Abstract
Current treatments for adolescent alcohol use disorder (AUD) are mainly psychosocial and limited in their efficacy. As such, pharmacotherapies are being investigated as potential adjunctive treatments to bolster treatment outcomes. N-acetylcysteine is a promising candidate pharmacotherapy for adolescent AUD because of its tolerability and demonstrated ability to modulate glutamatergic, GABAergic, and glutathione systems. The primary objective of this double-blind, placebo-controlled, within-subjects crossover preliminary investigation was to measure potential changes within glutamate + glutamine (Glx), GABA, and glutathione levels in the dorsal anterior cingulate cortex (dACC) using proton magnetic resonance spectroscopy during 10-days of N-acetylcysteine (1200 mg twice daily) compared to 10-days of placebo in non-treatment seeking adolescents who use alcohol heavily (N = 31; 55% female). Medication adherence was confirmed via video. Effects on alcohol use were measured using Timeline Follow-Back as an exploratory aim. Linear mixed effects models controlling for baseline metabolite levels, brain tissue composition, alcohol use, cannabis use, and medication adherence found no significant differences in Glx, GABA, or glutathione levels in the dACC after N-acetylcysteine compared to placebo. There were also no measurable effects on alcohol use; however, this finding was underpowered. Findings were consistent in the subsample of participants who met criteria for AUD (n = 19). The preliminary null findings in brain metabolite levels may be due to the young age of participants, relatively low severity of alcohol use, and non-treatment seeking status of the population investigated. Future studies can use these findings to conduct larger, well-powered studies within adolescents with AUD.
Subject terms: Drug discovery, Addiction, Addiction
Introduction
Alcohol remains the most used substance in adolescence [1, 2]. Patterns of alcohol misuse, such as heavy drinking (≥4–8 drinking occasions/month with 3–4 drinks/occasion) [3, 4] and binge drinking (4+ drinks for girls, 5+ drinks for boys on an occasion) [5], are related to worse outcomes in adolescents compared to adults, including being more likely to transition into an early and persistent alcohol use disorder (AUD) [1, 6–10]. Thus, youth who drink heavily represent an important target population for identifying more effective pathways to alcohol intervention.
Currently available interventions for adolescents with AUD are exclusively psychosocial [11] and only modestly effective [12, 13], with up to 50% of individuals returning to alcohol or other substance use within a year of treatment [14–16]. Adjunctive pharmacotherapies may bolster psychosocial treatment outcomes for adolescents with AUD. While there are four FDA-approved medications for treating adults with AUD, there are no FDA-approved medications for treating AUD in adolescents. It is important to investigate possible adjunctive pharmacotherapies specifically within the adolescent population, as safety and efficacy data should not be inferred from the adult AUD literature [17]. Natural or alternative medicine options may be particularly appealing for this age group [18, 19].
N-acetylcysteine is an antioxidant and pro-cystine supplement with the potential to reduce adolescent alcohol use. N-acetylcysteine is particularly appealing for adolescents with AUD since it is accessible as an over-the-counter supplement, has minimal side-effects in populations of adolescents who use substances [20], and has been FDA-approved for use as a respiratory drug in children since 1963, giving it a long-standing safety record. Based on preclinical findings, glutamate (Glu) has emerged as a potential pharmacotherapeutic target in the treatment of substance use disorders (SUD) [21–23]. N-acetylcysteine has glutamatergic properties and is thought to restore glutamatergic homeostasis that is disrupted during SUD [22–25]. Several preclinical studies have shown that N-acetylcysteine administration significantly reduces intake of multiple substances including alcohol [26, 27], cocaine [28], and nicotine [29]. Furthermore, treatment with N-acetylcysteine decreases reinstatement of alcohol (70–80% decrease in alcohol consumption [26, 27]), cocaine, heroin, and nicotine seeking [25, 30] in rodents.
Few studies have examined the effects of N-acetylcysteine in humans with SUD with mixed results [25, 31]; although limited, the findings have been promising in adolescents. Two clinical trials have shown potential in reducing cannabis and alcohol use in youth with cannabis use disorder (CUD). In youth with CUD (ages 15–21), those randomized to N-acetylcysteine had double the odds of a negative cannabinoid urine test as compared to placebo after 8 weeks [20]. In a secondary analysis of the trial, participants randomized to N-acetylcysteine who reduced their cannabis use also reduced their alcohol use. This relationship was not present in the placebo group, suggesting that N-acetylcysteine’s effects extended to alcohol use [32]. The ancillary finding was derived from a behavioral platform intervention solely focused on cannabis. While the follow-up trial in adults with CUD (ages 18–50) did not find a significant effect of N-acetylcysteine on cannabis use, post-hoc analyses within participants ages 18–21 (ages that overlapped with the previous trial) also revealed double the numerical rates of abstinence [33], suggesting N-acetylcysteine may be particularly effective in youth [20, 24, 32]. More research is needed to better understand the biological mechanisms of behavioral change, since research dedicated to investigating pharmacotherapeutic mechanisms of action in humans is a fundamental stage in the medication development pipeline before transitioning candidate pharmacotherapies into larger clinical trials [34, 35].
This can be accomplished by using in vivo neuroimaging techniques, such as proton magnetic resonance spectroscopy (1H-MRS). 1H-MRS is a non-invasive brain imaging technique that quantifies neurochemistry, allowing for the measurement of local changes in brain metabolite levels associated with alcohol misuse and assessing the therapeutic effects of medications on affected neurometabolites [36–39]. A recent meta-analysis of 1H-MRS studies on alcohol misuse and AUD [38] found a reduction in almost every measurable brain metabolite. Importantly, this analysis identified no studies in individuals younger than 21 years old [38], meaning there is a lack of information regarding alcohol related brain metabolite changes in adolescents.
1H-MRS can measure three of the major brain metabolites thought to underpin the mechanisms of SUD [21–23], shown to be modulated by N-acetylcysteine [40–42], and proposed as medication targets in adults with AUD [43–48]: glutamate (Glu; excitatory neurotransmitter), gamma-aminobutyric acid (GABA; inhibitory neurotransmitter), and glutathione (GSH; endogenous antioxidant). There is a biological connection between these metabolites and AUD. Briefly, alcohol and other substances lead to dysregulation in glutamatergic homeostasis [23], which can lead to changes in the excitatory/inhibitory balance (affecting GABA) and deplete GSH through oxidative stress [49]. An 1H-MRS open-label study in adults who use cocaine found that an acute dose of N-acetylcysteine reduced glutamate levels in the dorsal anterior cingulate cortex (dACC) without affecting glutamate levels in healthy controls [40]. However, no published study, to our knowledge, has reported on 1H-MRS specific effects of N-acetylcysteine on alcohol misuse or AUD.
The goal of this preliminary investigation was to examine the effects of N-acetylcysteine on Glu (as glutamate + glutamine, or Glx), GABA+ (GABA + macromolecules), and GSH systems in adolescents who drink heavily (Aim 1), as well as alcohol use (exploratory, underpowered Aim 2) in a randomized, double-blind, placebo-controlled within-subjects crossover study. In counterbalanced order, youth received a 10-day course of N-acetylcysteine (1200 mg twice daily) and matched placebo. Human laboratory and imaging procedures were conducted on the last day of each 10-day course of medication (last dose of medication taken 45 min before imaging session). For Aim 1, we hypothesized that youth who drink heavily would show lower levels of Glx and higher levels of GABA+ and GSH while taking N-acetylcysteine, as compared to placebo. Secondary metabolites of interest were Glu, total N-acetylaspartate (tNAA), total choline-containing metabolites (tCho), total creatine-containing metabolites (tCr), and myo-inositol (mI). Of note, Glx (glutamate + glutamine) is reported herein as the main glutamatergic metabolite of interest, rather than Glu (separated from glutamine), since the overlap between Glu and glutamine in the 1H-MRS spectrum is difficult to resolve into individual metabolite levels. Glu findings should be interpreted with caution for this reason. For exploratory Aim 2, we explored potential effects of N-acetylcysteine on alcohol use (quantity and frequency) and other substance use. We did not expect significant variation between courses of N-acetylcysteine and placebo since this was not a treatment-seeking population, and there was no treatment platform present to encourage behavior changes. However, omitting an exploratory examination of drinking outcomes in the context of this preliminary clinical trial would be a notable shortfall.
