Abstract
Objective: To find out the determinants of minimum dietary diversity (MDD) among under-five children in East Africa based on the 2017 revised indicator.
Methods: Secondary data from the demographic and health survey (DHS) of eight countries in East Africa were combined. A total of 27,223 weighted samples of children aged 6–59 months were included. Multi-level logistic regression analysis was employed to identify the determinants of dietary diversity.
Results: The magnitude of adequate MDD in East Africa was found to be 10.47% with 95% CI (10.12–10.84) with the lowest and highest magnitude in Ethiopia and Rwanda respectively. Having a mother in the age group of 35–49, having a mother with higher educational attainment, and having a post-natal check-up within 2 months were significant factors in determining adequate MDD.
Conclusion: The magnitude of adequate MDD intake among children aged 6–59 months in East Africa is relatively low. Therefore, strengthening interventions focused on improving the economic status of households, the educational status of mothers, and diversified food consumption of children aged 6–59 months should get priority to improve the recommended feeding practice of children.
Keywords: multilevel analysis, dietary diversity, East Africa, DHS, 6–59 months
Introduction
Dietary diversity is a quantitative number of food items or groups used as a method of determining the variety and nutrient adequacy of diets for an individual [1]. The world health organization (WHO) recommends that children should consume a diverse range of nutritionally balanced, suitable, and healthy foods to meet their nutritional requirements [2]. However, globally, less than a quarter of children aged 6–23 months satisfy the recommended standards for dietary diversity, and only a small portion of them receive a diet that is nutritionally sufficient [3].
Of the total 10.9 million deaths among children under the age of 5, malnutrition is directly or indirectly accountable for 60% of these fatalities. Each year, over 3.4 million children under the age of 5 die as a result of inadequate feeding practices. Among these deaths, approximately two-thirds are linked to inappropriate feeding practices that occur within the first 2 years of life [4, 5]. Less than one-quarter of children are reported to be malnourished in many nations, especially during the important first 1,000 days of life [6, 7]. The prevalence of adequate minimum dietary diversity was 46.5% in Nepal [8], 23%–63.85% in India [9, 10], 53.3% in Indonesia [11], and 71% in Sri Lanka [12].
In sub-Saharan African countries, the overall prevalence of minimum dietary diversity was 25.1%, with the highest and lowest in South Africa (43.9%) and Burkina Faso (5.6%), respectively [13] and in Ethiopia, it was 23.25% among children age 6–23 months [14].
The United Nations Sustainable Development Goals (SDGs) aim to enhance the health and wellbeing of all children by eliminating preventable deaths among newborns and children under the age of five by the year 2030. In order to achieve this objective, it is crucial for the SDGs to place a strong emphasis on nutrition priorities. Improvements in child nutrition play a significant role in attaining these sustainable development goals [15]. Dietary diversity is a good predictor of dietary quality and micronutrient density. Children residing in low and middle-income countries are known to experience micronutrient deficiencies, primarily caused by poor diet quality [16, 17]. Inadequate dietary diversity is a significant issue among impoverished populations in the developing world, particularly in Africa [18].
Attaining the minimum dietary diversity (MDD) for children under the age of five in sub-Saharan Africa (SSA) presents a major challenge due to the prevalence of poverty. Many parents in the region face low-income conditions, which hinder their ability to afford and provide appropriate complementary feeding practices for their infants, as well as meet the minimum requirements for dietary diversity among young children [19, 20]. For children who are not breastfed, it is recommended to provide them with meals four or five times a day, along with one to two snacks as desired. The frequency of meals serves as an indicator of energy intake from sources other than breast milk. Thus, when assessing feeding frequency for non-breastfed children, both milk feeds and solid or semi-solid feeds are taken into account [21, 22].
Shreds of evidence revealed maternal healthcare utilization status, sociodemographic factors like maternal education, residence, mother’s occupation, economic factors, and media exposure of the mothers were identified as determinants of dietary diversity [23–28].
So far, many small-scale studies have been conducted on child dietary diversity among children under five, but based on a cut-off of four out of seven food groups. The indicator was revised in 2017 to add breast milk as a separate food group, thereby increasing the total number of food groups to eight and increasing the cut-off to five groups. The indicator was revised because the previous indicator included infant formula but not breast milk. Therefore, this study is intended to find out the determinants of dietary diversity among children under five in East Africa based on the revised indicator.
Methods
Data Source, Tool, and Sampling Procedure
The data for this research was taken from the Measure Demographic and Health Survey (DHS) program, which can be accessed at www.measuredhs.com. The DHS program covers over 90 countries with low- and middle-income economies, allowing for a comprehensive collection of data. The data obtained from the program is designed to be comparable across different countries, enabling meaningful cross-country analysis and comparison. This study strictly adhered to the relevant statistical guidelines established by the DHS program. The program ensured uniformity by employing the same manual, data collection instrument, variable names, variable codes, and sampling process across all participating countries. By following these standardized protocols, the study maintains consistency and upholds the integrity of the data [29]. Data from the DHS were combined from 2015 to 2020 for eight countries in East Africa. During the designated period, the most recent DHS of Country-specific datasets were extracted. The 8 East Africa Countries from which data were extracted with their corresponding DHS year are Burundi (2017), Ethiopia (2016), Malawi (2016), Rwanda (2020), Tanzania (2016), Uganda (2016), Zambia (2018), and Zimbabwe (2015). The DHS program ensures the collection of comparable data across countries worldwide through the adoption of standardized methods. This includes the use of uniform questionnaires, manuals, and field procedures. The surveys conducted by DHS are nationally representative household surveys that involve face-to-face interviews with women aged 15–49 years. These surveys provide comprehensive information on various indicators related to population, health, and nutrition, making them valuable for monitoring and evaluating impacts in these areas. The surveys apply a stratified, multi-stage, random sampling design. Information was obtained from eligible women aged 15–49 years in each country. Survey methodology and sampling methods used to gather the data have been described in detail elsewhere [30].
Population and Sample Size
The source population for this study was all children aged 6–59 months in East Africa, whereas all children aged 6–59 months in East Africa who were in the selected countries were the study population. Specific children who have no record of the outcome variable were excluded.
The data sets were extracted from kids record (KR) files, which contain information about women and children, for this specific research, and extracting important variables related to adequate dietary diversity were performed from the data set. Accordingly, a total weighted sample of 27,223 who fulfilled the inclusion criteria was included in this study. This includes Burundi (3,592), Ethiopia (5,165), Malawi (2,388), Rwanda (1,895), Tanzania (4,938), Uganda (2,401), Zambia (4,370), and Zimbabwe (2,473).
Measurement
The outcome variable of this study was Minimum Dietary Diversity (MDD). MDD was measured as the percentage of children aged 6–59 months who have consumed foods and beverages from at least five out of eight defined food groups within the previous day. The outcome variable is binary, and it is coded as 1 if the children consumed at least five food categories in the last 24 h before an interview, were considered to have met the MDD requirements, and their MDD score was categorized as having adequate MDD. The variable is coded as 0 if the children consumed less than five food categories in the last 24 h before an interview, were considered to have not met the MDD requirements, and their MDD score was categorized as having inadequate MDD.
