Abstract
Objectives
Nuclear factor κB (NF-κB) is a superfamily of transcription factors that plays a key role in cancer genesis and progression. The present study aimed to examine the expression of NF-κB/p65 in breast cancer and its relationship with prognostic markers such as tumour grade, tumour size, hormone receptors, and HER-2.
Methods
Ninety-nine unselected formalin-fixed paraffin-embedded invasive ductal and lobular tissue sections were evaluated by immunohistochemistry methods to measure the expression of NF-κB/p65, oestrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER-2), and Ki-67. We assessed the correlation between NF-κB/p65 and clinicopathological parameters.
Results
NF-κB/p65 was found only in the cytoplasm and positively correlated with large tumours (≥2 cm) and high-grade tumours (p < 0.001 and p = 0.018, respectively). Other breast cancer markers, such as histological type (p = 0.766), HER-2 (p = 0.416), PR (p = 0.356), and ER (p = 0.606), had no significant link with the expression of NF-κB/p65. Furthermore, no significant relation with the Ki-67 marker was detected (p = 0.117).
Conclusions
The current study is indicative of a link between overexpression of NF-κB/p65 and both large tumour size and higher grade. This suggests that the expression of NF-κB/p65 is associated with aggressive biological activity in breast cancer; elucidating the mechanisms that lead to NF-κB/p65 cytoplasmic accumulation could lead to the development of novel therapeutic methods.
Keywords: NF-κB, Breast cancer, Immunohistochemistry, Tumour grade, Tumour size, Chemoresistance
Highlights of the Study
We studied the expression of NF-κB/p65 and its correlation with prognostic indicators, such as tumour grade, tumour size, hormone receptors, and HER-2 expression in breast cancer patients.
Both invasive ductal and lobular breast cancer tissues have higher levels of cytoplasmic NF-κB/p65 than non-tumour tissues; this may help explain the aggressive biological activity of breast cancer.
The association of high cytoplasmic NF-κB/p65 with larger and higher grade tumours suggests the involvement of NF-κB/p65 in tumour progression.
Introduction
Breast cancer is the most frequently diagnosed cancer in women and the second leading cause of cancer mortality in women worldwide [1]. In 2020, there were approximately 2.3 million new cases, accounting for 11.7% of all cases of cancer and approximately 685,000 deaths [2]. Breast cancer is the most common type of cancer in Kuwait, with 15 cases/100,000 population diagnosed each year, and it has increased more than 3-fold (50 cases/100,000 population every year) over the last 33 years [3]. Approximately 3.5 million breast cancer survivors worldwide are currently undertreated, including those who have completed treatment [1]. Conventional therapies currently available show a lack of efficacy in treating cancer as well as resistance to treatment and multiple side effects [1].
Recently, transcription factors have been recognized for their roles in cancer progression. NF-κB is a transcription factor that has been suggested to be a possible target in cancer. Several studies have established a link between NF-κB and breast cancer. Initially, an increase in NF-κB expression was detected in cultures of breast cancer cells [4]. Expression of NF-κB is reported to be associated with aggressive tumour biology in breast cancer, a poor prognosis, and a predictor of chemotherapy resistance [5, 6]. Additionally, HER-2 and epidermal growth factor (EGF) enhance tumour development by stimulating the NF-кB pathway [7, 8]. Moreover, NF-κB is constitutively active and detected in the nucleus of cancer cells in many tumours including breast cancer [9]. NF-κB is a homomeric or heterodimeric protein consisting of two of the following five possible subunits: p105/p50, RelA/p65, p100/p52, c-Rel, and RelB [10]. NF-κB is inactive in the cytoplasm and is bound to NF-κB (IκB), a specific inhibitory molecule [10]. Upon stimulation by external stimuli such as cytokines, bacteria, viral nucleic acids [11], and DNA damage-inducing agents [12], IκB proteins are degraded by the ubiquitin-proteasome pathway, and the active form of NF-κB is translocated into the nucleus, where it controls the expression of several target genes essential for proliferation, apoptosis, adhesion, angiogenesis, invasion, and chemoresistance [9, 13, 14]. As a result, it increases the ability of cells to metastasize and leads to resistance to radiotherapy or chemotherapy [6]. Experimental and clinical data suggest that the NF-κB gene is essential for the pathogenesis and progression of human tumours, which makes it a potential marker of malignant tumours and a potential therapeutic target.
