Abstract
Isolation and identification of two antimicrobial compounds, a phenyl pentyl ketone (CP1) and m-isobutyl methoxy benzoate (CP2), from Streptomyces chrestomyceticus ADP4 have been reported. Structure of the compounds were elucidated from analyses of spectral data that included LCMS/MS, NMR, FTIR and UV spectroscopies. Both the compounds displayed significant inhibition of albicans and non-albicans species of Candida (NAC) pathogens including C. auris, which is currently a pathogen of global concern. Also, the compounds showed potent antagonistic activity against Staphylococcus aureus, another significant human pathogen. No in-vitro cytotoxicity against HePG2 cells was observed with either of the compounds. Both displayed favourable drug likeness properties as determined by in-silico ADME and toxicological studies. Also, this is the first report on production of these anti-microbial compounds by an actinobacterium.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12088-023-01068-7.
Keywords: Streptomyces chrestomyceticus, Antimicrobial agents, Anti-Candida, Anti-S. aureus, ADME
Introduction
Candida spp. are a common component of human microbiota. They are infamous opportunistic pathogens that can cause vaginitis, oral candidiasis, cutaneous candidiasis, candidemia, and systemic invasive infections [1]. They have imposed enormous healthcare burden by accounting for 50–70% systemic fungal infections and almost 15% bloodstream infections [2]. C. albicans has been the most prevalent among the Candida pathogens but increasing incidences of non-albicans species of Candida across the world has drawn attention of scientific community since they are inherently drug resistant. This situation warranted urgent action for discovery and development of new and effective drugs [3, 4]. Currently the azoles are the most widely used anti-Candida drug. The polyenes, echinocandins, nucleoside analogs and allylamines are among the other antifungals being used depending on the type of infections, species specificity and the site of infections [5]. However, the Candida spp. have been fast gaining resistance against these classes of anti-mycotic drugs, which is alarming [6]. For a compound to be an ideal drug candidate, it should have desirable pharmacokinetic parameters, low toxicity, broad spectrum activity, low cost and effectivity by oral as well as parenteral route [7]. These criteria formed the basis of our current endeavour to find new drugs for difficult-to-treat Candida infections.
Streptomyces spp. under the phylum Actinobacteria are among the most valuable sources of bioactive secondary metabolites possessing vast structural and biological diversity [8]. Significant proportion of clinically relevant therapeutic compounds are derived from the metabolites produced by Streptomyces spp. [9]. S. chrestomyceticus has been reported for it’s prolific antimicrobial activity against various bacterial pathogens as well as for the production of metabolites belonging to different classes of compounds with therapeutic significance [10, 11]. In the present communication, two compounds CP1 and CP2 from S. chrestomyceticus ADP4 with potent activities against significant human pathogens are reported.
Material and Methods
Microorganisms
The isolation and identification of S. chrestomyceticus strain ADP4 has been reported in our earlier publication [12]. In brief, it was a soil actinobacterium grown at 28 °C maintained in laboratory condition at 4 °C, on Nutrient agar, pH 7.0 ± 0.2 (NA; Himedia, India). The anti-Candida activity was tested against different Candida spp. pathogens, which included C. albicans ATCC 10,231, C. krusei ATCC 6258, C. tropicalis ATCC 750, C. parapsilosis ATCC 90,018 and C. auris CBS 12,372.
Isolation of Compounds CP1 and CP2
The isolation of the compounds CP1 and CP2 was done from secondary metabolites produced by S. chrestomyceticus ADP4. The preparation of metabolite extract carried out by using the method described earlier [12]. Briefly, strain ADP4 was inoculated in the production medium i.e., Sabouraud Dextrose broth and was incubated at 28 °C and 150 rpm. The culture was harvested after 96 h and the cell-free broth was extracted with ethyl acetate (1:1, v/v) followed by complete evaporation of solvent to obtain dried extract of metabolite. Metabolite extract was then subjected to fractionation on HILIC column by applying a linear solvent gradient in order of increasing polarity. HILIC column was used since it resulted in efficient fractionation of compounds over a wide range of polarity varying from non-polar to polar. The bioactivity guided purification of these compounds was carried out by using bioautography followed by recovery of the pure compounds by preparative TLC [13]. Purity of the compounds was accessed by 2D-TLC and RP-HPLC on C18 column [14]. All the solvents used in this experiment were of HPLC grade and procured from Merck, India.
Structure Elucidation and Identification of the Compounds
Mass spectra (LC–MS/MS) of the compounds were collected from Waters SYNAPT G2-Si High-Definition Mass Spectrometry (M.A., USA). 1D and 2D-NMR data including 1H (500 MHz), 13C (125 MHz), Distortionless Enhancement by Polarization Transfer (DEPT-135), COSY (Correlation Spectroscopy), HSQC (Heteronuclear Single Quantum Correlation) and HMBC (Heteronuclear Multiple Bond Connectivity) were recorded on Bruker Ultrasheild 500 plus (Rheinstetten, 2 Germany). Deuterated methanol was used for dissolution of pure compounds to perform these experiments. UV absorption spectrum was recorded on Carry 60 UV–Vis Spectrophotometer (Agilent, California, USA). IR peaks were documented for determination of functional groups by attenuated total reflectance (ATR) mode of FTIR system (Agilent, California, USA). The chemical structures of the compounds were draw by using ChemDraw pro 8.0.
