Abstract
We have conducted this study to understand the impact of poor differentiation (PD), as a sole poor prognostic factor, in early oral cancers. This was a retrospective analysis of a prospectively maintained database of clinically node-negative early T stage OSCC patients operated between 2012 and 2014. Impact of PD on the survival and role of adjuvant therapy in these patients was noted. Out of 1172 patients screened, 280 patients were found to be eligible for the study. 11.4% patients had PDSCC. It was found to be associated with tongue cancers and peri-neural invasion. It had a significant impact on OS and DFS (48.7 months vs 81.4 months, p < 0.00 and 44.6 months vs 73.5 months, p < 0.00 respectively. Hazard ratio for DFS: 4.08. Although patients with PDSCC had better survival with radiotherapy, but this was not statistically significant. Poor differentiation as a stand-alone factor impacts survival in patients with early oral cancer. It may be seen more often in patients with tongue cancer and may have associated PNI. The role of adjuvant therapy in such patients is not clear.
Keywords: Poor differentiation, Oral cancer, Prognosis, adjuvant therapy, head and neck cancers
Introduction
The prognosis and survival of appropriately treated oral squamous cell carcinoma (OSCC) has improved over the years [1]. There are multitudes of poor prognostic factors which may affect survival in patients with oral cancers. Few of such factors include large tumor size (T3, T4), greater depth of invasion (DOI), perineural invasion (PNI), positive margins, lymph node positivity with or without extracapsular spread (ECS), etc. Adjuvant therapy in the form of radiotherapy (RT) or chemo-radiotherapy (CTRT) may be considered in the presence of certain adverse factors in the histopathological report. These may help in improving the local control as well as survival [2].
However, there is lack of clarity about the impact on survival and requirement for adjuvant therapy with respect to a few factors like poor differentiation (PD), lymphovascular emboli (LVE), etc. A majority of studies which have evaluated their role have done so in the presence of other adverse factors like advanced T stage, or presence of nodal metastasis, etc. There have hardly been any studies, which have looked at these factors in isolation. This bears more relevance in early oral cancers, when no other indication of adjuvant therapy exists. In fact, adjuvant therapy in such cases maybe considered an overkill and may also worsen the quality of life (QoL) of these patients [3]. Therefore, we conducted this study to evaluate the impact of PD on patients with early oral cancer and also to evaluate the role of adjuvant therapy in such cases.
Methodology
This study was a retrospective analysis of a prospectively maintained database where we screened 1172 patients of oral cancer operated between 2012 and 2014 at a tertiary care cancer center. We only included treatment naïve patients who had pathologically T1 and T2 tumors with pathologically N0 neck. The patients were staged according to AJCC 8th edition. So, patients with DOI more than 10 mm were also excluded from the study. Moreover, only patients with clear margins (> 5 mm) on final HPR were included. This was done so as to only include patients with early disease and no other risk factor which would have merited adjuvant radiation.
All the clinical details of the patients were obtained from the electronic medical records (EMR). The presence of various clinico-pathological factors like T stage, N stage, grade of differentiation, DOI, LVE, PNI, details of adjuvant therapy received, and disease status at the last follow-up were also recorded. Follow-up status of the patients was also updated from the EMR. Histopathology reporting was done by two head and neck pathologists, of which one was a senior faculty member. Decision regarding adjuvant therapy was made as per the multi-disciplinary team opinion as per the institutional policy. It was considered on case to case basis with following factors affecting decision—thicker tongue tumors (> 7-mm depth of invasion), presence of peri-neural invasion, and poor differentiation.
We evaluated the distribution of various clinico-pathological factors with respect to the differentiation of the tumor. The chi-square test was used to look for any association between the factors with p value less than 0.05 considered as significant.
