Abstract
Background
There are limited contemporary data on the epidemiology and outcomes of bacteremia in solid organ transplant recipients (SOTr).
Methods
Using the Swiss Transplant Cohort Study registry from 2008 to 2019, we performed a retrospective nested multicenter cohort study to describe the epidemiology of bacteremia in SOTr during the first year post-transplant.
Results
Of 4383 patients, 415 (9.5%) with 557 cases of bacteremia due to 627 pathogens were identified. One-year incidence was 9.5%, 12.8%, 11.4%, 9.8%, 8.3%, and 5.9% for all, heart, liver, lung, kidney, and kidney-pancreas SOTr, respectively (P = .003). Incidence decreased during the study period (hazard ratio, 0.66; P < .001). One-year incidence due to gram-negative bacilli (GNB), gram-positive cocci (GPC), and gram-positive bacilli (GPB) was 5.62%, 2.81%, and 0.23%, respectively. Seven (of 28, 25%) Staphylococcus aureus isolates were methicillin-resistant, 2/67 (3%) enterococci were vancomycin-resistant, and 32/250 (12.8%) GNB produced extended-spectrum beta-lactamases. Risk factors for bacteremia within 1 year post-transplant included age, diabetes, cardiopulmonary diseases, surgical/medical post-transplant complications, rejection, and fungal infections. Predictors for bacteremia during the first 30 days post-transplant included surgical post-transplant complications, rejection, deceased donor, and liver and lung transplantation. Transplantation in 2014–2019, CMV donor-negative/recipient-negative serology, and cotrimoxazole Pneumocystis prophylaxis were protective against bacteremia. Thirty-day mortality in SOTr with bacteremia was 3% and did not differ by SOT type.
Conclusions
Almost 1/10 SOTr may develop bacteremia during the first year post-transplant associated with low mortality. Lower bacteremia rates have been observed since 2014 and in patients receiving cotrimoxazole prophylaxis. Variabilities in incidence, timing, and pathogen of bacteremia across different SOT types may be used to tailor prophylactic and clinical approaches.
Keywords: bacteremia, bloodstream infection, clinical outcomes, epidemiology, risk factors, solid organ transplant, timing
Bacteremia represents a relatively common complication after a solid organ transplant (SOT) and is historically associated with high morbidity and mortality [1–9]. The incidence, pathogens, and risk factors of bacteremia in SOT recipients have been described in older series from the 1990s and early 2000s; however, contemporary data are lacking [2, 4–7, 10–21]. We have previously reported on infectious disease complications during the first year post-transplant with data on SOT recipients between 2008 and 2014 using the Swiss Transplant Cohort Study (STCS) registry [10]. Bacterial infections represented the bulk (63%) of infectious disease complications during the first year post-transplant, 22% of which were bacteremia. In that study, bacteremias were unequally distributed during the first year after an SOT, with 34% of bacteremias observed during the first month, and 44% and 22% between 1 and 6 and after 6 months, respectively. We performed a retrospective observational multicenter cohort study to describe the epidemiology, risk factors, and outcomes of bacteremia during the first year post-transplant using the STCS database between 2008 and 2019.
METHODS
Study Design
The STCS is a multicenter cohort study prospectively enrolling >95% of SOT recipients in all Swiss transplant centers since 2008 [11]. Data are prospectively collected at prespecified scheduled patient visits, are event-driven, for example, in case of rejection, graft failure, or infectious diseases leading to hospitalization, and are regularly monitored with quality audits, as previously described [10, 11]. All consecutive adult (≥18 years) patients who received a heart, liver, lung, kidney, or kidney-pancreas transplant between May 1, 2008, and December 31, 2019, with a minimum of 12-month follow-up and who signed an informed consent form were included in this study. For patients who received >1 transplant, only the first transplant was considered, and patient follow-up was stopped at the time of their second transplant. The study was approved by the STCS Scientific Committee and the relevant ethics committees.
Objectives
The primary objective was to describe the cumulative incidence and timing of bacteremia during the first year post-transplant, overall and according to the type of transplant. As secondary objectives, we described the (a) epidemiology of bacterial pathogens, (b) primary site of infection, (c) risk factors, and (d) all-cause mortality in SOT recipients with bacteremia during the first year post-transplant.
Definitions
Infection definitions were developed by the STCS Infectious Diseases Working Group, based on published recommendations and guidelines [10–13]. As previously described, proven bacteremia required isolation of a bacterial pathogen in a blood culture with associated clinical signs and symptoms and administration of appropriate antibiotic treatment [10, 11]. In case of potential skin contaminants (ie, coagulase-negative Staphylococcus spp., Bacillus spp.), a proven bacteremia was defined as identification of a pathogen in at least 2 positive blood cultures with associated clinical signs and symptoms prompting administration of appropriate antibiotic treatment [10, 11]. Patients could have 1 single or >1 bacteremia episode during the study period. For patients with >1 bacteremia episode, a bacteremia with the same pathogen was considered a new episode when it occurred >14 days after the primary bacteremia. Infections were defined as mono- and polybacterial if only 1 or >1 bacterial pathogen was identified in the same blood culture, respectively. Infections were considered very early, early, and late if they occurred 0–30 days (1 month), 31–180 days (2–6 months), and >180 days (>6 months) post-transplant, respectively. Data on bacterial drug resistance were systematically collected after 2012, and multidrug resistance was defined as previously reported [14]. Surgical post-transplant complications included vascular, anastomosis, biopsy-related bleeding, and surgical site complications. Medical post-transplant complications included diabetes mellitus, hypertension, metabolic disorders, cardiopulmonary disease, transplanted organ tumor, and/or primary disease recurrence.
Clinical Attitudes
Administration of antibacterial perioperative, viral, and Pneumocystis-related prophylaxis and immunosuppression were center-based and depended on local institutional protocols [10]. All SOT recipients received perioperative antibacterial prophylaxis and oral nystatin or amphotericin-B prophylaxis during the first 2 weeks post-transplant [10]. Administration of cotrimoxazole as primary Pneumocystis jirovecii pneumonia (PJP) prophylaxis is administered in all kidney, heart, lung, and high-risk liver transplant recipients for a minimum of 6 months post-transplant at all centers [15].
Statistical Analysis
Patient and donor characteristics were described as counts and percentages for qualitative data and as medians and interquartile ranges (IQRs) for quantitative data. The cumulative incidence method was used to estimate the probability of first bacteremia within 1 year post-transplant dealing with death, graft failure, and second transplant as competing events. The Gray test was used to compare cumulative incidence curves separated by transplant type, by number of pathogens (mono- vs polymicrobial bacteremia), and by type of pathogen for monomicrobial bacteremia. To visualize the temporal tendency of bacteremia infections, we analyzed the incidence proportions of patients infected during the first year after transplant date by transplant year via locally weighted scatterplot smoothing with a span of 1.5, a nonparametric local regression approach. Cause-specific Cox proportional hazard models were constructed to determine risk factors for bacteremia occurrence. The time from transplantation to each of the first bacteremia episodes within 30 days and within 1 year after transplantation was considered. Follow-up information observed after transplantation until first bacteremia, death, graft failure, or second transplantation, whichever came first, was modeled as time-dependent. Potential risk factors considered in the final multivariable model were chosen based on a combination of clinical relevance and consistency with transplant types. Models were applied in the total population and within subgroups defined by transplant types. To quantify 30-day mortality among patients with bacteremia, the Kaplan-Meier method was used, and Cox proportional hazard models were applied to identify risk factors for death. The Fleming-Harrington test was used to compare survival curves by transplant type, by number of pathogens (mono- vs polymicrobial bacteremia), and by type of pathogen for monomicrobial bacteremia. R, version 4.2.1, was used for statistical analysis.
