Abstract
To valorise the bioactive constituents abundant in leaves and other parts of medicinal plants with the objective to minimize the plant-based wastes, this study was undertaken. The main bioactive constituent of Andrographis paniculata, an Asian medicinal plant, is andrographolide (AG, a diterpenoid), which has shown promising results in the treatment of neurodegenerative illnesses. Continuous electrical activity in the brain is a hallmark of the abnormal neurological conditions such as epilepsy (EY). This can lead to neurological sequelae. In this study, we used GSE28674 as a microarray expression profiling dataset to identify DEGs associated with andrographolide and those with fold changes >1 and p-value <0.05 GEO2R. We obtained eight DEG datasets (two up and six down). There was marked enrichment under various Kyoto Encyclopaedia of Genes and Genomes (KEGG) and Gene Ontology (GO) terms for these DEGs (DUSP10, FN1, AR, PRKCE, CA12, RBP4, GABRG2, and GABRA2). Synaptic vesicles and plasma membranes were the predominant sites of DEG expression. AG acts as an antiepileptic agent by upregulating GABA levels. The low bioavailability of AG is a significant limitation of its application. To control these limitations, andrographolide nanoparticles (AGNPs) were prepared and their neuroprotective effect against pentylenetetrazol (PTZ)-induced kindling epilepsy was investigated using network pharmacology (NP) and docking studies to evaluate the antiepileptic multi-target mechanisms of AG. Andrographolide is associated with eight targets in the treatment of epilepsy. Nicotine addiction, GABAergic synapse, and morphine addiction were mainly related to epilepsy, according to KEGG pathway enrichment analysis (p < 0.05). A docking study showed that andrographolide interacted with the key targets. AG regulates epilepsy and exerts its therapeutic effects by stimulating GABA production. Rats received 80 mg/kg body weight of AG and AGNP, phenytoin and PTZ (30 mg/kg i.p. injection on alternate days), brain MDA, SOD, GSH, GABAand histological changes of hippocampus and cortex were observed. PTZ injected rats showed significantly (***p < 0.001) increased kindling behavior, increased MDA, decreased GSH, SOD, GABA activities, compared with normal rats, while treatment AGNPs significantly reduced kindling score and reversed oxidative damage. Finally, we conclude that the leaves and roots of A. Paniculata can be effectively utilized for its major bioactive constituent, andrographolide as a potent anti-epileptic agent. Furthermore, the findings of novel nanotherapeutic approach claim that nano-andrographolide can be successfully in the management of kindling seizures and neurodegenerative disorders.
Keywords: andrographolide, andrographolide nanoparticles, pentylenetetrazol, antikindiling, antioxidant, network pharmacology
Introduction
Food and medicinal plants are abundant in phytochemicals and bioactive components. Plant-based foods and medicinal products have commercial significance in agro-food, pharmaceutical and nutraceutical industries. However, the wastage of plant-based foods or bioactive substances could be attributed to be a major hurdle against the growth and sustainability of such natural resources. This issue can be resolved by maximizing the utilization of plant-based foods and medicinal constituents while minimizing their wastages. In medicinal practice, plant bioactives cannot be employed because of unsatisfactory physiological parameters or poor oral bioavailability. Therefore, the nanodelivery of bioactive components could thus maximize the medicinal/therapeutic benefits of the bioactive under investigations considering the safety and toxicity concerns. This is how biomaterials, food components and bioactive substances could have positive environmental impact through economic development and sustainability in the long run.
Medicinal plants rich in phytochemicals such as flavonoids, terpenoids, and coumarins have shown anticonvulsant activities in preclinical studies (1, 2). Andrographis paniculata (F. Acanthaceae) has been used in traditional medicines for curing various human ailments. The leaves of A. paniculata contain a diterpenoid called andrographolide (AG), which is the major bioactive component and it possesses a wide range of biological activities such as antioxidant, anti-inflammatory, neuroprotective, and anti-cancer properties. AG includes nicotine induces oxidative stress in the brain and protects against brain ischemia caused by dopamine-mediated neurotoxicity and inflammation-mediated neurodegeneration (3–5). The instability and poor water solubility of AG limits its clinical application because of its low bioavailability. Microemulsions, cyclodextrin inclusion complexes, liposomes, solid-lipid nanoparticles, niosomes etc. have been developed to enhance AG bioavailability; however, these systems have low loading capacity, poor stability and modest encapsulation efficiency (6, 7). A nanoparticle-based approach can be used to enhance the absorption, bioavailability, and biodistribution of flavonoids. In order to increase the solubility of hydrophobic drugs in water, nanoparticle drug delivery systems have been extensively used (8).
Approximating 1% of the world’s population is affected by epilepsy (EY), a complex neurological disorder, with considerable psychological, emotional and educational implications. Generally, an excessive glutamate concentration or a deficiency in GABA concentration in the central nervous system can cause a variety of pathological changes, which can be related to epilepsy (9). Several neurodegenerative conditions (for example, Alzheimer’s disease, Parkinson’s disease) are associated with oxidative stress, which causes neuronal damage. Molecular oxygen produces reactive oxygen species (ROS), which are generated by activating excitatory amino acids and releasing glutamate, causing long-term seizures and neuronal death. A direct effect of free radicals on seizures is seen when glutamate decarboxylase and glutamine synthase are deactivated, leading to a disproportionate amount of both excitatory (glutamate) and inhibitory (GABA) neurotransmitters (10–12). A scarcity of successful therapies for epilepsy exists around the world. It is possible to develop better antiepileptic treatments based on natural compounds. Patients with epilepsy may benefit from plants as a source of seizures and comorbid diseases (13).
Pentylenetetrazol kindling, a chronic epilepsy investigational model associated with seizures and neuronal plasticity, is typical in providing opportunities to study progressive behavioral variations closely resembling clinical epilepsy (14, 15). The present study was aimed to utilize andrographolide (AG) and it’s nanoformulation (AGN) as potential antikindling agent in PTZ-induced kindling rats.
Materials and methods
Potential targets of andrographolide
We identified the potential targets of the active compounds by analyzing the data collected from various databases. These include the TargetNet Database (TND), Comparative Toxicogenomic Database (CTD) (16), and Swiss Target Prediction (STP) (17). The chemical structures were then converted into a canonical version of SMILES using PubChem (17). The compound files were placed in the TargetNet and Swiss Target Prediction databases (16, 17). The results showed that the probability of a compound being produced was greater than 0.9. We verified that the targets were Homo sapiens using the UniProt database.
Epilepsy related targets and shared targets of andrographolide
With the help of Geo2R, the related targets of the EY can be retrieved. The tool is part of the GEO dataset, a collection of gene expression datasets. This study aimed to identify the most common genes, which were differentially shown in different sample groups. Gene chip GSE28674 has been frequently cited in literature (18, 19). The differentially revealed genes in the samples were identified by adjusting the p-values to <0.05 in analyzing the data in the geo2R database using Benjamini–Hochberg method. The criteria for determining the DEGs that should be screened were FDR > 0.05 and log FC > 1 (18). Furthermore, a volcano diagram was generated using ggplot2 and using the Venn package, the tool retrieved the related targets of the EY. We also performed a comprehensive analysis of potential targets of andrographolide.
Analysis of protein–protein interactions and hub targets
PPI analysis assists in identifying the hub targets related to AG on EY. The PPI network was constructed by using STRING with the “Homo sapiens” setting to retrieve the shared targets of andrographolide with ER (20). The network properties were analyzed using Cytoscape 3.7.2 software by selecting the “Analysis Network” function. The degree of freedom (DOF) plays an influential role in a PPI network because points overhead the average DOF typically play a significant role (21).