Methods
Participants
Randomized participants (N = 39) were between the ages of 17–19 years. The age of interest corresponds to a time when many adolescents initiate and escalate heavy drinking [50–52]. All participants met criteria for heavy drinking, based on quantity and frequency (minimum: 4–8 drinking occasions/month, consuming 3–4 drinks/occasion; see Fig. S1) [53, 54]. Frequency and quantity measures were used, as opposed to AUD diagnosis, as diagnostic criteria may not be optimal for capturing problematic drinking in youth [54]. For exclusion criteria, see Supplementary Materials. Participants were recruited from the community and schools using a mix of social media advertising, school and community event recruitment, and clinical recruitment through partnerships with local primary care clinics. All participants provided written informed consent/assent, and written permission was obtained from parents/guardians of individuals under the age of 18 years.
Consent and screening interview
Adolescents completed a screening visit, which captured demographic and background information, medication use, physical health, neurological history, family history of substance use, and personal substance use history. The Youth Structured Clinical Interview [53] was administered to ascertain information on psychosocial functioning (e.g., academic/social functioning) and health and medication history. The Mini International Neuropsychiatric Interview (MINI) was administered to ascertain current or lifetime history of the major psychiatric disorders in the DSM-5 and ICD-10 [55, 56].
Procedures
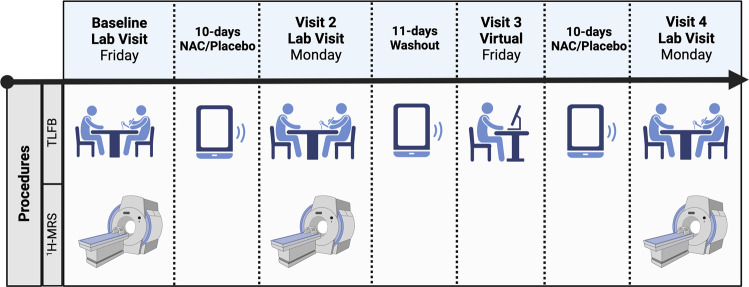
All procedures were approved by the local IRB and registered on Clinicaltrials.gov (NCT03238300). A randomized, double-blind, placebo-controlled within-subjects crossover design was utilized (Fig. 1). Human laboratory and imaging procedures were conducted after each 10-day course of medication. Participants completed baseline assessments to adjust for inter-individual differences prior to medication initiation.
Fig. 1. Experimental design of the clinical trial.
NAC N-acetylcysteine, TLFB timeline follow-back (completed using in-person interview, phone text messages, or virtual visits). Figure created in BioRender.com.
N-acetylcysteine or placebo administration
In counterbalanced order (randomization completed by statistician), adolescents received a 10-day course of N-acetylcysteine (two capsules, twice daily, totaling 2400 mg/day; IND approval #78,927) and a 10-day course of matched placebo (allocated in sequentially numbered containers provided by pharmacy), separated by an 11-day washout period to allow for the clearance of N-acetylcysteine. This was a longer washout period than previous studies [57] to avoid carryover effects. Participants took the last dose of each medication at the beginning of visit 2 and visit 4 (45 min before imaging procedures). Participants started the protocol on a Friday and captured two weekend drinking periods per condition [58]. Medication tolerability/adverse events were assessed at each clinic visit.
Medication adherence
Daily reminders to take the medication were sent via email or text. Medication compliance was measured in two ways: pill count and medication videos submitted via REDCap [59]. Participants were asked to submit medication videos via text showing them taking the pills each morning and evening [59]. Additionally, participants could save the video on their phone and show it with the timestamp to a study staff member if video upload failed.
Substance use
Recent substance use histories were assessed using a Timeline Follow-Back [60] (TLFB) to obtain information on typical use of alcohol, nicotine, cannabis, and other substances. TLFB was completed for the 90 days prior to the screening visit to establish eligibility for the study, then over the 10-days of either N-acetylcysteine or placebo and during the 11-day washout period. From the TLFB, the following substance use variables were calculated: (1) binge drinking episodes (defined previously), (2) average number of standard drinks on drinking days, (3) total number of alcohol use days, (4) total number of standard drinks, (5) nicotine use days, and (6) cannabis use days (summed over various methods of use for nicotine and cannabis).
Proton magnetic resonance spectroscopy
Medication was taken in front of study staff 45 min prior to the medication scans. All scans were performed with a Siemens 3.0T Prismafit MR scanner with actively shielded magnet and high-performance gradients (80 mT/m, 200 T/m-s) with a 32-channel head coil. A high-resolution anatomical scan (magnetization prepared rapid gradient echo; MPRAGE) was acquired, to allow subsequent registration to region-of-interest (ROI) definition (parameters: repetition/echo time (TR/TE) = 2250/4.18 ms; flip angle (FA) = 9°; field of view (FOV) = 256 mm2; voxel size = 1 mm2; 176 contiguous 1-mm-thick slices).
The 1H-MRS protocol was based on previously published methods [61, 62]. The dACC voxel for 1H-MRS was placed on midsagittal T1-weighted images (Fig. S2), anterior to the genu of the corpus callosum, with the ventral edge of the voxel aligned with the dorsal edge of the genu [63], with a voxel size of (30 × 25 × 25) mm3 [63, 64]. Following FAST(EST)MAP shimming [65], single-voxel water-suppressed (water suppression bandwidth 50 Hz for Glx and GSH or 100 Hz for GABA+, spectral bandwidth 2000 Hz, 1024 spectral points) 1H-MRS spectra is acquired with the following sequences: (1) Glx and other metabolites: SIEMENS Point Resolved Spectroscopy (PRESS) sequence: Repetition Time (TR) = 2000 ms; Echo Time (TE) = 40 ms; number of averages = 256; (2) GABA+ : SIEMENS WIP MEGA-PRESS sequence: Edit ON(OFF) = 1.90 (7.46) ppm; TR = 2000 ms, TE = 68 ms; number of averages = 160; (3) GSH: Universal MEGA-PRESS sequence [66]: Edit ON(OFF) = 4.56 (10.00) ppm; TR = 2000 ms, TE = 120 ms; number of averages = 320. Unsuppressed water spectra were co-acquired and scaled for partial volume effects and relaxation and used as a concentration reference. Six saturation bands (41 mm thickness) were placed 0.8 mm from each voxel face for outer volume suppression.
MRS data were processed using Osprey [67]. Osprey is an open-access, all-in-one MATLAB-based software. Standard parameters were used for Glx and GSH processing (metabolite fit range: 0.2 to 4.2 ppm; water fit range: 2.0 to 7.4 ppm; knot spacing = 0.4), while recommended parameters were used to optimize GABA+ processing (metabolite fit range: 0.5 to 4.0 ppm; water fit range: 2.0 to 7.4 ppm; knot spacing = 0.55; MM09 hard modeling) [68]. T1-weighted images were segmented using SPM12 [69] within Osprey, and voxel tissue fractions were saved [gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF)]. Metabolite quantifications were derived using fully tissue-and-relaxation-corrected molal concentration estimates [70]. The primary metabolites of interest were Glx, GABA+, and GSH. Secondary metabolites of interest were Glu, tNAA, tCho, tCr, and mI. Glu (separated from glutamine) was included as a secondary metabolite since it can be difficult to completely resolved from glutamine with the acquisition parameters used in this study. There was significant overlap between Glu and glutamine within the data (see Supplementary Materials); thus, Glx is presented as the primary metabolite and Glu findings should be interpreted with caution.
