Abstract
Decreased ATP Binding Cassette Transporter A1 (ABCA1) expression and caspase-4-mediated noncanonical inflammasome contribution have been described in podocytes in diabetic kidney disease (DKD). To investigate a link between these pathways, we evaluated pyroptosis-related mediators in human podocytes with stable knockdown of ABCA1 (siABCA1) and found that mRNA levels of IRF1, caspase-4, GSDMD, caspase-1 and IL1β were significantly increased in siABCA1 compared to control podocytes and that protein levels of caspase-4, GSDMD and IL1β were equally increased. IRF1 knockdown in siABCA1 podocytes prevented increases in caspase-4, GSDMD and IL1β. Whereas TLR4 inhibition did not decrease mRNA levels of IRF1 and caspase-4, APE1 protein expression increased in siABCA1 podocytes and an APE1 redox inhibitor abrogated siABCA1-induced expression of IRF1 and caspase-4. RELA knockdown also offset the pyroptosis priming, but ChIP did not demonstrate increased binding of NFκB to IRF1 promoter in siABCA1 podocytes. Finally, the APE1/IRF1/Casp1 axis was investigated in vivo. APE1 IF staining and mRNA levels of IRF1 and caspase 11 were increased in glomeruli of BTBR ob/ob compared to wildtype. In conclusion, ABCA1 deficiency in podocytes caused APE1 accumulation, which reduces transcription factors to increase the expression of IRF1 and IRF1 target inflammasome-related genes, leading to pyroptosispriming.
Subject terms: Biochemistry, Cell biology, Endocrinology, Nephrology
Introduction
Diabetic kidney disease (DKD) is the one of the main causes of end-stage kidney disease and among the top ten causes of death globally1. It also constitutes an independent risk factor of mortality and cardiovascular events in patients with diabetes2. Podocytes, which are terminally differentiated epithelial cells with low ability of self-renewal or proliferation, are key constituents of the glomerular filtration barrier. Clinical and experimental data suggest that podocyte loss correlates with the development of albuminuria, indicating the importance of podocyte injury in DKD3,4.
We previously demonstrated decreased ATP-binding cassette A1 (ABCA1) expression in glomerular transcripts from patients with DKD and in human podocytes exposed to sera from patients with DKD when compared to patients with diabetes and without DKD. Podocyte-specific Abca1 KO mice were sensitized to DKD injury, and both genetic and pharmacological induction of ABCA1 ameliorated DKD5,6. While the primary function of ABCA1 has been largely recognized as a cholesterol/phospholipid exporter, it has become clear that ABCA1 also exports reduction–oxidation factor 1 (Ref-1)/Apurinic/apyrimidinic endonuclease 1 (APE1), a transcription factor reducer and AP endonuclease7.
Pyroptosis, a form of inflammatory cell death, was found to contribute to podocyte loss in DKD with the main focus on canonical pyroptosis8. Pyroptosis requires two steps: priming and activation of the inflammasome. The priming step, in which the inflammasome components are transcriptionally upregulated, allows for the subsequent activation of inflammasome. In canonical pyroptosis, this transcriptional upregulation is induced through the binding of pathogen- and damage-associated molecular patterns (PAMPs and DAMPs) to pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) or through cytokines such as tumor necrosis factor (TNF) and IL1β, thus leading to NFκB activation9. Non-canonical pyroptosis is initiated by caspase-4/5 in human or caspase-11 in mice, and eventually causes downstream canonical inflammasome formation10. Non-canonical pyroptosis was recently found to mediate podocyte injury in DKD11. The main inducers of non-canonical pyroptosis known today are lipopolysaccharide (LPS) and activation of TLR4 signaling9. Indeed, activation of TLR4 signaling was shown to enhance pyroptosis in tubular cells in experimental DKD12. However, the mechanisms leading to non-canonical pyroptosis priming in podocytes remain unclear.
In this study, we demonstrate that ABCA1 deficiency in DKD contributes to the increase in non-canonical pyroptosis-related genes such as caspase-4/11, gasdermin D, caspase-1 and IL1β but not NLRP3. We also show that ABCA1 deficiency-induced priming of non-canonical pyroptosis is mediated by accumulated APE1, subsequent activation of transcription factors via reduction by APE1 and the enhancement of IRF1 transcription. Therefore, we propose the ABCA1/APE1/IRF1 axis to be a novel inducer of non-canonical pyroptosis in DKD.
Results
Non-canonical pyroptosis-related genes are upregulated in ABCA1 KD podocytes
We previously described that decreased ABCA1 expression in podocytes precedes podocyte injury in DKD and sensitizes podocytes to it5,6. Pyroptosis was recently identified as a novel mechanism of podocyte injury in DKD. However, the link between ABCA1 deficiency and pyroptosis has not been investigated8. To examine whether ABCA1 deficiency contributes to pyroptosis in podocytes, we measured mRNA levels of genes involved in pyroptosis in ABCA1 knockdown podocytes (siABCA1). mRNA expression of caspase-4, GSDMD, caspase-1 and IL1β was significantly increased in siABCA1 podocytes compared to control (p = 0.005 for caspase-4, 0.004 for GSDMD, 0.008 for caspase-1 and 0.01 for IL1β), whereas NLR family pyrin domain-containing protein 3 (NLRP3) expression decreased (Fig. 1a), in contrast to the previous reports indicating that NLRP3 expression is increased in podocytes treated with high glucose and in experimental DKD8,13. At the protein level, caspase-4 and GSDMD were significantly increased in siABCA1 compared to siCO podocytes (p = 0.007 for pro caspase-4 and 0.0001 for FL-GSDMD), while caspase-1 expression was increased but at a level that did not reach statistical significance (p = 0.08) (Fig. 1b). Although IL1β protein in cell lysates did not increase in siABCA1, we found increased IL1β precursor in the supernatants of siABCA1 podocytes when compared to siCO (Fig. 1c). Caspase-4 and GSDMD also increased and caspase-1 had the tendency to increase in their non-cleaved inactive forms in siABCA1 podocytes (Fig. 1a,b). These data demonstrated that ABCA1 knockdown in podocytes primes cells for non-canonical pyroptosis but is not sufficient to activate pyroptosis, which is consistent with our previous observation that ABCA1 deficiency contributes to DKD progression but is not sufficient to cause podocyte injury by itself6.