RESULTS
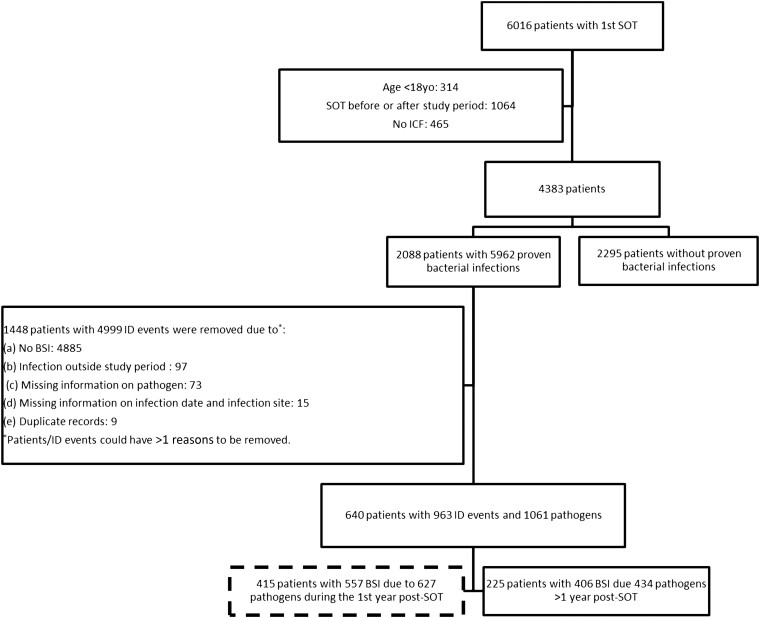
Of 6016 SOT recipients enrolled in the STCS during the study period, 4383 patients were included, with 415 (9.5%) patients with 557 bacteremia episodes due to 627 pathogens during the first year post-transplant (Figure 1). The patient baseline characteristics are described in Table 1.
Figure 1.
Study flowchart. Abbreviations: BSI, bloodstream infection; ICF, informed consent form; ID, infectious disease; SOT, solid organ transplant.
Table 1.
Patient Baseline Characteristics of Solid Organ Transplant Recipients Overall and Based on the Presence of a Bloodstream Infection During the First Year Post-transplant
| All Patients (n = 4383), No. (%) |
BSI (n = 415), No. (%) |
No BSI (n = 3968), No. (%) |
||
|---|---|---|---|---|
| Recipient demographics | ||||
| Median age (IQR), y | … | 55.7 (45.3–62.7) | 58.6 (49.7–64.5) | 55.3 (44.8–62.5) |
| Sex, female | … | 1535 (35.0) | 150 (36.1) | 1385 (34.9) |
| Recipient comorbidities | ||||
| Renal failure | … | 208 (11.3) | 32 (15.5) | 176 (10.8) |
| Diabetes mellitus | … | 921 (21.9) | 112 (27.0) | 809 (20.4) |
| Hypertension | … | 2643 (60.3) | 246 (59.3) | 2397 (60.4) |
| Cardiovascular/pulmonary disease | … | 2482 (56.6) | 275 (66.3) | 2207 (55.6) |
| Metabolic/endocrine disease | … | 3496 (79.8) | 332 (80.0) | 3164 (79.7) |
| Malignancy | … | 977 (22.3) | 93 (22.4) | 884 (22.3) |
| Donor | ||||
| Median age (IQR), y | … | 54 (42–63) | 55 (43–65) | 53 (42–63) |
| Sex, female | … | 2053 (47.1) | 189 (45.6) | 1864 (47.2) |
| Donor status | ||||
| … | Living | 1083 (24.7) | 76 (18.3) | 1007 (25.4) |
| … | DBD | 2933 (66.9) | 310 (74.7) | 2623 (66.1) |
| … | DCD | 367 (8.4) | 29 (7) | 338 (8.5) |
| Transplant information | ||||
| Transplant year | ||||
| … | 2008–2013 | 1937 (44.2) | 234 (56.4) | 1703 (42.9) |
| … | 2014–2019 | 2446 (55.8) | 181 (43.6) | 2265 (57.1) |
| Median cold ischemia time (IQR), median days | … | 356 (150–520) | 369 (185–515) | 354 (148–520) |
| Transplant type | ||||
| … | Heart | 359 (8.2) | 46 (11.1) | 313 (7.9) |
| … | Kidney | 2437 (55.6) | 201 (48.4) | 2236 (56.3) |
| … | Liver | 1044 (23.8) | 119 (28.7) | 925 (23.3) |
| … | Lung | 441 (10.1) | 43 (10.4) | 398 (10.0) |
| … | Kidney-pancreas | 102 (2.3) | 6 (1.5) | 96 (2.4) |
| Induction immunosuppression | Basiliximab | 3241 (80.9) | 289 (79.0) | 2952 (81.1) |
| … | Thymoglobulin | 862 (21.5) | 85 (23.2) | 777 (21.3) |
| … | Other | 285 (7.1) | 36 (9.8) | 249 (6.8) |
| Donor-recipient information | ||||
| Blood group incompatibility | … | 166 (3.8) | 22 (5.3) | 144 (3.6) |
| CMV D/R serology status | ||||
| … | CMV R+ | 2600 (60.2) | 264 (64.4) | 2336 (59.8) |
| … | CMV D+R- | 855 (19.8) | 90 (21.9) | 765 (19.6) |
| … | CMV D-R- | 861 (19.9) | 56 (13.7) | 805 (20.6) |
Abbreviations: BSI, bloodstream infection; CMV, cytomegalovirus; D, donor; DBD, donor after brainstem death; DCD, donor after circulatory death; HLA, human leucocyte antigen; IQR, interquartile range; R, recipient.
Incidence
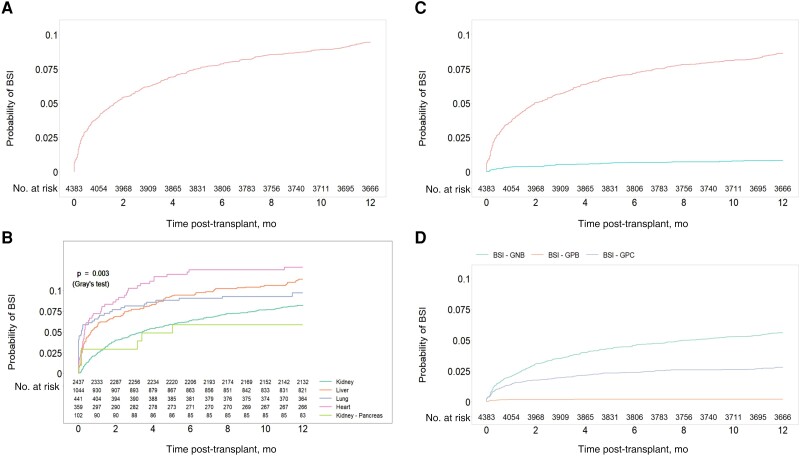
The 1-year cumulative incidence of bacteremia was 9.5% for all SOT recipients (95% CI, 8.63%–10.36%): 12.8% for heart (95% CI, 9.6%–16.5%), 11.4% for liver (95% CI, 9.6%–13.4%), 9.8% for lung (95% CI, 7.2%–12.7%), 8.3% for kidney (95% CI, 7.2%–9.4%), and 5.9% for kidney-pancreas transplant recipients (95% CI, 2.4%–11.6%; P = .003) (Figure 2A, B). Most patients (317/415, 76.4%) had 1 single bacteremia episode during the study period, while 98 (23.6%) patients were diagnosed with >1 distinct bacteremia episode during the first year post-transplant: 69, 19, 5, and 5 patients with 2, 3, 4, and 5 bacteremia episodes, respectively. More than 1 bacteremia episode was more frequently observed in heart (34.8%, 16/46), liver (26.9%, 32/119), and kidney (22.9%, 46/201), as compared with lung (9.3%, 4/43), transplant recipients. Monomicrobial bacteremia was observed in 379 patients (91.3%), with a 1-year incidence of 8.65% compared with 0.82% for polymicrobial bacteremia (Figure 2C). Cumulative incidence for monomicrobial bacteremia due to gram-negative bacilli (GNB), gram-positive cocci (GPC), and gram-positive bacilli (GPB) was 5.62%, 2.81%, and 0.23%, respectively (Figure 2D). Incidence significantly decreased during the study period from 2008–2013 to 2014–2019 (hazard ratio [HR], 0.66; P < .001).
Figure 2.
Estimated cumulative incidence of bacteremia during the first year post-transplant: (A) in the overall cohort; (B) per transplant type; (C) based on the number of pathogens identified: mono- vs polymicrobial bacteremia; and (D) based on the type of pathogen for monomicrobial bacteremia events (gram-positive cocci vs gram-negative bacteria vs gram-positive bacteria). The temporal tendency of bacteremia during the study period is presented in (E), showing the incidence proportions of patients infected during the first year after transplant date by transplant year via locally weighted scatterplot smoothing with span 1.5, a nonparametric local regression approach. Abbreviation: BSI, bloodstream infection.
