Abstract
Mucoepidermoid carcinoma (MEC) is the commonest malignant salivary gland tumor affecting the parotid gland in adults and children. In children and adolescents, there is a peak incidence in the second decade. We came across a 6-year-old girl with intermediate-grade MEC parotid gland, which is very unusual below 10 years of age. A global literature search revealed only 3 other similar cases in children below 10 years of age. She presented with a 2-year history of left parotid gradually increasing hard swelling involving the overlying skin and underlying sternocleidomastoid muscle which was confirmed on a contrast-enhanced computed tomography (CECT) scan of the face and neck as well as a core biopsy to be a MEC left parotid. The patient underwent a left radical parotidectomy sacrificing the main trunk of the facial nerve while carefully preserving its distal branches along with a left selective neck dissection (SND) followed by facial reanimation using primary neurorrhaphy. Histopathology confirmed an intermediate-grade MEC pT4aN2bMx with close deep lobe margin warranting adjuvant radiotherapy. Albeit very rare, salivary gland neoplasms may occur in children in the first decade of life. Appropriate planning regarding oncological resection with/without facial reanimation, appropriate rehabilitation followed by adjuvant treatment based on histopathology ensures a good prognosis.
Keywords: Salivary gland tumors, Mucoepidermoid carcinoma, Parotid malignancy, Pediatric mucoepidermoid carcinoma, Facial reanimation, Primary neurorrhaphy
Introduction
Salivary gland tumors account for < 10% of all pediatric head and neck tumors with a peak incidence in the second decade of life. Eighty percent of salivary gland tumors in the pediatric population are benign [1]. MEC is the commonest primary salivary gland malignancy in children constituting approximately 50%, mainly affecting the parotid gland followed by palatal minor salivary glands and the submandibular gland [2]. A Harvard case series reported 7 cases of pediatric MEC parotid, of which only 1 patient was < 10 years old [1]. Limited literature on MEC parotid < 10 years restrict standardized management protocols. We present India’s first ever case report of MEC parotid gland in a 6-year old.
Case Description
A 6-year-old girl presented with a 2-year-old left parotid swelling that gradually increased into a 4 cm hard mass without signs of facial nerve palsy. A contrast-enhanced computed tomography (CECT) confirmed a mass involving both lobes of the left parotid gland extending into the sternocleidomastoid muscle and skin (Fig. 1A). Core biopsy confirmed a low-grade MEC, and she was planned for a radical parotidectomy with SND with/without facial nerve resection and facial reanimation.
Fig. 1.
A CECT showing a left parotid lesion involving both lobes of the parotid with widening of the stylo-mandibular tunnel (marked by red arrow). B Carefully dissected and preserved distal branches of the facial nerve, namely, marginal mandibular branch (yellow arrow), lower buccal branch (white arrow), upper buccal branch (blue arrow), and zygomatico-temporal branch (green arrow). C Reinnervated facial nerve function using spinal accessory nerve to innervate the marginal mandibular nerve. D Reinnervated masseteric nerve to innervate the zygomatico-temporal nerve branch/eye branch
The surgical approach was through a standard modified Blair’s incision encompassing the overlying indurated skin at the erstwhile core biopsy site. In view of our patient being 6 years old, we had to keep in mind the fact that we were likely to encounter her facial nerve in a more superficial plane just deep to the subcutaneous tissue as compared to that in adults. Intraoperatively, the tumor involved both the superficial and deep lobes of the parotid, encasing the main trunk of the facial nerve, with uninvolved distal branches which were carefully dissected and preserved (Fig. 1B). Proximally, the facial nerve was identified in the fallopian canal by mastoid drilling and sacrificed to obtain an R0 resection, while saving maximum length of the uninvolved distal branches for facial reanimation. Left selective neck dissection was performed. Facial reanimation via neurorrhaphy using the masseteric nerve to reinnervate the zygomatic branch/eye branch and a few fascicles of the spinal accessory nerve to reinnervate the marginal mandibular nerve was performed. Her postoperative recovery was good and the reinnervated nerve function demonstrated a House Brackmann Score II.
Considering our patient’s age, the rehabilitation though challenging, was eased to a large extent by the patient herself with the help of our motivating Physiotherapy and Rehabilitation department. We initiated early facial nerve stimulating physiotherapy exercises a week after surgery, while electrical nerve stimulation of the re-innervated facial nerve was initiated 3 weeks after surgery to obtain near normal function with a House Brackmann Score between I and II (Fig. 1 C and D).
Histopathology confirmed a 4-cm intermediate-grade MEC involving the facial nerve with lympho-vascular invasion, 2/50 positive lymph nodes, extra-parenchymal involvement of the adjacent soft tissue, i.e., sternocleidomastoid muscle and tissue around the mastoid process, close deep lobe margin which was 1 mm away from the tumor while all the other margins including the facial nerve resection margin were free and at least 5 mm away, staged pT4aN2bMx warranting adjuvant radiation.
