Abstract
Objectives
Large-cell lung carcinoma (LCLC) is generally poorly differentiated with a poor prognosis. This study aimed to explore the impact of chemotherapy on the prognosis of patients with stage II–IV LCLC and to construct nomograms to predict overall survival (OS) and cancer-specific survival (CSS).
Methods
Propensity score matching analysis was used to balance the effects of baseline characteristics. The Kaplan-Meier method was used to analyze the prognostic impact of chemotherapy on LCLC patients. Cox regression analysis was used to identify prognostic risk factors, and then nomograms were constructed and validated.
Results
Overall, we identified 2,532 patients with LCLC from the Surveillance, Epidemiology, and End Results (SEER) database. The chemotherapy group showed better OS and CSS compared to the non-/unknown chemotherapy group for stage II–IV LCLC patients (p < 0.05). Two nomograms were plotted based on the results of Cox regression analysis. The areas under the curves (AUCs) of 1-, 3-, and 5-year OS were 0.786, 0.824, and 0.837, and the AUCs of CSS were 0.785, 0.821, and 0.836, respectively. The calibration curves showed excellent agreement between the prediction and the actual observation, and the decision curve analysis demonstrated good clinical utility.
Conclusions
Chemotherapy could improve the prognosis among stage II–IV LCLC patients. In addition, the nomograms showed good predictive ability, which could be useful in making clinical decisions.
Keywords: Large-cell lung carcinoma, SEER database, Chemotherapy, Nomogram
Highlights of the Study
Chemotherapy plays an important role in the prognosis of large-cell lung carcinoma, especially for stage II–IV patients.
The propensity score matching analysis balances the influence of other baseline features.
The nomogram models we constructed have good predictive ability for survival and prognosis of stage II–IV patients.
Introduction
Non-small cell lung cancer (NSCLC) is classified into adenocarcinoma, squamous cell carcinoma, and large-cell lung carcinoma (LCLC) [1]. LCLC accounts for about 5–10% of all lung cancers, and according to the 2015 World Health Organization classification of lung cancer, lung cancers previously defined as LCLC were reclassified as adenocarcinoma and squamous cell carcinoma, resulting in a significant decrease in the incidence of LCLC [2, 3]. Nevertheless, compared with other subtypes, the mortality of LCLC is higher, and LCLC is generally poorly differentiated with a poor prognosis [4, 5]. The diagnosis of LCLC is more difficult due to the small sample size obtained by transbronchial lung biopsy and the fact that LCLC can only be diagnosed in surgically resected tumors [2, 6]. Some previous studies have shown that the prognostic factors affecting lung cancer are mainly the American Joint Committee on Cancer (AJCC) stage, surgery, tumor size, chemotherapy, and level of differentiation [7, 8].
Generally, surgery remains the standard of care for stage I lung cancer patients, and stereotactic radiotherapy is the standard for those who cannot undergo surgical resection [9]. Surgery combined with cisplatin-based adjuvant chemotherapy is the current standard of care for stage II and stage IIIA NSCLC [10], and radiotherapy combined with chemotherapy is the standard treatment for patients with stage IIIB [11]. Chemotherapy is the main treatment for patients with advanced NSCLC, with different chemotherapeutic drugs for different tumor subtypes [12].
Is it possible to predict patient survival more accurately based on known characteristics? The nomogram is a simple scoring tool widely used in the study of cancer prognosis, which can transform the prediction model into the probability of a single event (such as death or recurrence) [13]. Previous studies have reported applications of nomograms to evaluate the prognosis of various lung cancers [14, 15]. Still, few studies have shown whether patients with stage II–IV LCLC benefit from chemotherapy, and no accurate predictive nomogram for stage II–IV LCLC has been found.
This study used data from the Surveillance, Epidemiology, and End Results (SEER) database to analyze the effect of chemotherapy on the survival and prognosis of stage II–IV LCLC patients. In addition, we attempted to construct and validate novel nomogram model for patients with stage II–IV LCLC.
Methods
Data Source
Data were acquired from the SEER database (https://seer.cancer.gov/) using SEER*STAT 8.3.9 software. The included LCLC patients met the following criteria: (i) the patient's diagnoses were made between 2004 and 2015; (ii) included patients had lung tumors (C34.0–C34.9), and the following International Classification of Diseases for Oncology (ICD-O) topography codes were designated as LCLC: “8012.3” (large-cell carcinoma, NOS) and “8014.3” (large-cell carcinoma with rhabdoid phenotype); and (iii) all patients had primary LCLC and no other tumors. Exclusion criteria were as follows: (i) clinicopathological characteristics of the patient (race, marital status, grade, laterality, surgery, staging information, tumor size, etc.) were missing or unknown; (ii) the survival time was less than 1 month or was unknown; and (iii) the information regarding causes of death was unclear. The detailed process of selection is shown in online supplementary Figure 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000529202).