Figure 1.
mRNA expression and uncleaved protein levels of non-canonical pyroptosis-related genes are increased in siABCA1 podocytes. (a) mRNA levels of non-canonical pyroptosis-related genes in siCO and siABCA1 podocytes. (b) Protein levels of uncleaevd or pro- form of non-canonical pyroptosis-related genes in siCO and siABCA1 podocytes. The bands indicated with *(around 35 kD) in the FL-GSDMD blot are nonspecific bands. The uncropped blots are in the Supplementary Fig. S1a–c. (c) Protein levels of pro-IL1b in cell lysates and supernatants of siCO and siABCA1 podocytes. N = 3 for each group except for IL1β mRNA, which is N = 4. The uncropped blots are in the Supplementary Fig. S1a,d.
Non-canonical pyroptosis activation induced by LPS electroporation is enhanced in ABCA1 KD podocytes
Next, we investigated whether the upregulation of pyroptosis-related genes in ABCA1 KD podocytes contributes to non-canonical pyroptosis activation. Caspase-4 and GSDMD is cleaved and excreted into medium by LPS electroporation, which is compatible with previous reports demonstrating non-canonical pyroptosis activation by LPS electroporation14,15. Pan-caspase inhibitor zVAD-fmk reverses the cleavage (Fig. 2a,b). ABCA1 KD significantly enhanced the cleavage (p = 0.002 for cleaved caspase-4 (32 kD), 0.0001 for cleaved caspase-4 (40 kD) and 0.0009 for N terminal-GSDMD (N-GSDMD)).
Figure 2.
Protein levels of cleaved caspase-4 and N-GSDMD activated by LPS electroporation are increased in siABCA1 podocytes. (a) Protein levels of cleaved caspase-4 in siCO and siABCA1 podocytes. (b) Protein levels of N-GSDMD in siCO and siABCA1 podocytes. The uncropped blots are in the Supplementary Fig. S2a,b. N = 3 for each group. Each lane contains medium from the same cell number.
IRF1 orchestrates the upregulation of non-canonical pyroptosis-related genes in ABCA1 KD podocytes
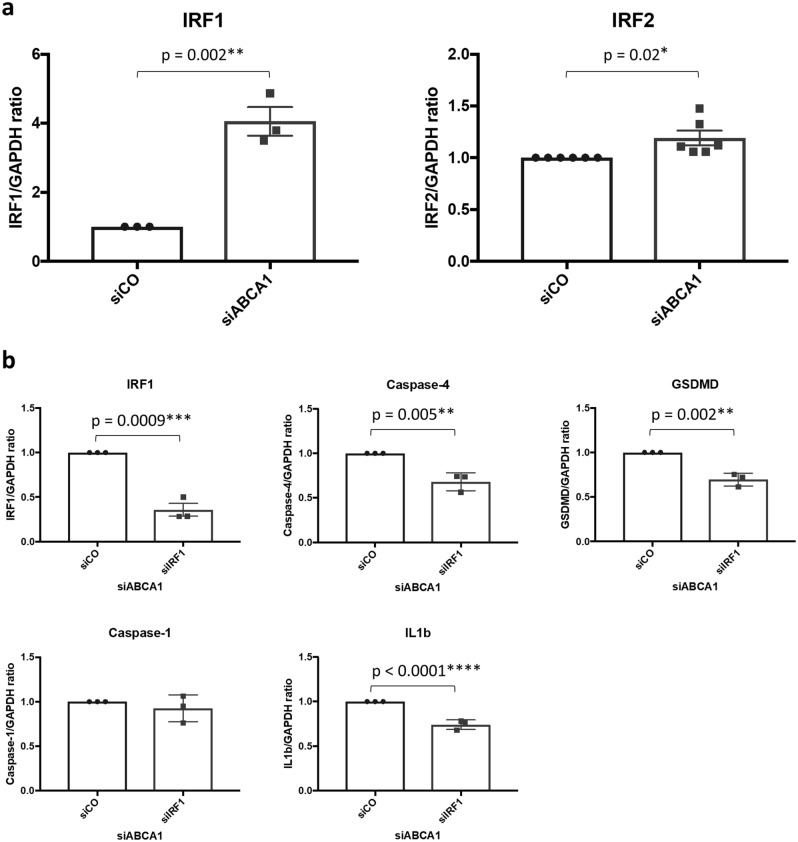
Interferon regulatory factor 1 and 2 (IRF1 and 2) are the transcription factors which regulate non-canonical pyroptosis with cell-type specific target genes. For example, in human U937 cells, IRF2 regulates caspase-4 and -1 but not GSDMD and in human EA.hy926 hybrid endothelial cells IRF2 regulates GSDMD but not caspase-4 nor -116,17. IRF1 compensates for IRF2 deficiency18. To explore whether IRF1 or 2 plays a role in regulating non-canonical pyroptosis-related genes in siABCA1 podocytes, we conducted qPCR for IRF1 and 2 gene expression in siABCA1 podocytes. Whereas the expression of IRF2 augmented significantly with larger n but to a small extent in siABCA1 podocytes (siABCA1/siCO ratio: 1.191 ± 0.0699, p = 0.02, N = 6), that of IRF1 were substantially increased (siABCA1/siCO ratio: 3.052 ± 0.4136, p = 0.002, N = 3) (Fig. 3a). In order to establish if IRF1 mediates the association between ABCA1 deficiency and pyroptosis, we next investigated whether siRNA knockdown for IRF1 in siABCA1 podocytes would offset the ABCA1 knockdown-induced changes in gene expression. We found mRNA levels of caspase-4, GSDMD and IL1β were significantly decreased in siIRF1 in siABCA1 podocytes (p = 0.005 for caspase-4, 0.002 for GSDMD, < 0.0001 for IL1β) while those of caspase-1 were not affected (Fig. 3b). Thus, we concluded that increased IRF1 is responsible for the upregulation of caspase-4, GSDMD and IL1β in siABCA1 podocytes.