Nuclear NF-κB expression has previously been found to be an important predictor of resistance of breast cancer to neoadjuvant treatment [6]. Thus, the inhibition of NF-κB might provide an effective strategy for cancer treatment. Inhibition of Akt/NF-κB signalling resulted in chemosensitization of breast cancer cells to taxanes in vivo and in vitro, which resulted in increased inhibition of growth and death of breast cancer cells [15]. Inhibition of NF-κB was then tested and found to be promising. Sato et al. [16] reported that the inhibition of NF-κB led to suppression of peritoneal metastasis of pancreatic cancer in response to Anoikis induction. In addition, silencing of IKKα or IKKβ significantly reduced the capacity of doxorubicin to trigger NF-κB activation, which in turn led to an increase in the apoptosis of human fibrosarcoma cells in comparison to doxorubicin alone [17]. The aim of this study was to examine the role of NF-κB as a biological marker and to detect any correlations between the expression of NF-κB and clinical and histopathological variables.
Material and Methods
Patient Data and Tissue Specimens
The study was approved by the Standing Committee for Coordination of Health and Medical Research, Ministry of Health, Kuwait (2017/538), and carried out according to Ministry guidelines. For clinicopathological correlation, we retrieved 99 retrospective unselected, formalin-fixed, paraffin-embedded invasive ductal and lobular breast cancer samples obtained from patients (median age 50 years) with biopsy-proven breast cancer at Al-Adan Hospital, Kuwait, between 2006 and 2020. The pathological parameters examined for each patient included age at diagnosis; histopathological subtype; grade; lymph node status; immunohistochemical profile of hormone receptors, including ER and PR, Ki-67, and HER-2. To confirm the diagnosis, all haematoxylin and eosin-stained slides, pathology reports, and other medical records were examined. The size of the tumour at lumpectomy or mastectomy was employed. Tumours were divided into three categories based on their size: 2 cm, >2–5 cm, and >5 cm. The established Nottingham histological grading method was used for the assessment of tumour grade [18]. The Ki-67 proliferation index cut-off was employed as published previously [19]. Invasive malignant cells having a 1% nuclear staining/immunoreactivity were considered ER-positive or PR-positive. HER-2 was scored according to Herceptin test guidelines (from 0 to 3+).
Immunohistochemistry
Deparaffinized sections from paraffin blocks (4 µm thickness) were prepared for loading into a BenchMark XT Ultra machine (Ventana Inc., USA). Slides were pre-treated for retrieval of antigens in Benchmark Ultra using their EZ prep (Ref No: 950-102) and Ultra Cell Conditioner 1 (Ultra CC1, Ref No: 950-224) reagents. The incubation time for antigen retrieval was standardized by validating the default protocol with different combinations of incubation times by comparing the intensity and quality of the reaction. Different dilutions in the recommended range were prepared and run with known control slides, and the results were compared. An optimal dilution of 1:400 for NF-κB/p65 (L8F6) mouse monoclonal antibody (Cell Signaling Technology, Inc., Danvers, MA, USA) was used, and the corresponding incubation time was determined. Staining was performed using a Ventana ultraView and DAB Detection system, which includes peroxidase inhibitor, HRP multimer, DAB chromogen, hydrogen peroxide, and ultraView DAB copper. Mouse monoclonal antibodies against ER, PR, HER-2/neu, and Ki-67 (Cell Marque, Rocklin, CA, USA, or Ventana) were used according to the manufacturer's recommendations.
Interpretation of Immunohistochemical Staining
NF-κB/p65 expression in lymphocytes was used as a positive internal control. Slides were evaluated by a pathologist, and cells were counted in 15 fields of vision for each segment using a double-blind approach at high Olympus CX51 light microscope magnification (×400). The average number of stained cells per 100 cells counted was used to compute the percentage of positive cells. The presence of a brownish-yellow colour in immunohistochemistry staining of NF-κB/p65 was considered positive. The scoring criteria were as follows: 0 points for negative, 1 point for pale yellow, 2 points for yellow, and 3 points for brownish-yellow.