Osprey provides data quality outcomes, including signal-to-noise ratio (Cr SNR; ratio between amplitude of Cr peak and standard deviation of detrended noise) and linewidth for Cr [full-width half-maximum (FWHM) of single-Lorentzian fit to Cr peak] and water (H2O; FWHM of single-Lorentzian fit to H2O reference peak). All spectra were visually inspected for artifacts (A.E.K.). Cases were excluded if the Cr SNR was >3 standard deviations away from the group mean or if the linewidth was >11 Hz. This resulted in the identification of 2 participants at baseline with poor data quality (PRESS n = 1, MEGA-PRESS GSH n = 1). See “Statistical analysis” section for more details. Additionally, the coefficient of variation (COV) was determined (standard deviation divided by the mean, where lower values indicated higher quality data) [71].
Statistical analyses
An a priori power analysis was conducted to ensure power to detect differences in Glu levels within the dACC. With 31 participants, the study has 99% power to detect such effects on Glu levels (calculated based on d = 1.13 (reported in [40]), α = 0.05, two-tailed) [40, 72]. It should be noted that the a priori power analysis was conducted using a t-test, while the included analyses required more complex models. Further, the power analysis was conducted on Glu levels, not Glx levels. Glu (not Glx) was originally proposed as a main metabolite of interest; however, as mentioned previously, Glu was not able to be completely resolved from glutamine which limited its validity and interpretability. The study was not powered for changes in other metabolites or changes in alcohol or other substance use.
All analyses were conducted in R Statistical Software (v4.2.0; R Core Team 2022) and run with participants who completed the full study. Medication adherence was summarized for both pill count and medication video submission (percentage for each medication condition). Medication effects on medication adherence were analyzed using a t-test. For 1H-MRS data quality, repeated measures ANOVA was used with the main effect of visit (baseline vs. N-acetylcysteine vs. placebo). Post-hoc tests were used to examine any significant differences.
The primary aim of this study was to examine the effects of N-acetylcysteine compared to placebo on concentrations of brain metabolites (Glx, GABA+, and GSH). We used linear mixed effects models (lme4 package [73], model assumptions were checked with Performance package [74]) containing the main effect of medication (N-acetylcysteine vs. placebo), visit (scan 1 during visit 2 vs. scan 2 during visit 4), and sequence (N-acetylcysteine/placebo vs. placebo/N-acetylcysteine) to ensure the crossover design and washout period were successful. Baseline metabolite levels and brain tissue composition [GM:BM = GM/(GM + WM)] were included as covariates. Random intercepts were included to account for individual differences. As mentioned, 2 participants had 1H-MRS data deemed excluded due to poor data quality. Multiple imputations (MICE package [75]) were run to replace the excluded data, and sensitivity analyses were conducted to ensure the imputed data did not affect the overall results. The effect size for medication (N-acetylcysteine vs. placebo) was reported using partial eta squared with 95% confidence intervals (Effectsize package [76]).
For exploratory Aim 2, the same linear mixed effect models were used to assess for effects of N-acetylcysteine on alcohol (binge drinking episodes, average standard drinks per drinking day, total drinking days, and total standard drinks), cannabis use days, and nicotine use days over the 10-day course of N-acetylcysteine and placebo. Baseline substance use (from 90-day TLFB at screening) were included as covariates in their respective models.
Other demographic covariates (age, sex, and race) and medication adherence (pill count) were assessed for their impact on the models for Aim 1 and 2 using likelihood-ratio test (lrtest package [77]); none of the demographic variables impacted the models and thus were not included as covariates. Medication adherence (as pill count) was shown to impact three substance use outcomes (drinks per drinking day, total standard drinks, and cannabis use days) and was included as a covariate for these models. To account for potential effects of specific domains of alcohol use on each brain metabolite level, and to avoid multicollinearity, models were run with each potential alcohol use covariate and compared in the Performance package [74]. The package ranks models based on R-squared, intraclass correlation coefficient (ICC), Akaike Information Criterion values (AIC), AIC weights, Bayesian Information Criterion (BIC) weights, and root-mean-square deviation (RMSE). The highest performing model with a single alcohol use domain (e.g., binge drinking episodes) was chosen as the final model to reduce multicollinearity. Covariates of interest included: alcohol use (number of binge episodes, average standard drinks per drinking day, number of drinking days, and total number of standard drinks), AUD status (Yes/No by DSM-5 criteria at screening) or AUD severity (none, mild, moderate, or severe by DSM-5 criteria at screening). Since cannabis use was common in this population, all metabolite models included a cannabis use days covariate. Finally, a sub-group analysis replicating the above methods was conducted in participants who met DSM-5 criteria for AUD (n = 19). See Supplemental Materials for full model comparisons of covariates and the AUD sub-group analysis.
Results
Participant characteristics
Thirty-one participants completed the full protocol (Table 1 and Fig. S3 for consort diagram). Fewer participants had MEGA-PRESS scans for GSH (n = 28), as this was added later in the study.
Table 1.
Sample characteristics (N = 31).
| Mean | SD | Range | |
|---|---|---|---|
| Demographics | |||
| Age | 18.79 | 0.65 | 17.57–19.90 |
| N | % | – | |
| Sex | |||
| Female | 17 | 54.8% | |
| Male | 14 | 45.2% | |
| Race | |||
| Asian | 2 | 6.5% | |
| White | 29 | 93.5% | |
| Grade completed | |||
| 10th | 1 | 3.2% | |
| 11th | 7 | 22.6% | |
| 12th or GED equivalent | 16 | 51.6% | |
| 1st year of college | 7 | 22.6% | |
| Substance use | |||
| Alcohola | |||
| Binge drinking episodes | 14.4 | 10.0 | 0–36 |
| Drinks per drinking days | 4.9 | 1.6 | 2.5–8.6 |
| Drinking days | 23.7 | 11.1 | 11–57 |
| Total number of drinks | 118.5 | 72.1 | 44–325 |
| Nicotine use daysa | 31.8 | 36.8 | 0–91 |
| Cannabis use daysa | 16.5 | 28.8 | 0–89 |
| N | % | – | |
| % Using Nicotine | 19 | 61.3% | |
| % Using Cannabis | 19 | 61.3% | |
| MINI AUD | |||
| None | 12 | 38.7% | |
| Mild | 10 | 32.3% | |
| Moderate | 6 | 19.4% | |
| Severe | 3 | 9.7% | |
MINI Mini International Neuropsychiatric Interview.
aSubstance use was obtained from the 90-day Timeline Follow Back (TLFB) obtained at screening visit.
Medication adherence
For N-acetylcysteine, on average 90.5% of pills were taken based on pill count (range: 33–100%) and 79.4% of medication videos were submitted (15–100%). For placebo, on average 90.5% of pills were taken based on pill count (range: 48–100%) and 80.3% of medication videos were submitted (range: 35–100%). There were no statistical differences in adherence between medications.
Magnetic resonance spectroscopy (1H-MRS)
1H-MRS data quality
Dorsal ACC voxel tissue fractions were consistent across visits (Table 2). For the Glx scans, there was a significant effect of visit on the linewidth for Cr, where it was narrower during the placebo visits (p = 0.02) compared to baseline, indicating better data quality (Table 2 and Fig. S4). For representative spectra, see Supplementary Materials (Fig. S5).
Table 2.
1H-MRS data quality.
| Baseline | N-acetylcysteine | Placebo | ANOVA | |
|---|---|---|---|---|
| Tissue fractions of dACC | ||||
| Gray matter (GM) | 0.61 (0.02) | 0.60 (0.02) | 0.60 (0.03) | F = 0.27, p = 0.76 |
| White matter (WM) | 0.26 (0.03) | 0.26 (0.03) | 0.26 (0.03) | F = 0.02, p = 0.99 |
| Cerebrospinal fluid (CSF) | 0.13 (0.03) | 0.13 (0.02) | 0.14 (0.02) | F = 0.43, p = 0.65 |
| GM:BM | 0.70 (0.03) | 0.70 (0.03) | 0.70 (0.04) | F = 0.02, p = 0.98 |
| SNR (Cr) | ||||
| Glx | 263.31 (47.51) | 261.23 (36.05) | 281.89 (43.97) | F = 2.19, p = 0.12 |
| GABA+ | 207.11 (34.29) | 205.06 (29.28) | 220.69 (31.45) | F = 2.22, p = 0.12 |
| GSH | 124.38 (16.39) | 123.37 (19.49) | 129.83 (14.40) | F = 1.18, p = 0.31 |
| Linewidth (Cr) | ||||
| Glx | 4.93 (0.87) | 4.63 (0.36) | 4.53 (0.33) | F = 3.93, p = 0.02 |
| GABA+ | 4.43 (0.66) | 4.23 (0.25) | 4.25 (0.25) | F = 2.02, p = 0.14 |
| GSH | 4.20 (0.45) | 4.06 (0.27) | 4.12 (0.31) | F = 1.07, p = 0.34 |
| Linewidth (H2O) | ||||
| Glx | 5.78 (0.40) | 5.90 (0.87) | 5.79 (0.45) | F = 0.40, p = 0.67 |
| GABA+ | 5.74 (0.43) | 5.64 (0.26) | 5.58 (0.33) | F = 1.54, p = 0.22 |
| GSH | 5.48 (0.33) | 5.35 (0.26) | 5.43 (0.38) | F = 1.13, p = 0.33 |
Baseline: Glx n = 30, GABA+ n = 31, GSH n = 27; N-acetylcysteine and placebo: Glx and GABA+ n = 31, GSH n = 28.
