in September 2020 [11].
We sent specimens from Nashville to CDC (Atlanta, GA) and specimens from San Francisco to UCSF for viral culture. Specimens meeting prespecified cycle threshold (Ct) cutoffs (Nashville, Ct <40 for any target; or San Francisco, Ct <40 for nucleocapsid and envelope gene targets) were inoculated onto Vero E6-TMRPSS2 cells with (UCSF) or without (CDC) coexpression of hACE2 and assessed for cytopathic effect 2–7 days after inoculation (Figure 1). Results were reported as culture positive or culture negative and RT-qPCR was used to confirm SARS-CoV-2 in presumptive positive cultures [7, 12].
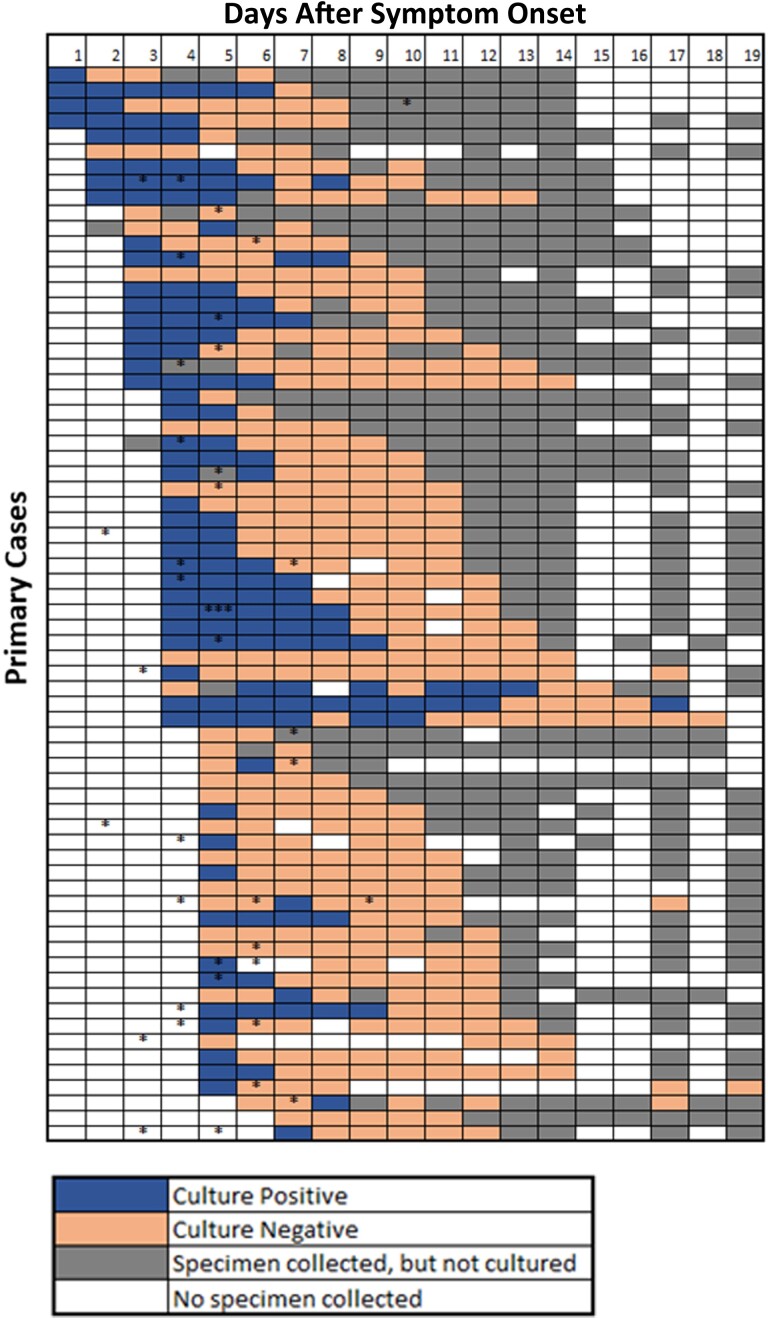
Figure 1.
