Introduction
Sarcomas of the extremities are relatively infrequent tumors, accounting for 1% of adult malignancies [1]. Multiple histological subtypes, diverse clinical presentation, and variable biological activity depict the spectrum of soft tissue sarcomas (STS) [2]. Surgery remains the mainstay modality of treatment of most subtypes of STS of the extremity [3]. The procedure of choice has evolved from amputation to limb salvage largely due to refinement in surgical techniques and imaging technology advancement [4–6]. Various prospective randomized studies [7] found no statistically significant differences in survival rates when comparing limb-sparing surgery plus radiotherapy with limb amputation. Achieving adequate resection margins is of paramount importance, although there is a sparsity of standardized guidelines that define ideal margins. The objective is to perform a wide resection with microscopic negative margins; hence, sometimes it is inevitable to resort to amputation of the limb to achieve acceptable margins of resection. This brings the “concept of spare parts” which is defined as reutilizing tissues from unsalvageable limbs to reconstruct large defects or to obtain an optimal proximal stump with the needed length for subsequent prosthetization without exploiting the rest of the body for any fresh resources [8].
The use of discarded tissue for reconstruction is referred to as “spare-part surgery,” also known as tissue scavenging surgery, and is an important strategy to utilize tissues otherwise wasted. Although there are no core principles or techniques, it falls within the scheme of primary reconstruction of the limbs. A successful application of this technique can be made by understanding the basic concepts of flaps and grafts.
This publication aims to perform a complete systematic revision of the main techniques encompassed in this concept, as well as incorporate our own experience with the surgical approach.
Concept of Spare-Part Surgery
Spare-part surgery does not have a standardized algorithm but falls within the scheme of primary reconstruction of the limbs. The unique aspect of spare-part surgery in STS is that the surgeon has to assess and create a strategy on the table to maximize the use of tissues that otherwise would be disposed of off. This can be achieved by surgical expertise, experience, and planning at the time of primary surgery. Certain factors should be taken into consideration in the decision-making, the first being prerequisites that must be satisfied when the surgeon contemplates spare-part surgery.
Prerequisites for Spare-Part Surgery
The spare parts need to have anatomical integrity and should be free of tumor infiltration, and the necessary imaging techniques should be performed to ensure it.
Ischemia time should be within salvageable limits for revascularization for composite spare parts.
Reconstruction should offer a better global function than primary amputation.
The spare part should serve a greater function when used for reconstruction than being replanted in its anatomical location.
Use of spare parts should include harvesting healthy tissue if it is necessary to obtain optimal reconstruction quality.
General condition of the patient should be satisfactory to enable a prolonged spare-part surgery.
Reconstructive Techniques
Spare-part reconstruction is a very broad term. Spare-part surgery can be stratified into various levels of complexity and incorporates procedures such as skin grafting for a simple skin defect to more complex microvascular free flaps and transposition of digits. The goal is to optimize oncologic outcomes and maximize functional restoration. Various forms of spare-part surgery include reconstruction utilizing one of the followings:
-
A.
Non-vascularized grafting of spare parts (split or full-thickness skin grafts, arterial or vein grafts, nerve grafts, or tendon or bone grafts)
-
B.Flaps which can be
- Fasciocutaneous flaps
- Musculocutaneous flaps
- Osteocutaneous fillet flaps
- Rotationplasty
Microsurgical techniques allow for complex reconstruction in spare-part surgery. Once successful, these surgeries may obviate the need for major secondary reconstructive procedures as well as donor sites. This useful concept has been frequently applied in hand injuries and major limb trauma, as well as major amputations in tumor surgery. Katsaros has described an extreme example of a free filleted “whole arm flap” in tumor surgery [9]. Free vascularized flaps in non-salvageable major amputations have been described by various authors [10–13].
