Abstract
Background
Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) is characterized by immune system dysregulation after exposure to adjuvants, such as aluminum. Although cases of autoimmune thyroid diseases caused by ASIA have been reported, Graves' disease is one of the rarer diseases. There are some reports that vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cause ASIA. Here, we describe a case of Graves’ disease following SARS-CoV-2 vaccination and a review of the literature.
Case presentation
A 41-year-old woman was admitted to our hospital because of palpitations and fatigue. Two weeks after receiving the second SARS-CoV-2 vaccine (BNT162b2, Coronavirus Modified Uridine messenger RNA (mRNA) Vaccine, Pfizer), she developed fatigue and gradually worsened. On admission, she exhibited thyrotoxicosis (thyroid-stimulating hormone (TSH) < 0.01 mIU/L (0.08–0.54), free triiodothyronine (FT3) 33.2 pmol/L (3.8–6.3), and free thyroxine (FT4) 72.1 pmol/L (11.6–19.3)) and palpitations associated with atrial fibrillation. TSH receptor antibody (TRAb) was positive (TRAb 5.0 IU/L (< 2.0)), and 99mTc scintigraphy showed diffuse uptake in the thyroid gland, suggesting that the thyrotoxicosis in this case was caused by Graves’ disease. Thiamazole was prescribed to correct her condition, and soon after this treatment was initiated, her symptoms and thyroid hormone levels were significantly reduced.
Conclusions
This case report reinforces the potential correlation between ASIA affecting the thyroid and SARS-CoV-2 mRNA vaccines. The clinical course suggests that it is essential to consider the possibility of developing ASIA, such as Graves' disease, after exposure to the SARS-CoV-2 vaccine.
Keywords: Graves’ disease, COVID-19, Vaccine, Autoimmune/inflammatory syndrome induced by adjuvants
Background
Graves’ disease is an autoimmune thyroid disease (AITD) and is recognized by the presence of thyroid-stimulating hormone (TSH) receptor antibody (TRAb) [1, 2]. That constitutively activates the thyroid gland, leading to a state of diffuse hyperthyroidism. Graves’ disease is the most common cause of hyperthyroidism. The underlying factors involved in the development of Graves' disease remain uncertain. A few genetic loci have been suggested, as well as smoking, stress, and exposure to great amounts of iodine, despite a lack of conclusive causal relationships [3].
Adjuvants encompass several substances commonly used in vaccines to boost immune reactivity toward antigens, and autoimmune/inflammatory syndrome induced by adjuvants (ASIA) is characterized by innate and adaptive immune system dysregulation after exposure to adjuvants, such as aluminum [4, 5]. Although cases of AITD caused by ASIA have been reported, most of them were destructive thyroiditis, and Graves' disease is a rare occurrence [6–8]. COVID-19 poses a significant threat to the entire world. Vaccines of different technologies and types have been developed to reduce COVID-19 infection and severity [9]. However, there are some reports that vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cause ASIA, including cases of Graves’ disease [10, 11].
Here, we present a case of Graves’ disease following SARS-CoV-2 vaccination and describe other cases found through a literature review.
Case presentation
A 41-year-old woman was admitted to our hospital because of palpitations and fatigue. Three months prior to hospitalization, she received the first vaccine for SARS-CoV-2 (BNT162b2, Coronavirus Modified Uridine messenger RNA (mRNA) Vaccine, Pfizer), and only mild pain at the vaccination site was observed. Three weeks later, she received the second dose of the vaccine. The next day, she had a fever and took acetaminophen orally, and the fever disappeared in one day. Two weeks later, she developed fatigue and gradually worsened. A week before admission, she went to the emergency department because of palpitations associated with atrial fibrillation and demonstrated thyrotoxicosis (TSH < 0.01 mIU/L (0.08–0.54, CLEIA method), free triiodothyronine (FT3) 28.3 pmol/L (3.8–6.3, CLEIA method), and free thyroxine (FT4) 61.8 pmol/L (11.6–19.3, CLEIA method)). At that time, she went home having received only symptomatic treatment. At the follow-up visit, her general status was not improved, and she was hospitalized to treat thyrotoxicosis.
