Abstract
Second-line chemotherapy is recommended for patients who have disease progression after first-line chemotherapy and have a good performance status. The aim of our study is thus to determine which chemotherapy regimen is more appropriate for second-line gastric cancer treatment. Patients were included if they met the following inclusion criteria: metastatic gastric adenocarcinoma pathology; no previous treatment for local gastric cancer (surgery, chemotherapy, or radiotherapy); received first-line chemotherapy for metastatic gastric cancer and had the disease progress afterward; had adequate organ functions for second-line chemotherapy; had an Eastern Cooperative Oncology Group (ECOG) score 0–2; and were HER-2 negative. The patients were examined in three groups according to the second-line chemotherapy regimen they received. These three groups were compared in terms of overall and progression-free survival. The three groups were statistically similar in overall survival, which was the primary endpoint of the study; the median overall survival was 5 months in the FOLFIRI group (n = 79), 6.5 months in the platinum-based group (n = 55), and 5.6 months in the taxane-based group (n = 40) (p = 0.554). There was no statistical difference between the groups’ progression-free survival either; the median progression-free survival time was 3.43 months in the FOLFIRI group, 4 months in the platinum-based group, and 2.77 months in the taxane-based group (p = 0.546). There was no statistically significant difference between the three irinotecan-based, platinum-based, and taxane-based treatments. According to our study’s results, the chemotherapy given in second-line treatment should be decided on an individual basis according to toxicity and cost.
Keywords: Gastric cancer, FOLFIRI, Platinum
Introduction
Gastric cancer is a lethal disease, especially when it becomes metastatic. Disease recurrence occurs in almost half of patients who undergo curative surgery for this disease [1]. The most common treatment for metastatic patients is palliative chemotherapy, which aims to prolong survival and improve their quality of life [2] . Second-line chemotherapy is also recommended for patients who have disease progression after first-line chemotherapy and have a good performance status. Many studies have demonstrated the contribution of second-line chemotherapy to overall patient survival [3–5] . A combination of chemotherapy and biological treatments has also been used in second-line therapy; combining ramucirumab, which blocks vascular endothelial growth factor receptor-2, and paclitaxel especially prolongs overall survival compared to paclitaxel therapy alone [6] .
Although studies have reported some positive results about the effects of second-line gastric cancer treatment, there is still no gold standard for this therapy. For this reason, clinicians choose the drugs for the second-line treatment by looking at specific criteria, such as the treatment, cost, and toxicity a patient previously received in first-line treatment.
To date, studies to identify specific second-line treatments have usually compared one chemotherapy regimen to another [7] . In this study, we instead gathered all the treatments used in clinical practice under main groups and compared them. The aim of our study is thus to determine which chemotherapy regimen is more appropriate for second-line gastric cancer treatment.
Materials and Methods
In this retrospective study, we examined the files of patients admitted to five centers in Turkey between 2015 and 2020. The inclusion criteria were as follows: metastatic gastric adenocarcinoma pathology; no previous treatment for local gastric cancer (surgery, chemotherapy, or radiotherapy); progressive disease after front line chemotherapy; had adequate organ functions for second-line chemotherapy; had an Eastern Cooperative Oncology Group (ECOG) score 0–2; and were HER-2 negative.
The patients were examined in three groups according to the second-line chemotherapy regimen they received. These were those who received irinotecan-based therapy (FOLFIRI), those who received platinum-based therapy (cisplatin/carboplatin or oxaliplatin combinations), and those who received taxane-based therapy (docetaxel or paclitaxel single agent or paclitaxel-ramucirumab). These three groups were compared in terms of overall and progression-free survival. We defined overall survival as the time from the start of second-line therapy to the patient’s death or last follow-up examination. In turn, we defined progression-free survival as the time from the start of second-line therapy to the date of disease progression, patient death, or the last follow-up examination. Tumor progression was evaluated according to RECIST criteria.
We also investigated the effects of age (over and under 65 years), gender, metastasis site (liver, peritoneum, lung, lymph node, bone), and the number of drugs in the first-line chemotherapy regimen (triplet or doublet) on survival. The distributions of the parameters affecting the groups’ overall survival were examined as well. Our study was performed in accordance with the Declaration of Helsinki and was reviewed and approved by Manisa Celal Bayar University local ethics committee (no: 143, 22.03.2021).
All analyses were performed using the statistical software package SPSS version 20.0 for Windows. We used the chi-square test to analyze differences in clinical characteristics between the three groups. Overall and progression-free survival were calculated with the log-rank test. We then used the Kaplan–Meier method to draw survival curves and the Cox proportional hazards regression model to determine statistically significant variables related to overall survival. Differences were considered significant when p < 0.05.