Overall survival (OS) was calculated from date of registration to the date of last known follow-up or the date of the death of the patient. Disease-free survival (DFS) was calculated from the date of registration to date of diagnosis of recurrence. Impact of degree of differentiation, more specifically PD on the survival, was noted. Impact of adjuvant therapy on survival in these patients was also evaluated. Multivariate analysis was done using cox regression analysis. Hazard ratio (HR) was calculated for the impact of poor differentiation on OS. Normally, for survival calculations, we look at the time period when 50% patients would have died. However, as the majority of the patients were alive at the end of the follow-up period, median value was not reached; hence, for calculations, we used the time period when at least 10% patients would have died.
As this was a retrospective analysis of the prospectively collected data with no intervention upon the patients, institutional review board approval was not sought for. The study was in compliance with Helsinki declaration. All statistical analyses were performed using SPSS version 21 (IBM corp).
Results
Out of 1172 patients screened, 280 patients were found to be eligible for the study. The mean age of the cohort was 51.4 years (range 26–60 years). 78.9% patients were males. Fifty percent patients had tongue cancer and 29.3% had buccal mucosa cancer. Sixty percent patients had pT2 disease and the remaining had pT1 disease. All the patients were pathologically node negative. 98.6% patients underwent unilateral neck dissection; remaining 1.4% underwent bilateral neck dissection. 11.4% (32/280) patients had poorly differentiated squamous cell carcinoma (PDSCC). 7.9% (22/280) had PNI and 0.7% had LVE (2/280). PNI and PD were both present in 2.1% (6/280) of the patients. 26.1% (73/280) patients received adjuvant RT. Among those with PD, 68.8% patients received adjuvant therapy (Table 1).
Table 1.
Distribution of various clinico-pathological factors in the cohort along with distribution when stratified based upon the differentiation of the tumor along with p values
| Factor | Total number of patients (percentage) n = 280 | PDSCC (n = 32, 11.4%) | Non-poorly differentiated (n = 248, 88.6%) | p value | |
|---|---|---|---|---|---|
| WDSCC (n = 77, 27.5%) | MDSCC (n = 171, 61.1%) | ||||
| Gender | .645 | ||||
| Male | 221 (78.9%) | 24 (75.0%) | 63 (81.8%) | 134 (78.4%) | |
| Female | 59 (21.1%) | 8 (25.0%) | 14 (18.2%) | 37 (21.6%) | |
| pT stage | .180 | ||||
| pT1 | 112 (40%) | 9 (28.1%) | 36 (46.8%) | 67 (39.2%) | |
| pT2 | 168 (60%) | 23 (71.9%) | 41 (53.2%) | 104 (60.8%) | |
| Sub-site | .001 | ||||
| Buccal mucosa | 82 (29.3%) | 5 (15.6%) | 27 (35.1%) | 50 (29.2%) | |
| Tongue | 140 (50%) | 25 (78.1%) | 27 (35.1%) | 88 (51.5%) | |
| Other sites | 58 (20.6%) | 2 (6.3%) | 23 (29.9%) | 33 (19.3%) | |
| Perineural invasion | .027 | ||||
| Yes | 22 (7.9%) | 6 (18.7%) | 04 (05.2%) | 12 (7.0%) | |
| No | 258 (92.1%) | 26 (81.3%) | 73 (94.8%) | 159 (93.0%) | |
| Lymphovascular emboli | 1.000 | ||||
| Yes | 2 (0.7%) | 0 (0%) | 0 (0%) | 2 (1.2%) | |
| No | 278 (99.3%) | 32 (100%) | 77 (100%) | 169 (98.8%) | |
| Adjuvant radiotherapy | .000 | ||||
| Yes | 73 (26.1%) | 22 (68.8%) | 08 (10.4%) | 43 (25.1%) | |
| No | 207 (73.9%) | 10 (31.2%) | 69 (89.6%) | 128 (74.9%) | |
WDSCC well-differentiated squamous cell carcinoma, MDSCC moderately differentiated squamous cell carcinoma, PDSCC poorly differentiated squamous cell carcinoma
Poorly differentiated tumors were more likely to pT2 and arise from tongue. They had higher association with PNI and were more likely to receive adjuvant therapy. The association was statistically significant for sub-site, PNI, and adjuvant therapy only (Table 1).