The sample sizes for lung cancer and LCLC were 461,905 and 8,907, respectively. LCLC sample size is approximately 1.928% of lung cancer. The optimal threshold for tumor size classification was determined by X-tile software (Yale University, New Haven, CT, USA) [16], and then the tumor size was stratified into three levels of ≤3.5 cm, 3.6–6.5 cm, and ≥6.6 cm (online suppl. Fig. 2). Overall survival (OS) was defined as the time from the date of diagnosis until the date of death from any cause, and cancer-specific survival (CSS) was defined as the duration from the date of diagnosis to the date of death caused by cancer.
Statistical Analyses
Differences between groups for categorical and continuous variables were analyzed by χ2 test, Fisher's exact tests, and Mann-Whitney U test, respectively. The LCLC patients were divided into the chemotherapy group and the non-/unknown chemotherapy group, and the propensity score matching (PSM) analysis was used to reduce the bias between the two groups. The Kaplan-Meier analysis demonstrated whether chemotherapy could affect survival outcomes. Among stage II–IV LCLC patients, 70% of them were randomly selected as the training set and the rest as the validation set. In the training set, univariable and multivariable Cox regression analyses were used to determine the independent prognostic factors of OS and CSS. Afterward, the factors that were identified from the multivariable Cox regression analysis were selected to construct nomograms. In the validation set, the model was evaluated by receiver operating characteristic curve, calibration curves, and decision curve analysis. The categorical variables that were significant for univariable analysis were selected as subgroup variables, and the results of subgroup analysis were presented using the forest plot method. All p values were two-sided, and p < 0.05 was considered statistically significant. All analyses were performed using R software (version 4.1.2) and X-tile software (version 3.6.1).
Results
Baseline Characteristics of Patients
Of the 2,532 patients with LCLC in the SEER database inducted into our study between 2004 and 2015, 1,202 (47.5%) were in the chemotherapy group and 1,330 (52.5%) were in the non-/unknown chemotherapy group. After PSM analysis, 1,456 patients were successfully matched; no statistically significant differences were found in the baseline characteristics between the two groups. The demographics and clinicopathological characteristics before and after PSM are displayed in Table 1.
Table 1.
Description of baseline characteristics of patients with LCLC
| Variable | Cases (N = 2532), n (%) | Data before PSM (chemotherapy) |
Data after PSM (chemotherapy) |
||||
| no/unknown (N = 1,330), n (%) | yes (N = 1,202), n (%) | p value | no/unknown (N = 728), n (%) | yes (N = 728), n (%) | p value | ||
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Female | 1,062 (41.9) | 565 (42.5) | 497 (41.3) | 0.591 | 291 (40.0) | 300 (41.2) | 0.669 |
| Male | 1,470 (58.1) | 765 (57.5) | 705 (58.7) | 437 (60.0) | 428 (58.8) | ||
| Year of diagnosis | |||||||
| 2004–2009 | 1,874 (74.0) | 1,004 (75.5) | 870 (72.4) | 0.083 | 544 (74.7) | 522 (71.7) | 0.214 |
| 2010–2015 | 658 (26.0) | 326 (24.5) | 332 (27.6) | 184 (25.3) | 206 (28.3) | ||
| Race | |||||||
| White | 2,048 (80.9) | 1,085 (81.6) | 963 (80.1) | 0.059 | 586 (80.5) | 587 (80.6) | 0.997 |
| Black | 353 (13.9) | 168 (12.6) | 185 (15.4) | 107 (14.7) | 106 (14.6) | ||
| Other | 131 (5.2) | 77 (5.8) | 54 (4.5) | 35 (4.8) | 35 (4.8) | ||
| Marital status | |||||||