Results
A total of 174 patients were included in our study. Of these, 79 patients were in the FOLFIRI group, 55 in the platinum-based treatment group, and 40 in the taxane treatment group. Table 1 shows the patients’ characteristics. The three groups were statistically similar in overall survival (Fig. 1); the median overall survival was 5 months in the FOLFIRI group, 6.5 months in the platinum-based group, and 5.6 months in the taxane-based group (p = 0.554). There was no statistical difference between the groups’ progression-free survival either (Fig. 2); the median progression-free survival time was 3.43 months in the FOLFIRI group, 4 months in the platinum-based group, and 2.77 months in the taxane-based group (p = 0.546).
Table 1.
General characteristics of the patients
| Platinum group N = 55 (%31.6) |
Taxane group N = 40 (%23) |
FOLFIRI group N = 79 (%45.4) |
p | |
|---|---|---|---|---|
|
Gender Male Female |
37 (%67.3) 18 (%32.7) |
27 (%67.5) 13 (%32.5) |
57 (%72.2) 22 (%27.8) |
0.792 |
| Age (mean) | 58.8 | 63.1 | 57.5 | 0.630 |
|
Metastatic sites (n) Liver Peritoneum Lung Lymph node Bone |
15 17 8 40 5 |
20 15 11 30 4 |
31 23 9 65 16 |
0.433 0.316 0.091 0.120 0.038 |
Fig. 1.
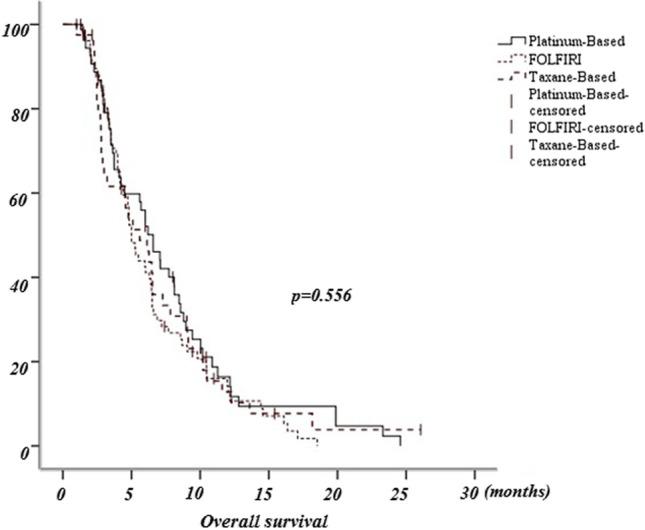
Overall survival
Fig. 2.

Progression-free survival
We observed that the variables of age (over and under 65 years old), gender, metastasis site (liver, peritoneum, lung, lymph node, bone), and first-line chemotherapy treatment (double and triple) were not independent predictors of overall survival after initiation of second-line chemotherapy (p = 0.261). When examined individually, we found that only the peritoneal metastasis variable had a statistically significant effect on overall survival (p = 0.041; Table 2). Table 1 shows the distribution of variables among the groups. No statistically significant differences arose between the distribution of peritoneal metastasis in the three groups. However, the proportion of patients with bone metastases and who received first-line triple chemotherapy treatment was statistically significantly higher in the FOLFIRI group (Tables 2 and 3).
Table 2.
Cox regression analysis. The effect of variables on overall survival
| Sig (p) |
Hazard ratio | 95.0% CI for Exp(B) | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 0.572 | 1.113 | 0.768 | 1.612 |
| Gender | 0.928 | 0.982 | 0.667 | 1.447 |
| Liver_metastasis | 0.657 | 0.924 | 0.653 | 1.309 |
| Lung_metastasis | 0.268 | 0.770 | 0.485 | 1.223 |
| Bone_metastasis | 0.065 | 1.610 | 0.971 | 2.671 |
| Lymphnode_metastasis | 0.771 | 1.064 | 0.702 | 1.612 |
| Peritonium_metastasis | 0.041 | 0.641 | 0.419 | 0.981 |
| Firstline_chemotherapy | 0.092 | 1.353 | 0.951 | 1.924 |
Table 3.
First-line therapy of patients
| First-line chemotherapy | FOLFIRI Group | Platinum-based group | Taxan-based group | Median progression-free survival (first line) | Significance p |
|---|---|---|---|---|---|
| Triplet therapy | 56 | 22 | 14 | 7.83 (month) | < 0.001 |
| mDCF | 44 | 16 | 8 | ||
| EOX | - | 1 | 3 | ||
| FLOT | 12 | 5 | 3 | ||
| Doublet therapy | 21 | 32 | 25 | 5.87 (month) | < 0.001 |
| FOLFOX-Xelox | 17 | 20 | 14 | ||
| Carboplatin-paklitaksel | - | 2 | 1 | ||
| Cisplatin-Xeloda | 4 | 9 | 9 | ||
| Others | - | 1 | 1 |
mmDCF, docetaxel-cisplatin-fluorouracil-leucovorin; EOX, epirubicin-oxaliplatine-capecitabine; FLOT, fluorouracil-leucovorin-oxaliplatin-docetaxel; FOLFOX, fluorouracil-leucovorin-oxaliplatin; Xelox, oxaliplatin-capecitabine; Xeloda, capecitabine
Discussion
The second-line treatment of gastric cancer has been the subject of many previous studies, though few have showed significant results. Ramucirumab combined with paclitaxel is especially recommended as a second-line therapy because of the significant results obtained after a phase 3 study. However, due to the fact that this regimen is expensive and access to the drug is limited, it is not used much in clinical practice. Physicians instead prefer conventional chemotherapy due to its low cost. Accordingly, many previous studies have compared the effectiveness of different chemotherapy regimens, though with the exception of some meta-analyses [8, 9] , it is still rare that several regimens are examined together, as in our study.
