Abstract
STUDY QUESTION
Does cancer itself, before any gonadotoxic treatment, affect ovarian function in reproductive-aged patients?
SUMMARY ANSWER
Our study revealed that women with cancer may have decreased ovarian reserve markers even before cancer therapy.
WHAT IS KNOWN ALREADY
With the field ‘oncofertility’ improving rapidly, cancer therapy-mediated ovarian damage is well characterized. However, there is a controversy about whether cancer itself affects ovarian function before gonadotoxic treatment.
STUDY DESIGN, SIZE, DURATION
We conducted a systematic meta-analysis investigating the association between cancer and ovarian function prior to gonadotoxic treatment. Titles or abstracts related to ovarian reserve (e.g. anti-Müllerian hormone (AMH), antral follicle count (AFC), or basal follicle-stimulating hormone (FSH)) combined with titles or abstracts related to the exposure (e.g. cancer*, oncolog*, or malignan*) were searched in PubMed, Embase, and Web of Science databases from inception to 1 February 2022.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We included cohort, case-control, and cross-sectional studies in English that examined ovarian reserve in reproductive-aged patients (18–45 years) with cancer compared to age-matched controls before cancer treatment. The quality of the included studies was assessed by ROBINS-I. Fixed or random effects were conducted to estimate standard or weighted mean difference (SMD or WMD, respectively) and CI. Heterogeneity was assessed by the Q test and I2 statistics, and publication bias was evaluated by Egger’s and Begg’s tests.
MAIN RESULTS AND THE ROLE OF CHANCE
The review identified 17 eligible studies for inclusion. The results showed that cancer patients had lower serum AMH levels compared to healthy controls (SMD = −0.19, 95% CI = −0.34 to −0.03, P = 0.001), especially women with hematological malignancies (SMD = −0.62, 95% CI = −0.99 to −0.24, P = 0.001). The AFC was also decreased in patients with cancer (WMD = −0.93, 95% CI = −1.79 to −0.07, P = 0.033) compared to controls, while inhibin B and basal FSH levels showed no statistically significant differences.
LIMITATIONS, REASONS FOR CAUTION
Serum AMH and basal FSH levels in this meta-analysis showed high heterogeneity, and the small number of studies contributing to most subgroup analyses limited the heterogeneity analysis. Moreover, the studies for specific cancer subtypes may be too small to draw conclusions; more studies are needed to investigate the possible impact of cancer type and stage on ovarian function.
WIDER IMPLICATIONS OF THE FINDINGS
Our study confirmed the findings that cancer per se, especially hematological malignancies, negatively affects serum AMH level, and AFC values of reproductive-aged women. However, the lower AMH levels and AFC values may also be due to the changes in ovarian physiology under oncological conditions, rather than actual lower ovarian reserves. Based on the meta-analysis, clinicians should raise awareness about the possible need for personalized approaches for young women with cancer who are interested in pursuing fertility preservation strategies before anticancer treatments.
STUDY FUNDING/COMPETING INTEREST(S)
This work was financially supported by the National Natural Science Foundation of China (nos 81873824, 82001514, and 81902669) and the Applied Basic Research Program of Wuhan Municipal Bureau of Science and Technology (2019020701011436). The authors declare that they have no conflicts of interest.
REGISTRATION NUMBER
PROSPERO (CRD42021235954).
Keywords: ovarian reserve, ovarian function, AMH, inhibin B, AFC, FSH, cancer, fertility preservation, oncofertility, reproductive age
WHAT DOES THIS MEAN FOR PATIENTS?
Over the past decades, the incidence of cancer in young women has increased constantly. Cancer therapy-mediated damage to the ovary is well characterized, but whether cancer itself, prior to cytotoxic treatment, affects ovarian function is controversial. Ovarian reserve is generally defined as the quantity of oocytes remaining in the ovary, and markers of ovarian reserve include the hormone levels of anti-Müllerian hormone (AMH), inhibin B, and basal follicle-stimulating hormone (FSH) and the sonographically measured antral follicle count (AFC). Among these, AMH and AFC are the most favorable indicators of ovarian reserve. Based on the results of this meta-analysis, patients with cancer had significant decreased serum AMH levels and AFC values before cancer treatment, while basal FSH levels and inhibin B levels showed no difference between cancer patients and control groups. From these results, we suggest that cancer itself may have negative effects on ovarian reserve. These findings may provide valuable information to clinicians and patients for choosing effective fertility preservation measures before cancer treatment.
Introduction
The incidence of cancer has increased over the past decades, and there is a rising trend in young women (Pilleron et al., 2021). A well-known long-term adverse effect of cancer treatment in reproductive-aged female cancer survivors is gonadal dysfunction, which often results in premature ovarian insufficiency (POI) (Spears et al., 2019; Xiong et al., 2021). Currently, a growing body of clinical evidence suggests that cancer itself may have negative effects on reproductive function. A previous study reported that cancer, especially lymphoma, adversely affects the male reproductive system prior to the initiation of therapy (Fitoussi et al., 2000). This relationship may be due to a direct cytotoxic effect of the cancer itself on testicular function or alterations of the immunological response (Rueffer et al., 2001). However, whether cancer per se impairs ovarian reserve remains controversial.
Ovarian reserve is generally defined as the quantity of oocytes remaining in the ovary (Broer et al., 2014). Markers of ovarian reserve include hormone levels (serum anti-Müllerian hormone (AMH), inhibin B, basal follicle-stimulating hormone (FSH)) and sonographically measured features (antral follicle count (AFC)) of the ovaries (Steiner et al., 2017; Tal and Seifer, 2017; Younis et al., 2020). Among them, AMH and AFC have demonstrated the most favorable analytical and performance characteristics as indicators of ovarian reserve (Iliodromiti et al., 2015). Serum AMH levels remain consistent throughout the menstrual cycle, with no significant variability between the follicular and luteal phases, and levels are not affected by short-term oral contraception (OCP) (Broer et al., 2014; Dewailly et al., 2014). In contrast, AFCs vary across cycles and exhibit limited utility for studies in cancer patients (Iliodromiti et al., 2015). However, both AMH and AFC show considerable variability in their clinical definitions and technical methods, which have not been standardized internationally (Practice Committee of the American Society for Reproductive Medicine, 2020). Researchers have recently focused on the effects of cancer on ovarian reserve. Some clinical studies have observed lower AMH levels in reproductive-aged women with cancer before treatment compared with unaffected women (Lutchman Singh et al., 2007; Lie Fong et al., 2008; Bala et al., 2016; Naasan et al., 2016; Paradisi et al., 2016a,b; Decanter et al., 2018; Porcu et al., 2020). Similarly, Decanter et al. (2018) showed that AFC was significantly lower in cancer patients. In contrast, other studies have reached the opposite conclusion, having observed no effect of cancer on ovarian reserve (Paradisi et al., 2016a,b; Goldrat et al., 2019; Gunnala et al., 2019; Hussein et al., 2021). Therefore, no definite conclusion about ovarian reserve before gonadotoxic treatments in patients with cancer can be drawn.