Our Experience
Case 1
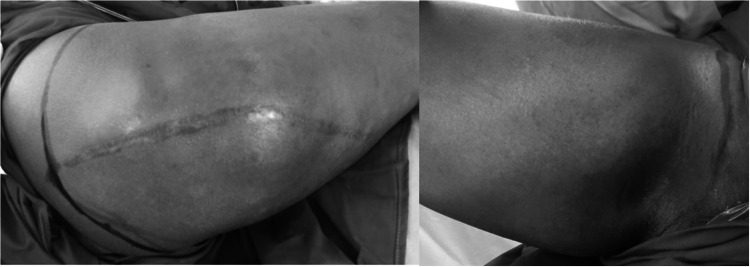
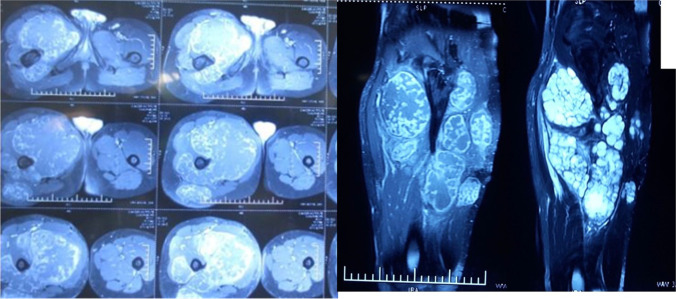
A 33-year-old gentleman presented with a recurrent swelling in the right proximal thigh after wide excision for the tumor over the same location 2 years back elsewhere. Examination revealed a 20 × 19 cm bony hard mass in the proximal thigh, involving the posterior, medial, and anterior compartments with a 27 cm vertical scar in the posterior compartment of the right thigh extending into the gluteal region (Fig. 1). MRI of the right leg was suggestive of a heterogeneous mass involving all compartments of the proximal thigh (Fig. 2). Core needle biopsy revealed chondrosarcoma. As all compartments of the thigh were involved, the patient was not a candidate for limb salvage. Extension of tumor into both the anterior and posterior thigh prevented use of anterior/posterior flaps for soft tissue cover. Right hip disarticulation was performed (Fig. 3). A free flap harvested from the amputated limb was planned for achieving soft tissue cover, avoiding donor site morbidity (Figs. 4 and 5). The fasciocutaneous flap was harvested from the posterior aspect of the leg based on the posterior tibial vessels (Fig. 6). End-to-side arterial anastomosis between posterior tibial and femoral artery and end-to-end venous anastomosis (posterior tibial vein and femoral vein) were performed (Fig. 7) after which skin was approximated (Fig. 8). The total duration of the procedure was approximately 18 h with a warm ischemic time of 4 h and 22 min. The patient had an uneventful postoperative stay. Histopathology revealed a 20 × 17 × 12 cm tumor, suggestive of chondrosarcoma, and resected edges were free of tumor. The patient is doing well at 6 years of follow-up.
Fig. 1.
Mass in the proximal thigh involving all compartments
Fig. 2.
MRI heterogeneous mass involving all compartments of the right proximal thigh
Fig. 3.
Right hip disarticulation status
Fig. 4.
Amputated stump with marked normal skin for covering the defect
Fig. 5.

Flap harvest showing donor vessels
Fig. 6.
Harvested fasciocutaneous flap
Fig. 7.

Vascular anastomosis
Fig. 8.

Closure
Case 2
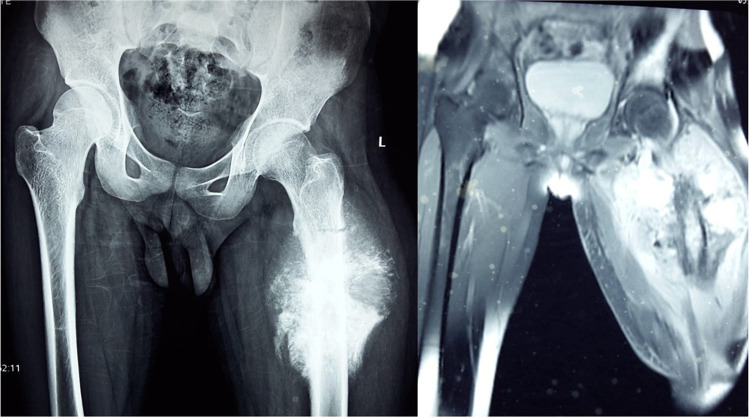
A 20-year-old gentleman was evaluated for pain and swelling over the left thigh for 4 months. Examination revealed an 18 × 17 cm swelling over the proximal part of the left thigh, involving all the compartments of the thigh without any distal neurovascular deficit. Tissue diagnosis was suggestive of osteosarcoma. Metastatic workup was negative. Given the extensive soft tissue involvement and coexisting pathological fracture, the patient was not considered for limb salvage surgery. The femoral vessels were found free and were dissected out, looped, and divided. After resection the size of the soft tissue defect was around 20 × 10 cm. As the tumor was located proximally in the thigh, both anterior/posterior flaps were not feasible to cover the soft tissue defect after disarticulation (Fig. 9). Left hip disarticulation was performed (Fig. 10). The patient underwent spare-part surgery as above with a harvest of the flap from the amputated limb (Figs. 11 and 12). Warm ischemia time was 3 h and 42 min. Arterial anastomosis was posterior tibial artery to the left deep inferior epigastric artery in an end to end fashion. Two venous anastomosis were performed. The posterior tibial vein to the left femoral vein (end to side anastomosis) and the great saphenous vein to a tributary of the femoral vein (end to end anastomosis). Skin was approximated after this (Fig. 13). Postoperative histopathology revealed a 12 × 11 × 12 cm tumor in the proximal femur with features suggestive of chondroblastic variant of osteosarcoma, with all resected edges free of tumor. The patient completed adjuvant chemotherapy and is doing well at 5-year follow-up.