She had no family history of endocrine diseases, including thyroid disease. She was a nonsmoker and nondrinker. She was not exposed to intense stress or excess iodine. She had no past history and did not have a preceding cold. She did not have a prior COVID-19 infection. Two months before the appearance of her symptoms, the electrocardiogram showed sinus rhythm, and 18 months before, her thyroid hormone levels were normal. Her body weight had decreased by 3 kg in the three months before admission. Her menstruation was regular before hospitalization, and she was not pregnant. She had irregularly taken iron supplements due to iron deficiency anemia.
Her height was 163 cm, her body weight was 39 kg, and her body mass index (BMI) was 14.7 kg/m2. Her consciousness was clear. Her blood pressure was 107/52 mmHg, her pulse rate was 100 beats/min and irregular, her body temperature was 36.7 °C, and her oxygen saturation (SpO2) was 100%. Her anterior neck was not swollen and without pain. She had a tremor. She had diarrhea without stomach pain. The other physical findings were unremarkable. She did not have eye symptoms.
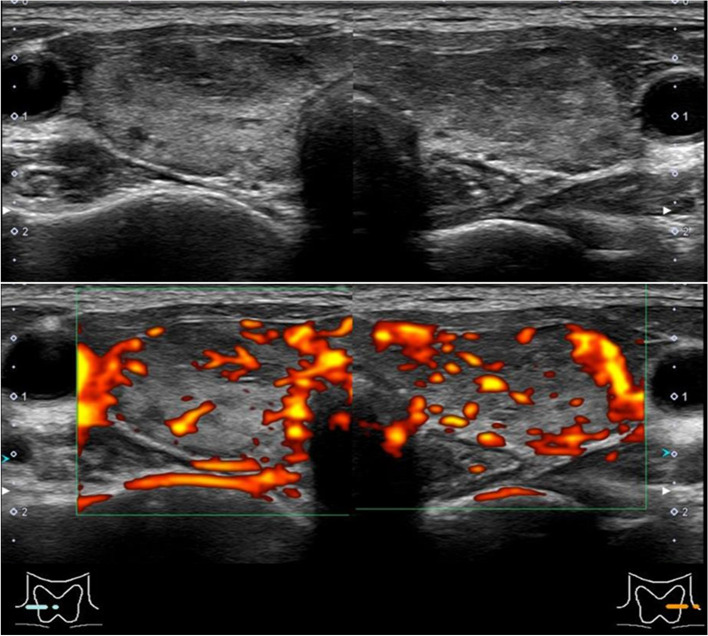
The laboratory findings on admission are shown in Table 1. She had severe thyrotoxicosis (TSH < 0.01 mIU/L, FT3 33.2 pmol/L, FT4 72.1 pmol/L). The presence of only gastrointestinal manifestation (diarrhea) suggested that she did not have a thyroid crisis. TRAb and thyroid-stimulating antibody (TSAb) were positive (TRAb 5.0 IU/L (< 2.0, CLEIA method) and TSAb 262% (< 120, EIA method)). Inflammatory markers were not elevated. Thyroglobulin antibody (Tg-Ab) and thyroid peroxidase antibody (TPO-Ab) were negative. Antinuclear antibody, which was screened to check for other autoimmune diseases, was also negative. Ultrasound revealed heterogeneous tissue and increased blood flow throughout the thyroid gland without diffuse goiter (Fig. 1). 99mTc pertechnetate scintigraphy showed diffuse uptake in the thyroid gland, and 99mTc uptake was 11.0% (0.5–3.5) (Fig. 2). These findings suggested that thyrotoxicosis in this case was caused by Graves’ disease, not destructive thyroiditis. An electrocardiogram showed slight tachycardia due to atrial fibrillation. Echocardiography revealed no abnormalities.
Table 1.
Baseline laboratory data
| Parameter | Observed | Reference range |
|---|---|---|
| Serum characteristics | ||
| CRP, mg/dL | 0.1 | < 0.3 |
| ESR (1 h), mm | 13 | 3–15 |
| FT3, pmol/L | 33.2 | 3.8–6.3 |
| FT4, pmol/L | 72.1 | 11.6–19.3 |
| TSH, mIU/L | < 0.01 | 0.08–0.54 |
| TRAb, IU/L | 5.0 | < 2.0 |
| TSAb, % | 262 | < 120 |
| Tg-Ab, IU/mL | 0.9 | < 4.1 |
| TPO-Ab, IU/mL | 2.2 | < 5.6 |
| Tg, ng/mL | 121 | < 34 |
CRP C-reactive protein, ESR erythrocyte sedimentation rate, FT3 free triiodothyronine, FT4 free thyroxine, TSH thyroid-stimulating hormone, TRAb TSH receptor antibody, TSAb thyroid stimulating antibody, Tg-Ab thyroglobulin antibody, TPO-Ab thyroid peroxidase antibody, Tg thyroglobulin
Fig. 1.