SARS-CoV-2 primary case daily culture status and timing of secondary infections from date of primary case illness onset through follow-up—household transmission studies in TN and CA, 2020–2022. Viral culture was performed on nasal specimens that met the following characteristics: (1) Nashville, TN, all SARS-CoV-2 RT-qPCR–positive (Ct < 40) specimens; and (2) San Francisco, CA, all SARS-CoV-2 RT-qPCR–positive (Ct < 40 for 2 gene targets, N and E) specimens up to day 14. After 14 days, SARS-CoV-2 RT-qPCR–positive (Ct < 40 for 2 gene targets, N and E) specimens were cultured until 3 consistent negative cultures were observed. Each asterisk (*) indicates days during follow-up when a household contact with SARS-CoV-2 infection had illness onset. Abbreviations: Ct, cycle threshold; RT-qPCR, reverse transcription quantitative polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Within each household, we defined the primary case as the SARS-CoV-2–infected participant with the earliest illness onset date, using the date of the first known SARS-CoV-2–positive test when symptom onset was unknown. We excluded 34 households with coprimary cases (primary case and an infected household contact with onset <2 days apart) and 6 households that did not have viral culture data available for the primary case. We considered household contacts as likely secondary cases if they had SARS-CoV-2 infection and either symptom onset or first PCR-positive specimen within 10 days of the primary case's onset. We calculated serial intervals, as a surrogate for generation time, defined as days between symptom onset in the primary case and an infected household contact.
We used Poisson regressions with generalized estimating equations, accounting for household clustering, to examine whether the risk of infection among household contacts was associated with culturable virus in the primary case. Each Poisson regression included the number of household members enrolled to account for crowding. We conducted 3 analyses using different definitions of exposure to culturable virus in the primary case: analysis 1, exposure was defined by whether the primary case had culturable virus detected at least once during follow-up (assumes cases with no culturable virus detected were never culture positive during their infection); analysis 2, exposure was days from illness onset to last culture-positive specimen, where primary cases who had no culturable virus detected during follow-up were assigned a duration equal to the days between their illness onset and study enrollment (allowing the maximum possible duration); and analysis 3, exposure was ≥6 days versus <6 days of culturable virus detected after illness onset, assigning primary cases with no culturable virus detected to <6 days. Using analysis 3, we explored confounding by age of the contact (<30 vs ≥ 30 years), study location, or study month. Seeing no evidence of confounding, we present all 3 analyses adjusted for household size only.
For sensitivity analyses, we repeated analysis 2 setting the duration for cases with no culturable virus detected during follow-up to 0 days. With analysis 3, we first modified group assignments assuming all primary cases had culturable virus for the full duration between illness onset and study enrollment and, second, included the 6 households previously excluded for lack of primary culture results, assigning households to groups assuming the primary case had culturable virus for the duration between illness onset and study enrollment. Finally, we constructed a 3-level variable for the primary case's duration of culturable-virus detection: ≥ 6 days postonset, 1–5 days postonset, and no culturable virus detected in study follow-up. Analyses were conducted using SAS version 9.4.
RESULTS
Seventy households (70 primary cases and 114 household contacts), enrolled from April 2020 to January 2022, met our inclusion criteria for analysis (Supplementary Figure A). Primary cases were infected with non-VOC/VOI (71%), Alpha (1%), Delta (23%), and Omicron (4%) lineage SARS-CoV-2 viruses (Supplementary Table A). Of the 70 primary cases (enrolled a median of 4 days after illness onset; interquartile range [IQR], 3–5 days; maximum, 7 days), 53 (76%) had at least 1 specimen with culturable virus detected during follow-up (median duration of culturable virus detection after illness onset, 6 days; IQR, 0–7 days; maximum, 18 days; Figure 1). Primary cases with culturable virus detected ≥6 days after illness onset (61%; 43/70) were slightly older but otherwise similar to those who had culturable virus detected for a shorter period of time (Supplementary Table A). Cough, sore throat, and fatigue were more prevalent among those with a longer duration of culturable virus (Supplementary Table A).