Timing of Bacteremia
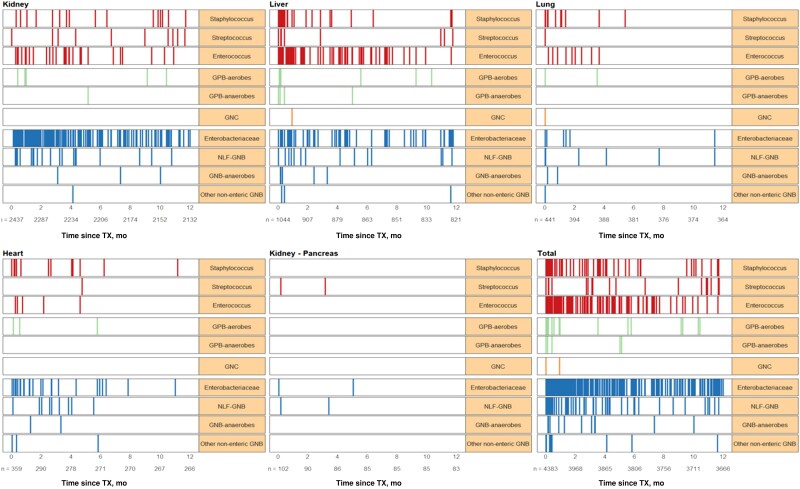
The median time from transplantation to the first bacteremia episode (IQR) was 71 (16–164) days. The median time to bacteremia (IQR) was shorter among lung (5.5 [0–53] days), compared with heart (37.5 [8–123] days), kidney-pancreas (50.5 [5–103] days), liver (67 [12–171] days), and kidney transplant recipients (95 [36–195] days; P < .001). Almost one-third of infections (189/557, 34%) were observed during the first month post-transplant, followed by 248/557 (44.5%) and 120/557 (21.5%) between 2–6 and 7–12 months post-transplant, respectively (Figure 3). More than half of infections in lung (30/48, 62.5%) and 47% (31/66) in heart transplant recipients were observed in the first month post-transplant.
Figure 3.
Infection density by pathogens causing bacteremia during the first year after a solid organ transplant overall and by transplant type.
Pathogen Distribution
Among 627 pathogens identified, there were 389 (62%), 215 (34.3%), 21 (3.4%), and 2 (0.3%) GNB, GPC, GPB, and GNC, respectively (Table 2). Enterobacterales were the most frequently identified pathogens in almost half of cases (48/627, 48%), followed by enterococci (121/327, 19.3%) and staphylococci (70/627, 11.2%), including 42/627 (6.7%) coagulase-negative Staphylococcus and 28/627 (4.5%) S. aureus. Overall, GNB were the most frequently encountered pathogens in heart (44/69, 63.8%), kidney (227/291, 78%), and kidney-pancreas (5/7, 71.4%) SOT recipients. Infections due to non-lactose-fermenting GNB were more frequently encountered in lung (16/54, 29.6%) and kidney-pancreas (3/7, 42.9%) transplant recipients. Proportionally, bacteremia episodes due to Staphylococcus spp. were more frequent in lung (14/54, 25.9%) and heart (15/69, 21.7%) transplant recipients.
Table 2.
Distribution of Bacterial Pathogens Among Solid Organ Transplant Recipients With a Bloodstream Infection During the First Year Post-transplant
| Overalla (n = 627), No. (%) |
Heart (n = 69), No. (%) |
Liver (n = 206), No. (%) |
Lung (n = 54), No. (%) |
Kidney (n = 291), No. (%) |
Kidney-Pancreas (n = 7), No. (%) |
|
|---|---|---|---|---|---|---|
| Gram-negative bacilli | 389 (62.0) | 44 (63.8) | 87 (42.2) | 26 (48.1) | 227 (78.0) | 5 (71.4) |
| Enterobacterales | 301 (48.0) | 30 (43.5) | 65 (31.6) | 7 (13) | 197 (67.7) | 2 (28.6) |
| E. coli | 182 (29.0) | 8 (11.6) | 36 (17.5) | 4 (7.4) | 133 (45.7) | 1 (14.3) |
| Enterobacter spp. | 21 (3.3) | 6 (8.7) | 7 (3.4) | 0 | 7 (2.4) | 1 (14.3) |
| Klebsiella spp. | 67 (10.7) | 8 (11.6) | 12 (5.8) | 1 (1.9) | 46 (15.8) | 0 |
| Proteus mirabilis | 5 (0.8) | 0 | 0 | 0 | 5 (1.7) | 0 |
| Serratia marcescens | 8 (1.3) | 4 (5.8) | 2 (1) | 0 | 2 (0.7) | 0 |
| Otherb | 18 (2.9) | 4 (5.8) | 8 (3.9) | 2 (3.7) | 4 (1.4) | 0 |
| Non-lactose-fermenting | 67 (10.7) | 9 (13) | 14 (6.8) | 16 (29.6) | 25 (8.6) | 3 (42.9) |
| Pseudomonas aeruginosa | 57 (9.1) | 9 (13) | 9 (4.4) | 13 (24.1) | 23 (7.9) | 3 (42.9) |
| Otherc | 8 (1.3) | 0 | 3 (1.5) | 3 (5.6) | 2 (0.7) | 0 |
| Other nonentericd | 8 (1.3) | 3 (4.3) | 3 (1.5) | 1 (1.9) | 1 (0.3) | 0 |
| Anaerobic | 13 (2.1) | 2 (2.9) | 5 (2.4) | 2 (3.7) | 4 (1.4) | 0 |
| Bacteroides fragilis | 4 (0.6) | 0 | 1 (0.5) | 1 (1.9) | 2 (0.7) | 0 |
| Othere | 9 (1.4) | 2 (2.9) | 4 (1.9) | 1 (1.9) | 2 (0.7) | 0 |
| Gram-negative coccif | 2 (0.3) | 0 | 1 (0.5) | 1 (1.9) | 0 | 0 |
| Gram-positive bacilli | 21 (3.4) | 3 (4.3) | 10 (4.9) | 2 (3.7) | 6 (2.1) | 0 |
| Aerobicg | 16 (2.6) | 3 (4.3) | 6 (2.9) | 2 (3.7) | 5 (1.7) | 0 |
| Anaerobich | 5 (0.8) | 0 | 4 (1.9) | 0 | 1 (0.3) | 0 |
| Gram-positive cocci | 215 (34.3) | 22 (31.9) | 108 (52.4) | 25 (46.3) | 58 (19.9) | 2 (28.6) |
| Staphylococcus spp. | 70 (11.2) | 15 (21.7) | 24 (11.7) | 14 (25.9) | 17 (5.8) | 0 |
| S. coagulase–negative | 42 (6.7) | 8 (11.6) | 14 (6.8) | 8 (14.8) | 12 (4.1) | 0 |
| S. aureus | 28 (4.5) | 7 (10.1) | 10 (4.9) | 6 (11.1) | 5 (1.7) | 0 |
| Enterococcus spp. | 121 (19.3) | 6 (8.7) | 75 (36.4) | 10 (18.5) | 30 (10.3) | 0 |
| Streptococcus spp.i | 24 (3.8) | 1 (1.4) | 9 (4.4) | 1 (1.9) | 11 (3.8) | 2 (28.6) |
Patients could have bloodstream infections with ≥1 bacterial pathogen.
Other Enterobacterales included Citrobacter braakii (2), Morganella morganii (3), Pantoea agglomerans (1), and other not-otherwise-specified organisms (12).
Other non-lactose-fermenting gram-negative bacilli included Acinetobacter spp. (3), Aeromonas hydrophila (1), Burkholderia cepacia (3), Pseudomonas (Flavimonas) oryzihabitans (1).
Other nonenteric gram-negative bacilli included Campylobacter spp. (1), Haemophilus influenzae (2), Haemophilus parainfluenzae (2), and other not-otherwise-specified organisms (3).
Other anaerobic gram-negative bacilli included Bacteroides spp. (3), Parabacteroides distasonis (1), and other not-otherwise-specified organisms (5).