Such an advanced stage presentation has not been reported in this age group previously in the literature. The patient underwent skin sparing adjuvant intensity modulated radiation therapy 60 Gy in 30 fractions which enabled us to continue with the regular facial exercises as well as electrical nerve stimulation without any major challenges and effects on functional outcomes during as well as post treatment except mild grade I radiation-related skin changes. She is currently doing well 18 months post treatment.
Discussion
Salivary gland neoplasms account for 1% of all pediatric neoplasms and < 10% of all pediatric head and neck tumors [2]. MEC is the commonest malignant salivary gland tumor, rarely found in children < 10 years [2]. Eveson and Cawson reviewed 2410 salivary gland tumor cases, of which only 6% were < 20 years and mainly adolescents [3]. MEC was extremely rare, with a good prognosis in low- to intermediate-grade tumors [3].
Salivary gland tumors characteristically present as a firm to hard mass gradually increasing in size as seen in our patient [4]. Pain, regional lymphadenopathy, and/or cranial nerve involvement may distinguish malignant and benign tumors [4].
CECT and/or a magnetic resonance imaging (MRI) are recommended to assess the extent of the lesion and regional lymph nodal involvement as was done in our patient. MRI also enables identification of facial nerve involvement [1].
Despite 84–97% accuracy of fine needle aspiration cytology in salivary gland tumors, a core biopsy was performed in our patient revealing a low-grade MEC parotid which is extremely unusual < 10 years [1]. Armed Forces Institute of Pathology (AFIP) criteria, followed by our institution uses histological features like cystic component (< 25%), mitosis per 10 high power field (> 4%), tumor necrosis, neural invasion and nuclear/cellular atypia to classify MEC into low, intermediate, and high grade [1, 4].
Despite no specific identifiable etiology, ionizing radiation exposure and genetic mutations have been implicated, which was ruled out in our patient. The 3 common genetic pathways include CRTC1-MAML2 or CRTC3-MAML2 Fusion, EGFR pathway, and p53 mutation [5].
The treatment of choice for MEC parotid is an en bloc R0 resection of the tumor with SND and facial reanimation in case of facial nerve resection even in the pediatric age group, as seen in the similar cases reported in the reviewed literature worldwide (Table 1). Chiaravalli et al. analyzed 17 patients with pediatric salivary gland tumors of which only 1 was diagnosed with low-grade MEC treated with left superficial parotidectomy with selective neck dissection followed by adjuvant RT in view of involved margins [6]. Another 4-year-old boy with low-grade MEC was treated by Zabin et al. with an adequate parotidectomy alone but underwent superficial parotidectomy and chemotherapy at recurrence later on [2]. Rahbar et al. reported 7 pediatric cases with low-grade MEC of which, only one was a 3-year-old girl (< 10 years) treated with total parotidectomy preserving the facial nerve and is disease free for over 5 years [ 3].
Table 1.
Review of literature of patients < 10 years old treated for muco-epidermoid carcinoma parotid
| Author | Age/sex | Grade (AFIP) | Stage | Surgery | Adjuvant treatment | Recurrence | Further management |
|---|---|---|---|---|---|---|---|
| Our case | 6/F | Intermediate | pT4aN2bMx | Left radical parotidectomy + SND + facial reanimation (close margin) | IMRT (60 Gy/30 fractions) | No | NA |
| Chiaravalli et al. (2014) 1/17 | 8/F | Low | pT1N1Mx | Left superficial parotidectomy + SND (involved margins) | RT (54 Gy) | No | NA |
| Zabin et al. (2009) | 4/M | Low | pT1NxMx | Superficial parotidectomy | None | Yes | Superficial parotidectomy + chemotherapy |
| Rahbar et al. | 3/F | Low | pT2N0Mx | Total parotidectomy preserving the facial nerve | None | No | NA |
Overall incidence of lymph node metastases in MEC parotid range between 18 and 28% [1]. Elective neck dissection is warranted in the presence of regional nodal metastasis, high TNM staging, tumors larger than 3 cm, high histologic grade, facial paralysis, extra-glandular extension, and lympho-vascular invasion [1].
An important point to remember during surgery for this 6-year-old girl was the difference in approach for parotid surgery compared to that in adults on account of differences in extra-cranial course of the facial nerve due to relative absence of the mastoid process in young children. The mastoid antrum is the first air cell to develop, recognized at 21–22 weeks of gestation and fully develops by 34 weeks. At birth, the fully developed mastoid antrum measures 2.9 cm2 and grows approximately at the rate of 1 cm2 per year up to the age of 6 years, after which there is a gradual increase till it reaches the adult size of 12 cm2. With increase in size and volume of the mastoid air cell system, the facial nerve subsequently takes a more medial and protected course behind the mastoid tip and tragal cartilage, making it critical to consider patient age and the extent of mastoid development when dissecting towards the expected course of the facial nerve in pediatric patients [7].