Fig. 9.
X-ray and MRI: left lower limb—mass involving all compartments with pathological fracture
Fig. 10.
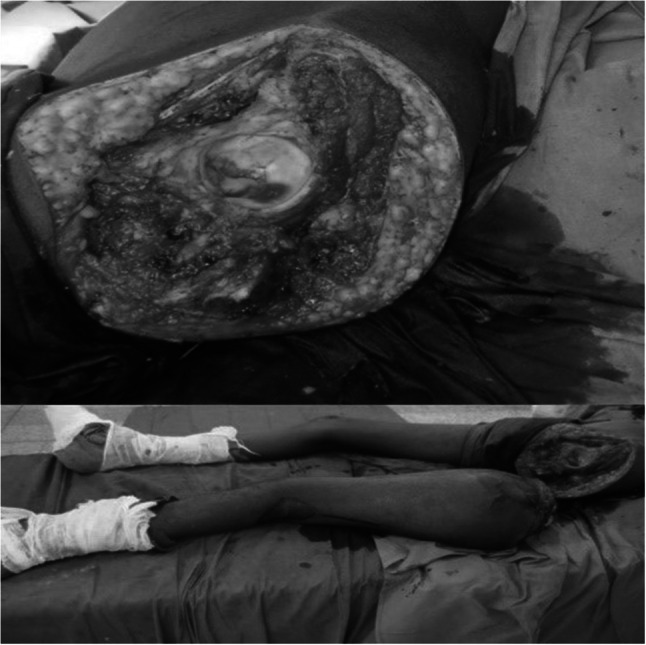
Amputated stump
Fig. 11.
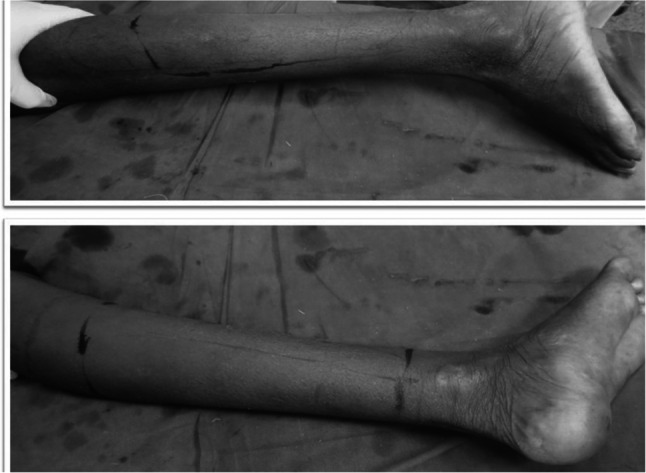
Surface marking for flap harvest
Fig. 12.