Forty (35% of 114; Supplementary Table B) household contacts were infected with SARS-CoV-2 within 10 days of the primary case's illness onset; 28 (70% of 40) had serial intervals <6 days, and 12 (30% of 40) contacts had serial intervals of 6–10 days. Serial intervals were similar between infection pairs regardless of the duration of culturable virus in the primary case (Supplementary Table C).
Forty percent (34/85) of household contacts of primary cases with culturable virus detected became infected with SARS-CoV-2, compared with 21% (6/29) of household contacts of primary cases with no culturable virus detected (adjusted risk ratio [aRR], 1.93; 95% confidence interval [CI], .87–4.27; Table 1). There was a nonsignificant increase in a contact's risk of becoming infected with SARS-CoV-2 with every additional day that a primary case had culturable virus (aRR, 1.02; 95% CI, .88–1.18). Contacts of primary cases with culturable virus for ≥6 days post-onset were more likely to be infected than contacts of primary cases with culturable virus detected for <6 days (41% vs 26%; aRR, 1.59; 95% CI, .84–3.01), although not statistically significant. These trends remained across analyses and when the model was additionally adjusted for age, site, or calendar time (Supplementary Table D).
Table 1.
Risk of Secondary SARS-CoV-2 Infection Among Household Contacts, by Viral Culture Positivity of the Household Primary Case—TN and CA, 2020–2022
| Viral Culture Positivity of the Household Primary Case | No. of Households | No. of Household Contacts | No. of Household Contacts With SARS-CoV-2 Infection (% of Household Contacts) | Risk Ratio (95% Confidence Interval) a | P Valueb |
|---|---|---|---|---|---|
| Overall | 70 | 114 | 40 (35.1) | – | – |
| Analysis 1 | |||||
| Positive viral culture ≥1 d during follow-up | 53 | 85 | 34 (40.0) | 1.93 (.87–4.27) | .11 |
| No positive viral cultures during follow-up | 17 | 29 | 6 (20.7) | Reference | |
| Analysis 2 | |||||
| No. of days culture positive after illness onset | 70 | 114 | – | 1.02, for a 1-d change in duration of positivity (.88–1.18) | .77 |
| Analysis 3 | |||||
| Positive viral cultures detected ≥ 6 d after illness onset | 43 | 71 | 29 (40.8) | 1.59 (.84–3.01) | .16 |
| Positive viral cultures detected < 6 d after illness onset, including never | 27 | 43 | 11 (25.6) | Reference | |
All analyses are adjusted for household size.
P value for generalized estimating equation (GEE) for Poisson regression model.
DISCUSSION
In a combined analysis of 2 single-site studies that rapidly recruited households after SARS-CoV-2 introduction and included daily follow-up, most primary cases had culturable virus detected at least once after illness onset and more than half remained culture positive 6 or more days after onset. We observed a high proportion of infection among household contacts, with a trend towards greater risk of infection among contacts when we detected culturable virus in a primary case or when a primary case had culturable virus for longer. We did not, however, see that prolonged detection of culturable virus in primary cases affected the timing of when household contacts became ill; transmission events still occurred early after the primary case was infected. Therefore, prolonged detection of culturable virus may be serving as a proxy for early infectious virus dynamics (such as higher infectious viral loads or timing of the peak viral load relative to infection or onset). Overall, these data reaffirm the need for rapid detection and isolation of SARS-CoV-2–infected persons, but also highlight the need for infected persons to continue using infection control measures after the first week of illness.
Although small, these studies add to the literature on the relationship of viral culture positivity with SARS-CoV-2 infectiousness. Having a laboratory marker of infectiousness would be helpful for studying predictors of, or assessing the impact of, vaccines and antivirals on infectiousness. Further work could focus on finding other markers of infectiousness, as viral cultures are complex assays that require access to biosafety level 3 laboratories. Longitudinal studies of acute SARS-CoV-2 infection that pair viral culture testing with widely available rapid antigen tests, for example, would be helpful. Host characteristics such as age or vaccination status, or their illness, such as symptoms or time since onset, as suggested by our study, may be useful in predicting duration of infectiousness. Further information on when and for how long a person infected with SARS-CoV-2 is infectious is needed and would enable more selective and effective prevention and control guidance [12, 13]. Furthermore, quantitative infectious viral load data, inclusive of the peak, will be necessary to understand the effects of virologic dynamics on infectiousness and transmission in future studies.