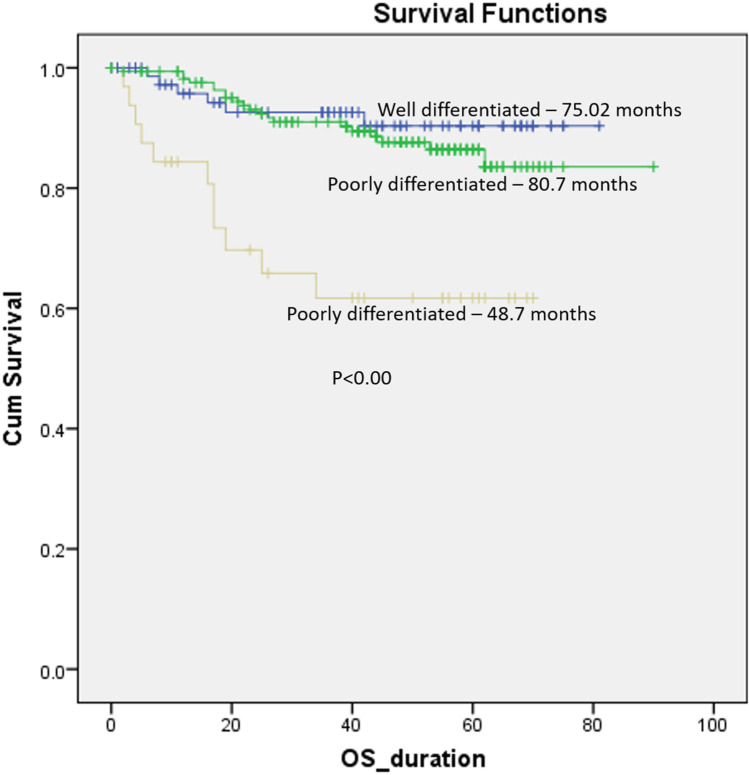
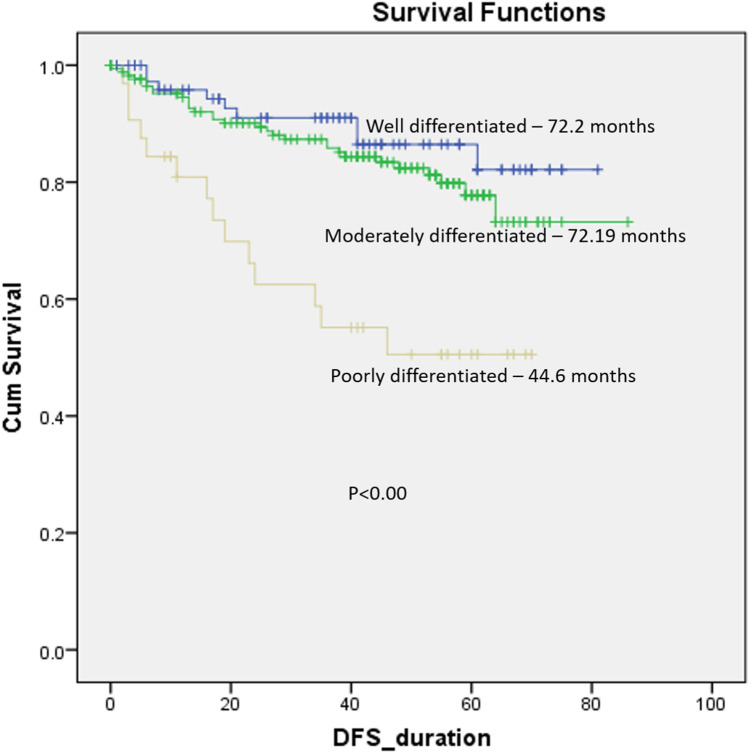
Mean OS and DFS of the cohort were 79.1 months and 71.1 months respectively. Five-year DFS for entire cohort was 86.1%. When stratified based upon the differentiation of the tumor, the patients with poor differentiation had statistically worse OS and DFS (p < 0.00) (Figs. 1, 2).