| Single | 338 (13.3) | 175 (13.2) | 163 (13.6) | <0.001 | 94 (12.9) | 93 (12.8) | 0.798 |
| Married | 1,381 (54.5) | 673 (50.6) | 708 (58.9) | 404 (55.5) | 416 (57.1) | ||
| Other | 813 (32.1) | 482 (36.2) | 331 (27.5) | 230 (31.6) | 219 (30.1) | ||
| Grade | |||||||
| I | 9 (0.4) | 6 (0.5) | 3 (0.2) | 0.473 | 3 (0.4) | 2 (0.3) | 0.265 |
| II | 35 (1.4) | 20 (1.5) | 15 (1.2) | 4 (0.5) | 8 (1.1) | ||
| III | 1,220 (48.2) | 624 (46.9) | 596 (49.6) | 337 (46.3) | 364 (50.0) | ||
| IV | 1,268 (50.1) | 680 (51.1) | 588 (48.9) | 384 (52.7) | 354 (48.6) | ||
| Laterality | |||||||
| Left | 1,021 (40.3) | 542 (40.8) | 479 (39.9) | 0.674 | 290 (39.8) | 296 (40.7) | 0.789 |
| Right | 1,511 (59.7) | 788 (59.2) | 723 (60.1) | 438 (60.2) | 432 (59.3) | ||
| Stage | |||||||
| I | 681 (26.9) | 536 (40.3) | 145 (12.1) | <0.001 | 120 (16.5) | 135 (18.5) | 0.735 |
| II | 218 (8.6) | 109 (8.2) | 109 (9.1) | 75 (10.3) | 73 (10.0) | ||
| III | 686 (27.1) | 247 (18.6) | 439 (36.5) | 204 (28.0) | 192 (26.4) | ||
| IV | 947 (37.4) | 438 (32.9) | 509 (42.3) | 329 (45.2) | 328 (45.1) | ||
| T | |||||||
| T1 | 484 (19.1) | 332 (25.0) | 152 (12.6) | <0.001 | 104 (14.3) | 115 (15.8) | 0.832 |
| T2 | 1,054 (41.6) | 565 (42.5) | 489 (40.7) | 297 (40.8) | 300 (41.2) | ||
| T3 | 235 (9.3) | 100 (7.5) | 135 (11.2) | 76 (10.4) | 73 (10.0) | ||
| T4 | 759 (30.0) | 333 (25.0) | 426 (35.4) | 251 (34.5) | 240 (33.0) | ||
| N | |||||||
| N0 | 1,125 (44.4) | 758 (57.0) | 367 (30.5) | <0.001 | 270 (37.1) | 287 (39.4) | 0.829 |
| N1 | 263 (10.4) | 119 (8.9) | 144 (12.0) | 91 (12.5) | 87 (12.0) | ||
| N2 | 886 (35.0) | 359 (27.0) | 527 (43.8) | 289 (39.7) | 281 (38.6) | ||
| N3 | 258 (10.2) | 94 (7.1) | 164 (13.6) | 78 (10.7) | 73 (10.0) | ||
| M | |||||||
| M0 | 1,585 (62.6) | 892 (67.1) | 693 (57.7) | <0.001 | 399 (54.8) | 400 (54.9) | 1.000 |
| M1 | 947 (37.4) | 438 (32.9) | 509 (42.3) | 329 (45.2) | 328 (45.1) | ||
| Surgery | |||||||
| No | 1,529 (60.4) | 670 (50.4) | 859 (71.5) | <0.001 | 491 (67.4) | 475 (65.2) | 0.405 |
| Yes | 1,003 (39.6) | 660 (49.6) | 343 (28.5) | 237 (32.6) | 253 (34.8) | ||
| Radiation | |||||||
| None/unknown | 1,383 (54.6) | 939 (70.6) | 444 (36.9) | <0.001 | 386 (53.0) | 385 (52.9) | 1.000 |
| Yes | 1,149 (45.4) | 391 (29.4) | 758 (63.1) | 342 (47.0) | 343 (47.1) | ||
| Tumor size, cm | |||||||
| ≤3.5 | 959 (37.9) | 592 (44.5) | 367 (30.5) | <0.001 | 241 (33.1) | 250 (34.3) | 0.629 |
| [3.6, 6.5] | 944 (37.3) | 450 (33.8) | 494 (41.1) | 290 (39.8) | 297 (40.8) | ||
| ≥6.6 | 629 (24.8) | 288 (21.7) | 341 (28.4) | 197 (27.1) | 181 (24.9) | ||
| Age (Q1, Q3) | 67 (59, 75) | 70 (62, 78) | 64 (56, 71) | <0.001 | 67 (59, 75) | 66.5 (59, 73) | 0.189 |
PSM, propensity score matching; LCLC, large-cell lung carcinoma.
Survival Analysis of Chemotherapy and Non-/Unknown Chemotherapy Groups
Before PSM, no statistically significant differences were observed in OS (p = 0.36, Fig. 1a) and CSS (p = 0.084, Fig. 1c) between chemotherapy and non-/unknown chemotherapy groups. After PSM, the patients on chemotherapy had a significantly better OS compared with the non-/unknown chemotherapy patients (5-year survival rate of 18.2% vs. 12.0%, p < 0.001, Fig. 1b). The results demonstrated that CSS of patients was better in the chemotherapy group (5-year survival rate of 21.8% vs. 16.0%, p < 0.001, Fig. 1d). Chemotherapy had no significant effects on OS and CSS in patients with stage I LCLC before and after PSM (p > 0.05, online suppl. Fig. 3). However, among the stage II–IV patients, there were statistically significant differences between the two groups (p < 0.05, online suppl. Fig. 3).