Figure 3.
IRF1 regulates non-canonical pyroptosis-related genes in siABCA podocytes. (a) mRNA levels of IRFs in siCO and siABCA1 podocytes. (b) mRNA levels of non-canonical pyroptosis-related genes in siABCA1 podocytes transiently transfected with siCO and siIRF1. N = 3 for each group except for IRF2, which is N = 6.
TLR4 does not mediate IRF1 upregulation in ABCA1 KD podocytes
TLR4 signaling contributes to non-canonical pyroptosis priming9. ABCA1 deficiency in macrophages was found to enhance TLRs signaling by increasing TLRs (including TLR4) trafficking to plasma membrane lipid rafts12,19. To explore whether TLR4 signaling contributes to IRF1 and its downstream gene expression changes we observed in siABCA1 podocytes, we treated podocytes with TAK-242, a TLR4 inhibitor. The mRNA levels of IRF1 and caspase-4 were not significantly influenced by the inhibition of TLR4 (Fig. 4). These data suggest that TLR4 signaling does not contribute to ABCA1 deficiency-induced non-canonical pyroptosis priming in podocytes in our system.
Figure 4.
TLR4 is not involved in the regulation of IRF1 or caspase-4. mRNA levels of IRF1 and caspase-4 in siCO and siABCA1 cells treated with TAK-242 (TLR4 inhibitor). N = 3 for each group.
Reducing ability of APE1 mediates IRF1 upregulation in ABCA1 KD podocytes
In addition to its role in reverse cholesterol transport, ABCA1 also has a role in exporting APE17. APE1, also known as Ref-1 has two functions20. First, APE1 reduces transcription factors to activation form and oxidizes itself. Among the transcription factors reduced by APE1 are HIF1α, NFκB, STAT3, AP1 and p5321. Second, APE1 works as an apurinic/apyrimidinic endonuclease to nick the abasic site of DNA to prep it for repair. To examine the amount of APE1 in whole cell and nuclei where it functions, we conducted Western blotting for APE1 in whole cell lysate and nuclear fractions of control and siABCA1 podocytes and found that APE1 was increased in siABCA1 podocytes compared to control both in whole cell lysate and nuclear fractions (Fig. 5a,b). Next, we used APX3330, a specific-inhibitor of oxidation–reduction function of APE1, to address the question if the reduction of transcription factors by APE1 contributes to the priming of non-canonical pyroptosis in siABCA1 podocytes. The APE1 redox inhibitor offset the increase of siABCA1-induced mRNA transcription of IRF1 and caspase-4, suggesting a role for APE1 in this process (Fig. 5c).
Figure 5.
APE1 and NFκB regulates the mRNA expression of IRF1 and caspase-4. (a) Protein levels of APE1 in whole cell lysate of siCO and siABCA1 podocytes. The uncropped blots are in the Supplementary Fig. S3a. (b) Protein levels of APE1 in nuclear fractions of siCO and siABCA1 podocytes. The uncropped blots are in the Supplementary Fig. S3b. (c) mRNA levels of IRF1, caspase-4 and caspase-1 in siCO and siABCA1 podocytes treated with DMSO or APX3330 (APE1 redox inhibitor). (d) siRELA knockdown efficiency in siCO podocytes. N = 3 for each group. (e) mRNA levels of IRF1 and caspase-4 in siABCA1 podocytes transiently transfected with siCO and siRELA. (f) The result of ChIP qPCR for siCO podocytes treated with DMSO, siABCA1 podocytes with DMSO and siABCA1 podocytes with APX3330. Neph3 was used as a positive control and HOXA13 as a negative control for NFκB binding to DNA. N = 3 for each group. APE1 apurinic/apyrimidinic endonuclease-1, Neph3 kirre like nephrin family adhesion molecule 2, HOXA13 Homeobox A13.
NFκB is involved in IRF1 upregulation induced by ABCA1 downregulation
NFκB is a transcription factor that plays an important role in pyroptosis priming9. Several signaling pathways activate NFκB, including reactive oxygen species or TLR4 signaling22–24. NFκB can directly induce IRF1 transcription in immune cells while IRF1 can indirectly affect the regulation of NFκB25,26. To investigate whether NFκB is involved in the upregulation of IRF1 in siABCA1 podocytes, we used siRNA knockdown for RELA, the NFκB p65 subunit, in siCO and siABCA1 podocytes (Fig. 5d). We found a significant statistical interaction with siABCA1 and siRELA in IRF1 and caspase-4 (p_int = 0.02 for IRF1 and 0.006 for caspase-4), which indicated the role of NFκB in siABCA1-induced upregulation of IRF1 and caspase-4 (Fig. 5e). However, we were unable to confirm that the binding of NFκB to binding sites in IRF1 was increased with siABCA1 podocytes (Fig. 5f).