Statistical Analysis
Data are presented as the mean ± standard deviation. To compare the two groups from independent samples, Student's t test (two-tailed) was performed, assuming equal variances among all experimental datasets. The correlation between expression of NF-κB/p65 and clinicopathological variables was evaluated using Pearson's χ2 test (χ2). P ≤ 0.05 was considered statistically significant in all tests. All tests were two-sided.
Results
Expression of NF-κB/p65 Protein in Breast Cancer Patients
We studied specimens from 99 breast cancer patients to evaluate the clinical relevance of NF-κB/p65 in breast cancer disease progression. Demographic details are presented in Table 1). Subjects in their forties made up the largest age group, accounting for 75.8% of the subjects. According to tumour grading, most cases were second/moderate grade (46.9%), followed by third/high grade (45.8%) and first/low grade (7.3%). We evaluated the expression of NF-κB/p65 protein by immunohistochemistry (Fig. 1). We observed that NF-κB/p65 immunoreactivity was observed only in the cytoplasm of both invasive ductal and lobular breast cancer cells when compared to the negatively stained ductal carcinoma cells used as a control (Fig. 1).
Table 1.
Association of NF-κB/p65 protein and clinical parameters in breast cancer patients
| Variable | NF-κB/p65 expression |
χ2 | p value | |||
|---|---|---|---|---|---|---|
| negative, n (%) | low, n (%) | moderate, n (%) | high, n (%) | |||
| Age groups | 2.736 | 0.434 | ||||
| ≤40 (n = 24) | 1 (4.1) | 5 (20.8) | 9 (37.5) | 9 (37.5) | ||
| >40 (n = 75) | 12 (16) | 17 (22.7) | 20 (26.7) | 26 (34.7) | ||
| Histologic grade | 24.9 | <0.001 | ||||
| Grade 1 (n = 7) | 5 (71.4) | 0 (0) | 2 (28.6) | 0 (0) | ||
| Grade 2 (n = 45) | 5 (11.1) | 8 (17.8) | 14 (31.1) | 18 (40) | ||
| Grade 3 (n = 44) | 3 (6.8) | 13 (29.5) | 12 (27.3) | 16 (36.4) | ||
| Tumour type | 1.144 | 0.766 | ||||
| Ductal (n = 97) | 13 (13.4) | 22 (22.7) | 28 (28.9) | 34 (35.1) | ||
| Lobular (n = 2) | 0 (0) | 0 (0) | 1 (50) | 1 (50) | ||
| ER | 1.839 | 0.606 | ||||
| Negative (n = 20) | 3 (15) | 3 (15) | 8 (40) | 6 (30) | ||
| Positive (n = 79) | 10 (12.7) | 19 (24.1) | 21 (26.6) | 29 (36.7) | ||
| PR | 3.243 | 0.356 | ||||
| Negative (n = 31) | 5 (16.1) | 8 (25.8) | 11 (35.5) | 7 (22.6) | ||
| Positive (n = 68) | 8 (11.8) | 14 (20.6) | 18 (26.5) | 28 (41.2) | ||
| HER-2 Score | 9.236 | 0.416 | ||||
| Negative (n = 46) | 6 (13) | 11 (24) | 13 (28.2) | 16 (35.8) | ||