Anti-Candida Activity of the Compounds
The MIC90 values of the compounds CP1 and CP2 against C. albicans ATCC 10,231, C. krusei ATCC 6258, C. tropicalis ATCC 750, C. parapsilosis ATCC 90,018, C. auris CBS 12,372 and S. aureus ATCC 29,213 were determined by using microtiter broth dilution method as per CLS guidelines [15]. Briefly, 0.5 McFarland suspension was prepared from 24 h freshly grown pathogenic cultures. 1:1000-fold dilution of Candida pathogens and 1:1 dilution of S. aureus was used in the assays. Stock solution of the compound CP1 and CP2 was prepared (1 mg/mL in 2% DMSO) in RPMI (Sigma, USA) supplemented with 2% dextrose (Merck, India). The effect of the compounds was investigated at a concentration range from 1140–8.9 µM and 1040–8.12 µM respectively for CP1 and CP2. 100 µL of the culture suspension was added to the 100 µL of compounds in 96 well microtiter plate. 2% DMSO was used as solvent control. Following incubation at 37 ℃ for 48 h and 24 h for Candida spp. and S. aureus respectively, 50 µL of 5 mg/mL INT (Iodonitrotetrazolium chloride, Merck, India) was added to each well and the incubated for further 5 h at 37 ℃ and the reading was taken at 490 nm on ELISA plate reader (Synergy, H1M multimode reader, Biotek USA). The concentration at which 90% of the inhibition was observed was considered as MIC90 value. Fluconazole was used as the control drug in the assays.
Anti-Biofilm Activity
Effect of different concentrations of the compounds (1140–8.9 µM and 1040–8.12 µM respectively for CP1 and CP2) was investigated against biofilms of different stages (0 h, 2 h and 24 h) formed by using 0.5 McFarland culture suspension of C. albicans ATCC 10,231 as per the method described elsewhere [11]. The biofilm preparations were treated with 100 µL of compound dilutions for 48 h at 37 ℃. Subsequently, the wells were washed with PBS and 100 µL INT was added to each well. The absorbance (at 490 nm) was taken following incubation for 5 h to calculate the minimum biofilm inhibitory concentration (MBIC).
Cytotoxicity Assessment of CP1 and CP2
The cytotoxicity of compounds CP1 and CP2 was evaluated on HepG2 cell line by using the MTT assay in the concentration range of 1300–20.31 µM as described previously [16]. 50% inhibition of cell growth was determined and presented as CC50 value.
In-silico ADME/T Study
Sketches of the structures were drawn by using ChemDraw 8.0. The ADME prediction tool, SwissADME (http://swissadme.ch/), was used to evaluate physicochemical properties, pharmacokinetics, drug-likeness and medicinal chemistry of the molecules. Prediction of physicochemical parameters and the drug-likeness were concluded based on the widely used Lipinski rule of five [17].
In-silico toxicity of the compounds was determined by TEST software against rat model. The bioaccumulative tendency, developmental toxicity and genotoxicity of the compounds were also investigated using this software [18].
Statistical Analysis
The data was expressed as mean ± standard deviation and all the experiments were performed in triplicates. The statistical analysis and significance of the test were performed by analysis of variance (ANOVA), Graph Pad Prism 5.01 software.
Results
Isolation and Identification Compounds CP1 and CP2
In view of prominent antagonistic activities of the metabolites of S. chrestomyceticus ADP4 against significant Candida spp. pathogens [11, 12], isolation and characterization of such anti-Candida compounds had been the focus of the present work. Fractionation of the metabolite extract on HILIC column resulted in recovery of the compounds, CP1 and CP2, in the fractions that eluted in the linear solvent gradient comprising of 35% Hexane and 65% Ethyl acetate. Analysis of relevant fractions on TLC suggested them to be still impure where further purification was necessary to obtain the compounds in pure form. Therefore, the compounds recovered from the said fractions were further fractionated on preparative TLC, which led to isolation of CP1 and CP2 with the yield of 5 mg/g and 12.2 mg/g respectively. The former was isolated as a light-yellow sticky compound while the later was found to be off-white dusty powder. Description of the spectral data for compounds is given in Table S1 and S2. The compound CP1 showed UV absorption maximum at 286 nm (Fig. S1) for an aromatic ring and IR absorption bands for carbonyl group (1673 cm−1) and aromatic ring (1558 cm−1) (Fig. S2). On the basis of mass spectra and 13C NMR spectra, its molecular ion peak was determined at m/z 176.1209 (Fig. S3) consistent with a molecular formula of a phenyl alkyl ketone, C12H16O. A prominent ion peak arising at m/z 105.0105 appears to have been produced as result of C7- C1′ fission, which indicated that a phenyl ketone unit was present at one of the terminals of the aliphatic pentyl chain. The 1H NMR spectrum of CP1 (Fig. S4a) displayed two one-proton deshielded doublets at δ 7.77 (J = 8.5 Hz) and 7.76 (J = 8.5 Hz) assigned to aromatic H-2 and H-6 protons, respectively. Three one-proton multiplets at δ 7.44, 7.37 and 7.34 were ascribed correspondingly to aromatic H-4, H-3 and H-5 protons. Four two-proton multiplets (Fig. S4b) at δ 2.08, 1.93, 1.24 and 0.99 were attributed to methylene H2-1′, H2-2′, H2-3′ and H2-4′ protons, respectively. A three-proton triplet at δ 0.79 (J = 7.0 Hz) was accounted to primary C-5′ methyl protons as shown in Fig. S4b. The 13C NMR spectrum of CP1 (Fig. S5) exhibited signals for aromatic carbons between δ 133.54–127.25, carbonyl carbon at δ 173.04 (C-7), methylene carbons from δ 29.32 to 19.87 and methyl carbon at δ 17.25 (C-5′).
The DEPT spectrum of CP1 (Fig. S6) showed the presence of five methine, four methylene carbons and one each methyl and quaternary carbons. The 1H-1H COSY spectrum of CP1 (Fig. S7) exhibited correlations of H-2, H-6, H-3 and H-5 with H-4; H-2, H-6, and H2-2′ with H2-1′; and H2-3′ and H2-4′ with H3-5′. The HMBC spectrum of CP1 (Fig. S8) displayed interactions of H-2, H-3, H-5 and H-6 with C-1; H-2, H-6, H2-1′ and H2-2′ with C-7; and H2-3′ and H2-4′ with C-5′. The HSQC spectrum of CP1 (Fig. S9) indicated that aromatic proton signals between δ 7.77–7.34 interacted with the respective carbon signals from ẟ 131.55 to 128.73; methylene proton signals at ẟ 2.08 ( H2-1′), 1.93 (H2-2′), 1.24 (H2-3′) and 0.99 (H2-4′) with the corresponding carbon signals at ẟ 29.32 (C-1′), 25.14 (C-2′), 23.08 (C-3′) and 19.87 (C-4′) and methyl proton signal at ẟ 0.79 (Me-5′) with the methyl carbon signal at δ 17.25 (C-5′). On the basis of the aforementioned spectral data analyses, the structure of CP1 has been elucidated as phenyl pentyl ketone (Fig. 1a).