Fig. 1.
a-d Kaplan-Meier survival curves of OS and CSS. OS, overall survival; CSS, cancer-specific survival; PSM, propensity score matching.
Prognostic Factors of OS and CSS in Patients with Stage II–IV LCLC
The stage II–IV patients were divided in a proportion of 7:3 into the training set (N = 1,296) and the validation set (N = 555). Univariable and multivariable Cox analysis were first performed in the training set. In the univariable analysis, factors including age, T status, N status, M status, surgery, tumor size, and chemotherapy (p < 0.05) were associated with OS in stage II–IV LCLC patients. Subsequently, the multivariable analysis indicated that age, M status, surgery, T status, tumor size, and chemotherapy were significant independent predictors of OS (Table 2). When analyzing CSS, age, T status, N status, M status, surgery, tumor size, and chemotherapy (all p < 0.05) were included in the multivariable Cox regression model. In the multivariable Cox regression model, age, M status, chemotherapy, T status, tumor size, and surgery were independent prognostic factors to improve CSS in stage II–IV patients (Table 3).
Table 2.
Univariable and multivariable Cox regression model analysis of OS
| Variable | Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Gender | ||||||
| Female | Reference | |||||
| Male | 1.103 | 0.982–1.238 | 0.097 | |||
| Year of diagnosis | ||||||
| 2004–2009 | Reference | |||||
| 2010–2015 | 0.958 | 0.841–1.092 | 0.524 | |||
| Race | ||||||
| White | Reference | |||||
| Black | 0.924 | 0.786–1.085 | 0.335 | |||
| Other | 0.837 | 0.656–1.069 | 0.154 | |||
| Marital status | ||||||
| Single | Reference | |||||
| Married | 0.987 | 0.834–1.168 | 0.878 | |||
| Other | 1.198 | 1.000–1.436 | 0.050 | |||
| Grade | ||||||
| I | Reference | |||||
| II | 1.042 | 0.228–4.761 | 0.958 | |||
| III | 1.507 | 0.375–6.053 | 0.563 | |||
| IV | 1.621 | 0.404–6.511 | 0.496 | |||
| Laterality | ||||||
| Left | Reference | |||||
| Right | 0.914 | 0.815–1.026 | 0.128 | |||
| T | ||||||
| T1 | Reference | Reference | ||||
| T2 | 1.343 | 1.106–1.631 | 0.003 | 1.147 | 0.910–1.444 | 0.245 |
| T3 | 1.104 | 0.870–1.402 | 0.417 | 1.062 | 0.805–1.400 | 0.671 |
| T4 | 1.806 | 1.492–2.186 | <0.001 | 1.322 | 1.055–1.656 | 0.015 |
| N | ||||||
| N0 | Reference | Reference | ||||
| N1 | 0.689 | 0.570–0.834 | <0.001 | 0.944 | 0.772–1.154 | 0.574 |
| N2 | 1.060 | 0.918–1.223 | 0.429 | 1.114 | 0.959–1.294 | 0.159 |
| N3 | 1.045 | 0.859–1.271 | 0.661 | 0.907 | 0.741–1.111 | 0.347 |
| M | ||||||
| M0 | Reference | Reference | ||||
| M1 | 1.987 | 1.770–2.230 | <0.001 | 1.631 | 1.440–1.847 | <0.001 |
| Surgery | ||||||
| No | Reference | Reference | ||||
| Yes | 0.436 | 0.380–0.501 | <0.001 | 0.528 | 0.450–0.619 | <0.001 |
| Radiation | ||||||
| None/unknown | Reference | |||||
| Yes | 0.966 | 0.862–1.082 | 0.546 | |||
| Chemotherapy | ||||||
| No/unknown | Reference | Reference | ||||
| Yes | 0.589 | 0.526–0.661 | <0.001 | 0.574 | 0.509–0.647 | <0.001 |
| Tumor size, cm | ||||||
| ≤3.5 | Reference | Reference | ||||
| [3.6, 6.5] | 1.205 | 1.050–1.383 | 0.008 | 1.064 | 0.903–1.254 | 0.457 |
| ≥6.6 | 1.464 | 1.264–1.696 | <0.001 | 1.318 | 1.111–1.563 | 0.002 |
| Age | 1.017 | 1.012–1.023 | <0.001 | 1.011 | 1.005–1.016 | <0.001 |
HR, hazard ratio; CI, confidence interval; OS, overall survival.
Table 3.