Currently, CDC recommends that SARS-CoV-2–infected individuals isolate for at least 5 days after illness onset, after which the individual should continue to wear a well-fitting mask when around others until 10 days postonset or after 2 sequential negative antigen tests taken 48 hours apart [14]. Most illnesses among household contacts in our studies occurred within 5 days of the primary case's onset, underscoring that current isolation guidance targets the period when most transmission occurred in households. However, it was common that a primary case had culturable virus detected for longer than 5 days; thus, continued use of mitigation measures, such as masking, is critical 6–10 days after onset to further prevent community transmission [1, 2, 15].
While our data support current public health guidance, they were underpowered to definitively demonstrate increased household transmission from primary cases with culturable virus. The interpretation of our data is further limited in that we did not know whether primary cases were culture positive between their first clinic test and study enrollment and thus have made assumptions about culture positivity during those days. Also, our estimate of transmission events that occurred on day 6 or later could be an overestimate because we know that the true generation time is likely shorter given the variability of the incubation period with SARS-CoV-2. Additionally, viral culture can be insensitive, particularly near the end of the infectious period; therefore, the duration of culture positivity in our analysis may be truncated. Next, there were differences in viral culture cell lines between laboratories; however, we did not observe a significant difference in the duration of culturable virus by site among the primary cases. Finally, these analyses may not be representative of infection and transmission dynamics such as serial intervals of future variants or of transmission within community settings.
In conclusion, these 2 studies provide unique data on the duration of culture positivity and secondary transmission risk within households. We found that the risk of secondary infection among household contacts may be greater when primary cases had culturable virus detected for longer than 5 days, although the majority of transmission occurred in the first 5 days of the primary case's illness, irrespective of the duration of culture positivity. Our findings reaffirm the need for rapid isolation and mitigation measures used over the course of illness in the home to reduce transmission.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Jessica E Deyoe, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
J Daniel Kelly, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA; Institute for Global Health Sciences, University of California, San Francisco, California, USA; San Francisco VA Medical Center, San Francisco, California, USA; F.I. Proctor Foundation, University of California, San Francisco, California, USA.
Carlos G Grijalva, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Gaston Bonenfant, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Scott Lu, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA; Institute for Global Health Sciences, University of California, San Francisco, California, USA.
Khamal Anglin, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA; Institute for Global Health Sciences, University of California, San Francisco, California, USA.
Miguel Garcia-Knight, Department of Microbiology and Immunology, University of California, San Francisco, California, USA.
Jesus Pineda-Ramirez, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA; Institute for Global Health Sciences, University of California, San Francisco, California, USA.
Melissa Briggs Hagen, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Sharon Saydah, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Glen R Abedi, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Sarah A Goldberg, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA; Institute for Global Health Sciences, University of California, San Francisco, California, USA.
Michel Tassetto, Department of Microbiology and Immunology, University of California, San Francisco, California, USA.
Amethyst Zhang, Department of Microbiology and Immunology, University of California, San Francisco, California, USA.
Kevin C Donohue, School of Medicine, University of California, San Francisco, California, USA.
Michelle C Davidson, School of Medicine, University of California, San Francisco, California, USA.
Ruth Diaz Sanchez, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA; Institute for Global Health Sciences, University of California, San Francisco, California, USA.
Manuella Djomaleu, Department of Microbiology and Immunology, University of California, San Francisco, California, USA.
Sujata Mathur, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA; Institute for Global Health Sciences, University of California, San Francisco, California, USA.
Joshua R Shak, San Francisco VA Medical Center, San Francisco, California, USA; School of Medicine, University of California, San Francisco, California, USA.