Gram-negative cocci included Moraxella spp. (1), Veillonella spp. (1).
Aerobic gram-positive bacilli included Bacillus spp. (1), Corynebacterium spp. (1), Lactobacillus spp. (2), Lactococcus lactis (1), Listeria monocytogenes (1), Micrococcus luteus (1), Pedicoccus acidilacti (1), Ruminococcus spp. (1), Spiroplasma spp. (1), and other not-otherwise-specified organisms (6).
Anaerobic gram-positive bacilli included Bifidobacterium spp. (1), Clostridium spp. (3), Cutibacterium acnes (1).
In addition to Streptococcus spp., Gemella spp. (1) and Granulicatella spp. (1) were also included.
Antibiotic Resistance
Data on antibiotic resistance were not systematically collected until 2012 and were missing for some patients thereafter. Overall, multidrug-resistant (MDR) pathogens, including MDR and extended-spectrum beta-lactamase (ESBL)–producing GNB, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant enterococci (VRE), remained relatively stable during the study period (Figure 4A, B). Among 67 of 121 (55.4%) isolates of Enterococcus spp. with known resistance patterns, 36/67 (53.7%), 29/67 (43.3%), and 2/67 (3%) were pan-susceptible, penicillin-resistant, and VRE, respectively. There were 28 isolates of S. aureus, 7 (25%) of which were MRSA. Resistance data were available for 250/389 (64.3%) GNB: 17/250 (6.8%) and 32/250 (12.8%) were MDR and ESBL-producing pathogens, respectively. Among the 32 ESBL-producing GNB, there were 20, 11, and 1 E. coli, Klebsiella spp., and Enterobacter spp., respectively, observed in kidney (18/32, 56.3%), liver (10/32, 31.3%), heart (3/32, 9.4%), and lung (1/32, 3%) transplant recipients. There were no carbapenemase-producing GNB identified.
Figure 4.
A, Distribution of MDR pathogens through the study period. MDR pathogens included MDR and ESBL-producing GNB, MRSA, and VRE. MDR GNB definitions were based on previously published guidelines [14]. Results are presented as stacked bar charts including the proportions of MDR pathogens, non-MDR pathogens, and pathogens with unknown resistance patterns. B, Distribution of resistant pathogens through the study period, including MDR and ESBL GNB, MRSA, and VRE. MDR GNB definitions were based on previously published guidelines [14]. Results are presented as the proportion of resistant pathogens over the number of pathogens with known antibiotic susceptibilities per year. Data on bacterial drug resistance were collected after 2012; hence results are presented between 2012 and 2019. Antimicrobial resistance patterns were not available for all pathogens between 2012 and 2019. Abbreviations: BSI, bloodstream infection; ESBL, extended-spectrum beta-lactamase; MDR, multidrug-resistant; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
Bacteremia Source
The source of bacteremia was recorded in 302/557 (54.2%) bacteremia events. In 279/302 (92.4%), 21/302 (7%), and 2/302 (0.7%) events, there were 1, 2, and 3 sources reported, respectively, for a total of 327 different sources. Overall, urinary tract infections were the most frequently identified bacteremia source (156/327, 47.7%), followed by liver (35/327, 10.7%), gastrointestinal tract (34/327, 10.4%), respiratory tract (30/327, 9.2%), catheter-related (30/327, 9.2%), surgical site (28/327, 8.6%), mucocutaneous (10/327, 3.1%), bone and joint (2/327, 0.6%), and heart (2/327, 0.6%) infections.
Risk Factors
Risk factor analysis for bacteremia was performed to identify bacteremia predictors during the first year post-transplant (Table 3). Recipient age (HR, 1.19; P < .001), diabetes (HR, 1.82; P = .02) and cardiopulmonary comorbidities before and/or at transplant (HR, 1.38; P = .005), surgical (HR, 3.13; P < .001) and medical (HR, 2.32; P = .001) post-transplant complications, acute rejection (HR, 2.24; P < .001), and invasive fungal infections (HR, 3.55; P < .001) were identified as significant risk factors. In contrast, transplantation between 2014 and 2019 (HR, 0.61; P < .001), CMV donor-negative/recipient-negative serology status (HR, 0.66; P = .006), and administration of cotrimoxazole as prophylaxis (HR, 0.55; P < .001) were protective against bacteremia. Similar analyses were performed to identify predictors of very early bacteremia during the first month post-transplant. Liver (HR, 1.69, P = .03) and lung transplant recipients (HR, 2.78; P < .001), surgical complications post-transplant (HR, 2.45; P < .001), and acute rejection (HR, 2.76; P < .001) were independent predictors of early bacteremia. Transplantation between 2014 and 2019 (HR, 0.67; P = .009) and administration of cotrimoxazole (HR, 0.41; P < .001) were protective against very early bacteremia.
Table 3.
Results of Multivariable Analyses to Identify Risk Factors for Bloodstream Infections During the First Year Post-transplant: (A) Overall, (B) Very Early (0–30 Days) vs Later; for the Purposes of Those Analyses, Kidney-Pancreas Transplant Recipients Were Considered With Kidney Transplant Recipients, due to the Small Number of Kidney-Pancreas Transplants
| Overall | Very Early BSIa | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Demographics recipient/donor | ||||||
| Recipient age, 10 y | 1.16 | 1.05–1.29 | 0.003 | 0.99 | 0.86–1.14 | 0.9 |
| Recipient sex, male vs female | 0.86 | 0.69–1.08 | .2 | … | … | … |
| Donor age, 10 y | 1.05 | 0.98–1.12 | .14 | 1.03 | 0.93–1.13 | .6 |
| Baseline comorbidities | ||||||
| Diabetes mellitus, yes vs no | 1.42 | 1.12–1.8 | .004 | 1.33 | 0.92–1.91 | .18 |
| Hypertension, yes vs no | 1.05 | 0.81–1.35 | .7 | 0.98 | 0.67–1.45 | >.9 |
| Cardiopulmonary disease, yes vs no | 1.39 | 1.09–1.77 | .007 | 1.21 | 0.83–1.77 | .3 |
| Malignancy, yes vs no | 0.79 | 0.6–1.06 | .12 | … | … | … |
| Transplant-related variables | ||||||
| Donor status, deceased vs living | 1.28 | 0.94–1.74 | .11 | 1.88 | 1.02–3.44 | .04 |
| Transplant year, 2014–2019 vs 2009–2013 | 0.66 | 0.54–0.82 | <.001 | 0.73 | 0.53–1.01 | .05 |
| Induction immunosuppression, Thymoglobulin vs other | 0.98 | 0.73–1.32 | >.9 | 1.28 | 0.8–2.07 | .3 |
| CMV serology status, D-/R- vs R+ | 0.62 | 0.46–0.86 | .003 | 0.57 | 0.35–0.94 | .03 |
| CMV serology status, D+/R- vs R+ | 1.04 | 0.8–1.34 | .8 | 0.9 | 0.6–1.35 | .6 |
| Transplant type (compared with kidney) | ||||||
| Heart | 1.1 | 0.72–1.67 | .7 | 1.54 | 0.85–2.81 | .2 |
| Liver | 0.98 | 0.69–1.39 | >.9 | 1.52 | 0.9–2.56 | .11 |
| Lungs | 1.02 | 0.68–1.53 | >.9 | 2.65 | 1.5–4.67 | <.001 |
| Post-transplant time-dependent variables | ||||||
| Surgical complications,b yes vs no | 3.09 | 2.21–4.33 | <.001 | 2.4 | 1.59–3.61 | <.001 |
| Medical complications,c yes vs no | 2.27 | 1.3–3.98 | .004 | … | … | … |
| Rejection,d yes vs no | 2.37 | 1.76–3.19 | <.001 | 2.77 | 1.66–4.63 | <.001 |
| Invasive fungal infection,d yes vs no | 3.14 | 1.57–6.27 | .001 | … | … | … |
| Any viral infection (excluding CMV),d yes vs no | 1.08 | 0.39–2.96 | .9 | … | … | … |
| CMV infection,d yes vs no | 1.8 | 0.66–4.87 | .2 | … | … | … |
| Administration of TMP/SMX as prophylaxis, yes vs no | 0.5 | 0.38–0.65 | <.001 | 0.4 | 0.27–0.6 | <.001 |
Abbreviations: BSI, bloodstream infection; CMV, cytomegalovirus; D/R, donor/recipient; HR, hazard ratio; TMP/SMX, trimethoprim/sulfamethoxazole.