APE1, IRF1 and caspase-11 are increased in glomeruli of BTBR ob/ob mice
Finally, to investigate whether our in vitro observations translate in vivo, we used BTBR ob/ob mice, a mouse model of DKD. We previously demonstrated that decreased ABCA1 expression in podocytes plays an important role in DKD progression5,6. Immunofluorescence staining demonstrated increased APE1 expression in glomeruli of BTBR ob/ob compared to WT mice (Fig. 6a). In support, we found increased mRNA levels of IRF1 and caspase-11, which is compatible with the activation of the ABCA1/APE1/IRF1 axis in vivo as well as in vitro in DKD (Fig. 6b).
Figure 6.
APE1, IRF1 and non-canonical pyroptosis-related genes are increased in glomeruli of ob/ob mice. (a) Representative images of immunofluorescence staining for APE1 (green), synaptopodin (red) and nuclei (blue) in kidney tissue sections of wt and BTBR ob/ob mice and average APE1 positive area/synaptopodin positive area of 10 glomeruli in each mouse. N = 3 for each group. (b) mRNA levels of IRF1 and non-canonical pyroptosis-related genes in wt and ob/ob mouse glomeruli. Caspase-11 is the mouse ortholog of caspase-4 in human. N = 9 for each group except for caspase-1 and IL1β in ob/ob, which is n = 8.
Discussion
In this study, we demonstrated that ABCA1 deficiency in podocytes, which we previously showed to contribute to the progression of DKD, induced non-canonical pyroptosis priming in association with nuclear APE1 accumulation and subsequent activation of transcription factors by redox reaction (Fig. 7). Among the non-canonical pyroptosis-related genes upregulated in siABCA1 podocytes, caspase-4/11, GSDMD and IL1β were identified to be regulated by IRF1, while caspase-1 was not regulated by IRF1 but increased by APE1 redox reaction.
Figure 7.

Schematic of the hypothesis. ABCA1 deficiency in DKD causes accumulation of APE1 in nuclei, which activates transcription factors including NFκB via redox reaction. Activated transcription factors (TFs) increases IRF1 expression and IRF1 subsequently increases the expression of non-canonical pyroptosis-related genes such as caspase-4/11, GSDMD and IL1β. TFs: transcription factors.
Although the function of ABCA1 has been largely attributed to cholesterol efflux, ABCA1 is also involved in other cellular export mechanisms27. ABCA1 participates in the secretion of phospholipids such as cardiolipin, lysophosphatidylcholine and phosphatidylserine, extracellular vesicles and proteins associated with anti-inflammatory response and apoptotic cell clearance28–31. Recently, a role for ABCA1 in APE1 export has been described7.
APE1 mainly has two functions, an endonuclease and a redox function20,32. In its redox function, APE1 is oxidized and a transcription factor is reduced through a thiol/sulfide exchange and activated by facilitated DNA binding. Those transcription factors include NFκB, Hypoxia Inducible Factor (HIF), Signal Transducer and Activator of Transcription 3 (STAT3), Activator Protein 1 (AP-1) and cAMP-response element binding protein (CREB). So far, in canonical and non-canonical pyroptosis priming, NFκB was shown to be activated via phosphorylation of inhibitor of NFκB (IκB) kinases by TLR4 signaling22,33,34. The canonical pyroptosis pathway involving NLRP3 contributes to DKD but a contribution of non-canonical pyroptosis was also recently reported11,35. This study demonstrates that ABCA1 deficiency in podocytes, which we previously demonstrated to play an important role in DKD progression5,6, leads to APE1 accumulation and activation of transcription factors by redox reaction of APE1, not by TLR4, in the absence of NLRP3 increase. Thus, this pathway could represent a new mechanism of non-canonical pyroptosis priming in DKD. Our data suggest that APE1 activates several transcription factors and that the combination of their activation could lead to a distinct pattern of gene expression in siABCA1 podocytes. However, further investigation to elucidate the exact details of this process are warranted. It can also be speculated that the involvement of APE1 in mitochondrial function via the regulation of mitochondrial RNA may contribute to DKD32.
Priming of pyroptosis in the absence of an activation or cleavage of pyroptosis-related proteins occurred in siABCA1 podocytes, consistent with our previous observation in vitro and in vivo that ABCA1 downregulation itself is not sufficient to cause injury6. Nevertheless, ABCA1 induction has been explored as a treatment for DKD36. APE1 has been investigated as a treatment target of malignancy, ocular diseases and inflammatory bowel disease20,32, and this study indicates the possibility that it could be a target of DKD as well as IRF1.
We have previously published that oxidized cardiolipin is suggested to be implicated in the sensitization of siABCA1 podocytes to DKD.6 Some relationship between oxidized phospholipids (oxPLs) and non-canonical pyroptosis are described so far. Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (oxPAPC) can bind caspase-11 and activate it like LPS37. On the other hand, although we do not know specifically whether oxidized cardiolipin induces pyroptosis, some other oxPLs are demonstrated to sensitize bone marrow-derived macrophages to N-GSDMD-induced cytotoxicity38. It is possible that oxPLs and non-canonical pyroptosis mutually affect each other in ABCA1 downregulated podocytes.