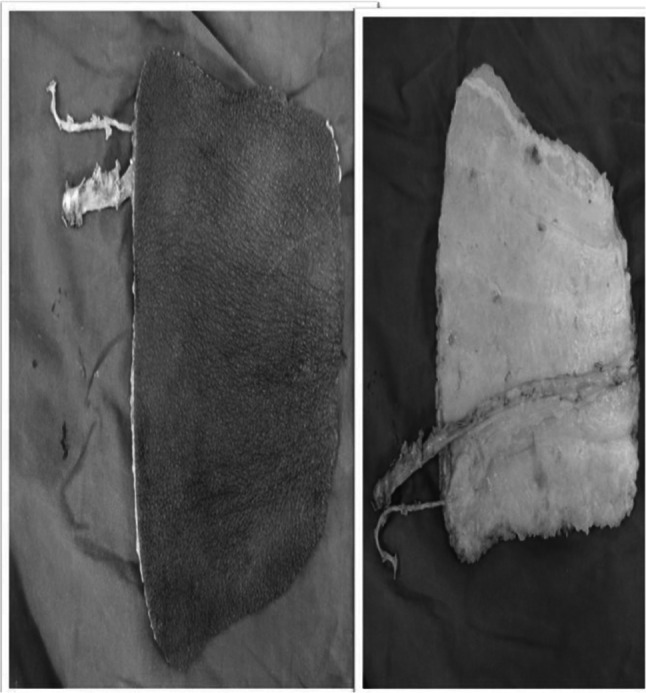
Harvested fasciocutaneous flap
Fig. 13.

Closure
Discussion
In patients with sarcomas of the extremities, it is often necessary to plan wide surgical excision procedures, which may sometimes inevitably lead to amputation. In these cases, defect reconstruction with free tissue transfers from another anatomical region may lead to additional morbidity in the potential donor area. The use of the spare-part technique provides the advantage of reduced donor site morbidity.
The spare-part concept is useful in this situation to address primary reconstruction which obviates unnecessary donor-site morbidity and should be used whenever possible. It is limited by the availability of healthy tissue in the non-salvageable segments and depends greatly on the surgeon’s expertise and the ability to plan primary reconstruction. As emphasized earlier, spare-part surgery can be used alone or in combination with other reconstructive procedures.
When deciding on the reconstructive technique, free flaps are a better option after massive resection as reported by Samant et al., in which a total of 50 patients who underwent reconstruction after a massive oncologic resection were analyzed. In this study, it was found that patients undergoing free flap reconstruction had significantly fewer complications compared with reconstructions using other techniques. This may be because free flaps provide a large amount of soft tissue, often distant from the location of the tumor and zone of injury from prior [14].
Spare-part surgery not only covers massive oncologic resection defects but can also be used in lengthening and optimization of proximal amputation stumps. Thus, in patients with lower limb sarcomas with a short proximal tibial stump after amputation, an osteocutaneous fillet flap may be advised. The sole osteocutaneous fillet flap is ideal for weight-bearing and can allow coverage for a relatively large area. Moreover, the septae between the skin and the plantar fascia prevent shearing forces from being transmitted on soft tissue over the plantar aspect when the patient is walking with the [10]. Also, the sole carries plantar innervated skin that improves prosthesis compliance and reduces complications such as ulcers and painful neuromas [15].
Concerning the probability of occurrence and severity of neuropathic pain and phantom limb symptomatology associated with spare-part surgery techniques, it is important to keep in mind that, when the distance between two severed injured or sectioned nerves is large, axon regeneration and regrowth occur in an unorganized pattern. If neurorrhaphy of the severed ends is not attempted, about 61% of patients report residual limb pain, of which approximately 48.7% are to be caused by a sensitized neuroma [16]. Therefore, the maintenance of nerves in continuity is essential, and whenever nerve sectioning is required, neurorrhaphy of the distal nerve to the proximal amputated trunk must be performed whenever possible. These strategies help reduce the severity of neuropathic pain.
Conclusions
The above-mentioned principles are only some aspects of the multiple possibilities of reconstruction and functional improvement after the application of the spare-part surgery concept. Any reconstruction modality can be utilized either alone or in combination and adapted to each case. In addition, meticulous knowledge of anatomy empowers new designs, which contributes to the continuous improvement of this field of reconstructive surgery. However, despite the numerous advantages for the patient described previously, the application of these techniques has the drawbacks of a prolonged surgical time and greater technical complexity, compared to usual amputation techniques. These aspects are partially solved if a multidisciplinary approach is carried out in reference centers, by an experienced team.
Declarations
Ethics Approval and Consent to Participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Institution where the work was conducted: Cancer Institute (WIA), 38, Sardar Patel Road, Chennai: 600036 India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anand Raja, Email: dr_anand@yahoo.com.