Fig. 1.
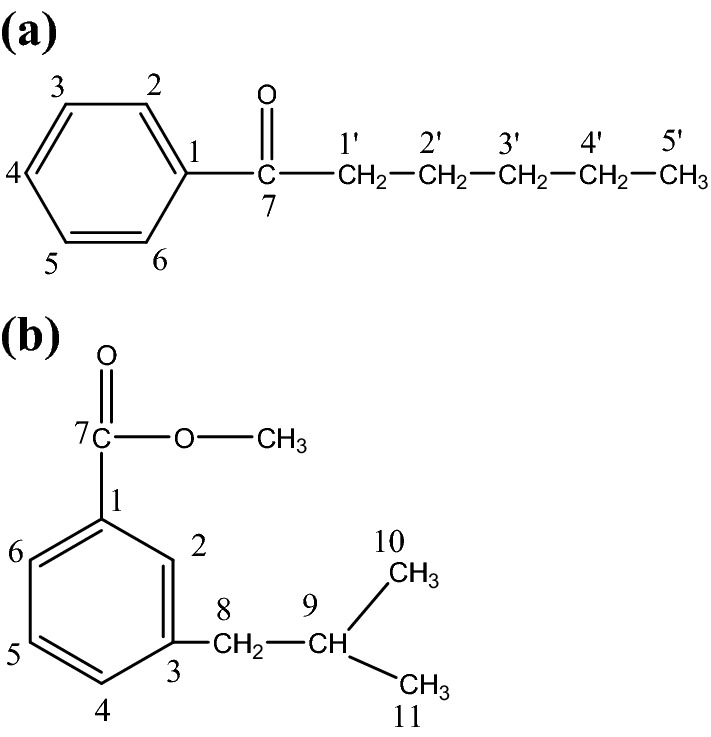
Structure of the compounds. CP1 was identified as phenyl pentyl ketone (a) and CP2 was found to be m-isobutyl methoxy benzoate (b)
The compound CP2 had an UV absorption maximum at 294 nm for an aromatic ring (Fig. S10) and IR absorption bands for an ester group (1748 cm−1) and aromatic ring (1509 cm−1) (Fig. S11). Its molecular ion peak was established at m/z 192.115 based on mass spectra and 13C NMR corresponding with a molecular formula of an alkyl methoxy benzoate, C12H16O2. An ion peak at m/z 148.7835 [C8- C9 fission, C6H5-COOCH3-(CH2)] + (Fig. S12) was formed due to removal of isopropyl unit suggesting that an isobutyl unit was attached to methoxy benzoate unit. The 1H NMR spectrum of CP2 (Fig. S13) showed two one-proton deshielded doublets at δ 7.39 (J = 8.0 Hz) and 7.33 (J = 7.5 Hz) assigned to ortho-coupled aromatic H-6 and H-4 protons, respectively. A one-proton double doublet at δ 7.18 (J = 8.0, 7.5 Hz) and a one-proton singlet δ 6.77 were ascribed correspondingly to aromatic H-5 and H-2 protons. A three-proton singlet at δ 3.99 was accounted to methoxy protons. A two-proton multiplet at δ 1.79, a one-proton multiplet at δ 1.64 and two three-proton doublets at δ 0.89 (J = 7.0 Hz, H3-10) and 0.87 (J = 6.5 Hz, H3-11) were attributed to methylene H2-8, methine H-9 and secondary methyl H3-10 and H3-11 protons, respectively, of the isobutyl moiety. The 13C NMR spectrum of CP2 (Fig. S14) exhibited signals for aromatic carbons from δ 133.94 to 116.20, carboxyl carbon at δ 169.30 (C-7), methylene carbon at δ 43.93 (C-8), methine carbon at δ 47.54 (C-9), methyl carbons at δ 23.86 (C-10) and 21.90 (C-11) and methoxy carbon at δ 54.15 (OCH3). The DEPT spectrum of CP2 (Fig. S15) showed the presence of five methine, one methylene, three methyl and three quaternary carbons. The 1H-1H COSY spectrum of CP2 (Fig. S16) exhibited correlations of H-4 and H-6 with H-2 and H-5; and H-8, H3-10 and H3-11 with H-9. The HMBC spectrum of CP2 (Fig. S17) displayed interactions of H-2, H-5 and H-6 with C-1; H-2, H-4 and H2-8 with C-3; and H2-8, H3-10 and H3-11 with C-9. The HSQC spectrum of CP2 (Fig. S18) indicated that aromatic proton signals between δ 7.39–7.18 interacted with the respective carbon signals from ẟ 128.71 to 116.20; methylene proton signal at ẟ 1.79 (H2-8) with the methylene carbon signal at ẟ 43.93 (C-8), methyl proton signals at ẟ 0.89 (H3-10) and 0.87 (H3-11) with the corresponding methyl carbon signals at δ 23.86 (C-10) and 21.90 (C-11), methine proton at ẟ 1.64 (H-9) with methine carbon at ẟ 47.54 (C-9) and methoxy protons signal at ẟ 3.99 (OCH3) with carbon signal at ẟ 54.15 (OCH3). On the basis of above-mentioned discussion, the structure of CP2 was elucidated as m-isobutyl methoxy benzoate, an aromatic ester derivative (Fig. 1b).