Thyroid ultrasonography. Right lobe: 15 × 50 × 13 mm. Left lobe: 13 × 50 × 11 mm. Increased blood flow throughout the thyroid gland without diffuse goiter was revealed
Fig. 2.
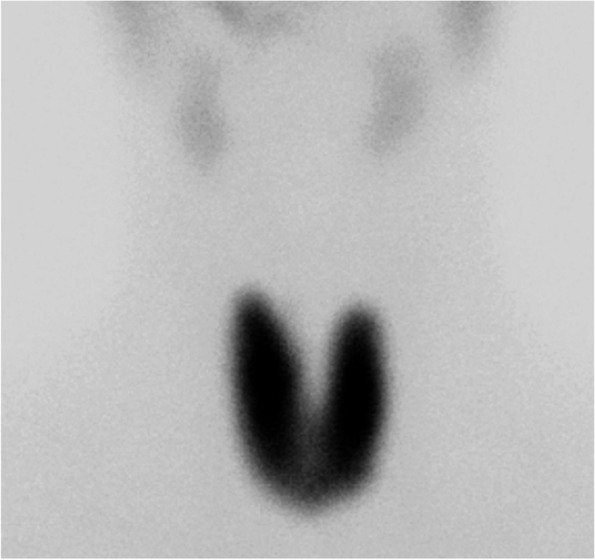
99mTc scintigraphy. Diffuse uptake in the thyroid gland was revealed
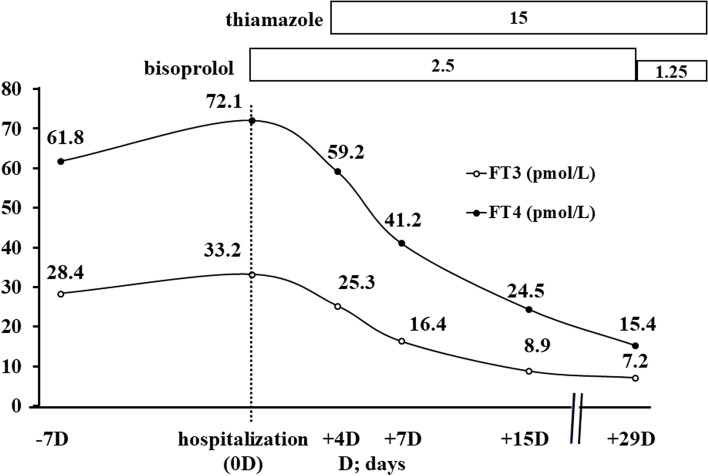
Thiamazole and bisoprolol were prescribed to correct her condition (Fig. 3). Soon after this treatment was initiated, her palpitations and fatigue were significantly relieved, and she was discharged from the hospital. After that, her thyroid hormone levels improved, and the bisoprolol dose was reduced. There were no side effects associated with the treatment.
Fig. 3.
Clinical course. When the patient went to the emergency department because of palpitations associated with atrial fibrillation, her TSH was under the detection limit, and her thyroid hormone levels were elevated. After admission, she was absolutely rested and treatment with bisoprolol was initiated. After diagnosis with Graves’ disease, treatment with thiamazole was initiated and her symptoms and thyroid hormones improved dramatically
Discussion and conclusions
We reported a case of Graves’ disease following SARS-CoV-2 vaccination. Treatment with thiamazole was significantly effective for her condition.