Based on different kinds of literature we have included two types of independent variables that are individual-level and community-level variables. The individual-level variables are the age of a child, sex of the child, age of the mother, mother’s age at 1st birth, marital status, educational status of the mother, occupational status of the mother, parity, family size, wealth index, number of ANC visits, media exposure, birth order, place of delivery, and child meal frequency. The community-level variables are residence, community-level education, and country.
Media Exposure: was determined based on the respondents’ exposure to newspapers/magazines, radio, and television. If the respondents reported using at least one of these media sources, they were considered to have media exposure.
Community-level Education: was constructed by combining individual-level variables to capture the impact of the neighborhood or community on minimum dietary diversity. Individual-level values were aggregated at the cluster level to create the community-level variable. The aggregated variables were then categorized as either “high” or “low” based on the distribution of proportion values calculated for each cluster or community.
Data Management and Analysis
To ensure the survey’s representativeness and account for the sampling design when calculating standard errors and providing valid statistical estimates, the data were weighted using various factors. These factors include sampling weights, primary sampling units, and strata. Before any statistical analysis was conducted, these weights were applied to the data. We have used the sampling weight from DHS individual sample weights which are generated by dividing (v005) by 1,000,000 before using it to approximate the number of cases. STATA version 17 was used for analysis.
Multi-Level Analysis
Given the hierarchical nature of DHS data, there is a possibility of a cluster effect, which can violate the assumption of independent observations and equal variance across clusters. To account for this, and considering the binary nature of the outcome variable, a two-level mixed-effects logistic regression analysis was conducted. This approach allows for the consideration of between-cluster variability and helps to obtain reliable standard errors and unbiased estimates. By incorporating random effects at the cluster level, the analysis properly addresses the potential clustering effect, providing a more accurate assessment of the relationship between variables and the outcome of interest [31, 32]. In the analysis of determinants for Minimum Dietary Diversity (MDD), the individual and community-level variables were initially examined independently using a bivariable multilevel logistic regression model. In this step, variables were assessed for their association with MDD, and those that demonstrated statistical significance at a p-value of 0.2 in the bivariable analysis were selected for the individual and community level model adjustments. Finally, a multivariable multilevel mixed-effects analysis was conducted to identify the significant determinants of Minimum Dietary Diversity (MDD). In this analysis, variables that exhibited a p-value of ≤0.05 were considered statistically significant determinants of MDD.
Model Building
Four models were fitted in the analysis. The first model, referred to as the null model, did not include any exposure variables. Its purpose was to assess the variation at the community level and provide evidence to evaluate random effects at the community level. The second model was the adjustment of the multiple variable model for individual variables and the third model was adjusted to consider factors at the community level. Whereas, in the fourth model, potential candidate variables from individual and community variables were adjusted to the outcome variable. The model with smaller AIC and BIC was considered as a parsimonious model.
Parameter Estimation Method
Fixed effects were employed to estimate the association between the probability of Minimum Dietary Diversity (MDD) and explanatory variables at both the community and individual levels. The results of this estimation were expressed as odds ratios along with their corresponding 95% confidence intervals.
In assessing the measures of variation or random effects in the analysis, several indicators were utilized, including the intra-cluster correlation coefficient (ICC), the proportional change in variance (PCV), and the median odds ratio (MOR).
The intra-cluster correlation coefficient (ICC) measures the proportion of the total variation in Minimum Dietary Diversity (MDD) outcomes that is attributable to differences between communities. A higher ICC value indicates greater clustering or similarity of MDD outcomes within communities.
The proportional change in variance (PCV) quantifies the percentage of variation in MDD outcomes explained by the inclusion of explanatory variables in the model. It provides insights into the extent to which the individual and community-level variables account for the observed variation in MDD.
The median odds ratio (MOR) is a measure that assesses the extent of between-community variation by comparing the odds of MDD for two randomly chosen individuals from different communities. It provides an understanding of the potential influence of unmeasured community-level factors on MDD.
The purpose of the Median Odds Ratio (MOR) is to translate the area level variance in the widely used odds ratio (OR) scale that has a consistent and intuitive interpretation.
It is computed by;
Where; VA is the area level variance, and 0.6745 is the 75th centile of the cumulative distribution function of the normal distribution with mean 0 and variance 1. See elsewhere for a more detailed explanation. Whereas the PCV is calculated as:
| (1) |
Where; where = variance of the initial model, and = variance of the model with more terms.
By using these measures of variation, the study aimed to evaluate the clustering effect and assess the impact of individual and community-level variables on the observed variation in MDD outcomes.
Results
Socio-Demographic and Economic Characteristics
A total of 27,223 children aged 6–59 months were included in this study. Most of the children 9,650 (35.45%) were between the age of 6–15 months, and the mean age of the child with a standard deviation is 25.83 ± 16.12 months. Exactly, half 50.0% of the children’s mothers had a primary education. The majority of the children 22,130 (81.29%) were from rural areas. The largest number of children 5,165 (18.97%) were from Ethiopia, while the smallest number of children 1,895 (6.96%) were from Rwanda (Table 1).
TABLE 1.
Socio-demographic and economic characteristics of under-five children in East Africa countries from 2015 to 2020.
| Variables | Weighted frequency | Percentage (%) |
|---|---|---|
| Age of Child (Months) | ||
| 6–23 | 16,578 | 61.09 |
| 24–59 | 10,559 | 38.91 |
| Sex of Child | ||
| Female | 13,602 | 49.97 |
| Male | 13,620 | 50.03 |
| Mothers Age (Years) | ||
| 15–24 | 8,215 | 30.18 |
| 25–34 | 13,290 | 48.82 |
| 35–49 | 5,717 | 21.00 |
| Marital Status | ||
| Not Married | 1,180 | 4.33 |
| Ever Married | 26,042 | 95.67 |
| Mothers Education | ||
| No Education | 7,430 | 27.30 |
| Primary | 13,611 | 50.00 |
| Secondary | 5,467 | 20.08 |
| Higher | 714 | 2.62 |
| Mothers occupation | ||
| No Occupation | 8,883 | 32.63 |
| Had Occupation | 18,339 | 67.37 |
| Sex of HH Head | ||
| Female | 21,899 | 80.45 |
| Male | 5,323 | 19.55 |
| Wealth Index | ||
| Poor | 13,110 | 48.16 |
| Middle | 5,359 | 19.69 |
| Rich | 8,753 | 32.15 |
| Media Exposure | ||
| No | 11,414 | 41.93 |
| Yes | 15,808 | 58.07 |
| Partner Education (24,209) | ||
| No Education | 5,394 | 22.28 |
| Primary | 12,042 | 49.74 |
| Secondary | 5,612 | 23.18 |
| Higher | 1,161 | 4.79 |
| Number of HH Members | ||
| ≤5 | 12,135 | 44.58 |
| 6 and Above | 15,087 | 55.42 |
| Number of under 5 children | ||
| ≤2 | 20,741 | 76.19 |
| 3 and Above | 6,481 | 23.81 |
| Age at 1st birth | ||
| <20 | 16,324 | 59.00 |
| 20–30 | 11,079 | 40.05 |
| 31–49 | 263 | 0.95 |
| Community Level Education | ||
| Lower | 6,379 | 23.43 |
| Higher | 20,843 | 76.57 |
| Residence | ||
| Rural | 22,130 | 81.29 |
| Urban | 5,092 | 18.71 |
| Country | ||
| Burundi | 3,592 | 13.20 |
| Ethiopia | 5,165 | 18.97 |
| Malawi | 2,388 | 8.77 |
| Rwanda | 1,895 | 6.96 |
| Tanzania | 4,938 | 18.14 |
| Uganda | 2,401 | 8.82 |
| Zambia | 4,370 | 16.05 |
| Zimbabwe | 2,473 | 9.08 |
Service-Related Characteristics
Nearly half (45.43%) of the mothers did not have any ANC visits at all. The majority of the mothers (18,563, 68.19%) delivered at a health facility. Only 5,885 (36.35%) mothers of children aged 6–59 months had Post-natal checks within 2 months. Most of the children (5,990, 36.89%) had their meal twice per day (Table 2).