To shed light on this issue, we performed a systematic review and meta-analysis to clarify the relationship between cancer and ovarian reserve before anticancer treatment. We aimed to analyze ovarian reserve function in patients with different types of cancer prior to treatment. To our knowledge, this is the first meta-analysis of case-controlled studies assessing ovarian reserve in patients with cancer and comparing the outcomes with those in age-matched infertile or healthy women. Our results may provide some guidance on fertility protection for patients even before cancer treatment.
Materials and methods
We performed this systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The protocol for this systematic review and meta-analysis was registered in PROSPERO (Registration ID Number CRD42021235954).
Literature search
We performed a broad-range search strategy for eligible research articles in English in the PubMed, Embase and Web of Science databases from inception to 1 February 2022. Titles or abstracts related to ovarian reserve (e.g. AMH, AFC, or FSH) were combined with titles or abstracts related to exposure (e.g. cancer*, oncolog*, or malignan*). The full search strategy is shown in Supplementary Table S1. We also searched the reference lists of selected publications to retrieve additional studies that were not identified in the database search.
Study selection
Cohort, case–control, and cross-sectional studies that examined ovarian reserve between patients with cancer and age-matched controls in the same setting, overall and/or by cancer type were included. The inclusion criteria were patients with mean age 18–45 years, no chemotherapy and/or radiotherapy, and no ovarian surgery, such as ovarian cystectomy or unilateral oophorectomy. Women taking OCP which may influence the ovarian reserve were excluded. Studies about women with a history of ovarian cancer, endometriosis, polycystic syndrome, or other ovarian diseases were excluded. Case series, case reports, reviews, meta-analyses, commentaries, letters, and animal studies were also excluded. Studies that compared ovarian reserve in women with cancer to a non-age-matched control group or those without a control group (non-cancer healthy population) were excluded. Studies that presented exclusively without full-text publication, with inappropriate raw data, or as conference abstracts were excluded. When the same study population was used in different publications, we selected the study with the largest number of participants.
Data extraction
General information was collected from each study (first author’s last name, publication year, geographical, and clinical setting), study characteristics (study period, design, and inclusions/exclusions), and participants' characteristics (sample size, mean age, cancer type, fertility status, number of participants, control population, and adjustment factors). Serum AMH, inhibin B, basal FSH hormonal (follicular phase FSH) levels, and AFC values (mean or median and SD, SEM, 95% CI, and range) were extracted. Four reviewers (Q.Q.Z., M.W., Y.C.G,. and Y.B.H.) performed the primary evaluation by reviewing titles, abstracts, and keywords for relevance to cancer and ovarian reserve. The full text of selected articles was retrieved to provide the list of potentially eligible studies. Two authors (Q.Q.Z. and M.W.) performed the final assessment of the study eligibility. Q.Q.Z. extracted the data independently using a standard data extraction Excel form. M.W. checked the extractions for accuracy.
Heterogeneity
Heterogeneity between studies was assessed using Cochran’s Q and I2 statistics (Higgins and Thompson, 2002), and I2 with values of 0%, 25%, 50%, and 75% provided evidence for no, low, moderate, and high between-heterogeneity, respectively. Sensitivity analyses were performed by excluding one study at a time to clarify whether the results were driven by one large study or a study with an extreme result. Additional sensitivity analyses were performed for subgroup analyses whenever possible to evaluate potential sources of heterogeneity based on study characteristics, such as study design (i.e. cohort, case-control, and cross-sectional), geographic location, study quality per the ROBINS-I tool (‘low’ or ‘moderate’ versus ‘serious’ or ‘critical’), cancer type or biochemical measurements of the ovarian reserve markers. The subgroup differences were examined using meta-regression. For all analyses, the impact of the study sample size was accounted for in the statistical modeling and is represented by the width of the 95% CI.
Publication bias
The publication bias across studies was evaluated by using funnel plots for asymmetry, Egger’s, and Begg’s tests.
Risk of bias assessment
Based on the extracted data, we used the risk of bias in non-randomized studies of interventions (ROBINS-I) tool to assess the quality of the eligible studies (Sterne et al., 2016). The tool contains seven domains: bias due to confounding, bias in study participant selection, bias in exposure measurement, bias due to misclassification of exposure during follow-up, bias due to missing data, bias in outcome measurement, and bias in the selection of reported results. A study was deemed to have a low risk of bias when it was rated as probably at risk for one domain, and a study was considered at serious or critical risk of bias when rated as high risk for more than one domain or critical risk in at least one domain, respectively. ‘No information’ was applied to a study when information was missing in at least two domains. Two authors (Q.Q.Z. and Y.C.G.) performed the evaluation independently. Any disagreements were resolved via consensus or mediation by a third author (M.W.).
Outcomes and statistical analysis
Quantitative results in all settings were extracted or converted into means and SD. If mean or SD values were unavailable and the median, SEM, range, and 95% CI were reported; we estimated the mean and SD using the formulas suggested for meta-analysis to pool data (Wan et al., 2014; Luo et al., 2018). When there were multiple cancer groups and a single control group, the age between the two independent case–control groups was compared using Student’s t test. For continuous variables with consistent units, including inhibin B, basal FSH, and AFC, we calculated the weighted mean difference (WMD) with the associated 95% CIs between the study and control groups for the effect of cancer on ovarian reserve in the meta-analysis. For AMH variables with inconsistent units, we calculated the standard mean difference (SMD). Fixed or random effects meta-analysis was used according to the heterogeneity of pooled data. The statistical analyses were performed using Stata software (Stata Corp, College Station, TX, USA).