Univariable and multivariable Cox regression model analysis of CSS
| Variable | Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Gender | ||||||
| Female | Reference | |||||
| Male | 1.064 | 0.944–1.199 | 0.312 | |||
| Year of diagnosis | ||||||
| 2004–2009 | Reference | |||||
| 2010–2015 | 0.973 | 0.851–1.114 | 0.694 | |||
| Race | ||||||
| White | Reference | |||||
| Black | 0.933 | 0.790–1.103 | 0.417 | |||
| Other | 0.845 | 0.655–1.089 | 0.192 | |||
| Marital status | ||||||
| Single | Reference | |||||
| Married | 0.954 | 0.802–1.135 | 0.594 | |||
| Other | 1.172 | 0.973–1.412 | 0.094 | |||
| Grade | ||||||
| I | Reference | |||||
| II | 0.938 | 0.205–4.285 | 0.934 | |||
| III | 1.230 | 0.306–4.936 | 0.771 | |||
| IV | 1.365 | 0.340–5.480 | 0.661 | |||
| Laterality | ||||||
| Left | Reference | |||||
| Right | 0.933 | 0.827–1.052 | 0.256 | |||
| T | ||||||
| T1 | Reference | Reference | ||||
| T2 | 1.321 | 1.078–1.619 | 0.007 | 1.105 | 0.867–1.407 | 0.421 |
| T3 | 1.132 | 0.884–1.451 | 0.325 | 1.073 | 0.805–1.430 | 0.631 |
| T4 | 1.805 | 1.479–2.204 | <0.001 | 1.281 | 1.012–1.623 | 0.040 |
| N | ||||||
| N0 | Reference | Reference | ||||
| N1 | 0.657 | 0.537–0.804 | <0.001 | 0.939 | 0.759–1.161 | 0.560 |
| N2 | 1.061 | 0.914–1.231 | 0.440 | 1.130 | 0.968–1.321 | 0.123 |
| N3 | 1.106 | 0.906–1.351 | 0.322 | 0.968 | 0.787–1.190 | 0.758 |
| M | ||||||
| M0 | Reference | Reference | ||||
| M1 | 2.127 | 1.886–2.399 | <0.001 | 1.727 | 1.517–1.965 | <0.001 |
| Surgery | ||||||
| No | Reference | Reference | ||||
| Yes | 0.412 | 0.356–0.478 | <0.001 | 0.515 | 0.436–0.610 | <0.001 |
| Radiation | ||||||
| None/unknown | Reference | |||||
| Yes | 1.005 | 0.893–1.131 | 0.931 | |||
| Chemotherapy | ||||||
| No/unknown | Reference | Reference | ||||
| Yes | 0.593 | 0.527–0.668 | <0.001 | 0.568 | 0.502–0.643 | <0.001 |
| Tumor size (cm) | ||||||
| ≤3.5 | Reference | Reference | ||||
| [3.6, 6.5] | 1.229 | 1.064–1.419 | 0.005 | 1.093 | 0.921–1.298 | 0.309 |
| ≥6.6 | 1.506 | 1.293–1.755 | <0.001 | 1.359 | 1.137–1.625 | <0.001 |
| Age | 1.014 | 1.009–1.020 | <0.001 | 1.008 | 1.003–1.014 | 0.004 |
HR, hazard ratio; CI, confidence interval; CSS, cancer-specific survival.
Establishment and Validation of Nomograms for OS and CSS
Two nomograms were constructed based on prognostic factors derived from Cox regression (Fig. 2a, b). For a given LCLC patient, in the nomogram model, each variable corresponded to a score, and all variable scores were then summed to obtain a total score, which corresponds vertically to the following OS and CSS to obtain the 1-, 3-, and 5-year OS and CSS probabilities for the patient (online suppl. Table 1).
Fig. 2.
Nomogram for predicting 1-, 3-, and 5-year OS (a) and CSS (b) for stage II–IV LCLC, and the ROC curve of validation cohort for predicting 1-, 3-, and 5-year OS (c) and CSS (d). OS, overall survival; CSS, cancer-specific survival; LCLC, large-cell lung carcinoma; ROC, receiver operating characteristic. *p <0.05, **p <0.01, ***p <0.001.
The areas under the curves (AUCs) of 1-, 3-, and 5-year OS (Fig. 2c) were 0.786, 0.824, and 0.837, respectively, and the AUCs of 1-, 3-, and 5-year CSS (Fig. 2d) were 0.785, 0.821, and 0.836, respectively. They were all greater compared to the AUC values of the TNM staging system (online suppl. Fig. 4), indicating that nomograms had better discriminatory ability. Calibration curves of the 1-, 3-, and 5-year OS (online suppl. Fig. 5A–C) and CSS (online suppl. Fig. 5D–F) nomograms were close to the 45° standard line, indicating good consistency between the predicted and actual probabilities. In addition, decision curve analysis showed better positive net benefits in the predictive model at 1-, 3-, and 5-year OS (online suppl. Fig. 5G–I) and CSS (online suppl. Fig. 5J–L) than TNM staging model, demonstrating the favorable potential clinical effect of the predictive model.