TABLE 2.
Service related characteristics of children under five in East Africa countries from 2015 to 2020.
| Variables | Weighted frequency | Percentage (%) |
|---|---|---|
| Number of ANC Visit | ||
| No Visit | 12,366 | 45.43 |
| 1–3 Visit | 6,268 | 23.02 |
| 4 and above visit | 8,588 | 31.55 |
| Child Twin | ||
| No | 26,585 | 97.66 |
| Yes | 637 | 2.34 |
| Birth Order | ||
| First Order | 6,350 | 23.33 |
| Two to Fourth order | 13,104 | 48.14 |
| Fifth and above order | 7,768 | 28.54 |
| Place of Delivery | ||
| Home | 8,659 | 31.81 |
| Health Institution | 18,563 | 68.19 |
| Post-natal check with in 2 months (n = 16,189) | ||
| No | 10,304 | 63.65 |
| Yes | 5,885 | 36.35 |
| Meal Frequency (n = 16,236) | ||
| One Time | 3,792 | 23.35 |
| Two Time | 5,990 | 36.89 |
| Three Time | 4,419 | 27.22 |
| Four and above | 2,035 | 12.54 |
The Proportion of Each of the Eight Food Groups
The following figure presents the proportion of dietary consumption for each of the eight food groups. The majority of children aged 6–59 months in East Africa (88.99%) and (83.76%) cannot eat eggs and dairy products, respectively. However, children aged 6–59 months in East Africa have better access to breast milk (85.89%) and cereals and roots (61.16%) (Figure 1).
FIGURE 1.
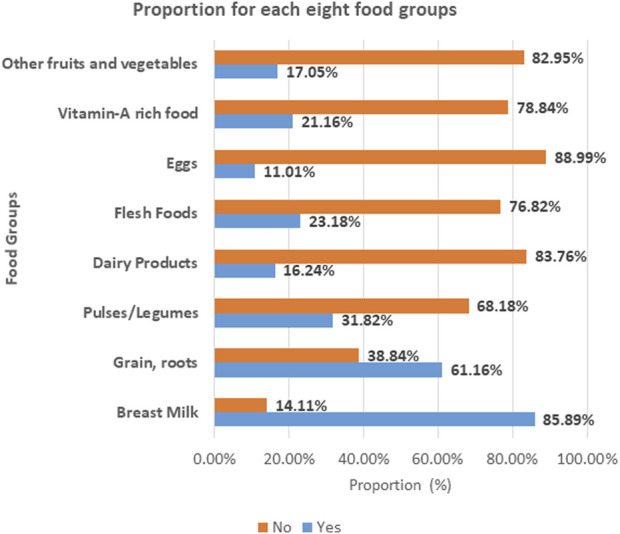
The proportion of each of the eight food groups in East Africa (2015–2020).
Minimum Dietary Diversity Proportion
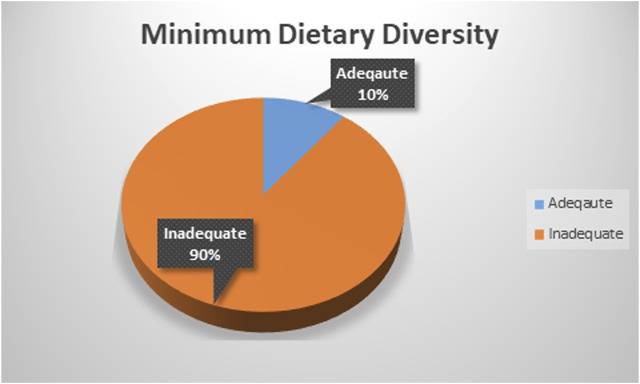
The magnitude of adequate minimum dietary diversity in East Africa was found to be 10.47% with 95% CI (10.12–10.84) ranging from 6.81% with 95% CI (6.15–7.52) in Ethiopia to 16.22% with 95% CI (14.63–17.94) in Rwanda (Figure 2).
FIGURE 2.
Magnitude of adequate minimum dietary diversity in East Africa (2015–2020).
Bi-Variable Model
Most of the children who had a mother aged 25–34 years had adequate MDD. The highest adequate MDD was reported for children whose mothers had a primary education and, educational attainment was significant in determining the MDD. In addition, the highest adequate MDD was reported among children whose families were rich and the wealth index was significant in determining the MDD (Table 3).
TABLE 3.
Minimum Dietary Diversity of Children aged 6-59 months in East Africa countries from 2015 to 2020 and Bi-Variable Multilevel Mixed-Effect Logistic Regression in COR (n = 27,655).