| Equivocal (n = 18) | 1 (5.6) | 2 (11.1) | 5 (27.8) | 10 (55.6) | ||
| Positive (n = 34) | 6 (17.6) | 9 (26.5) | 10 (24.9) | 9 (26.5) | ||
| Ki-67 Expression | 5.895 | 0.117 | ||||
| Low (n = 28) | 7 (25) | 7 (25) | 5 (17.9) | 9 (32.1) | ||
| High (n = 63) | 5 (7.9) | 14 (22.2) | 20 (31.7) | 24 (38.1) | ||
| Tumor size | 15.328 | 0.018 | ||||
| ≤2 cm (n = 9) | 2 (22.2) | 3 (33.3) | 1 (11.1) | 3 (33.3) | ||
| >2 cm − 5 cm (n = 75) | 8 (25.8) | 4 (12.9) | 13 (41.9) | 6 (19.4) | ||
| >5 cm (n = 12) | 0 (0) | 3 (25) | 1 (8.3) | 8 (66.7) | ||
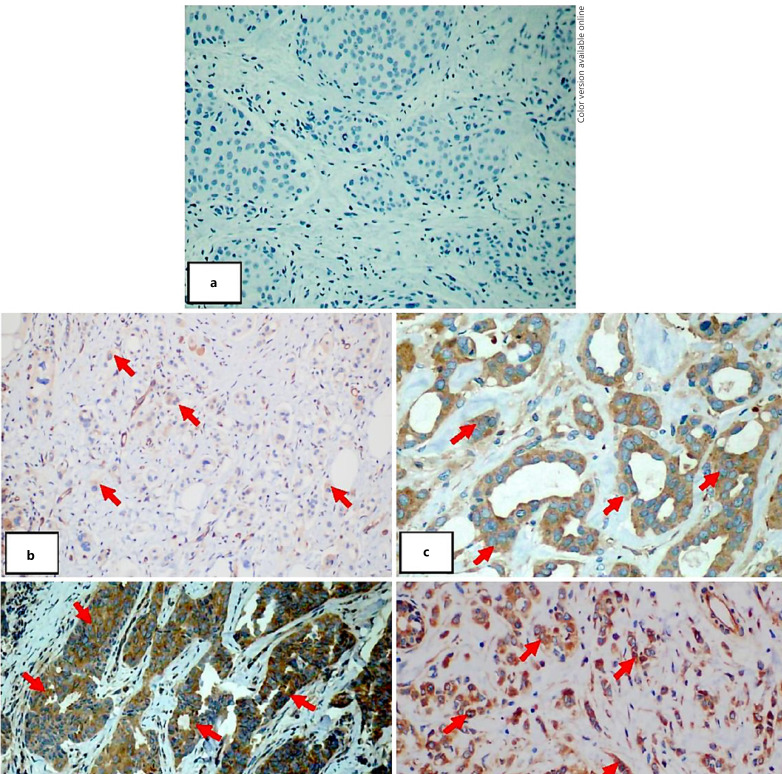
Fig. 1.
NF-κB/p65 protein expression in breast carcinoma cases. a Invasive ductal carcinoma grade 2 with weak NF-κB/p65 expression, ×200. b Invasive ductal carcinoma grade 2 with moderate NF-κB/p65 expression, ×200. c Invasive ductal carcinoma grade 2 with moderate NF-κB/p65 expression, ×200. d Invasive ductal carcinoma grade 2 with high NF-κB/p65 expression, ×200. e Invasive lobular carcinoma grade 2 with moderate NF-κB/p65 expression, ×400.
In invasive ductal cancer, thirteen specimens (13.1%) were found to be negative for NF-κB/p65, 22 (22.7%) were weakly positive, 29 (28.9%) were moderately positive, and 35 (35.1%) were strongly positive. NF-κB/p65 expression was found in all invasive lobular specimens. We found no relationship between age and NF-κB/p65 expression (p = 0.434).
Relationship between NF-κB/P65 Immunostaining and Clinical Parameters in Breast Cancer
The clinicopathological characteristics of all 99 patients with primary invasive breast cancer are listed in Table 1. We performed statistical analyses to find any possible link between NF-κB/p65 staining and various histopathological parameters (Table 1). NF-κB/p65 expression showed no significant correlation with prognostic factors of breast cancer, i.e., histological type (p = 0.766), HER-2 (p = 0.416), PR (p = 0.356), or ER (p = 0.606) (online suppl. Materials; for all online suppl. 1, see www.karger.com/doi/10.1159/000527828). Furthermore, values of the proliferation indicator Ki-67 were not associated with NF-κB/p65 expression (p = 0.117). Nevertheless, a strong correlation was observed between NF-B/p65 expression and samples with a high tumour grade (p < 0.001). 93.2% of NF-κB/p65 expressions were in the third/high grade, while 88.8% of the cases were in the second/moderate grade. Moreover, there was a significant relationship between NF-κB/p65 expression and tumour size (p = 0.018); 81.4% of the patients had positive staining in tumours larger than 2 cm.