Madhuri Nutakki, Email: nutakki.madhuri90@gmail.com.
Chandra Kumar Krishnan, Email: chandrubcbs@gmail.com.
Viswamadesh Ramachandran, Email: umadesh_2000@yahoo.com.
Sivakumar Mahalingam, Email: itzdrsiva@gmail.com.
Kanuj Malik, Email: malikkanuj03@gmail.com.
Narayanaswamy Kathiresan, Email: nkathiresan@yahoo.com.
References
- 1.Morrison BA. Soft tissue sarcomas of the extremities. Proc (Bayl Univ Med Cent) 2003;16(3):285–290. doi: 10.1080/08998280.2003.11927915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR (2017) AJCC Cancer Staging Manual. 8th edition. Springer
- 3.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353(7):701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 4.Suit HD, Spiro I (1994) Role of radiation in the management of adult patients with sarcoma of soft tissue. Semin Surg Oncol 10(5):347–56. 10.1002/ssu.2980100507 [DOI] [PubMed]
- 5.Tiong SS, Dickie C, Haas RL, O'Sullivan B. The role of radiotherapy in the management of localized soft tissue sarcomas. Cancer Biol Med. 2016;13(3):373–383. doi: 10.20892/j.issn.2095-3941.2016.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindberg RD, Martin RG, Romsdahl MM, Barkley HT., Jr Conservative surgery and postoperative radiotherapy in 300 adults with soft-tissue sarcomas. Cancer. 1981;47(10):2391–2397. doi: 10.1002/1097-0142(19810515)47:10<2391::aid-cncr2820471012>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, DeMoss EV, Seipp C, Sindelar WF, Sugarbaker P, Wesley R. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196(3):305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng YP, Lahiri A. Spare-part surgery. Semin Plast Surg. 2013;27(4):190–197. doi: 10.1055/s-0033-1360586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsaros J (1992) Indications for free soft-tissue flap transfer to the upper limb and the role of alternative procedures. Hand Clin 8(3):479–507 [PubMed]
- 10.Ghali S, Harris PA, Khan U, Pearse M, Nanchahal J. Leg length preservation with pedicled fillet of foot flaps after traumatic amputations. Plast Reconstr Surg. 2005;115(2):498–505. doi: 10.1097/01.prs.0000149482.96729.11. [DOI] [PubMed] [Google Scholar]
- 11.Kayikçioğlu A, Ağaoğlu G, Nasir S, Keçik A. Crossover replantation and fillet flap coverage of the stump after ectopic implantation: a case of bilateral leg amputation. Plast Reconstr Surg. 2000;106(4):868–873. doi: 10.1097/00006534-200009020-00019. [DOI] [PubMed] [Google Scholar]
- 12.Cavadas PC, Raimondi P. Free fillet flap of the hand for elbow preservation in nonreplantable forearm amputation. J Reconstr Microsurg. 2004;20(5):363–366. doi: 10.1055/s-2004-829999. [DOI] [PubMed] [Google Scholar]
- 13.Baek RM, Eun SC, Heo CY, Baek SM. Amputation stump salvage using a free forearm flap from the amputated part. J Plast Reconstr Aesthet Surg. 2009;62(10):e398–400. doi: 10.1016/j.bjps.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Samant M, Chang EI, Petrungaro J, Ver Halen JP, Yu P, Skoracki RJ, Chang DW. Reconstruction of massive oncologic defects following extremity amputation: a 10-year experience. Ann Plast Surg. 2012;68(5):467–471. doi: 10.1097/SAP.0b013e318232b096. [DOI] [PubMed] [Google Scholar]
- 15.Laporta R, Atzeni M, Longo B, Santanelli di Pompeo F (2016) Double free fillet foot flap: sole of foot and dorsalis pedis in severe bilateral lower extremity trauma, a 10-year follow-up case report. Case Reports Plast Surg Hand Surg 3(1):62–65. 10.1080/23320885.2016.1221730. [DOI] [PMC free article] [PubMed]
- 16.Oliveira KMC, Pindur L, Han Z, Bhavsar MB, Barker JH, Leppik L. Time course of traumatic neuroma development. PLoS ONE. 2018;13(7):e0200548. doi: 10.1371/journal.pone.0200548. [DOI] [PMC free article] [PubMed] [Google Scholar]