Glx glutamate + glutamine and secondary metabolites from PRESS (40 ms) acquisition, GABA+ GABA+ from MEGA-PRESS (68 ms) acquisition, GSH glutathione from the MEGA-PRESS (120 ms) acquisition, Tissue fractions percent of brain tissue (GM, WM, or CSF) in the dACC voxel, GM:BM brain tissue composition [GM/(GM + WM)], SNR (Cr) ratio between amplitude of Cr peak and standard deviation of detrended noise, Linewidth (Cr) FWHM of single-Lorentzian fit to Cr peak, Linewidth (H2O) FWHM of single-Lorentzian fit to H2O reference peak.
Bold values indicate statistical significance.
N-acetylcysteine vs. placebo: primary metabolites (primary aim) and secondary metabolites
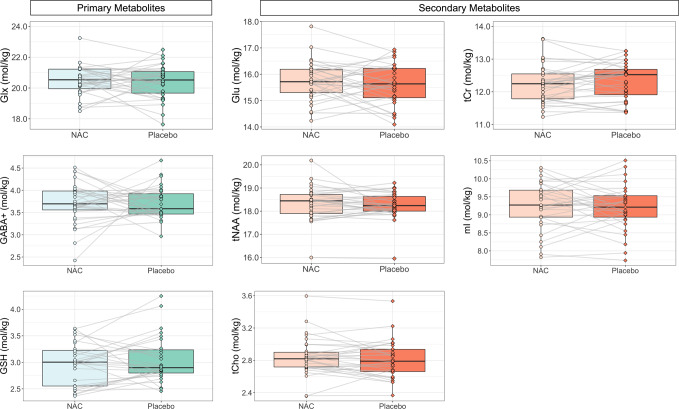
For descriptive statistics and COV of all metabolites, see Table S1. There were no significant differences between N-acetylcysteine and placebo for the primary metabolite levels (Glx, GABA+, or GSH) or secondary metabolite levels (Table 3 and Fig. 2). Partial eta squared statistics indicated very small effect sizes for medication for all metabolites (η2p range: <0.01 to 0.03; 95% CI upper threshold range: 0.03–0.24). The average absolute difference between N-acetylcysteine and placebo was less than 0.13 mol/kg (difference range: 0.01–0.13 mol/kg; Fig. S6), with Glu having the largest average difference (0.13 mol/kg) and GABA+ having the smallest average difference (0.01 mol/kg). Sub-group analysis of participants meeting criteria for AUD showed similar results for model significance and effect sizes (Table S2; η2p range: <0.01–0.1; 95% CI upper threshold range: 0.03–0.45).
Table 3.
Linear mixed effects models results comparing N-acetylcysteine to placebo on primary and secondary brain metabolite levels in the dorsal anterior cingulate cortex of adolescents who drink heavily.
| Metabolite | Fixed effect | Estimate | Std. error | DF | T-value | p value | η2p (95% CI) |
|---|---|---|---|---|---|---|---|
| Glx | |||||||
| Medication | −0.03 | 0.23 | 29.31 | −0.14 | 0.89 | <0.01 (0.00–0.09) | |
| Sequence | 0.09 | 0.37 | 25.45 | 0.24 | 0.81 | ||
| Visit | 0.10 | 0.12 | 30.39 | 0.85 | 0.40 | ||
| Baseline Glx | 0.07 | 0.08 | 25.49 | 0.79 | 0.44 | ||
| GM:BM | −2.81 | 4.68 | 27.66 | −0.60 | 0.55 | ||
| Cannabis use days | −0.18 | 0.30 | 36.47 | −0.58 | 0.57 | ||
| AUD | 0.33 | 0.35 | 25.44 | 0.95 | 0.35 | ||
| GABA+a | |||||||
| Medication | 0.01 | 0.11 | 54.00 | 0.07 | 0.94 | <0.01 (0.00–0.03) | |
| Sequence | 0.04 | 0.11 | 54.00 | 0.34 | 0.74 | ||
| Visit | −0.06 | 0.05 | 54.00 | −1.16 | 0.25 | ||
| Baseline GABA+ | 0.11 | 0.13 | 54.00 | 0.86 | 0.39 | ||
| GM:BM | −2.39 | 1.67 | 54.00 | −1.43 | 0.16 | ||
| Cannabis use days | −0.01 | 0.12 | 54.00 | −0.07 | 0.95 | ||
| AUD severity | −0.07 | 0.06 | 54.00 | −1.26 | 0.21 | ||
| GSH | |||||||
| Medication | 0.07 | 0.08 | 25.20 | 0.84 | 0.41 | 0.03 (0.00–0.24) | |
| Sequence | −0.06 | 0.14 | 22.91 | −0.43 | 0.67 | ||
| Visit | 0.05 | 0.04 | 27.18 | 1.26 | 0.22 | ||
| Baseline GSH | 0.08 | 0.19 | 23.18 | 0.42 | 0.68 | ||
| GM:BM | −1.17 | 1.99 | 26.14 | −0.59 | 0.56 | ||
| Cannabis use days | 0.02 | 0.14 | 45.91 | 0.11 | 0.91 | ||
| Binge drinking episodes | 0.07 | 0.04 | 47.99 | 1.81 | 0.08 | ||
| Glu | |||||||
| Medication | −0.13 | 0.16 | 28.11 | −0.82 | 0.42 | 0.02 (0.00–0.22) | |
| Sequence | −0.00 | 0.25 | 24.97 | 0.01 | 0.99 | ||
| Visit | 0.04 | 0.08 | 29.67 | 0.52 | 0.61 | ||
| Baseline Glu | 0.10 | 0.08 | 25.19 | 1.28 | 0.21 | ||
| GM:BM | −4.20 | 3.33 | 27.48 | −1.26 | 0.22 | ||
| Cannabis use days | 0.09 | 0.22 | 39.64 | 0.40 | 0.69 | ||
| Binge drinking episodes | −0.11 | 0.06 | 46.72 | −1.76 | 0.08 | ||
| tNAA | |||||||
| Medication | −0.10 | 0.10 | 29.38 | −1.02 | 0.31 | 0.03 (0.00–0.23) | |
| Sequence | −0.22 | 0.16 | 25.64 | −1.34 | 0.19 | ||
| Visit | −0.04 | 0.05 | 30.63 | −0.87 | 0.39 | ||
| Baseline tNAA | 0.62 | 0.11 | 25.61 | 5.60 | <0.001 | ||
| GM:BM | −1.09 | 2.32 | 28.36 | −0.47 | 0.64 | ||
| Cannabis use days | 0.13 | 0.15 | 29.29 | 0.90 | 0.37 | ||
| AUD | −0.34 | 0.17 | 25.57 | −1.99 | 0.06 | ||
| tCho | |||||||
| Medication | −0.03 | 0.03 | 29.36 | −0.91 | 0.37 | 0.03 (0.00–0.21) | |
| Sequence | −0.03 | 0.04 | 25.60 | −0.68 | 0.50 | ||
| Visit | 0.01 | 0.01 | 30.49 | 0.81 | 0.42 | ||
| Baseline tCho | 0.98 | 0.11 | 26.20 | 9.16 | <0.001 | ||
| GM:BM | −1.92 | 0.60 | 27.97 | −3.18 | 0.004 | ||
| Cannabis use days | −0.04 | 0.04 | 28.06 | −0.90 | 0.37 | ||
| AUD | −0.08 | 0.04 | 25.51 | −1.83 | 0.08 | ||
| tCr | |||||||
| Medication | 0.08 | 0.08 | 29.48 | 0.94 | 0.35 | 0.03 (0.00–0.22) | |
| Sequence | −0.14 | 0.16 | 26.81 | −0.87 | 0.39 | ||
| Visit | 0.00 | 0.04 | 30.41 | 0.04 | 0.97 | ||
| Baseline tCr | 0.59 | 0.15 | 27.13 | 3.93 | <0.001 | ||
| GM:BM | −3.05 | 2.33 | 31.90 | −1.31 | 0.20 | ||
| Cannabis use days | 0.07 | 0.14 | 47.79 | 0.49 | 0.62 | ||
| Drinks per drinking day | −0.04 | 0.03 | 50.29 | −1.73 | 0.09 | ||
| mI | |||||||
| Medication | −0.07 | 0.11 | 29.42 | −0.59 | 0.56 | 0.01 (0.00–0.18) | |
| Sequence | −0.18 | 0.19 | 26.42 | −0.97 | 0.34 | ||
| Visit | −0.04 | 0.06 | 30.12 | −0.78 | 0.44 | ||
| Baseline mI | 0.51 | 0.14 | 26.56 | 3.58 | 0.001 | ||
| GM:BM | −0.64 | 2.64 | 30.20 | −0.24 | 0.81 | ||
| Cannabis use days | 0.01 | 0.17 | 42.69 | 0.06 | 0.95 | ||
| Drinks per drinking day | −0.02 | 0.03 | 53.26 | −0.68 | 0.50 | ||
n = 31 for glutamate + glutamine (Glx), GABA+, and other secondary metabolites; n = 28 for glutathione (GSH). References: medication = placebo; sequence = placebo/N-acetylcysteine; visit = visit 4 (last MRI visit).
Glu glutamate, tNAA total N-acetylaspartate, tCho total choline-containing metabolites, tCr total creatine-containing metabolites, mI myo-inositol, AUD alcohol use disorder, GM:BM brain tissue composition [GM/(GM + WM)], η2p partial eta squared (reported only for medication).
Bold values indicate statistical significance.
aRandom intercepts were not appropriate for this model, resulting in higher degrees of freedom.
Fig. 2. Differences between N-acetylcysteine vs. placebo in primary and secondary metabolite levels in the dorsal anterior cingulate cortex of adolescents who drink heavily (raw data).
There were no significant differences for any metabolite. Primary metabolites: Glx (glutamate + glutamine), GABA+ (GABA + macromolecules), and Glutathione (GSH). Secondary metabolites: Glutamate (Glu), total N-acetylaspartate (tNAA), total choline-containing metabolites (tCho), total creatine-containing metabolites (tCr), and myo-inositol (mI). NAC N-acetylcysteine.
N-acetylcysteine vs. placebo: Alcohol and other substance use (exploratory aim)
There were no significant effects of N-acetylcysteine on alcohol or other substance use (Tables S3 and S4). Medication adherence (as pill count) was included as a covariate for standard drinks per drinking day, total standard drinks, and cannabis use days models; however, adding medication adherence did not change the overall interpretation of these models (i.e., insignificant effect of medication). Partial eta squared statistics indicated very small effect sizes for medication for all alcohol and other substance use outcomes (η2p range: <0.01–0.04; 95% CI upper threshold range: 0.03–0.24). Sub-group analysis of participants meeting criteria for AUD showed consistent results for model significance and effect sizes (Table S5; η2p range: <0.01–0.1; 95% CI upper threshold range: 0.10–0.42).
Discussion
The primary objective of this preliminary investigation was to assess the biological mechanisms of N-acetylcysteine in adolescents who drink heavily using magnetic resonance spectroscopy. Due to the underlying mechanisms of action of N-acetylcysteine, our main metabolites of interest were Glx, GABA+, and GSH in the dACC. The 10-day course of N-acetylcysteine, as compared to placebo, did not result in significant alterations in concentrations of Glx, GABA+, GSH, or any of the secondary metabolites of interest. For our exploratory aim regarding alcohol and other substance use, there were also no significant differences in alcohol or other substance use, likely because this was a non-treatment seeking population, there was no behavioral platform targeting alcohol use behaviors, and the short duration of medication administration (10 days). Further, results held up in a sub-group analysis in participants meeting DSM-5 criteria for AUD.
No published studies have tested the effects of N-acetylcysteine administration on brain metabolite levels in adolescents who drink heavily. One 1H-MRS publication assessed the effects of an acute dose of N-acetylcysteine (2400 mg given 1 h before scan) in a small sample of male individuals dependent on cocaine (n = 8, average age = 35.1 years), which found lower levels of Glu in the ACC after N-acetylcysteine [40]. Another study randomized individuals who smoked cigarettes to either N-acetylcysteine (n = 24, average age = 37.0 years, 2400 mg over 14 days) or placebo (n = 24, average age = 33.1 years), but did not find treatment effects on Glu or GABA levels within the ACC [78]. Our study design had participants take N-acetylcysteine for 10 days, in addition to taking the final dose in front of study staff 45 min prior to the start of the scan so that peak plasma concentration was reached during the scanning session [79]. Additionally, we documented high medication adherence based on pill count and medication video submissions across both the N-acetylcysteine and placebo study periods, and medication adherence (as pill count) was included as a covariate in final models when appropriate. Therefore, we believe the null findings are not due to ineffective medication dosing.
A recent meta-analysis investigated brain metabolite level alterations associated with alcohol misuse and AUD, finding widespread differences across metabolites and brain regions [38], with no studies included in populations younger than 21 years old. One study since assessed differences in metabolite levels between young adults who drank heavily and controls (average age 22.3 years) and reported lower levels of GABA+ in the occipital lobe; dACC metabolite levels were not assessed [80]. It is possible that alcohol-related brain metabolite level abnormalities were not present within our sample, negating the ability to detect differences from N-acetylcysteine administration, as our study participants were much younger (17–19 years old) than any other populations reported. Furthermore, our sample was a non-treatment seeking group, which can impact severity of alcohol misuse [38, 80–82] and alterations in brain metabolite levels [83–85]. N-acetylcysteine may work by alleviating glutamatergic or GABAergic imbalances [21–24], which may only exist in more severely affected substance-using populations.
Beyond N-acetylcysteine, other pharmacotherapy studies utilizing 1H-MRS have demonstrated treatment changes in neurometabolite levels. One crossover study in individuals with bipolar disorder and CUD (n = 22, average age = 37.6 years) found that 1 week of gabapentin (1200 mg/day) resulted in higher levels of Glu in the ACC and the right basal ganglia, as compared to placebo [86]. Studies of medication interventions within depression [87, 88], pediatric obsessive-compulsive disorder [89], social anxiety disorder [90], and psychosis patients [91, 92] have also shown changes in brain metabolite levels after treatment. These findings suggest that 1H-MRS can detect neurometabolite changes associated with pharmacological interventions. Alternatively, N-acetylcysteine may act on other processes that are not captured with 1H-MRS, like reducing acute reactivity to alcohol cues [93].
There are several strengths to this study. First, we used two forms of medication adherence assessments to ascertain confidence in our dosing regimen. The use of twice daily text messages and medication video submissions is a valid and reliable way to verify and increase medication adherence [59]. Additionally, participants took their last dose of medication 45 min before the scan for peak plasma concentration [40, 79]. Second, we used edited sequences to obtain GABA+ and GSH metabolite levels, which is recommended based on current expert consensus [94], and the PRESS sequence used for Glx was shown to be replicable and reliable [95]. Even so, isolating Glu from glutamine within the spectrum is difficult, which is why Glx was presented as the primary glutamatergic metabolite since there was significant overlap between Glu and glutamine within the data, limiting the interpretability of isolated Glu. The results were similar across both measures (Glx and Glu), increasing our confidence that N-acetylcysteine did not affect glutamatergic metabolites in this study. We also used Osprey [67], a state-of-the-art and standardized processing software, reducing the chance of user error or incorrect spectral modeling. Finally, alcohol clinical trials historically underrecruit female participants (representing only 29% of AUD trials) [96]. Due to our targeted recruitment efforts, female participants made up 55% of our final sample, which is important considering rates of heavy drinking are similar between sexes at these ages [50].
There are also some limitations to this preliminary work. This was a non-treatment seeking population that did not have to meet criteria for AUD, which does not perfectly represent the group of people who could eventually receive N-acetylcysteine as a treatment option for adolescent AUD. However, 61% of our sample did meet criteria for AUD, and the null findings remained in the sub-group analysis. The medication period was shorter than in the existing clinical trials examining N-acetylcysteine for CUD (8–12 weeks vs. 10-day dosing for this trial), which could have limited our ability to observe any changes in alcohol or other substance use, especially since a treatment platform was not present in our studies that could encourage such change. Co-use of cannabis and nicotine (usually through e-cigarettes or vaping) is common among this age group. Cannabis use was included as a covariate for all models, but it did not influence the overall effects of N-acetylcysteine. Consistent with alcohol, we did not see any alterations in cannabis or nicotine use. The study was not powered to detect substance use differences in exploratory Aim 2, so these results should be interpreted with caution. Lastly, the study was powered using only one small and homogenous study [40] and was powered to detect simple within subject differences in dACC Glu levels. The Schmaal et al. [40] study reported a large effect size for Glu (d = 1.13; as isolated Glu), while our detectable effects were much smaller across all metabolites (d range: 0.02–0.16; η2p range: <0.01–0.03). We used more complex models with more covariates in the final models presented, limiting the translational capacity of the a priori power analysis. A priori power analyses were not conducted on the other reported metabolites or on substance use outcomes. Since the sample studied by Schmaal et al. [40] (older, using cocaine, all male) differed vastly from the current sample, future studies in adolescents who use substances will now have additional information for power analyses. Future work would benefit from looking at effects of N-acetylcysteine in a potentially more severely affected population of treatment-seeking adolescents with AUD to expose any brain metabolite abnormalities (as compared to non-drinking controls) and changes in brain metabolites or substance use behaviors.
Adolescents who drink heavily did not show significant effects of N-acetylcysteine on neurometabolite levels measured with 1H-MRS, nor on alcohol use in this preliminary investigation. However, N-acetylcysteine has shown promise in reducing alcohol and cannabis use in adolescents, which may be due to its effects on other biological systems or downstream effects on behavioral and psychological determinants (e.g., changes in mood, craving, cue reactivity). Therefore, it is important to further identify suitable treatment populations to examine biological mechanisms and behavioral indicators of change in candidate pharmacotherapies such as N-acetylcysteine before moving into larger, expensive clinical trials to reduce the “valley of death” between preclinical and clinical trials research in translational science.
Supplementary information
Author contributions
All authors made significant contributions to this work. The project was conceived by LMS, KMG and RLT. LMS received funding for this project. DJM, JJP, RM, KTB and KMG were mentors for LMS K23. AMM, HL, BDB and AEK acquired data. AEK, PLF, and RG analyzed the data. The manuscript was written by AEK, BDB, RG, PLF, LMS and revised by all authors. All authors approved the final version of the manuscript.
Funding
This work was supported by the National Institute of Alcohol and Alcoholism (NIAAA-K23-AA025399). AEK is supported by F32-AA029930-01A1, BDB is supported by T32DA007288, RG is supported by T32AA007474.
Competing interests
KMG has provided consultation to Jazz Pharmaceuticals and has received research funding from Aelis Farma. RLT has received funding from the American Society of Addiction Medicine. The authors declare no other competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01553-z.
References
- 1.Lees B, Meredith LR, Kirkland AE, Bryant BE, Squeglia LM. Effect of alcohol use on the adolescent brain and behavior. Pharmacol Biochem Behav. 2020;192:172906. doi: 10.1016/j.pbb.2020.172906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global status report on alcohol and health 2018: World Health Organization; 2019.
- 3.Sun D, Adduru VR, Phillips RD, Bouchard HC, Sotiras A, Michael AM, et al. Adolescent alcohol use is linked to disruptions in age-appropriate cortical thinning: an unsupervised machine learning approach. Neuropsychopharmacology. 2022;11:1–10. [DOI] [PMC free article] [PubMed]
- 4.Lees B, Debenham J, Squeglia LM. Alcohol and cannabis use and the developing brain. Alcohol Res: Curr Rev. 2021;41:11. [DOI] [PMC free article] [PubMed]
- 5.Lees B, Mewton L, Stapinski LA, Squeglia LM, Rae CD, Teesson M. Neurobiological and cognitive profile of young binge drinkers: a systematic review and meta-analysis. Neuropsychol Rev. 2019;29:357–85. doi: 10.1007/s11065-019-09411-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, et al. Mechanisms of persistent neurobiological changes following adolescent alcohol exposure: NADIA consortium findings. Alcohol: Clin Exp Res. 2019;43:1806–22. doi: 10.1111/acer.14154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094–101. doi: 10.1176/appi.ajp.2017.16101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Addolorato G, Vassallo GA, Antonelli G, Antonelli M, Tarli C, Mirijello A, et al. Binge drinking among adolescents is related to the development of alcohol use disorders: results from a cross-sectional study. Sci Rep. 2018;8:1–9. doi: 10.1038/s41598-018-29311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsson CA, Romaniuk H, Salinger J, Staiger PK, Bonomo Y, Hulbert C, et al. Drinking patterns of adolescents who develop alcohol use disorders: results from the Victorian Adolescent Health Cohort Study. BMJ Open. 2016;6:e010455. doi: 10.1136/bmjopen-2015-010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlers CL, Stouffer GM, Gilder DA. Associations between a history of binge drinking during adolescence and self‐reported responses to alcohol in young adult Native and Mexican Americans. Alcohol: Clin Exp Res. 2014;38:2039–47. doi: 10.1111/acer.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fadus MC, Squeglia LM, Valadez EA, Tomko RL, Bryant BE, Gray KM. Adolescent substance use disorder treatment: an update on evidence-based strategies. Curr Psychiatry Rep. 2019;21:1–10. doi: 10.1007/s11920-019-1086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen CD, Cushing CC, Aylward BS, Craig JT, Sorell DM, Steele RG. Effectiveness of motivational interviewing interventions for adolescent substance use behavior change: a meta-analytic review. J Consult Clin Psychol. 2011;79:433. doi: 10.1037/a0023992. [DOI] [PubMed] [Google Scholar]
- 13.Tripodi SJ, Bender K, Litschge C, Vaughn MG. Interventions for reducing adolescent alcohol abuse: a meta-analytic review. Arch Pediatr Adolesc Med. 2010;164:85–91. doi: 10.1001/archpediatrics.2009.235. [DOI] [PubMed] [Google Scholar]
- 14.Tanner-Smith EE, Lipsey MW. Brief alcohol interventions for adolescents and young adults: a systematic review and meta-analysis. J Subst Abus Treat. 2015;51:1–18. doi: 10.1016/j.jsat.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung T, Maisto SA. Relapse to alcohol and other drug use in treated adolescents: review and reconsideration of relapse as a change point in clinical course. Clin Psychol Rev. 2006;26:149–61. doi: 10.1016/j.cpr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Winters KC, Tanner-Smith EE, Bresani E, Meyers K. Current advances in the treatment of adolescent drug use. Adolesc Health, Med Ther. 2014;5:199. doi: 10.2147/AHMT.S48053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–96. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- 18.Balog-Way DH, Evensen D, Löfstedt RE. Pharmaceutical benefit–risk perception and age differences in the USA and Germany. Drug Saf. 2020;43:1141–56. doi: 10.1007/s40264-020-00977-6. [DOI] [PubMed] [Google Scholar]
- 19.O’Callaghan FV, Jordan N. Postmodern values, attitudes and the use of complementary medicine. Complement Ther Med. 2003;11:28–32. doi: 10.1016/S0965-2299(02)00109-7. [DOI] [PubMed] [Google Scholar]
- 20.Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169:805–12. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalivas P, Volkow N. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–86. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 23.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 24.McClure EA, Gipson CD, Malcolm RJ, Kalivas PW, Gray KM. Potential role of N-acetylcysteine in the management of substance use disorders. CNS Drugs. 2014;28:95–106. doi: 10.1007/s40263-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slattery J, Kumar N, Delhey L, Berk M, Dean O, Spielholz C, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294–321. doi: 10.1016/j.neubiorev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Lebourgeois S, González‐Marín MC, Jeanblanc J, Naassila M, Vilpoux C. Effect of N‐acetylcysteine on motivation, seeking and relapse to ethanol self‐administration. Addict Biol. 2018;23:643–52. doi: 10.1111/adb.12521. [DOI] [PubMed] [Google Scholar]
- 27.Quintanilla ME, Rivera‐Meza M, Berríos‐Cárcamo P, Salinas‐Luypaert C, Herrera‐Marschitz M, Israel Y. Beyond the “first hit”: marked inhibition by N‐acetyl cysteine of chronic ethanol intake but not of early ethanol intake. Parallel effects on ethanol‐induced saccharin motivation. Alcohol: Clin Exp Res. 2016;40:1044–51. doi: 10.1111/acer.13031. [DOI] [PubMed] [Google Scholar]
- 28.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–76. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez-Niño AM, D’Souza MS, Markou A. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology. 2013;225:473–82. doi: 10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–10. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smaga I, Frankowska M, Filip M. N-acetylcysteine in substance use disorder: a lesson from preclinical and clinical research. Pharmacol Rep. 2021;73:1205–19. doi: 10.1007/s43440-021-00283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Squeglia LM, Baker NL, McClure EA, Tomko RL, Adisetiyo V, Gray KM. Alcohol use during a trial of N-acetylcysteine for adolescent marijuana cessation. Addict Behav. 2016;63:172–7. doi: 10.1016/j.addbeh.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Squeglia LM, Tomko RL, Baker NL, McClure EA, Book GA, Gray KM. The effect of N-acetylcysteine on alcohol use during a cannabis cessation trial. Drug Alcohol Depend. 2018;185:17–22. doi: 10.1016/j.drugalcdep.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray LA, Bujarski S, Roche DJO, Magill M. Overcoming the “Valley of Death” in medications development for alcohol use disorder. Alcohol: Clin Exp Res. 2018;42:1612–22. doi: 10.1111/acer.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seyhan AA. Lost in translation: the valley of death across preclinical and clinical divide–identification of problems and overcoming obstacles. Transl Med Commun. 2019;4:1–19. doi: 10.1186/s41231-019-0050-7. [DOI] [Google Scholar]
- 36.Cohen-Gilbert JE, Jensen JE, Silveri MM. Contributions of magnetic resonance spectroscopy to understanding development: potential applications in the study of adolescent alcohol use and abuse. Dev Psychopathol. 2014;26:405–23. doi: 10.1017/S0954579414000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohut SJ, Kaufman MJ. Magnetic resonance spectroscopy studies of substance use disorders: current landscape and potential future directions. Pharmacol Biochem Behav. 2021;200:173090. doi: 10.1016/j.pbb.2020.173090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkland AE, Browning BD, Green R, Leggio L, Meyerhoff DJ, Squeglia LM. Brain metabolite alterations related to alcohol use: a meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry. 2022;27:3223–36. [DOI] [PMC free article] [PubMed]
- 39.Bustillo JR. Use of proton magnetic resonance spectroscopy in the treatment of psychiatric disorders: a critical update. Dialogues Clin Neurosci. 2022;15:329–37. [DOI] [PMC free article] [PubMed]
- 40.Schmaal L, Veltman DJ, Nederveen A, Van Den Brink W, Goudriaan AE. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology/ 2012;37:2143–52. doi: 10.1038/npp.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coles LD, Tuite PJ, Öz G, Mishra UR, Kartha RV, Sullivan KM, et al. Repeated‐dose oral N‐acetylcysteine in Parkinson’s disease: pharmacokinetics and effect on brain glutathione and oxidative stress. J Clin Pharmacol. 2018;58:158–67. doi: 10.1002/jcph.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuura ROG, Warnock G, Ametamey S, Treyer V, Noeske R, Buck A, et al. Imaging glutamate redistribution after acute N-acetylcysteine administration: a simultaneous PET/MR study. NeuroImage. 2019;184:826–33. doi: 10.1016/j.neuroimage.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 43.Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–25. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 44.Koob GF. A role for GABA in alcohol dependence1. Adv Pharmacol. 2006;54:205–29. doi: 10.1016/S1054-3589(06)54009-8. [DOI] [PubMed] [Google Scholar]
- 45.Ozaras R, Tahan V, Aydin S, Uzun H, Kaya S, Senturk H. N-acetylcysteine attenuates alcohol-induced oxidative stress in the rat. World J Gastroenterol. 2003;9:125. doi: 10.3748/wjg.v9.i1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider R, Bandiera S, Souza DG, Bellaver B, Caletti G, Quincozes-Santos A, et al. N-acetylcysteine prevents alcohol related neuroinflammation in rats. Neurochem Res. 2017;42:2135–41. doi: 10.1007/s11064-017-2218-8. [DOI] [PubMed] [Google Scholar]
- 47.Liang J, Olsen RW. Alcohol use disorders and current pharmacological therapies: the role of GABA A receptors. Acta Pharmacol Sin. 2014;35:981–93. doi: 10.1038/aps.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27:113–24. doi: 10.1080/13543784.2018.1417383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uys JD, Mulholland PJ, Townsend DM. Glutathione and redox signaling in substance abuse. Biomed Pharmacother. 2014;68:799–807. doi: 10.1016/j.biopha.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the future national survey results on drug use, 1975-2020: overview, key findings on adolescent drug use. Institute for Social Research; 2021.
- 51.Johnston LD, O’Malley PM, Bachman JG. Monitoring the future: national results on adolescent drug use: overview of key findings. Focus. 2003;1:213–34. doi: 10.1176/foc.1.2.213. [DOI] [Google Scholar]
- 52.Miech RA, Johnston LD, O’malley PM, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975-2014. Volume 1, Secondary School Students. Institute for Social Research; 2015.
- 53.Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology. 2012;220:529–39. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winters KC. Commentary on O’Brien: substance use disorders in DSM-V when applied to adolescents. Addiction. 2011;106:882. doi: 10.1111/j.1360-0443.2010.03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 56.Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID) J Clin Psychiatry. 2010;71:17393. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- 57.Stoops WW, Strickland JC, Hays LR, Rayapati AO, Lile JA, Rush CR. Influence of n-acetylcysteine maintenance on the pharmacodynamic effects of oral ethanol. Pharmacol Biochem Behav. 2020;198:173037. doi: 10.1016/j.pbb.2020.173037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lau-Barraco C, Braitman AL, Linden-Carmichael AN, Stamates AL. Differences in weekday versus weekend drinking among nonstudent emerging adults. Exp Clin Psychopharmacol. 2016;24:100. doi: 10.1037/pha0000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomko RL, Gray KM, Oppenheimer SR, Wahlquist AE, McClure EA. Using REDCap for ambulatory assessment: Implementation in a clinical trial for smoking cessation to augment in-person data collection. Am J Drug Alcohol Abuse. 2019;45:26–41. doi: 10.1080/00952990.2018.1437445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. Measuring alcohol consumption: Psychosocial and biochemical methods. 1992:41–72.