Fig. 1.
Overall survival stratified according to the differentiation of tumor
Fig. 2.
Disease-free survival stratified according to the differentiation of tumor
In order to assess the impact of grade of differentiation, we did two comparisons where we clubbed well (WDSCC) and moderately differentiated (MDSCC) tumors together and compared against poorly differentiated tumors. In this case, poorly differentiated tumors had statistically worse OS and DFS (48.7 months vs 81.4 months, p < 0.00 and 44.6 months vs 73.5 months, p < 0.00 respectively) as compared to well and moderately differentiated ones clubbed together.
Secondly, we clubbed MDSCC as well as PDSCC tumors together and compared them against well-differentiated ones. In this case, there was no statistically significant difference in OS and DFS between the two groups (77.6 months vs 75 months, p < 0.15 and 72.2 months vs 69.2 months, p 0.09 respectively). This clearly demonstrates that poor differentiation is a significant factor and moderate and well-differentiated tumors have similar prognosis and may be clubbed together. When comparing well, moderate, and poorly differentiated tumors individually, the graphs for well and moderate differentiation nearly overlapped, and poor differentiation had significantly worse survival. (OS: 75 months, 80.7 months, and 48.7 months respectively, p value < 0.00) (DFS: 72.2 months, 72.2 months, and 44.6 months respectively, p < 0.00).
Multivariate analysis revealed that only differentiation and PNI impacted overall survival in a significant manner.
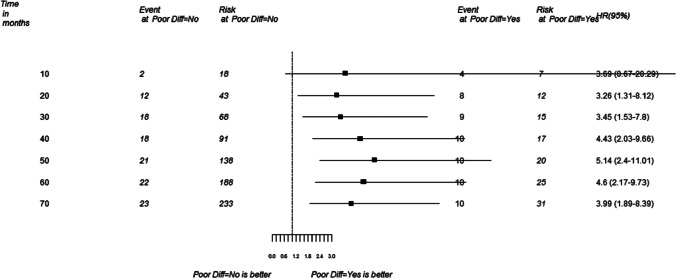
As the majority of the patients were alive at the end of the follow up period, we used the time period when at least 10% patients would have died, for hazard ratio calculation. HR for poor differentiation was 4.08 (Fig. 3). HR for peri-neural invasion was 4.35.
Fig. 3.
Hazards ratio (HR) for death in patients with early oral cancer with poor differentiation HR = 4.08 (CI 1.71–10.71)
Patients who received adjuvant RT had significantly worse OS and DFS as compared to those who did not receive RT (OS: 69.5 months vs 74.5 months, p < 0.01; DFS: 60.4 months vs 70.7 months, p < 0.001). For adjuvant radiotherapy, HR for death was 2.4 as compared to those who did not receive adjuvant therapy. This shows that though adjuvant therapy may improve survival in individuals with higher risk (even in early oral cancers) but still their survival is less as compared to patients without risk factors.
Among the subset of patients with PD, those who received radiotherapy had better OS and DFS as compared to those who did not (OS: 49.3 months vs 46.2 months, p < 0.73; DFS: 45.8 months vs 41.3 months, p < 0.74), though these were not statistically significant, probably because of less number of patients in the subsets. Five-year overall survival of patients with PD receiving and not receiving adjuvant radiotherapy was 65% and 60% respectively.