Results
Study selection
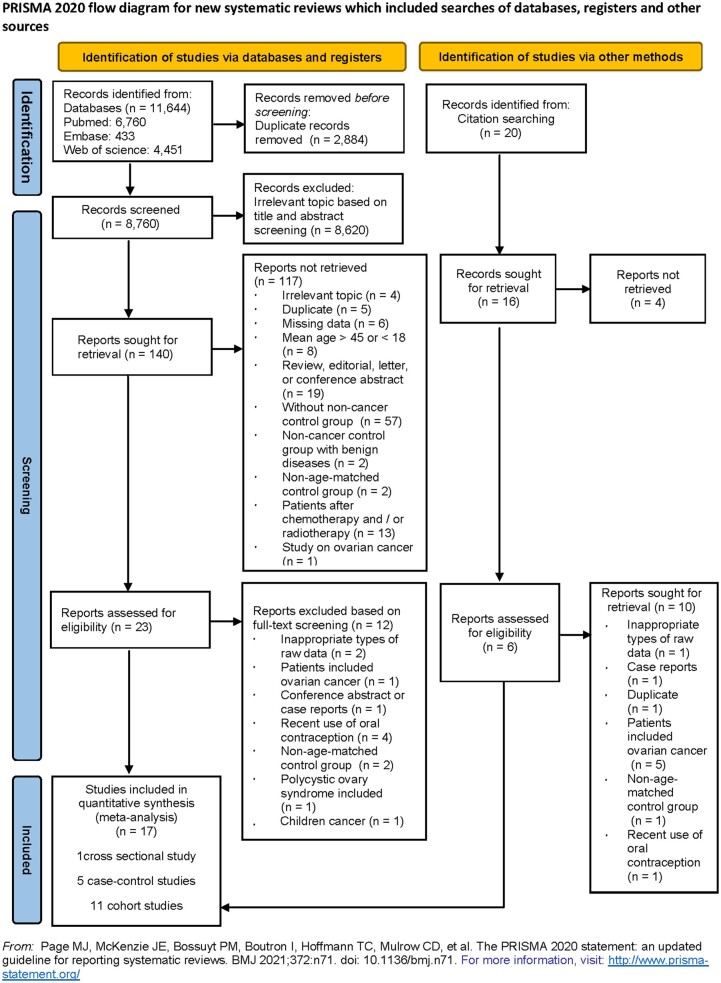
The initial search identified 11 644 records: including 6760 from PubMed, 433 from Embase, 4451 from Web of Science, and 20 from additional records from other sources. After excluding studies that did not meet the inclusion criteria, a total of 17 publications (11 cohort, 5 case–control, and 1 cross-sectional studies) were considered eligible for quantitative synthesis (Oktay et al., 2006; Lutchman Singh et al., 2007; Lie Fong et al., 2008; Quintero et al., 2010; Yu et al., 2010; Das et al., 2011; Johnson et al., 2013; Bala et al., 2016; Naasan et al., 2016; Paradisi et al., 2016a,b; Pereira et al., 2016; Quinn et al., 2017; Decanter et al., 2018; Goldrat et al., 2019; Gunnala et al., 2019; Porcu et al., 2020) (Fig. 1). The characteristics and results of the included studies are presented in Supplementary Table S2.
Figure 1.
Flow chart of study selection for a systematic review and meta-analysis of cancer and ovarian reserve.
Study characteristics
The 17 studies consisted of 8150 women of reproductive age with 1197 and 6953 in the cancer and control groups, respectively. To comprehensively understand the effects of cancer on ovarian reserve, we pooled all of the literature on corresponding ovarian reserve biomarkers for an overall cancer analysis regardless of cancer type.
Of these, there were enough data for subtype analyses of breast cancer and hematological malignancies, and so the corresponding data were combined for subsequent analyses on cancer types. Eight studies related to breast cancer (Oktay et al., 2006; Lutchman Singh et al., 2007; Yu et al., 2010; Bala et al., 2016; Pereira et al., 2016; Quinn et al., 2017; Goldrat et al., 2019; Porcu et al., 2020), with four studies reporting serum AMH levels (Yu et al., 2010; Bala et al., 2016; Pereira et al., 2016; Goldrat et al., 2019), two studies separately reporting basal FSH hormone levels and AFC values (Oktay et al., 2006; Quinn et al., 2017), and two studies reporting multiple ovarian reserve parameters (e.g. AMH, inhibin B, and AFC) (Lutchman Singh et al., 2007; Porcu et al., 2020). Hematological malignancies were analyzed in another two studies (Lie Fong et al., 2008; Paradisi et al., 2016a,b), both of which reported serum AMH levels. Three additional studies evaluated overall cancers without describing the cancer type in detail (Johnson et al., 2013; Paradisi et al., 2016a,b; Decanter et al., 2018), with AMH and AFC levels analyzed. Another three studies focused on multiple cancer types (e.g. breast cancer, endometrial cancer, brain cancer, and bone cancer) (Das et al., 2011; Naasan et al., 2016; Gunnala et al., 2019), for which serum AMH, basal FSH, and AFC were reported, respectively. One particular study reported on both overall cancer and breast cancer (Quintero et al., 2010), and basal FSH levels for each were analyzed.
Quality of evidence and risk of bias assessment
According to the ROBINS-I tool, three studies had a low risk of bias (Das et al., 2011; Paradisi et al., 2016a,b; Decanter et al., 2018), five studies had a moderate risk of bias (Lutchman Singh et al., 2007; Bala et al., 2016; Naasan et al., 2016; Paradisi et al., 2016a,b; Pereira et al., 2016), and nine studies had a serious risk of bias (Oktay et al., 2006; Lie Fong et al., 2008; Quintero et al., 2010; Yu et al., 2010; Johnson et al., 2013; Quinn et al., 2017; Goldrat et al., 2019; Gunnala et al., 2019; Porcu et al., 2020) (Table 1).
Table 1.
Risk of bias for the 17 included studies (2006–2020) based on the ROBINS-I tool (low, moderate, serious, and critical).
| Author, year, location | Type of bias |
Overall rating | ||||||
|---|---|---|---|---|---|---|---|---|
| Bias due to confounding | Bias due to selection of participants | Bias due to exposure assessment | Bias due to misclassification during follow-up | Bias due to missing data | Bias due to measurement of the outcome | Bias due to selective reporting of the results | ||
| Oktay et al., 2006, USA | Serious | Low | Low | Serious | Low | Moderate | Serious | Serious |
| Lutchman Singh et al., 2007, UK | Low | Low | Low | Low | Moderate | Low | Moderate | Moderate |
| Lie Fong et al., 2008, The Netherlands | Serious | Low | Serious | Serious | Serious | Moderate | Moderate | Serious |
| Quintero et al., 2010, USA | Serious | Low | Low | Serious | Low | Low | Moderate | Serious |
| Yu et al., 2010, USA | Serious | Low | Serious | Low | Low | Low | Moderate | Serious |
| Das et al., 2011, Canada | Low | Low | Low | Low | Low | Low | Low | Low |
| Johnson et al., 2013, USA | Serious | Low | Serious | Moderate | Low | Low | Moderate | Serious |
| Bala et al., 2016, India | Moderate | Low | Low | Low | No information | Moderate | Moderate | Moderate |
| Naasan et al., 2016, Ireland | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Paradisi et al., 2016a, Italy | Moderate | Low | Moderate | Low | No information | Low | Moderate | Moderate |
| Paradisi et al., 2016b, Italy | Low | Low | Low | Low | Low | Low | Low | Low |
| Pereira et al., 2016, USA | Low | Low | Low | Low | No information | Low | Moderate | Moderate |
| Quinn et al., 2017, USA | Serious | Serious | Low | Moderate | Low | Moderate | Moderate | Serious |
| Decanter et al., 2018, France | Low | Low | Low | Low | Low | Low | Low | Low |
| Goldrat et al., 2019, Belgium | Serious | Low | Low | Moderate | Low | Low | Moderate | Serious |
| Gunnala et al., 2019, USA | Serious | Low | Serious | Moderate | Low | Low | Moderate | Serious |
| Porcu et al., 2020, Italy | Serious | Low | Low | Serious | Low | Low | Moderate | Serious |
ROBINS-I tool, risk of bias in non-randomized studies of interventions tool.