In summary, we demonstrated that ABCA1 deficiency in podocytes, which contributes to DKD progression, induces the expression of non-canonical pyroptosis-related genes such as caspase-4/11, GSDMD, caspase-1 and IL1β via nuclear APE1 accumulation and subsequent reduction of transcription factors. Thus, non-canonical pyroptosis priming by ABCA1/APE1/IRF1 axis could represent a novel pathway to be targeted for the treatment of patients with DKD.
Materials and methods
Cell culture
Human podocytes (gift from Dr. Moin Saleem, University of Bristol, Bristol, England) were grown at 33 °C under permissive conditions in RPMI culture medium containing 10% FBS and 1% penicillin/streptomycin and 0.01 mg/ml recombinant human insulin, 0.0055 mg/ml human transferrin (substantially iron-free), and 0.005 μg/ml sodium selenite39. Human podocytes were then thermoshifted and differentiated for 14 days at 37 °C in RPMI medium 10% FBS and 1%penicillin/streptomycin. ABCA1 siRNA knockdown (siABCA1) and non-targeting siRNA control (siCO) podocytes were generated and validated as previously described40. On day 10–14 of differentiation, podocytes were collected and analyzed. For APE1 inhibition, podocytes were treated with 1 uM TAK-242 (Milipore Sigma, St. Louis MO, USA) for 24 h or 75 uM APX3330 (NOVUS biologicals, Centennial, CO, USA) for 48 h.
Animals
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Miami. All experiments were performed in accordance with the guidelines and regulations of IACUC at the University of Miami. Mice were sacrificed at 20 weeks by isoflurane inhalation. The authors complied with the ARRIVE guidelines. All the samples used for this manuscript including mRNA of glomeruli and frozen sections of kidneys of wildtype (wt) and BTBR ob/ob mice were collected and stored for our previous paper6 and were newly analyzed. The detailed methods are described previously6. 9 mice (5 females and 4 males) each were included in wt and ob/ob group. For immunofluorescence, 3 mice from each group which show typical pathological findings of normal or diabetic kidney disease in paraffin sections were selected and tissue in OCT from those 6 mice were freshly cut and analyzed. For qPCR, all the samples were analyzed, but because of the shortage of one sample, only 8 samples were analyzed for caspase-1 and IL1β in ob/ob group.
siRNA transfection
We used commercial siRNAs against human IRF1 (Silencer Select, s7501, s7503, Life Technologies Corporation, Carlsbad, CA, USA), RELA (Silencer Select, s11914, Life Technologies Corporation, Carlsbad, CA, USA) and a negative control siRNA (SIC001, Milipore Sigma, St. Louis MO, USA and Silencer Select#1, Life Technologies Corporation, Carlsbad, CA, USA). These siRNAs were introduced into podocytes using HiPerfect (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions.
Real-time quantitative PCR (qPCR)
Cell RNA was isolated with RNeasy Plus Mini Kit (QIAGEN, Hilden, Germany) and reverse transcribed using qScript DNA SuperMix (Quantabio, Beverly, MA, USA). PCR was performed on a StepOnePlus system (Applied biosystems, Waltham, MA, USA) with PerfeCTa SYBER Green SuperMix (Quantabio, Beverly, MA, USA). The data were normalized to GAPDH. Primer sequences are listed in Table 1. We repeated at least 3 transient transfections in siRNA KD. Each point per group represents a different transfection of cells. For IL1b mRNA expression and IRF2 mRNA expression we repeated the experiment 4 or 6 times respectively to reach statistical significance because of wide standard deviation.
Table 1.
Primers used in the article.
| Gene | Use | Species | Sequence | |
|---|---|---|---|---|
| Caspase-4 | RT-PCR | Human | Forward | AGATGCCCTCAAGCTTTGTC |
| Reverse | TGCGGTTGTTTCTCTCCTTT | |||
| GSDMD | RT-PCR | Human | Forward | GCCTCCACAACTTCCTGACAGATG |
| Reverse | GGTCTCCACCTCTGCCCGTAG | |||
| Caspase-1 | RT-PCR | Human | Forward | CACACCGCCCAGAGCACAAG |
| Reverse | TCCCACAAATGCCTTCCCGAATAC | |||
| IL1b | RT-PCR | Human | Forward | TTCGACACATGGGATAACGAGG |
| Reverse | TTTTTGCTGTGAGTCCCGGAG | |||
| NLRP3 | RT-PCR | Human | Forward | CTTCTCTGATGAGGCCCAAG |
| Reverse | GCAGCAAACTGGAAAGGAAG | |||
| IRF1 | RT-PCR | Human | Forward | CCAGAGCAGGAACAAGGG |
| Reverse | GGTCATCAGGCAGAGTGGA | |||
| IRF2 | RT-PCR | Human | Forward | TGGATGCATGCGGCTAGA |
| Reverse | CATCTGAAATTCGCCTTCC | |||
| GAPDH | RT-PCR | Human | Forward | TGCACCACCAACTGCTTAGC |
| Reverse | GGCATGGACTGTGGTCATGAG | |||
| Caspase-11 | RT-PCR | Mouse | Forward | CCTGAAGAGTTCACAAGGCTT |
| Reverse | CCTTTCGTGTACGGCCATTG | |||
| GSDMD | RT-PCR | Mouse | Forward | CCATCGGCCTTTGAGAAAGTG |
| Reverse | ACACATGAATAACGGGGTTTCC | |||
| Caspase-1 | RT-PCR | Mouse | Forward | AATGAAGTTGCTGCTGGAGGA |
| Reverse | CAGAAGTCTTGTGCTCTGGGC | |||
| IL1b | RT-PCR | Mouse | Forward | GCAACTGTTCCTGAACTCAACT |
| Reverse | ATCTTTTGGGGTCCGTCAACT | |||
| IRF1 | RT-PCR | Mouse | Forward | CTTCGTCGAGGTAGGACGTG |
| Reverse | CTTTGCTGCAGGAGCGATTC | |||
| GAPDH | RT-PCR | Mouse | Forward | GAAGGGCTCATGACCACA |
| Reverse | GGATGCAGGGATGATGTTCT | |||
| HOXA13 | ChIP | Human | Forward | GCTTCTTTCTCCCCCTCCTA |
| Reverse | CCGATCCCTGTGTAACTTGC | |||
| Neph3 | ChIP | Human | Forward | GAGTTTCTCAACGGGAAGAG |
| Reverse | GCCTCTGACGCTCTGAAAC | |||
| IRF1 | ChIP | Human | Forward | CCACCGAGCAATCCAAACAC |
| Reverse | GCCTGATTTCCCCGAAATGAC |
Sequences of the primers used for RT-PCR and ChIP in the article.