Discussion
The role of PDSCC has always been controversial and a matter of debate for long. The degree of differentiation between different tumors varies. There have been several attempts to classify the tumors based upon differentiation. Broder had initially classified tumors as well, moderately, poorly differentiated, and anaplastic in order of decreasing degree of differentiation and keratinization. Anaplastic/grade IV tumor differentiation has been discontinued now in several classifications. In 1987, Anneroth introduced a multifactorial grading system, with scoring for various risk factors (keratinization, nuclear pleomorphism, mitoses, pattern of invasion, stage of invasion, and lymphoplasmacytic infiltration). The sum of these scores was used for grading tumor differentiation. Bryne further modified Broder’s and Anneroth’s criteria and suggested that invasion should be checked at the deepest margins, and not in the whole thickness of tumor [4]. Although several authors and organizations have given their own sets of criteria for tumor grading, a consensus still lacks. None of the criteria is universally followed and interpretation between pathologists differs from each other, thus making the tumor grading system very subjective. High grade/PDSCC are characterized by a dissociated growth with invasion by smaller nests, clusters, and individual cells that have markedly abnormal nuclei, usually with minimal keratin [5].
The 5-year survival rate in early-stage OSCC may reach 80–90%, whereas for advanced stage oral squamous cell carcinoma it remains about 40% [6]. In our study, 86% patients were alive and disease free at the end of 5 years. A SEER (Surveillance, Epidemiology, and End Results) database review of 18,115 cases of OSCC evaluated the survival outcomes on basis of tumor stage and histopathological grading. Their cohort had 18% cases of PDSCC, whereas in our study PDSCC was seen in 11.4% patients. They found the risk of mortality in patients with early-stage node-negative tumors with PDSCC to be more than double the risk (2.66) for WDSCC after adjusting for all the confounders. This study has failed to adjust the hazards for PNI or DOI, both of which are important adverse prognostic factors [7].
Few studies have found higher odds (after adjustment for confounders) for developing occult metastasis in the neck with PDSCC (aOR = 6.25; p < .001) [8, 9]. Even otherwise, PD is known to have increased rate of nodal metastasis [7]. However, the poorer prognosis of PDSCC may be attributed to increased probability of developing lymph node metastasis, and not due to PDSCC per se, so its prognosis in a node-negative neck remains unclear.
Due to higher propensity for nodal metastasis, many studies have even advocated addressing the neck in early-stage node-negative tumors (cN0) for PDSCC [7]. A retrospective analysis observed a higher risk of nodal metastasis with MDSCC and PDSCC compared to WDSCC (RR 7; < 0.0001) [10]. The study did not look at the PDSCC separately, which has a different prognostic spectrum and poorer survival compared to MDSCC [11]. The controversy about elective neck dissection has been settled by an RCT, which clearly suggested better survival and loco-regional control in patients undergoing elective neck dissection [12]. So, irrespective of the tumor grade, neck dissection is to be considered for all cases with oral cancer.
Another retrospective study of 1240 Chinese patients with oral cancer reported PDSCC to have significantly inferior overall survival outcomes compared to WDSCC (OR = 1.46; CI = 1.04–2.06 in multivariate analysis). This study had included both early (49.45%) as well as advanced (50.55%) oral cancers. Since the study did not consider DOI in staging, the number of advanced cases would further increase if staged as per the latest staging system [13]. A similar study of 1047 oral cancer patients found increase in tumor grade to be associated with significantly worse prognosis. But after adjusting the confounders, tumor grading was found to be not significant for prognostication. Interestingly, 26% of the study population had advanced disease (pT3,4), and more than 30% had nodal metastasis. Also, the DOI has not been taken into account [14]. PD undoubtedly worsens the prognosis, but associations with other poor prognostic factors (advanced stage, greater DOI, nodal positivity) dilute its effect, hence giving a biased result.