| Variables | Minimum dietary diversity | COR (95% CI) | |
|---|---|---|---|
| Not adequate | Adequate | ||
| Age of Child (Months) | |||
| 6–23 | 14,593 | 1,985 | 1.80 (1.63–1.99)** |
| 24–59 | 9,766 | 793 | Ref. |
| Mothers Age (Years) | |||
| 15–24 | 7,396 | 9,820 | Ref. |
| 25–34 | 11,778 | 1,511 | 1.13 (1.01–1.27)* |
| 35–49 | 5,196 | 521 | 0.82 (0.71–0.94)* |
| Mothers Education | |||
| No Education | 7,053 | 378 | Ref. |
| Primary | 12,294 | 1,317 | 1.97 (1.69–2.29)** |
| Secondary | 4,548 | 918 | 4.14 (3.47–4.94)** |
| Higher | 475 | 239 | 10.68 (8.05–14.16)** |
| Mothers occupation | |||
| No Occupation | 8,100 | 783 | Ref. |
| Had Occupation | 16,270 | 2,069 | 1.38 (1.23–1.56)** |
| Sex of HH Head | |||
| Female | 4,847 | 476 | Ref. |
| Male | 19,523 | 2,376 | 1.44 (1.26–1.63)** |
| Wealth Index | |||
| Poor | 12,318 | 792 | Ref. |
| Middle | 4,916 | 443 | 1.44 (1.24–1.67)** |
| Rich | 7,136 | 1,617 | 3.75 (3.28–4.29)** |
| Media Exposure | |||
| No | 10,744 | 670 | Ref. |
| Yes | 13,626 | 2,182 | 2.50 (2.22–2.82)** |
| Partner Education (n = 24,209) | |||
| No Education | 5,122 | 272 | Ref. |
| Primary | 10,878 | 1,164 | 1.93 (1.62–2.29)** |
| Secondary | 4,791 | 821 | 3.41 (2.80–4.15)** |
| Higher | 829 | 332 | 8.94 (6.86–11.64)** |
| Number of HH Members | |||
| ≤5 | 10,688 | 1,447 | Ref. |
| 6 and Above | 13,682 | 1,405 | 0.77 (0.70–0.85)** |
| Number of under 5 children | |||
| ≤2 | 18,344 | 2,397 | Ref. |
| 3 and Above | 6,026 | 455 | 0.55 (0.49–0.63)** |
| Age at 1st birth | |||
| <20 | 14,640 | 1,395 | Ref. |
| 20–30 | 9,518 | 1,409 | 1.55 (1.39–1.71)** |
| 31–49 | 212 | 48 | 1.91 (1.22–2.98)** |
| Community Level Education | |||
| Lower | 6,019 | 360 | Ref. |
| Higher | 18,351 | 2,492 | 2.21 (1.79–2.70)** |
| Residence | |||
| Rural | 20,295 | 1,834 | Ref. |
| Urban | 4,075 | 1,018 | 3.55 (2.96–4.26)** |
| Country | |||
| Burundi | 3,281 | 311 | 1.48 (1.04–2.10) * |
| Ethiopia | 4,813 | 352 | Ref. |
| Malawi | 2,048 | 340 | 3.14 (2.23–4.41)** |
| Rwanda | 1,588 | 307 | 4.14 (2.89–5.92)** |
| Tanzania | 4,407 | 531 | 2.70 (1.94–3.76)** |
| Uganda | 2,056 | 345 | 2.79 (1.97–3.3.95)** |
| Zambia | 3,949 | 421 | 1.58 (1.12–2.23)** |
| Zimbabwe | 2,228 | 245 | 1.77 (1.21–2.59)** |
| Number of ANC Visit | |||
| No Visit | 11,436 | 931 | Ref. |
| 1–3 Visit | 5,550 | 717 | 1.66 (1.47–1.88)** |
| 4 and above visit | 7,384 | 1,204 | 1.98 (1.77–2.21)** |
| Child Twin | |||
| No | 23,782 | 2,803 | Ref. |
| Yes | 588 | 49 | 0.51 (0.35–0.73)** |
| Birth Order | |||
| First Order | 5,563 | 787 | Ref. |
| Two to Fourth order | 11,670 | 1,433 | 0.83 (0.74–0.92)** |
| Fifth and above order | 7,137 | 632 | 0.64 (0.56–0.74)** |
| Place of Delivery | |||
| Home | 8,174 | 486 | Ref. |
| Health Institution | 16,196 | 2,366 | 2.17 (1.88–2.50)** |
| Post-natal check with in 2 months (n = 16,189) | |||
| No | 9,142 | 1,164 | Ref. |
| Yes | 5,023 | 862 | 1.30 (1.15–1.46)** |
| Meal Frequency (n = 16,458) | |||
| One Time | 3,464 | 328 | Ref. |
| Two Time | 5,340 | 649 | 1.38 (1.17–1.62)** |
| Three Time | 3,791 | 628 | 2.14 (1.80–2.54)** |
| Four and above | 1,613 | 423 | 3.44 (2.84–4.17)** |
The symbol “**” implies the statistical significance with p-value less than 0.001 and the symbol “*, **” implies the statistical significance with p-value less than 0.05.
Determinants of Dietary Diversity
Random Effects
The results of the null model revealed that there was statistically significant variability in the odds of MDD with community variance (S.E.) of 3.47 (0.20). Likewise, the ICC in the null model showed that 51.38% of the total variance was due to differences between communities. The MOR was also 5.87 which is significant. This suggests that the likelihood of having adequate MDD was 5.87 times higher when respondents moved from low-risk to high-risk communities. This has shown a significant heterogeneity in MDD between different communities. In the full model (adjusted for individual and community factors), the community variance (community variance = 1.56; SE = 0.14) remained significant but decreased. Approximately 32.20% of the total variance of adequate MDD that can be attributed to contextual factors remained significant even after accounting for some contextual risk factors. The PCV in this model was 55.04%, indicating that 55.04% of the community variance observed in the null model was explained by the community as well as individual variables (Table 4).
TABLE 4.
Multivariable multilevel mixed-effect logistic regression analysis of determinants of dietary diversity in East Africa countries from 2015 to 2020.
| Variables | Models | |||
|---|---|---|---|---|
| Null model | Model-I | Model-II | Model-II | |
| AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| Age of Child (Months) | ||||
| 6–23 | — | 1.33 (0.18–9.96) | — | 1.21 (0.16–9.11) |
| 24–59 | — | Ref. | — | Ref. |
| Child Twin | ||||
| No | — | Ref. | — | Ref. |
| Yes | — | 0.59 (0.33–1.05) | — | 0.55 (0.31–0.98)* |
| Mothers Age (Years) | ||||
| 15–24 | — | Ref. | — | Ref. |
| 25–34 | — | 1.01 (0.84–1.21) | — | 1.02 (0.84–1.22) |
| 35–49 | — | 0.70 (0.54–0.92)* | — | 0.70 (0.53–0.91)* |
| Mothers Education | ||||
| No Education | — | Ref. | — | Ref. |
| Primary | — | 1.47 (1.20–1.80)** | — | 1.41 (1.12–1.77)** |
| Secondary | — | 1.90 (1.48–2.46)** | — | 1.98 (1.50–2.63)** |
| Higher | 3.03 (2.02–4.53)** | — | 3.04 (1.99–4.64)** | |
| Mothers occupation | ||||
| No Occupation | — | Ref. | — | Ref. |
| Had Occupation | — | 1.41 (1.22–1.62)** | — | 1.29 (1.11–1.49)** |
| Birth Order | ||||
| First Order | — | Ref. | — | Ref. |
| Two to Fourth order | — | 0.99 (0.83–1.20) | — | 1.01 (0.84–1.22) |
| Fifth and above order | — | 1.24 (0.93–1.64) | — | 1.28 (0.96–1.70) |
| Sex of HH Head | ||||
| Female | — | Ref. | — | Ref. |
| Male | — | 1.42 (1.18–1.70)** | — | 1.41 (1.17–1.69)** |
| Wealth Index | ||||
| Poor | — | Ref. | — | Ref. |
| Middle | — | 1.29 (1.07–1.54)* | — | 1.27 (1.05–1.52)* |
| Rich | — | 2.07 (1.74–2.46)** | — | 1.85 (1.53–2.24)** |
| Media Exposure | ||||
| No | — | Ref. | — | Ref. |
| Yes | — | 1.53 (1.32–1.79)** | — | 1.44 (1.23–1.68)** |
| Partner Education | ||||
| No Education | — | Ref. | — | Ref. |
| Primary | — | 1.29 (1.04–1.60)* | — | 1.25 (1.01–1.55)* |
| Secondary | — | 1.38 (1.07–1.77)* | — | 1.50 (1.16–1.93)** |
| Higher | — | 2.00 (1.42–2.83)* | — | 2.03 (1.43–2.89)** |
| Number of HH Members | ||||
| ≤5 | — | Ref. | — | Ref. |
| 6 and Above | — | 0.92 (0.79–1.07) | — | 0.81 (0.85–1.10) |
| Number of under 5 children | ||||
| ≤2 | — | Ref. | — | Ref. |
| 3 and Above | — | 0.86 (0.71–01.06) | — | 0.88 (0.72–1.08) |
| Age at 1st birth | ||||
| <20 | — | Ref. | — | Ref. |
| 20–30 | — | 1.38 (1.19–1.59)** | — | 1.32 (1.14–1.52)** |