Kyoto Encyclopaedia of Genes and Genomes analysis and Gene Ontology enrichment
KEGG pathway enrichment analysis and GO function enrichment analysis were performed using DAVID with Homo sapiens as the selected species. Visualization was performed using the online tool Weishengxin (2). A threshold level of p < 0.05 is used for all GO enrichment and pathway analyses. The final pathway map was created by combining pathways with the highest scores.
Network construction
By using Cytoscape 3.7.2 software, a primary regulatory network was used to construct in order to visualize the “drug–target–disease” correlation between AG and EY. The nodes in this network are the shared targets, active compounds, and pathways. The edges show how compounds, targets, and pathways interact.
Molecular docking
Andrographolide was retrieved from the 3D.sdf file of the PubChem database. Using OpenBabel-2.3, the SDF files were converted to pdb files, which were then converted into pdbqt files using AutoDock Tools (version 1.5.6) (22–24). The x-ray crystal structure of the gamma-aminobutyric acid receptor GABA(A)R-beta3 (PDB ID 4COF) (25–33) was obtained from the RCSB Protein Data Bank. MGL AutoDock Tools were used to prepare the protein, including removing crystal water and ligands and adding Kollman charges and polar hydrogen atoms. The PDB structured proteins were transformed to the pdbqt format utilizing Autodock. Ten distinct poses of the ligand molecules were acquired after docking with target proteins (x, y, z = 34.55, 56.37, 23.86) by employing AutoDock Vina’s standard settings (34–39). The Biovia Discovery Studio was chosen to illustrate ligand-protein interactions (40–47).
Preparation of andrographolide nanoparticles
An antisolvent (n-hexane) was added to absolute ethanol (15 mg/mL) to obtain the nanosuspension of andrographolide. The n-hexane to ethanol ratio was 10:1 to facilitate the process of nanosuspension. Subsequently, the nanosuspension of the medication was placed into a round-bottom flask and rotated at 90 rpm at a temperature of 40°C and a pressure of 300 mbar, and the solvent was then removed using a rotary evaporator. Next, the solid in the flask was evaporated and dried (48). The particle sizes of the prepared AGN and AG particles were measured at an angle of 900° using the dynamic light scattering technique.
Animals
The albino Wistar male rats were weighed between 150–200 grams, and they were held in a room with a temperature of 25°C, 55% relative humidity, and a 12/12 h light/dark cycle, which complies with CPCSEA regulations. Experimental rats were fed with pellet diet, and water ad libitum. The IAEC (1725/GO/a/13/CPCSEA) evaluated and accepted the current experimental protocol.
Treatment protocol
Kindling induction
Rats were treated with sub-convulsant dose of PTZ (35 mg/kg/b.wt. i.p./alternative days) until kindling was developed. The Racine scale was used to monitor the intensity of seizures was monitored for around 30 min following each injection (49). Kindling intensity is monitor based on the following score:
Score 0 = No response,
Score = 1 facial and mouth jerks,
Score = 2 myoclonic body jerksor nodding,
Score = 3 forelimb clonus, rearing, hindlimb clonus, falling down and forelimbtonus.
Score = 4 tonic extension of the hindlimb,
Score = 5 status epilepticus and/or death.
In the study, rats with convulsions on the first day were excluded. When rats exhibit stage 5 seizures, they are considered fully kindled. The doses (50), phenytoin (51), and andrographolide (52) have been determined in previous studies.
Rats were divided in to five groups, each group contain six animals.
Group I: Received vehicle saline i.p.
Group II: PTZ (35 mg/kg/b.wt. i.p./alternative days).
Group III: Phenytoin (35 mg/kg/b.wt. i.p./daily) + PTZ (35 mg/kg/b.wt. i.p./alternative days) treatment.
Group IV: Phenytoin (35 mg/kg/b.wt. i.p.) + AG (80 mg/kg. p.o) + PTZ (35 mg/kg/b.wt. i.p./alternative days) treatment.
Group V: PTZ + phenytoin (35 mg/kg/b.wt. i.p.) + AGN (80 mg/kg.p./o) + PTZ (35 mg/kg/b.wt. i.p./alternative days).
The ends of the treated rats were executed under ether anesthesia, and the brains were quickly removed, cleaned with ice-cold saline, and subjected to estimation of malonaldehyde (MDA), reduced glutathione (GSH) (53), superoxide dismutase (SOD) (54), and gamma-aminobutyric acid (GABA) (53).
Histopathological examination
Toluidine blue and hematoxylin and eosin were used for staining the sections (55). The hippocampal and cortical regions of the slides were photographed digitally under a microscope.
Statistical analysis
The mean value and standard error of the mean (SEM) for data gathered from three trials is presented. A significant difference (p < 0.001) was identified between the kindling rats and treatment rats when using one-way ANOVA followed by Dunnett’s comparison test.
Results
Screening of potential targets in andrographolide
This study identified 423 targets of andrographolide. It is possible that andrographolide may have similar biological effects as andrographide and that when combined, these effects may be synergistic. The integration of all targets resulted in 371 targets corresponding to andrographolide.
Related targets of andrographolide and shared targets of andrographolide against epilepsy
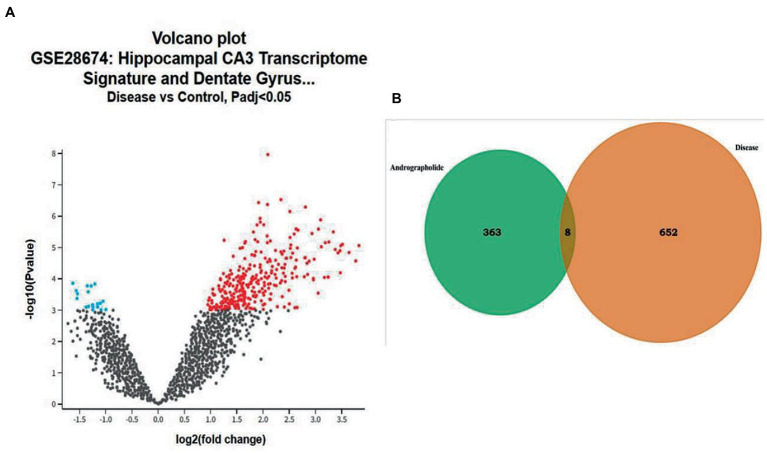
Gene chip GSE28674 was used to examine the impact of dentate gyrus MRI features and hippocampal CA3 transcriptome signature in relation to initial precipitating injury in refractory temporal lobe epilepsy, including twelve normal samples (hippocampus no febrile seizures) and six samples (hippocampus febrile seizures). As shown in Figure 1A, 660 DEGs were identified between normal samples and disease samples using the above screening criteria of FDR > 0.05 and log FC > 1. To further understand andrographolide’s mechanism of action in treating EY, a Venn diagram (Figure 1B) was used to identify eight shared targets (Table 1) between 660 DEGs and 371 potential targets.
Figure 1.
Genes with differential expression in epilepsy (EY). (A) A red or blue gene represents an upregulated gene, whereas a blue gene represents a downregulated gene, based on the standard FDR of 0.05 and log FC > 1. (B) Potential targets of andrographolide and differentially expressed genes in EY.
Table 1.