Western blotting
Cells lysates were prepared using RIPA buffer. Protein concentration was measured with the bicinchoninic acid (BCA) reagent (Thermo Fisher Scientific Inc., Waltham, MA, USA). 20–30 μg of protein extract was loaded onto 4 to 20% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) gels (Bio-Rad Laboratories Inc., Hercules, A, USA) and transferred to Immobilon-P PVDF membranes (Bio-Rad Laboratories Inc., Hercules, A, USA). Western blot analysis was performed using following primary antibodies: caspase-4 ($4450, rabbit, 1:1000), caspase-1 (#3866, rabbit, 1:1000 all from Cell Signaling Technology Inc.), IL1β (AF-201-NA, goat, 1:1000, R and D systems), GSDMD (ab210070, rabbit, 1:1000, Abcam PLC), N-GSDMD (ab215203, rabbit, 1:1000, Abcam PLC), APE1 (NB100-116, mouse, 1:1000, NOVUS biologicals), GAPDH (CB1001, mouse, 1:10,000, Sigma-Aldrich), beta actin (A3854, mouse, 1:10,000, Sigma-Aldrich); or secondary antibodies: anti-mouse IgG HRP (Promega, W402B, 1:10,000), anti-rabbit IgG HRP (Promega, W401B, 1:10,000), anti-goat IgG HRP (Promega, V8051, 1:10,000). Signal was detected with Radiance ECL (Azure Biosystems Inc., Dublin, CA, USA) or WesternBright ECL (Advansta Inc., San Jose, CA, USA) using Azure c600 Imaging System.
Protein from supernatants were isolated using Spin-X UF Concentrator (Corning Inc., Corning, NY, USA) and the values were normalized to the protein concentration of cells in the medium.
Nuclear fractions were obtained using a Cell Fractionation Kit (ab109719, Abcam PLC, Cambridge, UK) according to the manufacturer’s instructions.
LPS electroporation
LPS wea electroporated into podocytes using program T-020 in Nucleofector 2b (Lonza, Valais, Switzerland) and Amexa Basic Nucleofector Kit for Primary Mammalian epithelial cells (VPI-1005) according to manufacturer’s instruction. LPS-EB ultrapure (Invivogen, San Diego, CA, USA) was added to differentiated podocytes at a concentration of 3.0 ng/400,000 cells. The supernatants were collected at 4 h after electroporation and protein from them were collected using Amicon Ultra (Merck, Darmstadt, Germany) and analyzed with western blotting. Samples were normalized to mL/number of cells in the medium. Z-VAD-fmk (Selleck Biotech, Tokyo, Japan) was added 1 h prior to, during and after electroporation at a concentration of 20 nM.
Chromatin Immunoprecipitation (ChIP)
The binding of NFκB to the IRF1 promoter was assessed using the chromatin immunoprecipitation assay. Podocytes were cross-linked for 10 min using 1% paraformaldehyde and sonicated into fragments. The fragmented DNA forming a complex with NFκB was immunoprecipitated with an anti- NFκB rabbit polyclonal antibody (Cell Signaling Technology, Inc. Danvers, MA, USA) and Dynabeads M-280 Sheep Anti-Rabbit IgG (Invitrogen, St. Louis MO, USA). The precipitated DNA was used as a template for PCR reactions with the primers listed in Table 1. Five NFκB binding sites in IRF promoter region (− 500 to + 1500 from the transcription starting point) were predicted by FIMO database and the one nearest to the transcription starting site was selected, which was consistent with the publicly available ChIP-seq data which showed a strong peak near the transcription starting site in B-lymphocytes (GSM935478, GSM935526, GSM935285, GSM5529, GSM935531, GSM935273 and GSM935279)25. Neph3 (also known as kirre like nephrin family adhesion molecule 2) promoter region has known NFκB-binding site, and was used as a positive control41. Homeobox A13 (HOXA13) is the negative-control primer, which was designed for the promoter regions of chromosome 20.
Immunofluorescence
For immunofluorescence staining of kidneys from mice, fresh cut 4 μm-thick tissue in OCT was used. No fixation agent was applied. Permeabilization was performed using 0.3% Triton X-100 in 1 × PBS for 15 min at room temperature. A blocking step was performed using Power Block Universal Blocking Reagent (BioGenex, Fremont CA, USA) for 10 min at room temperature. Primary antibodies (for APE1 (NB100-101, rabbit, 1:500, NOVUS biologicals) and synaptopodin (sc-21537, goat, 1:500, Santa Cruz Biotechnolgy Inc.) were used for 24 h at 4 °C. Secondary antibodies (anti-rabbit Alexa Flour 488 (A32790, donkey, Invitrogen) and anti-goat Alexa Flour 568 (A11057, donkey, Invitrogen)) were used for 1 h at room temperature. The anti-caspase-11 antibody was conjugated with Alexa Flour 594 and used without secondary antibodies. To detect nucleus, ProLong Gold antifade reagent with DAPI (Invitrogen, St. Louis MO, USA) was applied.