| 31–49 | — | 1.41 (0.80–2.47) | — | 1.28 (0.73–2.25) |
| Number of ANC Visit | ||||
| No Visit | — | Ref. | — | Ref. |
| 1–3 Visit | — | 1.03 (0.75–1.40) | — | 0.92 (0.66–1.26) |
| 4 and above visit | — | 0.99 (0.0.73–1.36) | — | 0.95 (0.69–1.30) |
| Place of Delivery | ||||
| Home | — | Ref. | — | Ref. |
| Health Institution | — | 1.36 (1.13–1.65)** | — | 1.26 (1.04–1.54)* |
| Post-natal check with in 2 months | ||||
| No | — | Ref. | — | Ref. |
| Yes | — | 1.09 (0.95–1.26) | — | 1.24 (1.07–1.45)** |
| Meal Frequency | ||||
| One Time | — | Ref. | — | Ref. |
| Two Time | — | 1.38 (1.15–1.65)** | — | 1.37 (1.14–1.64)** |
| Three Time | — | 2.07 (1.72–2.50)** | — | 2.09 (1.73–2.52)** |
| Four and above | — | 2.83 (2.29–3.50)** | — | 2.96 (2.39–3.66)** |
| Community Level Education | ||||
| Lower | — | — | Ref. | Ref. |
| Higher | — | — | 1.60 (1.30–1.97)** | 0.95 (0.72–1.25) |
| Residence | ||||
| Rural | — | — | Ref. | Ref. |
| Urban | — | — | 3.62 (3.02–4.34)** | 1.33 (1.08–1.63)** |
| Country | ||||
| Ethiopia | — | — | Ref. | Ref. |
| Burundi | — | — | 1.47 (1.05–2.07)* | 1.29 (0.91–1.83) |
| Malawi | — | — | 2.92 (2.09–4.08)** | 2.04 (1.44–2.89)** |
| Rwanda | — | — | 3.52 (2.48–5.01)** | 2.08 (1.44–3.00)** |
| Tanzania | — | — | 2.17 (1.58–2.99)** | 1.45 (1.05–2.01)* |
| Uganda | — | — | 2.38 (1.70–3.35)** | 1.63 (1.15–2.33)** |
| Zambia | — | — | 1.13 (0.82–1.59) | 0.77 (0.54–1.09) |
| Zimbabwe | — | — | 1.23 (0.85–1.79) | 0.65 (0.44–0.96)* |
| Random Effects | ||||
| Community Variance (SE) | 3.47 (0.22) | 1.63 (0.15) | 2.74 (0.18) | 1.56 (0.14) |
| ICC(%) | 51.38% | 33.17% | 45.45% | 32.20% |
| PCV(%) | Ref. | 53.02% | 21.04% | 55.04% |
| MOR | 5.87 | 3.36 | 4.82 | 3.27 |
| Model Comparison | ||||
| AIC | 16060.65 | 9313.72 | 15750.3 | 9251.57 |
| BIC | 16077.07 | 9532.01 | 15840.6 | 9531.60 |
The bold value indicates AIC and BIC implies the Model with the smallest AIC and BIC which suggests it is the best parsimonious model in this study.
The symbol “**” implies the statistical significance with p-value less than 0.001 and the symbol “*, **” implies the statistical significance with p-value less than 0.05.
Fixed Effects
The model with smaller AIC and BIC was considered as a parsimonious model, and the interpretation of the fixed effects was based on this model. Model-III was adjusted for both individual and community-level factors that have small AIC and BIC, compared to other models, and this model fits the data well. In the multivariable analysis, respondent’s twin child, mother’s age, mother’s education, mother’s occupation, wealth index, sex of HH head, media exposure, partner education, age at 1st birth, place of delivery, meal frequency, place of residence, and country of origin were significant determinants of MDD in East Africa at a 5% level of significance.
The odds of having adequate minimum dietary diversity were 30% less likely among children whose mothers were in the age group of 35–49, compared to those whose mothers were in the age group of 15–19 (AOR = 0.70; 95% CI; 0.53–0.91). Twin children were 45% less likely to have adequate minimum dietary diversity, compared to their counterparts (AOR = 0.55; 95% CI; 0.31–0.91). Children whose mothers had higher educational attainment were 3.04 times more likely to have adequate minimum dietary diversity, compared to those whose mothers had no formal education (AOR = 3.04; 95% CI; 1.99–4.64). Children who had a post-natal check within 2 months had 24% higher odds of reaching adequate minimum dietary diversity, compared to their counterparts (AOR = 1.24; 95% CI; 1.07–1.45). Urban children had 33% higher odds of having adequate minimum dietary diversity, compared to the rural (AOR = 1.33; 95% CI; 1.08–1.63). The odds of reaching adequate minimum dietary diversity were 2.08 (AOR = 2.08; 95% CI; 1.44–3.00) times higher among the children who live in Rwanda, compared to those who live in Ethiopia. However, the odds of having adequate minimum dietary diversity were 0.65 (AOR = 0.65; 95% CI: 0.44–0.96) times lower among children living in Zimbabwe, compared to those living in Ethiopia.
Discussion
This study assessed the minimum dietary diversity among children aged 6–59 months in East African Countries. This study found that the magnitude of Adequate minimum dietary diversity (MDD) among children aged 6–59 months was 10.47% (95% CI: 10.12–10.84), and women’s age, mother’s education level, mother’s occupation, partner educational level, media exposure, wealth index, place of delivery, meal frequency, residence, and country in which the mothers live determines the adequate MDD.
The magnitude of adequate dietary diversity among children aged 6–59 months in East Africa was found to be 10.47 (95% CI; 10.12–10.84). This finding is lower than the study conducted in different (sub-Saharan African countries (25.1%), (43.9%) in South Africa, (23.25%) in Ethiopia) [14], Dabat district (17%) in Ethiopia [23], Gedeo Zone (29.9%) [33] in Southern Ethiopia, Sinan district (13%) in Ethiopia [34], Dejen district (13.6%) in Ethiopia [4], Rwanda (23%), and Burundi (16%) [35], and it is also lower than the study conducted in Bangladesh (41.9%) [36] and India (15.2%) [37]. However, this finding is higher than a study conducted using 2011 EDHS in Ethiopia (10.8%) [38], EDHS 2016 (24%) in Ethiopia [39], Gorche district (10.6%) [40], and Burkina Faso (5.6%) [13].
The observed discrepancy in Minimum Dietary Diversity (MDD) rates may be attributed to various factors, including socioeconomic differences [41], variations in child feeding habits, differences in study settings, and variations in the age and size of study samples.
Socioeconomic differences play a significant role in shaping child feeding habits and dietary patterns. Communities with different economic conditions may have distinct food availability, access, and cultural preferences, which can influence the diversity of foods consumed by children [41].
Child feeding habits can also vary across societies based on their dominant agricultural practices or reliance on animal products. Some communities may have a tradition of predominantly plant-based diets, while others may heavily rely on animal-derived foods.
The differences in study settings, such as geographical location or cultural context, can further contribute to variations in child feeding practices. These variations may be influenced by regional traditions, dietary norms, and availability of food resources.
Furthermore, the age range and sample size of the study can impact the findings. Different age groups may have different dietary needs and behaviors, leading to variations in MDD rates. Additionally, the size of the study sample can affect the generalizability of the findings to the larger population.