The eight shared targets between the andrographolide and epilepsy with FDR < 0.05 and log FC > 1.
| Gene | log FC | FDR |
|---|---|---|
| DUSP10 | −1.00026 | 0.010221 |
| FN1 | 1.571008 | 0.014388 |
| AR | −1.1762 | 0.010014 |
| PRKCE | 1.284686 | 0.045194 |
| CA12 | 1.315168 | 0.026297 |
| RBP4 | 2.214523 | 0.020294 |
| GABRG2 | 2.807807 | 0.004511 |
| GABRA2 | 1.089175 | 0.014506 |
Kyoto Encyclopaedia of Genes and Genomes pathway enrichment and Gene Ontology functional annotation analysis
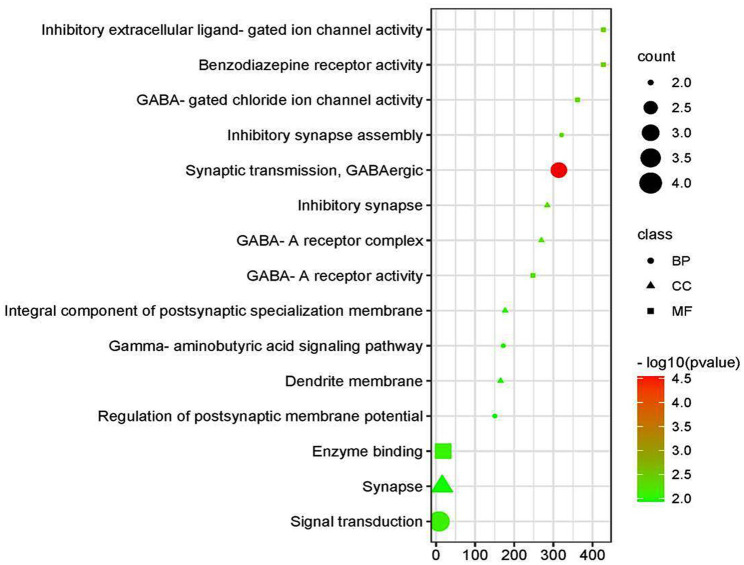
Based on the GO functional annotation analysis, 11 CC terms, 16 BP terms, and 10 MF terms predominated across the eight shared targets. As shown in Figure 2 and Table 2, the top five enrichment results are presented for each part. Synaptic transmission, GABAergic inhibitory synapse assembly, GABA signaling pathway, signal transduction, and regulation of postsynaptic membrane potential are closely connected to BP. Concerning CC, higher enrichment was found in the synapse, dendrite membrane (GABA-A receptor complex), postsynaptic specialization membrane, and plasma membrane. The main terms of EY in MF included benzodiazepine receptor activity, GABA-gated chloride ion channel activity, GABA-A receptor activity, inhibitory extracellular ligand-gated ion channel activity, and enzyme binding activities. The current analysis exhibited that these targets were closely associated to the activation of GABA ion channels, causing an influx of chloride ions and leading to hyperpolarization and decreased excitability.
Figure 2.
Bubble chart showing the relationship between KEGG pathways and associated genes.
Table 2.
Gene Ontology (GO) enrichment analysis performed on eight common genes.
| Term | Description | Count | p-value | Genes | Fold enrichment | Bonferroni |
|---|---|---|---|---|---|---|
| Biological process | ||||||
| GO:0051932 | Synaptic transmission, GABAergic | 3 | 2.85549E−05 | GABRA2, PRKCE, GABRG2 | 313.9565217 | 0.005126771 |
| GO:1904862 | Inhibitory synapse assembly | 2 | 0.005440965 | GABRA2, GABRG2 | 320.9333333 | 0.625456681 |
| GO:0007165 | Signal transduction | 4 | 0.008137434 | GABRA2, AR, PRKCE, GABRG2 | 7.599052881 | 0.770242346 |
| GO:0007214 | GABA signalling pathway | 2 | 0.010135923 | GABRA2, GABRG2 | 171.9285714 | 0.84019093 |
| GO:0060078 | Regulation of postsynaptic membrane potential | 2 | 0.011576698 | GABRA2, GABRG2 | 150.4375 | 0.877048021 |
| Cellular components | ||||||
| GO:0060077 | Inhibitory synapse | 2 | 0.006139434 | GABRA2, GABRG2 | 284.3333333 | 0.242041103 |
| GO:1902711 | GABA-A receptor complex | 2 | 0.006479565 | GABRA2, GABRG2 | 269.3684211 | 0.253626525 |
| GO:0099060 | Integral component of postsynaptic specialization membrane | 2 | 0.009875383 | GABRA2, GABRG2 | 176.4827586 | 0.360200909 |
| GO:0032590 | Dendrite membrane | 2 | 0.010553352 | GABRA2, GABRG2 | 165.0967742 | 0.379620876 |
| GO:0045202 | Synapse | 3 | 0.010999223 | GABRA2, PRKCE, GABRG2 | 15.73155738 | 0.392077144 |
| Molecular function | ||||||
| GO:0008503 | Benzodiazepine receptor activity | 2 | 0.004086826 | GABRA2, GABRG2 | 427.5227273 | 0.255360015 |
| GO:0005237 | Inhibitory extracellular ligand-gated ion channel activity | 2 | 0.004086826 | GABRA2, GABRG2 | 427.5227273 | 0.255360015 |
| GO:0022851 | GABA-gated chloride ion channel activity | 2 | 0.004828345 | GABRA2, GABRG2 | 361.75 | 0.294241982 |
| GO:0004890 | GABA-A receptor activity | 2 | 0.007050064 | GABRA2, GABRG2 | 247.5131579 | 0.399145455 |
| GO:0019899 | Enzyme binding | 3 | 0.008403619 | AR, PRKCE, FN1 | 18.0875 | 0.455352909 |
GO analysis showed 16 entries for biological processes, 11 for cell components, and 10 for molecular functions (p < 0.05). The top five entries with the most significant p-values are shown.