Graves’ disease is an AITD marked by the presence of TRAb, with manifestations of diffuse goiter, thyrotoxicosis, and ophthalmopathy [1, 2]. The most common cause of hyperthyroidism is Graves’ disease. The causes of Graves’ disease are a loss of immunotolerance and the development of autoantibodies that stimulate thyroid follicular cells by binding to the TSH receptor. Our patient displayed symptoms due to excess thyroid hormones, such as palpitations due to atrial fibrillation, weight loss, fatigue, tremor, diarrhea, and menstrual disturbances. In addition, she demonstrated thyrotoxicosis with TSH levels under the detection limit and elevated levels of thyroid hormone, TRAb and TSAb positivity, and diffuse uptake in the thyroid gland on 99mTc scintigraphy, so her symptoms were consistent with a diagnosis of Graves’ disease. She did not display diffuse goiter or ophthalmopathy. The low BMI found in our case was already present prior to the vaccination and is not part of the present discussion.
COVID-19 is caused by the novel beta-coronavirus SARS-CoV-2 [12, 13]. There are some reports of cases with Graves’ disease after COVID-19. The spike proteins covering SARS-CoV-2 bind to angiotensin-converting enzyme 2 (ACE2) receptors for its initial entry [14]. ACE2 expression levels are higher in the small intestine, testis, kidneys, heart, lung, adipose tissue, and thyroid [15]. SARS-CoV-2 may affect the thyroid via ACE2, the viral receptor, resulting in the onset of AITD, such as Graves’ disease [12, 13]. The cytokine storm associated with COVID-19 infection may also induce thyroid dysfunction.
An immunologic adjuvant is a substance that enhances the antigen-specific immune response without triggering one on its own [4, 5]. Adjuvants are commonly used to boost an immune response to treatments such as vaccination. ASIA was first coined in 2011 with the aim of codifying disorders characterized by innate and adaptive immune system dysregulation after adjuvant exposure [4]. Although the diagnostic criteria of ASIA are not clearly determined, the major signs of exposure to a preceding external stimulus (infection, vaccine, silicone, and adjuvant), ‘typical’ clinical manifestations, and improvement induced by removal of the inciting agent suggest a diagnosis of ASIA. In addition, the appearance of autoantibodies or antibodies directed at the suspected adjuvant is a minor diagnostic sign. Genetic predispositions for vaccine reactions such as ASIA remain uncertain [4]. ASIA includes various autoimmune diseases, such as collagen disease, blood disease, hepatic disease, neurological disease, and endocrine disease [5]. Most cases of AITD caused by ASIA are autoimmune subacute thyroiditis, which appear most often after exposure to human papillomavirus (HPV) vaccine, followed by influenza vaccine [8]. There are some reports of autoimmune Hashimoto's thyroiditis as an AITD; however, there are few reports of Graves' disease [6–8]. Our patient was exposed to the SARS-CoV-2 vaccine prior to clinical manifestations due to excess thyroid hormone and the appearance of thyroid antibodies of Graves’ disease. These findings suggested that the thyrotoxicosis in this case was due to Graves’ disease caused by ASIA.
To date, 11 different vaccines have been granted an emergency use listing by the World Health Organization and used worldwide, including mRNA, protein subunit, viral vector, and inactivated vaccines [9]. Similar to other vaccines, there are some reports of the development of autoimmune diseases after exposure to the SARS-CoV-2 vaccine, such as collagen disease, heart disease, blood disease, hepatic disease, and endocrine disease [10, 16, 17]. No predictive factors have been associated with SARS-CoV-2 vaccine-associated ASIA. Side effects after exposure to the SARS-CoV-2 vaccine were reported to each appropriate authority worldwide, and reports of Graves' disease caused by ASIA were included in these reports. The cases that had been published in journals at the time of submission are shown in Table 2. We identified a total of 62 cases of Graves’ disease following SARS-CoV-2 vaccination, excluding our case. All patients showed positive TRAb or thyroid-stimulating immunoglobulin, and TRAb-positive cases without thyrotoxicosis were excluded. The table was categorized according to the sales company of the vaccines. We identified 32 patients who received the Pfizer mRNA vaccine; [11, 18–36] six patients who received the Moderna mRNA vaccine; [20, 28, 31, 37–39] 12 patients who received mRNA vaccines from unknown companies; [40] ten patients who received the AstraZeneca vaccine, including those from the Serum Institute of India; [37, 41–44] and one patient each who received Johnson & Johnson and Sinovac vaccines [27, 38]. Characteristically, across all companies, most patients with Graves’ disease are relatively young women, which is consistent with Graves’ disease in general. In most cases, symptoms developed within several days after vaccination. Inconsistent with the common clinical signs of Graves’ disease, there were some patients without goiter. Almost all confirmed cases showed good treatment responsiveness. According to previous reports, patients and their immunological background were not related. There were few descriptions of ophthalmopathy and past history of COVID-19 infection, and we were not able to confirm these data. Comparing patients who received mRNA vaccines with those from other companies, we recognized frequent onset of symptoms after not only the first but also the second and later mRNA vaccinations.