Subgroup Analysis
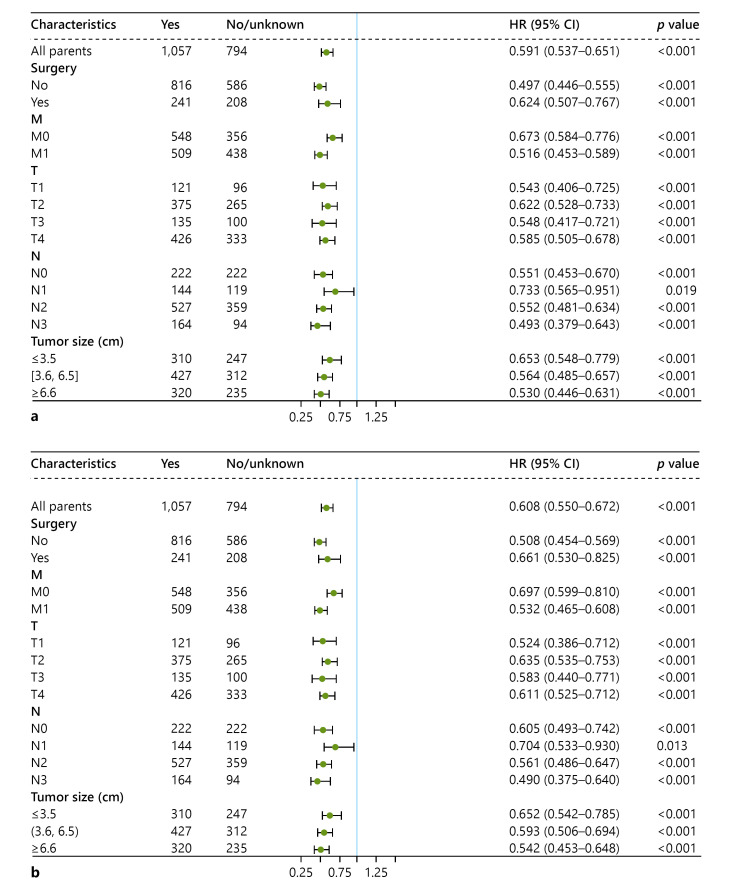
Our results showed that in all subgroups, chemotherapy had better effect on OS and CSS in patients with stage II–IV LCLC (all p < 0.05). See Figure 3 for more details. Surgery combined with chemotherapy (Fig. 4a, b) had a better survival benefit compared to surgery alone (all p < 0.05). Patients who received chemoradiotherapy (Fig. 4c, d) survived better than patients treated with radiotherapy (all p < 0.05). Surgery combined with chemoradiotherapy (Fig. 4e, f) resulted in better OS and CSS compared to surgery combined with radiotherapy (all p < 0.05).
Fig. 3.
Subgroup analysis of OS (a) and CSS (b). OS, overall survival; CSS, cancer-specific survival.
Fig. 4.
Survival analysis of chemotherapy in specific populations. a, b OS and CSS for the surgical population. c, d OS and CSS for the radiotherapy population. e, f OS and CSS for the surgical plus radiotherapy population. OS, overall survival; CSS, cancer-specific survival.
Discussion
LCLC is a highly metastatic malignant tumor [17]. To our knowledge, few studies have explored the effects of treatment methods, especially the effect of chemotherapy, on the survival and prognosis of LCLC. We observed that, after PSM, chemotherapy provided good survival benefits for LCLC patients. Bi et al. [18] reported that chemotherapy was the only predictor associated with prognosis in patients with LCLC, significantly improving patient survival. However, the sample size in this study was relatively small, and was thus less representative. One of our important findings was that patients with stage II–IV LCLC who received chemotherapy had good survival, while the efficacy of chemotherapy was not significant for stage I patients; this finding ought to help clinicians to better use chemotherapy treatment modalities. There was still no certain conclusion as to whether chemotherapy was beneficial for stage I LCLC patients. Hu et al. [19] found that patients with stage IA LCLC did not benefit from postoperative chemotherapy for OS, whereas in patients with stages IB and II, postoperative chemotherapy patients had better OS than those who had surgery alone. On the contrary, some previous studies indicated that chemotherapy was beneficial to the survival of stage I lung cancer patients [20, 21].
The results of our Cox regression analysis suggest that age may be an important independent prognostic influence in patients with stage II–IV LCLC. A study of the clinical characteristics of LCNEC showed that age was an unfavorable prognostic factor and that survival rates were lower in older adults than in younger adults [22]. This was probably because young patients compared with elderly patients have better physical fitness, stronger immunity [23], and fewer complications after receiving treatment [24].