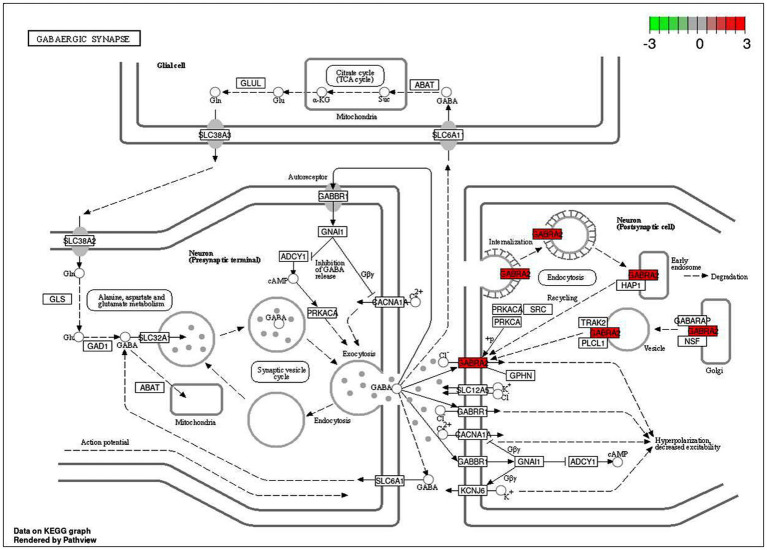
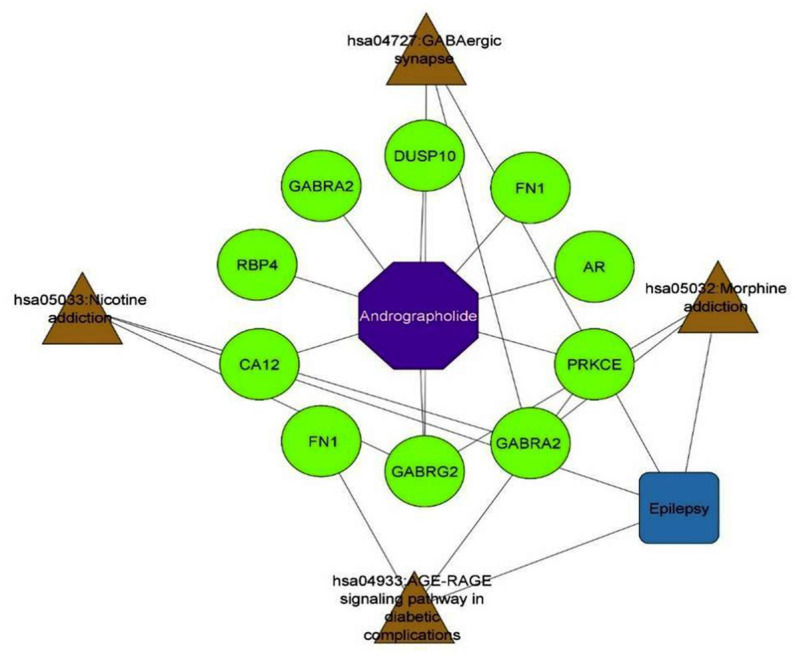
An intensive KEGG enrichment analysis was conducted on the eight shared targets, targeting the anti-EY pathway of andrographolide, and results showed an FDR of <0.05. The outcomes are presented in Figure 3. The four enriched pathways were GABAergic synapse (hsa04727), nicotine addiction (hsa05033), morphine addiction (hsa05032), and the AGE-RAGE signaling pathway in diabetic complications (hsa04933) (Table 3). For instance, GABRA2 and GABRG2 appear in three pathways, indicating that andrographolide mainly acts by activating GABA ion channel receptors (Figure 4).
Figure 3.
Component-target-signal pathway network.
Table 3.
Target genes in 4 signaling pathways enrichment related to EY.
| Description | Count | p-value | Genes | Fold enrichment | Bonferroni |
|---|---|---|---|---|---|
| hsa05033:Nicotine addiction | 2 | 0.029076553 | GABRA2, GABRG2 | 58.25714286 | 0.654331654 |
| hsa04727:GABAergic synapse | 2 | 0.033731908 | GABRA2, GABRG2 | 26.18298555 | 0.906586848 |
| hsa05032:Morphine addiction | 2 | 0.045124214 | GABRA2, GABRG2 | 25.60753532 | 0.911459737 |
| hsa04933:AGE-RAGE signaling pathway in diabetic complications | 2 | 0.05136826 | PRKCE, FN1 | 23.30285714 | 0.930438835 |
Figure 4.
Mechanisms of action of andrographolide against EY.
An enrichment analysis of andrographolide’s anti-EY pathway was conducted with KEGG, with FDR < 0.05. Figure 5 illustrates the results. The four enriched pathways were GABAergic synapse (hsa04727), nicotine addiction (hsa05033), morphine addiction (hsa05032), and the AGE-RAGE signaling pathway in diabetic complications (hsa04933). For instance, GABRA2 and GABRG2 appear in three pathways, indicating that andrographolide mainly acts by activating GABA ion channel receptors.
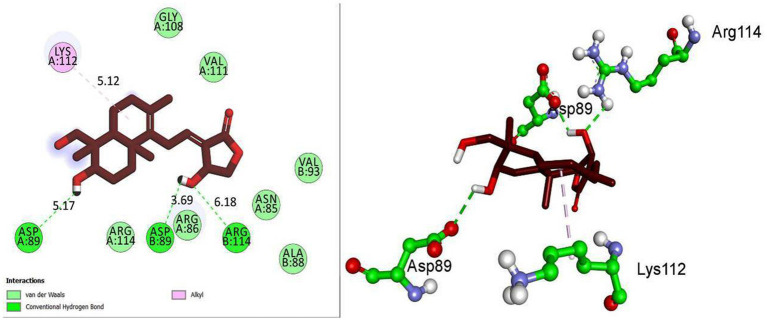
Figure 5.
Molecular docking interactions of andrographolide in 2D and 3D representations.
Molecular docking
Based on KEGG enrichment analysis, GABAergic synapse signaling pathways appear to be a hub signaling pathway of andrographolide against EY. The GABRA2 and GABRG2 genes were related to three signaling pathways, including hsa05033, nicotine addiction; hsa04727, GABAergic synapse; and hsa05032, morphine addiction. Therefore, GABA is considered to be a prominent target of andrographolide in the treatment of epilepsy.
Based on molecular docking studies, andrographolide binds to the GABA receptor with a binding energy of −6.8 kcal/mol. It formed three hydrogen bonds with ASP A:89, ASP B:89, and ARG B:114, with distances of 5.17, 3.69, and 6.18 A°. It also interacted with LYS A:112 via hydrophobic interactions at a distance of 5.12 A° (Figure 5). This suggests that andrographolide may be an important ligand for controlling glucose homeostasis.
Particle size and charge of andrographolide nanoparticles
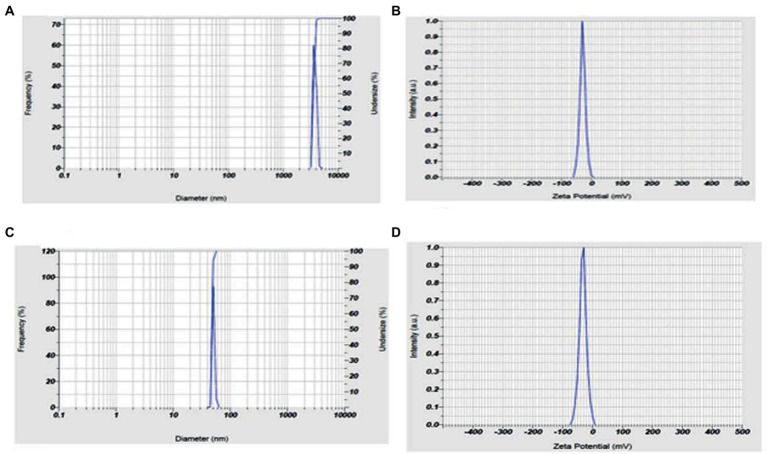
The andrographolide particles were found to have a size of 3587.8 nm (3.6 microns), indicating their large particle nature. On the other hand, the size of the prepared nano-andrographolide was found to be 47.9 nm indicating that the prepared nanoparticles were of good size. The nano-andrographolide particles exhibited −32.3 mV charge indicating that the particles remained stable and free of agglomeration (Figure 6).
Figure 6.
(A) Andrographolides particle size, (B) andrographolides particle charge, (C) andrographolides nanoparticle size, (D) andrographolides nanoparticle charge.
Effect of andrographolide nanoparticle treatment on pentylenetetrazol-induced kindling model
After three injections, PTZ-treated rats reached seizure severity stage 5 and died. In contrast, the phenytoin +AG combination did not cause severe seizures in rats. Up to the ninth PTZ injection, AGN + phenytoin combinations were seizure-free, but the severity of the seizures increased following the eleventh PTZ injection. Our results agree with those of various studies in which PTZ was frequently used to induce seizure severity (56–58). In this study, the number of animals that developed kindling significantly decreased after AGN treatment (Tables 4–7).
Table 4.