Pooled AMH results and heterogeneity analyses
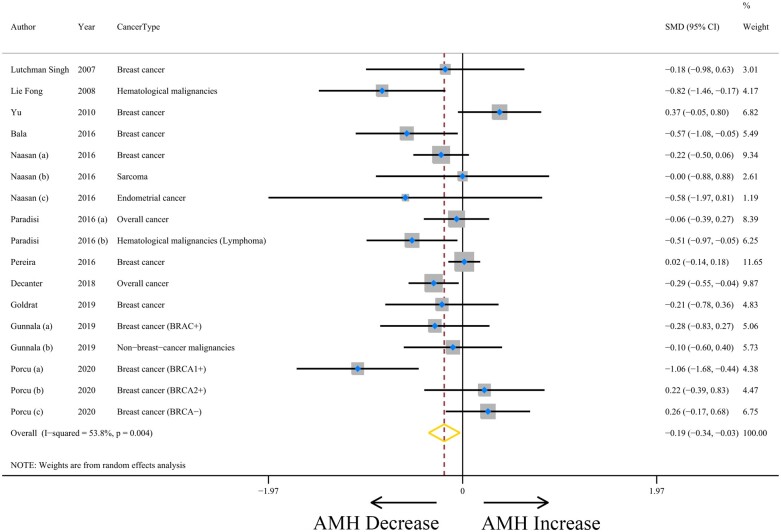
The meta-analysis included 12 studies that evaluated the association between cancer and serum AMH levels (Lutchman Singh et al., 2007; Lie Fong et al., 2008; Yu et al., 2010; Bala et al., 2016; Naasan et al., 2016; Paradisi et al., 2016a,b; Pereira et al., 2016; Decanter et al., 2018; Goldrat et al., 2019; Gunnala et al., 2019; Porcu et al., 2020) (Supplementary Table S3). For overall cancer, serum AMH levels, in 17 analyses, were lower overall compared to that of age-matched control participants (SMD = −0.19, 95% CI = −0.34 to −0.03, P = 0.021). However, there was moderate heterogeneity among the included studies (I2 = 53.8%, P = 0.004) (Fig. 2 and Table 2). We carried out subgroup analyses to detect the source of heterogeneity. However, this heterogeneity was not explained by cohort (SMD = −0.09, 95% CI = −0.31 to 0.13, I2 = 63.8%, P = 0.005), case–control (SMD = −0.32, 95% CI = −0.51 to −0.12, I2 = 17.1%, P = 0.300), or cross-sectional study design (SMD = −0.18, 95% CI = −0.98 to 0.63; Pheterogeneity = 0.223). The magnitude of heterogeneity was low when stratified by geographic location and cancer type except for studies of breast cancer (SMD = −0.13, 95% CI = −0.35 to 0.10, I2 = 62.2%, P = 0.005). There was a moderate level of heterogeneity within the categories of the AMH assay (IOT: SMD = −0.54, 95% CI = −1.16 to 0.08; DSL: SMD = 0.37, 95% CI = −0.05 to 0.80; AMH Gen II: SMD = −0.27, 95% CI = −0.43 to −0.11; DSL and AMH Gen II: SMD = −0.21, 95% CI = −0.47 to 0.05; Not reported: SMD = −0.11, 95% CI = −0.47 to 0.25; Pheterogeneity = 0.500). The observed heterogeneity could not be eliminated by the two ROBINS-I risk of bias categories (low or moderate: SMD = −0.19, 95% CI = −0.34 to −0.04; serious or critical: SMD = −0.17, 95% CI = −0.51 to 0.17; Pheterogeneity = 0.656) (Supplementary Table S4). No statistically significant heterogeneity was found through the above subgroup analyses. We speculated that control groups with infertility history (e.g. tubal and/or idiopathic factor) may explain the potential heterogeneity to some extent (Naasan et al., 2016; Goldrat et al., 2019). The influence analysis generated stable summary estimates without detecting influential publications (Supplementary Fig. S1). There was no evidence of publication bias causing overestimation of the association between the cancer effect and AMH values (Supplementary Fig. S2), as shown by Egger’s (P = 0.172) and Begg’s test (P = 0.303).
Figure 2.
The association between cancer and serum anti-Müllerian hormone (AMH) levels. WMD, weighted mean difference.
Table 2.
Results of the meta-analysis examining the association between cancer and ovarian reserve outcome measures.
| Overall cancer |
Breast cancer |
Hematological malignancies |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| na | Effect estimate | P | Heterogeneity | na | Effect estimate | P | Heterogeneity | na | Effect estimate | P | Heterogeneity | |
| (95% CI) | I 2, Pb | (95% CI) | I 2, Pb | (95% CI) | I 2, Pb | |||||||
| AMHc | 17 | −0.19 (−0.34, −0.03)R | 0.021 | 53.8%, 0.004 | 10 | −0.13 (−0.35, 0.10)R | 0.266 | 62.2%, 0.005 | 2 | −0.62 (−0.99, −0.24) | 0.001 | 0.0%, 0.452 |
| Inhibin Bd | 2 | −5.35 (−19.16, 8.47) | 0.448 | 0.0%, 0.876 | NC | NC | NC | NC | NC | NC | NC | NC |
| Basal FSHd | 11 | 0.00 (−0.85,0.85)R | 0.997 | 79.3%, 0.000 | 7 | 0.21 (−0.99, 1.41)R | 0.727 | 84.1%, 0.000 | NC | NC | NC | NC |
| AFCd | 16 | −0.93 (−1.79, −0.07) | 0.033 | 37.4%, 0.066 | 9 | −0.66 (−1.72, 0.41) | 0.225 | 0.0%, 0.842 | NC | NC | NC | NC |
Bold records denote statistically significant associations. All pooled effect estimates are derived from fixed-effects analyses, except for records marked with R(random-effects).
AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone; AFC, antral follicle count; CI, confidence interval; NC, not calculated.
aNumber of publications.
b P-value derived from Cochran Q statistic.
cAll analyses were based on SMD.
dAll analyses were based on WMD.