Due to the limited number of BSI events during the first month post-transplant, a smaller number of independent variables was considered based on clinical plausibility and available literature data, excluding the following variables from this model: malignancy before transplant, post-transplant medical complications, invasive fungal infections, and viral infections.
Surgical post-transplant complications within 30 days of BSI included vascular, anastomosis, biopsy, bleeding, and surgical site complications.
Medical transplant-related complications within 1 year of BSI included diabetes mellitus, hypertension, metabolic disorders, cardiopulmonary disease, transplanted organ tumor, and/or primary disease recurrence.
Rejection and invasive fungal or viral/CMV infections were considered within 90 days of BSI.
Additional organ-specific risk factor analyses were performed, including variables considering clinical plausibility and limitations associated with the smaller number of events per SOT type (Table 4). Recipient age (HR, 1.29; P < .001), deceased donors (HR, 1.58; P = .004), surgical (HR, 2.58; P = .002) and medical (HR, 4.06; P = .002) post-transplant complications, and rejection (HR, 2.79; P < .001) were risk factors for bacteremia in kidney transplant recipients. In contrast, transplantation between 2014 and 2019 (HR, 0.65; P = .003) and administration of cotrimoxazole as prophylaxis (HR, 0.41; P < .001) were protective against bacteremia. For liver transplant recipients, surgical complications (HR, 2.74; P < .001) and rejection (HR, 2.58; P < .001) predicted bacteremia, while bacteremia were less likely if transplantation occurred between 2014 and 2019 (HR, 0.46; P < .001). For thoracic organ transplant recipients, surgical (HR, 3.23; P < .001) and medical (HR, 3.03; P = .02) post-transplant complications and CMV infection (HR, 7.52; P = .05) were independent risk factors for bacteremia. CMV serology donor-negative/recipient-negative (HR, 0.43; P = .01) and cotrimoxazole prophylaxis (HR, 0.46; P = .01) were protective against bacteremia.
Table 4.
Results of Multivariable Analyses to Identify Risk Factors for Bloodstream Infections During the First Year Post-transplant for (A) Kidney, (B) Liver, and (C) Heart and Lung Transplant Recipients
| Kidneya | Livera | Heart and Lunga | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Demographics Recipient/donor | |||||||||
| Recipient age, 10 y | 1.29 | 1.14–1.47 | <.001 | 1.16 | 0.97–1.39 | .11 | 1.02 | 0.86–1.2 | .9 |
| Recipient sex, male vs female | 0.79 | 0.59–1.06 | .12 | … | … | … | … | … | … |
| Donor age, 10 y | 1.09 | 0.99–1.2 | .07 | … | … | … | … | … | … |
| Transplant-related variables | |||||||||
| Donor status, deceased vs living | 1.57 | 1.13–2.18 | .007 | … | … | … | … | … | … |
| Transplant year, 2014–2019 vs 2009–2013 | 0.74 | 0.56–1.0 | .05 | 0.46 | 0.32–0.68 | <.001 | 0.7 | 0.45–1.07 | .1 |
| Induction immunosuppression, thymoglobulin vs other | 1.16 | 0.84–1.6 | .4 | … | … | … | … | … | … |
| CMV serology status, D-/R- vs R+ | 0.73 | 0.48–1.09 | .13 | 0.67 | 0.37–1.21 | .2 | 0.43 | 0.22–0.85 | .01 |
| CMV serology status, D+/R- vs R+ | 0.97 | 0.67–1.41 | .9 | 1.14 | 0.72–1.81 | .6 | 0.99 | 0.61–1.59 | >.9 |
| Post-transplant time-dependent variables | |||||||||
| Surgical complications,b yes vs no | 2.79 | 1.54–5.04 | <.001 | 2.74 | 1.65–4.54 | <.001 | 3.23 | 1.83–5.71 | <.001 |
| Medical complications,c yes vs no | 4.23 | 1.71–1.05 | .002 | 1.64 | 0.83–3.23 | .2 | 3.03 | 1.18–7.74 | .02 |
| Rejection,d yes vs no | 3.05 | 2.08–4.47 | <.001 | 2.58 | 1.54–4.32 | <.001 | 1.61 | 0.84–3.07 | .15 |
| Invasive fungal infection,d yes vs no | … | … | … | … | … | 1.97 | 0.61–6.34 | .3 | |
| Any viral infection (excluding CMV),d yes vs no | 0.89 | 0.12–6.37 | >.9 | … | … | … | … | … | … |
| CMV infection,d yes vs no | 0.66 | 0.09–4.76 | .7 | 2.77 | 0.66–11.6 | .2 | 7.52 | 1.02–55.7 | .05 |
| Administration of TMP/SMX as prophylaxis, yes vs no | 0.37 | 0.25–0.55 | <.001 | 0.83 | 0.56–1.24 | .4 | 0.46 | 0.25–0.84 | .01 |
Abbreviations: BSI, bloodstream infection; CMV, cytomegalovirus; D/R, donor/recipient; HR, hazard ratio; TMP/SMX, trimethoprim/sulfamethoxazole.
Due to the limited number of BSI events for each individual organ transplantation, different numbers of independent variables were considered based on clinical plausibility and available literature data.
Surgical post-transplant complications within 30 days of BSI included vascular, anastomosis, biopsy, bleeding, and surgical site complications.
Medical transplant-related complications within 1 year of BSI included diabetes mellitus, hypertension, metabolic disorders, cardiopulmonary disease, transplanted organ tumor, and/or primary disease recurrence.
Rejection and invasive fungal or viral/CMV infections were considered within 90 days of BSI.
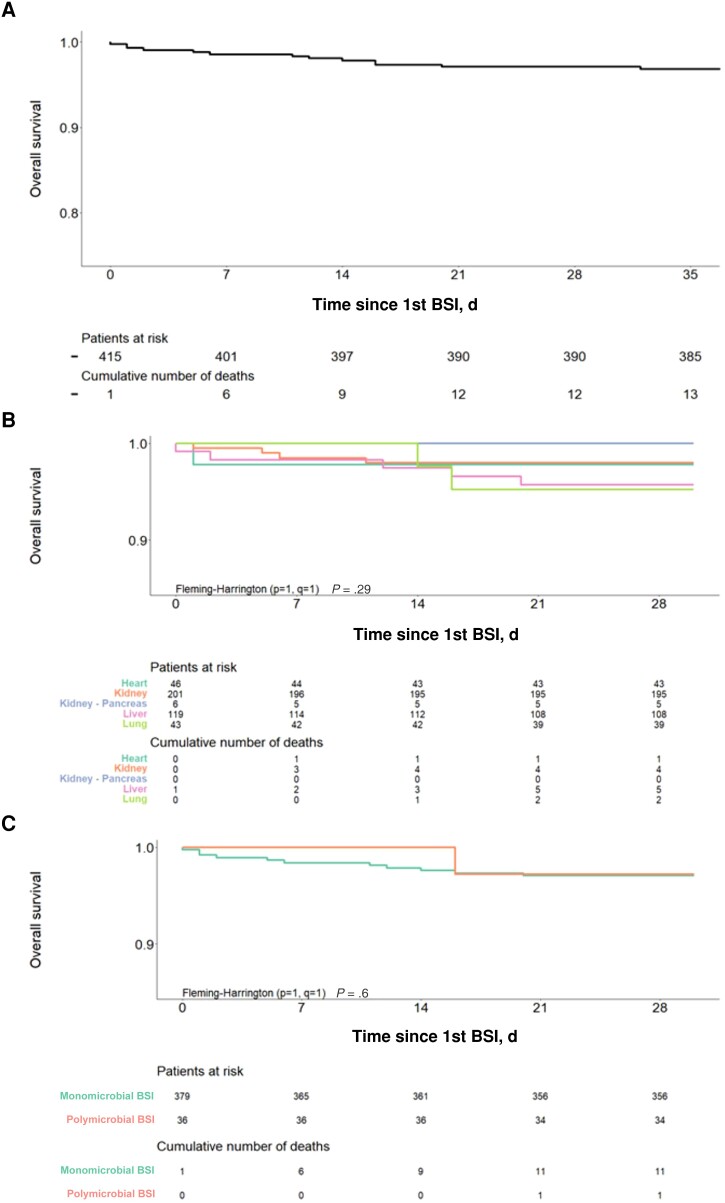
Outcomes
All-cause 30-day mortality in SOT recipients with bacteremia was 3% (12/415) (Figure 5A) and did not significantly differ based on SOT type: 2% (1/46), 2% (4/201), 4% (5/119), 5% (2/43), and 0% (0/6) for heart, kidney, liver, lung, and kidney-pancreas SOTr, respectively (P = .29) (Figure 5B). All-cause 30-day mortality was 3% (11/379) and 3% (1/36) in patients with mono- vs polymicrobial bacteremia (P = .6) (Figure 5C). No predictors, including SOT type, time from SOT to bacteremia, or GNB bacteremia, were found to significantly affect mortality postbacteremia (data not shown).