Table 2.
Reported cases of Graves’ disease after exposure to SARS-CoV-2 vaccination
| Our case | Pfizer (n = 32) |
Moderna (n = 6) |
Pfizer and Moderna (n = 50) |
AstraZeneca (n = 10) |
Johnson & Johnson (n = 1) |
Sinovac (n = 1) |
|
|---|---|---|---|---|---|---|---|
| Vaccine Type | mRNA (Pfizer) | mRNA | mRNA | mRNA | Viral vector | Viral vector | Inactivated |
| Age, year | 41 | 44.8 (22–74) | 45.8 (36–63) | 43.4 (22–74) | 43.3 (19–70) | 68 | 44 |
| Male, n (%) | Female | 9 (28) | 2 (33) | 12 (24) | 3 (30) | Female | Female |
| Time of symptom onset after vaccination, day | 14 | 18.5 (1–120) | 16.3 (2–46) | 18.7 (1–120) | 13.2 (2–31) | 32 | 7 |
| Dose, n (%) | Second |
First 21 (66) Second 10 (31) Third 1 (3) |
First 3 (50) Second 3 (50) |
First 29 (58) Second 20 (40) Third 1 (2) |
First 9 (90) Second 1 (10) |
First | First |
| Enlarged thyroid, n (%) | No | 18/24 (75) | 2/4 (50) | 32/40 (80) | 1/8 (13) | NA | NA |
| Relapse, n (%) | No | 4 (13) | 1/5 (20) | 11/49 (22) | 2 (20) | No | Yes |
| FT3, pmol/L | 33.2 | 19.8 (7.8–44.1) | 27.5 (n = 1) | 21.3 (6.3–44.1) | 30.7 (n = 1) | 21.2 | 14.8 |
| FT4, pmol/L | 72.1 | 48.4 (21–108) | 50.4 (20–77) | 48.2 (14–108) | 43.7 (29–61) | 46.3 | 34.4 |
| Positive Tg-Ab, n (%) | Yes | 14/22 (64) | 3/4 (75) | 17/26 (65) | 3/3 (100) | NA | Yes |
| Positive TPO-Ab, n (%) | Yes | 17/26 (65) | 5/5 (100) | 22/31 (71) | 7/8 (83) | Yes | Yes |
mRNA messenger RNA, FT3 free triiodothyronine, FT4 free thyroxine, Tg-Ab thyroglobulin antibody, TPO-Ab thyroid peroxidase antibody, NA not available
The mRNA-based SARS-CoV-2 vaccine (Pfizer and Moderna) uses lipid nanoparticles to facilitate the transport of mRNA into cells and is widely used worldwide, including in our patient [45, 46]. The vaccine contains a number of excipients and lipids, one of which is based on polyethylene glycol (PEG), which may induce an immune response in specific individuals in rare cases [46]. In a viral vector vaccine (AstraZeneca and Johnson & Johnson), this role could be played by polysorbate 80; in an inactivated vaccine (Sinovac), this role could be played by aluminum salts [47, 48]. On the other hand, Vojdani et al. showed that many thyroid peroxidase (TPO) peptide sequences shared homology or similarity with sequences in various SARS-CoV-2 proteins. Furthermore, the SARS-CoV-2 spike protein, nucleoprotein, and membrane protein all cross-reacted with TPO. This suggests that cross-recognition between the modified SARS-CoV-2 spike protein encoded in the mRNA vaccine and thyroid target proteins may promote AITD [49, 50].
In summary, we reported a case of Graves’ disease after exposure to the SARS-CoV-2 vaccine. Treatment with thiamazole was significantly effective for her condition. This case report reinforces the potential correlation between ASIA affecting the thyroid and SARS-CoV-2 vaccination. The clinical course suggests that it is essential to consider the possibility of developing ASIA, such as Graves' disease, after exposure to the SARS-CoV-2 vaccine.