Because there was a significant difference between the standard mean AMH of overall cancer and healthy controls, we examined which type of cancer contributed to the decreased AMH value. Using the control individuals as a reference, the SMD in breast cancer was −0.13 (95% CI = −0.35 to 0.10, P = 0.266), with moderate heterogeneity (I2 = 62.2%, P = 0.005) (Table 2; Supplementary Fig. S3). Patients with hematological malignancies had significantly lower levels of serum AMH, with an estimated SMD of −0.62 (95% CI = −0.99 to −0.24, P = 0.001) and without heterogeneity (I2 = 0.0%, P = 0.452) (Table 2; Supplementary Fig. S4). The results show that breast cancer may not damage ovarian function, but hematological malignancies may lead to lower levels of AMH. However, further studies are needed to investigate the possible impact of cancer type and stage on the level of AMH.
Pooled inhibin B results
The analysis of the association between cancer and inhibin B levels included two studies (Lutchman Singh et al., 2007; Paradisi et al., 2016a,b) (Supplementary Table S3). There was no significant difference in serum inhibin B levels between patients with cancer and healthy controls with a WMD of −5.35 (95% CI = −19.16 to 8.47, P = 0.448). There was no evidence of heterogeneity (I2 = 0.0%, P = 0.876) (Table 2; Supplementary Fig. S5). No testing for publication bias was performed because of the small number of included studies.
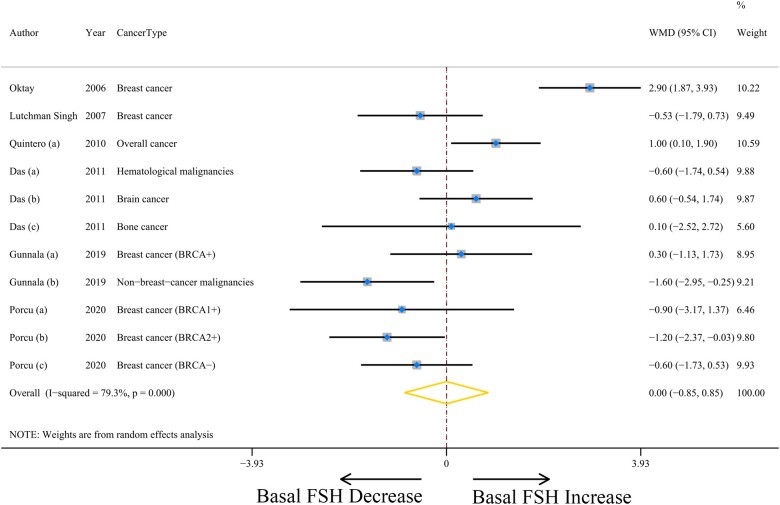
Pooled basal FSH results and heterogeneity analyses
A total of six studies were included in the analysis of basal FSH levels (Oktay et al., 2006; Lutchman Singh et al., 2007; Quintero et al., 2010; Das et al., 2011; Gunnala et al., 2019; Porcu et al., 2020) (Supplementary Table S3), and the weighted mean basal FSH values were not statistically significant between the cancer patients and control groups (WMD = 0.00, 95% CI = −0.85 to 0.85, P = 0.997) (Fig. 3). Heterogeneity between the studies was high (I2 = 79.3%, P = 0.000) (Table 2). There was a high level of heterogeneity within study designs, geographic location, ROBINS-I risk of bias, and cancer type (Supplementary Table S4), while no influential studies were found based on sensitivity analyses (Supplementary Fig. S6). There was no publication bias in eligible studies (all Egger’s or Begg’s test P > 0.28; Supplementary Fig. S7).
Figure 3.
The association between cancer and serum basal FSH. WMD, weighted mean difference.
Next, we performed subgroup analyses to find the association between types of cancer and basal FSH. Four studies focused on breast cancer were included in the analysis (Oktay et al., 2006; Lutchman Singh et al., 2007; Quintero et al., 2010; Porcu et al., 2020). A non-statistically significant WMD of 0.21 (95% CI = −0.99 to 1.41, P = 0.727) was found for breast cancer (Supplementary Fig. S8). Heterogeneity between these studies was high (I2 = 84.1%, P = 0.000) (Table 2).
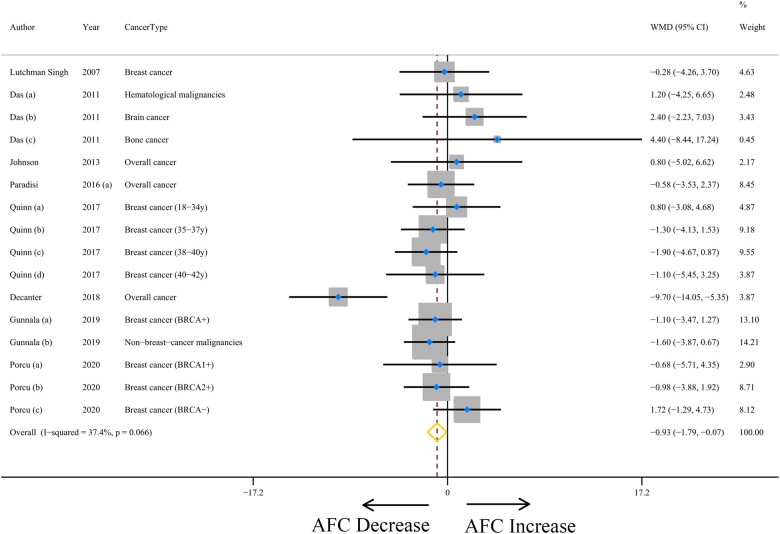
Pooled AFC results
Eight studies evaluating the impact of cancer on AFC values were included (Lutchman Singh et al., 2007; Das et al., 2011; Johnson et al., 2013; Paradisi et al., 2016a,b; Quinn et al., 2017; Decanter et al., 2018; Gunnala et al., 2019; Porcu et al., 2020) (Supplementary Table S3). When compared to controls, cancer patients had significantly lower weighted mean AFC values (WMD: −0.93, with 95% CI = −1.79 to −0.07; P = 0.033) (Fig. 4 and Table 2) with slight level of heterogeneity for this comparison (I2 = 37.4%, P = 0.066). No evidence of publication bias was found by Egger’s test (P = 0.524), although moderate publication bias was found by Begg’s test (P = 0.034) (Supplementary Fig. S9).
Figure 4.
The association between cancer and antral follicle count (AFC). WMD, weighted mean difference.
For the cancer type categories, there was an indication of a lower tendency in the mean AFC in patients with breast cancer compared with the controls, with a non-statistically significant difference (WMD: −0.66, with 95% CI = −1.72 to 0.41; P = 0.225). There was no evidence of heterogeneity (I2 = 0.0%, P = 0.842) (Table 2; Supplementary Fig. S10).