Discussion
In this study, we validated the level of NF-κB/p65 expression and its relationship with clinical and other molecular markers in 99 breast cancer patients. NF-κB/p65 was initially identified as a critical component in the establishment of immune responses but is currently being investigated as a possible target for cancer therapy. This is based on the fact that NF-κB/p65 works as a transcription catalyst for target genes involved in carcinogenesis; it induces cancer cell growth without the requirement for exogenous stimulation; it induces resistance to growth inhibition signals, uncontrolled proliferative ability, angiogenesis, invasion, or metastasis; and it decreases apoptotic ability, all of which are hallmarks of cancer [6, 9, 14]. However, many factors affect the activation of NF-κB/p65, such as the expression of several proteins that can control its functional activity. Our results revealed high expression of NF-κB/p65 in breast cancer tissues, as judged by immunostaining, in comparison with adjacent non-tumour tissues. NF-κB/p65 staining was found only in the cytoplasm. NF-κB is present as a dimer that binds to specific inhibitors (IκBs), after which the released NF-κB dimers translocate to the nucleus and activate various target genes [9, 14].
Despite the fact that prior research has linked nuclear NF-κB/p65 staining to chemoresistance [6], no link has been found between nuclear expression of NF-κB/p65 and response to chemotherapy response or prognosis [20]. However, nuclear NF-κB expression was recently found to be a predictive marker for the response to cyclophosphamide, doxorubicin, and fluorouracil chemotherapy regimens [21]. Additionally, Sampepajung et al. [22] reported that patients with low nuclear NF-κB usually show a better response to chemotherapy than those with high levels of NF-κB. The function of cytoplasmic NF-κB has yet to be determined. It is believed that there is an association between increase in NF-κB expression and the molecular and physiological changes that subsequently contribute to its activation; inactivation of NF-κB is thought to retain it in the cytoplasm, which leads to a better prognosis in triple-negative breast cancer. According to one study, patient tumours with cytoplasmic expression of NF-κB/p65 prior to chemotherapy reacted effectively to treatment and became disease-free [20].
Published literature on the association between NF-κB/p65 expression and other molecular and clinical aspects of breast cancer is inconsistent. In breast cancer, tumour grading has proven value as a predictive and prognostic factor; patients with grades 2 and 3 were shown to have a poorer prognosis than those with grade 1 in the first 5 years [23]. Furthermore, a significant association was found between histological grading, pathological response, overall survival, and disease-free survival in patients with locally advanced stage breast cancer who underwent chemotherapy, indicating the relevance of histopathological grading as a predictor of response to chemotherapy [24]. We observed that the highest level of NF-κB/p65 expression is linked to large tumour size and higher grade. Our findings are consistent with an earlier report [5]. In contrast, Tannahill et al. [25] demonstrated that both cytoplasmic and nuclear NF-κB expression showed no correlation with cancer grade, size, or lymph node. Our findings support the aggressive role for NF-κB/p65 in breast cancer patients and suggest that this could contribute to breast cancer progression.
The present work showed no association between NF-κB/p65 expression and histological type, hormonal status, or levels of Ki-67. A previous study also found no significant association between cytoplasmic or nuclear NF-κB/p65 expression and nodal status, histological type, or hormonal status [26]. On the other hand, several studies document the existence of an association between NF-κB/p65 expression and hormonal status or levels of Ki-67 markers. An association between high total expression of NF-κB/p65 and ER-positive and PR-positive breast cancer was observed [25]. Another study found that NF-κB/p65 expression was linked to a low Ki-67 score and negative ER [27]. According to Shapochka et al. [28], an increase in nuclear NF-κB expression is associated with an increase in p53 accumulation. Moreover, NF-κB was linked to negative ER, negative PR, and positive HER-2 [5]. In breast cancer patients, a link between NF-κB expression and negative ER status and overexpression of HER-2 was discovered [26, 29, 30].
Conclusions
Taken together, the correlation of NF-κB/p65 immunostaining with larger and higher grade tumours in the current study suggests the involvement of NF-κB/p65 in tumour progression. More molecular in vitro and in vivo investigations are needed to evaluate NF-κB/p65 as a possible molecular marker of metastases and to assess the plausibility of its inclusion as a novel targeted therapeutic target.
Statement of Ethics
This study was approved by The Standing Committee for Coordination of Health and Medical Research, Ministry of Health, Kuwait (2017/538).
Conflict of Interest Statement
The authors declare no conflict of interest.