Anti-Microbial Activity of the Compounds
The compounds were tested for antagonistic activities against fungal (Candida spp.) and bacterial human pathogens. As displayed in Fig. 2a and b, the compound CP1 and CP2 showed concentration-dependent inhibition of the Candida spp. The compound CP1 was better than CP2 against C. albicans with MIC90 value of 200 ± 1.98 µM. However, compound CP2 was found to be more potent against non-albicans species of Candida with MIC90 values of 161.20 ± 4.53, 585.16 ± 21.92, 184.06 ± 17.65 and 144.06 ± 10.88 µM against C. krusei, C. tropicalis, C. parapsilosis and C. auris respectively. The MIC90 values of CP1 and CP2 for inhibition of various Candida spp. pathogens are presented in Table 1, which showed that potency of both the compounds were much better for C. auris in comparison to the other Candida spp. The data also demonstrated that in respect of C. krusei, the antagonistic activities of CP2 was even better than the standard anti-fungal drug, fluconazole, which is an important finding of the present work. The compounds also inhibited S. aureus with MIC90 values of 168.07 ± 22.04 and 172.34 ± 20.36 µM for CP1 and CP2 respectively.
Fig. 2.
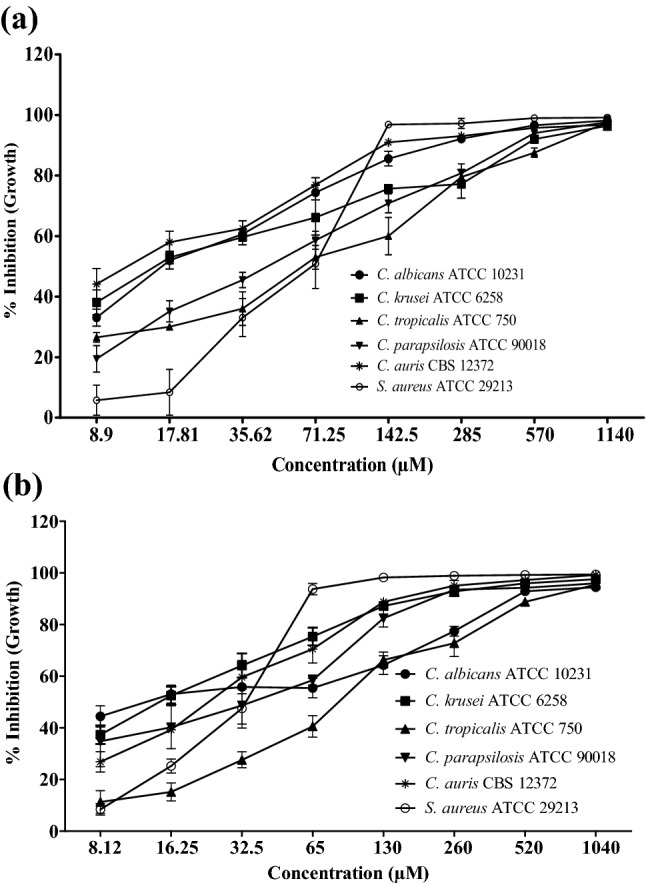
Anti-microbial activity of the compounds CP1 and CP2. The Effect of different concentration of CP1 (a) and CP2 (b) on growth inhibition of Candida spp. and S. aureus ATCC 29213 is presented as mean ± SEM. The experiments were performed in triplicates and the error bars represented the SEM values, p value < 0.005
Table 1.
MIC90 and MBIC90 value of the compounds CP1 and CP2
| Compounds | MIC90 (µM) | MBIC90(µM) | ||||||
|---|---|---|---|---|---|---|---|---|
| C. albicans ATCC 10,231 | C. krusei ATCC 6258 | C. tropicalis ATCC 750 | C. parapsilosis ATCC 90,018 | C. auris CBS 12,372 | 0 h biofilm | 2 h biofilm | 24 h biofilm | |
| CP1 | 200 ± 1.98 | 511.25 ± 2.89 | 659.20 ± 6.36 | 523.75 ± 11.53 | 137.72 ± 2.32 | 142.27 ± 4.54 | 405 ± 26.98 | 500.28 ± 26.98 |
| CP2 | 429.90 ± 4.58 | 161.20 ± 4.53 | 585.16 ± 21.92 | 184.06 ± 17.65 | 144.06 ± 10.88 | 354.90 ± 16.66 | 657.45 ± 39.74 | > 1000 |
| Fluconazole | 2.54 ± 0.36 | > 210 | > 210 | 7.77 ± 2.57 | 103.85 ± 0.69 | 14.98 ± 0.98 | > 700 | > 700 |
Anti-Biofilm Properties of the Compounds
The compounds CP1 and CP2 significantly inhibited the Candida-biofilms at different stages. Biofilm disruption potential of CP1 was significantly better than CP2 as well as control drug fluconazole. The MBIC90 value for CP1 was observed at 142.27 ± 4.54, 405 ± 26.98 and 500.28 ± 26.98 µM for inhibition of 0 h, 2 h and 24 h biofilms. The MBIC90 value for inhibition of biofilm formation was calculated to be 354.90 ± 16.66 µM for CP2. However, the CP2 and the control drug fluconazole were unable to inhibit 2 h and 24 h-old preformed biofilms. The effects of different concentrations of CP1 and CP2 on 0 h, 2 h and 24 h biofilms formed by C. albicans are shown in Fig. 3a and b.
Fig. 3.