Discussion
Principal findings
Previous publications have revealed conflicting results when examining the association between cancer and ovarian reserve prior to therapy. To our knowledge, this study is the first comprehensive meta-analysis investigating ovarian reserve in patients with cancer before gonadal toxicity treatment. Collectively, the eligible studies include a large sample size of population, including 8150 women of reproductive age, with 1197 and 6953 in the cancer and control groups, respectively. The present study revealed that patients with cancer had significant decreased serum AMH levels and AFC values before cancer treatment, especially patients with hematological malignancies. Basal FSH levels and inhibin B levels showed no difference between cancer patients and control groups. These findings, which need to be confirmed in larger sample sizes, should be considered and may influence the oncofertility counseling of this specific patient population.
Comparison with existing literature
Breast cancer is the most common malignancy in women of reproductive age (Pashayan et al., 2020), and we investigated the association between breast cancer and ovarian reserve. Seven studies reported no significantly differences in AMH and AFC levels in patients with breast cancer (Lutchman Singh et al., 2007; Yu et al., 2010; Naasan et al., 2016; Pereira et al., 2016; Quinn et al., 2017; Goldrat et al., 2019; Gunnala et al., 2019). Conversely, only one study reported significantly lower AMH levels (Bala et al., 2016). Our meta-analysis also found that women with breast cancer showed no significant difference in serum AMH levels and AFC values compared with healthy controls. There were two studies that showed higher basal FSH levels (Oktay et al., 2006; Quintero et al., 2010), while the other two reported no difference in patients with breast cancer (Lutchman Singh et al., 2007; Gunnala et al., 2019). We revealed that the basal FSH levels also showed no significant difference between patients with breast cancer and healthy controls. Hence, our results revealed that breast cancer has no negative impact on ovarian reserve, while more original studies are needed to confirm this conclusion. Interestingly, an included study by Porcu et al. (2020) reported that breast cancer patients with BRCA1 mutations, but not BRCA2 mutations, had significantly lower AMH levels. Some studies suggested that BRCA1 or BRCA2 mutations were involved in infertility or POI (Oktay et al., 2010; Titus et al., 2013; Wang et al., 2014; Lambertini et al., 2018; Son et al., 2019; Turan et al., 2021). Therefore, it can be hypothesized that the missing BRCA1 repair proteins dramatically prevent breast cancer patients having enough ovarian reserve to enable a potential conception. However, due to the small number of articles, a meta-analysis on the relationship between BRCA mutations, breast cancer and ovarian reserve was not possible.
In recent decades, the survival rate after hematological malignancies has been improved significantly, but ovarian function is negatively affected by the detrimental effect of hematological malignancies on follicular health before the administration of chemotherapy or radiotherapy (Lie Fong et al., 2008; Lawrenz et al., 2012; van Dorp et al., 2014; Paradisi et al., 2016a,b). Lie Fong et al. (2008) and Paradisi et al. (2016a,b) reported lower AMH levels in patients with hematological malignancies before treatment compared to healthy controls. Patients with lymphoma had much lower AMH and AFC levels than healthy controls and patients with other malignancies (Lekovich et al., 2016). Another study also showed decreased serum AMH levels in pubertal girls with newly diagnosed leukemia and lymphoma before treatment (van Dorp et al., 2014). Higher concentrations of proinflammatory cytokines may lead to the reduction of ovarian reserve in lymphoma patients (Paradisi et al., 2016a,b). Similarly, our study revealed that AMH levels in patients with hematological malignancies were significantly lower than in controls. These findings highlighted that patients with hematological malignancies experience decreased AMH levels, although more studies should be performed to confirm this conclusion.
Few studies have explored other types of cancers and ovarian reserve, and some studies have analyzed the relationship between overall cancers and ovarian reserve (Das et al., 2011; Paradisi et al., 2016a,b; Dolinko et al., 2018). Das et al. (2011) and Dolinko et al. (2018) found no significant difference in AFC levels between women with overall cancers (including breast cancer, gynecological cancer, hematologic cancer, gastrointestinal cancer, brain cancer, and bone cancer) and their control groups. Johnson et al. (2013) also found that cancer patients and control subjects did not differ in ovarian reserve markers. In contrast, a decreased serum AMH concentration was shown in women with gliomas (Nordan et al., 2020). Therefore, further original studies are needed to determine whether there is a difference in ovarian reserve between women with other types of cancers and healthy controls.
The reason for the decrease of ovarian reserve in patients with cancer is not clear. Previous studies have indicated that cancer was often characterized by anorexia, an increased catabolic state and malnutrition which results in weight loss (Chapman et al., 2009; Kuokkanen et al., 2016). Meczekalski et al. (2006) showed lower AMH levels in patients with weight loss-related amenorrhea. Cancer-associated stress hormone secretion is also responsible for a depleted ovarian reserve (Valsamakis et al., 2019). Another potential cause of the decreased ovarian reserve may be impaired DNA repair mechanisms. Genetic variation in DNA repair genes is associated with cancer and ovarian aging (Turan and Oktay, 2020). BRCA mutations increase the risk of breast malignancy and ovarian cancer (Roy et al., 2011). Recent laboratory data also indicate that patients with BRCA mutations, especially BRCA1 mutations, generally have reduced AMH concentrations (Daum et al., 2018; Porcu et al., 2020; Turan and Oktay, 2020). Other factors, such as immune inflammation caused by tumors, cannot be excluded. Paradisi et al. (2016a,b) noted that patients with lymphoma with lower AMH levels had higher concentrations of soluble IL-2 receptor (SIL-2R), IL-6, IL-8, and TNF-α compared to healthy volunteers, which indicated that cytokines may be the causal factor in the reduction of ovarian reserve. According to the conclusions reported in the literature, the tumor-associated changes in physical status, stress, genetic variation, and/or immune inflammation may explain for the decreased ovarian reserve prior to cancer treatments.
Strengths and limitations
Our systematic review and meta-analysis is the first study targeted female ovarian reserve prior to gonadotoxic treatments, comparing patients with cancer to healthy controls, and showing significant decreases in serum AMH levels and AFC values. However, in oncological conditions, the physiology of the ovary may change. The equilibrium between the remnant follicular pool and the number of growing follicles may be tempered so that one has lower AMH or AFC, but not lower ovarian reserve. High levels of heterogeneity were found in some indexes, i.e. serum AMH and basal FSH levels. The heterogeneity remained high in AMH and basal FSH levels when subgroup analyses were delimited to studies design, geographic location, assay method, risk of bias, or cancer type. Due to the small number of studies contributing to most subgroup analyses, the power to find statistically significant heterogeneity among categories was extremely limited, which will adversely affect the current conclusions. In addition, surgery, chemotherapy, or radiotherapy treatments are required when tumor are diagnosed, so high quality randomized controlled trials or prospective cohorts about ovarian function before cancer treatment are lacking. Many of the studies included were of moderate to high risk of bias, which may lead to the high heterogeneity and imprecise results. Moreover, the number of studies for specific cancer subtypes may be too small to draw conclusions, and more studies are needed to investigate the possible impact of cancer type and stage on ovarian function.