Effect of PTZ (35 mg/kg).
| Days | PTZ inj. | Score 1 (time) | Score 2 (time) | Score 3 (time) | Score 4 (time) | Score 5 (time) | |
|---|---|---|---|---|---|---|---|
| 1 | 1 | OA | 4 min | NO | NO | NO | NO |
| DA | 8 min | NO | NO | NO | NO | ||
| 3 | 2 | OA | NO | NO | 3 min | NO | NO |
| DA | NO | NO | 12 min | NO | NO | ||
| 5 | 3 | OA | NO | NO | NO | NO | 3 min followed by death (100%) |
| DA | NO | NO | NO | NO |
Table 7.
Andrographolide nanoparticles (50 mg/kg) + PTZ (35 mg/kg).
| Days | PTZ inj. | Score 1 (time) | Score 2 (time) | Score 3 (time) | Score 4 (time) | Score 5 (time) | |
|---|---|---|---|---|---|---|---|
| 1 | 1 | OA | 6 min | NO | NO | NO | NO |
| DA | 10 min | NO | NO | NO | NO | ||
| 3 | 2 | OA | 5 min | NO | NO | NO | NO |
| DA | 11 min | NO | NO | NO | NO | ||
| 5 | 3 | OA | NO | 5 min | NO | NO | NO |
| DA | NO | 12 min | NO | NO | NO | ||
| 7 | 4 | OA | NO | 4 min | NO | NO | NO |
| DA | NO | 15 min | NO | NO | NO | ||
| 9 | 5 | OA | NO | NO | 4 min | NO | NO |
| DA | NO | NO | 16 min | NO | NO | ||
| 11 | 6 | OA | NO | NO | 8 min | NO | NO |
| DA | NO | NO | 20 min | NO | NO | ||
| 13 | 7 | OA | NO | NO | NO | 15 min | NO |
| DA | NO | NO | NO | 5 min | NO | ||
| 15 | 8 | OA | NO | NO | NO | 20 min | NO |
| DA | NO | NO | NO | 7 min | NO | ||
| 17 | 9 | OA | NO | NO | NO | 10 min | NO |
| DA | NO | NO | NO | 9 min | NO | ||
| 19 | 10 | OA | NO | NO | NO | NO | 15 min |
| DA | NO | NO | NO | NO | 5 min | ||
| 21 | 11 | OA | NO | NO | NO | NO | 20 min |
| DA | NO | NO | NO | NO | 7 min |
NO, not observed; OA, onset of action; DA, duration of action.
Table 5.
Effect of PTZ (35 mg/kg) + phenytoin (35 mg/kg).
| Days | PTZ inj. | Score 1 (time) | Score 2 (time) | Score 3 (time) | Score 4 (time) | Score 5 (time) | |
|---|---|---|---|---|---|---|---|
| 1 | 1 | OA | NO | NO | NO | NO | NO |
| DA | NO | NO | NO | NO | NO | ||
| 3 | 2 | OA | 3 min | NO | NO | NO | NO |
| DA | 9 min | NO | NO | NO | NO | ||
| 5 | 3 | OA | NO | NO | 5 min | NO | NO |
| DA | NO | NO | 13 min | NO | NO | ||
| 7 | 4 | OA | NO | NO | NO | 9 min | NO |
| DA | NO | NO | NO | 15 min | NO | ||
| 9 | 5 | OA | NO | NO | NO | NO | 5 min followed death (83.3%) |
| DA | NO | NO | NO | NO |
Table 6.
Andrographolide (50 mg/kg) + PTZ (35 mg/kg).
| Days | PTZ inj. | Score 1 (Time) | Score 2 (Time) | Score 3 (Time) | Score 4 (Time) | Score 5 (Time) | |
|---|---|---|---|---|---|---|---|
| 1 | 1 | OA | 5 min | NO | NO | NO | NO |
| DA | 8 min | NO | NO | NO | NO | ||
| 3 | 2 | OA | 4 min | NO | NO | NO | NO |
| DA | 11 min | NO | NO | NO | NO | ||
| 5 | 3 | OA | NO | 3 min | NO | NO | NO |
| DA | NO | 15 min | NO | NO | NO | ||
| 7 | 4 | OA | NO | NO | 3 min | NO | NO |
| DA | NO | NO | 20 min | NO | NO | ||
| 9 | 5 | OA | NO | NO | NO | NO | 10 min followed death (66.6%) |
| DA | NO | NO | NO | NO |
Effect of andrographolides nanoparticles on pentylenetetrazol kindling-induced brain oxidative biomarkers
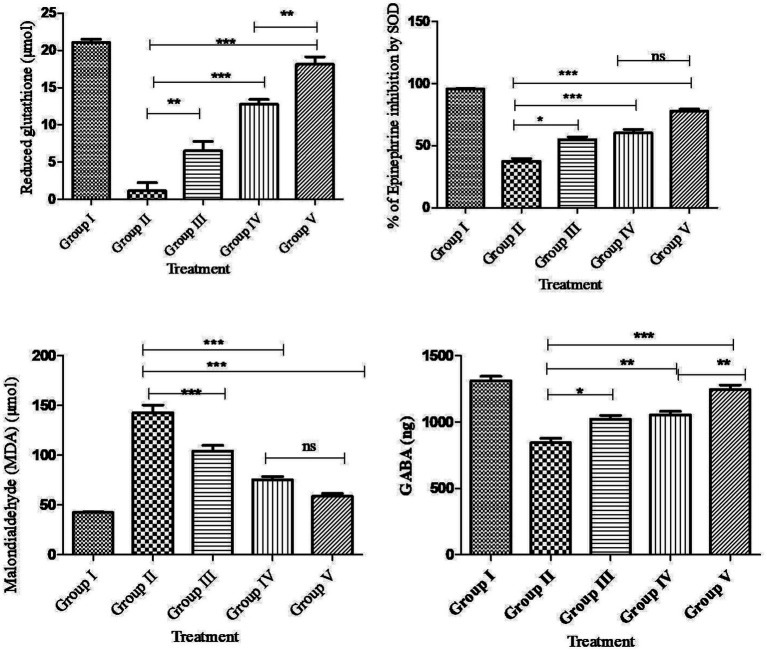
Compared to the normal control rats, PTZ treatment had a significant (p < 0.001***) decrease in SOD, GSH, GABA and an increase in MDA levels in the rat brain. Compared to PTZ alone treatment and AG treatment, AGN treatment had the effect of significantly (p < 0.001***) increasing SOD, GSH, GABA, and reducing MDA levels in the brain (Figure 7).
Figure 7.
Brain biochemical parameters. p<0.001***.
PTZ selectively blocks the chloride ionophore complex of the GABA receptor. Following repeated or single administration, its convulsant effects affect GABAergic, adenosinergic, and glutamatergic systems. In this study, PTZ treatment significantly decreased GABA activity, whereas the combination of AGN with phenytoin increased GABA activity compared with AG with phenytoin and phenytoin treatment. In this context, PTZ has also been shown to activate nucleases, phospholipases, and membrane proteases that result in the degradation of cytoskeletal proteins, membrane phospholipids, and protein phosphorylation (58–60). After PTZ-induced seizures, significant reductionin GSH and SOD activity (61) and increased MDA activity (62) have been observed in animal brain homogenates. Reactive oxygen species are produced in an unreliable manner as a result of antiepileptic medications including phenytoin, valproic acid, and carbamazepine (63). Epilepsy and associated neurological comorbidities can be improved when these drugs are combined with antioxidants (64, 65). An examination of histology showed a decrease in cell count and cell death in the cortex, CA1 and CA3 region of the rat’s hippocampus. The group receiving PTZ showed a significant increase in dead cells and a decreased density of cells compared with the control value. Supplementary Figures S1–S3 showed that the phenytoin + AGN + PTZ group had significantly fewer dead cells and greater cell density in the hippocampus and cortex in comparison with the AGN group. However, the combination of AGN and phenytoin treatment restored cellular antioxidant enzymes when compared with the combination of AG and phenytoin treatment in the PTZ group.