The lower proportion of MDD observed in the present study aligns with the findings from the Afrobarometer SDG Scorecards report. The report indicates a decline in performance, by more than 3 percentage points, in achieving targets related to SDG1 (no poverty), SDG2 (zero hunger), and SDG3 (good health and wellbeing) in most East African countries between the 6th and 8th round surveys conducted from 2014/15 to 2019/21 [42].
Overall, these factors collectively contribute to the observed discrepancies in MDD rates, highlighting the complex interplay between socioeconomic factors, cultural practices, and regional trends in achieving sustainable development goals related to poverty, hunger, and health. Addressing these variations and challenges requires targeted interventions and policy efforts to improve child nutrition and wellbeing in diverse.
The odds of having adequate dietary diversity were 30% lower among children whose mothers were in the age group of 35–49, compared to those whose mothers were in the age group of 15–19, which is supported by a study done in Ethiopia [23] and Bangladesh [43]. As the age of the mother increased, the probability of children getting adequate minimum dietary diversity of food is decreased [40, 43]. This may be related to the increase in maternal age and increase in family size that can affect the economic status of households to provide a variety of nutrients for all family members as per recommendations. In addition, the effect of age on MDD was supported by the previous report which found that as children get older, their dietary diversity gradually increases [44]. Furthermore, this finding is crucial for policymakers to work effectively on meeting the SDGs target as adequate MDD is associated with a lower risk of stunted growth and wasting among children [13].
Children aged 6–59 months living in households within the rich wealth index were associated with significantly higher odds of adequate Dietary diversity compared to those living in households within the poor wealth index which is consistent with the study conducted in Ethiopia and other East African countries [38, 45, 46] and Bangladesh [36]. This may be related to higher economic status being associated with improved access to information, finance, and other resources which improve dietary diversity of the household. The lower wealth quintile was associated with unmet minimum dietary diversity and it is a limiting factor for the mother to provide an adequate variety of nutritious food to their children daily [47, 48]. Therefore, improvement in all wealth quintiles may have a significant effect on appropriate child-feeding practices to meet their nutritional requirements.
Children aged 0–59 months from mothers who delivered at health institutions were significantly associated with adequate dietary diversity. This is similar to studies conducted in Ethiopia [45]. This might be due to attending institutional delivery providing the opportunity of being counseled by health professionals on child care which influences child feeding.
This study indicated that children aged 6–59 months from mothers who lived in urban areas were significantly associated with adequate dietary diversity which converges with a study done in Indonesia [49]. This could be because individuals from urban areas are better educated, have better access to information or have better awareness, and have better access to health facilities which influences their child feeding practices [50]. Additionally, it is known that urban areas offer better opportunities to aid mothers’ understanding and access to a varied dietary diversity due to the cumulative effect of a series of more favorable conditions, including better socioeconomic and educational conditions, in turn leading to better caring practices for children [51]. Thus, children who lived in rural residences can have a higher probability of inadequate MDD.
In this study, exposure to mass media showed a significant association with adequate dietary diversity among children aged 6–59 months. This finding is consistent with a study conducted in Ethiopia [14, 23, 33, 34, 40, 45]. This might be due to nutritional-related health education and promotion delivered by mass media can improve the feeding practices of children as recommended. Another possible reason may be that having media access in the household can help mothers obtain information about varied food options for children. This indicates that mass media can be used as an effective source of information for promoting the feeding practice of infants and young children [14]. In addition, previous reports showed that Television media currently promotes and shows the practice of Infant and Young Child Feeding (IYCF) which includes breastfeeding, a healthy diet, and good nutrition for children [52]. Thus, this can increase food variations in children aged 6–23 months.
Mothers’ occupational and educational status had a significant association with achieving adequate minimum dietary diversity among children aged 6–59 months which parallels a study conducted in the east African region [24, 38, 46, 53]. This could be because a higher educational level allows mothers to have better knowledge about nutrition and food groups consumed by children [48]. Another justification might be that educated mothers have a better chance to be employed. This gives more opportunities to have a higher income for purchasing diverse foods for their children, to have more information and understand educational messages delivered through different media outlets that can have a positive impact on overall health and child feeding practice [10]. In addition, better education is also associated with women’s empowerment which will influence better care for their children.
Place of delivery was significantly associated with adequate child dietary diversity which was consistent with studies from Ethiopia [14, 34]. This might be because attending an institutional delivery may provide more opportunities to receive effective nutrition education and counseling provided during ANC visits and after delivery at health institutes that might contribute to being knowledgeable on dietary diversity [54]. Therefore, this may imply that encouraging the utilization of maternity services and integrating them with infant and young child feeding helps to improve a variety of diet supplementation and child feeding practices.
Conclusion
The magnitude of adequate MDD among children aged 6–59 months in East Africa was found to be 10.47%. This finding is lower than the expected level. Therefore, more effort is needed in nutrition-specific interventions and strengthening the existing country-based strategies aimed to achieve the recommended minimum dietary diversity intake for all children aged between 6 and 59 months. Factors such as maternal education, maternal employment, exposure to media, wealth, post-natal checks, health institution delivery, and place of residence have a positive association with adequate MDD. However, having twin children and having more than five family members have a negative association with adequate MDD intake in East Africa.
Therefore, strengthening interventions focused on improving the economic status of households, the educational status of mothers, and diversified food consumption of children aged 6–59 months should be prioritized to improve the recommended feeding practice of children in east Africa. In addition, government support must be enhanced in all wealth quintiles to ensure that children receive a variety of foods. Furthermore, future country-based studies may be needed to examine the barriers to adequate MDD specifically in each country for better specific recommendations.
Acknowledgments
The authors would like to acknowledge the measure DHS program for permitting data access to the recent DHS dataset of SSA countries.
Data Availability Statement
Data for this study were sourced from Demographic and Health surveys (DHS) and available at: https://www.dhsprogram.com.
Ethics Statement
Since the data for this study is secondary and available in the public domain, ethics approval was not necessary. We registered with the DHS online archive, requested the dataset for our study, and were given permission to access and download the data files.
Author Contributions
The conception of the work, design of the work, acquisition of data, analysis, and interpretation of data was done by TR, BM, GA, and LR. Data curation, drafting of the article, revising it critically for intellectual content, validation and final approval of the version to be published was done by TR, BM, AD, GA, AB, BN, MG, LR, and KR. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Abbreviations
AIC, akaike information criteria; ANC, ante-natal care; AOR, adjusted odds ratio; BIC, Bayesian information criteria, CI, confidence interval; COR, crude odds ratio; DHS, demographic and health survey; ICC, intra-cluster correlation coefficient; MDD, minimum dietary diversity; MOR, median odds ratio; SDGs, sustainable development goals; SSA, Sub-Saharan Africa; PCV, proportional change in variance; WHO, world health organization.