Figure 5.
All-cause 30-day mortality of SOT recipients with bacteremia: (A) overall, (B) by SOT type, (C) number of pathogens identified (mono- vs polymicrobial). Abbreviation: SOT, solid organ transplant.
DISCUSSION
In this 12-year cohort study, almost 1 in 10 patients developed a bacteremia during the first year post-transplant, consistent with previously reported rates of an incidence ranging between 7% and 35%, depending on different transplant types [5, 16–18]. We observed the highest incidence in heart transplant recipients, as previously reported, with incidence rates up to 15% [5, 17]. A significant decline in bacteremia in the latter study period was observed. This may, in part, be attributed to improved clinical practices in surgical and medical management, but also improved follow-up and monitoring of patients through the years. Notably, multivariable analyses confirmed a protective effect against bacteremia for SOT occurring during 2014–2019 compared with 2008–2013.
In line with reduced numbers of bacteremia in the most recent part of the study period, mortality among patients with bacteremia in this cohort was low at 3%. This is in contrast to previously published data with mortality rates as high as 25% to 50% [2, 5, 6, 8, 17, 19, 20]. Although we were not able to definitively assess attributable mortality in this retrospective study, we report 30-day all-cause mortality, considering this relatively representative of a potential effect of bacteremia on survival outcomes. Improved contemporary transplant practices with close patient follow-up leading to early diagnosis of infectious complications and prompt treatment initiation might have, in part, contributed to the lower mortality rates observed in our cohort.
Older age has been previously identified as a bacteremia predictor in kidney and liver transplant recipients, suggesting that older patients may be more fragile, with more comorbidities, and at higher risk to present complications post-transplant, such as bacteremia [21, 22]. In alignment with prior published data, we found that diabetes and cardiopulmonary complications were significant risk factors for bacteremia [5, 8, 21, 23]. Similarly, surgical complications were identified as an important predictor of bacteremia, as has already been identified in previous studies, particularly in liver and lung transplant recipients [4, 16, 18, 24–28]. Acute rejection and medical complications post-transplant were found to be significantly associated with bacteremia during the first year as well. Rejection has been previously identified as a significant risk factor for bacteremia in both kidney and liver transplant recipients [21, 26]. It is likely that the impact of those complications on bacteremia may be related to their direct and/or indirect effects on the net immunosuppression status of those patients. Liver and lung transplant recipients were more likely to develop an early bacteremia compared with other organs, consistent with the high risk for infectious complications early post-transplant in those patient categories.
CMV donor-negative/recipient-negative serostatus was associated with fewer bacteremia episodes—overall, very early, and for thoracic organ transplant recipients in particular, likely due to fewer hospitalizations of those patients associated with CMV reactivation and infections and also fewer CMV-treatment associated toxicities, predominately cytopenias, associated with (val)ganciclovir. Notably, CMV infection was associated with higher bacteremia rates only in thoracic organ transplant recipients. It is known that heart and particularly lung transplant recipients are at high risk for CMV reactivation, which is associated with dismal clinical outcomes [29]. CMV reactivation may have direct, but also indirect, effects on rejection and graft loss and on the net state of immunosuppression of those patients, and hence increase their susceptibility to opportunistic infections [30–32]. Our data support this already established body of literature suggesting that CMV reactivation post–heart/lung SOT may predispose patients to other complications, including bacteremia.
Administration of cotrimoxazole prophylaxis against PJP was found to be protective against bacteremia overall but also in the early 30-day post-transplant period and for kidney and thoracic organ SOT recipients. Most data on the effect of cotrimoxazole prophylaxis on bacterial infection prevention come from the kidney transplant literature. For instance, administration of cotrimoxazole PJP prophylaxis was shown to have no significant effect on decreasing asymptomatic bacteriuria, cystitis, or allograft pyelonephritis in a single-center before-and-after retrospective study on kidney transplant recipients [33]. Similarly, in another single-center retrospective study on kidney transplant recipients, when PJP prophylaxis with cotrimoxazole was compared with dapsone, there was no difference in risk for urinary tract infections in the overall patient population [34]. However, cotrimoxazole had to be discontinued in almost half of patients. When analysis was performed in the subset of older (>47-year-old) patients who received dapsone (either up front or as a switch from cotrimoxazole), a potential benefit of cotrimoxazole prophylaxis in reducing urinary tract infections was noted. This observation was consistent with another smaller, single-center retrospective study on renal transplant recipients showing lower incidence of urinary tract infections in patients who received cotrimoxazole vs aerosolized pentamidine as PJP prophylaxis [35]. Similarly, administration of cotrimoxazole as primary prophylaxis during the first months after a kidney transplant to prevent urinary tract infections has been shown to be an effective and relatively safe approach, albeit at doses higher than PJP prophylactic doses [36, 37]. Our data suggest that administration of cotrimoxazole as PJP prophylaxis may contribute to lower bacteremia rates, not only very early, during the first month, but also extending through the first year post-transplant.
Collectively, GNB were more commonly identified, representing the majority of bacteremia episodes in heart, kidney, lung, and kidney-pancreas transplant recipients, as previously reported [5, 17, 38–40]. Consistent with prior reports, GPC were frequent pathogens in liver transplant recipients, with Enterococcus spp. encountered quite often [16, 17]. The latter might, in part, be related to frequent colonization of the biliary tract of liver transplant recipients with enterococci, leading to higher numbers of bacteremia due to those pathogens [41]. In contrast to prior reports on the emergence of MDR bacterial pathogens in SOT recipients, there were only 2 infections due to vancomycin-resistant enterococci, while a large number of Enterococcus spp. were still susceptible to penicillin [4, 41]. Similarly, rates of bacteremia due to other non-Enterococcus MDR pathogens were relatively low, with few infections due to ESBL-producing GNB and no infections identified due to carbapenemase-producing GNB. This is in contrast to MDR pathogen rates as high as 20%–25%, 8%, and 57% in kidney, liver, and lung transplant recipients, respectively [4, 6, 39, 42]. The low MDR incidence in SOT recipients in our cohort reflects the general trend of infections due to MDR bacterial pathogens in Switzerland, as reported by the ANRESIS network [43, 44]. Although those data are in accordance with the general epidemiology in our country, they also suggest that close control and monitoring of transplant prophylactic and treatment strategies by experienced transplant infectious disease teams in all transplant centers in Switzerland might have contributed to keeping antibiotic resistance low in this fragile patient population. Continuous surveillance and monitoring of MDR bacterial pathogen epidemiology in SOT recipients should be maintained in order to identify timely potential trends of MDR development in the future, which could affect clinical practices and patient outcomes.
Despite its limitations, associated with its retrospective design and occasional lack of resistance and other data, our findings complement the existing body of literature with results spanning a 12-year study period reporting on >4000 SOT recipients. In conclusion, we report low bacteremia incidence associated with low mortality rates during the first year after SOT. Improved clinical practices might have led to lower rates of bacteremia in the latter part of the study period and the observed low mortality rates. Continuous review and assessment of the epidemiology and risk factors of bacteremia may impact prophylactic and other approaches and allow for even better clinical outcomes in the future.
Acknowledgments
We would like to thank all our patients for their trust in and support for the Swiss Transplant Cohort Study since 2009. We would also like to sincerely thank all the nurses, coordinators, and other staff who care for all solid organ transplant recipients at all transplant centers in Switzerland.