Funding Sources
The work was supported by Kuwait University Research Sector (Grant NM01/14)
Author Contributions
Mashael Saqer Al-Mutairi: concept, design of experiments, data analysis, and manuscript drafting and Hany Onsy Habashy: supervision of immunostaining, histological examination, data analysis, and manuscript drafting.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Supplementary Material
Supplementary data
Acknowledgments
The authors thank Mr. Sanil C S and Sultan A. Khan for the histological and immunoassay work and Ms. Ibtehal Matar for helping with the manuscript.
Funding Statement
The work was supported by Kuwait University Research Sector (Grant NM01/14)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2020;70((1)):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020 GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71((3)):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Elbasmi A, Al-Asfour A, Al-Nesf Y, Al-Awadi A. Cancer in Kuwait magnitude of the problem. Gulf J Oncolog. 2010;1((8)):7–14. [PubMed] [Google Scholar]
- 4.Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, et al. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100((12)):2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jana D, Das S, Sarkar DK, Mandal S, Maji A, Mukhopadhyay M. Role of nuclear factor-κB in female breast cancer a study in Indian patients. Asian Pac J Cancer Prev. 2012;13((11)):5511–5515. doi: 10.7314/apjcp.2012.13.11.5511. [DOI] [PubMed] [Google Scholar]
- 6.Montagut C, Tusquets I, Ferrer B, Corominas JM, Bellosillo B, Campas C, et al. Activation of nuclear factor-kappa B is linked to resistance to neoadjuvant chemotherapy in breast cancer patients. Endocr Relat Cancer. 2006;13((2)):607–616. doi: 10.1677/erc.1.01171. [DOI] [PubMed] [Google Scholar]
- 7.Henson ES, Johnston JB, Los M, Gibson SB. Clinical activities of the epidermal growth factor receptor family inhibitors in breast cancer. Biologics. 2007;1((3)):229–239. [PMC free article] [PubMed] [Google Scholar]
- 8.Shah D, Osipo C. Cancer stem cells and HER2 positive breast cancer the story so far. Genes Dis. 2016;3((2)):114–123. doi: 10.1016/j.gendis.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Concetti J, Wilson CL. NFKB1 and cancer friend or foe? Cells. 2018;7((9)):133. doi: 10.3390/cells7090133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-κB a blossoming of relevance to human pathobiology. Cell. 2017;168((1–2)):37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Mani AM, Wu ZH. DNA damage-induced nuclear factor-kappa B activation and its roles in cancer progression. J Cancer Metastasis Treat. 2017;3:45–59. doi: 10.20517/2394-4722.2017.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu ZH, Miyamoto S. Many faces of NF-kappaB signaling induced by genotoxic stress. J Mol Med. 2007;85((11)):1187–1202. doi: 10.1007/s00109-007-0227-9. [DOI] [PubMed] [Google Scholar]
- 13.Zinatizadeh MR, Schock B, Chalbatani GM, Zarandi PK, Jalali SA, Miri SR. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021;8((3)):287–297. doi: 10.1016/j.gendis.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Li Y, Chen C, Ma J, Sun W, Tian Z, et al. NF-κB p65 Overexpression promotes bladder cancer cell migration via FBW7-mediated degradation of RhoGDIα protein. Neoplasia. 2017;19((9)):672–683. doi: 10.1016/j.neo.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman KMW, Ali S, Aboukameel A, Sarkar SH, Wang Z, Philip PA, et al. Inactivation of NF-kappaB by 3 3'-diindolylmethane contributes to increased apoptosis induced by chemotherapeutic agent in breast cancer cells. Mol Cancer Ther. 2007;6((10)):2757–2765. doi: 10.1158/1535-7163.MCT-07-0336. [DOI] [PubMed] [Google Scholar]
- 16.Sato M, Nakanishi K, Haga S, Fujiyoshi M, Baba M, Mino K, et al. Anoikis induction and inhibition of peritoneal metastasis of pancreatic cancer cells by a nuclear factor-κB inhibitor (-)-DHMEQ. Oncol Res. 2013;21((6)):333–343. doi: 10.3727/096504014X14024160459249. [DOI] [PubMed] [Google Scholar]