Acknowledgements
Not applicable.
Abbreviations
- AITD
Autoimmune thyroid disease
- TSH
Thyroid stimulating hormone
- TRAb
TSH receptor antibody
- ASIA
Autoimmune/inflammatory syndrome induced by adjuvants
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- mRNA
Messenger RNA
- FT3
Free triiodothyronine
- FT4
Free thyroxine
- BMI
Body mass index
- SpO2
Oxygen saturation
- TSAb
Thyroid stimulating antibody
- Tg-Ab
Thyroglobulin antibody
- TPO-Ab
Thyroid peroxidase antibody
- ACE2
Angiotensin-converting enzyme 2
- HPV
Human papillomavirus
- PEG
Polyethylene glycol
- TPO
Thyroid peroxidase
Authors’ contributions
KT, NI, YA, and KU managed the patient as the attending physicians. MN and KK evaluated the medical management strategies. KT, MN, and KK wrote the paper. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Consent for publication was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no competing interests in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Leo S, Lee SY, Braverman LE. Hyperthyroidism Lancet. 2016;388:906–918. doi: 10.1016/S0140-6736(16)00278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subekti I, Pramono LA. Current Diagnosis and Management of Graves’ Disease. Acta Med Indones. 2018;50:177–82. [PubMed]
- 3.Effraimidis G, Wiersinga WM. Mechanisms in endocrinology: autoimmune thyroid disease: old and new players. Eur J Endocrinol. 2014;170:R241–R252. doi: 10.1530/EJE-14-0047. [DOI] [PubMed] [Google Scholar]
- 4.Shoenfeld Y, Agmon-Levin N. “ASIA” - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36:4–8. [DOI] [PubMed]
- 5.Watad A, Bragazzi NL, McGonagle D, Adawi M, Bridgewood C, Damiani G, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) demonstrates distinct autoimmune and autoinflammatory disease associations according to the adjuvant subtype: Insights from an analysis of 500 cases. Clin Immunol. 2019;203:1–8. doi: 10.1016/j.clim.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Watad A, David P, Brown S, Shoenfeld Y. Autoimmune/Inflammatory Syndrome Induced by Adjuvants and Thyroid Autoimmunity. Front Endocrinol (Lausanne) 2016;7:150. doi: 10.3389/fendo.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leiva S, Madrazo J, Podesta C. Narcolepsy with cataplexy and hyperthyroidism sudden appeared after H1N1 vaccination. Sleep Sci. 2018;11:34–36. doi: 10.5935/1984-0063.20180008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bragazzi NL, Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metab. 2020;34:101412. doi: 10.1016/j.beem.2020.101412. [DOI] [PubMed] [Google Scholar]
- 9.COVID19 VACCINE TRACKER. https://covid19.trackvaccines.org/agency/who/. Accessed 5 March 2023.
- 10.Ishay Y, Kenig A, Tsemach-Toren T, Amer R, Rubin L, Hershkovitz YI, et al. Autoimmune phenomena following SARS-CoV-2 vaccination. Int Immunopharmacol. 2021;99:107970. doi: 10.1016/j.intimp.2021.107970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sánchez Valadez TI, Jara LJ. Two Cases of Graves' Disease Following SARS-CoV-2 Vaccination: An Autoimmune/Inflammatory Syndrome Induced by Adjuvants. Thyroid. 2021. [DOI] [PubMed]
- 12.Duntas LH, Jonklaas J. COVID-19 and Thyroid Diseases: A Bidirectional Impact. J Endocr Soc. 2021;5:bvab076. [DOI] [PMC free article] [PubMed]
- 13.Murugan AK, Alzahrani AS. SARS-CoV-2 plays a pivotal role in inducing hyperthyroidism of Graves’ disease. Endocrine. 2021;73:243–54. [DOI] [PMC free article] [PubMed]
- 14.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vuille-Lessard É, Montani M, Bosch J, Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J Autoimmun. 2021;123:102710. doi: 10.1016/j.jaut.2021.102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lui DTW, Lee KK, Lee CH, Lee ACH, Hung IFN, Tan KCB. Development of Graves’ Disease After SARS-CoV-2 mRNA Vaccination: A Case Report and Literature Review. Front Public Health. 2021;9: 778964. [DOI] [PMC free article] [PubMed]