References
- 1. Sealey-Potts C, Potts A. An Assessment of Dietary Diversity and Nutritional Status of Preschool Children. Austin J Nutr Food Sci (2014) 2(7):1040. [Google Scholar]
- 2. Kathryn D. Guiding Principles for Complementary Feeding of the Breastfed Child. Washington, DC: Pan American Health Organization (PAHO) (2003). [Google Scholar]
- 3. WHO. Nutrition Landscape Information System (NLiS). Geneva, Switzerland: World Health Organization (WHO) (2018). [Google Scholar]
- 4. Kumera G, Tsedal E, Ayana M. Dietary Diversity and Associated Factors Among Children of Orthodox Christian Mothers/caregivers during the Fasting Season in Dejen District, North West Ethiopia. Nutr Metab (2018) 15(1):16–9. 10.1186/s12986-018-0248-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. UNICEF. Monitoring the Situation of Children and Women. New York: UNICEF; (2013). [Google Scholar]
- 6. UNICEF. Children, Food and Nutrition and Growing Well in a Changing World. New York, NY: UNICEF; (2019). [Google Scholar]
- 7. Nkoka O, Mhone TG, Ntenda PAM. Factors Associated with Complementary Feeding Practices Among Children Aged 6-23 Mo in Malawi: an Analysis of the Demographic and Health Survey 2015-2016. Int Health (2018) 10(6):466–79. 10.1093/inthealth/ihy047 [DOI] [PubMed] [Google Scholar]
- 8. Baek Y, Chitekwe S. Sociodemographic Factors Associated with Inadequate Food Group Consumption and Dietary Diversity Among Infants and Young Children in Nepal. PloS one (2019) 14(3):e0213610. 10.1371/journal.pone.0213610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sekartaji R, Suza DE, Fauziningtyas R, Almutairi WM, Susanti IA, Astutik E, et al. Dietary Diversity and Associated Factors Among Children Aged 6-23 Months in Indonesia. J Pediatr Nurs (2021) 56:30–4. 10.1016/j.pedn.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 10. Agrawal S, Kim R, Gausman J, Sharma S, Sankar R, Joe W, et al. Socio-economic Patterning of Food Consumption and Dietary Diversity Among Indian Children: Evidence from NFHS-4. Eur J Clin Nutr (2019) 73(10):1361–72. 10.1038/s41430-019-0406-0 [DOI] [PubMed] [Google Scholar]
- 11. Paramashanti BA, Huda TM, Alam A, Dibley MJ. Trends and Determinants of Minimum Dietary Diversity Among Children Aged 6-23 Months: a Pooled Analysis of Indonesia Demographic and Health Surveys from 2007 to 2017. Public Health Nutr (2021) 25(7):1–12. 10.1017/S1368980021004559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Senarath U, Godakandage SS, Jayawickrama H, Siriwardena I, Dibley MJ. Determinants of Inappropriate Complementary Feeding Practices in Young Children in Sri Lanka: Secondary Data Analysis of Demographic and Health Survey 2006-2007. Matern child Nutr (2012) 8:60–77. 10.1111/j.1740-8709.2011.00375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aboagye RG, Seidu A-A, Ahinkorah BO, Arthur-Holmes F, Cadri A, Dadzie LK, et al. Dietary Diversity and Undernutrition in Children Aged 6–23 Months in Sub-saharan Africa. Nutrients (2021) 13(10):3431. 10.3390/nu13103431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Temesgen H, Negesse A, Woyraw W, Mekonnen N. Dietary Diversity Feeding Practice and its Associated Factors Among Children Age 6–23 Months in Ethiopia from 2011 up to 2018: a Systematic Review and Meta-Analysis. Ital J Pediatr (2018) 44(1):109–10. 10.1186/s13052-018-0567-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. UN. The UN’s Sustainable Development Goals Aim to End Poverty, Protect the Planet and Ensure prosperity for Everyone by 2030. New York, NY: United Nations Development Program (UNDP) report; (2015). [Google Scholar]
- 16. Bandoh DA, Kenu E. Dietary Diversity and Nutritional Adequacy of Under-fives in a Fishing Community in the central Region of Ghana. BMC Nutr (2017) 3(1):2–6. 10.1186/s40795-016-0120-4 [DOI] [Google Scholar]
- 17. Moursi MM, Arimond M, Dewey KG, Treche S, Ruel MT, Delpeuch F. Dietary Diversity Is a Good Predictor of the Micronutrient Density of the Diet of 6-to 23-Month-Old Children in Madagascar. J Nutr (2008) 138(12):2448–53. 10.3945/jn.108.093971 [DOI] [PubMed] [Google Scholar]
- 18. Leyna GH, Mmbaga EJ, Mnyika KS, Hussain A, Klepp K-I. Food Insecurity Is Associated with Food Consumption Patterns and Anthropometric Measures but Not Serum Micronutrient Levels in Adults in Rural Tanzania. Public Health Nutr (2010) 13(9):1438–44. 10.1017/S1368980010000327 [DOI] [PubMed] [Google Scholar]
- 19. Anin SK, Saaka M, Fischer F, Kraemer A. Association between Infant and Young Child Feeding (IYCF) Indicators and the Nutritional Status of Children (6–23 Months) in Northern Ghana. Nutrients (2020) 12(9):2565. 10.3390/nu12092565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saaka M, Wemakor A, Abizari A-R, Aryee P. How Well Do WHO Complementary Feeding Indicators Relate to Nutritional Status of Children Aged 6–23 Months in Rural Northern Ghana? BMC public health (2015) 15(1):1157–12. 10.1186/s12889-015-2494-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. WHO. Indicators for Assessing Infant and Young Child Feeding Practices: Part1 Definitions: Conclusions of a Consensus Meeting Held 6–8 November 2007 in Washington, DC, USA. Geneva, Switzerland: World Health Organization (WHO) (2008). [Google Scholar]
- 22. WHO. Indicators for Assessing Infant and Young Child Feeding Practices: Part3 Country Profiles. Geneva, Switzerland: World Health Organization (WHO) (2010). [Google Scholar]
- 23. Belew AK, Ali BM, Abebe Z, Dachew BA. Dietary Diversity and Meal Frequency Among Infant and Young Children: a Community Based Study. Ital J Pediatr (2017) 43(1):73–10. 10.1186/s13052-017-0384-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Solomon D, Aderaw Z, Tegegne TK. Minimum Dietary Diversity and Associated Factors Among Children Aged 6–23 Months in Addis Ababa, Ethiopia. Int J Equity Health (2017) 16(1):181–9. 10.1186/s12939-017-0680-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bedada Damtie S, Benti Tefera T, Tegegne Haile M. Dietary Diversity Practice and Associated Factors Among Children Aged 6–23 Months in Robe Town, Bale Zone, Ethiopia. J Nutr Metab (2020) 2020:9190458. 10.1155/2020/9190458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahoya B, Kavle JA, Straubinger S, Gathi CM. Accelerating Progress for Complementary Feeding in Kenya: Key Government Actions and the Way Forward. Matern Child Nutr (2019) 15:e12723, 10.1111/mcn.12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Issaka AI, Agho KE, Page AN, Burns PL, Stevens GJ, Dibley MJ. Determinants of Suboptimal Complementary Feeding Practices Among Children Aged 6-23 Months in Seven Francophone West African Countries. Matern Child Nutr (2015) 11:31–52. 10.1111/mcn.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ogbo FA, Page A, Idoko J, Claudio F, Agho KE. Trends in Complementary Feeding Indicators in Nigeria, 2003–2013. BMJ Open (2015) 5(10):e008467, 10.1136/bmjopen-2015-008467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Croft TN, Marshall AMJ, Allen CK. The DHS Program - Guide to DHS Statistics (English). Rockville, Maryland, USA: ICF; (2018).: [Google Scholar]