Conclusion
Based on the network pharmacology analysis, the andrographolide acts as an anti-epileptic agent by upregulating the GABA levels, and further molecular docking studies confirmed the same. PTZ administration revealed kindling development, greater oxidative stress, diminished antioxidant activity, augmented GABA levels, and neurodegeneration. However, the combination of phenytoin and andrographolide nanoparticles significantly reduced the seizure score (% of kindled animals). The above findings indicate that the potential anti-kindling effects of andrographolide nanoparticles may protect against oxidative stress and increase GABAergic activity in kindling seizures and thereby modulates neuroprotection in the cortex. Finally, we conclude that the leaves and roots of A. paniculata can be effectively utilized for its major bioactive constituent, andrographolide as a potent anti-epileptic agent. Furthermore, the findings of novel nanotherapeutic approach claim that nano-andrographolide can be successfully in the management of kindling seizures and neurodegenerative disorders.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Santhiram College of Pharmacy, JNTUA, Nandyal, Andhra Pradesh, India.
Author contributions
RB, DP, PP, SP, and VT: conceptualization, methodology, and software. SM, RK, MR, and JK: investigation, writing—original draft, review, and editing. PP, RK, MC, JKK, SA, BA, and SB: resources and supervision, validation, and formal analysis. JK, SA, BA, and SB: funding acquisition. SM and MR: critical analysis, final draft-review, and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the Deanship of Scientific Research at Majmaah University, Saudi Arabia for supporting this work under Project number (R-2023-279).
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1185236/full#supplementary-material
References
- 1.Costa JP, Ferreira PB, De Sousa DP, Jordan J, Freitas RM. Anticonvulsant effect of phytol in a pilocarpine model in mice. Neurosci Lett. (2012) 523:115–8. doi: 10.1016/j.neulet.2012.06.055, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. (2009) 4:44–57. doi: 10.1038/nprot.2008.211, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Chan SJ, Wong WF, Wong PT, Bian J-S. Neuroprotective effects of andrographolide in a rat model of permanent cerebral ischaemia. Br J Pharmacol. (2010) 161:668–79. doi: 10.1111/j.1476-5381.2010.00906.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tapia-Rojas C, Schüller A, Lindsay CB, Ureta RC, Mejías-Reyes C, Hancke J, et al. Andrographolide activates the canonical Wnt signalling pathway by a mechanism that implicates the non-ATP competitive inhibition of GSK-3β: autoregulation of GSK-3β in vivo. Biochem J. (2015) 466:415–30. doi: 10.1042/BJ20140207, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Wang T, Liu B, Zhang W, Wilson B, Hong J-S. Andrographolide reduces inflammation-mediated dopaminergic Neurodegeneration in Mesencephalic neuron-glia cultures by inhibiting microglial activation. J Pharmacol Exp Ther. (2004) 308:975–83. doi: 10.1124/jpet.103.059683, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Roy R, Das S, Bera T, Mondol S, Mukherjee A. Andrographolide nanoparticles in leishmaniasis: characterization and in vitro evaluations. Int J Nanomed. (2010) 5:1113–21. doi: 10.2147/IJN.S14787, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thingale AD, Shaikh KS, Channekar PR, Galgatte UC, Chaudhari PD, Bothiraja C. Enhanced hepatoprotective activity of andrographolide complexed with a biomaterial. Drug Deliv. (2015) 22:117–24. doi: 10.3109/10717544.2013.871602 [DOI] [PubMed] [Google Scholar]
- 8.Kheradmand E, Hajizadeh Moghaddam A, Zare M. Neuroprotective effect of hesperetin and nano-hesperetin on recognition memory impairment and the elevated oxygen stress in rat model of Alzheimer’s disease. Biomed Pharmacother. (2018) 97:1096–101. doi: 10.1016/j.biopha.2017.11.047, PMID: [DOI] [PubMed] [Google Scholar]
- 9.De Oliveira PA, Lino FL, Cappelari SE, Da Silva Brum LF, Picada JN, Pereira P. Effects of gamma-decanolactone on seizures induced by PTZ-kindling in mice. Exp Brain Res. (2008) 187:161–6. doi: 10.1007/s00221-008-1295-y, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Cho J. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch Pharm Res. (2006) 29:699–706. doi: 10.1007/BF02968255, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Hwang S-L, Shih P-H, Yen G-C. Neuroprotective effects of citrus flavonoids. J Agric Food Chem. (2012) 60:877–85. doi: 10.1021/jf204452y [DOI] [PubMed] [Google Scholar]
- 12.Sudha K, Rao AV, Rao A. Oxidative stress and antioxidants in epilepsy. Clin Chim Acta. (2001) 303:19–24. doi: 10.1016/S0009-8981(00)00337-5 [DOI] [PubMed] [Google Scholar]
- 13.Sahranavard S, Ghafari S, Mosaddegh M. Medicinal plants used in Iranian traditional medicine to treat epilepsy. Seizure. (2014) 23:328–32. doi: 10.1016/j.seizure.2014.01.013, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Gupta YK, Veerendra Kumar MH, Srivastava AK. Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacol Biochem Behav. (2003) 74:579–85. doi: 10.1016/S0091-3057(02)01044-4, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Shimada T, Yamagata K. Pentylenetetrazole-induced kindling mouse model. J Vis Exp. (2018). doi: 10.3791/56573, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis AP, Grondin CJ, Johnson RJ, Sciaky D, Wiegers J, Wiegers TC, et al. Comparative Toxicogenomics Database (CTD): update 2021. Nucleic Acids Res. (2021) 49:D1138–43. doi: 10.1093/nar/gkaa891, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji K-Y, Liu C, Liu Z-Q, Deng Y-F, Hou T-J, Cao D-S. Comprehensive assessment of nine target prediction web services: which should we choose for target fishing? Brief Bioinform. (2023) 24:bbad014. doi: 10.1093/bib/bbad014 [DOI] [PubMed] [Google Scholar]
- 18.Jiang XW, Lu HY, Xu Z, Liu TY, Wu Q, Yang Y, et al. In silico analyses for key genes and molecular genetic mechanism in epilepsy and Alzheimer’s disease. CNS Neurol Disord Drug Targets. (2018) 17:608–17. doi: 10.2174/1871527317666180724150839, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Wang C, Wang P, Li X, Zhou L. Effects of febrile seizures in mesial temporal lobe epilepsy with hippocampal sclerosis on gene expression using bioinformatical analysis. Acta Epileptol. (2020) 2:20. doi: 10.1186/s42494-020-00027-9 [DOI] [Google Scholar]