Images were acquired by laser scanning confocal microscopy using a Leica SP5 Inverted microscope, × 20 objective (Leica Microsystems CMS GmbH, Germany). Measuring of cell fluorescence was performed using Image J software.
Statistical analysis
All the data are reported as mean ± standard error of mean and as individual values in the dot plots. Two groups were compared using independent sample t-test and a two-way ANOVA test was used to analyze the effect of two independent factors on outcomes and investigate if there is an interaction effect between the two factors on the outcomes. A p-value < 0.05 was considered statistically significant for all tests. GraphPad Prism, version 7.0 (GraphPad Software Inc., San Diego, CA, USA) was used for data analyses.
Supplementary Information
Acknowledgements
We would like to thank Prof. Masaomi Nangaku for his valuable assistance with ChIP and electroporation experiments. MI draw Fig. 7.
Author contributions
M.I., S.M. and A.F. conceived and designed the analysis. M.I. collected the data, performed the analysis and wrote the original draft. G.M.D. collected the animal samples. J.D.M., J.V.S., S.K.M, J.J.K, M.G. A.M. and A.S. contributed data. M.I., S.M., I.M. and A.F. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
M.I. is supported by Manpei Suzuki Diabetes Foundation. A.F. and S.M. are inventors on pending (PCT/US2019/032215; US 17/057,247; PCT/US2019/041730; PCT/US2013/036484; US 17/259,883; US17/259,883; JP501309/2021, EU19834217.2; CN-201980060078.3; CA2,930,119; CA3,012,773; CA2,852,904) or issued patents (US10,183,038 and US10,052,345) aimed at preventing and treating renal disease. They stand to gain royalties from their future commercialization. A.F. is Vice-President of L&F Health LLC and is a consultant for ZyVersa Therapeutics, Inc. ZyVersa Therapeutics, Inc has licensed worldwide rights to develop and commercialize hydroxypropyl-beta-cyclodextrin from L&F Research for the treatment of kidney disease. A.F. also holds equities in Renal 3 River Corporation. S.M. holds indirect equity interest in, and potential royalty from, ZyVersa Therapeutics, Inc. by virtue of assignment and licensure of a patent estate. A.F. and S.M. are supported by Aurinia Pharmaceuticals Inc. Other authors do not possess any conflict of interest.
Data availability
The data underlying this article are available in the article. The datasets referred to in the current study are available in the the Gene Expression Omnibus (GEO) repository, GSM935478, GSM935526, GSM935285, GSM5529, GSM935531, GSM935273 and GSM935279.
Competing interests
M.I. is supported by Manpei Suzuki Diabetes Foundation. A.F. and S.M. are inventors on pending (PCT/US2019/032215; US 17/057,247; PCT/US2019/041730; PCT/US2013/036484; US 17/259,883; US17/259,883; JP501309/2021, EU19834217.2; CN-201980060078.3; CA2,930,119; CA3,012,773; CA2,852,904) or issued patents (US10,183,038 and US10,052,345) aimed at preventing and treating renal disease. They stand to gain royalties from their future commercialization. A.F. is Vice-President of L&F Health LLC and is a consultant for ZyVersa Therapeutics, Inc. ZyVersa Therapeutics, Inc has licensed worldwide rights to develop and commercialize hydroxypropyl-beta-cyclodextrin from L&F Research for the treatment of kidney disease. A.F. also holds equities in Renal 3 River Corporation. S.M. holds indirect equity interest in, and potential royalty from, ZyVersa Therapeutics, Inc. by virtue of assignment and licensure of a patent estate. A.F. and S.M. are supported by Aurinia Pharmaceuticals Inc. Other authors do not possess any conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marie Ito, Email: marieitou@gmail.com.
Alessia Fornoni, Email: afornoni@med.miami.edu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-35499-5.