Steven G Deeks, Division of HIV, Infectious Disease, and Global Medicine, University of California, San Francisco, California, USA.
Michael J Peluso, Division of HIV, Infectious Disease, and Global Medicine, University of California, San Francisco, California, USA.
Charles Y Chiu, Division of Infectious Diseases, University of California, San Francisco, California, USA.
Yuwei Zhu, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Natasha B Halasa, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
James D Chappell, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Alexandra Mellis, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Carrie Reed, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Raul Andino, Department of Microbiology and Immunology, University of California, San Francisco, California, USA.
Jeffrey N Martin, Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA.
Bin Zhou, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
H Keipp Talbot, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Claire M Midgley, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Melissa A Rolfes, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Notes
Acknowledgments . We acknowledge all the households that agreed in participate in the study.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC; cooperative agreement IP001083 and contract 75D30120C08009). The National Institute for Allergy and Infectious Diseases supported C. G. G. (grant number K24 AI148459) and J. D. K. (grant number K23 AI146268) during this study. The work used REDCap, which is supported by the National Center for Advancing Translational Sciences Clinical Translational Science Award Program (grant numbers UL1 TR002243 and 5UL1TR002243-03). C. Y. C. was supported in part by the CDC Epidemiology and Laboratory Capacity for Infectious Diseases (grant number 6NU50CK000539 to the California Department of Public Health [COVIDnet], the Innovative Genomics Institute at University of California Berkeley, and University of California San Francisco); and the CDC (grant number 75D30121C10991).
References
- 1. Boucau J, Marino C, Regan J, et al. Duration of shedding of culturable virus in SARS-CoV-2 Omicron (BA.1) infection. N Engl J Med 2022; 387:275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qutub M, Aldabbagh Y, Mehdawi F, et al. Duration of viable SARS-CoV-2 shedding from respiratory tract in different human hosts and its impact on isolation discontinuation polices revision; a narrative review. Clin Infect Pract 2022; 13:100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luna-Muschi A, Noguera SV, Borges IC, et al. Characterization of SARS-CoV-2 Omicron variant shedding and predictors of viral culture positivity on vaccinated healthcare workers with mild COVID-19. J Infect Dis 2022; 226:1726–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singanayagam A, Hakki S, Dunning J, et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis 2022; 22:183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Layan M, Gilboa M, Gonen T, et al. Impact of BNT162b2 vaccination and isolation on SARS-CoV-2 transmission in Israeli households: an observational study. Am J Epidemiol 2022; 191:1224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 infections in households—Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia-Knight M, Anglin K, Tassetto M, et al. Infectious viral shedding of SARS-CoV-2 delta following vaccination: a longitudinal cohort study. PLOS Pathog 2022; 18:e1010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quick J, Grubaugh ND, Pullan ST, et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat Protoc 2017; 12:1261–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tyson JR, James P, Stoddart D, et al. Improvements to the ARTIC multiplex PCR method for SARS-CoV-2 genome sequencing using nanopore. bioRxiv, https://doi.org/2020.09.04.283077, 4September2020, preprint: not peer reviewed. [Google Scholar]
- 10. Rambaut A, Holmes EC, O’Toole Á, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol 2020; 5:1403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization . Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-sars-cov-2-variants. Accessed 4 May 2022. [PubMed]
- 12. Bonenfant G, Deyoe JE, Wong T, et al. Surveillance and correlation of SARS-CoV-2 viral RNA, antigen, virus isolation, and self-reported symptoms in a longitudinal study with daily sampling. Clin Infect Dis 2022; 75:1698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Binnicker MJ. Can testing predict SARS-CoV-2 infectivity? The potential for certain methods to be surrogates for replication-competent virus. J Clin Microbiol 2021; 59:e0046921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. Isolation and Precautions for People with COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html. Accessed 4 May 2022.
- 15. Chu VT, Schwartz NG, Donnelly MAP, et al. Comparison of home antigen testing with RT-PCR and viral culture during the course of SARS-CoV-2 infection. JAMA Intern Med 2022; 182:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.