While well-defined landmarks are used to identify the main trunk of the facial nerve during parotid surgery in adults, poorly pneumatized mastoid bone causing an abrupt and horizontal course of the facial nerve from the stylomastoid foramen to the parotid gland as well as its superficial course, often lying just deep to the subcutaneous tissue obscure robust landmarks in the pediatric age [7]. Most important to note is the fact that the marginal mandibular branch of the facial nerve also takes a more superior course over the mandible in children compared to adults [7]. Farroir and Santini proposed identifying the facial nerve as it exits the stylomastoid foramen in a triangle bordered by the cartilaginous ear canal, the anterior border of the sternocleidomastoid muscle, and the digastric muscle. Landmark and approaches to identifying the main trunk of the facial nerve in children and adults have been enumerated in Table 2 [7]. In our patient, since the main trunk was involved by the tumor, we identified the peripheral branches and traced the main trunk posteriorly in addition to drilling the mastoid in order to obtain a clear resection margins on either side of the involved facial nerve segment.
Table 2.
Landmark and approaches to identifying the main trunk of the facial nerve in children and adults
| Young children | Older children and adults |
|---|---|
|
Nerve found in triangle bordered by: 1) Cartilaginous ear canal superiorly 2) Anterior border of the SCM posteriorly 3) Posterior belly of the digastric inferiorly |
1 cm anterior, inferior, and deep to the tragal pointer |
| Identification of the peripheral branches and trace posteriorly | 2–4 mm deep to the tympano-mastoid suture line |
| Nerve identification in the mastoid bone via otologic drill out | Nerve 5–14 mm superior to posterior belly of the digastric muscle |
| Nerve 2–4 mm deep to posterior auricular artery | |
| Nerve runs superficial and inferior to the palpated styloid process | |
| Identification of the peripheral branches and trace posteriorly | |
| Nerve identification in the mastoid bone via otologic drill out |
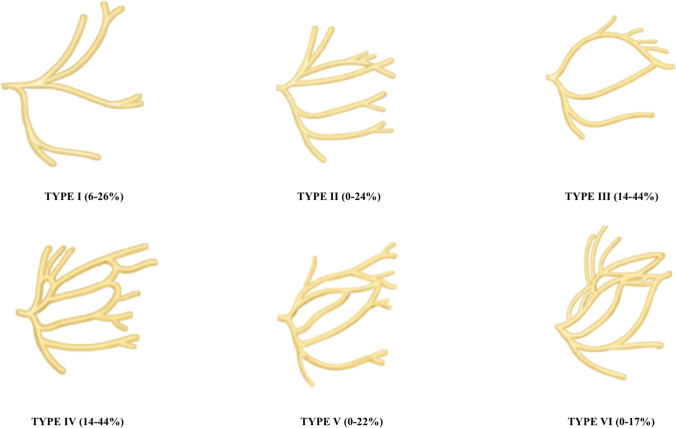
David et al. in 1956 described the 6 types of branching patterns of the facial nerve based on cadaveric dissections in 350 craniofacial halves as shown in Fig. 2: type I: no anastomosis between the upper and lower division; type II: anastomosis only between the branches of the upper division; type III: single anastomosis between the upper and lower divisions; type IV: combination of type II and type III; type V: double anastomosis between the upper and lower divisions; type VI: complex and numerous anastomoses between the two divisions. Our patient demonstrated type I branching pattern of the facial nerve [7].
Fig. 2.
Branching patterns of the facial nerve
Facial nerve sparing is advocated whenever feasible, and facial reanimation is vital in patients requiring facial nerve resection for oncological clearance. Primary neurorrhaphy for facial reanimation is the preferred method of choice ensuring at least Grade III House Brackmann Score [8]. The masseteric nerve, first described by Escat and Viela in 1925 for neurorrhaphy, is commonly used due to easy dissection and relatively lower donor site morbidity [8]. Partial transposition of the spinal accessory nerve using only the sternocleidomastoid branch was first described by Brandon and Gray in 1962 in order to prevent complete denervation of the Trapezius muscle [8]. Due to extensive involvement of the facial nerve main trunk by tumor tissue, our patient underwent a left radical parotidectomy with left SND and facial reanimation via neurorrhaphy using the masseteric nerve and few fascicles of the spinal accessory nerve.