References
- 1.Murray CJL, Lopez AD. Measuring the global burden of disease. N. Engl. J. Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 2.González-Pérez A, Saez M, Vizcaya D, Lind M, Garcia Rodriguez L. Incidence and risk factors for mortality and end-stage renal disease in people with type 2 diabetes and diabetic kidney disease: A population-based cohort study in the UK. BMJ Open Diabetes Res. Care. 2021;9:e002146. doi: 10.1136/bmjdrc-2021-002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 4.Wiggins JE, et al. Podocyte hypertrophy, ‘adaptation’, and ‘decompensation’ associated with glomerular enlargement and glomerulosclerosis in the aging rat: Prevention by calorie restriction. J. Am. Soc. Nephrol. 2005;16:2953–2966. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- 5.Merscher-Gomez S, et al. Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes. 2013;62:3817–3827. doi: 10.2337/db13-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ducasa GM, et al. ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J. Clin. Invest. 2019;130:3387–3400. doi: 10.1172/JCI125316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YR, et al. ATP binding cassette transporter A1 is involved in extracellular secretion of acetylated APE1/Ref-1. Int. J. Mol. Sci. 2019;20:3178. doi: 10.3390/ijms20133178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahzad K, et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015;87:74–84. doi: 10.1038/ki.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson KV, Deng M, Ting JPY. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downs KP, Nguyen H, Dorfleutner A, Stehlik C. An overview of the non-canonical inflammasome. Mol. Aspects Med. 2020;76:100924. doi: 10.1016/j.mam.2020.100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Q, et al. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol. Sin. 2021;42:954–963. doi: 10.1038/s41401-020-00525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, et al. TLR4/NF-κB signaling induces GSDMD-related pyroptosis in tubular cells in diabetic kidney disease. Front. Endocrinol. 2019;10:603. doi: 10.3389/fendo.2019.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Q, Zhang M, Qian L, Wen D, Wu G. Luteolin attenuates high glucose-induced podocyte injury via suppressing NLRP3 inflammasome pathway. Life Sci. 2019;225:1–7. doi: 10.1016/j.lfs.2019.03.073. [DOI] [PubMed] [Google Scholar]
- 14.Santos JC, et al. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat. Commun. 2020;11:1–15. doi: 10.1038/s41467-020-16889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K, et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180:941–955.e20. doi: 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Benaoudia S, et al. A genome-wide screen identifies IRF2 as a key regulator of caspase-4 in human cells. EMBO Rep. 2019;20:e48235. doi: 10.15252/embr.201948235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayagaki N, et al. IRF2 transcriptionally induces GSDMD expression for pyroptosis. Sci. Signal. 2019;12:4917. doi: 10.1126/scisignal.aax4917. [DOI] [PubMed] [Google Scholar]
- 18.Thygesen SJ, Katryn SJ. IRF1 and IRF2 regulate the non-canonical inflammasome. EMBO Rep. 2019;20:e48891. doi: 10.15252/embr.201948891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu X, et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah F, et al. Exploiting the Ref-1-APE1 node in cancer signaling and other diseases: From bench to clinic. NPJ Precis. Oncol. 2017;1:19. doi: 10.1038/s41698-017-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartman GD, Lambert-Cheatham NA, Kelley MR, Corson TW. Inhibition of ape1/ref-1 for neovascular eye diseases: From biology to therapy. Int. J. Mol. Sci. 2021;22:10279. doi: 10.3390/ijms221910279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rathinam VAK, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J, et al. New insights into the mechanisms of pyroptosis and implications for diabetic kidney disease. Int. J. Mol. Sci. 2020;21:1–23. doi: 10.3390/ijms21197057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Wang Y, Gao G. High glucose induces rat mesangial cells proliferation and MCP-1 expression via ROS-mediated activation of NF-κB pathway, which is inhibited by eleutheroside E. J. Recept. Signal Transduct. Res. 2016;36:152–157. doi: 10.3109/10799893.2015.1061002. [DOI] [PubMed] [Google Scholar]
- 25.Iwanaszko M, Kimmel M. NF-κB and IRF pathways: Cross-regulation on target genes promoter level. BMC Genom. 2015;16:307. doi: 10.1186/s12864-015-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghislat G, et al. NF-kB–dependent IRF1 activation programs cDC1 dendritic cells to drive antitumor immunity. Sci. Immunol. 2021;6:3570. doi: 10.1126/sciimmunol.abg3570. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Wang S, Xing D. New horizons for the roles and association of APE1/Ref-1 and ABCA1 in atherosclerosis. J. Inflamm. Res. 2021;14:5251–5271. doi: 10.2147/JIR.S330147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen X, Zhang S, Guo Z, Xing D, Chen W. The crosstalk of ABCA1 and ANXA1: A potential mechanism for protection against atherosclerosis. Mol. Med. 2020;26:1–8. doi: 10.1186/s10020-020-00213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hafiane A, Genest J. ATP binding cassette A1 (ABCA1) mediates microparticle formation during high-density lipoprotein (HDL) biogenesis. Atherosclerosis. 2017;257:90–99. doi: 10.1016/j.atherosclerosis.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Hamon Y, et al. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- 31.Morizawa YM, et al. Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat. Commun. 2017;8:28. doi: 10.1038/s41467-017-00037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caston RA, et al. The multifunctional APE1 DNA repair–redox signaling protein as a drug target in human disease. Drug Discov. Today. 2021;26:218–228. doi: 10.1016/j.drudis.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schauvliege R, Vanrobaeys J, Schotte P, Beyaert R. Caspase-11 gene expression in response to lipopolysaccharide and interferon-gamma requires nuclear factor-kappa B and signal transducer and activator of transcription (STAT) 1. J. Biol. Chem. 2002;277:41624–41630. doi: 10.1074/jbc.M207852200. [DOI] [PubMed] [Google Scholar]
- 34.Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020;5:312. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou Y, et al. NLRP3 inflammasome negatively regulates podocyte autophagy in diabetic nephropathy. Biochem. Biophys. Res. Commun. 2020;521:791–798. doi: 10.1016/j.bbrc.2019.10.194. [DOI] [PubMed] [Google Scholar]
- 36.Wright MB, et al. Compounds targeting OSBPL7 increase ABCA1-dependent cholesterol efflux preserving kidney function in two models of kidney disease. Nat. Commun. 2021;12:1–14. doi: 10.1038/s41467-021-24890-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zanoni I, et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352:1232–1236. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang R, et al. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe. 2018;24:97–108.e4. doi: 10.1016/j.chom.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saleem MA, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 40.Pedigo CE, et al. Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J. Clin. Invest. 2016;126:3336–3350. doi: 10.1172/JCI85939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ristola M, et al. Regulation of Neph3 gene in podocytes: Key roles of transcription factors NF-κB and Sp1. BMC Mol. Biol. 2009;10:1–12. doi: 10.1186/1471-2199-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article. The datasets referred to in the current study are available in the the Gene Expression Omnibus (GEO) repository, GSM935478, GSM935526, GSM935285, GSM5529, GSM935531, GSM935273 and GSM935279.