- 19.Patrizio A, Ferrari SM, Antonelli A, Fallahi P. A case of Graves’ disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J Autoimmun. 2021;125:102738. doi: 10.1016/j.jaut.2021.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weintraub MA, Ameer B, Sinha GN. Graves Disease Following the SARS-CoV-2 Vaccine: Case Series. J Investig Med High Impact Case Rep. 2021;9:23247096211063356. doi: 10.1177/23247096211063356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goblirsch TJ, Paulson AE, Tashko G, Mekonnen AJ. Graves’ disease following administration of second dose of SARS-CoV-2 vaccine. BMJ Case Rep. 2021;14:e246432. doi: 10.1136/bcr-2021-246432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto K, Mashiba T, Takano K, Suzuki T, Kami M, Takita M, et al. A Case of Exacerbation of Subclinical Hyperthyroidism after First Administration of BNT162b2 mRNA COVID-19 Vaccine. Vaccines (Basel) 2021;9:1108. doi: 10.3390/vaccines9101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pujol A, Gómez LA, Gallegos C, Nicolau J, Sanchís P, González-Freire M, et al. Thyroid as a target of adjuvant autoimmunity/infammatory syndrome due to mRNA-based SARS-CoV2 vaccination: from Graves’ disease to silent thyroiditis. Endocrinol Invest. 2022;45:875–882. doi: 10.1007/s40618-021-01707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manta R, Martin C, Muls V, Poppe KG. New-onset Graves’ disease following SARS-CoV-2 vaccination: a case report. Eur Thyroid J. 2022;11:e220049. doi: 10.1530/ETJ-22-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zettinig G, Krebs M. Two further cases of Graves' disease following SARS-Cov-2 vaccination. J Endocrinol Invest. 2021:1–2. [DOI] [PMC free article] [PubMed]
- 26.Sakai M, Takao K, Kato T, Ito K, Kubota S, Hirose T, et al. Graves’ Disease after Administration of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine in a Type 1 Diabetes Patient. Intern Med. 2022;61:1561–1565. doi: 10.2169/internalmedicine.9231-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bostan H, Ucan B, Kizilgul M, Calapkulu M, Hepsen S, Gul U, et al. Relapsed and newly diagnosed Graves’ disease due to immunization against COVID-19: A case series and review of the literature. J Autoimmun. 2022;128:102809. doi: 10.1016/j.jaut.2022.102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.PlaPeris B, Merchante Alfaro AÁ, MaravallRoyo FJ, AbellánGaliana P, Pérez Naranjo S, González BM. Thyrotoxicosis following SARS-COV-2 vaccination: a case series and discussion. J Endocrinol Invest. 2022;45:1071–1077. doi: 10.1007/s40618-022-01739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taieb A, Sawsen N, Asma BA, Ghada S, Hamza E, Yosra H, et al. A rare case of grave’s disease after SARS-CoV-2 vaccine: is it an adjuvant effect? Eur Rev Med Pharmacol Sci. 2022;26:2627–2630. doi: 10.26355/eurrev_202204_28500. [DOI] [PubMed] [Google Scholar]
- 30.Hamouche W, El Soufi Y, Alzaraq S, Okafor BV, Zhang F, Paras C. A case report of new onset graves’ disease induced by SARS-CoV-2 infection or vaccine? J Clin Transl Endocrinol Case Rep. 2022;23:100104. doi: 10.1016/j.jecr.2021.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chua MWJ. Graves’ disease after COVID-19 vaccination. Ann Acad Med Singap. 2022;51:127–128. doi: 10.47102/annals-acadmedsg.2021398. [DOI] [PubMed] [Google Scholar]