Conclusion
In conclusion, our study confirmed the findings that cancer negatively affects the serum AMH levels as well as the AFC values of reproductive-aged women. Based on our meta-analysis, clinicians should raise awareness about the possible need for personalized approaches for young women with cancer who are interested in pursuing fertility preservation strategies before anticancer treatments. Fertility counseling and preservation strategies should be tailored to not only patient age and the type of treatment they will receive, but also to their specific type of cancer and their associated baseline ovarian reserve.
Supplementary Material
Contributor Information
Meng Wu, Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Qingqing Zhu, Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Yibao Huang, Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Weicheng Tang, Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Jun Dai, Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Yican Guo, Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Jiaqiang Xiong, Department of Obstetrics and Gynecology, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China.
Jinjin Zhang, Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Su Zhou, Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Fangfang Fu, Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Mingfu Wu, Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Shixuan Wang, Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Data availability
All data are available in the main text or the supplementary materials.
Authors’ roles
S.X.W. and M.F.W. conceived the idea of this systematic review and meta-analysis. M.W. and Q.Q.Z. contributed to study design and construction of the protocol. M.W., Q.Q.Z., Y.B.H., J.D., and Y.C.G. contributed to the data extraction and executed the study, performed the analyses and data interpretation. M.W. and Q.Q.Z. drafted the manuscript. M.W., Q.Q.Z., Y.B.H., W.C.T., and Y.C.G. checked the data; J.Q.X., W.C.T., J.J.Z., S.Z. and F.F.F revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (nos 81873824, 82001514, and 81902669) and the Applied Basic Research Program of Wuhan Municipal Bureau of Science and Technology (2019020701011436).
Conflict of interest
The authors declare no conflicts of interest.
References
- Bala J, Seth S, Dhankhar R, Ghalaut VS.. Chemotherapy: impact on anti-Mullerian hormone levels in breast carcinoma. J Clin Diagn Res 2016;10:BC19–BC21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer SL, Broekmans FJ, Laven JS, Fauser BC.. Anti-Mullerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update 2014;20:688–701. [DOI] [PubMed] [Google Scholar]
- Chapman JF, McIntyre MH, Lipson SF, Ellison PT.. Weight change and ovarian steroid profiles in young women. Fertil Steril 2009;91:858–861. [DOI] [PubMed] [Google Scholar]
- Das M, Shehata F, Moria A, Holzer H, Son WY, Tulandi T.. Ovarian reserve, response to gonadotropins, and oocyte maturity in women with malignancy. Fertil Steril 2011;96:122–125. [DOI] [PubMed] [Google Scholar]
- Daum H, Peretz T, Laufer N.. BRCA mutations and reproduction. Fertil Steril 2018;109:33–38. [DOI] [PubMed] [Google Scholar]
- Decanter C, Robin G, Mailliez A, Sigala J, Morschhauser F, Ramdane N, Devos P, Dewailly D, Leroy-Martin B, Keller L.. Prospective assessment of follicular growth and the oocyte cohort after ovarian stimulation for fertility preservation in 90 cancer patients versus 180 matched controls. Reprod Biomed Online 2018;36:543–551. [DOI] [PubMed] [Google Scholar]
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, Griesinger G, Kelsey TW, La Marca A, Lambalk C. et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update 2014;20:370–385. [DOI] [PubMed] [Google Scholar]
- Dolinko AV, Farland LV, Missmer SA, Srouji SS, Racowsky C, Ginsburg ES.. Responses to fertility treatment among patients with cancer: a retrospective cohort study. Fertil Res Pract 2018;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitoussi, Eghbali H, Tchen N, Berjon JP, Soubeyran P, Hoerni B.. Semen analysis and cryoconservation before treatment in Hodgkin's disease. Ann Oncol 2000;11:679–684. [DOI] [PubMed] [Google Scholar]
- Goldrat O, Van Den Steen G, Gonzalez-Merino E, Dechene J, Gervy C, Delbaere A, Devreker F, De Maertelaer V, Demeestere I.. Letrozole-associated controlled ovarian hyperstimulation in breast cancer patients versus conventional controlled ovarian hyperstimulation in infertile patients: assessment of oocyte quality related biomarkers. Reprod Biol Endocrinol 2019;17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnala V, Fields J, Irani M, D'Angelo D, Xu K, Schattman G, Rosenwaks Z.. BRCA carriers have similar reproductive potential at baseline to noncarriers: comparisons in cancer and cancer-free cohorts undergoing fertility preservation. Fertil Steril 2019;111:363–371. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- Hussein RS, Zhao Y, Khan Z.. Does type of cancer affect ovarian response in oncofertility patients? J Gynecol Obstet Hum Reprod 2021;50:101944. [DOI] [PubMed] [Google Scholar]
- Iliodromiti S, Anderson RA, Nelson SM.. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update 2015;21:698–710. [DOI] [PubMed] [Google Scholar]
- Johnson LN, Dillon KE, Sammel MD, Efymow BL, Mainigi MA, Dokras A, Gracia CR.. Response to ovarian stimulation in patients facing gonadotoxic therapy. Reprod Biomed Online 2013;26:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuokkanen S, Polotsky AJ, Chosich J, Bradford AP, Jasinska A, Phang T, Santoro N, Appt SE.. Corpus luteum as a novel target of weight changes that contribute to impaired female reproductive physiology and function. Syst Biol Reprod Med 2016;62:227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini M, Goldrat O, Ferreira AR, Dechene J, Azim HA, Desir J, Delbaere A, t'Kint de Roodenbeke M-D, de Azambuja E, Ignatiadis M. et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol 2018;29:237–243. [DOI] [PubMed] [Google Scholar]
- Lawrenz B, Fehm T, von Wolff M, Soekler M, Huebner S, Henes J, Henes M; Centers of FertiPROTEKT Network. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma–evaluation by using antimullerian hormone and retrieved oocytes. Fertil Steril 2012;98:141–144. [DOI] [PubMed] [Google Scholar]
- Lekovich J, Lobel ALS, Stewart JD, Pereira N, Kligman I, Rosenwaks Z.. Female patients with lymphoma demonstrate diminished ovarian reserve even before initiation of chemotherapy when compared with healthy controls and patients with other malignancies. J Assist Reprod Genet 2016;33:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie Fong S, Lugtenburg PJ, Schipper I, Themmen AP, de Jong FH, Sonneveld P, Laven JS.. Anti-mullerian hormone as a marker of ovarian function in women after chemotherapy and radiotherapy for haematological malignancies. Hum Reprod 2008;23:674–678. [DOI] [PubMed] [Google Scholar]