Most phase 3 studies that have compared second-line chemotherapy are negative [10] . One of these compared irinotecan and paclitaxel, as in our study, but there was no statistically significant difference between these two chemotherapy treatments in terms of overall and progression-free survival [11] . Specifically, the median overall survival of the irinotecan group was 8.4 months, while the paclitaxel group was 9.5 months. In the same study, the median progression-free survival of the irinotecan group was 2.3 months, and the paclitaxel group was 3.6 months.
In addition to these negative studies, the most important study that featured statistically significant results that extend overall survival in second-line gastric cancer treatment is RAINBOW. According to this study, ramucirumab added to weekly paclitaxel treatment statistically significantly prolonged survival (6). Overall survival was 9.6 months in the paclitaxel-ramucirumab arm and 7.4 months in the paclitaxel arm, and progression-free survival was 4.4 months in the paclitaxel-ramucirumab arm and 2.9 months in the paclitaxel arm. Ultimately, however, while our study had progression-free survival rates almost similar to other studies, our overall survival results were lower. This may be attributed to our including patients with an ECOG score of 2, which may have shortened overall survival rates.
Due to our study’s retrospective nature, heterogeneities emerged between the three study groups. The presence of peritoneal metastases is the only parameter that has an independent effect on overall survival, according to multivariate analysis. However, no statistically significant difference was found between the peritoneal metastasis rates in the groups. Bone metastasis and first-line triple chemotherapy were especially effective in increasing overall survival, though patients with these parameters were statistically significantly more common in the FOLFIRI group. The excess of bone metastases also shortened overall survival, while the excess of first-line triple chemotherapy prolonged it.
Per these results, upon evaluating second-line chemotherapy, the overall and progression-free survival rates of conventional treatments are similar. The only agent that statistically prolongs survival is ramucirumab. If ramucirumab is unavailable, second-line chemotherapy can be planned according to the agents used in first-line chemotherapy and their side effects. In our study, the clinicians also chose treatment in this way; for instance, they preferred FOLFIRI in the second-line treatment of patients who used both platinum and taxane in their first-line treatment. Meanwhile, in cases where oxaliplatin or cisplatin/carboplatin was given in first-line treatment, the clinicians preferred taxanes as single agents in second-line treatment.
According to our study results, we could not demonstrate the superiority of certain conventional chemotherapies per progression-free and overall survival in the second-line treatment of gastric cancer. For this reason, it may be appropriate to make choices in second-line treatment according to the nature of the drugs given in first-line treatment and their side effects. Due to the small number of patients, we could not obtain definitive results either, which necessitates larger-scale studies.
Conclusion
To determine the most effective second-line treatment of metastatic gastric cancer, patients were divided into groups according to the chemotherapy they received and compared in terms of overall and progression-free survival. There was no statistically significant difference between the three irinotecan-based, platinum-based, and taxane-based treatments. According to our study’s results, the chemotherapy given in second-line treatment should be decided on an individual basis according to toxicity and cost. However, we do recommend larger studies to further assess this issue.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.D’Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–816. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 3.Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86. doi: 10.1016/S1470-2045(13)70549-7. [DOI] [PubMed] [Google Scholar]
- 4.Kang JH, Lee SI, Lim Do H, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 5.Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer–a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47:2306–2314. doi: 10.1016/j.ejca.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 7.Lee KW, Maeng CH, Kim TY, et al. A phase III study to compare the efficacy and safety of paclitaxel versus irinotecan in patients with metastatic or recurrent gastric cancer who failed in first-line therapy (KCSG ST10-01) Oncologist. 2019;1:18–e24. doi: 10.1634/theoncologist.2018-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wesolowski R, Lee C, Kim R. Is there a role for second-line chemotherapy in advanced gastric cancer? Lancet Oncol. 2009;10:903. doi: 10.1016/S1470-2045(09)70136-6. [DOI] [PubMed] [Google Scholar]
- 9.Thallinger CM, Raderer M, Hejna M. Esophageal cancer: a critical evaluation of systemic second-line therapy. J Clin Oncol. 2011;29:4709. doi: 10.1200/JCO.2011.36.7599. [DOI] [PubMed] [Google Scholar]
- 10.Shitara K, Takashima A, Fujitani K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;4:277–287. doi: 10.1016/S2468-1253(16)30219-9. [DOI] [PubMed] [Google Scholar]
- 11.Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438. doi: 10.1200/JCO.2012.48.5805. [DOI] [PubMed] [Google Scholar]