Members of the Swiss Transplant Cohort Study. Patrizia Amico, John-David Aubert, Vanessa Banz, Sonja Beckmann, Guido Beldi, Christoph Berger, Ekaterine Berishvili, Annalisa Berzigotti, Isabelle Binet, Pierre-Yves Bochud, Sanda Branca, Heiner Bucher, Thierry Carrel, Emmanuelle Catana, Anne Cairoli, Yves Chalandon, Sabina De Geest, Sophie De Seigneux, Michael Dickenmann, Joëlle Lynn Dreifuss, Michel Duchosal, Thomas Fehr, Sylvie Ferrari-Lacraz, Jaromil Frossard, Christian Garzoni, Déla Golshayan, Nicolas Goossens, Fadi Haidar, Jörg Halter, Dominik Heim, Christoph Hess, Sven Hillinger, Hans Hirsch, Patricia Hirt, Günther Hofbauer, Linard Hoessly, Uyen Huynh-Do, Franz Immer, Michael Koller, Bettina Laesser, Frédéric Lamoth, Roger Lehmann, Alexander Leichtle, Oriol Manuel, Hans-Peter Marti, Michele Martinelli, Valérie McLin, Katell Mellac, Aurélia Merçay, Karin Mettler, Nicolas Müller, Ulrike Müller-Arndt, Beat Müllhaupt, Mirjam Nägeli, Graziano Oldani, Manuel Pascual, Jakob Passweg, Rosemarie Pazeller, Klara Posfay-Barbe, Juliane Rick, Anne Rosselet, Simona Rossi, Silvia Rothlin, Frank Ruschitzka, Thomas Schachtner, Stefan Schaub, Alexandra Scherrer, Dominik Schneidawind, Aurelia Schnyder, Macé Schuurmans, Simon Schwab, Thierry Sengstag, Federico Simonetta, Jürg Steiger, Guido Stirnimann, Ueli Stürzinger, Christian Van Delden, Jean-Pierre Venetz, Jean Villard, Julien Vionnet, Madeleine Wick, Markus Wilhelm, Patrick Yerly.
Financial support. This study has been conducted in the framework of the Swiss Transplant Cohort Study, supported by the Swiss National Science Foundation and the Swiss University Hospitals and transplant centers. We thank all patients, doctors, and nurses associated with the Swiss Transplant Cohort Study (STCS). Data quality audits are funded by the Federal Office of Public Health of Switzerland.
Patient consent. All patients included in this study signed an informed consent form to participate in the STCT.
Ethical approval. This study has been approved by local ethical committees.
Contributor Information
Dionysios Neofytos, Transplant Infectious Diseases Unit, Division of Infectious Diseases, Geneva University Hospitals, Geneva, Switzerland.
Susanne Stampf, Clinic for Transplantation Immunology and Nephrology (Swiss Transplant Cohort Study), University Hospital of Basel, Basel, Switzerland.
Linard D Hoessly, Clinic for Transplantation Immunology and Nephrology (Swiss Transplant Cohort Study), University Hospital of Basel, Basel, Switzerland.
Matilde D’Asaro, Transplant Infectious Diseases Unit, Division of Infectious Diseases, Geneva University Hospitals, Geneva, Switzerland.
Gael Nguyen Tang, Transplant Infectious Diseases Unit, Division of Infectious Diseases, Geneva University Hospitals, Geneva, Switzerland.
Katia Boggian, Division of Infectious Diseases, Cantonal Hospital St Gallen, St Gallen, Switzerland.
Cedric Hirzel, Department of Infectious Diseases, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Nina Khanna, Division of Infectious Diseases and Hospital Epidemiology, University Hospital of Basel, Basel, Switzerland.
Oriol Manuel, Division of Infectious Diseases, University Hospital of Vaud, Lausanne, Switzerland.
Nicolas J Mueller, Division of Infectious Diseases and Hospital Epidemiology, University Hospital of Zurich, Switzerland.
Christian Van Delden, Transplant Infectious Diseases Unit, Division of Infectious Diseases, Geneva University Hospitals, Geneva, Switzerland.
for the Swiss Transplant Cohort Study:
Patrizia Amico, John-David Aubert, Vanessa Banz, Sonja Beckmann, Guido Beldi, Christoph Berger, Ekaterine Berishvili, Annalisa Berzigotti, Isabelle Binet, Pierre-Yves Bochud, Sanda Branca, Heiner Bucher, Thierry Carrel, Emmanuelle Catana, Anne Cairoli, Yves Chalandon, Sabina De Geest, Sophie De Seigneux, Michael Dickenmann, Joëlle Lynn Dreifuss, Michel Duchosal, Thomas Fehr, Sylvie Ferrari-Lacraz, Jaromil Frossard, Christian Garzoni, Déla Golshayan, Nicolas Goossens, Fadi Haidar, Jörg Halter, Dominik Heim, Christoph Hess, Sven Hillinger, Hans Hirsch, Patricia Hirt, Günther Hofbauer, Linard Hoessly, Uyen Huynh-Do, Franz Immer, Michael Koller, Bettina Laesser, Frédéric Lamoth, Roger Lehmann, Alexander Leichtle, Oriol Manuel, Hans-Peter Marti, Michele Martinelli, Valérie McLin, Katell Mellac, Aurélia Merçay, Karin Mettler, Nicolas Müller, Ulrike Müller-Arndt, Beat Müllhaupt, Mirjam Nägeli, Graziano Oldani, Manuel Pascual, Jakob Passweg, Rosemarie Pazeller, Klara Posfay-Barbe, Juliane Rick, Anne Rosselet, Simona Rossi, Silvia Rothlin, Frank Ruschitzka, Thomas Schachtner, Stefan Schaub, Alexandra Scherrer, Dominik Schneidawind, Aurelia Schnyder, Macé Schuurmans, Simon Schwab, Thierry Sengstag, Federico Simonetta, Jürg Steiger, Guido Stirnimann, Ueli Stürzinger, Christian Van Delden, Jean-Pierre Venetz, Jean Villard, Julien Vionnet, Madeleine Wick, Markus Wilhelm, and Patrick Yerly
References
- 1. Ye QF, Zhao J, Wan QQ, Qiao BB, Zhou JD. Frequency and clinical outcomes of ESKAPE bacteremia in solid organ transplantation and the risk factors for mortality. Transpl Infect Dis 2014; 16:767–74. [DOI] [PubMed] [Google Scholar]
- 2. Shao MJ, Wan QQ, Xie WZ, Ye QF. Bloodstream infections among solid organ transplant recipients: epidemiology, microbiology, associated risk factors for morbility and mortality. Transplant Rev-Orlan 2014; 28:176–81. [DOI] [PubMed] [Google Scholar]
- 3. Gavalda J, Aguado JM, Manuel O, Grossi P, Hirsch HH; ESCMID Study Group of Infection in Compromised Hosts . . A special issue on infections in solid organ transplant recipients. Clin Microbiol Infect 2014; 20(Suppl 7):1–3. [DOI] [PubMed] [Google Scholar]
- 4. Bodro M, Sabe N, Tubau F, et al. . Risk factors and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in solid-organ transplant recipients. Transplantation 2013; 96:843–9. [DOI] [PubMed] [Google Scholar]
- 5. Rodriguez C, Munoz P, Rodriguez-Creixems M, Yanez JF, Palomo JS, Bouza E. Bloodstream infections among heart transplant recipients. Transplantation 2006; 81:384–91. [DOI] [PubMed] [Google Scholar]
- 6. Husain S, Chan KM, Palmer SM, et al. . Bacteremia in lung transplant recipients in the current era. Am J Transplant 2006; 6:3000–7. [DOI] [PubMed] [Google Scholar]
- 7. Candel FJ, Grima E, Matesanz M, et al. . Bacteremia and septic shock after solid-organ transplantation. Transplant Proc 2005; 37:4097–9. [DOI] [PubMed] [Google Scholar]
- 8. Singh N, Paterson DL, Gayowski T, Wagener MM, Marino IR. Predicting bacteremia and bacteremic mortality in liver transplant recipients. Liver Transpl 2000; 6:54–61. [DOI] [PubMed] [Google Scholar]
- 9. Kritikos A, Manuel O. Bloodstream infections after solid-organ transplantation. Virulence 2016; 7:329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Delden C, Stampf S, Hirsch HH, et al. . Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss Transplant Cohort Study. Clin Infect Dis 2020; 71:e159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koller MT, van Delden C, Muller NJ, et al. . Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long-term follow-up cohort. Eur J Epidemiol 2013; 28:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donnelly JP, Chen SC, Kauffman CA, et al. . Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Humar A, Michaels M, AST ID Working Group on Infectious Disease Monitoring . American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant 2006; 6:262–74. [DOI] [PubMed] [Google Scholar]