In a multicentric study conducted in Finland, USA, and Brazil on early oral tongue cancers, the authors could not find any correlation between locoregional recurrence and grades of tumor. They found that the risk of cancer related death was higher for PDSCC compared to WDSCC; however, it was statistically not significant (HR 1.63, 95 % CI 0.85–3.13 for PDSCC vs WDSCC). They instead found DOI of and beyond 4 mm and worst pattern of invasion (WPOI) to have the highest prognostic value for both, locoregional recurrence and death due to OSCC in early tongue lesions [15]. Another study found that DOI > 10 mm had higher risk (RR 8; < 0.001) compared to PDSCC (RR 7; < 0.0001) [16]. DOI is a proven stronger prognostic factor compared to PDSCC. In fact, this has been recognized and included in the AJCC 8th edition. In our study, we have staged the patients as per AJCC 8th edition thus reducing the bias due to depth in our cohort of patients.
Most of studies, which have evaluated the role of PDSCC, have been done on a varied group of population, with majority of studies including patients with early as well as advanced disease. Most of these studies have included various other adverse factors, which deteriorate the survival outcomes (viz. large tumor size like T3, T4; metastatic lymph nodes; greater DOI; positive margins, etc.). In these studies, besides PD, other more “powerful” adverse prognostic factors were also present which merited adjuvant therapy. Thus, evaluating the actual impact of “poor differentiation” and role of adjuvant therapy in such cases is difficult through these studies [15–17].
In the present study, we chose a specific cohort of early-stage (pT1-2) tumors with pathologically node-negative (pN0) neck with clear margins. This allowed us to exclude other proven adverse prognostic factors, which would have merited adjuvant therapy and/or affected prognosis on their own.
We found that when different grades of tumor were evaluated separately, as well as when they were grouped together in different combinations, poorly differentiated tumors stood out because of statistically significant worse prognosis. These patients were more likely to have bigger (T2) tumors and have associated PNI. Tongue cancers were also found to be more frequently associated with PDSCC.
As mentioned earlier, the patients included in this study had excellent survival at 5 years. As a routine for survival analysis, we look at median values. As the number of events in the cohort were too less in view of good survival, we could not perform multivariate analysis or do match pair analysis in our study. Therefore, we looked at the time period when 10% of events took place and based upon that we calculated the HR. Overall HR for death with PDSCC was 4.08.
The role of adjuvant therapy in such cases (with PDSCC alone) is also controversial. A retrospective study has previously looked into this aspect. In it, out of 198 cases, PDSCC was present in 16.1%. Nearly half of the patients were treated with upfront RT or CTRT and not operated. Based upon DOI, only about 20% of the cases were truly early stage. Thus, this study does not provide a correct picture about the impact of PDSCC in patients with early oral cancers and the role of adjuvant therapy in such cases [11–18]. Most of the other existing studies have also not taken adjuvant treatment into consideration.
In the present study, we evaluated the impact of adjuvant treatment on the specific subset of PDSCC in early oral cancers. Though adjuvant radiation improved disease-free survival in patients with PDSCC, the difference was not statistically significant (RT arm vs observation arm, 45.8 months vs 41.3 months, p < 0.74). But, since the number of such patients was less due to highly specific subset, we cannot comment upon the role of adjuvant radiotherapy definitely.
This study has the drawback of being retrospective in nature. The subset of patients evaluated is very specific, and this has been reached after screening a large patient dataset. To the best of our knowledge, this is the only study that has brought forth the impact of poor differentiation alone in early oral cancers and the role of adjuvant therapy in such cases.
Conclusion
Poor differentiation as a stand-alone factor impacts survival in patients with early oral cancer. It may be seen more often in patients with tongue cancer and may have associated PNI. The role of adjuvant therapy in such patients is not clear.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rachit Mathur and Akshat Malik contributed equally to this work.