- 17.Bednarski BK, Ding X, Coombe K, Baldwin AS, Kim HJ. Active roles for inhibitory kappaB kinases alpha and beta in nuclear factor-kappaB-mediated chemoresistance to doxorubicin. Mol Cancer Ther. 2008;7:1827–1835. doi: 10.1158/1535-7163.MCT-08-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I The value of histological grade in breast cancer experience from a large study with long-term follow-up. Histopathology. 1991;19((5)):403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 19.Aman NA, Doukoure B, Koffi KD, Koui BS, Traore ZC, Kouyate M, et al. Immunohistochemical evaluation of Ki-67 and comparison with clinicopathologic factors in breast carcinomas. Asian Pac J Cancer Prev. 2019;20((1)):73–79. doi: 10.31557/APJCP.2019.20.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baba M, Takahashi M, Yamashiro K, Yokoo H, Fukai M, Sato M, et al. Strong cytoplasmic expression of NF-κB/p65 correlates with a good prognosis in patients with triple-negative breast cancer. Surg Today. 2016;46((7)):843–851. doi: 10.1007/s00595-015-1265-5. [DOI] [PubMed] [Google Scholar]
- 21.Indra MC, Manginstar C, Islam AA, Sampepajung D, Hamdani W, Bukhari A, et al. The relationship between NFKB ER expression and anthracycline -based neoadjuvan chemotherapy response in local advanced stadium breast cancer a cohort study in Eastern Indonesia. Ann Med Surg. 2021;63:102164. doi: 10.1016/j.amsu.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sampepajung E, Hamdani W, Sampepajung D, Prihantono P. Overexpression of NF-kB as a predictor of neoadjuvant chemotherapy response in breast cancer. Breast Dis. 2021;40((S1)):S45–S53. doi: 10.3233/BD-219007. [DOI] [PubMed] [Google Scholar]
- 23.Engstrøm MJ, Opdahl S, Hagen AI, Romundstad PR, Akslen LA, Haugen OA, et al. Molecular subtypes histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res Treat. 2013;140((3)):463–473. doi: 10.1007/s10549-013-2647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy prognostic significance and survival. Breast. 2003;12((5)):320–327. doi: 10.1016/s0960-9776(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 25.Tannahill C, Obondo C, Al-Murri A, Doughty J, Lannigan A, Wilson C, et al. The relationship between tumour NF-kB expression hormone status and clinicopathological factors in primary invasive breast cancer. Cancer Res. 2009;69((2_Suppl)):4038. [Google Scholar]
- 26.Sarkar DK, Jana D, Patil PS, Chaudhari KS, Chattopadhyay BK, Chikkala BR, et al. Role of NF-κB as a prognostic marker in breast cancer a pilot study in Indian patients. Indian J Surg Oncol. 2013;4((3)):242–247. doi: 10.1007/s13193-013-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones RL, Rojo F, A'Hern R, Villena N, Salter J, Corominas JM, et al. Nuclear NF-κB/p65 expression and response to neoadjuvant chemotherapy in breast cancer. J Clin Pathol. 2011;64((2)):130–135. doi: 10.1136/jcp.2010.082966. [DOI] [PubMed] [Google Scholar]
- 28.Shapochka DO, Zaletok SP, Gnidyuk MI. Relationship between NF-κB p53 expression in human breast cancer. Exp Oncol. 2012;34((4)):358–363. [PubMed] [Google Scholar]
- 29.Gershtein ES, Scherbakov AM, Platova AM, Tchemeris GY, Letyagin VP, Kushlinskii NE. The expression and DNA-binding activity of NF-κB nuclear transcription factor in the tumors of patients with breast cancer. Bull Exp Biol Med. 2010;150((1)):71–74. doi: 10.1007/s10517-010-1072-3. [DOI] [PubMed] [Google Scholar]
- 30.Cavalcante RS, Ishikawa U, Silva ES, Silva-Júnior AA, Araújo AA, Cruz LJ, et al. STAT3/NF-κB signalling disruption in M2 tumour-associated macrophages is a major target of PLGA nanocarriers/PD-L1 antibody immunomodulatory therapy in breast cancer. Br J Pharmacol. 2021;178((11)):2284–2304. doi: 10.1111/bph.15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data generated or analysed during this study are included in this published article.