- 14. Magiorakos AP, Srinivasan A, Carey RB, et al. . Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. [DOI] [PubMed] [Google Scholar]
- 15. Neofytos D, Hirzel C, Boely E, et al. . Pneumocystis jirovecii pneumonia in solid organ transplant recipients: a descriptive analysis for the Swiss Transplant Cohort. Transpl Infect Dis 2018; 20:e12984. [DOI] [PubMed] [Google Scholar]
- 16. Bert F, Larroque B, Paugam-Burtz C, et al. . Microbial epidemiology and outcome of bloodstream infections in liver transplant recipients: an analysis of 259 episodes. Liver Transpl 2010; 16:393–401. [DOI] [PubMed] [Google Scholar]
- 17. Moreno A, Cervera C, Gavalda J, et al. . Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am J Transplant 2007; 7:2579–86. [DOI] [PubMed] [Google Scholar]
- 18. Palmer SM, Alexander BD, Sanders LL, et al. . Significance of blood stream infection after lung transplantation: analysis in 176 consecutive patients. Transplantation 2000; 69:2360–6. [DOI] [PubMed] [Google Scholar]
- 19. Iida T, Kaido T, Yagi S, et al. . Posttransplant bacteremia in adult living donor liver transplant recipients. Liver Transpl 2010; 16:1379–85. [DOI] [PubMed] [Google Scholar]
- 20. Hsu RB, Chang CI, Fang CT, Chang SC, Wang SS, Chu SH. Bloodstream infection in heart transplant recipients: 12-year experience at a university hospital in Taiwan. Eur J Cardiothorac Surg 2011; 40:1362–7. [DOI] [PubMed] [Google Scholar]
- 21. Abbott KC, Oliver JD III, Hypolite I, et al. . Hospitalizations for bacterial septicemia after renal transplantation in the United States. Am J Nephrol 2001; 21:120–7. [DOI] [PubMed] [Google Scholar]
- 22. Kim SI, Kim YJ, Jun YH, et al. . Epidemiology and risk factors for bacteremia in 144 consecutive living-donor liver transplant recipients. Yonsei Med J 2009; 50:112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hashimoto M, Sugawara Y, Tamura S, et al. . Bloodstream infection after living donor liver transplantation. Scand J Infect Dis 2008; 40(6–7):509–16. [DOI] [PubMed] [Google Scholar]
- 24. Johnson LE, D'Agata EMC, Paterson DL, et al. . Pseudomonas aeruginosa bacteremia over a 10-year period: multidrug resistance and outcomes in transplant recipients. Transpl Infect Dis 2009; 11:227–34. [DOI] [PubMed] [Google Scholar]
- 25. Linares L, Cervera C, Hoyo I, et al. . Klebacteremiaella pneumoniae infection in solid organ transplant recipients: epidemiology and antibiotic resistance. Transplant Proc 2010; 42:2941–3. [DOI] [PubMed] [Google Scholar]
- 26. Shi SH, Kong HS, Xu J, et al. . Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transpl Infect Dis 2009; 11:405–12. [DOI] [PubMed] [Google Scholar]
- 27. Bedini A, Codeluppi M, Cocchi S, et al. . Gram-positive bloodstream infections in liver transplant recipients: incidence, risk factors, and impact on survival. Transplant Proc 2007; 39:1947–9. [DOI] [PubMed] [Google Scholar]
- 28. Lee SO, Kang SH, Abdel-Massih RC, Brown RA, Razonable RR. Spectrum of early-onset and late-onset bacteremias after liver transplantation: implications for management. Liver Transpl 2011; 17:733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonatti H, Tabarelli W, Ruttmann E, et al. . Impact of cytomegalovirus match on survival after cardiac and lung transplantation. Am Surg 2004; 70:710–4. [PubMed] [Google Scholar]
- 30. Estenne M, Maurer JR, Boehler A, et al. . Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002; 21:297–310. [DOI] [PubMed] [Google Scholar]
- 31. Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for Bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant 2002; 21:271–81. [DOI] [PubMed] [Google Scholar]
- 32. Zamora MR. Cytomegalovirus and lung transplantation. Am J Transplant 2004; 4:1219–26. [DOI] [PubMed] [Google Scholar]
- 33. Singh R, Bemelman FJ, Hodiamont CJ, Idu MM, Ten Berge IJ, Geerlings SE. The impact of trimethoprim-sulfamethoxazole as Pneumocystis jiroveci pneumonia prophylaxis on the occurrence of asymptomatic bacteriuria and urinary tract infections among renal allograft recipients: a retrospective before-after study. BMC Infect Dis 2016; 16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giullian JA, Cavanaugh K, Schaefer H. Lower risk of urinary tract infection with low-dose trimethoprim/sulfamethoxazole compared to dapsone prophylaxis in older renal transplant patients on a rapid steroid-withdrawal immunosuppression regimen. Clin Transplant 2010; 24:636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fu W, Barahona M, Harkness T, Cohen E, Reardon D, Yoo PS. Higher risk of urinary tract infections in renal transplant recipients receiving pentamidine versus trimethoprim-sulfamethoxazole (TMP-SMX) for Pneumocystis pneumonia prophylaxis. Clin Transplant 2020; 34:e14067. [DOI] [PubMed] [Google Scholar]
- 36. Tolkoff-Rubin NE, Cosimi AB, Russell PS, Rubin RH. A controlled study of trimethoprim-sulfamethoxazole prophylaxis of urinary tract infection in renal transplant recipients. Rev Infect Dis 1982; 4:614–8. [DOI] [PubMed] [Google Scholar]
- 37. Fox BC, Sollinger HW, Belzer FO, Maki DG. A prospective, randomized, double-blind study of trimethoprim-sulfamethoxazole for prophylaxis of infection in renal transplantation: clinical efficacy, absorption of trimethoprim-sulfamethoxazole, effects on the microflora, and the cost-benefit of prophylaxis. Am J Med 1990; 89:255–74. [DOI] [PubMed] [Google Scholar]
- 38. Al-Hasan MN, Razonable RR, Eckel-Passow JE, Baddour LM. Incidence rate and outcome of gram-negative bloodstream infection in solid organ transplant recipients. Am J Transplant 2009; 9:835–43. [DOI] [PubMed] [Google Scholar]
- 39. Daskalaki E, Koukoulaki M, Bakalis A, et al. . Blood stream infections in renal transplant recipients: a single-center study. Transplant Proc 2014; 46:3191–3. [DOI] [PubMed] [Google Scholar]
- 40. Silva M, Marra AR, Pereira CAP, Medina-Pestana JO, Camargo LFA. Bloodstream infection after kidney transplantation: epidemiology, microbiology, associated risk factors, and outcome. Transplantation 2010; 90:581–7. [DOI] [PubMed] [Google Scholar]
- 41. Rasmussen DB, Moller DL, Knudsen AD, et al. . Enterococcal infections the first year after liver transplantation—a prospective cohort study. Microorganisms 2021; 9:1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giannella M, Bartoletti M, Morelli MC, et al. . Risk factors for infection with carbapenem-resistant Klebacteremiaella pneumoniae after liver transplantation: the importance of pre- and posttransplant colonization. Am J Transplant 2015; 15:1708–15. [DOI] [PubMed] [Google Scholar]
- 43. Buetti N, Marschall J, Timsit JF, et al. . Distribution of pathogens and antimicrobial resistance in bacteraemia according to hospitalization duration: a nationwide surveillance study in Switzerland. Clin Microbiol Infect 2021; 27:1820–5. [DOI] [PubMed] [Google Scholar]
- 44. Barnsteiner S, Baty F, Albrich WC, et al. . Antimicrobial resistance and antibiotic consumption in intensive care units, Switzerland, 2009 to 2018. Euro Surveill 2021; 26:2001537.. [DOI] [PMC free article] [PubMed] [Google Scholar]