- 20.Doncheva NT, Morris JH, Gorodkin J, Jensen LJ. Cytoscape StringApp: network analysis and visualization of proteomics data. J Proteome Res. (2019) 18:623–32. doi: 10.1021/acs.jproteome.8b00702, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong H, Mason SP, Barabasi AL, Oltvai ZN. Lethality and centrality in protein networks. Nature. (2001) 411:41–2. doi: 10.1038/35075138 [DOI] [PubMed] [Google Scholar]
- 22.Badavath VN, Baysal I, Ucar G, Sinha BN, Jayaprakash V. Monoamine oxidase inhibitory activity of novel pyrazoline analogues: Curcumin based design and synthesis. ACS Med Chem Lett. (2016) 7:56–61. doi: 10.1021/acsmedchemLett.5b00326, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badavath VN, Ciftci-Yabanoglu S, Bhakat S, Timiri AK, Sinha BN, Ucar G, et al. Monoamine oxidase inhibitory activity of 2-aryl-4H-chromen-4-ones. Bioorg Chem. (2015) 58:72–80. doi: 10.1016/j.bioorg.2014.11.008, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Nayak BV, Ciftci-Yabanoglu S, Jadav SS, Jagrat M, Sinha BN, Ucar G, et al. Monoamine oxidase inhibitory activity of 3, 5-biaryl-4, 5-dihydro-1H-pyrazole-1-carboxylate derivatives. Eur J Med Chem. (2013) 69:762–7. doi: 10.1016/j.ejmech.2013.09.010, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Devasia J, Chinnam S, Khatana K, Shakya S, Joy F, Rudrapal M, et al. Synthesis, DFT, and in silico anti-COVID evaluation of novel tetrazole analogues. Polycycl Aromat Compd. (2022):1–16. doi: 10.1080/10406638.2022.2036778 [DOI] [Google Scholar]
- 26.Ghosh S, Chetia D, Gogoi N, Rudrapal M. Design, molecular docking, drug-likeness and molecular dynamics studies of 1,2,4-Trioxane derivatives as novel Plasmodium falciparum falcipain-2 (FP-2) inhibitors. Biotechnologia. (2021) 102:257–75. doi: 10.5114/bta.2021.108722, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James AE, Okoro UC, Ezeokonkwo MA, Bhimapaka CR, Rudrapal M, Ugwu DI, et al. Design, synthesis, molecular docking, molecular dynamics and in vivo antimalarial activity of new dipeptide-sulfonamides. ChemistrySelect. (2022) 7:e202103908. doi: 10.1002/slct.202103908 [DOI] [Google Scholar]
- 28.Junejo JA, Zaman K, Rudrapal M, Celik I, Attah EI. Antidiabetic bioactive compounds from Tetrastigma angustifolia (Roxb.) deb and Oxalis debilis Kunth.: validation of ethnomedicinal claim by in vitro and in silico studies. S Afr J Bot. (2021) 143:164–75. doi: 10.1016/j.sajb.2021.07.023 [DOI] [Google Scholar]
- 29.Kumar PP, Shaik RA, Khan J, Alaidarous MA, Rudrapal M, Khairnar SJ, et al. Cerebroprotective effect of aloe Emodin: in Silico and in vivo studies. Saudi J Biol Sci. (2022) 29:998–1005. doi: 10.1016/j.sjbs.2021.09.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasala PK, Siva Reddy SSL, Silvia N, Reddy DY, Sampath A, Dorababu N, et al. In vivo immunomodulatory activity and in silico study of Albizia procera bark extract on doxorubicin induced immunosuppressive rats. J King Saud Univ Sci. (2022) 34:101828. doi: 10.1016/j.jksus.2022.101828 [DOI] [Google Scholar]
- 31.Pasala PK, Uppara RK, Rudrapal M, Zothantluanga JH, Umar AK. Silybin Phytosomes attenuates cerebral ischemia-reperfusion injury in rats by suppressing oxidative stress and reducing inflammatory response: in vivo and in silico approaches. J Biochem Mol Toxicol. (2022) 36:e23072. doi: 10.1002/jbt.23073, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Puthenkalam R, Hieckel M, Simeone X, Suwattanasophon C, Feldbauer RV, Ecker GF, et al. Structural studies of GABAA receptor binding sites: which experimental structure tells us what? Front Mol Neurosci. (2016) 9:44. doi: 10.3389/fnmol.2016.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rashid IA, Mukelabai N, Agoni C, Rudrapal M, Aldosari SM, Ibrahim I, et al. Characterization of the binding of MRTX1133 as an avenue for the discovery of potential KRASG12D inhibitors for cancer therapy. Sci Rep. (2022) 12:17796. doi: 10.1038/s41598-022-22668-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudrapal M, Chetia D, Singh V. Novel series of 1,2,4-trioxane derivatives as antimalarial agents. J Enzyme Inhib Med Chem. (2017) 32:1159–73. doi: 10.1080/14756366.2017.1363742, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Annavarapu TR, Kamepalli S, Konidala SK, Kotra V, Challa SR, Rudrapal M, et al. Antioxidant and anti-inflammatory activities of 4-allylpyrocatechol and its derivatives with molecular docking and ADMET investigations. Bull Univ Karaganda-Chem. (2022) 105:50–9. doi: 10.31489/2022Ch1/50-59 [DOI] [Google Scholar]
- 36.Celik I, Rudrapal M, Yadalam PK, Chinnam S, Balaji TM, Varadarajan S, et al. Resveratrol and its natural analogues inhibit RNA dependant RNA polymerase (RdRp) of Rhizopus oryzae in mucormycosis through computational investigations. Polycycl Aromat Compd. (2022):1–18. doi: 10.1080/10406638.2022.2091618 [DOI] [Google Scholar]
- 37.Othman IMM, Mahross MH, Gad-Elkareem MAM, Rudrapal M, Gogoi N, Chetia D, et al. Toward a treatment of antibacterial and antifungal infections: design, synthesis and in vitro activity of novel arylhydrazothiazolylsulphonamide analogues and their insight of DFT, docking and molecular dynamics simulations. J Mol Struct. (2021) 1243:130862. doi: 10.1016/j.molstruc.2021.130862 [DOI] [Google Scholar]
- 38.Hussain N, Kakoti BB, Rudrapal M, Sarwa KK, Celik I, Attah EI, et al. Bioactive antidiabetic flavonoids from the stem bark of Cordia dichotoma Forst.: identification, docking and ADMET studies. Mol Ther. (2021) 2021:M1234. doi: 10.3390/M1234 [DOI] [Google Scholar]
- 39.Umar AK, Zothantluanga JH, Aswin SK, Maulana S, Zubair MS, Lalhlenmawia H, et al. Antiviral phytocompounds ‘ellagic acid’ and ‘(+)-sesamin’ of Bridelia retusa identified as potential inhibitors of SARS-CoV-2 3CL pro using extensive molecular docking, molecular dynamics simulation studies, and bioactivity prediction. Sruct Chem. (2022) 33:1445–65. doi: 10.1007/s11224-022-01959-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudrapal M, Celik I, Chinnam S, Çevik UA, Tallei TE, Nizam A, et al. Analgesic and anti-inflammatory potential of indole derivatives. Polycycl Aromat Compd. (2022):1–22. doi: 10.1080/10406638.2022.2139733 [DOI] [Google Scholar]