A few significant aspects of this study should be highlighted. First, our study on a large sample size is more representative and can provide some reference for clinicians to make decisions. We found that surgery combined with chemoradiotherapy had a much higher survival rate compared to surgery combined with radiotherapy. Second, as far as we know, this is the first study to develop two nomogram models to predict the survival rate of patients with stage II–IV LCLC. While previous studies have reported the development of nomograms for predicting OS of LCLC [25] and the prognosis of LCNEC [26], our nomogram models have some advantages. Our predictive model facilitates individualized prognostic assessment and provides an appropriate theoretical basis for clinical decision-making in evaluating patients. To improve survival of patients, it enables the monitoring of high-risk populations.
However, there are several limitations to this study that need to be considered. First, the exact details of the chemotherapy regimen were unknown, and the use of different chemotherapeutic agents had different efficacy. Second, the study of the SEER database data was retrospective, which may have led to some selection bias. The SEER database lacks data on body shape, tumor complexity, disease recurrence, and other factors, which may affect the choice of treatment strategy. Third, hospital characteristics such as hospital facilities [27] and number of hospital procedures [28] may affect survival and prognosis of patients with lung cancer. Furthermore, some variables related to prognosis, such as smoking history, targeted therapy, and imaging indicators, were not included. Finally, although we used a validation set to evaluate the performance of the model, external data are still preferable for further validation.
Conclusions
This study showed that the prognosis of patients with stage II–IV LCLC was closely related to chemotherapy. In addition, we found that age, surgery, chemotherapy, and tumor size were important factors affecting the prognosis of LCLC patients, and our nomogram model demonstrated better prediction performance than the TNM staging system. Finally, we found that chemotherapy had better efficacy in all specific subgroups of the population.
Statement of Ethics
This study did not require informed consent, as the data were extracted from public databases and the information is anonymous.
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Funding Sources
No funding was received for this study.
Author Contributions
Guoyan Hu conceived this study and participated in writing the manuscript. Guoyan Hu, Yane Liu, and Xinwei Li assisted in drafting the manuscript. Guoyan Hu, Mengdi Jin, and Xiangyu Liu collected data and analyzed the data. Qiong Yu directed the design of the project and reviewed the manuscript. All authors discussed the results and approved the final manuscript.
Data Availability Statement
The data were obtained from an open database, the SEER database.
Supplementary Material
Supplementary data
Funding Statement
No funding was received for this study.
References
- 1.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers a heterogeneous set of diseases. Nat Rev Cancer. 2014 Aug;14((8)):535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015 Sep;10((9)):1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019 Aug;94((8)):1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Saeed AM, Toonkel R, Glassberg MK, Nguyen D, Hu JJ, Zimmers TA, et al. The influence of Hispanic ethnicity on nonsmall cell lung cancer histology and patient survival an analysis of the Survival, Epidemiology, and End Results database. Cancer. 2012 Sep 15;118((18)):4495–4501. doi: 10.1002/cncr.26686. [DOI] [PubMed] [Google Scholar]
- 5.Rekhtman N, Travis WD. Large no more the journey of pulmonary large cell carcinoma from common to rare entity. J Thorac Oncol. 2019 Jul;14((7)):1125–1127. doi: 10.1016/j.jtho.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Hanagiri T, Oka S, Takenaka S, Baba T, Yasuda M, Ono K, et al. Results of surgical resection for patients with large cell carcinoma of the lung. Int J Surg. 2010;8((5)):391–394. doi: 10.1016/j.ijsu.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Chen W, Li C, Qi S, Zhou X, Zhang Y, et al. Clinicopathological characteristics and prediction of cancer-specific survival in large cell lung cancer a population-based study. J Thorac Dis. 2020 May;12((5)):2261–2269. doi: 10.21037/jtd.2020.04.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Ge L, Zhang G, Wang Z, Liu Y, Ren Y. Clinical characteristics and survival outcomes of patients with pneumonectomies a population-based study. Front Surg. 2022;9:948026. doi: 10.3389/fsurg.2022.948026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller M, Hanna N. Advances in systemic therapy for non-small cell lung cancer. BMJ. 2021 Nov 9;375:n2363. doi: 10.1136/bmj.n2363. [DOI] [PubMed] [Google Scholar]