References
- 1.Amit M, Yen TC, Liao CT, et al. Improvement in survival of patients with oral cavity squamous cell carcinoma: an international collaborative study. Cancer. 2013;119:4242–4248. doi: 10.1002/cncr.28357. [DOI] [PubMed] [Google Scholar]
- 2.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 3.Tirelli G, Gatto A, Bonini P, et al. Prognostic indicators of improved survival and quality of life in surgically treated oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126(1):31–40. doi: 10.1016/j.oooo.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Doshi Neena P, Shah Siddharth A, Patel Keyuri B, et al. Histological grading of oral cancer: a comparison of different systems and their relation to lymph node metastasis. Natl J Community Med. 2011;2:136–142. [Google Scholar]
- 5.Lester DR, Bishop JA. Head and neck pathology: a volume in the series foundations in diagnostic pathology. 3. Philadelphia: Elsevier; 2019. [Google Scholar]
- 6.Feller L, Lemmer J. Oral squamous cell carcinoma; epidemiology, clinical presentation and treatment. J Cancer Ther. 2012;3:263–268. doi: 10.4236/jct.2012.34037. [DOI] [Google Scholar]
- 7.Thomas B, Stedman M, Davies L. Grade as a prognostic factor in oral squamous cell carcinoma: a population-based analysis of the data. Laryngoscope. 2014;124:688–694. doi: 10.1002/lary.24357. [DOI] [PubMed] [Google Scholar]
- 8.Zhan KY, Morgan PF, Neskey DM, et al. Preoperative predictors of occult nodal disease in cT1N0 oral cavity squamous cell carcinoma: review of 2623 cases. Head Neck. 2018;40:1967–1976. doi: 10.1002/hed.25178. [DOI] [PubMed] [Google Scholar]
- 9.Liao CT, Wang HM, Ng SH, et al. Good tumor control and survivals of squamous cell carcinoma of buccal mucosa treated with radical surgery with or without neck dissection in Taiwan. Oral Oncol. 2006;42:800–809. doi: 10.1016/j.oraloncology.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Subramaniam N, Balasubramanian D, Reddy R, et al. Determinants of level Ib involvement in oral squamous cell carcinoma and implications for submandibular gland-sparing neck dissection. Int J Oral Maxillofac Surg. 2018;47:1507–1510. doi: 10.1016/j.ijom.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Padma R, Kalaivani A, Sundaresan S, et al. The relationship between histological differentiation and disease recurrence of primary oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2017;21:461. doi: 10.4103/jomfp.JOMFP_241_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015;373:521–529. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Chen F, Huang J, et al. Prospective study on factors affecting the prognosis of oral cancer in a Chinese population. Oncotarget. 2017;8(3):4352–4359. doi: 10.18632/oncotarget.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Wilmowsky C, Traxdorf M, Adler W, et al. Survival benefit for patients treated in a certified head and neck tumor center. Eur Rev Med Pharmacol Sci. 2019;23:2863–2869. doi: 10.26355/eurrev_201904_17564. [DOI] [PubMed] [Google Scholar]
- 15.Almangush A, Bello IO, Coletta RD, et al. For early-stage oral tongue cancer, depth of invasion and worst pattern of invasion are the strongest pathological predictors for locoregional recurrence and mortality. Virchows Arch. 2015;467(1):39–46. doi: 10.1007/s00428-015-1758-z. [DOI] [PubMed] [Google Scholar]
- 16.Subramaniam N, Balasubramanian D, Reddy R, et al. Determinants of level Ib involvement in oral squamous cell carcinoma and implications for submandibular gland-sparing neck dissection. Int J Oral Maxillofac Surg. 2018;47:1507–1510. doi: 10.1016/j.ijom.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Garavello W, Spreafico R, Gaini RM. Oral tongue cancer in young patients: a matched analysis. Oral Oncol. 2007;43(9):894–897. doi: 10.1016/j.oraloncology.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Padma R, Thilagavathi R, Sundaresan S. Survival outcomes of buccal mucosa carcinoma patients with multimodal therapy: an institutional study. Int J Nutr Pharmacol Neurol Dis. 2016;6:76–80. doi: 10.4103/2231-0738.179967. [DOI] [Google Scholar]