- 41.Rudrapal M, Celik I, Chinnam S, Ansari MA, Khan J, Alghamdi S, et al. Phytocompounds of Indian spices as inhibitors of SARS-CoV-2 Mpro and PLpro: molecular docking, molecular dynamics, and ADMET studies. Saudi J Biol Sci. (2022) 29:3456–65. doi: 10.1016/j.sjbs.2022.02.028, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudrapal M, Celik I, Khan J, Ismail RM, Ansari MA, Yadav R, et al. Identification of bioactive molecules from Triphala (Ayurvedic herbal formulation) as potential inhibitors of SARS-CoV-2 main protease (Mpro) through computational investigations. J King Saud Univ Sci. (2022) 34:101826. doi: 10.1016/j.jksus.2022.101826, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudrapal M, Gogoi N, Chetia D, Khan J, Banwas S, Alshehri B, et al. Repurposing of phytomedicine-derived bioactive compounds with promising anti-SARS-CoV-2 potential: molecular docking, MD simulations and drug-likeness/ADMET studies. Saudi J Biol Sci. (2022) 29:2432–46. doi: 10.1016/j.sjbs.2021.12.018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudrapal M, Rashid IA, Agoni C, Bendale AR, Nagar A, Soliman MES, et al. In silico screening of phytopolyphenolics for the identification of bioactive compounds as novel protease inhibitors effective against SARS-CoV-2. J Biomol Struct Dyn. (2022) 40:10437–53. doi: 10.1080/07391102.2021.1944909, PMID: [DOI] [PubMed] [Google Scholar]
- 45.Vikaraman PA, Armaković SJ, Armaković S, Celik I, Bhagyasree JB, Dinesh Babu KV, et al. Exploring the structural, photophysical and optoelectronic properties of a diaryl heptanoid curcumin derivative and identification as a SARS-COV-2 inhibitor. J Mol Struct. (2023) 1281:135110. doi: 10.1016/j.molstruc.2023.135110, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zothantluanga JH, Abdalla M, Rudrapal M, Tian Q, Chetia D, Li J. Computational investigations for identification of bioactive molecules from Baccaurea ramiflora and Bergenia ciliate as inhibitors of SARS-CoV-2 Mpro. Polycycl Aromat Compd. (2022):1–29. doi: 10.1080/10406638.2022.2046613 [DOI] [Google Scholar]
- 47.Zothantluanga JH, Aswin SK, Rudrapal M, Cheita D. Antimalarial flavonoid-glycoside from Acacia pennata against PfDHFR-TS: an in-silico study. Biointerface Res Appl Chem. (2022) 12:4871–87. doi: 10.33263/BRIAC124.48714887 [DOI] [Google Scholar]
- 48.Kakran M, Sahoo NG, Li L, Judeh Z. Fabrication of quercetin nanoparticles by anti-solvent precipitation method for enhanced dissolution. Powder Technol. (2012) 223:59–64. doi: 10.1016/j.powtec.2011.08.021 [DOI] [Google Scholar]
- 49.Giardina WJ, Gasior M. Acute seizure tests in epilepsy research: electroshock- and chemical-induced convulsions in the mouse. Curr Protoc Pharmacol. (2009) 45:5.22.1–5.22.37. doi: 10.1002/0471141755.ph0522s45 [DOI] [PubMed] [Google Scholar]
- 50.Hoeller A, De Carvalho C, Franco P, Formolo D, Imthon A, Dos Santos H, et al. Behavioral and neurochemical consequences of pentylenetetrazol-induced kindling in young and middle-aged rats. Pharmaceuticals. (2017) 10:75. doi: 10.3390/ph10030075, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sefil F, Arık AE, Acar MD, Bostancı MÖ, Bagirici F, Kozan R. Interaction between carbenoxolone and valproic acid on pentylenetetrazole kindling model of epilepsy. Int J Clin Exp Med. (2015) 8:10508–14. PMID: [PMC free article] [PubMed] [Google Scholar]
- 52.Ding L, Li J, Song B, Xiao X, Huang W, Zhang B, et al. Andrographolide prevents high-fat diet-induced obesity in C57BL/6 mice by suppressing the sterol regulatory element-binding protein pathway. J Pharmacol Exp Ther. (2014) 351:474–83. doi: 10.1124/jpet.114.217968, PMID: [DOI] [PubMed] [Google Scholar]
- 53.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. (1979) 95:351–8. doi: 10.1016/0003-2697(79)90738-3, PMID: [DOI] [PubMed] [Google Scholar]
- 54.Misra HP, Fridovich I. The oxidation of phenylhydrazine: superoxide and mechanism. Biochemistry. (1976) 15:681–7. doi: 10.1021/bi00648a036 [DOI] [PubMed] [Google Scholar]
- 55.Haggag BS, Hasanin AH, Raafat MH, Abdel Kawy HS. Lamotrigine decreased hippocampal damage and improved vascular risk markers in a rat model of Pentylenetetrazole induced kindling seizure. Korean J Physiol Pharmacol. (2014) 18:269–78. doi: 10.4196/kjpp.2014.18.3.269, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebrahimzadeh Bideskan A. Effects of soy extract on pentylenetetrazol-induced seizures in ovariectomized rats. J Chin Integr Med. (2011) 9:611–8. doi: 10.3736/jcim20110606 [DOI] [PubMed] [Google Scholar]
- 57.Hosseini M, Harandizadeh F, Niazmand S, Soukhtanloo M, Faizpour A, Ghasemabady M. The role for nitric oxide on the effects of hydroalcoholic extract of Achillea wilhelmsii on seizure. Avicenna J Phytomed. (2014) 4:251–9. PMID: [PMC free article] [PubMed] [Google Scholar]
- 58.Patsoukis N, Zervoudakis G, Georgiou CD, Angelatou F, Matsokis NA, Panagopoulos NT. Effect of pentylenetetrazol-induced epileptic seizure on thiol redox state in the mouse cerebral cortex. Epilepsy Res. (2004) 62:65–74. doi: 10.1016/j.eplepsyres.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 59.Akula KK, Dhir A, Kulkarni SK. Effect of various antiepileptic drugs in a pentylenetetrazol-induced seizure model in mice. Methods Find Exp Clin Pharmacol. (2009) 31:423–32. doi: 10.1358/mf.2009.31.7.1415891, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Celikyurt IK, Mutlu O, Ulak G, Akar FY, Erden F. Gabapentin, a GABA analogue, enhances cognitive performance in mice. Neurosci Lett. (2011) 492:124–8. doi: 10.1016/j.neulet.2011.01.072, PMID: [DOI] [PubMed] [Google Scholar]
- 61.Rauca C, Wiswedel I, Zerbe R, Keilhoff G, Krug M. The role of superoxide dismutase and α-tocopherol in the development of seizures and kindling induced by pentylenetetrazol - influence of the radical scavenger α-phenyl-N-tert-butyl nitrone. Brain Res. (2004) 1009:203–12. doi: 10.1016/j.brainres.2004.01.082, PMID: [DOI] [PubMed] [Google Scholar]
- 62.Bhardwaj M, Kumar A. Neuroprotective effect of lycopene against PTZ-induced kindling seizures in mice: possible behavioural, biochemical and mitochondrial dysfunction. Phytother Res. (2016) 30:306–13. doi: 10.1002/ptr.5533, PMID: [DOI] [PubMed] [Google Scholar]
- 63.Nazıroğlu M, Yürekli VA. Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements. Cell Mol Neurobiol. (2013) 33:589–99. doi: 10.1007/s10571-013-9936-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kola PK, Akula A, Nissankararao LS, Danduga RCSR. Protective effect of naringin on pentylenetetrazole (PTZ)-induced kindling; possible mechanisms of antikindling, memory improvement, and neuroprotection. Epilepsy Behav. (2017) 75:114–26. doi: 10.1016/j.yebeh.2017.07.011, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Kumar A, Lalitha S, Mishra J. Hesperidin potentiates the neuroprotective effects of diazepam and gabapentin against pentylenetetrazole-induced convulsions in mice: possible behavioral, biochemical and mitochondrial alterations. Indian J Pharmacol. (2014) 46:309–15. doi: 10.4103/0253-7613.132180, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.