- 10.Szeto CH, Shalata W, Yakobson A, Agbarya A. Neoadjuvant and adjuvant immunotherapy in early-stage non-small-cell lung cancer and future. J Clin Med. 2021 Nov 29;10((23)):5614. doi: 10.3390/jcm10235614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glatzer M, Elicin O, Ramella S, Nestle U, Putora PM. Radio(chemo)therapy in locally advanced nonsmall cell lung cancer. Eur Respir Rev. 2016 Mar;25((139)):65–70. doi: 10.1183/16000617.0053-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, et al. Non-small-cell lung cancer. Lancet. 2011 Nov 12;378((9804)):1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 13.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008 Mar 10;26((8)):1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 14.You H, Teng M, Gao CX, Yang B, Hu S, Wang T, et al. Construction of a nomogram for predicting survival in elderly patients with lung adenocarcinoma a retrospective cohort study. Front Med. 2021;8:680679. doi: 10.3389/fmed.2021.680679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin G, Qi K, Liu B, Liu H, Li J. A nomogram prognostic model for large cell lung cancer analysis from the Surveillance, Epidemiology and End Results Database. Transl Lung Cancer Res. 2021 Feb;10((2)):622–635. doi: 10.21037/tlcr-19-517b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camp RL, Dolled-Filhart M, Rimm DL. X-tile a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004 Nov 1;10((21)):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 17.Liang R, Chen TX, Wang ZQ, Jin KW, Zhang LY, Yan QN, et al. A retrospective analysis of the clinicopathological characteristics of large cell carcinoma of the lung. Exp Ther Med. 2015 Jan;9((1)):197–202. doi: 10.3892/etm.2014.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi Y, Qu Y, Liang Z, Liu Z, Zhang H, Liang X, et al. Clinicopathological analysis of large cell lung carcinomas definitely diagnosed according to the new World Health Organization criteria. Pathol Res Pract. 2018 Apr;214((4)):555–559. doi: 10.1016/j.prp.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Tai Q, Su B, Zhang L. Adjuvant chemotherapy improves the prognosis of early stage resectable pulmonary large cell carcinoma analysis of SEER data. Ann Palliat Med. 2020 Mar;9((2)):199–206. doi: 10.21037/apm.2020.02.10. [DOI] [PubMed] [Google Scholar]
- 20.Tu Z, Tian T, Chen Q, Li C. Overall survival analyses following adjuvant chemotherapy or nonadjuvant chemotherapy in patients with stage IB non-small-cell lung cancer. J Oncol. 2021;2021:8052752. doi: 10.1155/2021/8052752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreetti C, Ibrahim M, Gagliardi A, Poggi C, Maurizi G, Armillotta D, et al. Adjuvant chemotherapy extent of resection and immunoistochemical neuroendocrine markers as prognostic factors of early-stage large-cell neuroendocrine carcinoma. Thorac Cancer. 2022 Apr;13((7)):900–912. doi: 10.1111/1759-7714.14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao L, Li ZW, Wang M, Zhang TT, Bao B, Liu YP. Clinicopathological characteristics treatment and survival of pulmonary large cell neuroendocrine carcinoma a SEER population-based study. PeerJ. 2019;7:e6539. doi: 10.7717/peerj.6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd CM, Marsland BJ. Lung homeostasis influence of age, microbes, and the immune system. Immunity. 2017 Apr 18;46((4)):549–561. doi: 10.1016/j.immuni.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Cañizares Carretero M-Á, García Fontán E-M, Blanco Ramos M, Soro García J, Carrasco Rodríguez R, Peña González E, et al. Is age a predisposing factor of postoperative complications after lung resection for primary pulmonary neoplasms? Cir Esp. 2017;95((3)):160–166. doi: 10.1016/j.ciresp.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Tai QD, Zhang L, Hu XF. Clinical characteristics and treatments of large cell lung carcinoma a retrospective study using SEER data. Transl Cancer Res. 2020 Mar;9((3)):1455–1464. doi: 10.21037/tcr.2020.01.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia L, Wang L, Zhou Z, Han S. Treatment outcome and prognostic analysis of advanced large cell neuroendocrine carcinoma of the lung. Sci Rep. 2022 Oct 4;12((1)):16562. doi: 10.1038/s41598-022-18421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kommalapati A, Tella SH, Appiah AK, Smith L, Ganti AK. Association between treatment facility volume therapy types and overall survival in patients with stage IIIA non-small cell lung cancer. J Natl Compr Canc Netw. 2019 Mar 1;17((3)):229–236. doi: 10.6004/jnccn.2018.7086. [DOI] [PubMed] [Google Scholar]
- 28.Evers J, de Jaeger K, Hendriks LEL, van der Sangen M, Terhaard C, Siesling S, et al. Trends and variations in treatment of stage I–III non-small cell lung cancer from 2008 to 2018 a nationwide population-based study from The Netherlands. Lung Cancer. 2021 May;155:103–13. doi: 10.1016/j.lungcan.2021.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The data were obtained from an open database, the SEER database.