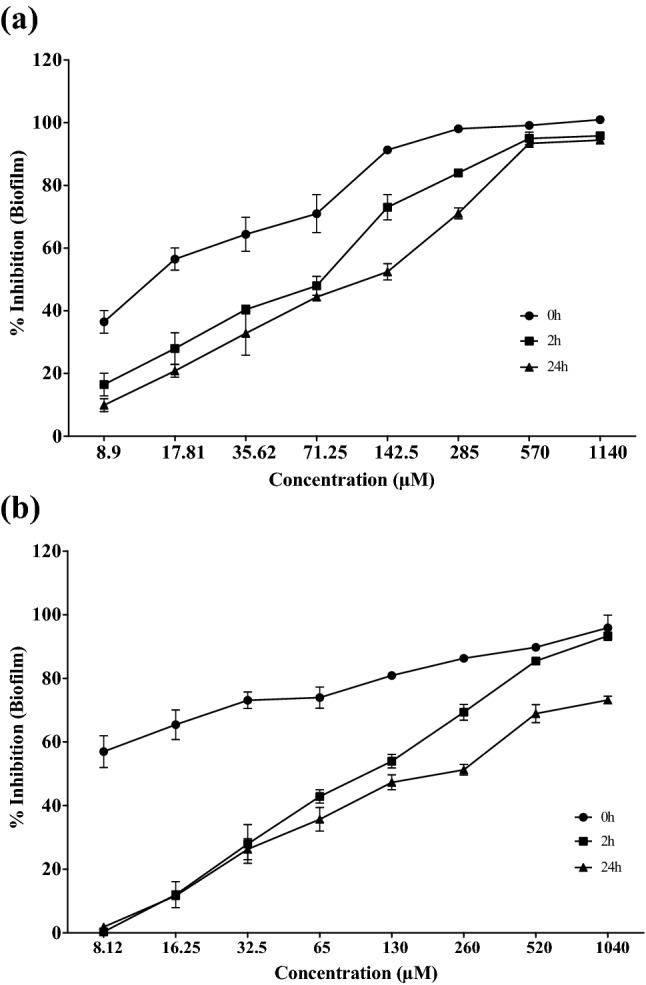
Anti-biofilm activities of the compounds CP1 and CP2. Inhibition of 0 h, 2 h and 24 h biofilm formed by Candida albicans ATCC 10231 at various concentrations of the compounds CP1 (a) and CP2 (b). The data is presented as mean ± SEM values with P value < 0.0001. The data is generated by performing experiments in triplicates
Cytotoxicity assessment of CP1 and CP2
The compounds CP1 and CP2 were found to be non-cytotoxic against HepG2 cell line at the concentrations on which they inhibited the growth of Candida spp. The CC50 value was observed at 1267.04 ± 94.65 µM for CP1. While for CP2, more than 70% survival of the HepG2 cells was observed even at the concentration of 1300 µM (Fig. 4).
Fig. 4.
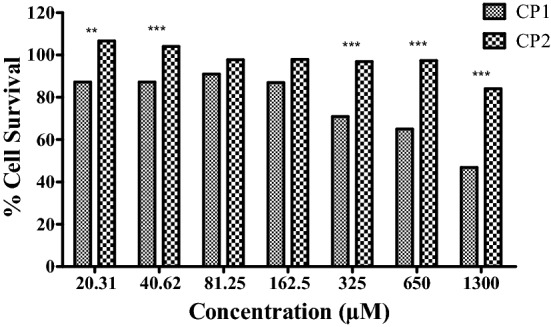
Cytotoxicity assessment of compounds CP1 and CP2. Effect of increasing concentration of compounds CP1 and CP2 on the viability of HePG2 cells. The data presented as mean ± SEM values of the experiments performed in triplicates with P value < 0.0001
In-silico ADME/T Study
Both the compounds displayed drug likeness properties with overall bioavailability value of 0.55 based on their favourable water solubility, lipophilicity, skin permeability and their pharmacokinetic properties like high GI absorption and blood brain barrier permeation properties. Both the compounds were P-gp non-inhibitor. Isoforms of cytochrome P450 i.e., CYP2C19, CYP2C9, CYP2D6, CYP3A4 were not inhibited by compounds CP1 and CP2. The values of these parameters are presented in Table 2. Both the compounds were found to be drug like according to the most widely accepted Lipinski rule without any violation. Also, No PAINS and BRENKS alert were observed for of these compounds. In-silico toxicity prediction showed that compounds showed higher LD50 values > 1000 mg/kg according to consensus as well as FDA method. Other toxicity effects like bioaccumulation and genotoxicity were negative for both, CP1 and CP2 (Table 3). In accordance with the FDA model, both the compounds were found to be developmental non-toxicants, which is an important factor to be considered for drug development.
Table 2.
ADME properties of the compounds CP1 and CP2
| Physiochemical properties | Water solubility | Lipophilicity | ||||||
|---|---|---|---|---|---|---|---|---|
| Attribute | CP1 | CP2 | Attribute | CP1 | CP2 | Attribute | CP1 | CP2 |
| Formula | C12H16O | C12H16O2 | Log S (ESOL) | − 3.37 | − 3.61 | Log Po/w (iLOGP) | 2.59 | 2.86 |
| Molecular weight | 176.25 g/mol | 192.25 g/mol | Solubility | 7.52e-02 mg/mL | 4.77e-02 mg/mL | Log Po/w (XLOGP3) | 3.85 | 4.00 |
| Num. heavy atoms | 13 | 14 | Class | Soluble | Soluble | Log Po/w (WLOGP) | 3.45 | 2.67 |
| Num. arom. heavy atoms | 6 | 6 | Log S (Ali) | − 3.90 | − 4.25 | Log Po/w (MLOGP) | 2.97 | 3.13 |
| Fraction Csp3 | 0.42 | 0.43 | Solubility | 2.19e-02 mg/mL | 1.07e-002 mg/mL | Log Po/w (SILICOS-IT) | 3.58 | 3.08 |
| Num. rotatable bonds | 5 | 4 | Class | Soluble | Moderately soluble | Consensus Log Po/w | 3.29 | 3.15 |
| Num. H-bond acceptors | 1 | 2 | Log S (SILICOS-IT) | − 4.35 | − 3.70 | |||
| Num. H-bond donors | 0 | 0 | Solubility | 7.89e-02 mg/mL | 3.80e-002 mg/mL | |||
| Molar Refractivity | 55.86 | 57.11 | Class | Moderately soluble | Soluble | |||
| TPSA | 17.07 Å | 26.30 Å | ||||||
| Pharmacokinetics | Drug likeness | Medicinal Chemistry | ||||||
| Attributes | CP1 | CP2 | Attributes | CP1 | CP2 | Attributes | CP1 | CP2 |
| GI absorption | High | High | Lipinski | Yes; 0 violations | Yes; 0 violations | PAINS | 0 alert | 0 alert |
| BBB permeant | Yes | Yes | Ghose | Yes | Yes | Brenk | 0 alert | 0 alert |
| P-gp substrate | No | No | Veber | Yes | Yes | Leadlikeness | No; 2 violations: MW < 200, Heteroatoms > 2 | No; 2 violations: MW < 200, XLOGP > 3.5 |
| CYP1A2 inhibitor | Yes | Yes | Egan | Yes | Yes | Synthetic accessibility | 1.33 | 1.64 |
| CYP2C19 inhibitor | No | No | Muegge | No; 2 violations: MW < 200, Heteroatoms > 2 | No; 1 violation: MW < 200, | |||
| CYP2C9 inhibitor | No | No | Bioavailability Score | 0.55 | 0.55 | |||
| CYP2D6 inhibitor | Yes | No | ||||||
| CYP3A4 inhibitor | No | No | ||||||
| Log Kp (skin permeation) | − 4.64 | − 4.63 | ||||||
Table 3.