- Luo D, Wan X, Liu J, Tong T.. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785–1805. [DOI] [PubMed] [Google Scholar]
- Lutchman Singh K, Muttukrishna S, Stein RC, McGarrigle HH, Patel A, Parikh B, Groome NP, Davies MC, Chatterjee R.. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer 2007;96:1808–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meczekalski B, Genazzani AR, Genazzani AD, Warenik-Szymankiewicz A, Luisi M.. Clinical evaluation of patients with weight loss-related amenorrhea: neuropeptide Y and luteinizing hormone pulsatility. Gynecol Endocrinol 2006;22:239–243. [DOI] [PubMed] [Google Scholar]
- Naasan M, Harrity C, Rajab H, Ranisavljevic N, Khalid S, Mocanu E.. Patients with cancer at the margins of reproductive age had reduced levels of anti-Mullerian hormone compared with patients experiencing infertility. Int J Gynaecol Obstet 2016;133:226–229. [DOI] [PubMed] [Google Scholar]
- Nordan T, Thomas AM, Ginsburg ES, Wen PY, Dolinko AV, Bortoletto P.. Fertility preservation outcomes in women with gliomas: a retrospective case-control study. J Neurooncol 2020;147:371–376. [DOI] [PubMed] [Google Scholar]
- Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, Bang H.. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab 2006;91:3885–3890. [DOI] [PubMed] [Google Scholar]
- Oktay K, Kim JY, Barad D, Babayev SN.. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol 2010;28:240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradisi R, Macciocca M, Vicenti R, Rossi S, Morselli-Labate AM, Mastroroberto M, Seracchioli R, Fabbri R.. New insights in the selection and management of cancer patients applicants for ovarian tissue cryopreservation. Gynecol Endocrinol 2016a;32:881–885. [DOI] [PubMed] [Google Scholar]
- Paradisi R, Vicenti R, Macciocca M, Seracchioli R, Rossi S, Fabbri R.. High cytokine expression and reduced ovarian reserve in patients with Hodgkin lymphoma or non-Hodgkin lymphoma. Fertil Steril 2016b;106:1176–1182. [DOI] [PubMed] [Google Scholar]
- Pashayan N, Antoniou AC, Ivanus U, Esserman LJ, Easton DF, French D, Sroczynski G, Hall P, Cuzick J, Evans DG. et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol 2020;17:687–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira N, Hancock K, Cordeiro CN, Lekovich JP, Schattman GL, Rosenwaks Z.. Comparison of ovarian stimulation response in patients with breast cancer undergoing ovarian stimulation with letrozole and gonadotropins to patients undergoing ovarian stimulation with gonadotropins alone for elective cryopreservation of oocytes. Gynecol Endocrinol 2016;32:823–826. [DOI] [PubMed] [Google Scholar]
- Pilleron S, Soto-Perez-de-Celis E, Vignat J, Ferlay J, Soerjomataram I, Bray F, Sarfati D.. Estimated global cancer incidence in the oldest adults in 2018 and projections to 2050. Int J Cancer 2021;148:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu E, Cillo GM, Cipriani L, Sacilotto F, Notarangelo L, Damiano G, Dirodi M, Roncarati I.. Impact of BRCA1 and BRCA2 mutations on ovarian reserve and fertility preservation outcomes in young women with breast cancer. J Assist Reprod Genet 2020;37:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril 2020;114:1151–1157. [DOI] [PubMed] [Google Scholar]
- Quinn MM, Cakmak H, Letourneau JM, Cedars MI, Rosen MP.. Response to ovarian stimulation is not impacted by a breast cancer diagnosis. Hum Reprod 2017;32:568–574. [DOI] [PubMed] [Google Scholar]
- Quintero RB, Helmer A, Huang JQ, Westphal LM.. Ovarian stimulation for fertility preservation in patients with cancer. Fertil Steril 2010;93:865–868. [DOI] [PubMed] [Google Scholar]
- Roy R, Chun J, Powell SN.. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer 2011;12:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueffer U, Breuer K, Josting A, Lathan B, Sieber M, Manzke O, Grotenhermen FJ, Tesch H, Bredenfeld H, Koch P. et al. Male gonadal dysfunction in patients with Hodgkin's disease prior to treatment. Ann Oncol 2001;12:1307–1311. [DOI] [PubMed] [Google Scholar]
- Son KA, Lee DY, Choi D.. Association of BRCA mutations and anti-Mullerian hormone level in young breast cancer patients. Front Endocrinol (Lausanne) 2019;10:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, Klinger FG.. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 2019;25:673–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, Baird DD.. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017;318:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal R, Seifer DB.. Ovarian reserve testing: a user's guide. Am J Obstet Gynecol 2017;217:129–140. [DOI] [PubMed] [Google Scholar]
- Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S. et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med 2013;5:172ra121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan V, Lambertini M, Lee DY, Wang E, Clatot F, Karlan BY, Demeestere I, Bang H, Oktay K.. Association of germline BRCA pathogenic variants with diminished ovarian reserve: a meta-analysis of individual patient-level data. J Clin Oncol 2021;39:2016–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan V, Oktay K.. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum Reprod Update 2020;26:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsamakis G, Chrousos G, Mastorakos G.. Stress, female reproduction and pregnancy. Psychoneuroendocrinology 2019;100:48–57. [DOI] [PubMed] [Google Scholar]
- van Dorp W, van den Heuvel-Eibrink MM, de Vries AC, Pluijm SM, Visser JA, Pieters R, Laven JS.. Decreased serum anti-Mullerian hormone levels in girls with newly diagnosed cancer. Hum Reprod 2014;29:337–342. [DOI] [PubMed] [Google Scholar]
- Wan X, Wang W, Liu J, Tong T.. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Pisarska MD, Bresee C, Chen YD, Lester J, Afshar Y, Alexander C, Karlan BY.. BRCA1 germline mutations may be associated with reduced ovarian reserve. Fertil Steril 2014;102:1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Xue L, Li Y, Tang W, Chen D, Zhang J, Dai J, Zhou S, Lu Z, Wu M. et al. THERAPY OF ENDOCRINE DISEASE: Novel protection and treatment strategies for chemotherapy-associated ovarian damage. Eur J Endocrinol 2021;184:R177–R192. [DOI] [PubMed] [Google Scholar]
- Younis JS, Iskander R, Fauser B, Izhaki I.. Does an association exist between menstrual cycle length within the normal range and ovarian reserve biomarkers during the reproductive years? A systematic review and meta-analysis. Hum Reprod Update 2020;26:904–928. [DOI] [PubMed] [Google Scholar]
- Yu B, Douglas N, Ferin MJ, Nakhuda GS, Crew K, Lobo RA, Hershman DL.. Changes in markers of ovarian reserve and endocrine function in young women with breast cancer undergoing adjuvant chemotherapy. Cancer 2010;116:2099–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials.