Abstract
Photocatalytic carboxylation of alkenes with CO2 is a promising and sustainable strategy to synthesize high value-added carboxylic acids. However, it is challenging and rarely investigated for unactivated alkenes due to their low reactivities. Herein, we report a visible-light photoredox-catalyzed arylcarboxylation of unactivated alkenes with CO2, delivering a variety of tetrahydronaphthalen-1-ylacetic acids, indan-1-ylacetic acids, indolin-3-ylacetic acids, chroman-4-ylacetic acids and thiochroman-4-ylacetic acids in moderate-to-good yields. This reaction features high chemo- and regio-selectivities, mild reaction conditions (1 atm, room temperature), broad substrate scope, good functional group compatibility, easy scalability and facile derivatization of products. Mechanistic studies indicate that in situ generation of carbon dioxide radical anion and following radical addition to unactivated alkenes might be involved in the process.
Subject terms: Synthetic chemistry methodology, Photocatalysis
Despite the importance of polycyclic carboxylic acids and derivatives in various fields, general methods for the arylcarboxylation of alkenes with CO2 remain elusive. Here, the authors transform unactivated alkenes into high value-added polycyclic carboxylic acids and derivatives via visible-light photoredox-catalysis, using CO2 as the one-carbon feedstock.
Introduction
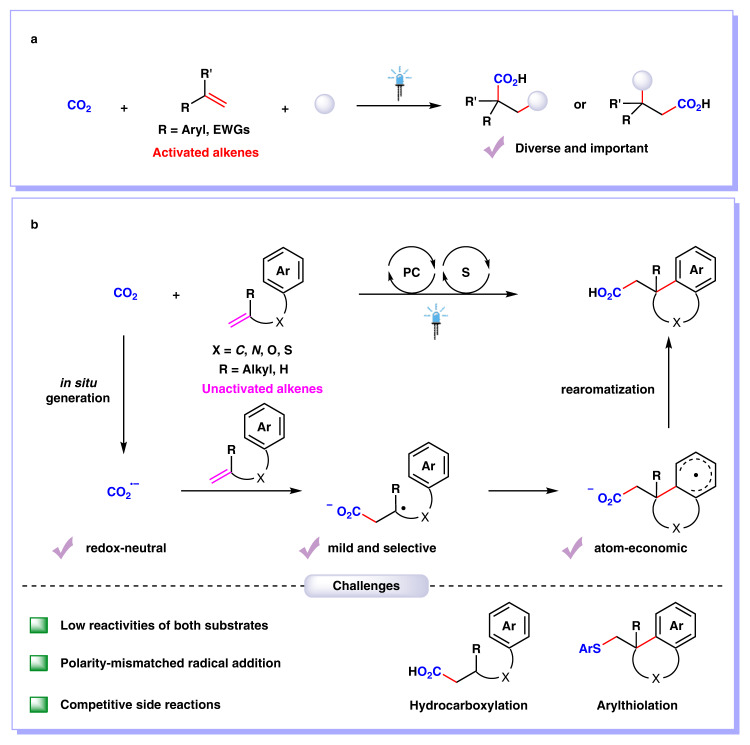
Carbon dioxide (CO2), which is inexpensive, non-toxic, and recyclable, has been regarded as an ideal one-carbon feedstock to engage in chemical transformations for the synthesis of high value-added chemicals1–4. As carboxylic acids are a privileged functional group in biochemistry and polymer chemistry, it is highly important to develop direct and flexible methods for carboxylation with CO25–9. In recent years, visible-light photocatalytic carboxylation with CO2 has attracted much attention as an efficient, versatile, and sustainable strategy10–15. As alkenes are common functional group in organic compounds and bulk chemicals in industry, visible-light photocatalytic carboxylation of alkenes with CO2 is of particular interest16–29. Notably, visible-light photoredox-catalyzed difunctionalizing carboxylation of alkenes with CO2 has recently emerged as an important access to valuable carboxylic acids with diverse functionality and high step economy22–29. Many groups, including Martin, Wu, Li, Xi, and our group, have reported visible-light photoredox-catalyzed 1,2-difunctionalizing carboxylation of alkenes with CO2 under mild conditions in high chemo- and regio-selectivities (Fig. 1a)22–29. However, these methods are mainly limited to activated alkenes, such as styrenes and acrylates. The photocatalytic 1,2-difunctionalizing carboxylation of unactivated alkenes with CO2 has not been disclosed yet.
Fig. 1. Visible-light photocatalytic 1,2-difunctionalizing carboxylation of alkenes with CO2.
a Visible-light photocatalytic 1,2-difunctionalizing carboxylation of activated alkenes with CO2. b Visible-light photocatalytic arylcarboxylation of unactivated alkenes with CO2. PC photocatalyst, EWGs electron-withdrawing groups.
As well known, unactivated alkenes are more abundant and easily available in nature and industry than activated alkenes. However, it is challenging for unactivated alkenes to undergo photocatalytic carboxylations with CO230–33, arising from high reductive potentials of both starting materials34–39 and sluggish radical addition onto unactivated alkenes to generate alkyl carbon radicals40–49, which are less stable than those from activated alkenes. Inspired by our recent work on hydrocarboxylation of unactivated alkenes with CO233, we further challenged us whether we could tune the chemoselectivity from C−H to C−C bonds formation based on similar carbon radical intermediates (Fig. 1b). We hypothesized the in situ generation of CO2 radical anion (CO2•−) and following radical addition to unactivated alkenes would result in unstabilized alkyl carbon radicals, which could be further trapped by arenes to generate the C−C bonds. Final rearomatization could give the desired arylcarboxylation products. If successful, it will realize 1,2-difunctionalizing carboxylation of unactivated alkenes with CO2. Moreover, as it is redox-neutral and atom-economic based on the C−H functionalization, it will also provide a practical and sustainable strategy to access a wide range of polycyclic carboxylic acids, which are highly important but not easy to obtain via other methods (Fig. 2). Nevertheless, many challenges remain. For example, it is challenging for conversion of CO2 into CO2•− due to the high reduction potential of CO2 [E1/2 (CO2/CO2•−) = −2.21 V vs SCE]50. Moreover, the addition of nucleophilic CO2•− to electron-rich unactivated alkenes is a polarity-mismatched process51. In addition, hydrocarboxylation, arylthiolation, and other competitive side reactions would also hamper the desired difunctionalizing carboxylation.
Fig. 2. Selected biologically active carboxylic acids and derivatives bearing polycyclic structures.
Examples of biologically active compounds possessing polycyclic acids and derivatives motifs.
Herein, we report our success in realizing the visible-light photoredox-catalyzed arylcarboxylation of unactivated alkenes with CO2 (Fig. 1b). A variety of tetrahydronaphthalen-1-ylacetic acids, indan-1-ylacetic acids, indolin-3-ylacetic acids, chroman-4-ylacetic acids and thiochroman-4-ylacetic acids are generated in high selectivities and moderate-to-good yields.
Results
Screening of reaction conditions
As carboxylic acids with polycyclic structures are widely found in natural products, drugs and bioactive compounds (Fig. 2)52–56, we initiated our project with 1a as standard substrate to generate tetrahydronaphthalen-1-ylacetic acid 2a as the desired product (Table 1). In the presence of fac-Ir(ppy)3 (Ir-1) as photocatalyst, 4-tert-butylthiophenol (T1) as hydrogen atom transfer (HAT) catalyst and Cs2CO3 as base (Please see the Supplementary Tables 1–5 in Supplementary Information (SI) for more details), the desired arylcarboxylation product 2a was obtained in 66% yield with high selectivity (Entry 1). Control experiments revealed that photocatalyst, thiol catalyst, Cs2CO3, visible light, and CO2 all played essential roles in the reaction (Entries 2–6). The use of p-tBuC6H4SK (T2) instead of p-tBuC6H4SH (T1) provided 2a in comparable yield (Entry 7). To our delight, PhMe2SiH turned to be a good additive that enhanced the yield of 2a to 86%, probably owing to the promotion of the CO2•− generation in the reaction (Entry 8)57. A variety of reaction conditions with other photocatalysts, solvents, HAT catalysts, bases, and silanes were also tested to give lower conversions and yields (Entries 9–14).
Table. 1.
Optimization of reaction conditionsa
 | ||
|---|---|---|
| Entry | Variations | Yield (%)b |
| 1 | none | 66 (62) |
| 2 | w/o Ir-1 | n.d. |
| 3 | w/o T1 | n.d. |
| 4 | w/o Cs2CO3 | n.d. |
| 5 | w/o light | n.d. |
| 6 | N2 instead of CO2 | n.d. |
| 7 | T2 instead of T1 | 62 |
| 8 | PhMe2SiH as an additive | 86 (83) |
| 9c | Ir-2 instead of Ir-1 | 60 |
| 10c | 4CzIPN instead of Ir-1 | n.d. |
| 11c | DMF instead of DMSO | 55 |
| 12c | tBuSH instead of T1 | 74 |
| 13c | K2CO3 instead of Cs2CO3 | 68 |
| 14c | PMHS instead of PhMe2SiH | 82 |
n.d. not detected, DMSO dimethyl sulfoxide, DMF N,N-dimethylformamide, ppy 2-phenylpyridine, dtbbpy 4,4’-di-tert-butyl-2,2’-bipyridine, 4CzIPN 2,4,5,6-tetra(carbazol-9-yl)isophthalonitrile, PMHS poly(methylhydrosiloxane).
aReaction conditions: 1a (0.2 mmol, 1.0 equiv), Ir-1 (1 mol%), T1 (20 mol%), Cs2CO3 (3.0 equiv.), DMSO (2 mL), irradiation by 30 W blue LEDs at room temperature (rt) under CO2 (1 atm) for 24 h.
bYield determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. Isolated yields in parentheses.
cPhMe2SiH (1.0 equiv.) was used.
Substrate scope
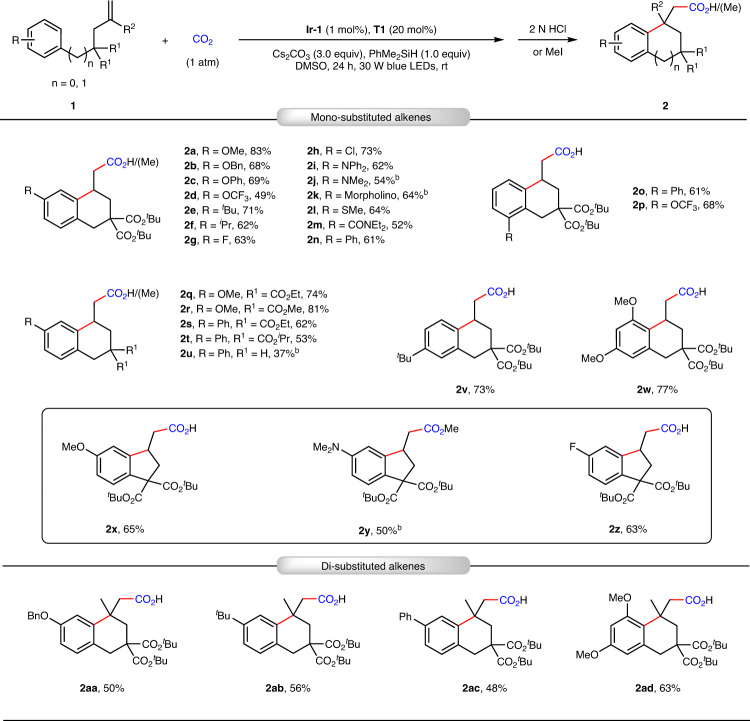
Having established the optimized reaction conditions, we investigated the substrate scope (Fig. 3). A wide variety of electron-donating groups (EDGs) and EWGs were tolerant at the para-positions of the arene moiety, providing the desired products 2a–2n in moderate-to-good yields. Substrates containing various functional groups, such as trifluoromethoxyl group (2d), fluoro (2g), amines (2i–2k), thioether (2l) and amide (2m), were smoothly converted to the corresponding products, thus allowing for downstream transformations. The efficiency of this protocol was not hampered by the ortho substituents on the phenyl ring, giving the corresponding arylcarboxylation products 2o–2p in moderate-to-good yields. Substrates with different substituents on the aliphatic chain were also suitable for such a transformation, furnishing products 2q–2t in 53–81% yields. When no ester group was present in the substrate, the carboxylative cyclization product 2u could also be obtained. To our delight, substrate 1v with tert-butyl group at the meta-position of the phenyl ring was tested in this reaction to give product 2v in 73% yield and sole regioselectivity owing to the steric hindrance effect. The substrate 1w bearing di-methoxyl groups also underwent the reaction smoothly to afford the arylcarboxylation product 2w in 77% yield. We were delighted to find that 5-exo cyclization process could also occur under such conditions, giving the indan-1-ylacetic acids 2x–2z in moderate-to-good yields. We next turned our attention to 1,1-disubstituted unactivated alkenes as CO2 coupling partners, which have rarely been used for photocatalytic cyclization reactions58. To our delight, this system also accomplished the 6-exo cyclizations to furnish carbocycles 2aa-2ad containing the quaternary carbon centers in 48–63% yields.
Fig. 3. Arylcarboxylation of unactivated alkenes with CO2 to construct tetrahydronaphthalen-1-ylacetic acid and indan-1-ylacetic acid derivatives.
aStandard reaction conditions (Table 1, Entry 8) with yields of isolated carboxylic acids or methyl esters. bEsterification by MeI (0.4 mmol, 2.0 equiv.), 65 °C, 3 h.
As indoline derivatives are privileged structural motifs found in alkaloids59 and clinical drugs60, seeking an efficient and simple approach for the construction of indolines is of continuous interest. Encouraged by the above results, we further turned our attention to selective carboxylation of N-protected allylanilines 3 with CO2 to afford indolin-3-ylacetic acid derivatives 4 (Fig. 4). Mono-substituents on the aromatic ring had a negligible impact on these reactions, as the corresponding indoline derivatives 4a–4g were obtained in satisfactory yields. Further investigations of the substrate scope showed that di- or tri-substituted N-protected allylanilines also delivered the corresponding indolin-3-ylacetic acid derivatives 4 h and 4i in synthetically useful yields.
Fig. 4. Arylcarboxylation of unactivated alkenes with CO2 to construct indolin-3-ylacetic acid derivatives.
aStandard reaction conditions (Table 1, Entry 8) with yields of isolated methyl esters.
Inspired by above results, we wondered whether other kinds of valuable polycyclic carboxylic acids could be formed using this strategy. As chromanes and thiochromanes are widely distributed in nature and display a broad range of biological and pharmaceutical activities61–63, we further tested phenol- and thiophenol-derived alkenes 5 under standard reaction conditions. Fortunately, these substrates were also reactive to furnish the desired chroman-4-ylacetic acid and thiochroman-4-ylacetic acid derivatives 6a–6d in 21–65% yields (Fig. 5).
Fig. 5. Arylcarboxylation of unactivated alkenes with CO2 to construct chroman-4-ylacetic acid and thiochromane-4-ylacetic acid derivatives.
aStandard reaction conditions (Table 1, Entry 8) with yields of isolated methyl esters.
Synthetic applications
In order to demonstrate the utility of this method, a gram-scale reaction and product derivatizations were performed (Fig. 6). The product 2a was obtained in 84% yield and gram scale, demonstrating the facile scalability of this reaction (Fig. 6a). Then, we carried out the derivatization of 2a to illustrate potential synthetic applications (Fig. 6b). Selective reduction of product 2a by using NaBH4 produced the alcohol 7 in 92% yield64. Condensation between 2a and methyl glycinate hydro-chloride gave cyclic amide 8 in an excellent yield65. A practical decarboxylation of primary carboxylic acid 2a via synergistic photoredox and HAT catalysis was achieved in excellent yield66. And 2a could also participate in decarboxylative trifluoromethylation to give compound 10 in moderate yield67. Notably, compound 2a was easily transformed to the redox-active ester 1168, which underwent C−P and C−S bonds formation through decarboxylative phosphination69 and arylthiolation70, respectively.
Fig. 6. Synthetic applications.
a Gram-scale reaction. b Product derivatizations. Please see SI for experimental details. Gly-OMe·HCl glycine methyl ester hydrochloride. HOBt 1-hydroxybenzotriazole, EDCI 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, NPhth phthalimidyl, BTMG 2-tert-butyl-1,1,3,3-tetramethylguanidine, DMAP 4-dimethylaminopyridine. DCC Dicyclohexylcarbodiimide, PMDTA Pentamethyldiethylenetriamine.
Mechanistic investigations
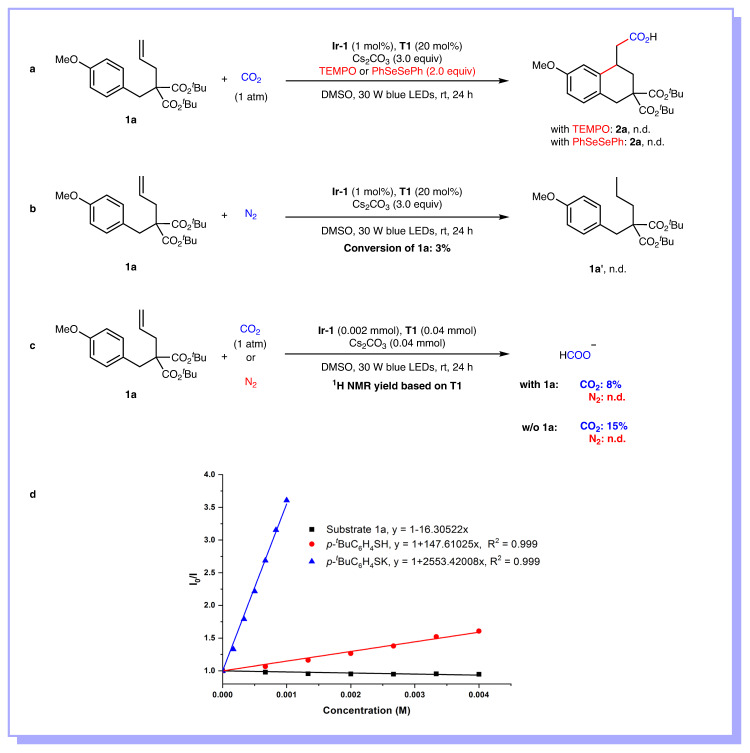
To gain more insight into this reaction, a series of control experiments were conducted (Fig. 7). When the reaction was performed in the presence of various radical scavengers, such as 2,2,6,6-tetramethyl-1-piperidiny-1-oxy (TEMPO) or diphenyldiselenide (PhSeSePh), the formation of product 2a was completely inhibited with almost full recovery of 1a, indicating that radical process might be involved (Fig. 7a). As the formation of reduction product 1a’ was not observed under nitrogen atmosphere, we believed that unactivated alkenes could not be reduced in the reaction (Fig. 7b). The results of detecting of formate (HCO2−) in the presence or absence of unactivated alkenes indicated that CO2•− could be generated from single electron reduction of CO2 in the reaction (Fig. 7c). Moreover, Stern-Volmer fluorescence quenching experiments showed that the excited state of the photocatalyst was quenched by the thiolate rather than unactivated alkenes (Fig. 7d).
Fig. 7. Mechanistic investigations.
a Radical trapping experiments. b Reduction of unactivated alkene 1a. c Detection of formate. d Stern-Volmer fluorescence quenching experiments.
Based on the control experiments and previous studies71–73, a possible mechanism for the overall transformation of 1a is proposed (Fig. 8). The irradiation of photocatalyst fac-IrIII(ppy)3 generates excited fac-*IrIII(ppy)3 (E1/2*III/II = +0.31 V vs SCE), which can be reductively quenched by a catalytic thiolate to furnish fac-IrII(ppy)3 and a thiyl radical. Then, the IrII species (E1/2III/II = −2.19 V vs SCE)72 may engage in reducing CO2 [E1/2 (CO2/CO2•−) = −2.21 V vs SCE]50 via SET event to deliver CO2•− along with regeneration of fac-IrIII(ppy)3 to close the photoredox catalytic cycle. The in situ generated CO2•− then undergoes radical addition to the C = C double bond of unactivated alkene of 1a to form an alkyl carbon radical A30,33, which is supposed to be quickly captured via cyclization to form the radical intermediate B. Finally, the carboxylate could be obtained via a HAT process of radical intermediate B with the thiyl radical, along with regeneration of the thiol catalyst74. The protonation during workup would afford the final product 2a. Meanwhile, the intermediate B might also undergo intermolecular HAT to deliver anti-Markovnikov hydrocarboxylation byproduct C33. In addition, we reason that the silane can serve as an additive to promote the generation of CO2•− from an alternative pathway (Please see Supplementary Fig. 18 in SI) 57. At this stage, we could not exclude other alternative pathways (Please see SI for details)75,76.
Fig. 8. Proposed mechanism.
Proposed catalytic cycle for this synergistic catalyzed arylcarboxylation of unactivated alkenes with CO2.
Discussion
In summary, we have developed the visible-light photoredox-catalyzed arylcarboxylation of unactivated alkenes with CO2. This protocol provides an efficient and facile approach to an array of high-valued polycyclic carboxylic acids, such as tetrahydronaphthalen-1-ylacetic acids, indan-1-ylacetic acids, indolin-3-ylacetic acids, chroman-4-ylacetic acids and thiochroman-4-ylacetic acids. This reaction features mild reaction conditions, broad substrate scope, and good functional group compatibility. Moreover, the derivatization of products could afford diverse valuable polycyclic compounds, which are difficult to access via other protocols. Further applications of CO2•− and difunctionalizing carboxylation of unactivated alkenes are undergoing in our group.
Methods
Synthesis of 2a-2z
To an oven-dried Schlenk tube (25 mL) equipped with a magnetic stir bar was added the unactivated alkenes (0.2 mmol, 1.0 equiv. for solid substrates) and fac-Ir(ppy)3 (1 mol%). The tube was moved into the glovebox where was added the Cs2CO3 (0.6 mmol, 195.5 mg, 3.0 equiv.). The tube was sealed and removed from the glovebox, then evacuated and back-filled with CO2 atmosphere for three times. liquid alkenes were added under CO2 atmosphere followed by anhydrous DMSO (2 mL), PhMe2SiH (0.2 mmol, 27.3 mg, 31 μL, 1.0 equiv.), 4-tert-butylthiophenol (0.04 mol, 6.7 mg, 7.0 μL, 20 mol%), and the tube was sealed at atmospheric pressure of CO2 (1 atm). The reaction was stirred and irradiated with a 30 W blue LED lamp (1 cm away, with a cooling fan to keep the reaction temperature at 25–30 °C and keeping the reaction region located in the center of LEDs lamp) for 24 h. Upon completion of the reaction, the reaction mixture was diluted with 3 mL ethyl ester (EA) and quenched by 3 mL 2 N HCl. After adding 10 mL of H2O, the mixture was extracted by EA for five times and the combined organic phases were concentrated in vacuo. The residue was purified by silica gel flash column chromatography (Petroleum/EA/AcOH 10/1/ ~ 5/1 ~ /5/10.2%) to give the pure desired product.
Synthesis of 2aa-2ad
To an oven-dried Schlenk tube (25 mL) equipped with a magnetic stir bar was added the unactivated alkenes (0.2 mmol, 1.0 equiv. for solid substrates) and fac-Ir(ppy)3 (1 mol%). The tube was moved into the glovebox where was added the Cs2CO3 (0.6 mmol, 195.5 mg, 3.0 equiv.). The tube was sealed and removed from the glovebox, then evacuated and back-filled with CO2 atmosphere for three times. liquid alkenes were added under CO2 atmosphere followed by anhydrous DMSO (2 mL), PhMe2SiH (0.2 mmol, 27.3 mg, 31 μL, 1.0 equiv.), 4-tert-butylthiophenol (0.04 mol, 6.7 mg, 7.0 μL, 20 mol%), and the tube was sealed at atmospheric pressure of CO2 (1 atm). The reaction was stirred and irradiated with a 30 W blue LED lamp (1 cm away, with a cooling fan to keep the reaction temperature at 25–30 °C and keeping the reaction region located in the center of LEDs lamp) for 24 h. Upon completion of the reaction, the reaction mixture was diluted with 3 mL EA and quenched by 3 mL 2 N HCl. After adding 10 mL of H2O, the mixture was extracted by EA for five times and the combined organic phases were concentrated in vacuo. The residue was purified by silica gel flash column chromatography (Petroleum/EA/AcOH 10/1/ ~ 5/1 ~ /5/10.2%) to give the pure desired product.
Synthesis of 4a-4i
To an oven-dried Schlenk tube (25 mL) equipped with a magnetic stir bar was added the unactivated alkenes (0.2 mmol, 1.0 equiv. for solid substrates) and fac-Ir(ppy)3 (1 mol%). The tube was moved into the glovebox where was added the Cs2CO3 (0.6 mmol, 195.5 mg, 3.0 equiv.). The tube was sealed and removed from the glovebox, then evacuated and back-filled with CO2 atmosphere for three times. liquid alkenes were added under CO2 atmosphere followed by anhydrous DMSO (2 mL), PhMe2SiH (0.2 mmol, 27.3 mg, 31 μL, 1.0 equiv.), 4-tert-butylthiophenol (0.04 mol, 6.7 mg, 7.0 μL, 20 mol%), and the tube was sealed at atmospheric pressure of CO2 (1 atm). The reaction was stirred and irradiated with a 30 W blue LED lamp (1 cm away, with a cooling fan to keep the reaction temperature at 25–30 °C and keeping the reaction region located in the center of LEDs lamp) for 24 h. Upon completion of the reaction, MeI (0.4 mmol, 25 μL, 2.0 equiv.) was added, the mixture was stirred at 65 oC for 3 h and then cooled to room temperature. The crude reaction mixture was diluted with 3 mL EA. After adding 10 mL of H2O, the mixture was extracted by EA for five times and the combined organic phases were concentrated in vacuo. The residue was purified by silica gel flash column chromatography (Petroleum/EA 60/1/ ~ 20/1) to give the pure desired product.
Synthesis of 6a–6d
To an oven-dried Schlenk tube (25 mL) equipped with a magnetic stir bar was added the unactivated alkenes (0.2 mmol, 1.0 equiv. for solid substrates) and fac-Ir(ppy)3 (1 mol%). The tube was moved into the glovebox where was added the Cs2CO3 (0.6 mmol, 195.5 mg, 3.0 equiv.). The tube was sealed and removed from the glovebox, then evacuated and back-filled with CO2 atmosphere for three times. liquid alkenes were added under CO2 atmosphere followed by anhydrous DMSO (2 mL), PhMe2SiH (0.2 mmol, 27.3 mg, 31 μL, 1.0 equiv.), 4-tert-butylthiophenol (0.04 mol, 6.7 mg, 7.0 μL, 20 mol%), and the tube was sealed at atmospheric pressure of CO2 (1 atm). The reaction was stirred and irradiated with a 30 W blue LED lamp (1 cm away, with a cooling fan to keep the reaction temperature at 25–30 °C and keeping the reaction region located in the center of LEDs lamp) for 24 h. Upon completion of the reaction, MeI (0.4 mmol, 25 μL, 2.0 equiv.) was added, the mixture was stirred at 65 oC for 3 h and then cooled to room temperature. The crude reaction mixture was diluted with 3 mL EA. After adding 10 mL of H2O, the mixture was extracted by EA for five times and the combined organic phases were concentrated in vacuo. The residue was first purified by silica gel flash column chromatography (Petroleum/EA 150/1/ ~ 60/1) to give the mixture and the yields were determined with CH2Br2 as an internal standard. The desired arylcarboxylation products were further purified by preparative HPLC.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Prof. Yu Lan for helpful discussions. Financial support is provided by the National Natural Science Foundation of China (22225106, for D.G.Y. 22101191, for W.Z. and 22201027 for L.L.L.), Sichuan Science and Technology Program (20CXTD0112, for D.G.Y. and 2021YJ0405 for W.Z.), Fundamental Research Funds from Sichuan University (2020SCUNL102). W.Z. was supported by the China Postdoctoral Science Foundation (2021M692261). We thank Central Government Funds of Guiding Local Scientific and Technological Development for Sichuan Province (2021ZYD0063) and the Fundamental Research Funds for the Central Universities. We also thank Xiaoyan Wang from the Analysis and Testing Center of Sichuan University as well as Jing Li, Qinfang Zhang, and Dongyan Deng from College of Chemistry at Sichuan University for compound testing.
Author contributions
D.G.Y. and J.H.Y. conceived and designed the study. W.Z., Z.C., Y.X.J., L.L.L., and W.W. performed the experiments, mechanistic studies and wrote the manuscript. All authors contributed to the analysis and interpretation of the data.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. Extra data are available from the author upon request. The Cartesian coordinates for the calculated structures are available within the Supplementary Data 1.
Competing interests
The authors declare the following competing financial interest(s): A Chinese Patent on this work has been applied with the number (202310600327.1). The authors declare no other competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jian-Heng Ye, Email: jhye@scu.edu.cn.
Da-Gang Yu, Email: dgyu@scu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-39240-8.
References
- 1.Aresta, M. Carbon dioxide as Chemical Feedstock. (Wiley-VCH, Weinheim, 2010).
- 2.Lu XB, Ren WM, Wu GP. CO2 copolymers from epoxides: catalyst activity, product selectivity, and stereochemistry control. Acc. Chem. Res. 2012;45:1721–1735. doi: 10.1021/ar300035z. [DOI] [PubMed] [Google Scholar]
- 3.Liu Q, Wu L, Jackstell R, Beller M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015;6:5933. doi: 10.1038/ncomms6933. [DOI] [PubMed] [Google Scholar]
- 4.He M, Sun Y, Han B. Green carbon science: efficient carbon resource processing, utilization, and recycling towards carbon neutrality. Angew. Chem. Int. Ed. 2022;61:e202112835. doi: 10.1002/anie.202112835. [DOI] [PubMed] [Google Scholar]
- 5.Maag, H. Prodrugs of Carboxylic Acids (Springer, New York, 2007).
- 6.Gooβen L, Rodríguez JN, Gooβen K. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed. 2008;47:3100–3120. doi: 10.1002/anie.200704782. [DOI] [PubMed] [Google Scholar]
- 7.Hong J, Li M, Zhang J, Sun B, Mo F. C−H bond carboxylation with carbon dioxide. ChemSusChem. 2019;12:6–39. doi: 10.1002/cssc.201802012. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Li Z, Takimoto M, Hou Z. Carboxylation reactions with carbon dioxide using N-heterocyclic carbene-copper catalysts. Chem. Rec. 2020;20:494–512. doi: 10.1002/tcr.201900060. [DOI] [PubMed] [Google Scholar]
- 9.Tortajada A, Börjesson M, Martin R. Nickel-catalyzed reductive carboxylation and amidation reactions. Acc. Chem. Res. 2021;54:3941–3952. doi: 10.1021/acs.accounts.1c00480. [DOI] [PubMed] [Google Scholar]
- 10.Cao Y, He X, Wang N, Li H-R, He L-N. Photochemical and electrochemical carbon dioxide utilization with organic compounds. Chin. J. Chem. 2018;36:644–659. doi: 10.1002/cjoc.201700742. [DOI] [Google Scholar]
- 11.Yeung CS. Photoredox catalysis as a strategy for CO2 incorporation: direct access to carboxylic acids from a renewable feedstock. Angew. Chem. Int. Ed. 2019;58:5492–5502. doi: 10.1002/anie.201806285. [DOI] [PubMed] [Google Scholar]
- 12.He X, Qiu L-Q, Wang W-J, Chen K-H, He L-N. Photocarboxylation with CO2: an appealing and sustainable strategy for CO2 fixation. Green. Chem. 2020;22:7301–7320. doi: 10.1039/D0GC02743J. [DOI] [Google Scholar]
- 13.Fan Z, Zhang Z, Xi C. Light-mediated carboxylation using carbon dioxide. ChemSusChem. 2020;13:6201–6218. doi: 10.1002/cssc.202001974. [DOI] [PubMed] [Google Scholar]
- 14.Cai B, Cheo HW, Liu T, Wu J. Light-promoted organic transformations utilizing carbon-based gas molecules as feedstocks. Angew. Chem. Int. Ed. 2021;60:2–33. doi: 10.1002/anie.202010710. [DOI] [PubMed] [Google Scholar]
- 15.Ye J-H, Ju T, Huang H, Liao L-L, Yu D-G. Radical carboxylative cyclizations and carboxylations with CO2. Acc. Chem. Res. 2021;54:2518–2531. doi: 10.1021/acs.accounts.1c00135. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, et al. Radical-type difunctionalization of alkenes with CO2. Acta Chim. Sin. 2019;77:783. doi: 10.6023/A19060208. [DOI] [Google Scholar]
- 17.Bertuzzi G, Cerveri A, Lombardi L, Bandini M. Tandem functionalization-carboxylation reactions of π-systems with CO2. Chin. J. Chem. 2021;39:3116–3126. doi: 10.1002/cjoc.202100450. [DOI] [Google Scholar]
- 18.Murata K, Numasawa N, Shimomaki K, Takaya J, Iwasawa N. Construction of a visible light-driven hydrocarboxylation cycle of alkenes by the combined use of Rh(I) and photoredox catalysts. Chem. Commun. 2017;53:3098–3101. doi: 10.1039/C7CC00678K. [DOI] [PubMed] [Google Scholar]
- 19.Meng Q-Y, Wang S, Huff GS, Konig B. Ligand controlled regioselective hydrocarboxylation of styrenes with CO2 by combining visible light and nickel catalysis. J. Am. Chem. Soc. 2018;140:3198–3201. doi: 10.1021/jacs.7b13448. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, et al. Visible light-driven anti-markovnikov hydrocarboxylation of acrylates and styrenes with CO2. CCS Chem. 2021;3:1746–1756. doi: 10.31635/ccschem.020.202000374. [DOI] [Google Scholar]
- 21.Jin Y, Caner J, Nishikawa S, Toriumi N, Iwasawa N. Catalytic direct hydrocarboxylation of styrenes with CO2 and H2. Nat. Commun. 2022;13:7584. doi: 10.1038/s41467-022-35293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yatham VR, Shen Y, Martin R. Catalytic intermolecular dicarbofunctionalization of styrenes with CO2 and radical precursors. Angew. Chem. Int. Ed. 2017;56:10915–10919. doi: 10.1002/anie.201706263. [DOI] [PubMed] [Google Scholar]
- 23.Ye J-H, et al. Visible-light-driven iron-promoted thiocarboxylation of styrenes and acrylates with CO2. Angew. Chem. Int. Ed. 2017;56:15416–15420. doi: 10.1002/anie.201707862. [DOI] [PubMed] [Google Scholar]
- 24.Hou J, et al. Visible-light-mediated metal-free difunctionalization of alkenes with CO2 and silanes or C(sp3)-H alkanes. Angew. Chem. Int. Ed. 2018;57:17220–17224. doi: 10.1002/anie.201811266. [DOI] [PubMed] [Google Scholar]
- 25.Fu Q, et al. Transition metal-free phosphonocarboxylation of alkenes with carbon dioxide via visible-light photoredox catalysis. Nat. Commun. 2019;10:3592. doi: 10.1038/s41467-019-11528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Gao Y, Zhou C, Li G. Visible-light-driven reductive carboarylation of styrenes with CO2 and aryl halides. J. Am. Chem. Soc. 2020;142:8122–8129. doi: 10.1021/jacs.0c03144. [DOI] [PubMed] [Google Scholar]
- 27.Ju T, et al. Dicarboxylation of alkenes, allenes, and (hetero)arenes with CO2 via visible-light photoredox catalysis. Nat. Catal. 2021;4:304–311. doi: 10.1038/s41929-021-00594-1. [DOI] [Google Scholar]
- 28.Liao L-L, et al. α-Amino acids and peptides as bifunctional reagents: carbocarboxylation of activated alkenes via recycling CO2. J. Am. Chem. Soc. 2021;143:2812–2821. doi: 10.1021/jacs.0c11896. [DOI] [PubMed] [Google Scholar]
- 29.Zhang B, Yi Y, Wu Z-Q, Chen C, Xi C-J. Photoredox-catalyzed dicarbofunctionalization of styrenes with amines and CO2: a convenient access to γ-amino acids. Green. Chem. 2020;22:5961–5965. doi: 10.1039/D0GC02254C. [DOI] [Google Scholar]
- 30.Morgenstern, D. A., Wittrig, R. E., Fanwick, P. E. & Kubiak, C. P. Photoreduction of carbon dioxide to its radical anion by [Ni3(μ3-I)2(dppm)3]: formation of two carbon–carbon bonds via addition of CO2•- to cyclohexene. J. Am. Chem. Soc. 115, 6470–6471 (1993).
- 31.Song L, et al. Visible-light photoredox-catalyzed remote difunctionalizing carboxylation of unactivated alkenes with CO2. Angew. Chem. Int. Ed. 2020;59:21121–21128. doi: 10.1002/anie.202008630. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Sakurazawa Y, Iwai A, Iwasawa N. Catalytic synthesis of a methylmalonate salt from ethylene and carbon dioxide through photoinduced activation and photoredox-catalyzed reduction of nickelalactones. ACS Catal. 2022;12:3776–3781. doi: 10.1021/acscatal.2c01053. [DOI] [Google Scholar]
- 33.Song L, et al. Visible-light photocatalytic di-and hydro-carboxylation of unactivated alkenes with CO2. Nat. Catal. 2022;5:832–838. doi: 10.1038/s41929-022-00841-z. [DOI] [Google Scholar]
- 34.Liao L-L, Song L, Yan S-S, Ye J-H, Yu D-G. Highly reductive photocatalytic systems in organic synthesis. Trend Chem. 2022;4:512–527. doi: 10.1016/j.trechm.2022.03.008. [DOI] [Google Scholar]
- 35.Seo H, Katcher MH, Jamison TF. Photoredox activation of carbon dioxide for amino acid synthesis in continuous flow. Nat. Chem. 2017;9:453–456. doi: 10.1038/nchem.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo H, Liu A, Jamison TF. Direct β-selective hydrocarboxylation of styrenes with CO2 enabled by continuous flow photoredox catalysis. J. Am. Chem. Soc. 2017;139:13969–13972. doi: 10.1021/jacs.7b05942. [DOI] [PubMed] [Google Scholar]
- 37.Alektiar SN, Wickens ZK. Photoinduced hydrocarboxylation via thiol-catalyzed delivery of formate across activated alkenes. J. Am. Chem. Soc. 2021;143:13022–13028. doi: 10.1021/jacs.1c07562. [DOI] [PubMed] [Google Scholar]
- 38.Kang G, Romo D. Photocatalyzed, β-selective hydrocarboxylation of α,β-unsaturated esters with CO2 under flow for β-lactone synthesis. ACS Catal. 2021;11:1309–1315. doi: 10.1021/acscatal.0c05050. [DOI] [Google Scholar]
- 39.Hayashi K, Griffin J, Harper KC, Kawamata Y, Baran PS. Chemoselective (hetero)arene electroreduction enabled by rapid alternating polarity. J. Am. Chem. Soc. 2022;144:5762–5768. doi: 10.1021/jacs.2c02102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giese B. Formation of CC bonds by addition of free radicals to alkenes. Angew. Chem. Int. Ed. Engl. 1983;22:753–764. doi: 10.1002/anie.198307531. [DOI] [Google Scholar]
- 41.Fischer H, Radom L. Factors controlling the addition of carbon-centered radicals to alkenes-an experimental and theoretical perspective. Angew. Chem. Int. Ed. 2001;40:1340–1371. doi: 10.1002/1521-3773(20010417)40:8<1340::AID-ANIE1340>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 42.Li W, Xu W, Xie J, Yu S, Zhu C. Distal radical migration strategy: an emerging synthetic means. Chem. Soc. Rev. 2018;47:654–667. doi: 10.1039/C7CS00507E. [DOI] [PubMed] [Google Scholar]
- 43.Wu X, Zhu C. Radical-mediated remote functional group migration. Acc. Chem. Res. 2020;53:1620–1636. doi: 10.1021/acs.accounts.0c00306. [DOI] [PubMed] [Google Scholar]
- 44.Wu Z, Ren R, Zhu C. Combination of a cyano migration strategy and alkene difunctionalization: the elusive selective azidocyanation of unactivated olefins. Angew. Chem. Int. Ed. 2016;55:10821–10824. doi: 10.1002/anie.201605130. [DOI] [PubMed] [Google Scholar]
- 45.Li Z-L, Li X-H, Wang N, Yang N-Y, Liu X-Y. Radical mediated 1,2-formyl/carbonyl functionalization of alkenes and application to the construction of medium-sized rings. Angew. Chem. Int. Ed. 2016;55:15100–15104. doi: 10.1002/anie.201608198. [DOI] [PubMed] [Google Scholar]
- 46.Wu Z, Wang D, Liu Y, Huan L, Zhu C. Chemo- and regioselective distal heteroaryl ipso-migration: a general protocol for heteroarylation of unactivated alkenes. J. Am. Chem. Soc. 2017;139:1388–1391. doi: 10.1021/jacs.6b11234. [DOI] [PubMed] [Google Scholar]
- 47.Tang X, Studer A. Alkene 1,2-difunctionalization by radical alkenyl migration. Angew. Chem. Int. Ed. 2018;57:814–817. doi: 10.1002/anie.201710397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon J, He Y-T, Shin S, Hong S. Visible-light-induced ortho-selective migration on pyridyl ring: trifluoromethylative pyridylation of unactivated alkenes. Angew. Chem. Int. Ed. 2020;59:281–285. doi: 10.1002/anie.201912746. [DOI] [PubMed] [Google Scholar]
- 49.Yu J, et al. Metal-free radical difunctionalization of ethylene. Chem. 2023;9:472–482. doi: 10.1016/j.chempr.2022.10.020. [DOI] [Google Scholar]
- 50.Koppenol WH, Rush JD. Reduction potential of the CO2/CO2•− couple. A comparison with other C1 radicals. J. Phys. Chem. 1987;91:4429–4430. doi: 10.1021/j100300a045. [DOI] [Google Scholar]
- 51.Domingo LR, Perez P. Global and local reactivity indices for electrophilic/nucleophilic free radicals. Org. Biomol. Chem. 2013;11:4350–4358. doi: 10.1039/c3ob40337h. [DOI] [PubMed] [Google Scholar]
- 52.Winter-Holt, J. J. et al. Fused thiazolopyrimidine derivatives as mnks inhibitors. US patent 10,669,284 B2 (2020).
- 53.Guan X, Borchardt RT. A convenient method for the synthesis of indole-3-acetic acids. Tetrahedron Lett. 1994;35:3013–3016. doi: 10.1016/S0040-4039(00)76815-8. [DOI] [Google Scholar]
- 54.Wickens P, et al. Indanylacetic acids as PPAR-δ activator insulin sensitizers. Bioorg. Med. Chem. Lett. 2007;17:4369–4373. doi: 10.1016/j.bmcl.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 55.Yasmin H, et al. Total synthesis and analgesic activity of 6-fluoroindan-1-acetic acid and its 3-oxo derivative. Med. Chem. 2009;5:468–473. doi: 10.2174/157340609789117831. [DOI] [PubMed] [Google Scholar]
- 56.Sahoo S, Pal S. Copper-catalyzed one-pot synthesis of quinazolinones from 2-nitrobenzaldehydes with aldehydes: application toward the synthesis of natural products. J. Org. Chem. 2021;86:18067–18080. doi: 10.1021/acs.joc.1c02343. [DOI] [PubMed] [Google Scholar]
- 57.Yan S-S, et al. Visible-light photoredox-catalyzed selective carboxylation of C(sp3)−F bonds with CO2. Chem. 2021;7:3099–3113. doi: 10.1016/j.chempr.2021.08.004. [DOI] [Google Scholar]
- 58.Zhang Z, Martinez H, Dolbier WR. Photoredox catalyzed intramolecular fluoroalkylarylation of unactivated alkenes. J. Org. Chem. 2017;82:2589–2598. doi: 10.1021/acs.joc.6b03012. [DOI] [PubMed] [Google Scholar]
- 59.Xu Z, Wang Q, Zhu J. Metamorphosis of cycloalkenes for the divergent total synthesis of polycyclic indole alkaloids. Chem. Soc. Rev. 2018;47:7882–7898. doi: 10.1039/C8CS00454D. [DOI] [PubMed] [Google Scholar]
- 60.Chadha N, Silakari O. Indoles as Therapeutics of Interest in Medicinal Chemistry: Bird’s Eye View. Eur. J. Med. Chem. 2017;134:159–184. doi: 10.1016/j.ejmech.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Vliet LA, et al. Synthesis and pharmacological evaluation of thiopyran analogues of the dopamine D3 receptor-selective agonist (4aR,10bR)-(+)-trans−3,4,4a,10b-tetrahydro-4-n-propyl-2H,5H-[1]benzopyrano[4,3-b]−1,4-oxazin-9-ol (PD 128907) J. Med. Chem. 2000;43:2871–2882. doi: 10.1021/jm0000113. [DOI] [PubMed] [Google Scholar]
- 62.Bolognesi ML, et al. Design, synthesis, and biological evaluation of conformationally restricted rivastigmine analogues. J. Med. Chem. 2004;47:5945–5952. doi: 10.1021/jm049782n. [DOI] [PubMed] [Google Scholar]
- 63.Pini E, et al. New chromane-based derivatives as inhibitors of Mycobacterium tuberculosis Salicylate Synthase (MbtI): preliminary biological evaluation and molecular modeling studies. Molecules. 2018;23:1506. doi: 10.3390/molecules23071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen L, et al. Photocatalytic carboxylation of C−N bonds in cyclic amines with CO2 by consecutive visible-light-induced electron transfer. Angew. Chem. Int. Ed. 2023;62:e202217918. doi: 10.1002/anie.202217918. [DOI] [PubMed] [Google Scholar]
- 65.Jiang Y-X, et al. Visible-light photoredox-catalyzed ring-opening carboxylation of cyclic oxime esters with CO2. ChemSusChem. 2020;13:6312–6317. doi: 10.1002/cssc.202002032. [DOI] [PubMed] [Google Scholar]
- 66.Li N, et al. A highly selective decarboxylative deuteration of carboxylic acids. Chem. Sci. 2021;12:5505–5510. doi: 10.1039/D1SC00528F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kautzky JA, Wang T, Evans RW, MacMillan DWC. Decarboxylative trifluoromethylation of aliphatic carboxylic acids. J. Am. Chem. Soc. 2018;140:6522–6526. doi: 10.1021/jacs.8b02650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huihui KMM, et al. Decarboxylative cross-electrophile coupling of N-hydroxyphthalimide esters with aryl iodides. J. Am. Chem. Soc. 2016;138:5016–5019. doi: 10.1021/jacs.6b01533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin S, et al. Decarboxylative phosphine synthesis: insights into the catalytic, autocatalytic, and inhibitory roles of additives and intermediates. ACS Catal. 2019;9:9764–9774. doi: 10.1021/acscatal.9b03366. [DOI] [Google Scholar]
- 70.Jin Y, Yang H, Fu H. An N-(acetoxy) phthalimide motif as a visible-light pro-photosensitizer in photoredox decarboxylative arylthiation. Chem. Commun. 2016;52:12909–12912. doi: 10.1039/C6CC06994K. [DOI] [PubMed] [Google Scholar]
- 71.Chen W, et al. Building congested ketone: substituted hantzsch ester and nitrile as alkylation reagents in photoredox catalysis. J. Am. Chem. Soc. 2016;138:12312–12315. doi: 10.1021/jacs.6b06379. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J, Li Y, Zhang FY, Hu CC, Chen YY. Generation of alkoxyl radicals by photoredox catalysis enables selective C(sp3)−H functionalization under mild reaction conditions. Angew. Chem. Int. Ed. 2016;55:1872–1875. doi: 10.1002/anie.201510014. [DOI] [PubMed] [Google Scholar]
- 73.Jiang M, Li H, Yang H, Fu H. Room-temperature arylation of thiols: breakthrough with aryl chlorides. Angew. Chem. Int. Ed. 2017;56:874–879. doi: 10.1002/anie.201610414. [DOI] [PubMed] [Google Scholar]
- 74.Huang CY, Li J, Liu W, Li CJ. Diacetyl as a ″traceless″ visible light photosensitizer in metal-free crossdehydrogenative coupling reactions. Chem. Sci. 2019;10:5018–5024. doi: 10.1039/C8SC05631E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giedyk M, et al. Photocatalytic activation of alkyl chlorides by assembly-promoted single electron transfer in microheterogeneous solutions. Nat. Cat. 2020;3:40–47. doi: 10.1038/s41929-019-0369-5. [DOI] [Google Scholar]
- 76.Schmalzbauer M, et al. Redox-neutral photocatalytic C−H carboxylation of arenes and styrenes with CO2. Chem. 2020;6:2658–2672. doi: 10.1016/j.chempr.2020.08.022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. Extra data are available from the author upon request. The Cartesian coordinates for the calculated structures are available within the Supplementary Data 1.