- 32.Brès F, Joyeux MA, Delemer B, Vitellius G, Barraud S. Three cases of thyroiditis after COVID-19 RNA-vaccine. Ann Endocrinol (Paris) 2022;83:262–264. doi: 10.1016/j.ando.2022.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruggeri RM, Giovanellla L, Campennì A. SARS-CoV-2 vaccine may trigger thyroid autoimmunity: real-life experience and review of the literature. J Endocrinol Invest. 2022;45:2283–2289. doi: 10.1007/s40618-022-01863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lioulios G, Tsouchnikas I, Dimitriadis C, Giamalis P, Pella E, Christodoulou M, et al. Two Cases of Autoimmune Thyroid Disorders after COVID Vaccination in Dialysis Patients. Int J Mol Sci. 2022;23:11492. doi: 10.3390/ijms231911492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oğuz SH, Şendur SN, İremli BG, Gürlek A, Erbas T, Ünlütürk U. SARS-CoV-2 Vaccine-induced Thyroiditis: Safety of Revaccinations and Clinical Follow-up. J Clin Endocrinol Metab. 2022;107:e1823–e1834. doi: 10.1210/clinem/dgac049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierman G, Delgrange E, Jonas C. Recurrence of Graves’ Disease (a Th1-type Cytokine Disease) Following SARS-CoV-2 mRNA Vaccine Administration: A Simple Coincidence? Eur J Case Rep Intern Med. 2021;8: 002807. [DOI] [PMC free article] [PubMed]
- 37.Shih SR, Wang CY. SARS-CoV-2 vaccination related hyperthyroidism of Graves’ disease. J Formos Med Assoc. 2022;121:1881–1882. doi: 10.1016/j.jfma.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh G, Howland T. Graves’ Disease Following COVID-19 Vaccination. Cureus. 2022;14: e24418. [DOI] [PMC free article] [PubMed]
- 39.Yasuda S, Suzuki S, Yanagisawa S, Morita H, Haisa A, Satomura A, et al. HLA typing of patients who developed subacute thyroiditis and Graves’ disease after SARS-CoV-2 vaccination: a case report. BMC Endocr Disord. 2023;23:54. doi: 10.1186/s12902-023-01287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chee YJ, Liew H, Hoi WH, Lee Y, Lim B, Chin HX, et al. SARS-CoV-2 mRNA Vaccination and Graves’ Disease: A Report of 12 Cases and Review of the Literature. J Clin Endocrinol Metab. 2022;107:e2324–e2330. doi: 10.1210/clinem/dgac119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sriphrapradang C, Shantavasinkul PC. Graves’ disease following SARS-CoV-2 vaccination. Endocrine. 2021;74:473–474. doi: 10.1007/s12020-021-02902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhary S, Dogra V, Walia R. Four cases of Graves’ disease following viral vector severe acute respiratory syndrome corona virus-2 (SARSCoV-2) vaccine. Endocr J. 2022;69:1431–1435. doi: 10.1507/endocrj.EJ22-0208. [DOI] [PubMed] [Google Scholar]
- 43.Cuenca D, Aguilar-Soto M, Mercado M. A Case of Graves’ Disease Following Vaccination with the Oxford-AstraZeneca SARS-CoV-2 Vaccine: Case Report and Review of the Literature. Eur J Case Rep Intern Med. 2022;9:003275. doi: 10.12890/2022_003275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan BC, Luo RR. Thyrotoxicosis in patients with a history of Graves’ disease after SARS-CoV-2 vaccination (adenovirus vector vaccine): Two case reports. World J Clin Cases. 2023;11:1122–1128. doi: 10.12998/wjcc.v11.i5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nanomedicine and the COVID-19 vaccines. Nat Nanotechnol. 2020;15:963. [DOI] [PMC free article] [PubMed]
- 46.Garvey LH, Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth. 2021;126:e106–e108. doi: 10.1016/j.bja.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borgsteede SD, Geersing TH, Tempels-Pavlica Ž. Other excipients than PEG might cause serious hypersensitivity reactions in COVID-19 vaccines. Allergy. 2021;76:1941–1942. doi: 10.1111/all.14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patrizio A, Ferrari SM, Elia G, Ragusa F, Paparo SR, Mazzi V, et al. Graves’ Disease Following SARS-CoV-2 Vaccination: A Systematic Review. Vaccines (Basel) 2022;10:1445. doi: 10.3390/vaccines10091445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vojdani A, Vojdani E, Kharrazian D. Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins With Tissue Antigens: Implications for Autoimmune Diseases. Front Immunol. 2021;11:617089. doi: 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Triantafyllidis KK, Giannos P, Stathi D, Kechagias KS. Graves’ disease following vaccination against SARS-CoV-2: A systematic review of the reported cases. Front Endocrinol (Lausanne). 2022;13: 938001. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.