In- silico toxicity prediction of the compounds CP1 and CP2
| Predictions attributes | Consensus method | FDA method | Hierarchical method | Nearest neighbor method | ||||
|---|---|---|---|---|---|---|---|---|
| CP1 | CP2 | CP1 | CP2 | CP1 | CP2 | CP1 | CP2 | |
| Oral rat LD50 mg/kg | 1304.61 (NT) | 1929.21 (NT) | 1907.22 (NT) | 3772.18 (NT) | 645.42 (NT) | 2400.36 (NT) | 1803.86 (NT) | 792.99 (NT) |
| Bioaccumulation factor | 26.02 (NB) | 12.38 (NB) | 23.32 (NB) | 2.39 (NB) | 23.81 (NB) | 30.45 (NB) | Not predicted | 4.27 (NB) |
| Developmental toxicant | 0.44 (NDT) | 0.62 (DT) | − 0.01 (NDT) | 0.41 (NDT) | 0.71 (DT) | 0.73 (DT) | 0.67 (DT) | 0.67 (DT) |
| Mutagenicity (positive/negative) | 0.14 (− ve) | 0.05 (− ve) | 0.00 (− ve) | − 0.14 − ve) | 0.00 (− ve) | − 0.06 (− ve) | 0.43 (− ve) | 0.33 (− ve) |
T Toxicity; NT No toxicity; N/A cannot be predicted; NB Non-bioaccumulative; DT Developmental toxicant; NDT developmental non-toxicant
Discussion
S. chrestomyceticus ADP4 had been reported to be a rich source of metabolites that displayed prominent anti-Candida activity against albicans and non-albicans strains of Candida spp. pathogens [11, 12, 19]. It was, thus, imperative to isolate the active molecules so that their potential as probable drug candidate for treatment of Candidiasis can be better understood. Towards this endeavour, the compounds CP1 and CP2 were isolated. Both of these compounds exhibited similar polarity as they co-eluted in the same fractions from the HILIC column. However, their physical properties were strikingly different. The compound CP1 was reported earlier as Hexanophenone or Caprofenona in PubChem with Chem Id 70,337 [20]. However, there had been no report on the production of this compound from any microbial sources. Similarly, for CP2, a related compound was mentioned earlier in chemical database PubChem with Chem Id 20,771,995 [21]. But again there had been no report on isolation of CP2 from any microbial source including Streptomyces spp. This is the first report on isolation of these compounds from a soil actinobacterium S. chrestomyceticus strain ADP4 and their structural–functional characterization.
The compounds showed broad spectrum activities against both yeast and bacterial pathogens. A scan through literature further suggested that there had been no report on antimicrobial properties of phenyl pentyl ketone (CP1) and m-isobutyl methoxy benzoate (CP2). However, some studies showed the use of m-isobutyl methoxy benzoate in treating cancer and inflammatory diseases [21]. Phenyl pentyl ketone was also investigated for anti-cancer property but found inactive [20]. As established in the present work, both the compounds have broad spectrum antimicrobial potency, which enhances their importance in the drug discovery [2]. C. albicans is among the most prominent fungal pathogens known to cause Invasive Candidiasis [22]. But a shift has been observed in the infections caused by non-albicans Candida spp. Among them, C. auris is of significant global concern since it developed resistance quickly against most of the current anti-fungal drugs in use [23]. The effectiveness of CP1 and CP2 against Candida spp. pathogens enhanced the significance of these compounds as antifungal agents.
C. albicans being the prevalent colonizing pathogen tends to adhere on tissues surfaces, which is associated with the formation of biofilms. The biofilms confer high degree of drug resistance to the pathogens and help them overcome adverse conditions [24]. Anti-biofilm properties of CP1 and CP2 added significance to their therapeutic potential. Therefore, further studies on these compounds as potent anti-biofilm agents can be undertaken since they could prevent the development of drug resistance in C. albicans through prevention and disruption of biofilms.
It is important to ascertain the cytotoxicity of antimicrobial compounds to find out their suitability as probable drug candidates. Since liver is the main detoxifying organ and accountable for drug metabolism and drug-drug interactions, use of HepG2 liver cells had been preferred to investigate cytotoxicity of CP1 and CP2. Also, the HepG2 liver cell line is among the most commonly used cell lines for this purpose [25]. Since liver is the main detoxifying organ and accountable for drug metabolism and drug-drug interactions, use of HepG2 liver cells had been preferred to investigate cytotoxicity of CP1 and CP2. The results of present investigation demonstrated the compounds to be safe for the HepG2 cells, hence can be explored further for their therapeutic applications through drug development pipeline [26]. However, the design and development of new drugs is both a time-consuming process and expensive, especially when it comes to experimental evaluation of pharmacokinetic profile. Computational approaches could be a preliminary but important step to analyze pharmacokinetic and cytotoxic properties of the compounds that may enable faster progression in drug discovery process [27]. The bioavailability radar of CP1 and CP2 is shown in Fig. 5a and b, wherein the properties shown in shaded region suggested better bioavailability of the compounds [28]. None of the molecules were substrate for P-gp, suggesting their improved absorption [29]. The non-inhibitory attributes of the compounds for any of the isoform of cytochrome P450 suggested that there will no accumulation of toxic by-products in body, which is a desirable property for a potential drug candidate [30]. Lipinski rule of five helps in finding the probability of failure or success of any molecule to be drug-like. Any molecule that complies with any two or more rules fall under drug-like category [31]. The PAINS and BRENK alert are important to identify in drug discovery for reproducibility and reliability of true hits and these results suggested the reliability and validation of the predictions [32]. In-silico toxicity of CP1 and CP2 suggested them to be safe for rat models based on their high lethal dose value [33]. The ADMET (Absorption, Digestion, Metabolism, excretion, and toxicity) parameters of these compounds were favourable for its pharmacokinetic, medicinal chemistry, drug likeness, in-vivo toxicity and overall bioavailability as shown in Tables 2 and 3, which further proved their suitability as potential candidates for better antifungal drugs [34]. While in-vitro anti-microbial potential of these compounds have been studied in the present work, detailed in-vivo studies and mechanism of action of these compounds need to be investigated further for evaluation of their potential as possible drug candidates.