- 30. Croft TN, Marshall AM, Allen CK, Arnold F, Assaf S, Balian S. Guide to DHS Statistics. Rockville, Maryland, USA; (2018). [Google Scholar]
- 31. Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CED, et al. A Brief Introduction to Mixed Effects Modelling and Multi-Model Inference in Ecology. PeerJ (2018) 6:e4794. 10.7717/peerj.4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merlo JCB, Yang M, Lynch J, Råstam L, Råstam L. A Brief Conceptual Tutorial on Multilevel Analysis in Social Epidemiology: Interpreting Neighbourhood Differences and the Effect of Neighbourhood Characteristics on Individual Health. J Epidemiol Community Health (2005) 59(12):1022–8. 10.1136/jech.2004.028035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Molla W, Adem DA, Tilahun R, Shumye S, Kabthymer RH, Kebede D, et al. Dietary Diversity and Associated Factors Among Children (6–23 Months) in Gedeo Zone, Ethiopia: Cross-Sectional Study. Ital J Pediatr (2021) 47(1):233–10. 10.1186/s13052-021-01181-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Temesgen H, Yeneabat T, Teshome M. Dietary Diversity and Associated Factors Among Children Aged 6–23 Months in Sinan Woreda, Northwest Ethiopia: a Cross-Sectional Study. BMC Nutr (2018) 4(1):5–8. 10.1186/s40795-018-0214-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Custodio E, Herrador Z, Nkunzimana T, Węziak-Białowolska D, Perez-Hoyos A, Kayitakire F. Children’s Dietary Diversity and Related Factors in Rwanda and Burundi: A Multilevel Analysis Using 2010 Demographic and Health Surveys. PLoS One (2019) 14(10):e0223237. 10.1371/journal.pone.0223237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kabir I, Khanam M, Agho KE, Mihrshahi S, Dibley MJ, Roy SK. Determinants of Inappropriate Complementary Feeding Practices in Infant and Young Children in Bangladesh: Secondary Data Analysis of Demographic Health Survey 2007. Matern Child Nutr (2012) 8:11–27. 10.1111/j.1740-8709.2011.00379.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patel A, Pusdekar Y, Badhoniya N, Borkar J, Agho KE, Dibley MJ. Determinants of Inappropriate Complementary Feeding Practices in Young Children in India: Secondary Analysis of National Family Health Survey 2005–2006. Matern Child Nutr (2012) 8:28–44. 10.1111/j.1740-8709.2011.00385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aemro M, Mesele M, Birhanu Z, Atenafu A. Dietary Diversity and Meal Frequency Practices Among Infant and Young Children Aged 6–23 Months in Ethiopia: a Secondary Analysis of Ethiopian Demographic and Health Survey 2011. J Nutr Metab (2013) 2013:782931. 10.1155/2013/782931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seboka BT, Hailegebreal S, Yehualashet DE, Gilano G, Kabthymer RH, Ewune HA, et al. Exploring Spatial Variations and Determinants of Dietary Diversity Among Children in Ethiopia: Spatial and Multilevel Analysis Using EDHS (2011–2016). J Multidiscip Healthc (2021) 14:2633–50. 10.2147/JMDH.S327456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dangura D, Gebremedhin S. Dietary Diversity and Associated Factors Among Children 6-23 Months of Age in Gorche District, Southern Ethiopia: Cross-Sectional Study. BMC Pediatr (2017) 17(1):6–7. 10.1186/s12887-016-0764-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Food and Agriculture Organization. Summary and Statistical Reports of the 2007: Population and Housing Census, Population Size by Age and Sex [2008]. Addis Ababa, Ethiopia: Food and Agriculture Organization, UNFPA; (2007). [Google Scholar]
- 42. Nkomo S. Tracking Progress in Africa toward Sustainable Development Goal. Addis Ababa, Ethiopia: United Nations Economic Commission for Africa report; (2021). [Google Scholar]
- 43. Kundu S, Sayeed A, Azene AG, Rezyona H, Banna MHA, Khan MSI. Exploring the Factors Associated with Dietary Diversity of Children Aged 6–59 Months in Some Rural and Slum Areas of Bangladesh amid the COVID-19 Pandemic: A Mixed-Effect Regression Analysis. Curr Dev Nutr (2022) 6(8):nzac109. 10.1093/cdn/nzac109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mengesha HG, Vatanparast H, Feng C, Petrucka P. Modeling the Predictors of Stunting in Ethiopia: Analysis of 2016 Ethiopian Demographic Health Survey Data (EDHS). BMC Nutr (2020) 6(1):52–11. 10.1186/s40795-020-00378-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beyene M, Worku AG, Wassie MM. Dietary Diversity, Meal Frequency and Associated Factors Among Infant and Young Children in Northwest Ethiopia: a Cross-Sectional Study. BMC public health (2015) 15(1):1007–9. 10.1186/s12889-015-2333-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gewa CA, Leslie TF. Distribution and Determinants of Young Child Feeding Practices in the East African Region: Demographic Health Survey Data Analysis from 2008-2011. J Health Popul Nutr (2015) 34(1):6–14. 10.1186/s41043-015-0008-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dafursa K, Gebremedhin S. Dietary Diversity Among Children Aged 6–23 Months in Aleta Wondo District, Southern Ethiopia. J Nutr Metab (2019) 2019:2869424. 10.1155/2019/2869424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Codjoe SNA, Okutu D, Abu M. Urban Household Characteristics and Dietary Diversity: An Analysis of Food Security in Accra, Ghana. Food Nutr Bull (2016) 37(2):202–18. 10.1177/0379572116631882 [DOI] [PubMed] [Google Scholar]
- 49. Ng CS, Dibley MJ, Ke A. Complementary Feeding Indicators and Determinants of Poor Feeding Practices in Indonesia: a Secondary Analysis of 2007 Demographic and Health Survey Data. Public Health Nutr (2012) 15(5):827–39. 10.1017/S1368980011002485 [DOI] [PubMed] [Google Scholar]
- 50. Lokossou YUA, Tambe AB, Azandjèmè C, Mbhenyane X. Socio-cultural Beliefs Influence Feeding Practices of Mothers and Their Children in Grand Popo, Benin. J Health Popul Nutr (2021) 40(1):33. 10.1186/s41043-021-00258-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kambale RM, Ngaboyeka GA, Kasengi JB, Niyitegeka S, Cinkenye BR, Baruti A, et al. Minimum Acceptable Diet Among Children Aged 6–23 Months in South Kivu, Democratic Republic of Congo: a Community-Based Cross-Sectional Study. BMC Pediatr (2021) 21(1):239–9. 10.1186/s12887-021-02713-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. UNICEF. Levels and Trends in Child Malnutrition. New York, US: United Nations Children's Fund, World Bank; (2018). eSocialSciences. [Google Scholar]
- 53. Senarath U, Dibley MJ. Complementary Feeding Practices in South Asia: Analyses of Recent National Survey Data by the South Asia Infant Feeding Research Network. Matern Child Nutr (2012) 8:5–10. 10.1111/j.1740-8709.2011.00371.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dhami MV, Ogbo FA, Osuagwu UL, Agho KE. Prevalence and Factors Associated with Complementary Feeding Practices Among Children Aged 6–23 Months in India: a Regional Analysis. BMC Public health (2019) 19(1):1034–16. 10.1186/s12889-019-7360-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study were sourced from Demographic and Health surveys (DHS) and available at: https://www.dhsprogram.com.