Post-surgical rehabilitation with facial exercises and electrical stimulation forms an important part of management in such patients, which is extremely challenging in this young age group. Literature search revealed no robust data as regards the appropriate time for initiation of rehabilitative measures. We initiated early facial exercises for our 6-year-old patient a week post-surgery, while electrical nerve stimulation was initiated 3 weeks post-surgery to ensure maximum healing post primary neurorrhaphy and was continued during as well as after adjuvant radiation for 6 months to improve and maintain a near normal facial nerve function facilitated by significant efforts of the patient, her parents, and our Physiotherapy and Rehabilitation department.
Judicious use of adjuvant radiotherapy is recommended in MECs with aggressive histologic features such as perineural invasion, soft tissue extension, and multiple nodal involvement, intermediate to high grade tumors, and unresectable residual disease to minimize the associated long-term adverse features seen in > 50% pediatric MEC patients [2]. Chemotherapy on the other hand has been reserved for patients with progressive local or metastatic disease that is not amenable to surgery or radiation therapy [2]. Our patient with an intermediate-grade MEC and lympho-vascular invasion, stage pT4aN2bMx underwent skin sparing adjuvant intensity modulated radiotherapy thereby minimizing the adverse reactions and chemotherapy was avoided. She is currently doing well 18 months post treatment.
Conclusion
With appropriate work up, detailed planning, counselling, and management, we were able to obtain good oncological clearance, primary facial reanimation, and adjuvant treatment in our 6-year-old patient ensuring a good quality of life. We emphasize the need for upfront facial nerve reanimation using primary neurorrhaphy when feasible in pediatric patients with MEC parotid requiring facial nerve sacrifice for R0 resection especially when adjuvant radiotherapy is warranted. Although under-utilized, an emphasis on using the masseteric nerve for primary neurorrhaphy in such cases is warranted. Physiotherapy and electrical stimulation post facial reanimation using primary neurorrhaphy ensure better outcomes.
Abbreviations
- AFIP
Armed Forces Institute of Pathology
- CECT
contrast-enhanced computed tomography
- MEC
mucoepidermoid carcinoma
- SND
selective neck dissection
Declarations
Informed Consent
An informed consent has been obtained from the patient and her parents for publication of this case report, accompanying images and photographs.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Presentations
Pune Grand Rounds 2022 & Tata Memorial Centre 2022 EBM.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vidita Powle, Email: viditapowle@gmail.com.
Taskeen Mannan Sikora, Email: taskeensikora@gmail.com.
Amit Mulay, Email: dramitmulay@gmail.com.
Nutan Jumle, Email: nutan.jumle@jehangirhospital.com.
Snita Sinukumar, Email: snitanag@gmail.com.
References
- 1.Rahbar R, Grimmer JF, Vargus SO, et al. Mucoepidermoid carcinoma of the parotid gland in children: A 10 year experience. Arch Otolaryngol Head Neck Surg. 2006;132:375–380. doi: 10.1001/archotol.132.4.375. [DOI] [PubMed] [Google Scholar]
- 2.Zabin M, Uddin MM, Chowdhury AR, et al. Malignant parotid tumor in a four years old boy- A case report. Bang Onc J. 2009;4(2):86–87. [Google Scholar]
- 3.Eveson JW, Cawson RA. Salivary gland tumours: A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol. 1985;146:51–58. doi: 10.1002/path.1711460106. [DOI] [PubMed] [Google Scholar]
- 4.Boahene DKO, Olsen KD, Lewis JE, et al. Mucoepidermoid carcinoma of the parotid gland: The Mayo Clinic experience. Arch Otolaryngol Head Neck Surg. 2004;130:849–856. doi: 10.1001/archotol.130.7.849. [DOI] [PubMed] [Google Scholar]
- 5.Yan K, Yesensky J, Hasina R, Agrawal N. Genomics of mucoepidermoid and adenoid cystic carcinomas. Laryngoscope Investig Otolaryngol. 2018;3:56–61. doi: 10.1002/lio2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiaravalli S, Guzzo M, Bisogno G, Pasquale MDD, et al. Salivary gland carcinomas in children and adolescents: The Italian TREP Project Experience. Pediatr Blood Cancer. 2014;61:1961–1968. doi: 10.1002/pbc.25139. [DOI] [PubMed] [Google Scholar]
- 7.Bann VD, Wilson M, et al. Pediatric Parotidectomy. In: Fliss DM, et al., editors. Atlas of pediatric head and neck and skull base surgery. Section II Head and Neck; 2021. pp. 101–103. [Google Scholar]
- 8.Biglioli F, Frigerio A, Colombo V, et al. Masseteric-facial nerve anastomosis for early facial reanimation. Journal of Cranio-Maxillo-Facial Surgery. 2012;40:149–155. doi: 10.1016/j.jcms.2011.03.005. [DOI] [PubMed] [Google Scholar]