Fig. 5.
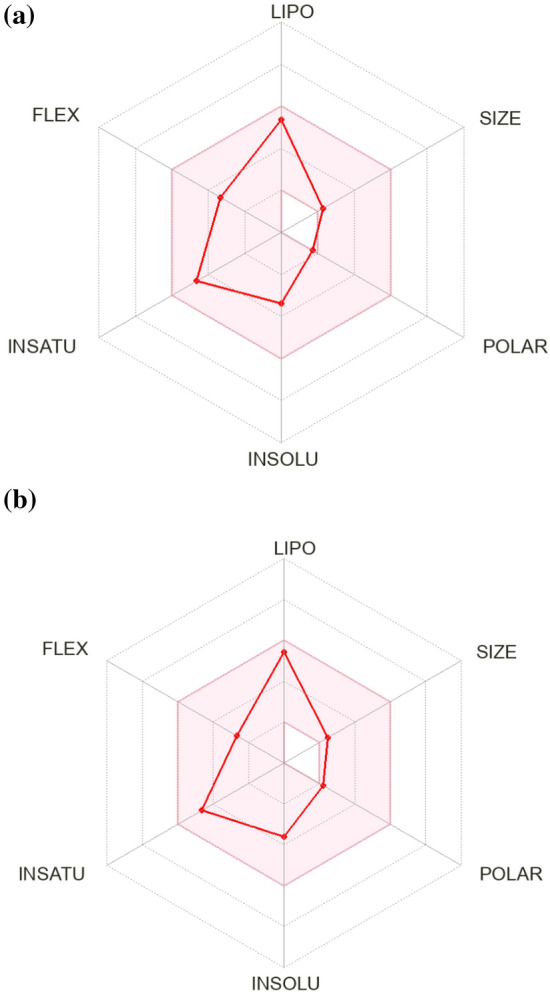
Overall bioavailability of compounds. Bioavailability radar of compounds CP1 (a) and CP2 (b) is presented where shaded region showed favourable physiochemical space for oral bioavailability (lipophilicity: XLOGP3 between − 0.7 and + 5.0, size: MW between 150 and 500 g/mol, polarity: TPSA between 20 and 130 Å, solubility: log S not higher than 6, saturation: fraction of carbons in the sp3 hybridization not less than 0.25, and flexibility: no more than 9 rotatable bonds)
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The work has been supported by Science and Engineering Research Board, Department of Science and Technology, Government of India through the Research Grant: SERB-EMR/2017/000254 to AKD.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wächtler B, Citiulo F, Jablonowski N, Förster S, Dalle F, Schaller M, Wilson D, Hube B. Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS ONE. 2012;7(5):e36952. doi: 10.1371/journal.pone.0036952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Oliveira Santos GC, Vasconcelos CC, Lopes AJ, de Sousa Cartágenes MDS, Filho AK, d0 Nascimento FR, de Andrade Monteiro C. Candida infections and therapeutic strategies mechanisms of action for traditional and alternative agents. Front Microbiol. 2018;9:1351. doi: 10.3389/fmicb.2018.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraga-Silva TFC, Munhoz-Alves N, Mimura LAN, Oliveira LRC, Figueiredo-Godoi LMA, Garcia MT, Oliveira ES, Ishikawa LLW, Zorzella-Pezavento SFG, Bonato VLD, Junqueira JC, Bagagli E, Sartori A. Systemic infection by non-albicans Candida Species affects the development of a murine model of multiple sclerosis. J Fungi Basel. 2022;8(4):386. doi: 10.3390/jof8040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deorukhkar SC, Saini S, Mathew S. Non-albicans Candida infection: an emerging threat. Interdiscip Perspect Infect Dis. 2014;2014:615958. doi: 10.1155/2014/615958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Clinical practice guideline for the management of candidiasis: 2016 Update by the infectious diseases society of America. Clin Infect Dis. 2016;62(4):1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun FJ, Li M, Gu L, Wang ML, Yang MH. Recent progress on anti-Candida natural products. Chin J Nat Med. 2021;19(8):561–579. doi: 10.1016/S1875-5364(21)60057-2. [DOI] [PubMed] [Google Scholar]
- 7.Escalante-Réndiz D, de la Rosa-García S, Tapia Tussell R, Martín J, Reyes F, Vicente F, Gamboa Angulo M. Molecular identification of selected Streptomyces strains isolated from Mexican tropical soils and their anti-Candida Activity. Int J Environ Res Public Health. 2019;16(11):1913. doi: 10.3390/ijerph16111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Procópio RE, Silva IR, Martins MK, Azevedo JL, Araújo JM. Antibiotics produced by Streptomyces. Braz J Infect Dis. 2012;16(5):466–471. doi: 10.1016/j.bjid.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Pusparajah P, Letchumanan V, Law JWF, Ab Mutalib NS, Ong YS, Goh BH, Tan LTH, Lee LH. Streptomyces sp. A treasure trove of weapons to combat methicillin-resistant Staphylococcus aureus biofilm associated with biomedical devices. Int J Mol Sci. 2021;22(17):9360. doi: 10.3390/ijms22179360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.She W, Ye W, Shi Y, Zhou L, Zhang Z, Chen F, Qian PY. A novel chresdihydrochalcone from Streptomyces chrestomyceticus exhibiting activity against Gram-positive bacteria. J Antibiot (Tokyo) 2020;73(7):429–434. doi: 10.1038/s41429-020-0298-1. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava V, Singla RK, Dubey AK. Inhibition of biofilm and virulence factors of Candida albicans by partially purified secondary metabolites of Streptomyces chrestomyceticus Strain ADP4. Curr Top Med Chem. 2018;18(11):925–945. doi: 10.2174/1568026618666180711154110. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava V, Dubey AK. Anti-biofilm activity of the metabolites of Streptomyces chrestomyceticus strain ADP4 against Candida albicans. J Biosci Bioeng. 2016;122(4):434–440. doi: 10.1016/j.jbiosc.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Šegan S, Živković-Radovanović V, Tosti T, Ristivojević P, Milojković-Opsenica D. Thin-layer chromatography in bioassays of antimicrobial compounds from plants. J Liq Chromatogr Relat Technol. 2021;44(9–20):507–518. doi: 10.1080/10826076.2021.1968429. [DOI] [Google Scholar]