- 61.Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J‐coupled metabolites. Magn Reson Med. 2008;60:964–9. doi: 10.1002/mrm.21696. [DOI] [PubMed] [Google Scholar]
- 62.Prisciandaro JJ, Mikkelsen M, Saleh MG, Edden RA. An evaluation of the reproducibility of 1H-MRS GABA and GSH levels acquired in healthy volunteers with J-difference editing sequences at varying echo times. Magn Reson Imaging. 2020;65:109–13. doi: 10.1016/j.mri.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Öngür D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, et al. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64:718–26. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frye MA, Hinton DJ, Karpyak VM, Biernacka JM, Gunderson LJ, Feeder SE, et al. Anterior cingulate glutamate is reduced by acamprosate treatment in patients with alcohol dependence. J Clin Psychopharmacol. 2016;36:669. doi: 10.1097/JCP.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echo‐planar techniques. Magn Reson Med. 2000;43:319–23. doi: 10.1002/(SICI)1522-2594(200002)43:2<319::AID-MRM22>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 66.Saleh MG, Rimbault D, Mikkelsen M, Oeltzschner G, Wang AM, Jiang D, et al. Multi-vendor standardized sequence for edited magnetic resonance spectroscopy. Neuroimage. 2019;189:425–31. doi: 10.1016/j.neuroimage.2019.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oeltzschner G, Zöllner HJ, Hui SC, Mikkelsen M, Saleh MG, Tapper S, et al. Osprey: open-source processing, reconstruction & estimation of magnetic resonance spectroscopy data. J Neurosci Methods. 2020;343:108827. doi: 10.1016/j.jneumeth.2020.108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zöllner HJ, Tapper S, Hui SC, Barker PB, Edden RA, Oeltzschner G. Comparison of linear combination modeling strategies for edited magnetic resonance spectroscopy at 3 T. NMR Biomed. 2022;35:e4618. doi: 10.1002/nbm.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210. doi: 10.1002/hbm.460020402. [DOI] [Google Scholar]
- 70.Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55:1219–26. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- 71.Smucny J, Carter CS, Maddock RJ. Medial prefrontal cortex glutamate is reduced in schizophrenia and moderated by measurement quality: a meta-analysis of 1H-MRS studies. Biol Psychiatry. 2021;90:643–51. [DOI] [PMC free article] [PubMed]
- 72.Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods Instrum Comput. 1996;28:1–11. doi: 10.3758/BF03203630. [DOI] [Google Scholar]
- 73.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 74.Lüdecke D, Ben-Shachar MS, Patil I, Waggoner P, Makowski D. performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw. 2021;6:3139.
- 75.Van Buuren S, Groothuis-Oudshoorn K, Robitzsch A. Package ‘mice’: multivariate imputation by chained equations. CRAN Repos. 2019.
- 76.Ben-Shachar MS, Lüdecke D, Makowski D. effectsize: Estimation of effect size indices and standardized parameters. J Open Source Softw. 2020;5:2815.
- 77.Kunzetsova A, Brockhoff P, Christensen R. lmerTest package: tests in linear mixed effect models. J Stat Softw. 2017;82:1–26. [Google Scholar]
- 78.Schulte M, Goudriaan A, Kaag A, Kooi D, Van Den Brink W, Wiers R, et al. The effect of N-acetylcysteine on brain glutamate and gamma-aminobutyric acid concentrations and on smoking cessation: a randomized, double-blind, placebo-controlled trial. J Psychopharmacol. 2017;31:1377–9. doi: 10.1177/0269881117730660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991;20:123–34. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
- 80.Marinkovic K, Myers ABA, Arienzo D, Sereno MI, Mason GF. Cortical GABA levels are reduced in young adult binge drinkers: association with recent alcohol consumption and sex. Neuroimage Clin. 2022;35:103091. doi: 10.1016/j.nicl.2022.103091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haass‐Koffler CL, Piacentino D, Li X, Long VM, Lee MR, Swift RM, et al. Differences in sociodemographic and alcohol‐related clinical characteristics between treatment seekers and nontreatment seekers and their role in predicting outcomes in the COMBINE study for alcohol use disorder. Alcohol: Clin Exp Res. 2020;44:2097–108. doi: 10.1111/acer.14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ray LA, Bujarski S, Yardley MM, Roche DJ, Hartwell EE. Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Abuse. 2017;43:703–10. doi: 10.1080/00952990.2017.1312423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyerhoff D, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, et al. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol: Clin Exp Res. 2004;28:650–61. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prisciandaro JJ, Schacht JP, Prescot AP, Brenner HM, Renshaw PF, Brown TR, et al. Intraindividual changes in brain GABA, glutamate, and glutamine during monitored abstinence from alcohol in treatment‐naive individuals with alcohol use disorder. Addict Biol. 2020;25:e12810. doi: 10.1111/adb.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prisciandaro JJ, Schacht JP, Prescot AP, Renshaw PF, Brown TR, Anton RF. Brain glutamate, GABA, and glutamine levels and associations with recent drinking in treatment‐naive individuals with alcohol use disorder versus light drinkers. Alcohol: Clin Exp Res. 2019;43:221–6. doi: 10.1111/acer.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prisciandaro JJ, Mellick W, Squeglia LM, Hix S, Arnold L, Tolliver BK. Results from a randomized, double‐blind, placebo‐controlled, crossover, multimodal‐MRI pilot study of gabapentin for co‐occurring bipolar and cannabis use disorders. Addict Biol. 2022;27:e13085. doi: 10.1111/adb.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abdallah CG, Niciu MJ, Fenton LR, Fasula MK, Jiang L, Black A, et al. Decreased occipital cortical glutamate levels in response to successful cognitive-behavioral therapy and pharmacotherapy for major depressive disorder. Psychother Psychosom. 2014;83:298–307. doi: 10.1159/000361078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Machado-Vieira R, Zanetti MV, Otaduy MC, De Sousa RT, Soeiro-de-Souza MG, Costa AC, et al. Increased brain lactate during depressive episodes and reversal effects by lithium monotherapy in drug-naive bipolar disorder: a 3T 1H-MRS study. J Clin Psychopharmacol. 2017;37:40. doi: 10.1097/JCP.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bolton J, Moore GJ, MacMillan S, Stewart CM, Rosenberg DR. Case study: caudate glutamatergic changes with paroxetine persist after medication discontinuation in pediatric OCD. J Am Acad Child Adolesc Psychiatry. 2001;40:903–6. doi: 10.1097/00004583-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 90.Pollack MH, Jensen JE, Simon NM, Kaufman RE, Renshaw PF. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: response to treatment with levetiracetam. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:739–43. doi: 10.1016/j.pnpbp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 91.de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Jung-Cook H, et al. Prefrontal and striatal gamma-aminobutyric acid levels and the effect of antipsychotic treatment in first-episode psychosis patients. Biol Psychiatry. 2018;83:475–83. [DOI] [PMC free article] [PubMed]
- 92.Ueno F, Nakajima S, Iwata Y, Honda S, Torres‐Carmona E, Mar W, et al. GABA levels in the midcingulate cortex and clozapine response in patients with treatment‐resistant schizophrenia: a 1H‐MRS study. Psychiatry Clin Neurosci. 2022;76:587–94. [DOI] [PubMed]
- 93.Back SE, McCauley JL, Korte KJ, Gros DF, Leavitt V, Gray KM, et al. A double-blind, randomized, controlled pilot trial of N-acetylcysteine in veterans with posttraumatic stress disorder and substance use disorders. J Clin Psychiatry. 2016;77:12406. doi: 10.4088/JCP.15m10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi IY, Andronesi OC, Barker P, Bogner W, Edden RA, Kaiser LG, et al. Spectral editing in 1H magnetic resonance spectroscopy: experts’ consensus recommendations. NMR Biomed. 2021;34:e4411. doi: 10.1002/nbm.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu X-L, Li L, Li J-N, Rong J-H, Liu B, Hu Z-X. Reliability of glutamate quantification in human nucleus accumbens using proton magnetic resonance spectroscopy at a 70-cm wide-bore clinical 3T MRI system. Front Neurosci. 2017;11:686. doi: 10.3389/fnins.2017.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gunn CM, Pankowska M, Harris M, Helsing E, Battaglia TA, Bagley SM. The representation of females in clinical trials for substance use disorder conducted in the United States (2010–19). Addiction. 2022;117:2583–90. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.