- 14.Singla RK, Dubey AK. Molecules and metabolites from natural products as inhibitors of biofilm in Candida spp. pathogens. Curr Top Med Chem. 2019;19(28):2567–2578. doi: 10.2174/1568026619666191025154834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CLSI (2017). Performance standards of antifungal susceptibility testing of yeasts. 1st edition. CLSI supplement M60. Wayne, PA: Clinical and Laboratory Standards, 2017
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Gautret P, Lagier J, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S, Colson P, Chabrière E, La Scola B, Rolain J, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label nonrandomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.U.S. EPA (2020). User’s Guide for T.E.S.T. (version 5.1) (Toxicity Estimation Software Tool): a program to estimate toxicity from molecular structure
- 19.Srivastava V, Singla RK, Dubey AK. Emerging Virulence, Drug Resistance and Future Anti-fungal Drugs for Candida Pathogens. Curr Top Med Chem. 2018;18(9):759–778. doi: 10.2174/1568026618666180528121707. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Biotechnology Information (2022a) PubChem Substance Record for SID 363595479, pentyl phenyl ketone, Source: MassBank of North America (MoNA). https://pubchem.ncbi.nlm.nih.gov/substance/363595479
- 21.National Center for Biotechnology Information (2022b) PubChem Compound Summary for CID 20771995, Methyl 3-isobutyl-benzoate. https://pubchem.ncbi.nlm.nih.gov/compound/Methyl-3-isobutyl-benzoate
- 22.Al-Hatmi AMS, Mohsin J, Al-Huraizi A, Khamis F. COVID-19 associated invasive candidiasis. J Infect. 2021;82(2):e45–e46. doi: 10.1016/j.jinf.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ademe M, Girma F. Candida auris: from multidrug resistance to pan-resistant strains. Infect Drug Resist. 2020;13:1287–1294. doi: 10.2147/IDR.S249864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atriwal T, Azeem K, Husain FM, Hussain A, Khan MN, Alajmi MF, Abid M. Mechanistic understanding of Candida albicans biofilm formation and approaches for its inhibition. Front Microbiol. 2021;12:638609. doi: 10.3389/fmicb.2021.638609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harjumäki R, Nugroho RWN, Zhang X, Lou YR, Yliperttula M, Valle-Delgado JJ, Österberg M. Quantified forces between HepG2 hepatocarcinoma and WA07 pluripotent stem cells with natural biomaterials correlate with in vitro cell behavior. Sci Rep. 2019;9:7354. doi: 10.1038/s41598-019-43669-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scorzoni L, de Paula e Silva ACA, Marcos CM, Assato PA, de Melo WCMA, de Oliveira HC, Costa Orlandi CB, Mendes Giannini MJS, Fusco Almeida AM, Antifungal therapy: new advances in the understanding and treatment of mycosis. Front Microbiol. 2017;8:36. doi: 10.3389/fmicb.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knoll KE, van der Walt MM, Loots DT. In Silico drug discovery strategies identified ADMET properties of Decoquinate RMB041 and its potential drug targets against Mycobacterium tuberculosis. Microbiol Spectr. 2022;10(2):e0231521. doi: 10.1128/spectrum.02315-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finch A, Pillans P. P-glycoprotein and its role in drug-drug interactions. Aust Prescr. 2014;37:137–139. doi: 10.18773/austprescr.2014.050. [DOI] [Google Scholar]
- 30.Bozina N, Bradamante V, Lovrić M. Genetic polymorphism of metabolic enzymes P450 (CYP) as a susceptibility factor for drug response, toxicity, and cancer risk. Arh za Hig Rada Toksikol. 2009;60(2):217–242. doi: 10.2478/10004-1254-60-2009-1885. [DOI] [PubMed] [Google Scholar]
- 31.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 32.Capuzzi SJ, Muratov EN, Tropsha A. Phantom PAINS: problems with the utility of alerts for pan- assay interference compounds. J Chem Inf Model. 2017;57:417–427. doi: 10.1021/acs.jcim.6b00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranjith SN, Kumar M, Ashwin Karthick N, Bhuvaneswari S, Udaya Prakash NK. In silico evaluation of multispecies toxicity of natural compounds. Drug Chem Toxicol. 2021;44(5):480–486. doi: 10.1080/01480545.2019.1614023. [DOI] [PubMed] [Google Scholar]
- 34.Padhi S, Masi M, Chourasia R, Rajashekar Y, Rai AK, Evidente A. ADMET profile and virtual screening of plant and microbial natural metabolites as SARS-CoV-2 S1 glycoprotein receptor binding domain and main protease inhibitors. Eur J Pharmacol. 2021;890:173648. doi: 10.1016/j.ejphar.2020.173648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


